Sex Steroids and Brain-Derived Neurotrophic Factor Interactions in the Nervous System: A Comprehensive Review of Scientific Data
Abstract
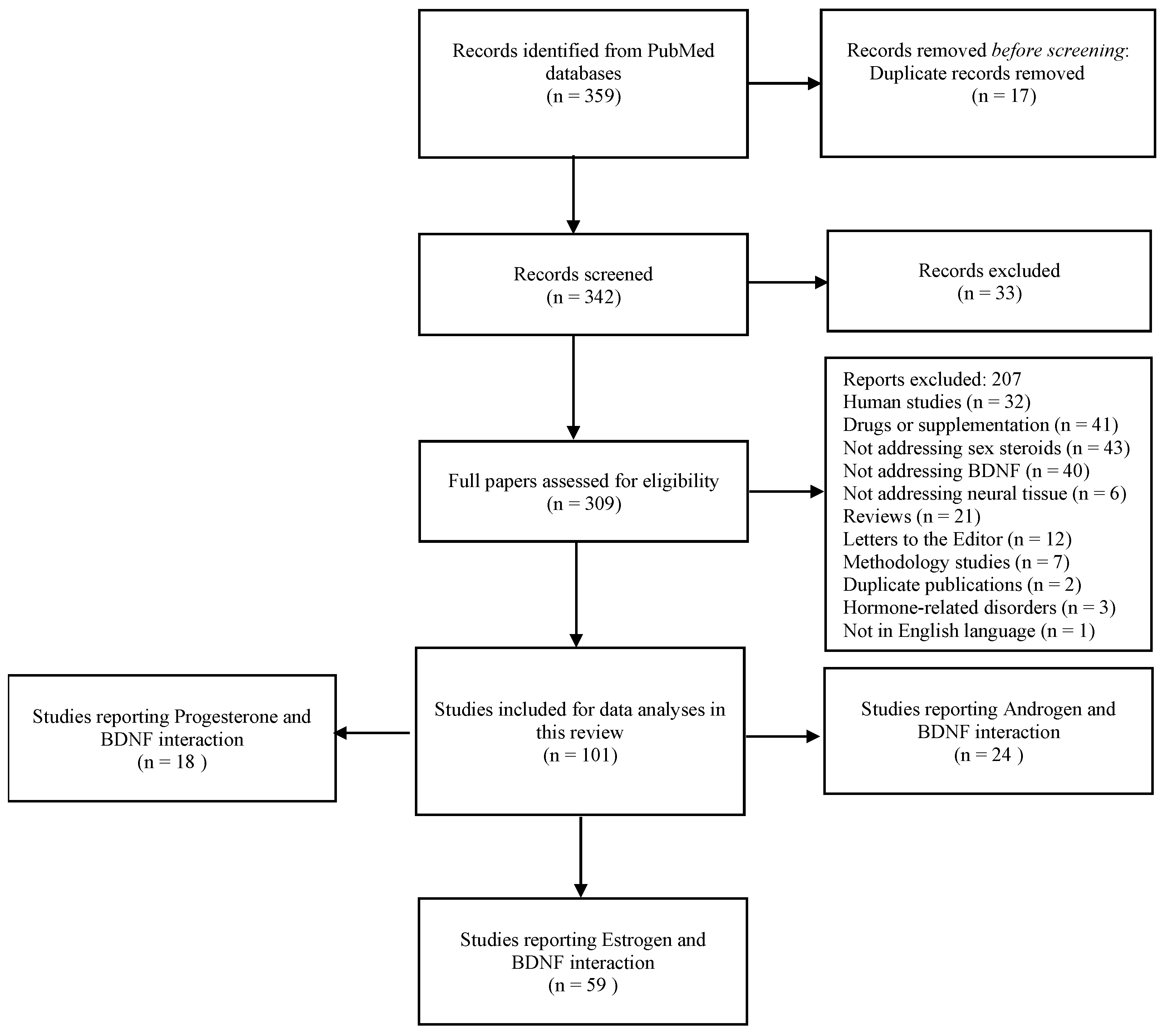
:1. Introduction
2. Methods
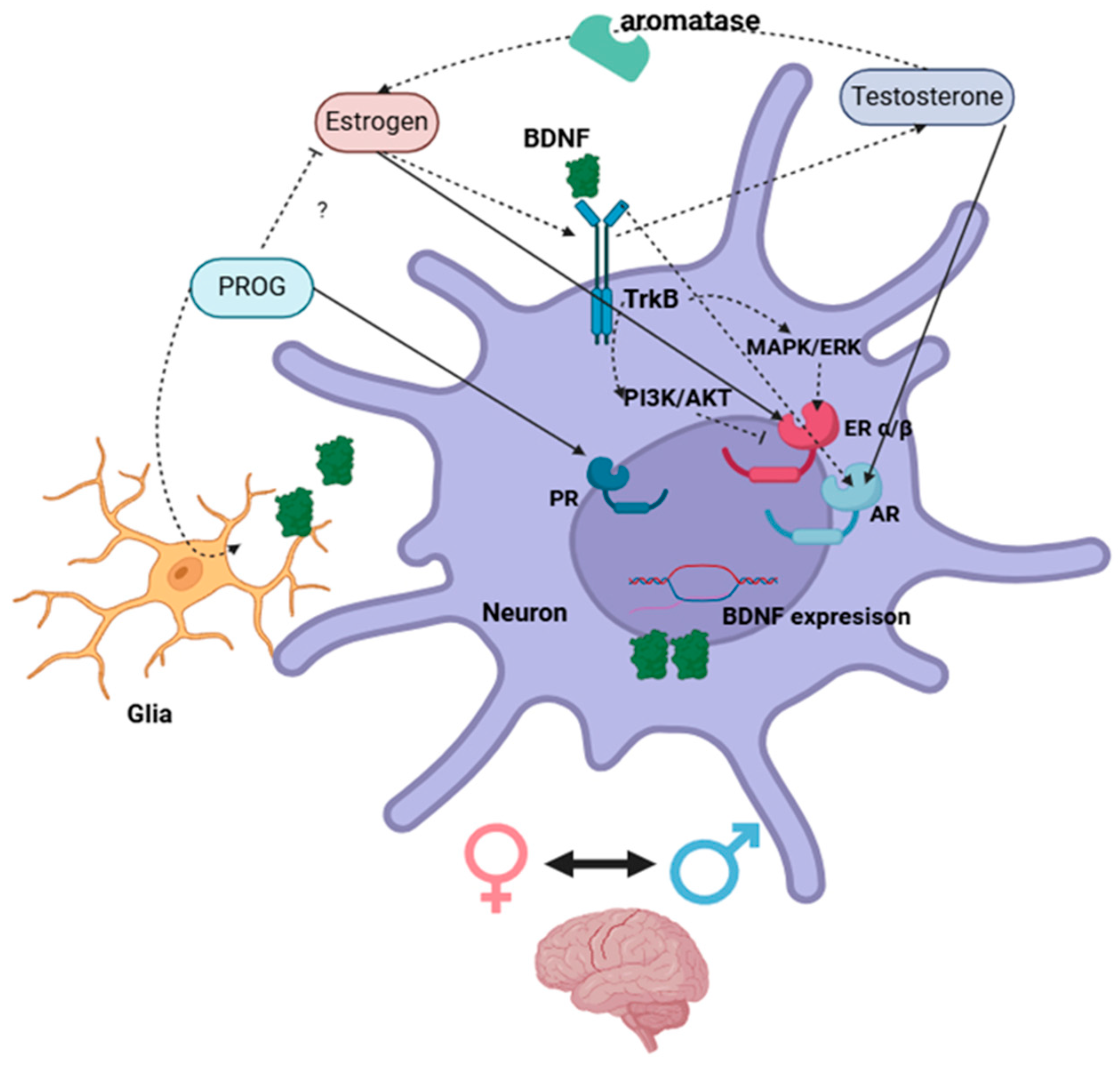
3. Estrogen and BDNF Interactions
4. Progesterone and BDNF Regulation
| Title | Design/Methods | Aim/Experiment | Main Findings | |
|---|---|---|---|---|
| 1 | Treatment with estrogen and progesterone affects relative levels of brain-derived neurotrophic factor mRNA and protein in different regions of the adult rat brain Gibbs, 1999. [95] | Ex vivo, RT-PCR, ELISA. | To examine the acute effects of estrogen and PROG on levels of BDNF expression and protein in different brain regions in adult mice. | Estrogen or estrogen + PROG increased BDNF expression and protein levels in the pyriform cortex of mice. Increases in BDNF expression in the hippocampus accompanied a decrease in BDNF protein. |
| 2 | Progesterone prevents estrogen-induced dendritic spine formation in cultured hippocampal neurons Murphy and Segal, 2000. [96] | In vitro, Hippocampal cultures, Immunocyto chemistry. | To examine PROG effects on estrogen-induced formation of dendritic spines in hippocampal cell cultures. | PROG did not affect the estrogen-induced downregulation of BDNF, but it did block the effect of estrogen on CREB phosphorylation. |
| 3 | Progesterone counteracts estrogen-induced increases in neurotrophins in the aged female rat brain Bimonte-Nelson, Nelson, and Granholm, 2004. [98] | Ex vivo, ELISA. | To test estrogen and estrogen + PROG effects on neurotrophin levels in cognitive brain regions in aged OVX mice. | Estrogen treatment increased BDNF, NGF, and NT3 levels in the mice’s entorhinal cortexes, and PROG abated these effects, and dropped BDNF levels in aged OVX non-treated mice. |
| 4 | Progesterone up-regulates neuronal brain-derived neurotrophic factor expression in the injured spinal cord González et al., 2004. [99] | Ex vivo, In situ hybridization, Immunocytochemistry. | To demonstrate that BDNF increases with PROG treatment in ventral horn motoneurons from spinal cord injured mice. | Spinal cord injury reduces BDNF expression levels by 50% in spinal motoneurons. PROG enhances BDNF in the motoneurons of lesioned spinal cord mice. |
| 5 | Progesterone treatment of spinal cord injury: Effects on Receptors, Neurotrophins, and Myelination De Nicola et al., 2006. [100] | In vitro, RT-PCR, In situ hybridization, Immunocytochemistry. | To describe the response of PR to injury and hormone treatment. | PROG increases BDNF expression and protein in motoneurons in injured rats. These increases were correlated with increased TrkB and phosphorylated CREB in motoneurons. |
| 6 | Progesterone increases Brain-Derived Neurotrophic Factor Expression and Protects Against Glutamate Toxicity in a Mitogen-Activated Protein Kinase-and Phosphoinositide-3 Kinase-Dependent Manner in Cerebral Cortical Explants Paramjit Kaur et al., 2007. [101] | In vitro, ELISa RT-PCR. | To examine frontal and cingulate cerebral cortex explants from mice treated with PROG in vitro. | PROG induces a 75% increase in BDNF expression in explants of the cerebral cortex, with a nearly identical effect on BDNF protein levels. |
| 7 | Progesterone modulates brain-derived neurotrophic factor and choline acetyltransferase in degenerating Wobbler motoneurons Gonzalez Deniselle et al., 2007. [102] | Ex vivo, In situ hybridization, Immunofluorescence. | To examine steroid and BDNF expression and protein in the spinal cord and in muscle atrophy in wobbler rodents. | BDNF expression was found in neurons of steroid-naïve wobbler mice compared to controls. PROG treatment increased BDNF expression in wobblers compared to untreated but not in controls. |
| 8 | Progesterone pre-treatment enhances serotonin-stimulated BDNF gene expression in rat C6 glioma cells through production of 5α-reduced neurosteroids Morita and Her, 2008. [103] | In vitro, Rat C6 glioma cells, RT-PCR. | To investigate the role of neurotransmitters on glial cell metabolism and function in rat glioma cells in vitro. | BDNF expression levels in both non-treated and PROG-pre-treated glioma cells were similarly elevated by serotonin treatment with a concentration-dependent effect of serotonin on BDNF gene expression. |
| 9 | The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression Jodhka et al., 2009. [104] | In vitro, Western blot, RT-PCR. | To determine which type of PROG receptor mediates the neuroprotective effect of PROG on BDNF. | PROG induces an increase in the BDNF protein levels in cerebral cortical explants in a concentration-dependent manner. PROG regulates BDNF expression through the classical PROG receptor. |
| 10 | Progesterone, BDNF and Neuroprotection in the Injured CNS Coughlan, Gibson, and Murphy, 2009. [105] | In vitro, RT-PCR. | To investigate the neuroprotective mechanism of PROG and BDNF. | PROG had no effect on BDNF expression in granule neurons. No neuroprotective role for PROG on BDNF was observed. |
| 11 | Progesterone inhibits estrogen-mediated neuroprotection against excitotoxicity by down-regulating estrogen receptor-β. Aguirre et al., 2010. [97] | Ex vivo, Western blot, RT-PCR. | To examine PROG and estrogen treatment in cultured hippocampal slices on levels of ERα and ERβ and BDNF. | Estrogen elevated ERβ expression and protein levels and did not modify ERα expression, but it did increase ERα protein levels and BDNF expression levels in hippocampal cells. PROG reversed the estrogen-elicited increases in ERβ, ERα protein, and BDNF expression levels. |
| 12 | Progesterone treatment alters neurotrophin/proneurotrophin balance and receptor expression in rats with traumatic brain injury Cekic et al., 2012. [106] | Ex vivo, Western blot. | To characterize the expression of BDNF isoforms following PROG treatment for traumatic brain injury. | PROG reduces levels of pro-BDNF and TrkB post-brain injury. Mature BDNF was decreased at 24 and 72 h. |
| 13 | Progesterone increases the release of brain-derived neurotrophic factor from glia via progesterone receptor membrane component 1 (Pgrmc1)-dependent ERK5 signaling. Su et al., 2012. [107] | In vitro, RT-PCR, ELISA. | To study PROG-induced BDNF release and the extracellular signal-regulated kinase 5. | PROG and the membrane-impermeable PROG both induced BDNF release from glial cells and primary astrocytes, which lack the classical nuclear/intracellular PROG receptor but express membrane-associated PROG receptors. |
| 14 | Progesterone effects on neuronal brain-derived neurotrophic factor and glial cells during progression of Wobbler mouse neurodegeneration. Meyer et al., 2012. [108] | Ex vivo, In situ hybridization, Immunohistochemistry. | To compare PROG regulation of BDNF in motoneurons and oligodendrocytes of wobbler mice. | PROG upregulated low levels of BDNF expression in gray matter regions at the symptomatic stage of the disease and increased BDNF expression in late-stage wobblers. BDNF protein was normal in steroid-naive symptomatic wobblers. |
| 15 | Progesterone attenuates several hippocampal abnormalities of the wobbler mouse. Meyer et al., 2013. [109] | Ex vivo, In situ hybridization. | To examine the hippocampus of wobbler mice and their changes in response to PROG treatment. | Wobbler mice display decreased BDNF expression. PROG did not change the normal parameters in control mice and attenuated hippocampal abnormalities in wobblers. |
| 16 | Progesterone in the treatment of neonatal arterial ischemic stroke and acute seizures: Role of BDNF/TrkB signaling. Atif, Yousuf, and Stein, 2016. [110] | Ex vivo, Western blot. | To examine the effects of PROG on BDNF-TrkB signaling and inflammation following neonatal arterial ischemic stroke in mice. | PROG suppresses the expression of BDNF in mice with seizures at day 1, but at day 3, BDNF expression is comparable to controls. PROG treatment first inhibited TrkB expression at day 1 then increased TrkB receptor expression at day 3. |
| 17 | Progesterone modulates post-traumatic epileptogenesis through regulation of BDNF-TrkB signaling and cell survival-related pathways in the rat hippocampus. Ghadiri et al., 2019. [111] | Ex vivo, Western blot. | To study the effect of PROG on post-traumatic epileptogenesis survival-related pathways. | The duration of seizures was reduced in PROG-treated animals which showed an enhanced amount of BDNF in the ipsilateral hippocampus. |
| 18 | Progesterone’s Effects on Cognitive Performance of Male Mice Are Independent of Progestin Receptors but Relate to Increases in GABAA Activity in the Hippocampus and Cortex. Frye, Lembo, and Walf, 2021. [112] | Ex vivo, ELISA. | To evaluate the effect of PROG on the hippocampal and cortical levels of BDNF in mice. | PROG increased BDNF levels in the hippocampus, but not in the cortex, of male mice. |
5. Androgen and BDNF Interactions
| Title | Design/Methods | Experiment | Main Findings | |
|---|---|---|---|---|
| 1 | Brain-derived neurotrophic factor regulates expression of androgen receptors in perineal motoneurons Al-Shamma and Arnold, 1997. [127] | In vitro, Immunohistochemistry. | To examine steroid receptor expression in motoneurons of the SNB in mice. | Axonal transport disruption downregulates AR expression in motoneurons, and BDNF treatment reverses it. |
