A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
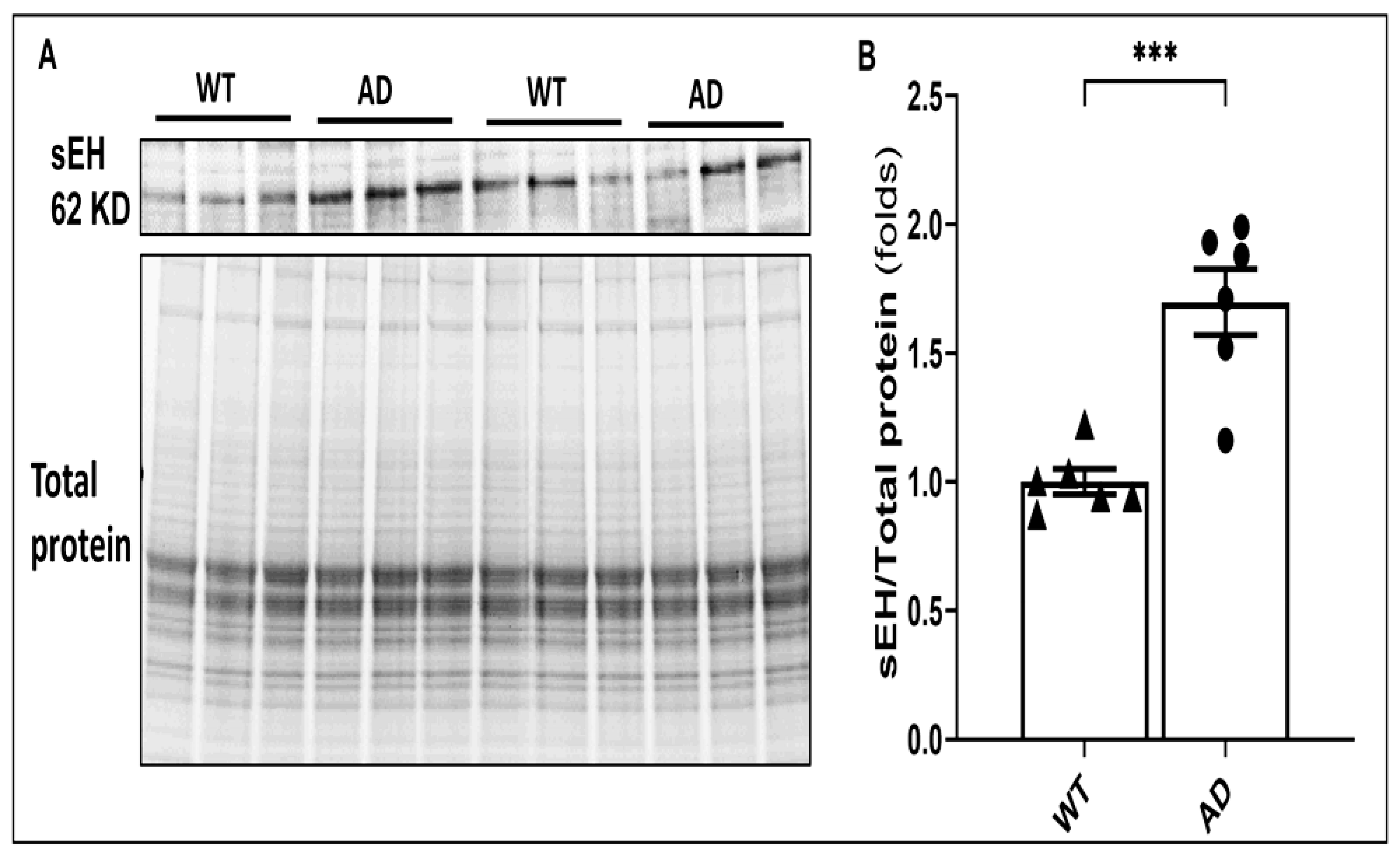
2.1. The Expression of sEH Is Elevated in AD Brains
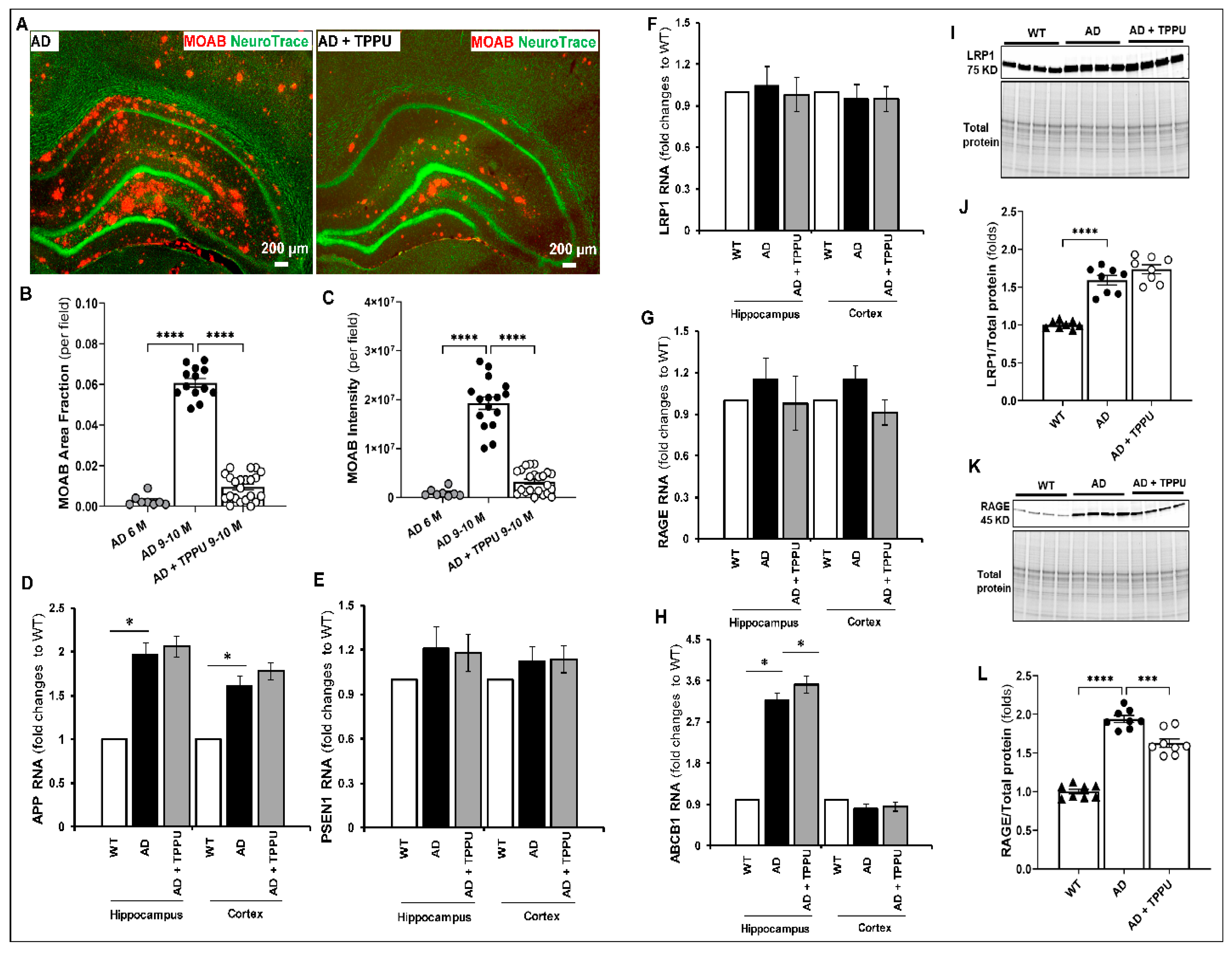
2.2. TPPU Attenuates Beta-Amyloid (Aβ) Accumulation in AD Rats
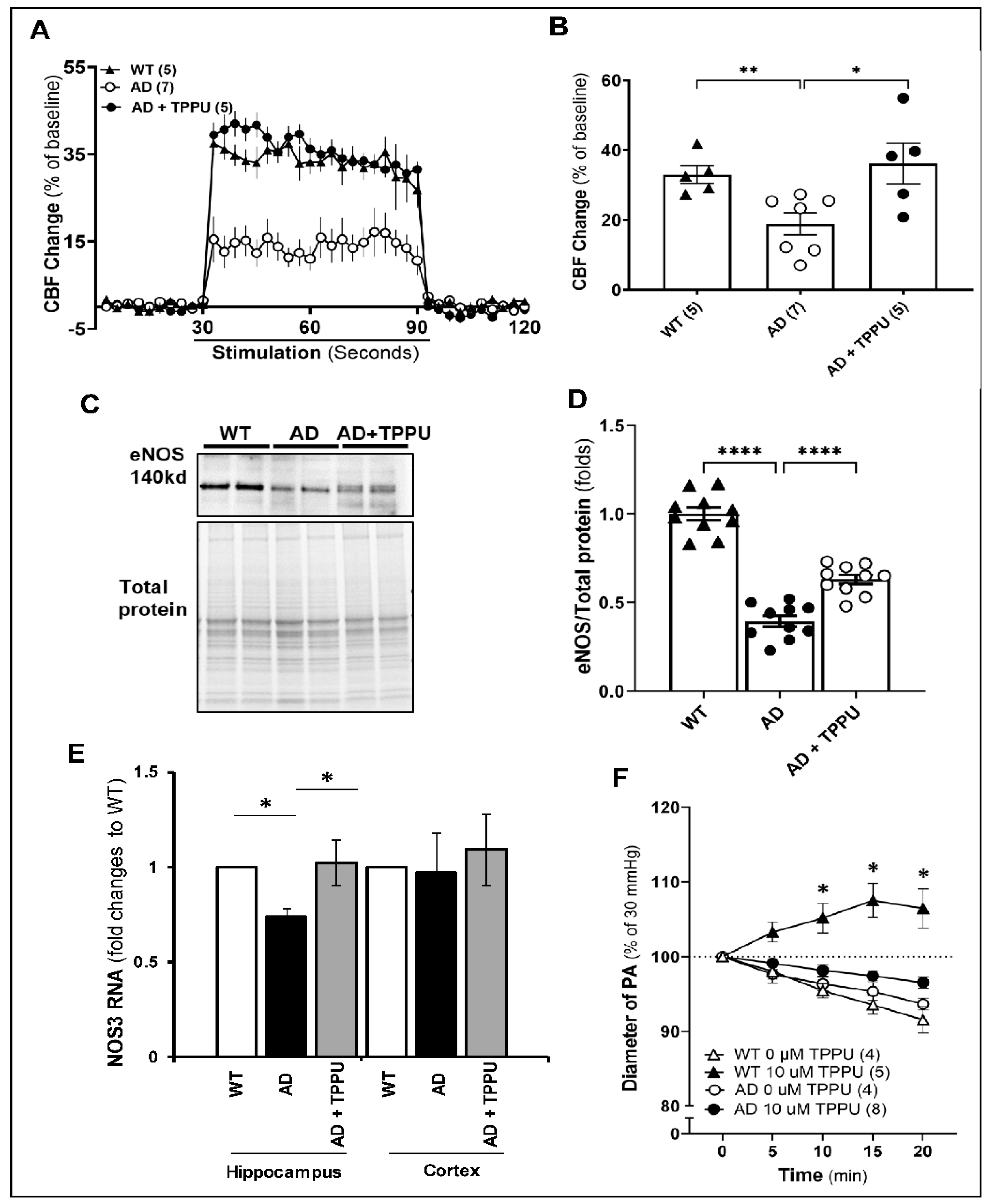
2.3. TPPU Normalizes Functional Hyperemic Responses in AD Rats
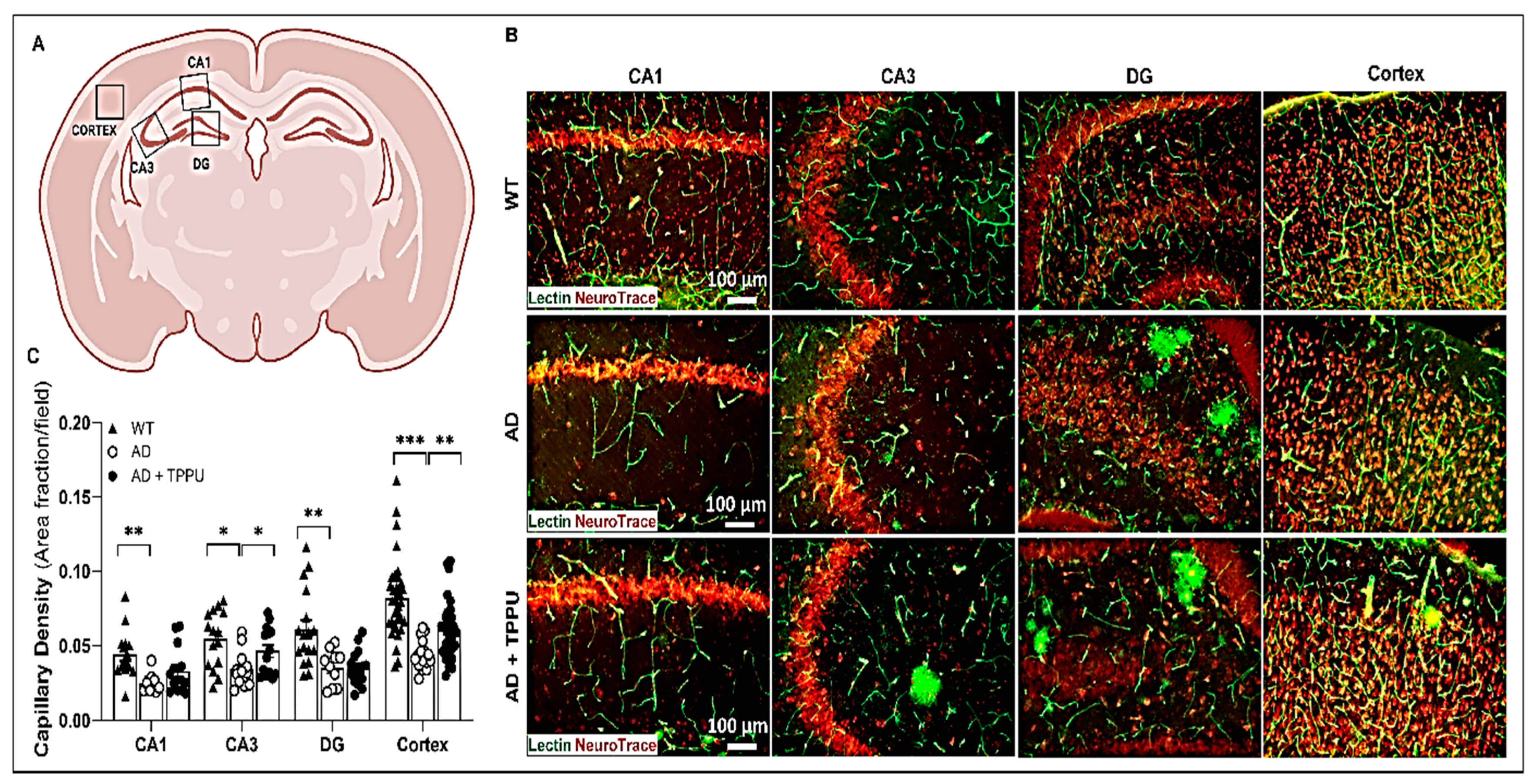
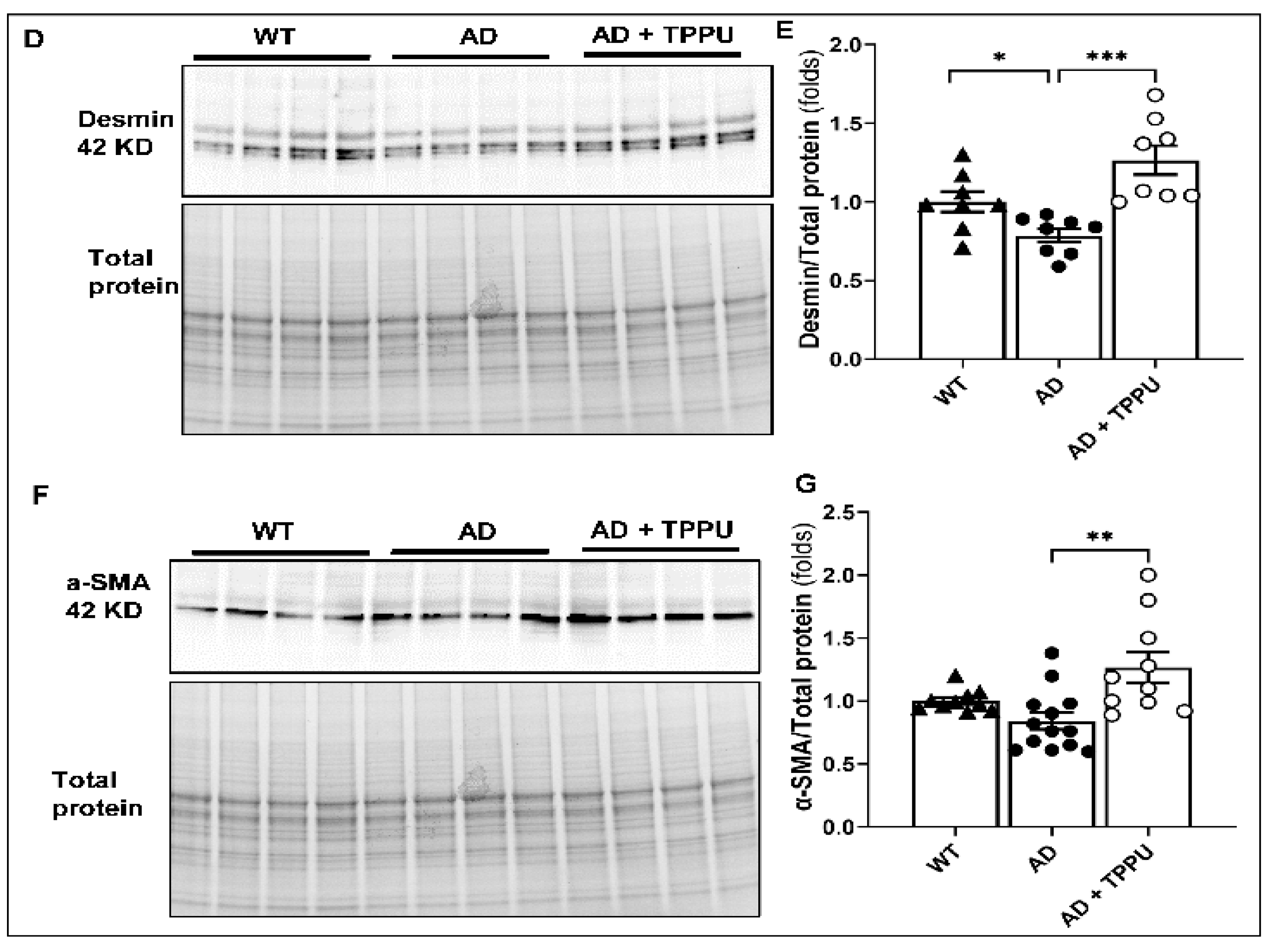
2.4. TPPU Attenuates Capillary Rarefaction in AD Rats
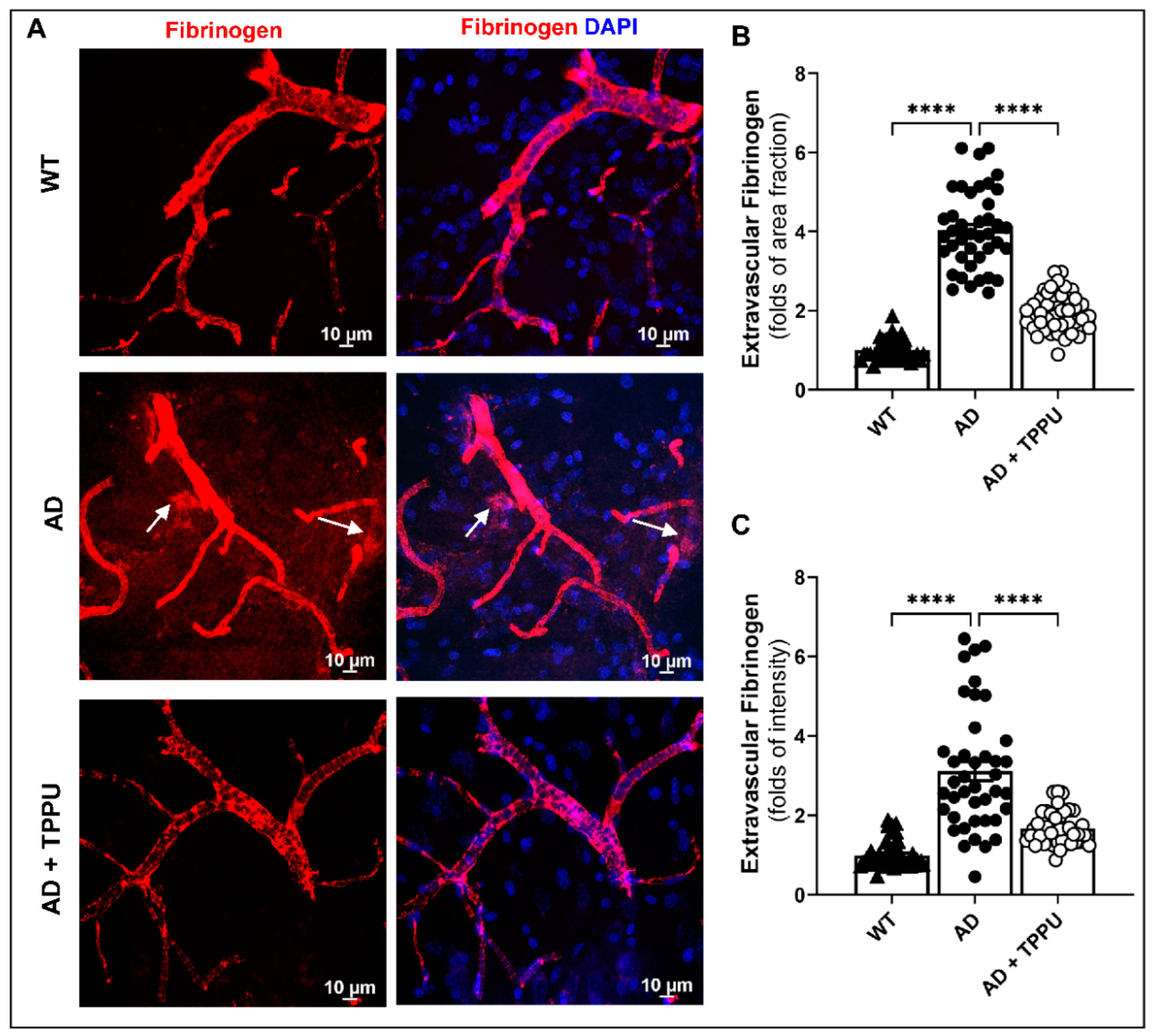
2.5. TPPU Improves Blood–Brain Barrier (BBB) Leakage in AD Rats
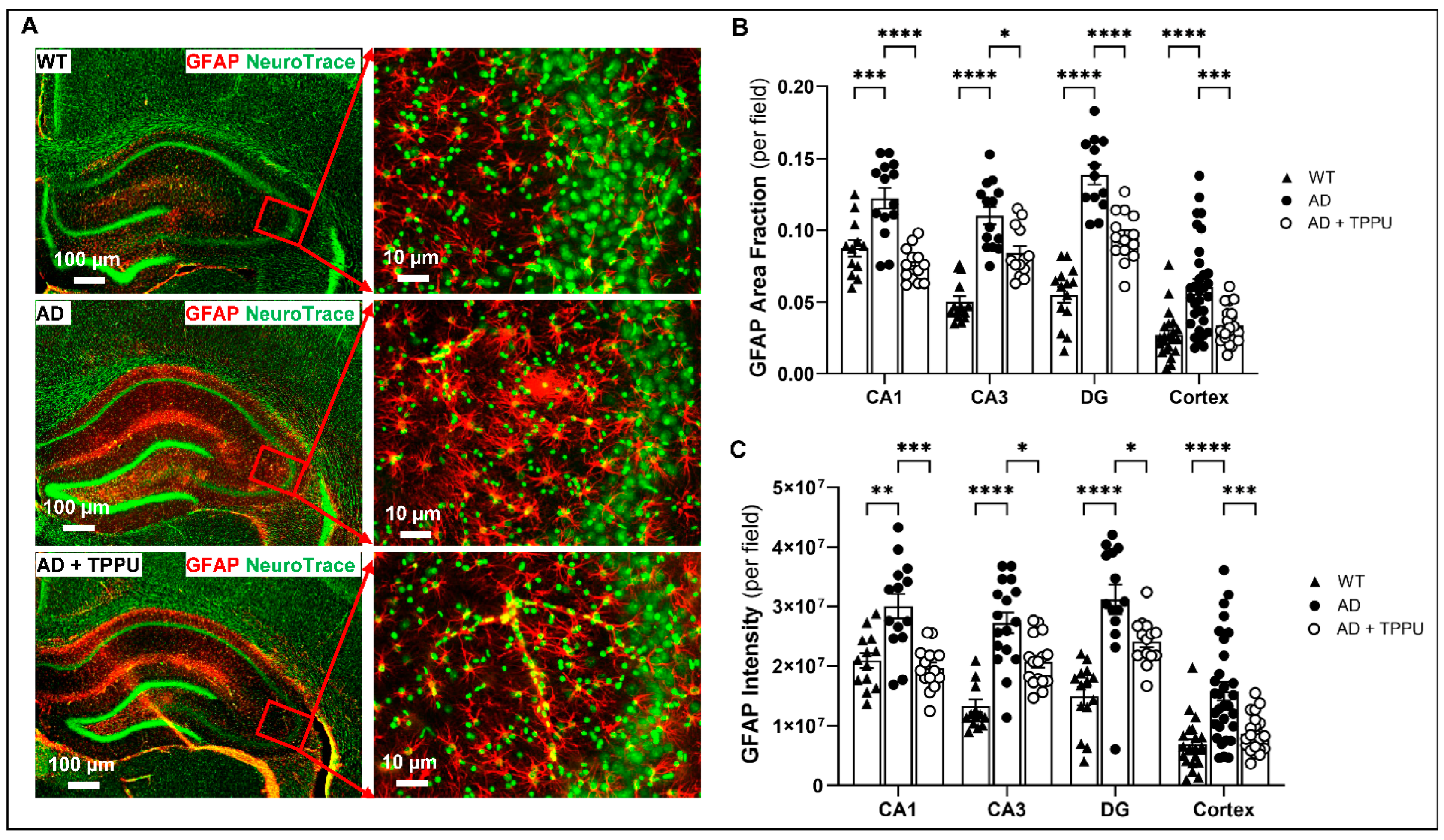
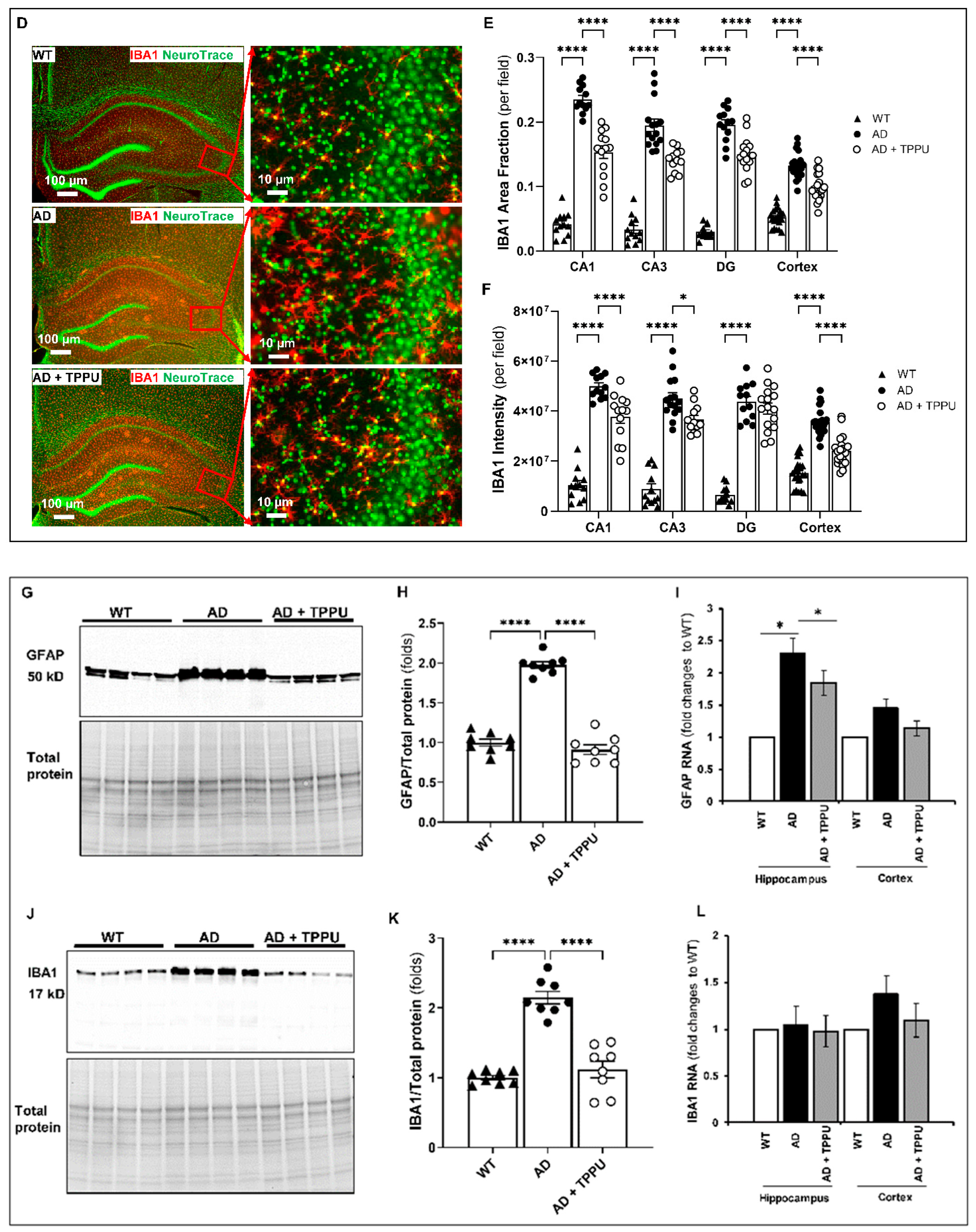
2.6. TPPU Attenuates Astrogliosis and Microgliosis in AD Rats
2.7. TPPU Improves Synaptic Deficits in AD Rats
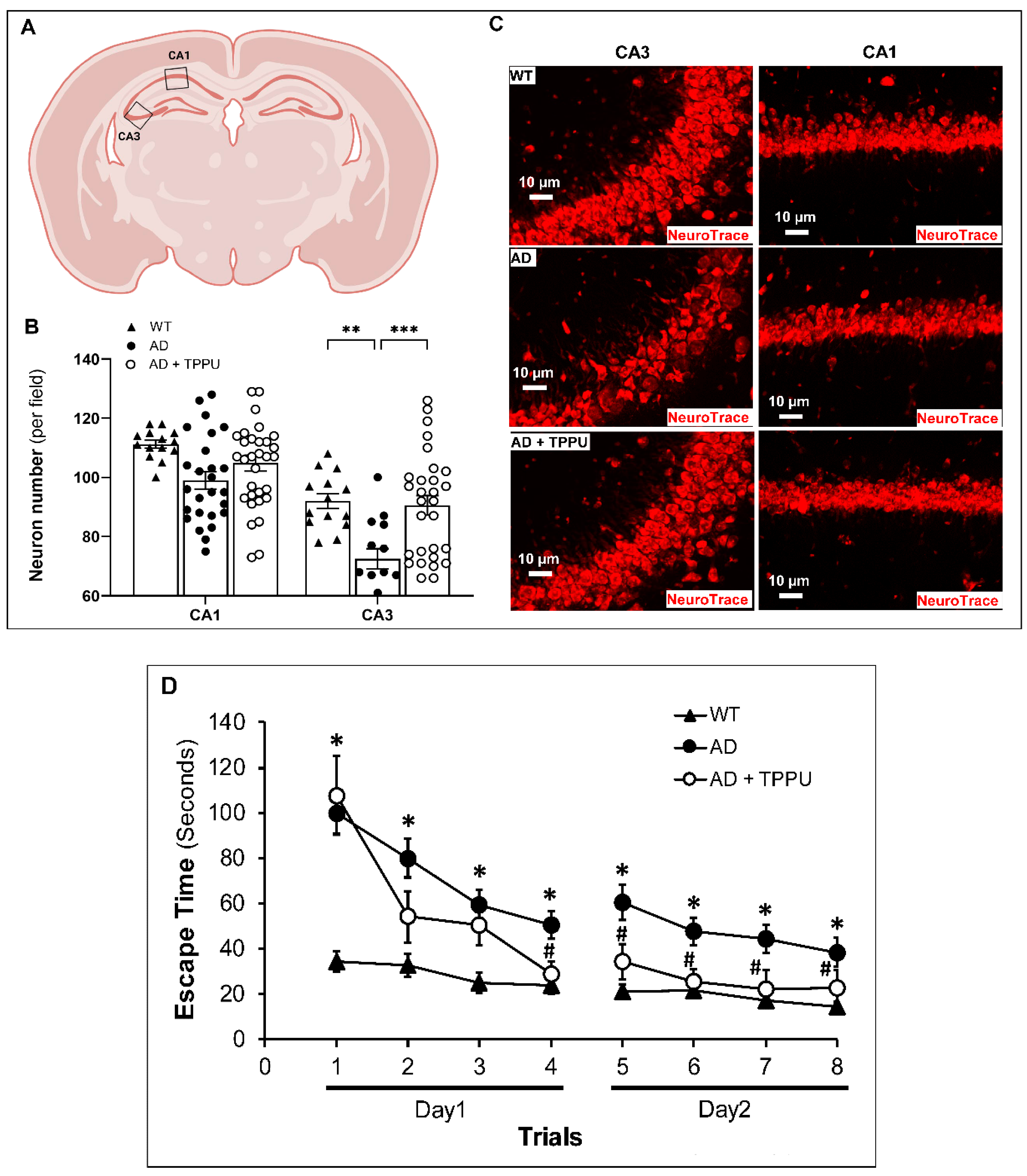
2.8. TPPU Improves Neurodegeneration and Cognitive Impairments in AD Rats
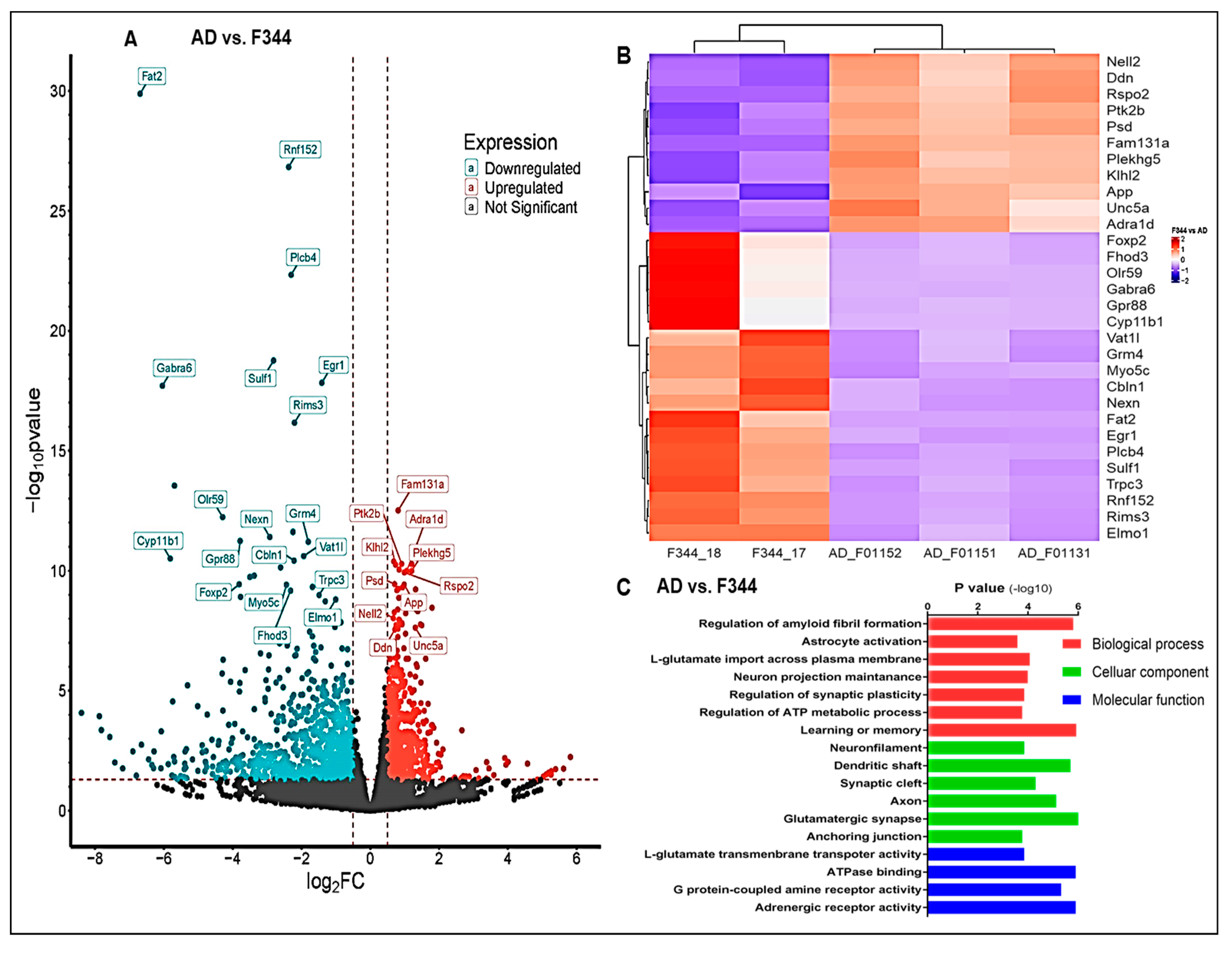
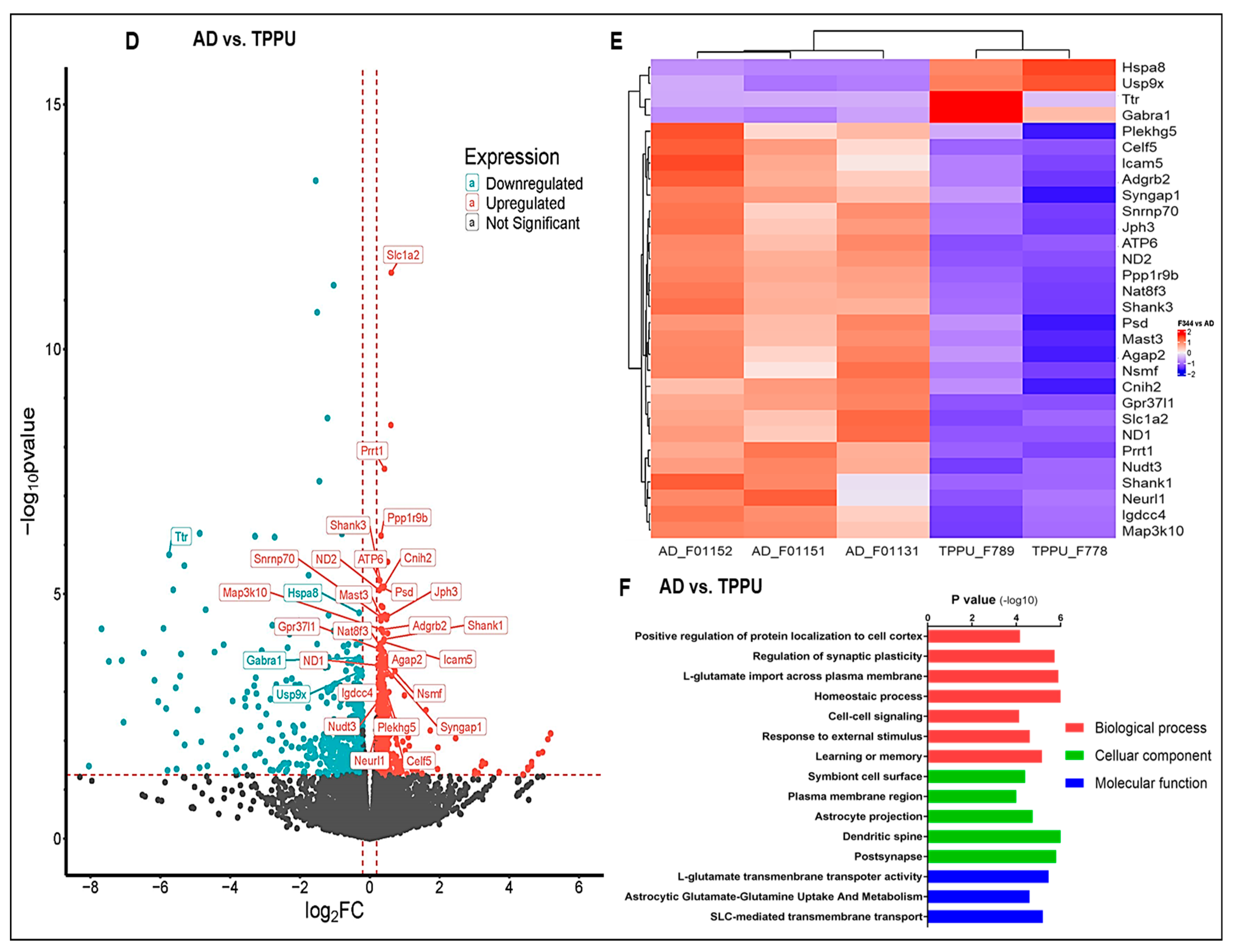
2.9. Transcriptome Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Study Design
4.2. Laser Speckle Imaging of Cerebral Hemodynamics In Vivo
4.3. Vascular Reactivity Ex Vivo
4.4. Western Blot
4.5. Immunohistochemistry
4.6. Eight-Arm Water Maze
4.7. RNA Sequencing and Bioinformatic Analysis
4.8. Statistics
4.9. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dementia. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 27 June 2024).
- Alzheimer’s Association Report. 2024 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2024, 20, 3708–3821. [Google Scholar] [CrossRef]
- Abanto, J.; Dwivedi, A.K.; Imbimbo, B.P.; Espay, A.J. Increases in amyloid-β42 slow cognitive and clinical decline in Alzheimer’s disease trials. Brain 2024, 147, 3513–3521. [Google Scholar] [CrossRef]
- Budd Haeberlein, S.; Aisen, P.S.; Barkhof, F.; Chalkias, S.; Chen, T.; Cohen, S.; Dent, G.; Hansson, O.; Harrison, K.; von Hehn, C.; et al. Two Randomized Phase 3 Studies of Aducanumab in Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2022, 9, 197–210. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, Y.; Ruan, S.; Hu, Y. Current Anti-Amyloid-beta Therapy for Alzheimer’s Disease Treatment: From Clinical Research to Nanomedicine. Int. J. Nanomed. 2023, 18, 7825–7845. [Google Scholar] [CrossRef] [PubMed]
