The Effects of Iridin and Irigenin on Cancer: Comparison with Well-Known Isoflavones in Breast, Prostate, and Gastric Cancers
Abstract
1. Introduction
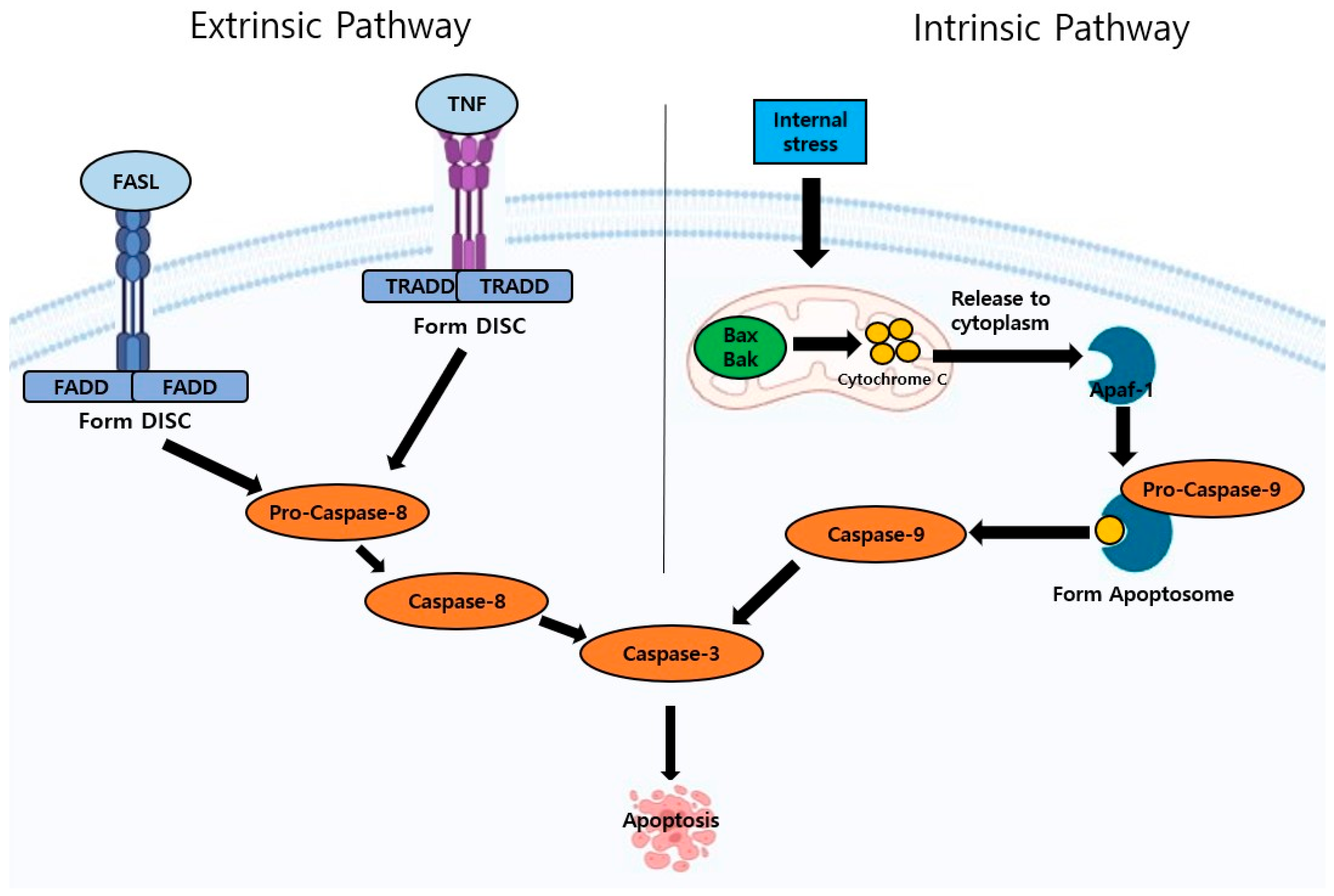
2. Apoptosis Pathway
3. Various Signaling Pathways That Are Involved in Apoptosis in Cancer
4. Anti-Cancer Effects of Various Isoflavones
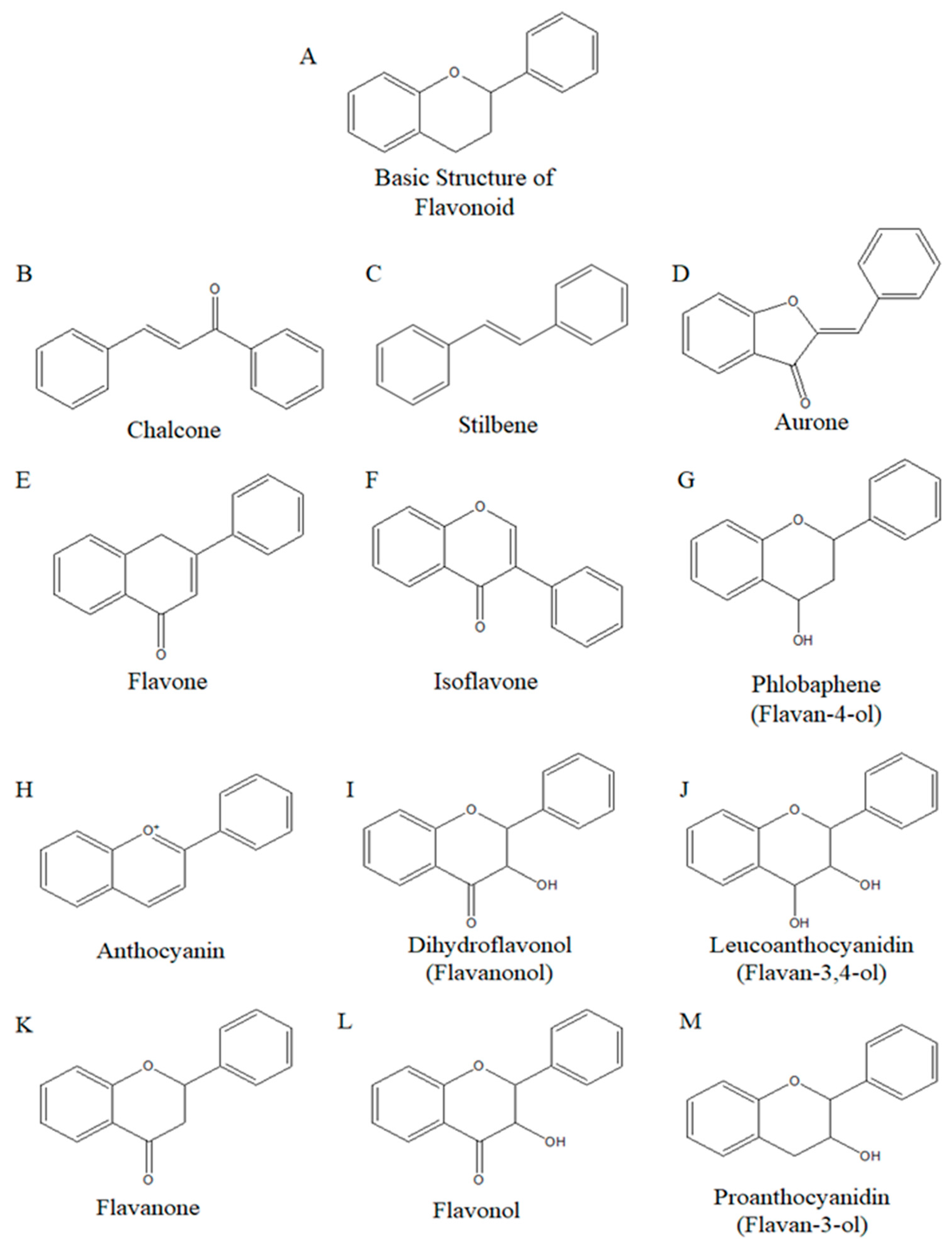
4.1. Isoflavones Structure and Their Role in Human Health
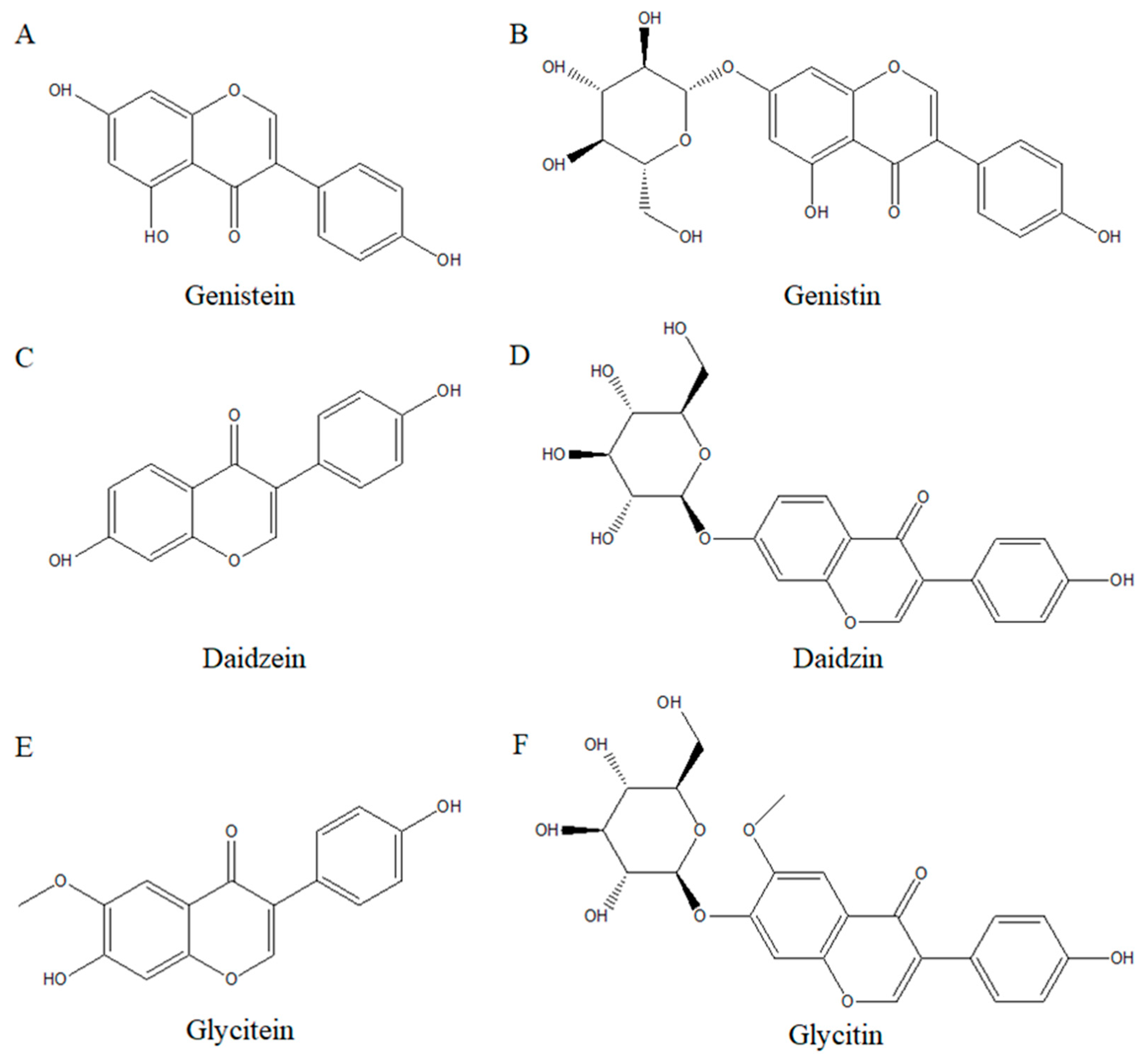
4.2. Various Isoflavones and Their Effects on Anti-Cancer
4.2.1. Genistein and Its Effects on Breast, Prostate, and Gastric Cancers
4.2.2. Daidzein and Its Effects on Breast, Prostate, and Gastric Cancers
4.2.3. Glycitein and Its Effects on Breast, Prostate, and Gastric Cancers
4.2.4. Irigenin/Iridin and Its Effects on Breast, Prostate, and Gastric Cancers
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hosseinzadeh, E.; Hassanzadeh, A.; Marofi, F.; Alivand, M.R.; Solali, S. Flavonoid-based cancer therapy: An updated review. Anti Cancer Agents Med. Chem. 2020, 20, 1398–1414. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global cancer incidence and mortality rates and trends—An update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Vineis, P.; Wild, C.P. Global cancer patterns: Causes and prevention. Lancet 2014, 383, 549–557. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Morana, O.; Wood, W.; Gregory, C.D. The apoptosis paradox in cancer. Int. J. Mol. Sci. 2022, 23, 1328. [Google Scholar] [CrossRef]
- Carneiro, B.A.; El-Deiry, W.S. Targeting apoptosis in cancer therapy. Nat. Rev. Clin. Oncol. 2020, 17, 395–417. [Google Scholar] [CrossRef]
- Jan, R. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205. [Google Scholar] [CrossRef]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar]
- Hazafa, A.; Rehman, K.-U.; Jahan, N.; Jabeen, Z. The role of polyphenol (flavonoids) compounds in the treatment of cancer cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef]
- Hsiao, Y.-H.; Ho, C.-T.; Pan, M.-H. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. J. Funct. Foods 2020, 74, 104164. [Google Scholar] [CrossRef]
- Pejčić, T.; Zeković, M.; Bumbaširević, U.; Kalaba, M.; Vovk, I.; Bensa, M.; Popović, L.; Tešić, Ž. The role of isoflavones in the prevention of breast cancer and prostate cancer. Antioxidants 2023, 12, 368. [Google Scholar] [CrossRef] [PubMed]
- Nakai, S.; Fujita, M.; Kamei, Y. Health promotion effects of soy isoflavones. J. Nutr. Sci. Vitaminol. 2020, 66, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhang, Y.J.; Zhu, G.Y.; Shi, X.C.; Chen, X.; Herrera-Balandrano, D.D.; Liu, F.Q.; Laborda, P. Occurrence of isoflavones in soybean sprouts and strategies to enhance their content: A review. J. Food Sci. 2022, 87, 1961–1982. [Google Scholar] [CrossRef]
- Nabi, R.; Alvi, S.S.; Shah, M.S.; Ahmad, S.; Faisal, M.; Alatar, A.A.; Khan, M.S. A biochemical & biophysical study on in-vitro anti-glycating potential of iridin against D-Ribose modified BSA. Arch. Biochem. Biophys. 2020, 686, 108373. [Google Scholar]
- Bhosale, P.-B.; Vetrivel, P.; Ha, S.-E.; Kim, H.-H.; Heo, J.-D.; Won, C.-K.; Kim, S.-M.; Kim, G.-S. Iridin induces G2/M phase cell cycle arrest and extrinsic apoptotic cell death through PI3K/AKT signaling pathway in AGS gastric cancer cells. Molecules 2021, 26, 2802. [Google Scholar] [CrossRef]