| 2 | Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch Dittrich et al., 1999. [93] | Ex vivo, In situ hybridization. | To examine the expression of AR, BDNF, and TrkB in the HVC, neostriatum, and archistriatum in zebra finches. | BDNF expression is increased in the HVC of male, but not female, zebra finches. Estrogen and aromatase inhibition induce premature stimulation and inhibition of the increased patterns of BDNF expression, respectively, in juvenile males. |
| 3 | BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain Rasika, Alvarez-Buylla, and Nottebohm, 1999. [123] | In vitro, Immunohistochemistry. | To examine BDNF responses to testosterone treatment in the HVC of male canaria. | Testosterone treatment increases BDNF levels in the HVC of adult canaria. BDNF antibodies block the testosterone-induced increase in new neurons. |
| 4 | BDNF regulation of androgen receptor expression in axotomized SNB motoneurons of adult male rats Yang and Arnold, 2000. [128] | In vitro, Autoradiography. | To examine BDNF effects on the axotomy-induced loss of AR expression in SNB motoneurons in rats. | The delayed application of BDNF to axotomized SNB motoneurons restored AR expression the intact levels. |
| 5 | Blockade of endogenous neurotrophic factors prevents the androgenic rescue of rat spinal motoneurons Xu et al., 2001. [116] | Ex vivo, Histology. | To exploit motoneuron cell death in the SNB of mice and the effect of androgen. | The blockage of TrkB activity prevented the androgenic sparing of SNB motoneurons. This did not reduce the SNB motoneuron number. |
| 6 | Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain Louissaint et al., 2002. [117] | In vitro, RT-PCR, Immunohistochemistry, ELISA. | To investigate testosterone-related angiogenesis and neuronal recruitment in adult songbird neostriatum. | HVC endothelial cells produce BDNF in a testosterone-dependent manner. |
| 7 | Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries Fusani et al., 2003. [118] | Ex vivo, In situ hybridization. | To investigate the role of estrogen in controlling the development of song structures (HVC) in female canaries. | The aromatase inhibition of testosterone-induced song motor development correlates with the inhibition of BDNF in HVC of adult female canaries and alters the song pattern. |
| 8 | Brain-Derived Neurotrophic Factor and Androgen Interact in the Maintenance of Dendritic Morphology in a Sexually Dimorphic Rat Spinal Nucleus Yang, Verhovshek, and Sengelaub, 2004. [129] | Ex vivo, Histochemistry. | To test BDNF and testosterone effects on dendritic morphology in motoneurons of the SNB in rats. | Testosterone or BDNF failed to support dendritic length or distribution. Treatment with testosterone plus BDNF restores dendritic morphology to the level of controls. |
| 9 | Androgen regulates trkB immunolabeling in spinal motoneurons Osborne, Verhovshek, and Sengelaub, 2007. [130] | Ex vivo, Immunohistochemistry. | To examine gonadal hormone regulation of BDNF systems in rodents’ spinal motoneurons. | TrkB receptor regulation is androgen-sensitive in motoneurons on the SNB. Castration-induced changes in SNB motoneurons are prevented by testosterone replacement. |
| 10 | Androgen-dependent regulation of brain-derived neurotrophic factor and tyrosine kinase B in the sexually dimorphic SNB Ottem et al., 2007. [131] | Ex vivo, In situ hybridization, RT-PCR. | To investigate the androgen regulation of BDNF protein in SNB motoneurons. | SNB motoneurons and the non-androgen-responsive motoneurons of the adjacent retrodorsolateral nucleus express BDNF and trkB. Testosterone regulates BDNF protein in SNB but not in the retrodorsolateral nucleus dendrites. |
| 11 | Differential expression and regulation of brain-derived neurotrophic factor mRNA isoforms in androgen-sensitive motoneurons of the rat lumbar spinal cord. Ottem et al., 2010. [119] | Ex vivo, In situ hybridization. | To examine the specific BDNF transcripts regulated by androgens in the SNB motoneurons of male rats. | BDNF isoforms containing exon VI were decreased in SNB motoneurons in an androgen-dependent manner but unaffected in retrodorsolateral motoneurons. |
| 12 | Androgen regulates brain-derived neurotrophic factor in spinal motoneurons and their target musculature. Verhovshek et al., 2010. [132] | Ex vivo, Immunohistochemistry, ELISA. | To examine the androgen regulation of BDNF in quadriceps and SNB motoneurons and their corresponding target musculature in male rats. | Castration reduced BDNF protein in the quadriceps, SNB motoneurons, and their target musculature, and this was prevented with testosterone replacement. |
| 13 | Modulatory Effects of Sex Steroid Hormones on Brain-Derived Neurotrophic Factor-Tyrosine Kinase B Expression during Adolescent Development in C57Bl/6 Mice. Hill et al., 2012. [133] | Ex vivo, Western blot. | To examine sex steroid hormones and neurotrophic signaling during adolescent development in a mouse model. | Castration and testosterone or DHT replacement had a receptor-dependent effect on BDNF-TrkB signaling in the forebrain and hippocampal regions of adolescent animals. Changes in BDNF-TrkB signaling in females did not align with changes in serum estrogen. |
| 14 | Androgen action at the target musculature regulates brain-derived neurotrophic factor protein in the SNB. Verhovshek and Sengelaub, 2013. [134] | Ex vivo, Immunohistochemistry. | To examine if testosterone regulates BDNF in SNB motoneurons of male rats by acting locally at the bulbocavernosus muscle. | Testosterone directly to the bulbocavernosus muscle maintains BDNF levels in SNB motoneurons intact after castration. AR blockage decreases BDNF compared with animals treated with intramuscular testosterone. |
| 15 | Regulatory mechanisms of testosterone-stimulated song in the sensorimotor nucleus HVC of female songbirds. Dittrich et al., 2014. [122] | Ex vivo, Microarray. | To examine the effects of testosterone on the anatomy and song control nucleus HVC of female European robins. | Testosterone induced differentiation, angiogenesis, and neuron projection morphogenesis. BDNF functions as a common mediator of the testosterone effects in HVC. |
| 16 | Testosterone enhances functional recovery after stroke through promotion of antioxidant defenses, BDNF levels and neurogenesis in male rats. Fanaei et al., 2014. [121] | Ex vivo, Immunohistochemistry. | To evaluate the effects of testosterone on BDNF and neurogenesis in a castrated male rat model of focal cerebral ischemia. | Testosterone increased BDNF levels and neurogenesis after focal cerebral ischemia. |
| 17 | The effect of adolescent testosterone on hippocampal BDNF and TrkB mRNA expression: Relationship with cell proliferation. Allen et al., 2015. [126] | Ex vivo, RT-PCR, In situ hybridization. | To examine the molecular mechanism underlying testosterone actions on postnatal neurogenesis and BDNF/TrkB levels in rhesus macaques and rats. | Gonadectomy or steroid replacement did not alter BDNF or TrkB expression levels in the hippocampus of young adult male rats or rhesus macaques. There was a positive correlation between cell proliferation and TrkB expression, but only when steroids were present. |
| 18 | Effects of testosterone on synaptic plasticity mediated by androgen receptors in male SAMP8 mice. Jia et al., 2016. [124] | Ex vivo, Western blot. | To study the protective role of testosterone on cognitive performance in an Alzheimer’s disease animal model. | The expression of BDNF and cyclic-AMP response element-binding protein (CREB)/CREB levels were elevated in testosterone-treated animals. |
| 19 | Hedonic sensitivity to low-dose ketamine is modulated by gonadal hormones in a sex-dependent manner. Saland, Schoepfer, and Kabbaj, 2016. [113] | Ex vivo, Western blot. | To investigate testosterone contribution in the rapid antidepressant-like effects of ketamine. | Testosterone treatment responsiveness was associated with higher hippocampal BDNF levels in female rats. |
| 20 | TrkB is necessary for male copulatory behavior in the Syrian Hamster (Mesocricetus auratus). Brague et al., 2018. [135] | Ex vivo, Western blot, RT-PCR. | To examine how TrkB and BDNF mediate testosterone effects on the medial preoptic nucleus in hamsters. | Testosterone treatment increased BDNF expression levels and, conversely, lowered the expression of TrkB receptors in the medial preoptic area of animals. |
| 21 | Prenatal Androgenization Induces Anxiety-Like Behavior in Female Rats, Associated with Reduction of Inhibitory Interneurons and Increased BDNF in Hippocampus and Cortex. Rankov Petrovic et al., 2019. [136] | Ex vivo, Immunohistochemistry, Western blot, ELISA. | To evaluate the influence of maternal hyperandrogenemia on offspring levels of BDNF in the hippocampus and cerebral cortex. | BDNF expression was increased in the hippocampus and cerebral cortex of prenatal hyperandrogenization offspring in comparison with the controls. |
| 22 | Deficiency in Androgen Receptor Aggravates the Depressive-Like Behaviors in Chronic Mild Stress Model of Depression. Hung et al., 2019. [137] | Ex vivo, Immunohistochemistry, Western blot, ELISA. | To exploit how AR and stress influence the onset of major depressive disorder. | Loss of AR affects depressive-like behaviors by modulating BDNF expression. |
| 23 | Effect of adolescent androgen manipulation on psychosis-like behaviour in adulthood in BDNF heterozygous and control mice. Du et al., 2019 [138] | Ex vivo, Western blot. | To examine how adolescent androgens influence psychosis-like behavior in adulthood and the role of BDNF in mice. | Testosterone and DHT treatment reduce the expression of dopamine transporter in the medial prefrontal cortex of mice. These effects are absent in BDNF heterozygous mice. |
