Improved Salt Tolerance in Brassica napus L. Overexpressing a Synthetic Deinocuccus Stress-Resistant Module DICW
Abstract
1. Introduction
2. Results
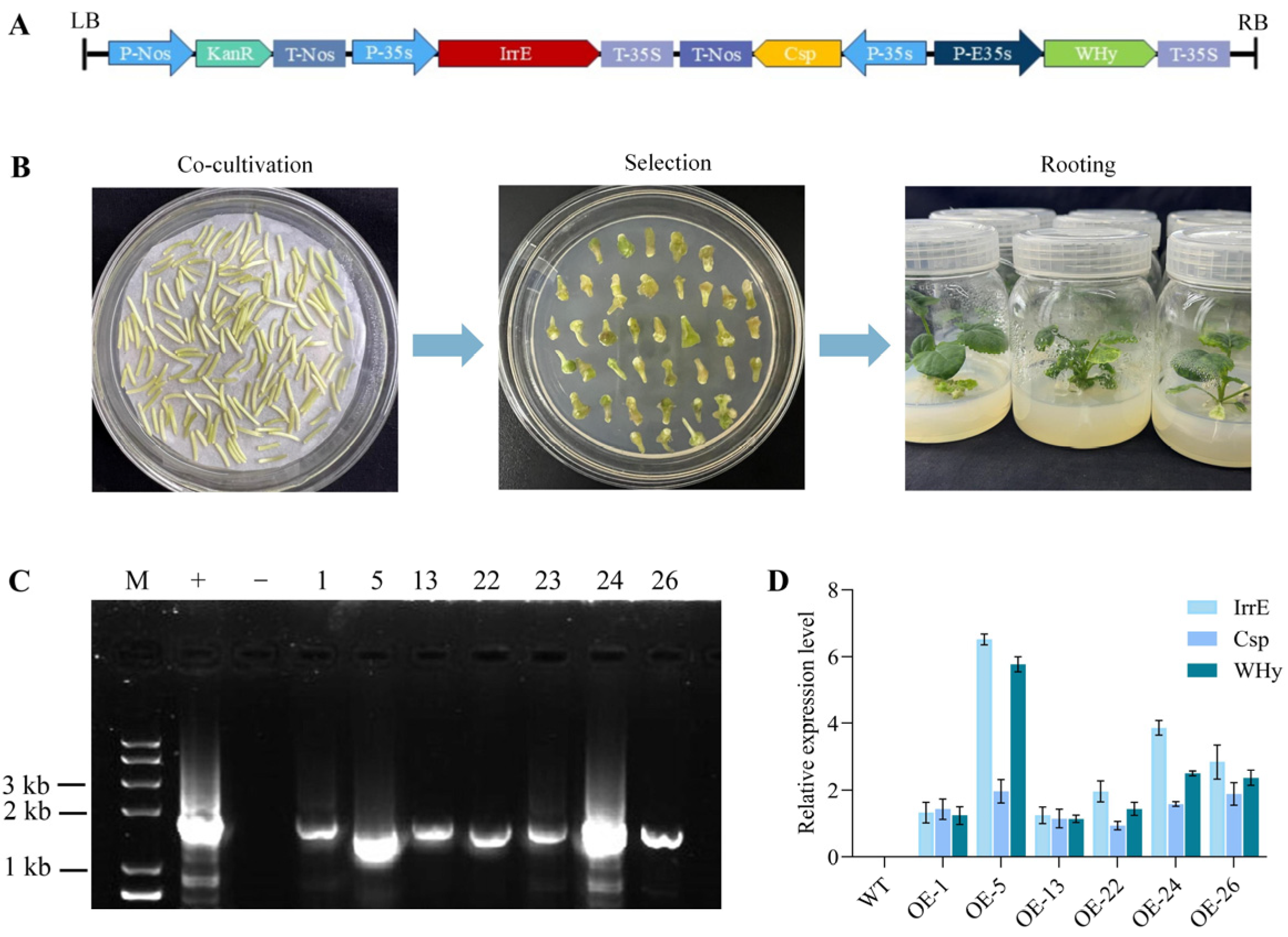
2.1. Generation of Transgenic B. napus Plants Overexpressing DICW
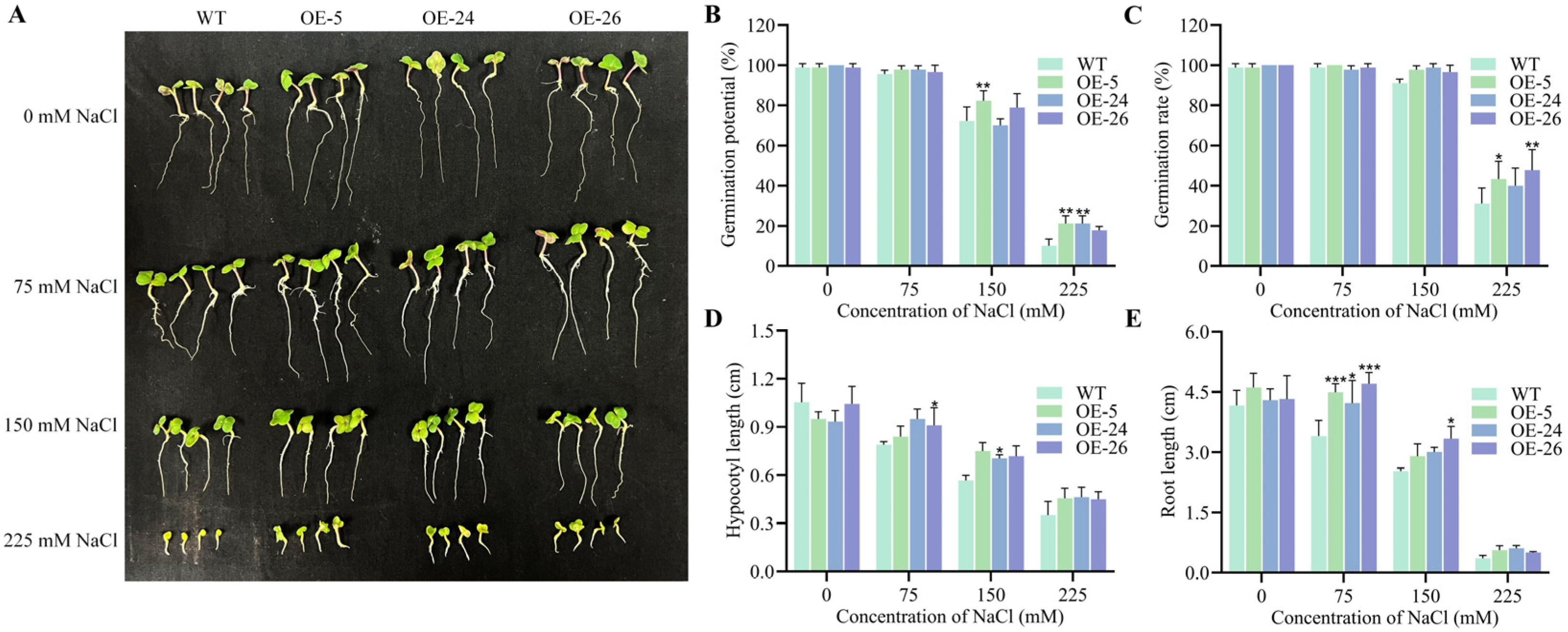
2.2. DICW Resistance-Module Promotes B. napus Growth Under Salt Stress
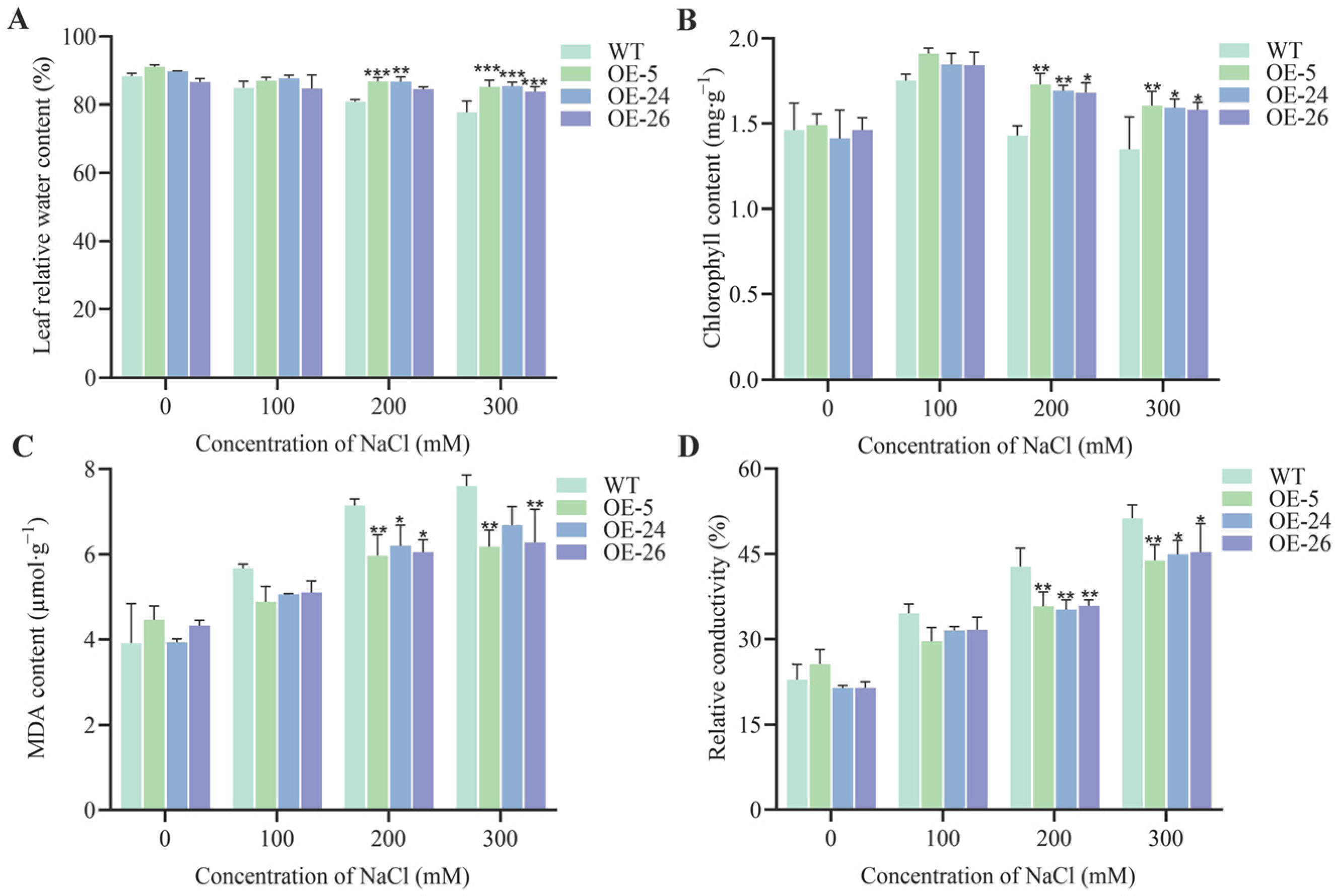
2.3. DICW Resistance Module Enhances Salt Tolerance in B. napus
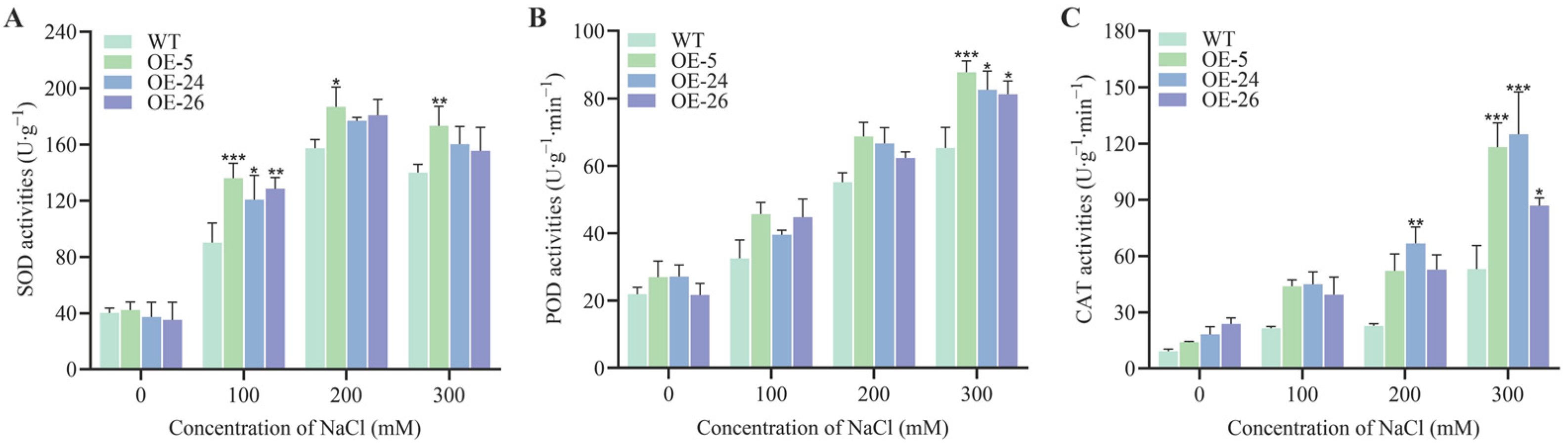
2.4. Antioxidant Capacity of B. napus Is Enhanced by the Resistance-Module DICW
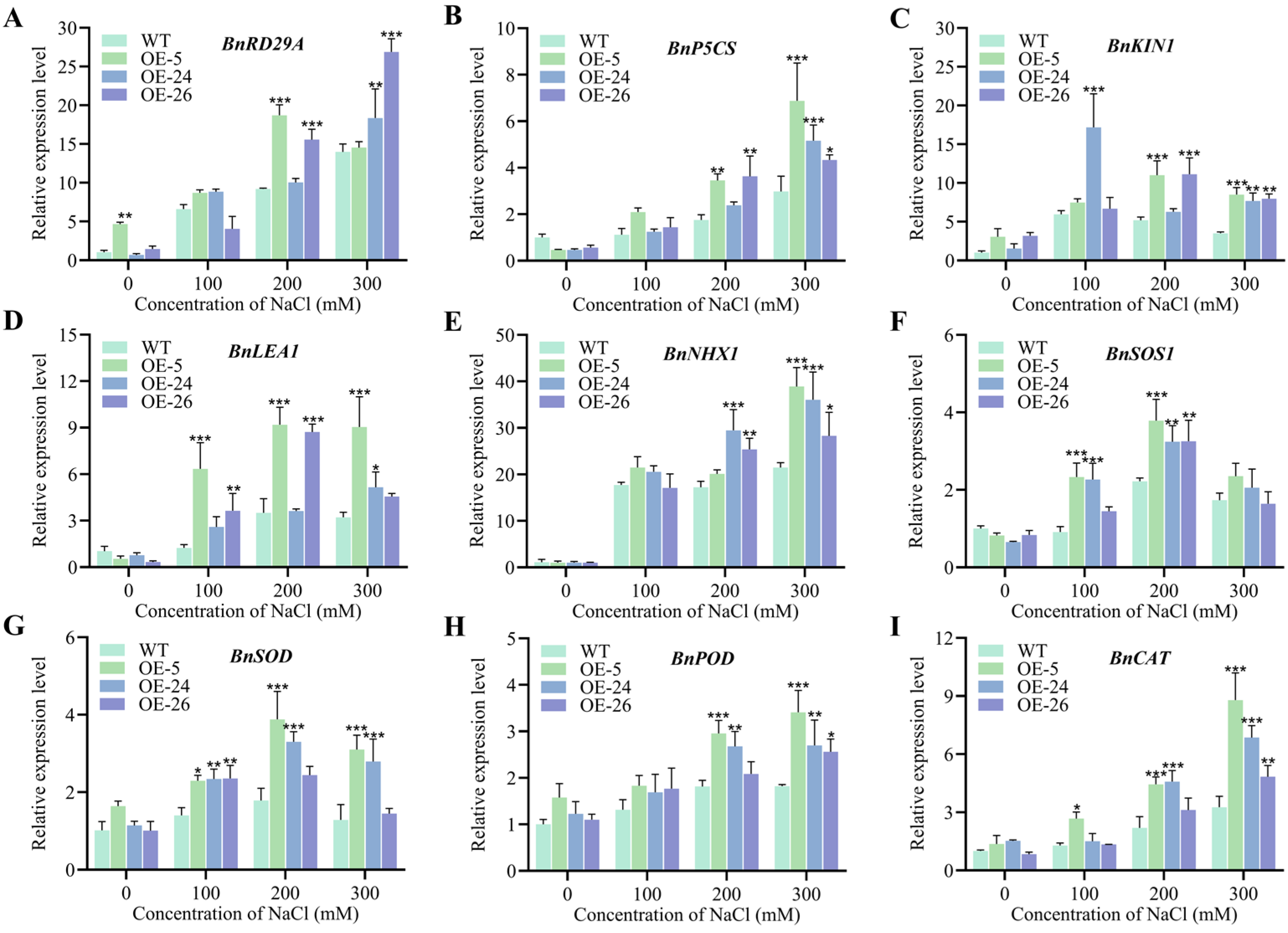
2.5. Expression of Stress-Related Genes in DICW Transgenic Oilseed Rape Lines Under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growing Conditions
4.2. Construction and Transformation of Expression Vectors
4.3. Positive Identification of Transgenic Plants
4.4. Determination of Seed Germination Index
4.5. Measurement of Physiological Indicators
4.6. qRT-PCR Analysis of Stress-Related Genes
4.7. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.Q.; Chai, Y.H.; Wang, X.S.; Huang, L.Y.; Luo, X.M.; Qiu, C.; Liu, Q.H.; Guan, X.Y. Bacterial Community in Saline Farmland Soil on the Tibetan Plateau: Responding to Salinization While Resisting Extreme Environments. BMC Microbiol. 2021, 21, 119. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.J.; Xu, X.X.; Zhang, R.D.; Chen, X.F.; Xing, Y.F.; Li, B.; Liu, C.; Zhou, Y.F. Effect of Short-Term Combined Alkaline Stress on Antioxidant Metabolism, Photosynthesis, and Leaf-Air Temperature Difference in Sorghum. Photosynthetica 2022, 60, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Duan, X.; Luo, L.; Dai, S.; Ding, Z.; Xia, G. How Plant Hormones Mediate Salt Stress Responses. Trends Plant Sci. 2020, 25, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Guo, Y. Elucidating the Molecular Mechanisms Mediating Plant Salt-Stress Responses. New Phytol. 2018, 217, 523–539. [Google Scholar] [CrossRef]
- Zhang, L.; Xing, L.; Dai, J.; Li, Z.; Zhang, A.; Wang, T.; Liu, W.; Li, X.; Han, D. Overexpression of a Grape WRKY Transcription Factor VhWRKY44 Improves the Resistance to Cold and Salt of Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 7437. [Google Scholar] [CrossRef]