- Ying, Z.-H.; Li, H.-M.; Yu, W.-Y.; Yu, C.-H. Iridin prevented against lipopolysaccharide-induced inflammatory responses of macrophages via inactivation of PKM2-mediated glycolytic pathways. J. Inflamm. Res. 2021, 14, 341–354. [Google Scholar] [CrossRef]
- Patel, D.K. Biological activities and therapeutic potential of irigenin on gastric, lung, prostate, breast, and endometrial cancer: Pharmacological and analytical aspects. Curr. Cancer Ther. Rev. 2022, 18, 172–180. [Google Scholar] [CrossRef]
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384. [Google Scholar] [CrossRef]
- Opferman, J.T.; Kothari, A. Anti-apoptotic BCL-2 family members in development. Cell Death Differ. 2018, 25, 37–45. [Google Scholar] [CrossRef]
- Peña-Blanco, A.; García-Sáez, A.J. Bax, Bak and beyond—Mitochondrial performance in apoptosis. FEBS J. 2018, 285, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Huang, Q.; Xie, L.; Qian, Z.; Yang, H.; Shi, X.; Huang, Z. Different effects of the chemically similar foodborne flavonoids genistein, genistin, and daidzein on the inhibition of proliferation and induction of apoptosis in U251 glioma cells. Res. Sq. 2022, preprint. [Google Scholar]
- Han, Y.-H.; Wang, Y.; Lee, S.-J.; Jin, M.-H.; Sun, H.-N.; Kwon, T. Regulation of anoikis by extrinsic death receptor pathways. Cell Commun. Signal. 2023, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Panda, D.; Ray, D.; Behera, D.; Tripathy, D.; Bhanja, D.; Sangeeta, S.; Acharya, D. A review on apoptosis: When death precedes life. Eur. J. Mol. Clin. Med. 2020, 7, 1174–1182. [Google Scholar]
- Glaviano, A.; Foo, A.S.; Lam, H.Y.; Yap, K.C.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 138. [Google Scholar] [CrossRef]
- Uko, N.E.; Güner, O.F.; Matesic, D.F.; Bowen, J.P. Akt pathway inhibitors. Curr. Top. Med. Chem. 2020, 20, 883–900. [Google Scholar] [CrossRef]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT pathway as a target for cancer treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Li, H.; Prever, L.; Hirsch, E.; Gulluni, F. Targeting PI3K/AKT/mTOR signaling pathway in breast cancer. Cancers 2021, 13, 3517. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.-Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.-F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Guo, Y.-J.; Pan, W.-W.; Liu, S.-B.; Shen, Z.-F.; Xu, Y.; Hu, L.-L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef]
- Yue, J.; López, J.M. Understanding MAPK signaling pathways in apoptosis. Int. J. Mol. Sci. 2020, 21, 2346. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.H.; Park, M.Y.; Bhosale, P.B.; Abusaliya, A.; Hwang, K.H.; Moon, Y.G.; Heo, J.D.; Seong, J.K.; Ahn, M. Potential Anticancer Effects of Isoflavone Prunetin and Prunetin Glycoside on Apoptosis Mechanisms. Int. J. Mol. Sci. 2024, 25, 11713. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Wang, J.; Wu, Q.; Qian, J.; Yang, C.; Bo, P. Genistein inhibits the growth and regulates the migration and invasion abilities of melanoma cells via the FAK/paxillin and MAPK pathways. Oncotarget 2017, 8, 21674. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Kong, S.; Fan, L.; Jiang, J. Irigenin exhibits anticancer activity against human colon cancer cells via autophagy, inhibition of cell migration and invasion, and targeting of ERK/MAPK signal pathway. Trop. J. Pharm. Res. 2021, 20, 1357–1363. [Google Scholar] [CrossRef]