| 24 | Dose-dependent effects of testosterone on spatial learning strategies and brain-derived neurotrophic factor in male rats. Zhang et al., 2020. [139] | Ex vivo, ELISA. | To investigate the effect of different doses of testosterone on spatial learning strategies in male rats. | Low testosterone doses increased total BDNF in the striatum, and high doses increased total BDNF in the hippocampus. |
6. Summary and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CREB | cAMP response element-binding protein |
References
- Lenz, K.M.; Nugent, B.M.; McCarthy, M.M. Sexual Differentiation of the Rodent Brain: Dogma and Beyond. Front. Neurosci. 2012, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Diviccaro, S.; Serafini, M.M.; Caruso, D.; Garcia-Segura, L.M.; Viviani, B.; Melcangi, R.C. Sex Differences in Steroid Levels and Steroidogenesis in the Nervous System: Physiopathological Role. Front. Neuroendocrinol. 2020, 56, 100804. [Google Scholar] [CrossRef] [PubMed]
- Corpechot, C.; Robel, P.; Axelson, M.; Sjövall, J.; Baulieu, E.E. Characterization and Measurement of Dehydroepiandrosterone Sulfate in Rat Brain. Proc. Natl. Acad. Sci. USA 1981, 78, 4704–4707. [Google Scholar] [CrossRef] [PubMed]
- Selvaraj, V.; Stocco, D.M.; Tu, L.N. Minireview: Translocator Protein (TSPO) and Steroidogenesis: A Reappraisal. Mol. Endocrinol. 2015, 29, 490–501. [Google Scholar] [CrossRef]
- Porcu, P.; Barron, A.M.; Frye, C.A.; Walf, A.A.; Yang, S.Y.; He, X.Y.; Morrow, A.L.; Panzica, G.C.; Melcangi, R.C. Neurosteroidogenesis Today: Novel Targets for Neuroactive Steroid Synthesis and Action and Their Relevance for Translational Research. J. Neuroendocrinol. 2016, 28, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Frick, K.M.; Kim, J.; Tuscher, J.J.; Fortress, A.M. Sex Steroid Hormones Matter for Learning and Memory: Estrogenic Regulation of Hippocampal Function in Male and Female Rodents. Learn. Mem. 2015, 22, 472–493. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.K.; Goodwin, S.E.; Schuppe, E.R.; Dawn, A.A.; Preininger, D.; Mangiamele, L.A.; Fuxjager, M.J. Activational vs. Organizational Effects of Sex Steroids and Their Role in the Evolution of Reproductive Behavior: Looking to Foot-Flagging Frogs and Beyond. Horm. Behav. 2022, 146, 105248. [Google Scholar] [CrossRef]
- Fleischer, A.W.; Frick, K.M. New Perspectives on Sex Differences in Learning and Memory. Trends Endocrinol. Metab. 2023, 34, 526–538. [Google Scholar] [CrossRef]
- Bouron, A.; Boisseau, S.; De Waard, M.; Peris, L. Differential Down-Regulation of Voltage-Gated Calcium Channel Currents by Glutamate and BDNF in Embryonic Cortical Neurons. Eur. J. Neurosci. 2006, 24, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.L.; Ratnu, V.S.; Gordillo, L.; Hwang, I.; Mariani, L.; Weinand, K.; Hammarén, H.M.; Heck, J.; Bulyk, M.L.; Savitski, M.M.; et al. Comparative Chromatin Accessibility upon BDNF Stimulation Delineates Neuronal Regulatory Elements. Mol. Syst. Biol. 2022, 18, e10473. [Google Scholar] [CrossRef]
- Reichlin, S. Neuroendocrinology. N. Engl. J. Med. 1963, 269, 1246–1250. [Google Scholar] [CrossRef] [PubMed]
- Hammes, S.R.; Levin, E.R. Impact of Estrogens in Males and Androgens in Females. J. Clin. Investig. 2019, 129, 1818–1826. [Google Scholar] [CrossRef]
- Segal, S. Hormones, Amino-Acid Transport and Protein Synthesis. Nature 1964, 203, 17–19. [Google Scholar] [CrossRef]
- Tomkins, G.M.; Maxwell, E.S. Some Aspects of Steroid Hormone Action. Annu. Rev. Biochem. 1963, 32, 677–708. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.P.; Gorski, R.A. Gonadal Steroid Induction of Structural Sex Differences in the Central Nervous System. Annu. Rev. Neurosci. 1984, 7, 413–442. [Google Scholar] [CrossRef] [PubMed]
- Toran-Allerand, C.D. On the Genesis of Sexual Differentiation of the Central Nervous System: Morphogenetic Consequences of Steroidal Exposure and Possible Role of α-Fetoprotein. Prog. Brain Res. 1984, 61, 63–98. [Google Scholar] [CrossRef] [PubMed]
- Miranda, R.C.; Sohrabji, F.; Toran-Allerand, D. Interactions of Estrogen with the Neurotrophins and Their Receptors during Neural Development. Horm. Behav. 1994, 28, 367–375. [Google Scholar] [CrossRef]
- Singh, M.; Meyer, E.M.; Simpkins, J.W. The Effect of Ovariectomy and Estradiol Replacement on Brain-Derived Neurotrophic Factor Messenger Ribonucleic Acid Expression in Cortical and Hippocampal Brain Regions of Female Sprague-Dawley Rats. Endocrinology 1995, 136, 2320–2324. [Google Scholar] [CrossRef] [PubMed]
- Levin, E.R. Plasma Membrane Estrogen Receptors. Trends Endocrinol. Metab. 2009, 20, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Hammes, S.R.; Levin, E.R. Extranuclear Steroid Receptors: Nature and Actions. Endocr. Rev. 2007, 28, 726–741. [Google Scholar] [CrossRef]
- Kulkoyluoglu, E.; Madak-Erdogan, Z. Nuclear and Extranuclear-Initiated Estrogen Receptor Signaling Crosstalk and Endocrine Resistance in Breast Cancer. Steroids 2016, 114, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Hans, A.; Bajramovic, J.J.; Syan, S.; Perret, E.; Dunia, I.; Brahic, M.; Gonzalez-Dunia, D. Persistent, Noncytolytic Infection of Neurons by Borna Disease Virus Interferes with ERK 1/2 Signaling and Abrogates BDNF-Induced Synaptogenesis. FASEB J. 2004, 18, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Su, C.; Ng, S. Non-Genomic Mechanisms of Progesterone Action in the Brain. Front. Neurosci. 2013, 7, 159. [Google Scholar] [CrossRef]
- Gibbs, R.B. Levels of TrkA and BDNF MRNA, but Not NGF MRNA, Fluctuate across the Estrous Cycle and Increase in Response to Acute Hormone Replacement. Brain Res. 1998, 787, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Murphy, D.D.; Cole, N.B.; Segal, M. Brain-Derived Neurotrophic Factor Mediates Estradiol-Induced Dendritic Spine Formation in Hippocampal Neurons. Proc. Natl. Acad. Sci. USA 1998, 95, 11412–11417. [Google Scholar] [CrossRef] [PubMed]
- Jezierski, M.K.; Sohrabji, F. Region- and Peptide-Specific Regulation of the Neurotrophins by Estrogen. Mol. Brain Res. 2000, 85, 77–84. [Google Scholar] [CrossRef]
- Jezierski, M.K.; Sohrabji, F. Neurotrophin Expression in the Reproductively Senescent Forebrain Is Refractory to Estrogen Stimulation. Neurobiol. Aging 2001, 22, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fowler, C.D.; Young, L.J.; Yan, Q.; Insel, T.R.; Wang, Z. Expression and Estrogen Regulation of Brain-Derived Neurotrophic Factor Gene and Protein in the Forebrain of Female Prairie Voles. J. Comp. Neurol. 2001, 433, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Berchtold, N.C.; Kesslak, J.P.; Pike, C.J.; Adlard, P.A.; Cotman, C.W. Estrogen and Exercise Interact to Regulate Brain-Derived Neurotrophic Factor MRNA and Protein Expression in the Hippocampus. Eur. J. Neurosci. 2001, 14, 1992–2002. [Google Scholar] [CrossRef]
- Ivanova, T.; Küppers, E.; Engele, J.; Beyer, C. Estrogen Stimulates Brain-derived Neurotrophic Factor Expression in Embryonic Mouse Midbrain Neurons through a Membrane-mediated and Calcium-dependent Mechanism. J. Neurosci. Res. 2001, 66, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Solum, D.T.; Handa, R.J. Estrogen Regulates the Development of Brain-Derived Neurotrophic Factor MRNA and Protein in the Rat Hippocampus. J. Neurosci. 2002, 22, 2650–2659. [Google Scholar] [CrossRef] [PubMed]
- Jezierski, M.K.; Sohrabji, F. Estrogen Enhances Retrograde Transport of Brain-Derived Neurotrophic Factor in the Rodent Forebrain. Endocrinology 2003, 144, 5022–5029. [Google Scholar] [CrossRef] [PubMed]
- Cavus, I.; Duman, R.S. Influence of Estradiol, Stress, and 5-HT2A Agonist Treatment on Brain-Derived Neurotrophic Factor Expression in Female Rats. Biol. Psychiatry 2003, 54, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Krizsan-Agbas, D.; Pedchenko, T.; Hasan, W.; Smith, P.G. Oestrogen Regulates Sympathetic Neurite Outgrowth by Modulating Brain Derived Neurotrophic Factor Synthesis and Release by the Rodent Uterus. Eur. J. Neurosci. 2003, 18, 2760–2768. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, J.; Guan, R.; Shen, Y.; Xu, P.; Xu, J. Estrogen Affects BDNF Expression Following Chronic Constriction Nerve Injury. Neuroreport 2003, 14, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Blurton-Jones, M.; Kuan, P.N.; Tuszynski, M.H. Anatomical Evidence for Transsynaptic Influences of Estrogen on Brain-Derived Neurotrophic Factor Expression. J. Comp. Neurol. 2004, 468, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Gresack, J.E.; Frick, K.M. Environmental Enrichment Reduces the Mnemonic and Neural Benefits of Estrogen. Neuroscience 2004, 128, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Brito, V.I.; Carrer, H.F.; Cambiasso, M.J. Inhibition of Tyrosine Kinase Receptor Type B Synthesis Blocks Axogenic Effect of Estradiol on Rat Hypothalamic Neurones in Vitro. Eur. J. Neurosci. 2004, 20, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, H.; Cohen, R.S.; Pandey, S.C. Effects of Estrogen Treatment on Expression of Brain-Derived Neurotrophic Factor and CAMP Response Element-Binding Protein Expression and Phosphorylation in Rat Amygdaloid and Hippocampal Structures. Neuroendocrinology 2005, 81, 294–310. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Rhodes, M.E.; Dudek, B. Estradiol to Aged Female or Male Mice Improves Learning in Inhibitory Avoidance and Water Maze Tasks. Brain Res. 2005, 1036, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Franklin, T.B.; Perrot-Sinal, T.S. Sex and Ovarian Steroids Modulate Brain-Derived Neurotrophic Factor (BDNF) Protein Levels in Rat Hippocampus under Stressful and Non-Stressful Conditions. Psychoneuroendocrinology 2006, 31, 38–48. [Google Scholar] [CrossRef]
