Identification of POU1F1 Variants in Vietnamese Patients with Combined Pituitary Hormone Deficiency
Abstract
1. Introduction
2. Results
2.1. Clinical Findings
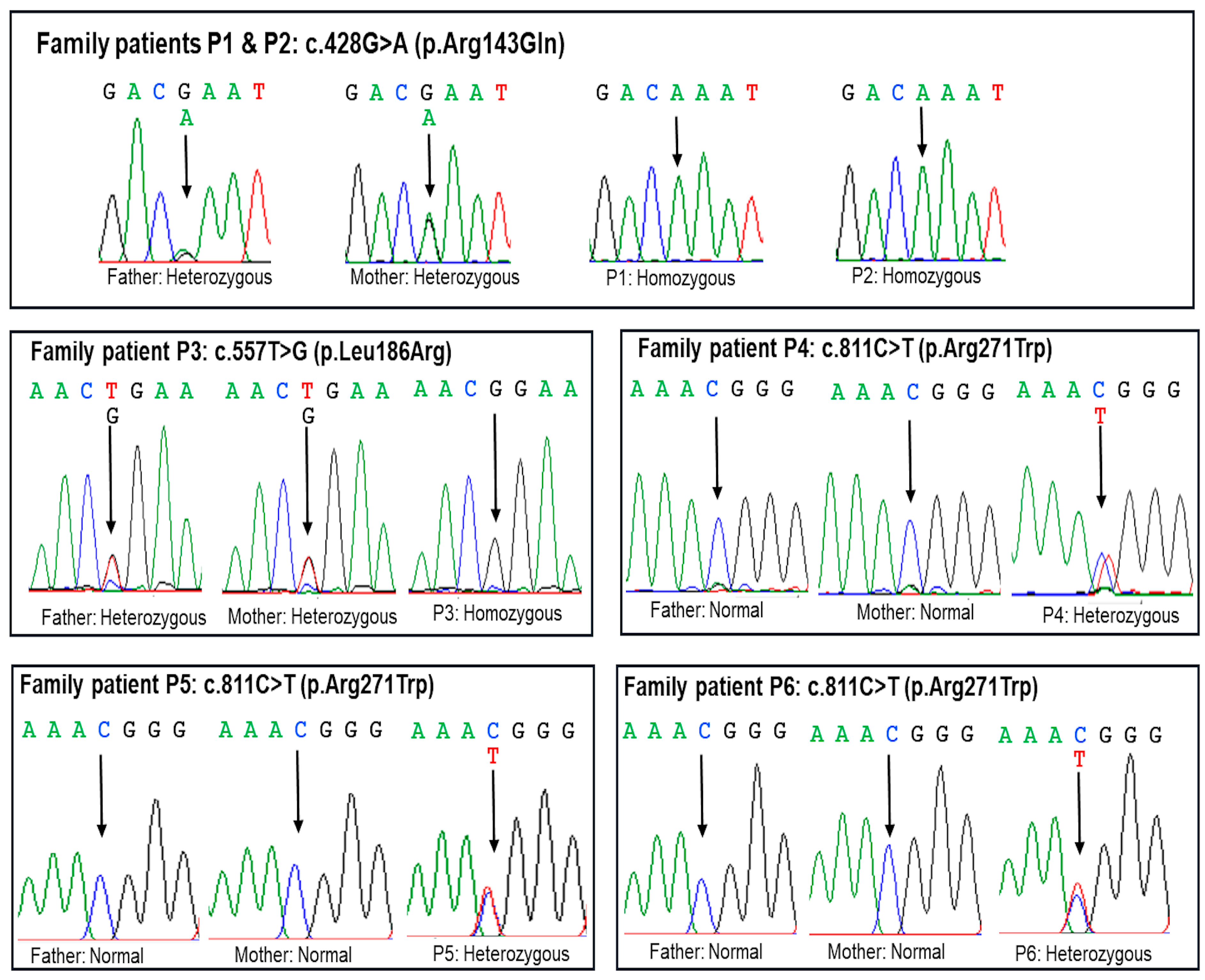
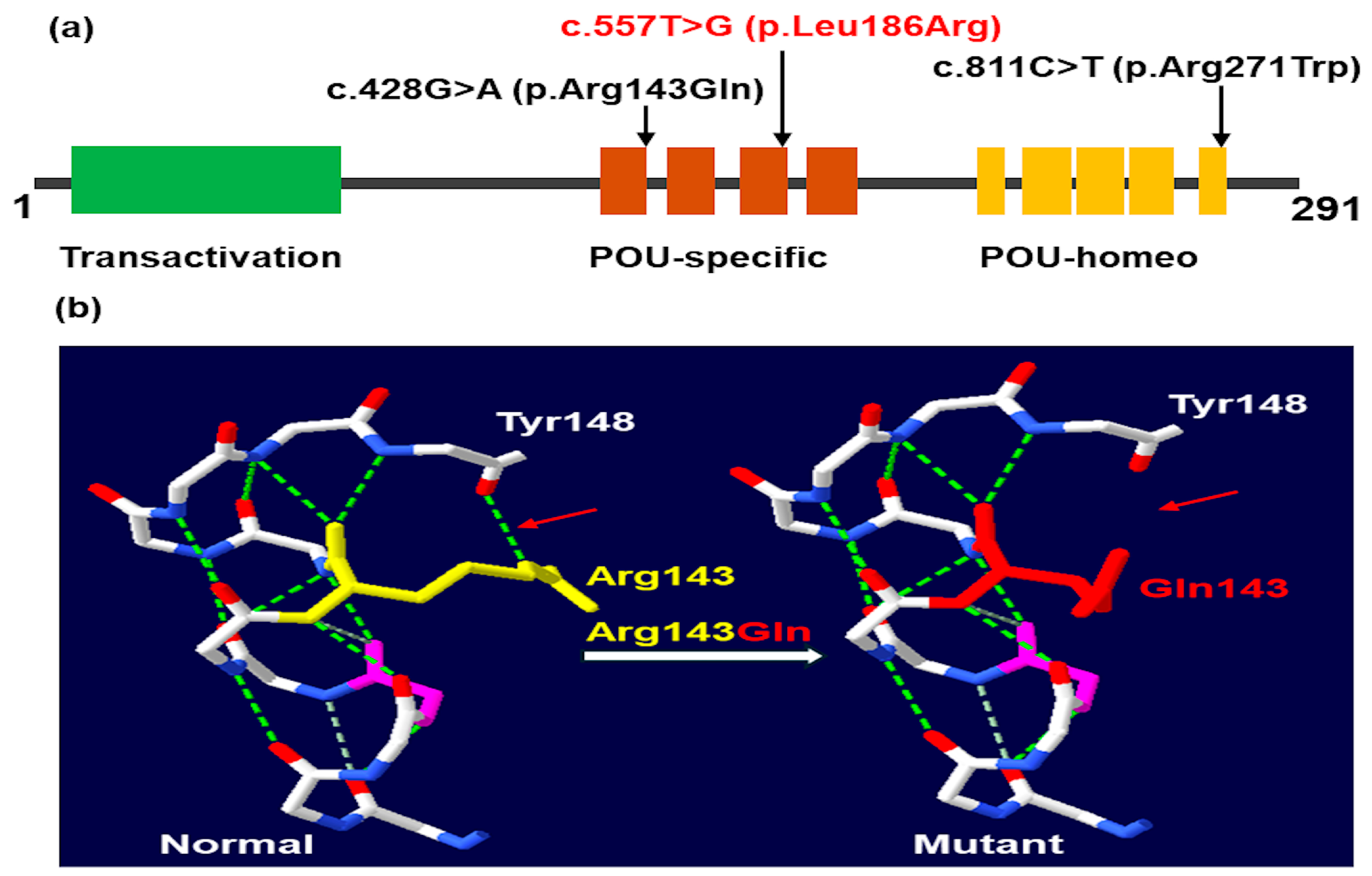
2.2. Molecular Findings
2.3. Outcomes
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bosch i Ara, L.; Katugampola, H.; Dattani, M.T. Congenital Hypopituitarism during the Neonatal Period: Epidemiology, Pathogenesis, Therapeutic Options, and Outcome. Front. Pediatr. 2020, 8, 600962. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, N.M.; Tadi, P. Physiology, Pituitary Hormones. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Majdoub, H.; Amselem, S.; Legendre, M.; Rath, S.; Bercovich, D.; Tenenbaum-Rakover, Y. Extreme Short Stature and Severe Neurological Impairment in a 17-Year-Old Male with Untreated Combined Pituitary Hormone Deficiency Due to POU1F1 Mutation. Front. Endocrinol. 2019, 10, e00381. [Google Scholar] [CrossRef] [PubMed]
- Parkin, K.; Kapoor, R.; Bhat, R.; Greenough, A. Genetic Causes of Hypopituitarism. Arch. Med. Sci. 2019, 16, 27–33. [Google Scholar] [CrossRef]
- Gregory, L.C.; Dattani, M.T. The Molecular Basis of Congenital Hypopituitarism and Related Disorders. J. Clin. Endocrinol. Metab. 2020, 105, e2103–e2120. [Google Scholar] [CrossRef] [PubMed]
- Gounden, V.; Anastasopoulou, C.; Jialal, I. Hypopituitarism. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Fang, Q.; George, A.S.; Brinkmeier, M.L.; Mortensen, A.H.; Gergics, P.; Cheung, L.Y.M.; Daly, A.Z.; Ajmal, A.; Pérez Millán, M.I.; Ozel, A.B.; et al. Genetics of Combined Pituitary Hormone Deficiency: Roadmap into the Genome Era. Endocr. Rev. 2016, 37, 636–675. [Google Scholar] [CrossRef]
- Martinez-Mayer, J.; Vishnopolska, S.; Perticarari, C.; Garcia, L.I.; Hackbartt, M.; Martinez, M.; Zaiat, J.; Jacome-Alvarado, A.; Braslavsky, D.; Keselman, A.; et al. Exome Sequencing Has a High Diagnostic Rate in Sporadic Congenital Hypopituitarism and Reveals Novel Candidate Genes. J. Clin. Endocrinol. Metab. 2024, 109, dgae320. [Google Scholar] [CrossRef]