- Iturria-Medina, Y.; Sotero, R.C.; Toussaint, P.J.; Mateos-Pérez, J.M.; Evans, A.C.; Weiner, M.W.; Aisen, P.; Petersen, R.; Jack, C.R.; Jagust, W.; et al. Early role of vascular dysregulation on late-onset Alzheimer’s disease based on multifactorial data-driven analysis. Nat. Commun. 2016, 7, 11934. [Google Scholar] [CrossRef] [PubMed]
- Arranz, A.M.; De Strooper, B. The role of astroglia in Alzheimer’s disease: Pathophysiology and clinical implications. Lancet Neurol. 2019, 18, 406–414. [Google Scholar] [CrossRef]
- Bracko, O.; Cruz Hernández, J.C.; Park, L.; Nishimura, N.; Schaffer, C.B. Causes and consequences of baseline cerebral blood flow reductions in Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2021, 41, 1501–1516. [Google Scholar] [CrossRef]
- Korte, N.; Nortley, R.; Attwell, D. Cerebral blood flow decrease as an early pathological mechanism in Alzheimer’s disease. Acta Neuropathol. 2020, 140, 793–810. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Kisler, K.; Montagne, A.; Toga, A.W.; Zlokovic, B.V. The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 2018, 21, 1318–1331. [Google Scholar] [CrossRef]
- Fang, X.; Fan, F.; Border, J.J.; Roman, R.J. Cerebrovascular Dysfunction in Alzheimer’s Disease and Transgenic Rodent Models. J. Exp. Neurol. 2024, 5, 42–64. [Google Scholar] [CrossRef]
- Iadecola, C.; Gottesman, R.F. Cerebrovascular Alterations in Alzheimer Disease. Circ. Res. 2018, 123, 406–408. [Google Scholar] [CrossRef]
- Zhu, W.M.; Neuhaus, A.; Beard, D.J.; Sutherland, B.A.; DeLuca, G.C. Neurovascular coupling mechanisms in health and neurovascular uncoupling in Alzheimer’s disease. Brain 2022, 145, 2276–2292. [Google Scholar] [CrossRef] [PubMed]
- Marques, F.; Sousa, J.C.; Sousa, N.; Palha, J.A. Blood-brain-barriers in aging and in Alzheimer’s disease. Mol. Neurodegener. 2013, 8, 38. [Google Scholar] [CrossRef]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood-brain barrier dysfunction? J. Exp. Med 2017, 214, 3151–3169. [Google Scholar] [CrossRef] [PubMed]