- Waheed, A.; Zhou, L.; Wang, M.; Hailiang, X.; Tong, Z.; Wang, C.; Aili, A. Integrative mechanisms of plant salt tolerance: Biological pathways, phytohormonal regulation, and technological innovations. Plant Stress 2024, 14, 100652. [Google Scholar] [CrossRef]
- Tan, Z.D.; Han, X.; Dai, C.; Lu, S.P.; He, H.Z.; Yao, X.; Chen, P.; Yang, C.; Zhao, L.; Yang, Q.Y.; et al. Functional Genomics of Brassica napus: Progresses, Challenges, and Perspectives. J. Integr. Plant Biol. 2024, 66, 484–509. [Google Scholar] [CrossRef]
- Huang, D.B.; Kosentka, P.Z.; Liu, W.S. Synthetic Biology Approaches in Regulation of Targeted Gene Expression. Curr. Opin. Plant Biol. 2021, 63, 102036. [Google Scholar] [CrossRef]
- Zhang, J.H.; Zhao, Y.S.; Cao, Y.X.; Yu, Z.P.; Wang, G.P.; Li, Y.Q.; Ye, X.Q.; Li, C.F.; Lin, X.; Song, H. Synthetic sRNA-Based Engineering of Escherichia coli for Enhanced Production of Full-Length Immunoglobulin G. Biotechnol. J. 2020, 15, 1900363. [Google Scholar] [CrossRef]
- Lee, S.B.; Lee, S.; Lee, H.; Kim, J.; Choi, H.; Lee, S.; Kim, B. Engineering Nicotiana benthamiana for Chrysoeriol Production Using Synthetic Biology Approaches. Front. Plant Sci. 2024, 15, 1458916. [Google Scholar] [CrossRef]
- Fan, Y.; Yin, X.; Xie, Q.; Xia, Y.; Wang, Z.; Song, J.; Zhou, Y.; Jiang, X. Co-expression of SpSOS1 and SpAHA1 in Transgenic Arabidopsis Plants Improves Salinity Tolerance. BMC Plant Biol. 2019, 19, 74. [Google Scholar] [CrossRef] [PubMed]
- Hua, Y.; Narumi, I.; Gao, G.; Tian, B.; Satoh, K.; Kitayama, S.; Shen, B. PprI: A General Switch Responsible for Extreme Radioresistance of Deinococcus radiodurans. Biochem. Biophys. Res. Commun. 2003, 306, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tan, Y.S.; Chen, K.; Ntakirutimana, S.; Liu, Z.H.; Li, B.Z.; Yuan, Y.J. Global Regulator IrrE on Stress Tolerance: A Review. Crit. Rev. Biotechnol. 2024, 44, 1439–1459. [Google Scholar] [CrossRef] [PubMed]