- Bando, H.; Urai, S.; Kanie, K.; Sasaki, Y.; Yamamoto, M.; Fukuoka, H.; Iguchi, G.; Camper, S.A. Novel Genes and Variants Associated with Congenital Pituitary Hormone Deficiency in the Era of Next-Generation Sequencing. Front. Endocrinol. 2022, 13, 1008306. [Google Scholar] [CrossRef]
- Giordano, M. Genetic Causes of Isolated and Combined Pituitary Hormone Deficiency. Best Pract. Res. Clin. Endocrinol. Metab. 2016, 30, 679–691. [Google Scholar] [CrossRef]
- Andersen, B.; Pearse, R.V.; Jenne, K.; Sornson, M.; Lin, S.C.; Bartke, A.; Rosenfeld, M.G. The Ames Dwarf Gene Is Required for Pit-1 Gene Activation. Dev. Biol. 1995, 172, 495–503. [Google Scholar] [CrossRef][Green Version]
- Jacobson, E.M.; Li, P.; Leon-del-Rio, A.; Rosenfeld, M.G.; Aggarwal, A.K. Structure of Pit-1 POU Domain Bound to DNA as a Dimer: Unexpected Arrangement and Flexibility. Genes. Dev. 1997, 11, 198–212. [Google Scholar] [CrossRef]
- Howard, P.W.; Jue, S.F.; Maurer, R.A. Expression of the Synaptotagmin I Gene Is Enhanced by Binding of the Pituitary-Specific Transcription Factor, POU1F1. Mol. Endocrinol. 2009, 23, 1563–1571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jadhav, S.; Diwaker, C.; Lila, A.R.; Gada, J.V.; Kale, S.; Sarathi, V.; Thadani, P.M.; Arya, S.; Patil, V.A.; Shah, N.S.; et al. POU1F1 Mutations in Combined Pituitary Hormone Deficiency: Differing Spectrum of Mutations in a Western-Indian Cohort and Systematic Analysis of World Literature. Pituitary 2021, 24, 657–669. [Google Scholar] [CrossRef]
- Rey, R.A.; Bergadá, I.; Ballerini, M.G.; Braslavsky, D.; Chiesa, A.; Freire, A.; Grinspon, R.P.; Keselman, A.; Arcari, A. Diagnosing and Treating Anterior Pituitary Hormone Deficiency in Pediatric Patients. Rev. Endocr. Metab. Disord. 2024, 25, 555–573. [Google Scholar] [CrossRef] [PubMed]
- Ayyar, V.S. History of Growth Hormone Therapy. Indian J. Endocrinol. Metab. 2011, 15, S162–S165. [Google Scholar] [CrossRef] [PubMed]
- Danowitz, M.; Grimberg, A. Clinical Indications for Growth Hormone Therapy. Adv. Pediatr. 2022, 69, 203–217. [Google Scholar] [CrossRef]