- Zenaro, E.; Piacentino, G.; Constantin, G. The blood-brain barrier in Alzheimer’s disease. Neurobiol. Dis. 2017, 107, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Tsartsalis, S.; Sleven, H.; Fancy, N.; Wessely, F.; Smith, A.M.; Willumsen, N.; Cheung, T.K.D.; Rokicki, M.J.; Chau, V.; Ifie, E.; et al. A single nuclear transcriptomic characterisation of mechanisms responsible for impaired angiogenesis and blood-brain barrier function in Alzheimer’s disease. Nat. Commun. 2024, 15, 2243. [Google Scholar] [CrossRef]
- Yue, Q.; Leng, X.; Xie, N.; Zhang, Z.; Yang, D.; Hoi, M.P.M. Endothelial Dysfunctions in Blood-Brain Barrier Breakdown in Alzheimer’s Disease: From Mechanisms to Potential Therapies. CNS Neurosci. Ther. 2024, 30, e70079. [Google Scholar] [CrossRef]
- Al-Ghraiybah, N.F.; Wang, J.; Alkhalifa, A.E.; Roberts, A.B.; Raj, R.; Yang, E.; Kaddoumi, A. Glial Cell-Mediated Neuroinflammation in Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23, 10572. [Google Scholar] [CrossRef]
- Huang, L.K.; Chao, S.P.; Hu, C.J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 18. [Google Scholar] [CrossRef]
- Kursun, O.; Karatas, H.; Bariskaner, H.; Ozturk, S. Arachidonic Acid Metabolites in Neurologic Disorders. CNS Neurol. Disord. Drug Targets 2022, 21, 150–159. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic acid metabolism in health and disease. Medcomm 2023, 4, e363. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Li, R.; Chen, G.; Hoopes, S.L.; Zeldin, D.C.; Wang, D.W. The Role of Cytochrome P450 Epoxygenases, Soluble Epoxide Hydrolase, and Epoxyeicosatrienoic Acids in Metabolic Diseases. Adv. Nutr. 2016, 7, 1122–1128. [Google Scholar] [CrossRef]
- Iyer, M.R.; Kundu, B.; Wood, C.M. Soluble epoxide hydrolase inhibitors: An overview and patent review from the last decade. Expert Opin. Ther. Pat. 2022, 32, 629–647. [Google Scholar] [CrossRef]
- Lee, H.T.; Lee, K.I.; Chen, C.H.; Lee, T.S. Genetic deletion of soluble epoxide hydrolase delays the progression of Alzheimer’s disease. J. Neuroinflammation 2019, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Comerota, M.M.; Wan, D.; Chen, F.; Propson, N.E.; Hwang, S.H.; Hammock, B.D.; Zheng, H. An epoxide hydrolase inhibitor reduces neuroinflammation in a mouse model of Alzheimer’s disease. Sci. Transl. Med. 2020, 12, eabb1206. [Google Scholar] [CrossRef] [PubMed]