- Hayat, R.; Manzoor, M.; Hussain, A. Wnt signaling pathway: A comprehensive review. Cell Biol. Int. 2022, 46, 863–877. [Google Scholar] [CrossRef]
- Rim, E.Y.; Clevers, H.; Nusse, R. The Wnt pathway: From signaling mechanisms to synthetic modulators. Annu. Rev. Biochem. 2022, 91, 571–598. [Google Scholar]
- Peppelenbosch, M.; Lebbink, J.; Smits, R.; Zhang, R.; Li, S.; Schippers, K.; Li, Y.; Eimers, B.; Lavrijsen, M.; Wang, L. Analysis of Tumor-Associated AXIN1 Missense Mutations Identifies Variants That Activate β-Catenin Signaling. J. Cancer Res. 2024, 84, 1443–1459. [Google Scholar]
- Taciak, B.; Pruszynska, I.; Kiraga, L.; Bialasek, M.; Król, M. Wnt signaling pathway in development and cancer. J Physiol Pharmacol 2018, 69, 185–196. [Google Scholar]
- de Almeida, G.C.; Oliveira, L.F.; Predes, D.; Fokoue, H.H.; Kuster, R.M.; Oliveira, F.L.; Mendes, F.A.; Abreu, J.G. Piperine suppresses the Wnt/β-catenin pathway and has anti-cancer effects on colorectal cancer cells. Sci. Rep. 2020, 10, 11681. [Google Scholar]
- Liu, J.; Wang, F.; Luo, F. The role of JAK/STAT pathway in fibrotic diseases: Molecular and cellular mechanisms. Biomolecules 2023, 13, 119. [Google Scholar] [CrossRef]
- Hu, Q.; Bian, Q.; Rong, D.; Wang, L.; Song, J.; Huang, H.-S.; Zeng, J.; Mei, J.; Wang, P.-Y. JAK/STAT pathway: Extracellular signals, diseases, immunity, and therapeutic regimens. Front. Bioeng. Biotechnol. 2023, 11, 1110765. [Google Scholar] [CrossRef] [PubMed]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Banerjee, K.; Resat, H. Constitutive activation of STAT 3 in breast cancer cells: A review. Int. J. Cancer 2016, 138, 2570–2578. [Google Scholar] [CrossRef]
- Ceccarelli, I.; Bioletti, L.; Peparini, S.; Solomita, E.; Ricci, C.; Casini, I.; Miceli, E.; Aloisi, A.M. Estrogens and phytoestrogens in body functions. Neurosci. Biobehav. Rev. 2022, 132, 648–663. [Google Scholar] [CrossRef]
- Chen, L.-R.; Ko, N.-Y.; Chen, K.-H. Isoflavone supplements for menopausal women: A systematic review. Nutrients 2019, 11, 2649. [Google Scholar] [CrossRef]
- Wang, J.-F.; Liu, S.-S.; Song, Z.-Q.; Xu, T.-C.; Liu, C.-S.; Hou, Y.-G.; Huang, R.; Wu, S.-H. Naturally occurring flavonoids and isoflavonoids and their microbial transformation: A review. Molecules 2020, 25, 5112. [Google Scholar] [CrossRef]
- Liu, W.; Feng, Y.; Yu, S.; Fan, Z.; Li, X.; Li, J.; Yin, H. The flavonoid biosynthesis network in plants. Int. J. Mol. Sci. 2021, 22, 12824. [Google Scholar] [CrossRef]
- Takaoka, O.; Mori, T.; Ito, F.; Okimura, H.; Kataoka, H.; Tanaka, Y.; Koshiba, A.; Kusuki, I.; Shigehiro, S.; Amami, T. Daidzein-rich isoflavone aglycones inhibit cell growth and inflammation in endometriosis. J. Steroid Biochem. Mol. Biol. 2018, 181, 125–132. [Google Scholar] [CrossRef]
- Křížová, L.; Dadáková, K.; Kašparovská, J.; Kašparovský, T. Isoflavones. Molecules 2019, 24, 1076. [Google Scholar] [CrossRef]
- Perna, S.; Peroni, G.; Miccono, A.; Riva, A.; Morazzoni, P.; Allegrini, P.; Preda, S.; Baldiraghi, V.; Guido, D.; Rondanelli, M. Multidimensional effects of soy isoflavone by food or supplements in menopause women: A systematic review and bibliometric analysis. Nat. Prod. Commun. 2016, 11, 1934578X1601101127. [Google Scholar] [CrossRef]
- Chadha, R.; Bhalla, Y.; Jain, A.; Chadha, K.; Karan, M. Dietary soy isoflavone: A mechanistic insight. Nat. Prod. Commun. 2017, 12, 1934578X1701200439. [Google Scholar] [CrossRef]