- Besci, Ö.; Deveci Sevim, R.; Yüksek Acinikli, K.; Akın Kağızmanlı, G.; Ersoy, S.; Demir, K.; Ünüvar, T.; Böber, E.; Anık, A.; Abacı, A. Growth Hormone Dosing Estimations Based on Body Weight versus Body Surface Area. J. Clin. Res. Pediatr. Endocrinol. 2023, 15, 268–275. [Google Scholar] [CrossRef]
- Drake, W.M.; Howell, S.J.; Monson, J.P.; Shalet, S.M. Optimizing GH Therapy in Adults and Children. Endocr. Rev. 2001, 22, 425–450. [Google Scholar] [CrossRef]
- Rachmiel, M.; Rota, V.; Atenafu, E.; Daneman, D.; Hamilton, J. Final Height in Children with Idiopathic Growth Hormone Deficiency Treated with a Fixed Dose of Recombinant Growth Hormone. Horm. Res. 2007, 68, 236–243. [Google Scholar] [CrossRef]
- Grimberg, A.; DiVall, S.A.; Polychronakos, C.; Allen, D.B.; Cohen, L.E.; Quintos, J.B.; Rossi, W.C.; Feudtner, C.; Murad, M.H.; on behalf of the Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society. Guidelines for Growth Hormone and Insulin-like Growth Factor-I Treatment in Children and Adolescents: Growth Hormone Deficiency, Idiopathic Short Stature, and Primary Insulin-like Growth Factor-I Deficiency. Horm. Res. Paediatr. 2016, 86, 361–397. [Google Scholar] [CrossRef]
- Ross, J.; Fridman, M.; Kelepouris, N.; Murray, K.; Krone, N.; Polak, M.; Rohrer, T.R.; Pietropoli, A.; Lawrence, N.; Backeljauw, P. Factors Associated With Response to Growth Hormone in Pediatric Growth Disorders: Results of a 5-Year Registry Analysis. J Endocr. Soc. 2023, 7, bvad026. [Google Scholar] [CrossRef]
- Ross, J.; Lee, P.A.; Gut, R.; Germak, J. Factors Influencing the One- and Two-Year Growth Response in Children Treated with Growth Hormone: Analysis from an Observational Study. Int. J. Pediatr. Endocrinol. 2010, 2010, 494656. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.; Blair, J.; Kotnik, P.; Pournara, E.; Pedersen, B.T.; Rohrer, T.R. Early Growth Hormone Treatment Start in Childhood Growth Hormone Deficiency Improves near Adult Height: Analysis from NordiNet® International Outcome Study. Eur. J. Endocrinol. 