- Di Lucente, J.; Freitas, H.R.; Wagner, K.M.; Hammock, B.D.; Maezawa, I.; Jin, L.-W. Efficacy of soluble epoxide hydrolase inhibition in a rat model of Alzheimer’s disease. Alzheimer’s Dement. 2021, 17, e054073. [Google Scholar] [CrossRef]
- Chen, W.; Wang, M.; Zhu, M.; Xiong, W.; Qin, X.; Zhu, X. 14,15-Epoxyeicosatrienoic Acid Alleviates Pathology in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2020, 40, 8188–8203. [Google Scholar] [CrossRef]
- Fan, F.; Border, J.J.; Tang, C.; Zhang, H.; Fang, X.; Liu, Y.; Hwang, S.H.; Hammock, B.D.; Roman, R.J. Abstract: Inhibition of Soluble Epoxide Hydrolase, a Potential Novel AD/ADRD Treatment? In Proceedings of the 19th International Winter Eicosanoid Conference, Baltimore, MD, USA, 15 October 2023. [Google Scholar]
- Node, K.; Huo, Y.; Ruan, X.; Yang, B.; Spiecker, M.; Ley, K.; Zeldin, D.C.; Liao, J.K. Anti-inflammatory properties of cytochrome P450 epoxygenase-derived eicosanoids. Science 1999, 285, 1276–1279. [Google Scholar] [CrossRef]
- Hu, J.; Dziumbla, S.; Lin, J.; Bibli, S.I.; Zukunft, S.; de Mos, J.; Awwad, K.; Fromel, T.; Jungmann, A.; Devraj, K.; et al. Inhibition of soluble epoxide hydrolase prevents diabetic retinopathy. Nature 2017, 552, 248–252. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Jarné-Ferrer, J.; Bellver-Sanchís, A.; Codony, S.; Puigoriol-Illamola, D.; Sanfeliu, C.; Oh, Y.; Lee, S.; Vázquez, S.; Pallàs, M. Novel molecular mechanism driving neuroprotection after soluble epoxide hydrolase inhibition: Insights for Alzheimer’s disease therapeutics. CNS Neurosci. Ther. 2024, 30, e14511. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Border, J.J.; Zhang, H.; Gregory, A.; Bai, S.; Fang, X.; Liu, Y.; Wang, S.; Hwang, S.H.; Gao, W.; et al. Inhibition of soluble epoxide hydrolase ameliorates cerebral blood flow autoregulation and cognition in Alzheimer’s disease and diabetes-related dementia rat models. Geroscience 2025, 47, 1–21. [Google Scholar] [CrossRef]