- Sivoňová, M.K.; Kaplán, P.; Tatarková, Z.; Lichardusová, L.; Dušenka, R.; Jurečeková, J. Androgen receptor and soy isoflavones in prostate cancer. Mol. Clin. Oncol. 2019, 10, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.S.; Prasad, S.K.; Shivamallu, C.; Prasad, K.S.; Syed, A.; Reddy, P.; Cull, C.A.; Amachawadi, R.G. Genistein: A potent anti-breast cancer agent. Curr. Issues Mol. Biol. 2021, 43, 1502–1517. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.R.; Shim, J.; Kim, M.J. Genistin: A novel potent anti-adipogenic and anti-lipogenic agent. Molecules 2020, 25, 2042. [Google Scholar] [CrossRef]
- Konstantinou, E.K.; Gioxari, A.; Dimitriou, M.; Panoutsopoulos, G.I.; Panagiotopoulos, A.A. Molecular Pathways of Genistein Activity in Breast Cancer Cells. Int. J. Mol. Sci. 2024, 25, 5556. [Google Scholar] [CrossRef]
- Park, C.; Cha, H.-J.; Lee, H.; Hwang-Bo, H.; Ji, S.Y.; Kim, M.Y.; Hong, S.H.; Jeong, J.-W.; Han, M.H.; Choi, S.H. Induction of G2/M cell cycle arrest and apoptosis by genistein in human bladder cancer T24 cells through inhibition of the ROS-dependent PI3k/Akt signal transduction pathway. Antioxidants 2019, 8, 327. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Imran, M.; Rauf, A.; Nadeem, M.; Gondal, T.A.; Ahmad, B.; Atif, M.; Mubarak, M.S.; Sytar, O. Genistein: An integrative overview of its mode of action, pharmacological properties, and health benefits. Oxidative Med. Cell. Longev. 2021, 2021, 3268136. [Google Scholar] [CrossRef]
- Pawlicka, M.A.; Zmorzyński, S.; Popek-Marciniec, S.; Filip, A.A. The effects of genistein at different concentrations on MCF-7 breast cancer cells and BJ dermal fibroblasts. Int. J. Mol. Sci. 2022, 23, 12360. [Google Scholar] [CrossRef]
- Sohel, M.; Biswas, P.; Al Amin, M.; Hossain, M.A.; Sultana, H.; Dey, D.; Aktar, S.; Setu, A.; Khan, M.S.; Paul, P. Genistein, a potential phytochemical against breast cancer treatment-insight into the molecular mechanisms. Processes 2022, 10, 415. [Google Scholar] [CrossRef]
- Alatawi, F.S.; Faridi, U. Anticancer and anti-metastasis activity of 1, 25 dihydroxycholecalciferols and genistein in MCF-7 and MDA-MB-231 breast cancer cell lines. Heliyon 2023, 9, e21975. [Google Scholar] [CrossRef]
- Kaushik, S.; Shyam, H.; Sharma, R.; Balapure, A.K. Genistein synergizes centchroman action in human breast cancer cells. Indian J. Pharmacol. 2016, 48, 637–642. [Google Scholar] [PubMed]
- Shafiee, G.; Saidijam, M.; Tayebinia, H.; Khodadadi, I. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem. 2022, 128, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Geng, D.; Li, S.; Chen, Z.; Zhao, W. LncRNA PART1 modulates toll-like receptor pathways to influence cell proliferation and apoptosis in prostate cancer cells. Biol. Chem. 2018, 399, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M.; El Oirdi, M.; Aatif, M.; Nahvi, I.; Muteeb, G.; Alam, M.W. Soy isoflavones induce cell death by copper-mediated mechanism: Understanding its anticancer properties. Molecules 2023, 28, 2925. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Zhang, G.-Q.; Yang, Y.; Zhang, C.-Y.; Fu, R.-X.; Yang, Y.-M. Genistein induces G2/M arrest in gastric cancer cells by increasing the tumor suppressor PTEN expression. Nutr. Cancer 2013, 65, 1034–1041. [Google Scholar] [CrossRef]