- Juntawong, P.; Sorenson, R.; Bailey-Serres, J. Cold Shock Protein 1 Chaperones mRNAs During Translation in Arabidopsis thaliana. Plant J. 2013, 74, 1016–1028. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, J.; Liu, X.; Liu, Y.; Guo, C.; Zhang, L.; Han, J.; Wu, X.; Xue, D.; Gomaa, A.E.; et al. DrwH, a Novel WHy Domain-Containing Hydrophobic LEA5C Protein from Deinococcus radiodurans, Protects Enzymatic Activity under Oxidative Stress. Sci. Rep. 2017, 7, 9281. [Google Scholar] [CrossRef]
- Ciccarelli, F.D.; Bork, P. The WHy Domain Mediates the Response to Desiccation in Plants and Bacteria. Bioinformatics 2005, 21, 1304–1307. [Google Scholar] [CrossRef]
- Yu, T.F.; Xu, Z.S.; Guo, J.K.; Wang, Y.X.; Abernathy, B.; Fu, J.D.; Chen, X.; Zhou, Y.B.; Chen, M.; Ye, X.G.; et al. Improved Drought Tolerance in Wheat Plants Overexpressing a Synthetic Bacterial Cold Shock Protein Gene SeCspA. Sci. Rep. 2017, 7, 44050. [Google Scholar] [CrossRef]
- Xia, W.; Zong, J.; Zheng, K.; Wang, Y.; Zhang, D.; Guo, S.; Sun, G. DgCspC Gene Overexpression Improves Cotton Yield and Tolerance to Drought and Salt Stress Comparison with Wild-Type Plants. Front. Plant Sci. 2022, 13, 985900. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Y.; Zhou, X.; Wang, C.; Wang, C.; Fu, J.; Wei, T. Overexpression of a Harpin-Encoding Gene popW from Ralstonia solanacearum Primed Antioxidant Defenses with Enhanced Drought Tolerance in Tobacco Plants. Plant Cell Rep. 2016, 35, 1333–1344. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Dai, Q.; Feng, B.; Zuo, K.; Lin, M. Salt Tolerance Conferred by Expression of a Global Regulator IrrE from Deinococcus radiodurans in Rapeseed. Mol. Breed. 2016, 36, 88. [Google Scholar] [CrossRef]
- Zhu, X.X.; Tang, C.; Zhang, T.; Zhang, S.L.; Wu, J.Y.; Wang, P. PbrCSP1, a Pollen Tube-specific Cold Shock Domain Protein, is Essential for the Growth and Cold Resistance of Pear Pollen Tubes. Mol. Breed. 2024, 44, 18. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Jiang, S.S.; Pan, J.W.; Cai, G.H.; Li, D.Q. Group 5 LEA Protein, ZmLEA5C, Enhance Tolerance to Osmotic and Low Temperature Stresses in Transgenic Tobacco and Yeast. Plant Physiol. Biochem. 