- Cohen, R.M.; Rezai-Zadeh, K.; Weitz, T.M.; Rentsendorj, A.; Gate, D.; Spivak, I.; Bholat, Y.; Vasilevko, V.; Glabe, C.G.; Breunig, J.J.; et al. A transgenic Alzheimer rat with plaques, tau pathology, behavioral impairment, oligomeric abeta, and frank neuronal loss. J. Neurosci. 2013, 33, 6245–6256. [Google Scholar] [CrossRef]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood-Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Fang, X.; Border, J.J.; Rivers, P.L.; Zhang, H.; Williams, J.M.; Fan, F.; Roman, R.J. Amyloid beta accumulation in TgF344-AD rats is associated with reduced cerebral capillary endothelial Kir2.1 expression and neurovascular uncoupling. Geroscience 2023, 45, 2909–2926. [Google Scholar] [CrossRef] [PubMed]
- Toth, P.; Tarantini, S.; Davila, A.; Valcarcel-Ares, M.N.; Tucsek, Z.; Varamini, B.; Ballabh, P.; Sonntag, W.E.; Baur, J.A.; Csiszar, A.; et al. Purinergic glio-endothelial coupling during neuronal activity: Role of P2Y1 receptors and eNOS in functional hyperemia in the mouse somatosensory cortex. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1837–H1845. [Google Scholar] [CrossRef] [PubMed]
- Hosford, P.S.; Gourine, A.V. What is the key mediator of the neurovascular coupling response? Neurosci. Biobehav. Rev. 2019, 96, 174–181. [Google Scholar] [CrossRef]
- Kitaguchi, H.; Ihara, M.; Saiki, H.; Takahashi, R.; Tomimoto, H. Capillary beds are decreased in Alzheimer’s disease but not in Binswanger’s disease. Neurosci. Lett. 2007, 417, 128–131. [Google Scholar] [CrossRef]
- Janota, C.S.; Brites, D.; Lemere, C.A.; Brito, M.A. Glio-vascular changes during ageing in wild-type and Alzheimer’s disease-like APP/PS1 mice. Brain Res. 2015, 1620, 153–168. [Google Scholar] [CrossRef][Green Version]
- Kouznetsova, E.; Klingner, M.; Sorger, D.; Sabri, O.; Grossmann, U.; Steinbach, J.; Scheunemann, M.; Schliebs, R. Developmental and amyloid plaque-related changes in cerebral cortical capillaries in transgenic Tg2576 Alzheimer mice. Int. J. Dev. Neurosci. 2006, 24, 187–193. [Google Scholar] [CrossRef]