- Hou, S. Genistein: Therapeutic and preventive effects, mechanisms, and clinical application in digestive tract tumor. Evid. Based Complement. Altern. Med. 2022, 2022, 5957378. [Google Scholar] [CrossRef]
- Kaushik, S.; Shyam, H.; Agarwal, S.; Sharma, R.; Nag, T.C.; Dwivedi, A.K.; Balapure, A.K. Genistein potentiates Centchroman induced antineoplasticity in breast cancer via PI3K/Akt deactivation and ROS dependent induction of apoptosis. Life Sci. 2019, 239, 117073. [Google Scholar] [CrossRef]
- Kumar, V.; Chauhan, S.S. Daidzein induces intrinsic pathway of apoptosis along with ER α/β ratio alteration and ROS production. Asian Pac. J. Cancer Prev. 2021, 22, 603. [Google Scholar] [CrossRef]
- Kaushik, S.; Shyam, H.; Sharma, R.; Balapure, A.K. Dietary isoflavone daidzein synergizes centchroman action via induction of apoptosis and inhibition of PI3K/Akt pathway in MCF-7/MDA MB-231 human breast cancer cells. Phytomedicine 2018, 40, 116–124. [Google Scholar] [CrossRef]
- Noh, S.; Choi, E.; Hwang, C.-H.; Jung, J.H.; Kim, S.-H.; Kim, B. Dietary compounds for targeting prostate cancer. Nutrients 2019, 11, 2401. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Liu, T.; Sun, R.; Yang, L.; An, R.; Xue, Y. Daidzein induces choriocarcinoma cell apoptosis in a dose-dependent manner via the mitochondrial apoptotic pathway. Mol. Med. Rep. 2018, 17, 6093–6099. [Google Scholar] [PubMed]
- Zhang, B.; Su, J.-P.; Bai, Y.; Li, J.; Liu, Y.-H. Inhibitory effects of O-methylated isoflavone glycitein on human breast cancer SKBR-3 cells. Int. J. Clin. Exp. Pathol. 2015, 8, 7809. [Google Scholar]
- Zhu, Y. Anticancer Effects of Soybean Bioactive Components and Anti-Inflammatory Activities of the Soybean Peptide Lunasin. Ph.D. Thesis, University of Liège, Liège, Belgium, 2018. [Google Scholar]
- Zhu, Y.; Yao, Y.; Shi, Z.; Everaert, N.; Ren, G. Synergistic effect of bioactive anticarcinogens from soybean on anti-proliferative activity in MDA-MB-231 and MCF-7 human breast cancer cells in vitro. Molecules 2018, 23, 1557. [Google Scholar] [CrossRef] [PubMed]
- Zang, Y.Q.; Feng, Y.Y.; Luo, Y.H.; Zhai, Y.Q.; Ju, X.Y.; Feng, Y.C.; Wang, J.R.; Yu, C.Q.; Jin, C.H. Glycitein induces reactive oxygen species-dependent apoptosis and G0/G1 cell cycle arrest through the MAPK/STAT3/NF-κB pathway in human gastric cancer cells. Drug Dev. Res. 2019, 80, 573–584. [Google Scholar]
- Monthakantirat, O.; De-Eknamkul, W.; Umehara, K.; Yoshinaga, Y.; Miyase, T.; Warashina, T.; Noguchi, H. Phenolic Constituents of the Rhizomes of the Thai Medicinal Plant Belamcanda c hinensis with Proliferative Activity for Two Breast Cancer Cell Lines. J. Nat. Prod. 2005, 68, 361–364. [Google Scholar]
- Morrissey, C.; Bektic, J.; Spengler, B.; Galvin, D.; Christoffel, V.; Klocker, H.; Fitzpatrick, J.M.; Watson, R.W.G. Phytoestrogens derived from Belamcanda chinensis have an antiproliferative effect on prostate cancer cells in vitro. J. Urol. 2004, 172, 2426–2433. [Google Scholar] [CrossRef]
- Xu, Y.; Gao, C.-C.; Pan, Z.-G.; Zhou, C.-W. Irigenin sensitizes TRAIL-induced apoptosis via enhancing pro-apoptotic molecules in gastric cancer cells. Biochem. Biophys. Res. Commun. 2018, 496, 998–1005. [Google Scholar]
- Alshehri, M.M.; Sharifi-Rad, J.; Herrera-Bravo, J.; Jara, E.L.; Salazar, L.A.; Kregiel, D.; Uprety, Y.; Akram, M.; Iqbal, M.; Martorell, M. Therapeutic potential of isoflavones with an emphasis on daidzein. Oxidative Med. Cell. Longev. 2021, 2021, 6331630. [Google Scholar]