2014, 84, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Huo, Q.; Gong, M.; Jiang, Y.; Yang, X.; Kong, M.; He, J.; Zhang, Q.; Song, J.; Li, X.; Han, W.; et al. Microencapsulated Microbial Seed Coating Could Improve Soil Environment and Maize Grain Yield in Saline Soil. Plants 2024, 13, 3139. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Shao, H.; Tang, X. Recent Advances in Utilizing Transcription Factors to Improve Plant Abiotic Stress Tolerance by Transgenic Technology. Front. Plant Sci. 2016, 7, 67. [Google Scholar] [CrossRef]
- Lindquist, J.A.; Mertens, P.R. Cold Shock Proteins: From Cellular Mechanisms to Pathophysiology and Disease. Cell Commun. Signal 2018, 16, 63. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, Q.; Liu, M.; Zhou, H.; Ma, C.; Wang, P. Regulation of Plant Responses to Salt Stress. Int. J. Mol. Sci. 2021, 22, 4609. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, J.; Linyerera, S.M.; Magwanga, R.O.; Hou, Y.; Wang, Y.; Xu, Y.; Khan, A.; Yu, S.; Zhou, Z.; et al. Overexpression and Knockdown of Cotton GhdadD Gene Reveals its Drought and Salt Stress Tolerance Role. iScience 2024, 27, 108664. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Chen, H.; Wang, P.; Wang, P.; Song, C.; Zhang, X.; Wang, D. Multi-Gene Co-Expression Can Improve Comprehensive Resistance to Multiple Abiotic Stresses in Brassica Napus L. Plant Sci. 2018, 274, 410–419. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Wijewardene, I.; Hu, R.B.; Shen, G.X.; Zhang, J.L.; Zhang, H. Co-overexpression of in Arabidopsis thaliana Greatly Increases Tolerance to Salt and Drought Stresses. Environ. Exp. Bot. 2022, 200, 104934. [Google Scholar] [CrossRef]
- Shen, Z.; Cheng, X.; Li, X.; Deng, X.; Dong, X.; Wang, S.; Pu, X. Effects of Silicon Application on Leaf Structure and Physiological Characteristics of Glycyrrhiza uralensis Fisch. and Glycyrrhiza inflata Bat. under Salt Treatment. BMC Plant Biol. 2022, 22, 390. [Google Scholar] [CrossRef]