- Takuma, K.; Matsuo, A.; Himeno, Y.; Hoshina, Y.; Ohno, Y.; Funatsu, Y.; Arai, S.; Kamei, H.; Mizoguchi, H.; Nagai, T.; et al. 17Β-Estradiol Attenuates Hippocampal Neuronal Loss and Cognitive Dysfunction Induced by Chronic Restraint Stress in Ovariectomized Rats. Neuroscience 2007, 146, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Sasahara, K.; Shikimi, H.; Haraguchi, S.; Sakamoto, H.; Honda, S.I.; Harada, N.; Tsutsui, K. Mode of Action and Functional Significance of Estrogen-Inducing Dendritic Growth, Spinogenesis, and Synaptogenesis in the Developing Purkinje Cell. J. Neurosci. 2007, 27, 7408–7417. [Google Scholar] [CrossRef]
- Aguado-Llera, D.; Arilla-Ferreiro, E.; Chowen, J.A.; Argente, J.; Puebla-Jiménez, L.; Frago, L.M.; Barrios, V. 17β-Estradiol Protects Depletion of Rat Temporal Cortex Somatostatinergic System by β-Amyloid. Neurobiol. Aging 2007, 28, 1396–1409. [Google Scholar] [CrossRef]
- Meltser, I.; Tahera, Y.; Simpson, E.; Hultcrantz, M.; Charitidi, K.; Gustafsson, J.Å.; Canlon, B. Estrogen Receptor β Protects against Acoustic Trauma in Mice. J. Clin. Investig. 2008, 118, 1563–1570. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Akaishi, T.; Matsuki, N.; Ohno, Y.; Nakazawa, K. β-Estradiol Induces Synaptogenesis in the Hippocampus by Enhancing Brain-Derived Neurotrophic Factor Release from Dentate Gyrus Granule Cells. Brain Res. 2007, 1150, 108–120. [Google Scholar] [CrossRef]
- Hartog, T.E.; Dittrich, F.; Pieneman, A.W.; Jansen, R.F.; Frankl-Vilches, C.; Lessmann, V.; Lilliehook, C.; Goldman, S.A.; Gahr, M. Brain-Derived Neurotrophic Factor Signaling in the HVC Is Required for Testosterone-Induced Song of Female Canaries. J. Neurosci. 2009, 29, 15511–15519. [Google Scholar] [CrossRef] [PubMed]
- Pietranera, L.; Lima, A.; Roig, P.; De Nicola, A.F. Involvement of Brain-Derived Neurotrophic Factor and Neurogenesis in Oestradiol Neuroprotection of the Hippocampus of Hypertensive Rats. J. Neuroendocrinol. 2010, 22, 1082–1092. [Google Scholar] [CrossRef] [PubMed]
- Tripanichkul, W.; Gerdprasert, O.; Jaroensuppaperch, E.O. Estrogen Reduces BDNF Level, but Maintains Dopaminergic Cell Density in the Striatum of MPTP Mouse Model. Int. J. Neurosci. 2010, 120, 489–495. [Google Scholar] [CrossRef]
- Wong, J.; Woon, H.G.; Weickert, C.S. Full Length TrkB Potentiates Estrogen Receptor Alpha Mediated Transcription Suggesting Convergence of Susceptibility Pathways in Schizophrenia. Mol. Cell. Neurosci. 2011, 46, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kiss, Á.; Delattre, A.M.; Pereira, S.I.R.; Carolino, R.G.; Szawka, R.E.; Anselmo-Franci, J.A.; Zanata, S.M.; Ferraz, A.C. 17β-Estradiol Replacement in Young, Adult and Middle-Aged Female Ovariectomized Rats Promotes Improvement of Spatial Reference Memory and an Antidepressant Effect and Alters Monoamines and BDNF Levels in Memory- and Depression-Related Brain Areas. Behav. Brain Res. 2012, 227, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.P.; Wade, J. 17β-Estradiol Regulates the Sexually Dimorphic Expression of BDNF and TrkB Proteins in the Song System of Juvenile Zebra Finches. PLoS ONE 2012, 7, e43687. [Google Scholar] [CrossRef]
- Spencer-Segal, J.L.; Tsuda, M.C.; Mattei, L.; Waters, E.M.; Romeo, R.D.; Milner, T.A.; McEwen, B.S.; Ogawa, S. Estradiol Acts via Estrogen Receptors Alpha and Beta on Pathways Important for Synaptic Plasticity in the Mouse Hippocampal Formation. Neuroscience 2012, 202, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Gatson, J.W.; Liu, M.M.; Abdelfattah, K.; Wigginton, J.G.; Smith, S.; Wolf, S.; Simpkins, J.W.; Minei, J.P. Estrone Is Neuroprotective in Rats after Traumatic Brain Injury. J. Neurotrauma 2012, 29, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, S.; Sasahara, K.; Shikimi, H.; Honda, S.I.; Harada, N.; Tsutsui, K. Estradiol Promotes Purkinje Dendritic Growth, Spinogenesis, and Synaptogenesis during Neonatal Life by Inducing the Expression of BDNF. Cerebellum 2012, 11, 416–417. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, X.; Senthil Kumar, S.P.D.; Zhang, J.; Shi, H. Central Expression and Anorectic Effect of Brain-Derived Neurotrophic Factor Are Regulated by Circulating Estradiol Levels. Horm. Behav. 2013, 63, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, S.; Atif, F.; Sayeed, I.; Wang, J.; Stein, D.G. Post-Stroke Infections Exacerbate Ischemic Brain Injury in Middle-Aged Rats: Immunomodulation and Neuroprotection by Progesterone. Neuroscience 2013, 239, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Piovano, G.S.; Varayoud, J.; Luque, E.H.; Ramos, J.G. Long-Term Ovariectomy Increases BDNF Gene Methylation Status in Mouse Hippocampus. J. Steroid Biochem. Mol. Biol. 2014, 144, 243–252. [Google Scholar] [CrossRef]
- Pietranera, L.; Brocca, M.E.; Roig, P.; Lima, A.; Garcia-Segura, L.M.; De Nicola, A.F. 17α-Oestradiol-Induced Neuroprotection in the Brain of Spontaneously Hypertensive Rats. J. Neuroendocrinol. 2014, 26, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Fortress, A.M.; Kim, J.; Poole, R.L.; Gould, T.J.; Frick, K.M. 17β-Estradiol Regulates Histone Alterations Associated with Memory Consolidation and Increases Bdnf Promoter Acetylation in Middle-Aged Female Mice. Learn. Mem. 2014, 21, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, P.; Subramanian, M.; Nunez, J.L.; MohanKumar, S.M.J.; MohanKumar, P.S. Chronic Estradiol Treatment Decreases Brain Derived Neurotrophic Factor (BDNF) Expression and Monoamine Levels in the Amygdala—Implications for Behavioral Disorders. Behav. Brain Res. 2014, 261, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.W.C.; Du, X.; Van Den Buuse, M.; Hill, R.A. Analyzing the Influence of BDNF Heterozygosity on Spatial Memory Response to 17β-Estradiol. Transl. Psychiatry 2015, 5, e498. [Google Scholar] [CrossRef] [PubMed]
- Munetomo, A.; Hojo, Y.; Higo, S.; Kato, A.; Yoshida, K.; Shirasawa, T.; Shimizu, T.; Barron, A.; Kimoto, T.; Kawato, S. Aging-Induced Changes in Sex-Steroidogenic Enzymes and Sex-Steroid Receptors in the Cortex, Hypothalamus and Cerebellum. J. Physiol. Sci. 2015, 65, 253–263. [Google Scholar] [CrossRef]
- Cikla, U.; Chanana, V.; Kintner, D.B.; Udho, E.; Eickhoff, J.; Sun, W.; Marquez, S.; Covert, L.; Otles, A.; Shapiro, R.A.; et al. ERα Signaling Is Required for TrkB-Mediated Hippocampal Neuroprotection in Female Neonatal Mice after Hypoxic Ischemic Encephalopathy. eNeuro 2016, 3, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Kim, C.S.; Kim, J.Y.; Kim, C.J.; Seo, J.H. Combined Exercise Ameliorates Ovariectomy-Induced Cognitive Impairment by Enhancing Cell Proliferation and Suppressing Apoptosis. Menopause 2016, 23, 18–26. [Google Scholar] [CrossRef]
- Pietranera, L.; Correa, J.; Brocca, M.E.; Roig, P.; Lima, A.; Di Giorgio, N.; Garcia-Segura, L.M.; De Nicola, A.F. Selective Oestrogen Receptor Agonists Rescued Hippocampus Parameters in Male Spontaneously Hypertensive Rats. J. Neuroendocrinol. 2016, 28. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhang, Q.; Kong, W.; Chen, J.; Ma, J.; Wang, L.; Wang, Y.; Liu, Y.; Li, Y.; Wen, J. Regulation of Endometrial Cell Proliferation by Estrogen-Induced BDNF Signaling Pathway. Gynecol. Endocrinol. 2017, 33, 485–489. [Google Scholar] [CrossRef]
- Karisetty, B.C.; Joshi, P.C.; Kumar, A.; Chakravarty, S. Sex Differences in the Effect of Chronic Mild Stress on Mouse Prefrontal Cortical BDNF Levels: A Role of Major Ovarian Hormones. Neuroscience 2017, 356, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Kight, K.E.; McCarthy, M.M. Sex Differences and Estrogen Regulation of BDNF Gene Expression, but Not Propeptide Content, in the Developing Hippocampus. J. Neurosci. Res. 2017, 95, 345–354. [Google Scholar] [CrossRef]
- Chhibber, A.; Woody, S.K.; Karim Rumi, M.A.; Soares, M.J.; Zhao, L. Estrogen Receptor β Deficiency Impairs BDNF–5-HT2A Signaling in the Hippocampus of Female Brain: A Possible Mechanism for Menopausal Depression. Psychoneuroendocrinology 2017, 82, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Li, B.; Fan, J.; Zhang, K.; Yang, W.; Ren, B.; Cui, R. Additive Antidepressant-like Effects of Fasting with β-Estradiol in Mice. J. Cell. Mol. Med. 2019, 23, 5508–5517. [Google Scholar] [CrossRef] [PubMed]
- Rashidy-Pour, A.; Bavarsad, K.; Miladi-Gorji, H.; Seraj, Z.; Vafaei, A.A. Voluntary Exercise and Estradiol Reverse Ovariectomy-Induced Spatial Learning and Memory Deficits and Reduction in Hippocampal Brain-Derived Neurotrophic Factor in Rats. Pharmacol. Biochem. Behav. 2019, 187, 172819. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, T.; Liang, S.; Li, W.; Hu, X.; Wu, X.; Jin, F. Sex-Dependent Aberrant PFC Development in the Adolescent Offspring Rats Exposed to Variable Prenatal Stress. Int. J. Dev. Neurosci. 2020, 80, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Salahinejad, A.; Attaran, A.; Niyogi, S.; Chivers, D.P. Rapid Effects of Estradiol and Its Receptor Agonists on Object Recognition and Object Placement in Adult Male Zebrafish. Behav. Brain Res. 2020, 384, 112514. [Google Scholar] [CrossRef] [PubMed]