2017, 177, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Maghnie, M.; Ranke, M.B.; Geffner, M.E.; Vlachopapadopoulou, E.; Ibáñez, L.; Carlsson, M.; Cutfield, W.; Rooman, R.; Gomez, R.; Wajnrajch, M.P.; et al. Safety and Efficacy of Pediatric Growth Hormone Therapy: Results from the Full KIGS Cohort. J. Clin. Endocrinol. Metab. 2022, 107, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Ranke, M.B.; Lindberg, A.; Tanaka, T.; Camacho-Hübner, C.; Dunger, D.B.; Geffner, M.E. Baseline Characteristics and Gender Differences in Prepubertal Children Treated with Growth Hormone in Europe, USA, and Japan: 25 Years’ KIGS® Experience (1987–2012) and Review. Horm. Res. Paediatr. 2017, 87, 30–41. [Google Scholar] [CrossRef]
- Turton, J.P.G.; Reynaud, R.; Mehta, A.; Torpiano, J.; Saveanu, A.; Woods, K.S.; Tiulpakov, A.; Zdravkovic, V.; Hamilton, J.; Attard-Montalto, S.; et al. Novel Mutations within the POU1F1 Gene Associated with Variable Combined Pituitary Hormone Deficiency. J. Clin. Endocrinol. Metab. 2005, 90, 4762–4770. [Google Scholar] [CrossRef]
- Chen, W.-Y.; Niu, D.-M.; Chen, L.-Z.; Yang, C.-F. Congenital Hypopituitarism Due to Novel Compound Heterozygous POU1F1 Gene Mutation: A Case Report and Review of the Literature. Mol. Genet. Metab. Rep. 2021, 29, 100819. [Google Scholar] [CrossRef]
- Binder, G.; Weber, K.; Rieflin, N.; Steinruck, L.; Blumenstock, G.; Janzen, N.; Franz, A.R. Diagnosis of Severe Growth Hormone Deficiency in the Newborn. Clin. Endocrinol. 2020, 93, 305–311. [Google Scholar] [CrossRef]
- Goodman, H.G.; Grumbach, M.M.; Kaplan, S.L. Growth and Growth Hormone. N. Engl. J. Med. 1968, 278, 57–68. [Google Scholar] [CrossRef]
- Cherella, C.E.; Cohen, L.E. Congenital Hypopituitarism in Neonates. NeoReviews 2018, 19, e742–e752. [Google Scholar] [CrossRef]
- Ohta, K.; Nobukuni, Y.; Mitsubuchi, H.; Fujimoto, S.; Matsuo, N.; Inagaki, H.; Endo, F.; Matsuda, I. Mutations in the Pit-1 Gene in Children with Combined Pituitary Hormone Deficiency. Biochem. Biophys. Res. Commun. 1992, 189, 851–855. [Google Scholar] [CrossRef]