- Majeed, J.A.; Bibi, S.; Mahmood, A.; Ali, L.; Safdar, M.E.; Seleiman, M.F.; Abidin, Z.U.; Alhammad, B.A.; Asghar, M.A. Optimizing Tomato (Lycopersicon esculentum) Yield Under Salt Stress: The Physiological and Biochemical Effects of Foliar Thiourea Application. Plants 2024, 13, 3318. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Tu, Y.; Liao, C.; Guo, P.; Tian, Y.; Zhou, Y.; Xie, Q.; Chen, G.; Hu, Z. Overexpression of SlALC Increases Drought and Salt Tolerance and Affects Fruit Dehiscence in Tomatoes. Int. J. Mol. Sci. 2024, 25, 9433. [Google Scholar] [CrossRef] [PubMed]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance During Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Khare, T.; Srivastava, A.K.; Suprasanna, P.; Kumar, V. Individual and Additive Stress Impacts of Na+ and Cl− on Proline Metabolism and Nitrosative Responses in Rice. Plant Physiol. Biochem. 2020, 152, 44–52. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, L.; Wei, Z.H.; Bai, Q.Y.; Zhao, C.A.; Zhang, S.H.; Pan, J.L.; Yu, J.X.; Zhang, S.; Wei, J. Gamma-aminobutyric Acid (GABA) Improves Salinity Stress Tolerance in Soybean Seedlings by Modulating their Mineral Nutrition, Osmolyte Contents, and Ascorbate-glutathione Cycle. BMC Plant Biol. 2024, 24, 365. [Google Scholar]
- Xu, J.; Wang, T.; Wang, X.; Yan, H.; Liu, P.; Hou, X.; Gao, Y.; Yang, L.; Zhang, L. Exogenous Eugenol Alleviates Salt Stress in Tobacco Seedlings by Regulating the Antioxidant System and Hormone Signaling. Int. J. Mol. Sci. 2024, 25, 6771. [Google Scholar] [CrossRef]
- Liang, X.; Li, Y.; Yao, A.; Liu, W.; Yang, T.; Zhao, M.; Zhang, B.; Han, D. Overexpression of MxbHLH18 Increased Iron and High Salinity Stress Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2022, 23, 8007. [Google Scholar] [CrossRef]
- Wei, H.; Ruan, J.; Zhou, R.; Bai, Y.; Liu, M.; Jiang, F.; Wu, Z. Screening and Verification of Aquaporin Gene AsPIP1-3 in Garlic (Allium sativum L.) under Salt and Drought Stress. Horticulturae 2024, 10, 738. [Google Scholar] [CrossRef]
- Tan, Y.S.; Li, J.H.; Wang, P.L.; Wang, D.N.; Liu, B.C.; Phetmany, S.; Li, Y.X.; Xie, Q.J.; Gao, C.Q. The PHD Transcription Factor ThPHD5 Regulates Antioxidant Enzyme Activity to Increase Salt Tolerance in Tamarix hispida. Plant Sci. 2025, 350, 112319. [Google Scholar] [CrossRef]