- Bohm-Levine, N.; Goldberg, A.R.; Mariani, M.; Frankfurt, M.; Thornton, J. Reducing Luteinizing Hormone Levels after Ovariectomy Improves Spatial Memory: Possible Role of Brain-Derived Neurotrophic Factor. Horm. Behav. 2020, 118, 104590. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, S.; Lim, J.; Chung, S.; Koo, T.S.; Ji, Y.G.; Suh, Y.G.; Son, W.S.; Kim, S.H.; Choi, H.J. ERRγ Ligand HPB2 Upregulates BDNF-TrkB and Enhances Dopaminergic Neuronal Phenotype. Pharmacol. Res. 2021, 165, 105423. [Google Scholar] [CrossRef]
- Baumgartner, N.E.; Black, K.L.; McQuillen, S.M.; Daniel, J.M. Previous Estradiol Treatment during Midlife Maintains Transcriptional Regulation of Memory-Related Proteins by ERα in the Hippocampus in a Rat Model of Menopause. Neurobiol. Aging 2021, 105, 365–373. [Google Scholar] [CrossRef]
- Gross, K.S.; Alf, R.L.; Polzin, T.R.; Frick, K.M. 17β-Estradiol Activation of Dorsal Hippocampal TrkB Is Independent of Increased Mature BDNF Expression and Is Required for Enhanced Memory Consolidation in Female Mice. Psychoneuroendocrinology 2021, 125, 105110. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.I.; Lim, J.; Choi, H.J.; Kim, S.H.; Choi, H.J. ERRγ Ligand Regulates Adult Neurogenesis and Depression-like Behavior in a LRRK2-G2019S-Associated Young Female Mouse Model of Parkinson’s Disease. Neurotherapeutics 2022, 19, 1298–1312. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, Y.; Wang, Z.; Zhang, Z.; Wang, J.; Zhu, G.; Yang, S. Activation of GPER1 by G1 Prevents PTSD-like Behaviors in Mice: Illustrating the Mechanisms from BDNF/TrkB to Mitochondria and Synaptic Connection. CNS Neurosci. Ther. 2024, 30, e14855. [Google Scholar] [CrossRef] [PubMed]
- van Noort, V.; Snel, B.; Huynen, M.A. Predicting Gene Function by Conserved Co-Expression. Trends Genet. 2003, 19, 238–242. [Google Scholar] [CrossRef]
- Teichmann, S.A.; Babu, M.M. Conservation of Gene Co-Regulation in Prokaryotes and Eukaryotes. Trends Biotechnol. 2002, 20, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Cambiasso, M.J.; Colombo, J.A.; Carrer, H.F. Differential Effect of Oestradiol and Astroglia-Conditioned Media on the Growth of Hypothalamic Neurons from Male and Female Rat Brains. Eur. J. Neurosci. 2000, 12, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Bourque, M.; Morissette, M.; Di Paolo, T. Neuroprotection in Parkinsonian-Treated Mice via Estrogen Receptor α Activation Requires G Protein-Coupled Estrogen Receptor 1. Neuropharmacology 2015, 95, 343–352. [Google Scholar] [CrossRef]
- Almey, A.; Milner, T.A.; Brake, W.G. Estrogen Receptors in the Central Nervous System and Their Implication for Dopamine-Dependent Cognition in Females. Horm. Behav. 2015, 74, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Dahlman-Wright, K.; Gustafsson, J.A. Estrogen Receptor Beta: An Overview and Update. Nucl. Recept. Signal. 2008, 6, nrs-06003. [Google Scholar] [CrossRef] [PubMed]
- Delaunay, F.; Pettersson, K.; Tujague, M.; Gustafsson, J.Å. Functional Differences between the Amino-Terminal Domains of Estrogen Receptors α and β. Mol. Pharmacol. 2000, 58, 584–590. [Google Scholar] [CrossRef]
- Hevener, A.L.; Ribas, V.; Moore, T.M.; Zhou, Z. The Impact of Skeletal Muscle ERα on Mitochondrial Function and Metabolic Health. Endocrinology 2020, 161, bqz017. [Google Scholar] [CrossRef] [PubMed]
- Tse, M.C.L.; Pang, B.P.S.; Bi, X.; Ooi, T.X.; Chan, W.S.; Zhang, J.; Chan, C.B. Estrogen Regulates Mitochondrial Activity Through Inducing Brain-Derived Neurotrophic Factor Expression in Skeletal Muscle. J. Cell. Physiol. 2025, 240, e31483. [Google Scholar] [CrossRef] [PubMed]
- Küppers, E.; Krust, A.; Chambon, P.; Beyer, C. Functional Alterations of the Nigrostriatal Dopamine System in Estrogen Receptor-α Knockout (ERKO) Mice. Psychoneuroendocrinology 2008, 33, 832–838. [Google Scholar] [CrossRef]
- de Assis, G.G.; Hoffman, J.R. The BDNF Val66Met Polymorphism Is a Relevant, But Not Determinant, Risk Factor in the Etiology of Neuropsychiatric Disorders—Current Advances in Human Studies: A Systematic Review. Brain Plast. 2022, 8, 133–142. [Google Scholar] [CrossRef]
- de Assis, G.G.; Hoffman, J.R.; Bojakowski, J.; Murawska-Ciałowicz, E.; Cięszczyk, P.; Gasanov, E.V. The Val66 and Met66 Alleles-Specific Expression of BDNF in Human Muscle and Their Metabolic Responsivity. Front. Mol. Neurosci. 2021, 14, 638176. [Google Scholar] [CrossRef] [PubMed]
- Dittrich, F.; Feng, Y.; Metzdorf, R.; Gahr, M. Estrogen-Inducible, Sex-Specific Expression of Brain-Derived Neurotrophic Factor MRNA in a Forebrain Song Control Nucleus of the Juvenile Zebra Finch. Proc. Natl. Acad. Sci. USA 1999, 96, 8241–8246. [Google Scholar] [CrossRef] [PubMed]
- Rashidy-Pour, A.; Derafshpour, L.; Vafaei, A.A.; Bandegi, A.R.; Kashefi, A.; Sameni, H.R.; Jashire-Nezhad, N.; Saboory, E.; Panahi, Y. Effects of Treadmill Exercise and Sex Hormones on Learning, Memory and Hippocampal Brain-Derived Neurotrophic Factor Levels in Transient Congenital Hypothyroid Rats. Behav. Pharmacol. 2020, 31, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, R.B. Treatment with Estrogen and Progesterone Affects Relative Levels of Brain-Derived Neurotrophic Factor MRNA and Protein in Different Regions of the Adult Rat Brain. Brain Res. 1999, 844, 20–27. [Google Scholar] [CrossRef]
- Murphy, D.D.; Segal, M. Progesterone Prevents Estradiol-Induced Dendritic Spine Formation in Cultured Hippocampal Neurons. Neuroendocrinology 2000, 72, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, C.; Jayaraman, A.; Pike, C.; Baudry, M. Progesterone Inhibits Estrogen-Mediated Neuroprotection against Excitotoxicity by down-Regulating Estrogen Receptor-β. J. Neurochem. 2010, 115, 1277–1287. [Google Scholar] [CrossRef] [PubMed]
- Bimonte-Nelson, H.A.; Nelson, M.E.; Granholm, A.C.E. Progesterone Counteracts Estrogen-Induced Increases in Neurotrophins in the Aged Female Rat Brain. Neuroreport 2004, 15, 2659–2663. [Google Scholar] [CrossRef] [PubMed]
- González, S.L.; Labombarda, F.; González Deniselle, M.C.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone Up-Regulates Neuronal Brain-Derived Neurotrophic Factor Expression in the Injured Spinal Cord. Neuroscience 2004, 125, 605–614. [Google Scholar] [CrossRef]
- De Nicola, A.F.; Gonzalez, S.L.; Labombarda, F.; Deniselle, M.C.G.; Garay, L.; Guennoun, R.; Schumacher, M. Progesterone Treatment of Spinal Cord Injury: Effects on Receptors, Neurotrophins, and Myelination. J. Mol. Neurosci. 2006, 28, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Jodhka, P.K.; Underwood, W.A.; Bowles, C.A.; De Fiebre, N.E.C.; De Fiebre, C.M.; Singh, M. Progesterone Increases Brain-Derived Neurotrophic Factor Expression and Protects against Glutamate Toxicity in a Mitogen-Activated Protein Kinase- and Phosphoinositide-3 Kinase-Dependent Manner in Cerebral Cortical Explants. J. Neurosci. Res. 2007, 85, 2441–2449. [Google Scholar] [CrossRef]
- Gonzalez Deniselle, M.C.; Garay, L.; Gonzalez, S.; Saravia, F.; Labombarda, F.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone Modulates Brain-Derived Neurotrophic Factor and Choline Acetyltransferase in Degenerating Wobbler Motoneurons. Exp. Neurol. 2007, 203, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Morita, K.; Her, S. Progesterone Pretreatment Enhances Serotonin-Stimulated BDNF Gene Expression in Rat C6 Glioma Cells through Production of 5α-Reduced Neurosteroids. J. Mol. Neurosci. 2008, 34, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Jodhka, P.K.; Kaur, P.; Underwood, W.; Lydon, J.P.; Singh, M. The Differences in Neuroprotective Efficacy of Progesterone and Medroxyprogesterone Acetate Correlate with Their Effects on Brain-Derived Neurotrophic Factor Expression. Endocrinology 2009, 150, 3162–3168. [Google Scholar] [CrossRef]
- Coughlan, T.; Gibson, C.; Murphy, S. Progesterone, BDNF and Neuroprotection in the Injured CNS. Int. J. Neurosci. 2009, 119, 1718–1740. [Google Scholar] [CrossRef] [PubMed]
- Cekic, M.; Johnson, S.J.; Bhatt, V.H.; Stein, D.G. Progesterone Treatment Alters Neurotrophin/Proneurotrophin Balance and Receptor Expression in Rats with Traumatic Brain Injury. Restor. Neurol. Neurosci. 2012, 30, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Cunningham, R.L.; Rybalchenko, N.; Singh, M. Progesterone Increases the Release of Brain-Derived Neurotrophic Factor from Glia via Progesterone Receptor Membrane Component 1 (Pgrmc1)-Dependent ERK5 Signaling. Endocrinology 2012, 153, 4389–4400. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Gonzalez Deniselle, M.C.; Gargiulo-Monachelli, G.; Garay, L.I.; Schumacher, M.; Guennoun, R.; De Nicola, A.F. Progesterone Effects on Neuronal Brain-Derived Neurotrophic Factor and Glial Cells during Progression of Wobbler Mouse Neurodegeneration. Neuroscience 2012, 201, 267–279. [Google Scholar] [CrossRef]
- Meyer, M.; Gonzalez Deniselle, M.C.; Gargiulo-Monachelli, G.; Lima, A.; Roig, P.; Guennoun, R.; Schumacher, M.; De Nicola, A.F. Progesterone Attenuates Several Hippocampal Abnormalities of the Wobbler Mouse. J. Neuroendocrinol. 2013, 25, 235–243. [Google Scholar] [CrossRef]
- Atif, F.; Yousuf, S.; Stein, D.G. Progesterone in the Treatment of Neonatal Arterial Ischemic Stroke and Acute Seizures: Role of BDNF/TrkB Signaling. Neuropharmacology 2016, 107, 317–328. [Google Scholar] [CrossRef]
- Ghadiri, T.; Vakilzadeh, G.; Hajali, V.; Khodagholi, F. Progesterone Modulates Post-Traumatic Epileptogenesis through Regulation of BDNF-TrkB Signaling and Cell Survival-Related Pathways in the Rat Hippocampus. Neurosci. Lett. 2019, 709, 134384. [Google Scholar] [CrossRef]