- Chan-Ling, T.; Page, M.P.; Gardiner, T.; Baxter, L.; Rosinova, E.; Hughes, S. Desmin ensheathment ratio as an indicator of vessel stability: Evidence in normal development and in retinopathy of prematurity. Am J Pathol 2004, 165, 1301–1313. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef]
- Zheng, Z.; Chopp, M.; Chen, J. Multifaceted roles of pericytes in central nervous system homeostasis and disease. J Cereb Blood Flow Metab 2020, 40, 1381–1401. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Tang, C.; Liu, Y.; Border, J.J.; Roman, R.J.; Fan, F. Impact of impaired cerebral blood flow autoregulation on cognitive impairment. Front. Aging 2022, 3, 1077302. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Tang, C.; Zhang, H.; Border, J.J.; Liu, Y.; Shin, S.M.; Yu, H.; Roman, R.J.; Fan, F. Longitudinal characterization of cerebral hemodynamics in the TgF344-AD rat model of Alzheimer’s disease. Geroscience 2023, 45, 1471–1490. [Google Scholar] [CrossRef]
- DeBay, D.R.; Phi, T.T.; Bowen, C.V.; Burrell, S.C.; Darvesh, S. No difference in cerebral perfusion between the wild-type and the 5XFAD mouse model of Alzheimer’s disease. Sci. Rep. 2022, 12, 22174. [Google Scholar] [CrossRef]
- Zhukov, O.; He, C.; Soylu-Kucharz, R.; Cai, C.; Lauritzen, A.D.; Aldana, B.I.; Björkqvist, M.; Lauritzen, M.; Kucharz, K. Preserved blood-brain barrier and neurovascular coupling in female 5xFAD model of Alzheimer’s disease. Front. Aging Neurosci. 2023, 15, 1089005. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Patten, K.T.; Valenzuela, A.; Lein, P.J.; Taha, A.Y. Probing changes in brain esterified oxylipin concentrations during the early stages of pathogenesis in Alzheimer’s Disease transgenic rats. Neurosci. Lett. 2022, 791, 136921. [Google Scholar] [CrossRef]
- Hennebelle, M.; Metherel, A.H.; Kitson, A.P.; Otoki, Y.; Yang, J.; Lee, K.S.S.; Hammock, B.D.; Bazinet, R.P.; Taha, A.Y. Brain oxylipin concentrations following hypercapnia/ischemia: Effects of brain dissection and dissection time. J. Lipid Res. 2019, 60, 671–682. [Google Scholar] [CrossRef]
- Hardy, J.; Allsop, D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991, 12, 383–388. [Google Scholar] [CrossRef]