- Radovick, S.; Nations, M.; Du, Y.; Berg, L.A.; Weintraub, B.D.; Wondisford, F.E. A Mutation in the POU-Homeodomain of Pit-1 Responsible for Combined Pituitary Hormone Deficiency. Science 1992, 257, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Darendeliler, F.; Lindberg, A.; Wilton, P. Response to Growth Hormone Treatment in Isolated Growth Hormone Deficiency versus Multiple Pituitary Hormone Deficiency. Horm. Res. Paediatr. 2011, 76 (Suppl. 1), 42–46. [Google Scholar] [CrossRef] [PubMed]
- Sävendahl, L.; Polak, M.; Backeljauw, P.; Blair, J.C.; Miller, B.S.; Rohrer, T.R.; Hokken-Koelega, A.; Pietropoli, A.; Kelepouris, N.; Ross, J. Long-Term Safety of Growth Hormone Treatment in Childhood: Two Large Observational Studies: NordiNet IOS and ANSWER. J. Clin. Endocrinol. Metab. 2021, 106, 1728–1741. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and Accurate Short Read Alignment with Burrows-Wheeler Transform. Bioinform. 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce Framework for Analyzing next-Generation DNA Sequencing Data. Genome. Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Cingolani, P.; Platts, A.; Wang, L.L.; Coon, M.; Nguyen, T.; Wang, L.; Land, S.J.; Lu, X.; Ruden, D.M. A Program for Annotating and Predicting the Effects of Single Nucleotide Polymorphisms, SnpEff: SNPs in the Genome of Drosophila Melanogaster Strain W1118; Iso-2; Iso-3. Fly 2012, 6, 80–92. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Cooper, D.N.; Schuelke, M.; Seelow, D. MutationTaster2: Mutation Prediction for the Deep-Sequencing Age. Nat. Methods 2014, 11, 361–362. [Google Scholar] [CrossRef]
- Rentzsch, P.; Schubach, M.; Shendure, J.; Kircher, M. CADD-Splice-Improving Genome-Wide Variant Effect Prediction Using Deep Learning-Derived Splice Scores. Genome Med. 2021, 13, 31. [Google Scholar] [CrossRef]
- Ng, P.C.; Henikoff, S. SIFT: Predicting Amino Acid Changes That Affect Protein Function. Nucleic Acids Res. 2003, 31, 3812–3814. [Google Scholar] [CrossRef]