- Koubaa, S.; Brini, F. Functional Analysis of a Wheat Group 3 Late Embryogenesis Abundant Protein (TdLEA3) in Arabidopsis thaliana under Abiotic and Biotic Stresses. Plant Physiol. Biochem. 2020, 156, 396–406. [Google Scholar] [CrossRef]
- Yu, C.; Zhou, F.; Wang, R.; Ran, Z.; Tan, W.; Jiang, L.; Cui, S.; Xie, Z.; Xiao, Y.; Zhou, Y.; et al. B2, an Abscisic Acid Mimic, Improves Salinity Tolerance in Winter Wheat Seedlings via Improving Activity of Antioxidant Enzymes. Front. Plant Sci. 2022, 13, 916287. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Tang, S.; Mei, F.; Peng, X.; Li, J.; Li, X.; Yan, X.; Zeng, X.; Liu, F.; Wu, Y.; et al. BnSIP1-1, a Trihelix Family Gene, Mediates Abiotic Stress Tolerance and ABA Signaling in Brassica napus. Front. Plant Sci. 2017, 8, 44. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, M.; Zhao, M.; Yu, M.; Zheng, X.; Tian, Y.; Sun, Z.; Liu, X.; Wang, C. The Δ1-pyrroline-5-carboxylate Synthetase Family Performs Diverse Physiological Functions in Stress Responses in Pear (Pyrus betulifolia). Front. Plant Sci. 2022, 13, 1066765. [Google Scholar] [CrossRef] [PubMed]
- Msanne, J.; Lin, J.; Stone, J.M.; Awada, T. Characterization of Abiotic Stress-Responsive Arabidopsis thaliana RD29A and RD29B Genes and Evaluation of Transgenes. Planta 2011, 234, 97–107. [Google Scholar] [CrossRef]
- Chen, M.; She, Z.; Aslam, M.; Liu, T.; Wang, Z.; Qi, J.; Niu, X. Genomic Insights of the WRKY Genes in Kenaf (Hibiscus cannabinus L.) Reveal That HcWRKY44 Improves the Plant’s Tolerance to the Salinity Stress. Front. Plant Sci. 2022, 13, 984233. [Google Scholar] [CrossRef]
- Pandey, M.; Paladi, R.K.; Srivastava, A.K.; Suprasanna, P. Thiourea and Hydrogen Peroxide Priming Improved K+ Retention and Source-Sink Relationship for Mitigating Salt Stress in Rice. Sci. Rep. 2021, 11, 3000. [Google Scholar] [CrossRef]
- Dubrovina, A.S.; Kiselev, K.V.; Khristenko, V.S.; Aleynova, O.A. VaCPK20, a Calcium-Dependent Protein Kinase Gene of Wild Grapevine Vitis amurensis Rupr., Mediates Cold and Drought Stress Tolerance. J. Plant Physiol. 2015, 185, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, P.; Bao, Z.; Ma, Q.; Duan, L.; Bao, A.; Zhang, J.; Wang, S. SOS1, HKT1;5, and NHX1 Synergistically Modulate Na+ Homeostasis in the Halophytic Grass Puccinellia tenuiflora. Front. Plant Sci. 2017, 8, 576. [Google Scholar] [CrossRef]