- Filosa, J.A.; Bonev, A.D.; Straub, S.V.; Meredith, A.L.; Wilkerson, M.K.; Aldrich, R.W.; Nelson, M.T. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat. Neurosci. 2006, 9, 1397–1403. [Google Scholar] [CrossRef]
- Longden, T.A.; Nelson, M.T. Vascular inward rectifier K+ channels as external K+ sensors in the control of cerebral blood flow. Microcirculation 2015, 22, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Longden, T.A.; Dabertrand, F.; Koide, M.; Gonzales, A.L.; Tykocki, N.R.; Brayden, J.E.; Hill-Eubanks, D.; Nelson, M.T. Capillary K+-sensing initiates retrograde hyperpolarization to increase local cerebral blood flow. Nat. Neurosci. 2017, 20, 717–726. [Google Scholar] [CrossRef]
- Attwell, D.; Buchan, A.M.; Charpak, S.; Lauritzen, M.; Macvicar, B.A.; Newman, E.A. Glial and neuronal control of brain blood flow. Nature 2010, 468, 232–243. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Ganesana, M.; Hwang, P.; Tan, X.; Kinkaid, M.M.; Sun, Y.Y.; Bian, E.; Weybright, A.; Sol-Church, K.; Eyo, U.B.; et al. Microglia modulate cerebral blood flow and neurovascular coupling through ectonucleotidase CD39. bioRxiv 2024. [Google Scholar] [CrossRef]
- Joo, I.L.; Lai, A.Y.; Bazzigaluppi, P.; Koletar, M.M.; Dorr, A.; Brown, M.E.; Thomason, L.A.; Sled, J.G.; McLaurin, J.; Stefanovic, B. Early neurovascular dysfunction in a transgenic rat model of Alzheimer’s disease. Sci. Rep. 2017, 7, 46427. [Google Scholar] [CrossRef]
- Wang, S.; Lv, W.; Zhang, H.; Liu, Y.; Li, L.; Jefferson, J.R.; Guo, Y.; Li, M.; Gao, W.; Fang, X.; et al. Aging exacerbates impairments of cerebral blood flow autoregulation and cognition in diabetic rats. Geroscience 2020, 42, 1387–1410. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Wang, S.; Guo, Y.; Fang, X.; Zheng, B.; Gao, W.; Yu, H.; Chen, Z.; Roman, R.J.; et al. Reduced pericyte and tight junction coverage in old diabetic rats are associated with hyperglycemia-induced cerebrovascular pericyte dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2021, 320, H549–H562. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 27 June 2024).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Chambers, J.; Dalgaard, P.; Gentleman, R.; Hornik, K.; Ihaka, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2008; Available online: https://cran.r-project.org/doc/manuals/r-release/ (accessed on 24 January 2024).