- Sun, M.-Y.; Ye, Y.; Xiao, L.; Rahman, K.; Xia, W.; Zhang, H. Daidzein: A review of pharmacological effects. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 117–132. [Google Scholar] [CrossRef]
- Ubaid, M.; Salauddin; Shadani, M.A.; Kawish, S.; Albratty, M.; Makeen, H.A.; Alhazmi, H.A.; Najmi, A.; Zoghebi, K.; Halawi, M.A. Daidzein from dietary supplement to a drug candidate: An evaluation of potential. ACS Omega 2023, 8, 32271–32293. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guo, R.; Wang, Y. Glycitin exerts anticancer effect on human lung cancer cells through induction of apoptosis, cell cycle arrest, and inhibition of PI3K/AKT signal pathway. Trop. J. Pharm. Res. 2022, 21, 943–950. [Google Scholar] [CrossRef]
- Stephens, B.R.; Bomser, J.A. Glycitein in Health. Master’s Thesis, Ohio State University, Columbus, OH, USA, 2012. [Google Scholar]
- Van der Eecken, H.; Joniau, S.; Berghen, C.; Rans, K.; De Meerleer, G. The use of soy isoflavones in the treatment of prostate cancer: A focus on the cellular effects. Nutrients 2023, 15, 4856. [Google Scholar] [CrossRef]
- Hu, T.; Ge, X.; Wang, J.; Zhang, N.; Diao, X.; Hu, L.; Wang, X. Metabolite identification of iridin in rats by using UHPLC-MS/MS and pharmacokinetic study of its metabolite irigenin. J. Chromatogr. B 2021, 1181, 122914. [Google Scholar] [CrossRef]
- Xu, J.; Sun, S.; Zhang, W.; Dong, J.; Huang, C.; Wang, X.; Jia, M.; Yang, H.; Wang, Y.; Jiang, Y. Irigenin inhibits glioblastoma progression through suppressing YAP/β-catenin signaling. Front. Pharmacol. 2022, 13, 1027577. [Google Scholar]
- Liu, D.; Wang, Q.; Yuan, W.; Wang, Q. Irigenin attenuates lipopolysaccharide-induced acute lung injury by inactivating the mitogen-activated protein kinase (MAPK) signaling pathway. Hum. Exp. Toxicol. 2023, 42, 09603271231155098. [Google Scholar]
- Zhang, L.; Wei, K.; Xu, J.; Yang, D.; Zhang, C.; Wang, Z.; Li, M. Belamcanda chinensis (L.) DC-An ethnopharmacological, phytochemical and pharmacological review. J. Ethnopharmacol. 2016, 186, 1–13. [Google Scholar] [CrossRef]
- Widowati, W.; Prahastuti, S.; Ekayanti, N.; Munshy, U.; Kusuma, H.; Wibowo, S.; Amalia, A.; Widodo, W.; Rizal, R. Anti-inflammation assay of black soybean extract and its compounds on lipopolysaccharide-induced RAW 264.7 cell. In Proceedings of the Journal of Physics: Conference Series, Malang, Indonesia, 11–12 July 2019; IOP Science: Bristol, UK, 2019; p. 012052. [Google Scholar]
- Jeong, J.-W.; Lee, H.H.; Han, M.H.; Kim, G.-Y.; Kim, W.-J.; Choi, Y.H. Anti-inflammatory effects of genistein via suppression of the toll-like receptor 4-mediated signaling pathway in lipopolysaccharide-stimulated BV2 microglia. Chem. Biol. Interact. 2014, 212, 30–39. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, X.; Cheang, W.S. Isoflavones daidzin and daidzein inhibit lipopolysaccharide-induced inflammation in RAW264. 7 macrophages. Chin. Med. 2022, 17, 95. [Google Scholar]