- Adzhubei, I.A.; Schmidt, S.; Peshkin, L.; Ramensky, V.E.; Gerasimova, A.; Bork, P.; Kondrashov, A.S.; Sunyaev, S.R. A Method and Server for Predicting Damaging Missense Mutations. Nat. Methods 2010, 7, 248–249. [Google Scholar] [CrossRef]
- Guex, N.; Peitsch, M.C. SWISS-MODEL and the Swiss-PdbViewer: An Environment for Comparative Protein Modeling. Electrophoresis 1997, 18, 2714–2723. [Google Scholar] [CrossRef] [PubMed]


| P1 | P2 | P3 | P4 | P5 | P6 | |
|---|---|---|---|---|---|---|
| Sociodemographic characteristics | ||||||
| Gender | Male | Male | Female | Female | Female | Female |
| Birth weight (g) | 2500 | 3000 | 3400 | 3200 | 2800 | 3100 |
| Family history | (+) | (+) | (−) | (−) | (−) | (−) |
| Clinical characteristics | ||||||
| Age at diagnosis | 1y7mo | 2mo | Neonate | 2y1mo | 9y4mo | 10mo |
| Intellectual disability | (+) | (−) | (−) | (+) | (+) | (−) |
| Pituitary dwarfism | (+) | (+) | (+) | (+) | (+) | (+) |
| Depressed nasal bridge | (+) | (+) | (+) | (+) | (+) | (+) |
| Constipation | (+) | (+) | (+) | (+) | (+) | (+) |
| Umbilical hernia | (+) | (−) | (−) | (−) | (−) | (−) |
| Prolonged neonatal jaundice | (−) | (+) | (+) | (−) | (−) | (−) |
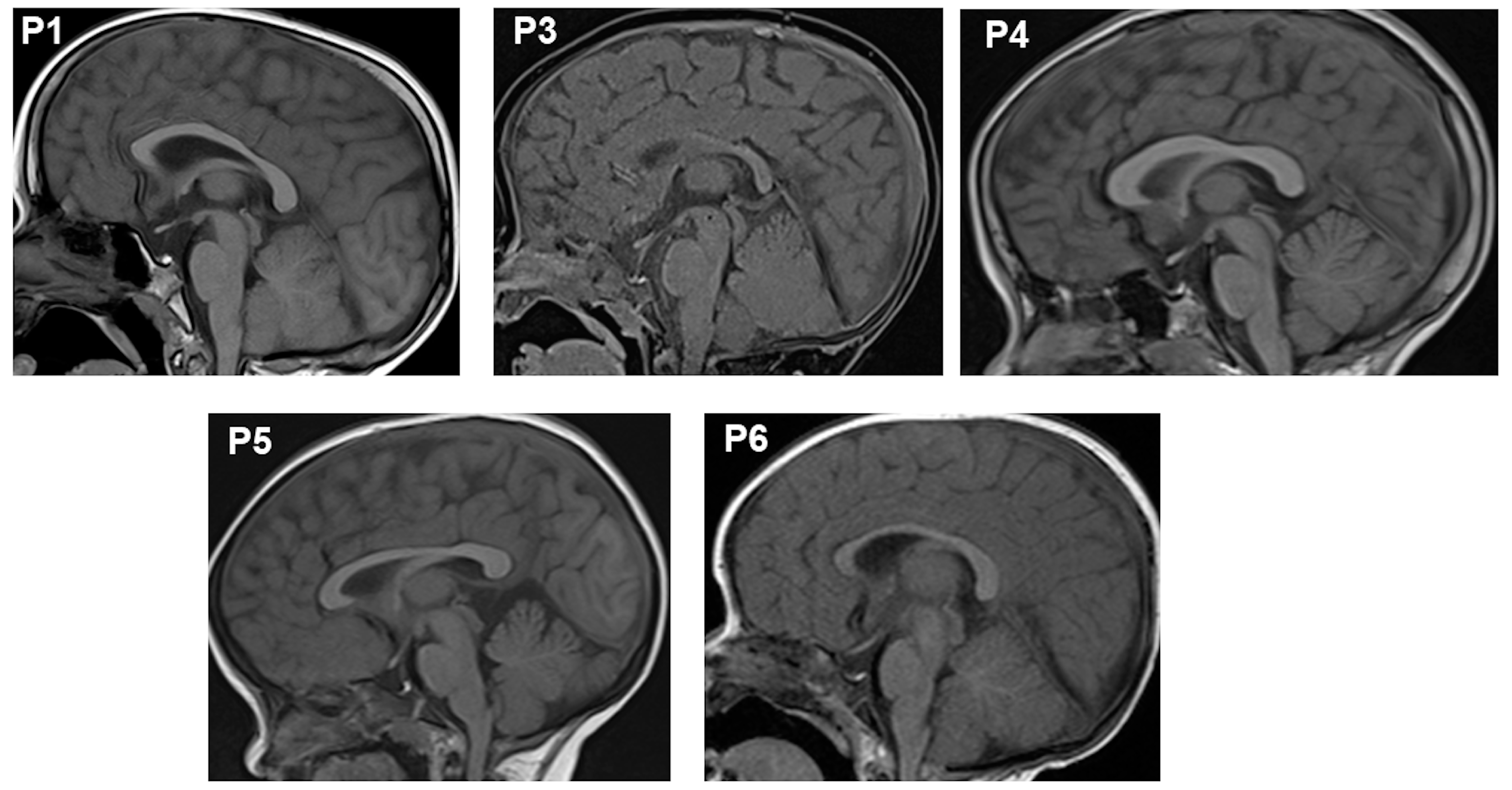
| Pituitary’s MRI | Anterior hypoplasia | Anterior hypoplasia | Anterior hypoplasia | Anterior hypoplasia | Anterior hypoplasia | Anterior hypoplasia |
| Hormonal profiles | ||||||
| Peak GH (pmol/L) | <0.30 (≥10) | n/a | 0.03 (≥10) | n/a | 0.03 (≥10) | n/a |
| TSH (mU/L) | 0.03 (0.70–6.40) | 0.01 (1.70–9.10) | 0.05 (1.70–9.10) | 1.55 (0.70–6.40) | 1.00 (0.70–6.40) | 1.32 (0.70–6.40) |
| ACTH (pg/mL) (Normal range) | 4.50 (1.60–13.90) | 40.16 (1.60–13.90) | 4.14 (1.60–13.90) | 5.00 (1.60–13.90) | 4.74 (1.60–13.90) | 51.50 (1.60–13.90) |
| LH (IU/L) (Normal range) | n/a | n/a | n/a | n/a | 4.02 * (0.37–6.23) | n/a |
| Prolactin (µIU/mL) (Normal range) | <2.00 (63.80–425.00) | <2.00 (63.80–532.00) | 2.79 (63.80–532.00) | 11.40 (63.80–425.00) | 2.38 (63.80–425.00) | 5.00 (63.80–532.00) |