- Frye, C.A.; Lembo, V.F.; Walf, A.A. Progesterone’s Effects on Cognitive Performance of Male Mice Are Independent of Progestin Receptors but Relate to Increases in GABAA Activity in the Hippocampus and Cortex. Front. Endocrinol. 2021, 11, 552805. [Google Scholar] [CrossRef]
- Saland, S.K.; Schoepfer, K.J.; Kabbaj, M. Hedonic Sensitivity to Low-Dose Ketamine Is Modulated by Gonadal Hormones in a Sex-Dependent Manner. Sci. Rep. 2016, 6, 21322. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.L.; Oñate, S.A.; Tsai, M.J.; O’Malley, B.W. CREB Binding Protein Acts Synergistically with Steroid Receptor Coactivator-1 to Enhance Steroid Receptor-Dependent Transcription. Proc. Natl. Acad. Sci. USA 1996, 93, 8884–8888. [Google Scholar] [CrossRef] [PubMed]
- Litim, N.; Morissette, M.; Di Paolo, T. Effects of Progesterone Administered after MPTP on Dopaminergic Neurons of Male Mice. Neuropharmacology 2017, 117, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Gingras, K.M.; Bengston, L.; Di Marco, A.; Forger, N.G. Blockade of Endogenous Neurotrophic Factors Prevents the Androgenic Rescue of Rat Spinal Motoneurons. J. Neurosci. 2001, 21, 4366–4372. [Google Scholar] [CrossRef]
- Louissaint, A.; Rao, S.; Leventhal, C.; Goldman, S.A. Coordinated Interaction of Neurogenesis and Angiogenesis in the Adult Songbird Brain. Neuron 2002, 34, 945–960. [Google Scholar] [CrossRef]
- Fusani, L.; Metzdorf, R.; Hutchison, J.B.; Gahr, M. Aromatase Inhibition Affects Testosterone-Induced Masculinization of Song and the Neural Song System in Female Canaries. J. Neurobiol. 2003, 54, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Ottem, E.N.; Poort, J.E.; Wang, H.; Jordan, C.L.; Breedlove, S.M. Differential Expression and Regulation of Brain-Derived Neurotrophic Factor (BDNF) MRNA Isoforms in Androgen-Sensitive Motoneurons of the Rat Lumbar Spinal Cord. Mol. Cell. Endocrinol. 2010, 328, 40–46. [Google Scholar] [CrossRef]
- Li, M.; Masugi-Tokita, M.; Takanami, K.; Yamada, S.; Kawata, M. Testosterone Has Sublayer-Specific Effects on Dendritic Spine Maturation Mediated by BDNF and PSD-95 in Pyramidal Neurons in the Hippocampus CA1 Area. Brain Res. 2012, 1484, 76–84. [Google Scholar] [CrossRef]
- Fanaei, H.; Karimian, S.M.; Sadeghipour, H.R.; Hassanzade, G.; Kasaeian, A.; Attari, F.; Khayat, S.; Ramezani, V.; Javadimehr, M. Testosterone Enhances Functional Recovery after Stroke through Promotion of Antioxidant Defenses, BDNF Levels and Neurogenesis in Male Rats. Brain Res. 2014, 1558, 74–83. [Google Scholar] [CrossRef]
- Dittrich, F.; Ramenda, C.; Grillitsch, D.; Frankl-Vilches, C.; Ko, M.C.; Hertel, M.; Goymann, W.; ter Maat, A.; Gahr, M. Regulatory Mechanisms of Testosterone-Stimulated Song in the Sensorimotor Nucleus HVC of Female Songbirds. BMC Neurosci. 2014, 15, 128. [Google Scholar] [CrossRef]
- Rasika, S.; Alvarez-Buylla, A.; Nottebohm, F. BDNF Mediates the Effects of Testosterone on the Survival of New Neurons in an Adult Brain. Neuron 1999, 22, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.-X.; Cui, C.-L.; Yan, X.-sS.; Zhang, B.-F.; Song, W.; Huo, D.-S.; Wang, H.; Yang, Z.-J. Effects of Testosterone on Synaptic Plasticity Mediated by Androgen Receptors in Male SAMP8 Mice. J. Toxicol. Environ. Health Part A 2016, 79, 849–855. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, R.; Goldman, S.A. Testosterone Modulation of Angiogenesis and Neurogenesis in the Adult Songbird Brain. Neuroscience 2013, 239, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.M.; Purves-Tyson, T.D.; Fung, S.J.; Shannon Weickert, C. The Effect of Adolescent Testosterone on Hippocampal BDNF and TrkB MRNA Expression: Relationship with Cell Proliferation. BMC Neurosci. 2015, 16, 4. [Google Scholar] [CrossRef]
- Al-Shamma, H.A.; Arnold, A.P. Brain-Derived Neurotrophic Factor Regulates Expression of Androgen Receptors in Perineal Motoneurons. Proc. Natl. Acad. Sci. USA 1997, 94, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Arnold, A.P. BDNF Regulation of Androgen Receptor Expression in Axotomized SNB Motoneurons of Adult Male Rats. Brain Res. 2000, 852, 127–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Verhovshek, T.; Sengelaub, D.R. Brain-Derived Neurotrophic Factor and Androgen Interact in the Maintenance of Dendritic Morphology in a Sexually Dimorphic Rat Spinal Nucleus. Endocrinology 2004, 145, 161–168. [Google Scholar] [CrossRef]
- Osborne, M.C.; Verhovshek, T.; Sengelaub, D.R. Androgen Regulates TrkB Immunolabeling in Spinal Motoneurons. J. Neurosci. Res. 2007, 85, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Ottem, E.N.; Beck, L.A.; Jordan, C.L.; Breedlove, S.M. Androgen-Dependent Regulation of Brain-Derived Neurotrophic Factor and Tyrosine Kinase B in the Sexually Dimorphic Spinal Nucleus of the Bulbocavernosus. Endocrinology 2007, 148, 3655–3665. [Google Scholar] [CrossRef] [PubMed]
- Verhovshek, T.; Cai, Y.; Osborne, M.C.; Sengelaub, D.R. Androgen Regulates Brain-Derived Neurotrophic Factor in Spinal Motoneurons and Their Target Musculature. Endocrinology 2010, 151, 253–261. [Google Scholar] [CrossRef]
- Hill, R.A.; Wu, Y.W.C.; Kwek, P.; Van den Buuse, M. Modulatory Effects of Sex Steroid Hormones on Brain-Derived Neurotrophic Factor-Tyrosine Kinase B Expression during Adolescent Development in C57Bl/6 Mice. J. Neuroendocrinol. 2012, 24, 774–788. [Google Scholar] [CrossRef]
- Verhovshek, T.; Sengelaub, D.R. Androgen Action at the Target Musculature Regulates Brain-Derived Neurotrophic Factor Protein in the Spinal Nucleus of the Bulbocavernosus. Dev. Neurobiol. 2013, 73, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Brague, J.C.; Zinn, C.R.; Granot, D.Y.; Feathers, C.T.; Swann, J.M. TrkB Is Necessary for Male Copulatory Behavior in the Syrian Hamster (Mesocricetus Auratus). Horm. Behav. 2018, 97, 162–169. [Google Scholar] [CrossRef]
- Rankov Petrovic, B.; Hrncic, D.; Mladenovic, D.; Simic, T.; Suvakov, S.; Jovanovic, D.; Puskas, N.; Zaletel, I.; Velimirovic, M.; Cirkovic, V.; et al. Prenatal Androgenization Induces Anxiety-Like Behavior in Female Rats, Associated with Reduction of Inhibitory Interneurons and Increased BDNF in Hippocampus and Cortex. Biomed Res. Int. 2019, 2019, 426092. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.Y.; Huang, Y.L.; Chang, C.; Kang, H.Y. Deficiency in Androgen Receptor Aggravates the Depressive-like Behaviors in Chronic Mild Stress Model of Depression. Cells 2019, 8, 1021. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; McCarthny, C.R.; Notaras, M.; van den Buuse, M.; Hill, R.A. Effect of Adolescent Androgen Manipulation on Psychosis-like Behaviour in Adulthood in BDNF Heterozygous and Control Mice. Horm. Behav. 2019, 112, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.J.; Ramdev, R.A.; Tuta, N.J.; Spritzer, M.D. Dose-Dependent Effects of Testosterone on Spatial Learning Strategies and Brain-Derived Neurotrophic Factor in Male Rats. Psychoneuroendocrinology 2020, 121, 104850. [Google Scholar] [CrossRef]
- Halievski, K.; Henley, C.L.; Domino, L.; Poort, J.E.; Fu, M.; Katsuno, M.; Adachi, H.; Sobue, G.; Breedlove, S.M.; Jordan, C.L. Androgen-Dependent Loss of Muscle BDNF MRNA in Two Mouse Models of SBMA. Exp. Neurol. 2015, 269, 224–232. [Google Scholar] [CrossRef]
- Lannigan, D.A. Estrogen Receptor Phosphorylation. Steroids 2003, 68, 1–9. [Google Scholar] [CrossRef]
- Anbalagan, M.; Rowan, B.G. Estrogen Receptor Alpha Phosphorylation and Its Functional Impact in Human Breast Cancer. Mol. Cell. Endocrinol. 2015, 418, 264–272. [Google Scholar] [CrossRef]
- Frank, L. BDNF Down-Regulates Neurotrophin Responsiveness, TrkB Protein and TrkB MRNA Levels in Cultured Rat Hippocampal Neurons. Eur. J. Neurosci. 1996, 8, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Anerillas, C.; Herman, A.B.; Munk, R.; Garrido, A.; Lam, K.W.G.; Payea, M.J.; Rossi, M.; Tsitsipatis, D.; Martindale, J.L.; Piao, Y.; et al. A BDNF-TrkB Autocrine Loop Enhances Senescent Cell Viability. Nat. Commun. 2022, 13, 6228. [Google Scholar] [CrossRef] [PubMed]
- Sohrabji, F.; Miranda, R.C.G.; Toran-Allerand, C.D. Identification of a Putative Estrogen Response Element in the Gene Encoding Brain-Derived Neurotrophic Factor. Proc. Natl. Acad. Sci. USA 1995, 92, 11110–11114. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Squillacioti, C.; Paone, I.; Ciarcia, R.; Russo, M.; Paino, G. Effects of Castration on the Expression of Brain-Derived Neurotrophic Factor (BDNF) in the Vas Deferens and Male Accessory Genital Glands of the Rat. Cell Tissue Res. 2006, 323, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Bezanilla, S.; Beard, D.J.; Hood, R.J.; Åberg, N.D.; Crock, P.; Walker, F.R.; Nilsson, M.; Isgaard, J.; Ong, L.K. Growth Hormone Increases BDNF and MTOR Expression in Specific Brain Regions after Photothrombotic Stroke in Mice. Neural Plast. 2022, 2022, 983042. [Google Scholar] [CrossRef] [PubMed]
- Eyileten, C.; Sharif, L.; Wicik, Z.; Jakubik, D.; Jarosz-Popek, J.; Soplinska, A.; Postula, M.; Czlonkowska, A.; Kaplon-Cieslicka, A.; Mirowska-Guzel, D. The Relation of the Brain-Derived Neurotrophic Factor with MicroRNAs in Neurodegenerative Diseases and Ischemic Stroke. Mol. Neurobiol. 2021, 58, 329–347. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.H.; Park, H.H. Neurogenesis in Stroke Recovery. Transl. Stroke Res. 2017, 8, 3–13. [Google Scholar] [CrossRef]