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kevin Blighe, S.R.; Lewis, M. EnhancedVolcano: Publication-Ready Volcano Plots with Enhanced Coloring and Labeling. Available online: https://bioconductor.org/packages/devel/bioc/vignettes/EnhancedVolcano/inst/doc/EnhancedVolcano.html (accessed on 27 June 2024).
- Kolde, R. pheatmap: Pretty Heatmaps. R Package Version 1.0.12. Available online: https://rdrr.io/cran/pheatmap/ (accessed on 27 June 2024).
- Xu, S.; Hu, E.; Cai, Y.; Xie, Z.; Luo, X.; Zhan, L.; Tang, W.; Wang, Q.; Liu, B.; Wang, R.; et al. Using clusterProfiler to characterize multiomics data. Nat. Protoc. 2024, 19, 3292–3320. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, L.; Raudvere, U.; Kuzmin, I.; Vilo, J.; Peterson, H. gprofiler2—an R package for gene list functional enrichment analysis and namespace conversion toolset g:Profiler. F1000Research 2020, 9, ELIXIR-709. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Border, J.J.; Zhang, H.; Challagundla, L.; Kaur, J.; Hwang, S.H.; Hammock, B.D.; Fan, F.; Roman, R.J. A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 2433. https://doi.org/10.3390/ijms26062433
Fang X, Border JJ, Zhang H, Challagundla L, Kaur J, Hwang SH, Hammock BD, Fan F, Roman RJ. A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(6):2433. https://doi.org/10.3390/ijms26062433
Chicago/Turabian StyleFang, Xing, Jane J. Border, Huawei Zhang, Lavanya Challagundla, Jasleen Kaur, Sung Hee Hwang, Bruce D. Hammock, Fan Fan, and Richard J. Roman. 2025. "A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 6: 2433. https://doi.org/10.3390/ijms26062433
APA StyleFang, X., Border, J. J., Zhang, H., Challagundla, L., Kaur, J., Hwang, S. H., Hammock, B. D., Fan, F., & Roman, R. J. (2025). A Soluble Epoxide Hydrolase Inhibitor Improves Cerebrovascular Dysfunction, Neuroinflammation, Amyloid Burden, and Cognitive Impairments in the hAPP/PS1 TgF344-AD Rat Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 26(6), 2433. https://doi.org/10.3390/ijms26062433