- Ding, C.; Zhang, W.; Li, D.; Dong, Y.; Liu, J.; Huang, Q.; Su, X. Effect of Overexpression of JERFs on Intracellular K+/Na+ Balance in Transgenic Poplar (Populus alba × P. berolinensis) Under Salt Stress. Front. Plant Sci. 2020, 11, 1192. [Google Scholar] [CrossRef]
- Rajput, V.D.; Harish; Singh, R.K.; Verma, K.K.; Sharma, L.; Quiroz-Figueroa, F.R.; Meena, M.; Gour, V.S.; Minkina, T.; Sushkova, S.; et al. Recent Developments in Enzymatic Antioxidant Defence Mechanism in Plants with Special Reference to Abiotic Stress. Biology 2021, 10, 267. [Google Scholar] [CrossRef]
- Yao, Y.; Yang, Y.; Pan, Y.; Liu, Z.; Hou, X.; Li, Y.; Zhang, H.; Wang, C.; Liao, W. Crucial Roles of Trehalose and 5-Azacytidine in Alleviating Salt Stress in Tomato: Both Synergistically and Independently. Plant Physiol. Biochem. 2023, 203, 108075. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc Oxide Nanoparticles Alleviate Chilling Stress in Rice (Oryza sativa L.) by Regulating Antioxidative System and Chilling Response Transcription Factors. Molecules 2021, 26, 2196. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alaraidh, I.A.; Alsahli, A.A.; Alamri, S.A.; Ali, H.M.; Alayafi, A.A. Bacillus firmus (SW5) Augments Salt Tolerance in Soybean (Glycine max L.) by Modulating Root System Architecture, Antioxidant Defense Systems and Stress-Responsive Genes Expression. Plant Physiol. Biochem. 2018, 132, 375–384. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Yao, X.; Liu, X.; Qiao, Z.; Liu, Y.; Li, X.; Jiang, X. Brassinolide Can Improve Drought Tolerance of Maize Seedlings under Drought Stress: By Inducing the Photosynthetic Performance, Antioxidant Capacity and ZmMYB Gene Expression of Maize Seedlings. J. Soil Sci. Plant Nutr. 2022, 22, 2092–2104. [Google Scholar] [CrossRef]
- Shao, Z.; Dai, L.; Liu, L.; Qiang, S.; Song, X. Stress Increases Ecological Risk of Glufosinate-Resistant Transgene Located on Alien Chromosomes in Hybrids Between Transgenic Brassica napus and Wild Brassica juncea. Plants 2025, 14, 572. [Google Scholar] [CrossRef]