- Chen, A.; Xingo, L.; Tong, Y.; Mao, M. The Neuroprotective Roles of BDNF in Hypoxic Ischemic Brain Injury. Biomed. Rep. 2013, 1, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Cole, R.; Pascal, L.E.; Wang, Z. The Classical and Updated Models of Androgen Receptor Nucleocytoplasmic Trafficking. Am. J. Clin. Exp. Urol. 2021, 9, 287–291. [Google Scholar]

| Title | Design/Methods | Aim/Experiment | Main Findings | |
|---|---|---|---|---|
| 1 | Interactions of estrogen with the neurotrophins and their receptors during neural development. Miranda, Sohrabji and Toran-Allerand, 1994. [17] | In vitro, Cell differentiation, P12 cells. | To examine interactions between estrogen and neurotrophins in cortical neurons. | Cortical neurons co-express BDNF, p75, and TrkB, and basal forebrain neurons only express neurotrophin receptors. |
| 2 | The effect of ovariectomy and estrogen replacement on brain-derived neurotrophic factor messenger ribonucleic acid expression in cortical and hippocampal brain regions of female Sprague Dawley rats. Singh, Meyer, and Simpkins, 1995. [18] | Ex vivo, mRNA analysis, In situ hybridization. | To investigate whether estradiol affects cholinergic function by modulating the levels of neurotrophic factors. | There was a reduction in BDNF levels in the rats’ frontal, parietal, and temporal cortices by 28 after OVX. |
| 3 | Levels of trkA and BDNF mRNA, but not NGF mRNA, fluctuate across the estrous cycle and increase in response to acute hormone replacement Gibbs, 1998. [24] | Ex vivo, mRNA analysis by In situ hybridization. | To examine the levels of BDNF in mice’s hippocampus and physiological changes in circulating sex steroids. | BDNF expression levels in CA1 and CA3/4 fluctuated across the estrous cycle. BDNF expression increased in the dentate granule cell layer, region CA1, and region CA3/4 in OVX animals 53 h after estrogen and 5 h after PROG treatment. |
| 4 | Brain-derived neurotrophic factor mediates estrogen-induced dendritic spine formation in hippocampal neurons Murphy, Cole, and Segal, 1998. [25] | In vitro, Cell differentiation, Cultured hippocampal neurons. | To examine estrogen and BDNF regulation of glutamic acid decarboxylase expression in hippocampal cultures. | Estrogen decreases BDNF expression in hippocampal neurons within 24 h, which suppresses inhibition and increases the excitatory tone, leading to an increase in dendritic spine density in pyramidal neurons. |
| 5 | Region- and peptide-specific regulation of the neurotrophins by estrogen Jezierski and Sohrabji, 2000. [26] | Ex vivo, Protein concentrations by Enzyme-Linked Immunosorbent Assay (ELISA) assay. | To examine the effect of estrogen on BDNF expression in the olfactory bulb and the cingulate cortex in OVX mice. | Estrogen regulation of BDNF is region-specific. It increases BDNF expression in mice olfactory bulbs and diagonal bands of Broca but decreases in the cingulate cortex. |
| 6 | Neurotrophin expression in the reproductively senescent forebrain is refractory to estrogen stimulation Jezierski and Sohrabji, 2001 [27] | Ex vivo, Protein concentrations, Western blot. | To compare the estrogen regulation of neurotrophin ligands and receptors in young adult and senescent mice diagonal bands of Broca. | Estrogen increases BDNF and TrKB expression in the olfactory bulb and horizontal limb and decreases p75NRT expression in young mice but increases it in senescent mice. Senescent mice have higher ERα expression but very low steroid receptor coactivator (SRC-1) expression in the olfactory bulb. |
| 7 | Expression and estrogen regulation of brain-derived neurotrophic factor gene and protein in the forebrain of female prairie voles Liu et al., 2001. [28] | Ex vivo, Protein immunoreactive staining and mRNA labeling. | To map BDNF immunoreactive staining and mRNA labeling throughout the forebrain in female prairie voles. | Estrogen-treated mice have higher levels of BDNF in the DG and CA3 regions of the hippocampus, as well as in the basolateral nucleus of the amygdala than controls. |
| 8 | Estrogen and exercise interact to regulate brain-derived neurotrophic factor mRNA and protein expression in the hippocampus Berchtold et al., 2001. [29] | Ex vivo, ELISA and in situ hybridization. | To investigate estrogen and exercise interaction in BDNF regulation. | Exercise increases hippocampal BDNF expression and protein levels in female mice, which is reduced in the absence of estrogen in a time-dependent manner. |
| 9 | Estrogen stimulates brain-derived neurotrophic factor expression in embryonic mouse midbrain neurons through a membrane-mediated and calcium-dependent mechanism Ivanova et al., 2001. [30] | Ex vivo and in vitro, ELISA, Cell culture and RT-PCR analyses. | To investigate if estrogen influences dopaminergic cell differentiation through a BDNF-dependent mechanism in the midbrains of mice. | Estrogen upregulates BDNF expression in the midbrains of mice and has a stimulatory effect on dopaminergic neuron differentiation by coordinating BDNF expression. |
| 10 | Estrogen Regulates the Development of Brain-Derived Neurotrophic Factor mRNA and Protein in the Rat Hippocampus Solum and Handa, 2002. [31] | Ex vivo, Immunocytochemistry, RT-PCR, Western blot analysis. | To examine gonadectomy and estrogen replacement effects on the BDNF system in mice’s developing hippocampus. | ERα and BDNF colocalize in cells within the developing hippocampus. BDNF expression levels reduce within 7 days in postnatal gonadectomized male rats, and estrogen treatment restores BDNF levels in intact animals. No changes were found in TrkB levels. |
| 11 | Estrogen enhances retrograde transport of Brain-Derived Neurotrophic Factor in the Rodent Forebrain Jezierski and Sohrabji, 2003. [32] | Ex vivo, Immunohisto-chemisthy. | To examine the effect of estrogen on the retrograde transport of BDNF in the diagonal band of Broca and its forebrain target in mice. | Estrogen-treated animals had greater numbers of neurons with retrogradely labeled BDNF than controls. |
| 12 | Influence of estradiol, stress, and 5-HT2A agonist treatment on brain-derived neurotrophic factor expression in female rats Cavus and Duman, 2003. [33] | Ex vivo, In situ hybridization. | To examine the estrous cycle and BDNF expression in the hippocampus and cortex of mice. | BDNF expression levels in the DG and the medial prefrontal cortex decrease when estradiol levels are highest. Acute estradiol treatment decreased hippocampal BDNF expression in acute OVX but did not affect chronic OVX animals. |
| 13 | Oestrogen regulates sympathetic neurite outgrowth by modulating brain derived neurotrophic factor synthesis and release by the rodent uterus Krizsan-Agbas et al., 2003. [34] | Ex vivo, ELISA, In situ hybridization. | To examine the role of neurotrophins and estrogen in uterine sympathetic nerve remodeling. | Estrogen increases BDNF expression and protein in the myometrium and endometrium of OVX mice. |
| 14 | Estrogen affects BDNF expression following chronic constriction nerve injury Zhao et al., 2003 [35] | Ex vivo, Radioimmunoassay, ELISA and RT-PCR. | To investigate the effect of estrogen on BDNF expression in neuropathic pain in a chronic constriction injury model of mice. | Mice with higher estrogen were more sensitive to thermal stimuli and had higher levels of BDNF expression and protein levels. |
| 15 | Anatomical evidence for transsynaptic influences of estrogen on brain-derived neurotrophic factor expression Blurton-Jones, Kuan, and Tuszynski, 2004. [36] | Ex vivo, Immunohistochemistry. | To examine the localization of estrogen receptors and BDNF in the brains of adult mice. | ERα and BDNF colocalize in the hypothalamus, amygdala, prelimbic cortex, and ventral hippocampus. ERβ and BDNF do not colocalize in any brain regions. |
| 16 | Environmental enrichment reduces the mnemonic and neural benefits of estrogen Gresack and Frick, 2004. [37] | Ex vivo, ELISA. | To observe environmental factors that influence mnemonic and neural response to estrogen in mice. | Estrogen decreased hippocampal BDNF in mice in standard conditions but not enriched ones. |
| 17 | Inhibition of tyrosine kinase receptor type B synthesis blocks axogenic effect of estrogen on rat hypothalamic neurones in vitro Brito, Carrer, and Cambiasso, 2004. [38] | In vitro, Western blot, Immunohistochemistry. | To examine the estrogen-induced axogenic response and upregulation of TrkB in neuronal and glial cultures of male mice. | An increase in TrkB is necessary for estrogen to exert its axogenic effect on male-derived neurons. |
| 18 | Effects of estrogen treatment on expression of brain-derived neurotrophic factor and cAMP response element-binding protein expression and phosphorylation in rat amygdaloid and hippocampal structures Zhou et al., 2005. [39] | Ex vivo, In situ PCR, Immunohistochemistry. | To examine the effect of estrogen on CREB expression, phosphorylation, and BDNF expression in the amygdala and hippocampus of mice. | Estrogen increased BDNF expression levels in mice’s amygdala, CA1, and CA3 regions of the hippocampus and increased pCREB in the medial and basomedial regions but not the central or basolateral amygdala. |
| 19 | Estradiol to aged female or male mice improves learning in inhibitory avoidance and water maze tasks Frye, Rhodes, and Dudek, 2005. [40] | Ex vivo, ELISA, Radioimmunoassay. | To evaluate the mnemonic effects of post-training estradiol in aged male mice. | BDNF levels decreased in the hippocampus of trained mice 1 h following estradiol exposure. |