| IGF1 (ng/mL) (Normal range) | <15.00 (33.9–183.9) | <15.00 (11.0–157.0) | 7.00 (17.9–125.6) | <15.00 (22.2–145.5) | <7.00 (67.2–349.4) | 7.00 (19.5–132.3) |
| FT4 (pmol/L) (Normal range) | T4: 50.50 (74.00–150.00) | 9.60 (14.00–23.00) | 0.11 (19.00–39.00) | 10.80 (12.00–22.00) | 4.24 (12.00–22.00) | 4.91 (14.00–23.00) |
| Patient | P1 and P2 | P3 | P4, P5, and P6 |
|---|---|---|---|
| Gene | POU1F1 | POU1F1 | POU1F1 |
| Locus | chr3:87313449C>T | chr3:87311268A>C | chr3:87309109G>A |
| Exon | 3 | 4 | 6 |
| c.DNA change (NM_000306.4) | c.428G>A | c.557T>G | c.811C>T |
| Amino acid change | p.(Arg143Gln) | p.(Leu186Arg) | p.(Arg271Trp) |
| Status in the patient | Homozygous | Homozygous | Heterozygous |
| Segregation | Paternal and maternal | Paternal and maternal | De novo |
| Inheritance pattern | Autosomal recessive | Autosomal recessive | Autosomal dominant |
| CADD (Phred score) | Damaging (33) | Damaging (31) | Damaging (28.7) |
| SIFT prediction | Deleterious | Deleterious | Deleterious |
| PolyPhen_2 | Probably damaging | Probably damaging | Probably damaging |
| Mutation Taster | Deleterious | Deleterious | Deleterious |
| Minor allele frequency | 0.000004–0.000008 | 0 | 0 |
| dbSNP154 | rs104893759 | - | rs104893755 |
| ClinVar | 13606 Pathogenic | - | 13603 Pathogenic |
| LOVD v3.0 | 0000886010 | - | - |
| GnomAD v2.1.1 | 1 Heterozygous | 0 | 0 |
| Pathogenicity (ACMG 2015) | Likely pathogenic PM2, PM3, PP3, PP4 | Likely pathogenic PM2, PM3, PP3, PP4 | Pathogenic PS2, PM1, PM2, PP3, PP4 |
| P1 | P2 | P3 | P4 | P5 | P6 | |
|---|---|---|---|---|---|---|
| Age at diagnosis | 1y7mo | 2mo | Neonate | 2y1mo | 9y4mo | 10mo |
| Age at thyroxine treatment | 1y7mo | 2mo | Neonate | 2y1mo | 9y4mo | 10mo |
| Age at rhGH treatment | 3y8mo | 23mo | 8mo | 2y1mo | 9y5mo | 12mo |
| Treatment | Levothyroxin + GH | Levothyroxin + GH | Levothyroxin + GH | Levothyroxin + GH | Levothyroxin + GH | Levothyroxin + GH |
| 5–10 | 5–10 | 5–10 | 5–10 | 5–10 | 5–10 |
| 25 | 25 | 25 | 25 | 25 | 25 |
| 27.5 | 27.5 | 25 | 32.6 | 22.5 | 28.3 |
| IGF1 (ng/mL) | ||||||
| 44.3 | 51.5 | 44.3 | 74.0 | 130.0 | 18.1 |