- Park, J.-S.; Woo, M.-S.; Kim, D.-H.; Hyun, J.-W.; Kim, W.-K.; Lee, J.-C.; Kim, H.-S. Anti-inflammatory mechanisms of isoflavone metabolites in lipopolysaccharide-stimulated microglial cells. J. Pharmacol. Exp. Ther. 2007, 320, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Danciu, C.; Avram, S.; Pavel, I.Z.; Ghiulai, R.; Dehelean, C.A.; Ersilia, A.; Minda, D.; Petrescu, C.; Moaca, E.-A.; Soica, C. Main isoflavones found in dietary sources as natural anti-inflammatory agents. Curr. Drug Targets 2018, 19, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V. Genistein: Its role in breast cancer growth and metastasis. Curr. Drug Metab. 2020, 21, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xu, H.; Hu, X.; Ma, W.; Zhang, J.; Li, Y.; Yu, M.; Zhang, Y.; Li, X.; Ye, X. Synergetic inhibition of daidzein and regular exercise on breast cancer in bearing-4T1 mice by regulating NK cells and apoptosis pathway. Life Sci. 2020, 245, 117387. [Google Scholar] [CrossRef]
- Jackson, I.L.; Pavlovic, R.; Alexander, A.A.; Connors, C.Q.; Newman, D.; Mahmood, J.; Eley, J.; Harvey, A.J.; Kaytor, M.D.; Vujaskovic, Z. BIO 300, a nanosuspension of genistein, mitigates radiation-induced erectile dysfunction and sensitizes human prostate cancer xenografts to radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2019, 105, 400–409. [Google Scholar] [CrossRef]
- Tang, Q.; Ma, J.; Sun, J.; Yang, L.; Yang, F.; Zhang, W.; Li, R.; Wang, L.; Wang, Y.; Wang, H. Genistein and AG1024 synergistically increase the radiosensitivity of prostate cancer cells. Oncol. Rep. 2018, 40, 579–588. [Google Scholar]
- Singh-Gupta, V.; Zhang, H.; Yunker, C.K.; Ahmad, Z.; Zwier, D.; Sarkar, F.H.; Hillman, G.G. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: Potentiation of radiotherapy. Pharm. Res. 2010, 27, 1115–1127. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Kimura, T.; Ikehara, S.; Cui, M.; Kawanishi, Y.; Kimura, T.; Ueda, K.; Iso, H. Soy consumption and incidence of gestational diabetes mellitus: The Japan Environment and Children’s Study. Eur. J. Nutr. 2021, 60, 897–904. [Google Scholar] [CrossRef]
- Mejia, S.B.; Messina, M.; Li, S.S.; Viguiliouk, E.; Chiavaroli, L.; Khan, T.A.; Srichaikul, K.; Mirrahimi, A.; Sievenpiper, J.L.; Kris-Etherton, P. A meta-analysis of 46 studies identified by the FDA demonstrates that soy protein decreases circulating LDL and total cholesterol concentrations in adults. J. Nutr. 2019, 149, 968–981. [Google Scholar]
- Miyanaga, N.; Akaza, H.; Hinotsu, S.; Fujioka, T.; Naito, S.; Namiki, M.; Takahashi, S.; Hirao, Y.; Horie, S.; Tsukamoto, T. Prostate cancer chemoprevention study: An investigative randomized control study using purified isoflavones in men with rising prostate-specific antigen. Cancer Sci. 2012, 103, 125–130. [Google Scholar] [CrossRef]
- Pendleton, J.M.; Tan, W.W.; Anai, S.; Chang, M.; Hou, W.; Shiverick, K.T.; Rosser, C.J. Phase II trial of isoflavone in prostate-specific antigen recurrent prostate cancer after previous local therapy. BMC Cancer 2008, 8, 132. [Google Scholar] [CrossRef]
- Pabich, M.; Materska, M. Biological effect of soy isoflavones in the prevention of civilization diseases. Nutrients 2019, 11, 1660. [Google Scholar] [CrossRef]



| Genistein | ||||
|---|---|---|---|---|
| Cancer | Cell Line | Treatment | Effect | Reference |
| Breast cancer | MCF-7 | 50, 100 µM | Arrested the growth at G2/M phase | [59] |
| MCF-7 MDA-MB-231 | 10, 25, and 50 µM | Decreased cell proliferation Increased BAX expression Decreased cell invasion/migration | [60] | |
| MDA-MB-231 MDA-MB-468 MCF-7 T-47D MCF-10A | 10 to 200 µM For 24/48 h | Viability of all cell line low | [61] | |
| MCF-7 | 150 µM | Downregulated Bcl-2 | [68] | |