- Patanè, C.; Cosentino, S.L.; Romano, D.; Toscano, S. Relative Water Content, Proline, and Antioxidant Enzymes in Leaves of Long Shelf-Life Tomatoes under Drought Stress and Rewatering. Plants 2022, 11, 3045. [Google Scholar] [CrossRef]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological Responses of Contrasting Rice Genotypes to Salt Stress at Reproductive Stage. Rice Sci. 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, W.; Tian, R.; Li, C.; Ji, Y.; Li, T.; Wei, C.; Chen, Z. Morphological, Physiological, and Secondary Metabolic Responses of Taraxacum officinale to Salt Stress. Plant Physiol. Biochem. 2022, 189, 71–82. [Google Scholar] [CrossRef]
- Li, W.; Li, P.; Chen, H.; Zhong, J.; Liang, X.; Wei, Y.; Zhang, L.; Wang, H.; Han, D. Overexpression of a Fragaria vesca 1R-MYB Transcription Factor Gene (FvMYB114) Increases Salt and Cold Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2023, 24, 5261. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Q.; Zhang, L.; Jiang, S.; Su, B.; Li, Z.; Shuai, Y.; Wang, J. Improved Salt Tolerance in Brassica napus L. Overexpressing a Synthetic Deinocuccus Stress-Resistant Module DICW. Int. J. Mol. Sci. 2025, 26, 2500. https://doi.org/10.3390/ijms26062500
Dai Q, Zhang L, Jiang S, Su B, Li Z, Shuai Y, Wang J. Improved Salt Tolerance in Brassica napus L. Overexpressing a Synthetic Deinocuccus Stress-Resistant Module DICW. International Journal of Molecular Sciences. 2025; 26(6):2500. https://doi.org/10.3390/ijms26062500
Chicago/Turabian StyleDai, Qilin, Lingling Zhang, Shijie Jiang, Bodan Su, Zhaoqin Li, Yinying Shuai, and Jin Wang. 2025. "Improved Salt Tolerance in Brassica napus L. Overexpressing a Synthetic Deinocuccus Stress-Resistant Module DICW" International Journal of Molecular Sciences 26, no. 6: 2500. https://doi.org/10.3390/ijms26062500
APA StyleDai, Q., Zhang, L., Jiang, S., Su, B., Li, Z., Shuai, Y., & Wang, J. (2025). Improved Salt Tolerance in Brassica napus L. Overexpressing a Synthetic Deinocuccus Stress-Resistant Module DICW. International Journal of Molecular Sciences, 26(6), 2500. https://doi.org/10.3390/ijms26062500