| 7.0 | 36.6 | 7.0 | 76.4 | 215.0 | 51.0 |
| 7.0 | 7.0 | 118.0 | 161.0 | 325.0 | |
| 147.0 | 386.0 | ||||
| 38.4 | 289.0 | ||||
| Height (cm) (SDS) | ||||||
| 71.0 (−7.3) | 71.0 (−4.8) | 62.0 (−3.6) | 64.0 (−6.6) | 79.0 (−9.2) | 57.5 (−6.4) |
| 82.6 (−5.58) | 82.0 (−2.69) | 86.0 (0.43) | 84.0 (−2.9) | 96.0 (−6.81) | 74.0 (−3.37) |
| 91 (−4.96) | 90 (−2.52) | 88 (−1.46) * | 94 (−2.18) | 108 (−5.63) | 86 (−2.12) |
| 99 (−4.37) | 100 (−1.74) | 100 (−0,47) | 101 (−2.08) | 124 (−4.33) | |
| 108 (−1.94) | 135 (−2.85) | ||||
| 116 (−1.4) | 143 (−2.55) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, H.T.; Nguyen, K.N.; Dien, T.M.; Can, T.B.N.; Nguyen, T.T.N.; Lien, N.T.K.; Tung, N.V.; Xuan, N.T.; Tao, N.T.; Nguyen, N.L.; et al. Identification of POU1F1 Variants in Vietnamese Patients with Combined Pituitary Hormone Deficiency. Int. J. Mol. Sci. 2025, 26, 2406. https://doi.org/10.3390/ijms26062406
Nguyen HT, Nguyen KN, Dien TM, Can TBN, Nguyen TTN, Lien NTK, Tung NV, Xuan NT, Tao NT, Nguyen NL, et al. Identification of POU1F1 Variants in Vietnamese Patients with Combined Pituitary Hormone Deficiency. International Journal of Molecular Sciences. 2025; 26(6):2406. https://doi.org/10.3390/ijms26062406
Chicago/Turabian StyleNguyen, Ha Thu, Khanh Ngoc Nguyen, Tran Minh Dien, Thi Bich Ngoc Can, Thi Thanh Ngan Nguyen, Nguyen Thi Kim Lien, Nguyen Van Tung, Nguyen Thi Xuan, Nguyen Thien Tao, Ngoc Lan Nguyen, and et al. 2025. "Identification of POU1F1 Variants in Vietnamese Patients with Combined Pituitary Hormone Deficiency" International Journal of Molecular Sciences 26, no. 6: 2406. https://doi.org/10.3390/ijms26062406
APA StyleNguyen, H. T., Nguyen, K. N., Dien, T. M., Can, T. B. N., Nguyen, T. T. N., Lien, N. T. K., Tung, N. V., Xuan, N. T., Tao, N. T., Nguyen, N. L., Tran, V. K., Mai, T. T. C., Tran, V. A., Nguyen, H. H., & Vu, C. D. (2025). Identification of POU1F1 Variants in Vietnamese Patients with Combined Pituitary Hormone Deficiency. International Journal of Molecular Sciences, 26(6), 2406. https://doi.org/10.3390/ijms26062406