| Prostate cancer | PC3 | 30, 50, 70 µM | Increased caspase-3 Inhibited p38 MAPK | [62] |
| LNCaP | 12.5, 25, 50, and 100 µmol/L | Inhibited PART-1 expression | [63] | |
| LNCaP DU145 | 0, 10, 25, 50 µM | Decreased cell proliferation | [64] | |
| Gastric cancer | BCG-823 | 20–80 µM | Inactivated AKT by upregulating PTEN | [65,67] |
| SGC-7901 BGC-823 | 10, 20, 40, 80 µM | Arrested the growth at G2/M phase | [66,67] | |
| Daidzein | ||||
| Cancer | Cell Line | Treatment | Effect | |
| Breast cancer | MCF-7 | 25, 50, 100 µM | Activated caspase-9 | [69,70] |
| MCF-7 | 50 µM | Inhibited cell proliferation Activated caspase-3, -7 | [69] | |
| MCF-7 MDA-MB-231 | 10 to 200 µM | Low viability Inhibited PI3K/AKT pathway | [70] | |
| Prostate cancer | PC3 | 50 µM | Increased Bax Decreased IAP | [71] |
| LNCaP | 12.5, 25, 50, and 100 µmol/L | Inhibited PART-1 expression | [63] | |
| LNCaP DU145 | 0, 10, 25, 50 µM | Decreased cell proliferation | [64] | |
| Gastric cancer | BGC-823 | 20, 80 µM | Regulated caspase-3, 9 Decreased Bcl-2 Increased Bax | [72] |
| Glycitein | ||||
| Cancer | Cell Line | Treatment | Effect | |
| Breast cancer | SKBR-3 | 5, 10, 20 µM | Increased membrane permeability | [32,73] |
| MDA-MB-231 | 0–200 µM | Inhibited proliferation | [74] | |
| MCF-7 | 0–200 µM | Downregulated phosphorylated STAT3, AKT, mTOR, and p38 | [75] | |
| Gastric cancer | AGS cell | 30 µM | Arrested the growth at G0/G1 phase Inhibited STAT3/NF- kB pathway Activated caspase cascade Activated MAPK pathway | [76] |
| Irigenin/Iridin | ||||
| Cancer | Cell Line | Treatment | Effect | |
| Breast cancer | MCF-7 T-47D | 10 nM to 100 μM | No effects | [18,77] |
| Prostate cancer | RWPE-1 LNCaP PC3 Cell | 50, 100 µM | Arrested G1 phase Inhibited p21, p27 protein | [78] |
| Gastric cancer | TRAIL-resistant gastric cancer cell AGS HaCaT | 12.5, 200 µM | Inhibited PI3K/AKT pathway Decreased caspase-3 and 8 | [16,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Won, Y.; Kim, H.-H.; Jeong, S.-H.; Bhosale, P.B.; Abusaliya, A.; Heo, J.-D.; Seong, J.-K.; Ahn, M.-J.; Kim, H.-J.; Kim, G.-S. The Effects of Iridin and Irigenin on Cancer: Comparison with Well-Known Isoflavones in Breast, Prostate, and Gastric Cancers. Int. J. Mol. Sci. 2025, 26, 2390. https://doi.org/10.3390/ijms26062390
Won Y, Kim H-H, Jeong S-H, Bhosale PB, Abusaliya A, Heo J-D, Seong J-K, Ahn M-J, Kim H-J, Kim G-S. The Effects of Iridin and Irigenin on Cancer: Comparison with Well-Known Isoflavones in Breast, Prostate, and Gastric Cancers. International Journal of Molecular Sciences. 2025; 26(6):2390. https://doi.org/10.3390/ijms26062390
Chicago/Turabian StyleWon, Yaeram, Hun-Hwan Kim, Se-Hyo Jeong, Pritam Bhagwan Bhosale, Abuyaseer Abusaliya, Jeong-Doo Heo, Je-Kyung Seong, Mee-Jung Ahn, Hye-Jung Kim, and Gon-Sup Kim. 2025. "The Effects of Iridin and Irigenin on Cancer: Comparison with Well-Known Isoflavones in Breast, Prostate, and Gastric Cancers" International Journal of Molecular Sciences 26, no. 6: 2390. https://doi.org/10.3390/ijms26062390
APA StyleWon, Y., Kim, H.-H., Jeong, S.-H., Bhosale, P. B., Abusaliya, A., Heo, J.-D., Seong, J.-K., Ahn, M.-J., Kim, H.-J., & Kim, G.-S. (2025). The Effects of Iridin and Irigenin on Cancer: Comparison with Well-Known Isoflavones in Breast, Prostate, and Gastric Cancers. International Journal of Molecular Sciences, 26(6), 2390. https://doi.org/10.3390/ijms26062390