| 20 | Sex and ovarian steroids modulate brain-derived neurotrophic factor (BDNF) protein levels in rat hippocampus under stressful and non-stressful conditions Franklin and Perrot-Sinal, 2006. [41] | Ex vivo, ELISA. | To investigate stress, sex hormones, and BDNF protein levels in CA1, CA3, and DG subregions of mice hippocampus. | Females have higher levels of BDNF in CA3 and lower levels in DG relative to males. Stress decreases BDNF in CA3 in all animals. Stress increases BDNF levels in the DG of PROG-treated OVX mice while decreasing in controls. |
| 21 | 17β-estrogen Attenuates Hippocampal Neuronal Loss and Cognitive Dysfunction Induced By Chronic Restraint Stress in Ovariectomized Rats Takuma et al., 2007. [42] | Ex vivo, ELISA, RT-PCR. | To evaluate the effect of estrogen on cognitive function in rodents under stress environments. | OVX or chronic stress decreases the levels of hippocampal BDNF expression in the CA3 region. Estrogen attenuates the stress-induced decrease in hippocampal BDNF expression levels in OVX rats. |
| 22 | Mode of action and functional significance of estrogen-inducing dendritic growth, spinogenesis, and synaptogenesis in the developing Purkinje cell Sasahara et al., 2007. [43] | Ex vivo, RT-PCR Immunocytochemistry. | To analyze the expression of BDNF and estrogen in Purkinje cells of neonatal and cytochrome P450 aromatase knock-out rodents. | Estrogen induces the expression of BDNF in mouse cerebella and promotes dendritic growth of Purkinje cells during development. |
| 23 | 17β-estrogen protects depletion of rat temporal cortex somatostatinergic system by β-amyloid Aguado-Llera et al., 2007. [44] | Ex vivo, ELISA RT-PCR Immunohistochemistry. | To investigate estrogen treatment in amyloid-beta-related changes in neuronal cells in the hippocampi of rodents. | Estrogen increased BDNF expression in hippocampal cells both in the absence or presence of estrogen. |
| 24 | Estrogen receptor β protects against acoustic trauma in mice Meltser et al., 2008. [45] | Ex vivo, Immunoassays, RT-PCR, Western blots. | To examine the role of ERs in response to auditory trauma. | BDNF expression was more pronounced in wild-type mice compared to ER-deficient mice. |
| 25 | β-estrogen induces synaptogenesis in the hippocampus by enhancing brain-derived neurotrophic factor release from dentate gyrus granule cells Sato et al., 2007. [46] | In vitro, hippocampal neuron cultures. | To examine the effect of estrogen on synaptogenesis in hippocampal neuronal cell cultures. | The effects of estrogen in the hippocampal and subregional hippocampal neurons were independent of nuclear ERs and dependent on BDNF. Estrogen enhanced BDNF release from DG granule cells via nuclear ER-independent and PKA-dependent mechanisms. |
| 26 | Brain-derived neurotrophic factor signaling in the HVC is required for testosterone-induced song of female canaries Hartog et al., 2009 [47] | Ex vivo, In situ transfection, Western blot. | To examine BDNF and T4-dependent development of the song system in female canaries. | The testosterone-induced song system is blocked by concurrent inhibition of the vascular endothelial growth factor receptor tyrosine kinase, which is reversed by BDNF. |
| 27 | Involvement of Brain-Derived Neurotrophic Factor and Neurogenesis in Oestrogen Neuroprotection of the Hippocampus of Hypertensive Rats. Pietranera et al., 2010. [48] | Ex vivo, ELISA. Immunocytochemistry. | To evaluate estrogen treatment in hypertensive rats and BDNF expression. | Hypertensive rats exhibit decreased expression and protein levels of BDNF in the DG without changes in CA1 or CA3 pyramidal cell layers. Estrogen increases BDNF expression in the DG and BDNF protein in the whole hippocampus. |
| 28 | Estrogen reduces BDNF level, but maintains dopaminergic cell density in the striatum of MPTP mouse model. Tripanichkul, Gerdprasert, and Jaroensuppaperch, 2010. [49] | Ex vivo, Immunohistochemistry. | To examine the effects of estrogen treatment on BDNF expression and the density of DA neurons in the striatum of MPTP mice. | Estrogen impaired dopaminergic denervation and decreased the striatal BDNF upregulation triggered by MPTP. |
| 29 | Full length TrkB potentiates estrogen receptor alpha mediated transcription suggesting convergence of susceptibility pathways in schizophrenia. Wong, Woon, and Weickert, 2011. [50] | In vitro, Cull culture, Immunofluorescence, Western blot. | To examine ERα interaction with TrkB in neuronal and non-neuronal cell lines. | TrkB activation increases transcription at EREs, independent of exogenous estrogen, and further potentiates the effect of estrogen-ERα-mediated transcription. |
| 30 | 17β-Estrogen replacement in young, adult and middle-aged female ovariectomized rats promotes improvement of spatial reference memory and an antidepressant effect and alters monoamines and BDNF levels in memory- and depression-related brain areas. Kiss et al., 2012 [51] | Ex vivo, ELISA. | To evaluate the effects of chronic treatment with estrogen on cognition and depressive-like behaviors in young, adult, and middle-aged female rats. | Both young mice and estrogen-treated OVX mice have higher BDNF levels. The young estrogen-treated OVX group presented higher BDNF levels compared to adult and middle-aged estrogen-treated animals. |
| 31 | 17β-estrogen Regulates the Sexually Dimorphic Expression of BDNF and TrkB Proteins in the Song System of Juvenile Zebra Finches. Tang and Wade, 2012. [52] | Ex vivo, Western blot. | To examine BDNF isoforms and TrkB expression in the developing song system of juvenile males and females zebra finch treated with estrogen. | Estrogen modulates BDNF expression and TrkB in the song system of juveniles of both sexes. |
| 32 | Estradiol acts via estrogen receptors alpha and beta on pathways important for synaptic plasticity in the mouse hippocampal formation. Spencer-Segal et al., 2012. [53] | Ex vivo, In situ hybridization, Western blot. | To study estradiol systems and pathways related to plasticity and learning. | Estradiol increased phosphorylated Akt phosphorylated TrkB receptor in the hippocampus. These effects were abolished in ERα and ERβ knockout mice. |
| 33 | Estrone is neuroprotective in rats after traumatic brain injury. Gatson et al., 2012. [54] | Ex vivo, Immunohistochemistry, Western blot. | To study the role of estrone in traumatic brain injury in rats. | Cortical levels of phospho-ERK1/2 are increased by estrone, which was associated with an increase in phospho-CREB levels and BDNF expression. |
| 34 | Estradiol promotes purkinje dendritic growth, spinogenesis, and synaptogenesis during neonatal life by inducing the expression of BDNF. Haraguchi et al., 2012. [55] | Ex vivo, Immunohistochemistry. | To study estradiol and cerebellar neuronal circuit formation, dendritic growth, spinogenesis, and synaptogenesis in the Purkinje cell of wild vs. aromatase KO mice. | Estradiol increased neuroplasticity in all Purkinje cells. ER antagonist decreases BDNF levels in all mice. BDNF administration to ER antagonist-treated mice increased Purkinje dendritic growth. |
| 35 | Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estrogen levels. Zhu et al., 2013. [56] | Ex vivo, RT-PCR. | To study if estradiol modulates the anorectic effect of BDNF in OVX rats. | BDNF expression is elevated in the hypothalamus during oestrus, following the estradiol peak, and after estradiol treatment. |
| 36 | Post-stroke infections exacerbate ischemic brain injury in middle-aged rats: Immunomodulation and neuroprotection by PROG. Yousuf et al., 2013 [57] | Ex vivo, ELISA. | To evaluate the effect of systemic inflammation on stroke outcomes and PROG neuroprotection in middle-aged rats. | Serum BDNF levels decrease in systemic inflammation conditions. PROG decreases cytokine levels and systemic inflammation and restores BDNF levels within 3 and 7 days post-stroke. |
| 37 | Long-term OVX increases BDNF gene methylation status in mouse hippocampus. Moreno-Piovano et al., 2014. [58] | Ex vivo, RT-PCR, Immunohistochemistry. Real-time quantitative methylation-specific PCR. | To determine if the post-OVX timeframe elapsed before E treatment is critical for the estrogen induction of neurotrophins BDNF in the rodents’ hippocampus. | Early estrogen-treated animals showed increased BDNF expression and a higher activity of BDNF II, IV, and V promoters. Late treated animals did not show estrogen induction of neurotrophins, and the methylation levels of the regulatory sequences of the BDNF gene were higher than in the early-treated animals. |
| 38 | 17α-Oestrogen-Induced Neuroprotection in the Brain of Spontaneously Hypertensive Rats. Pietranera et al., 2014. [59] | Ex vivo, Immunohistochemistry, In situ hybridization. | To investigate estrogen and pathological changes in the hippocampus and hypothalamus of hypertensive rats. | Estrogen treatment enhanced the number of cells and increased BDNF expression in the CA1 region and DG. |
| 39 | 17β-Estrogen regulates histone alterations associated with memory consolidation and increases Bdnf promoter acetylation in middle-aged female mice. Fortress et al., 2014 [60] | Ex vivo, RT-PCR, Western blot. | To examine the effects of estrogen infusion in the mice hippocampal on object recognition and spatial memory. | Estrogen specifically increased acetylation at BDNF promoters pII and pIV in the dorsal hippocampus of young and middle-aged mice despite age-related decreases. |
| 40 | Chronic estrogen treatment decreases Brain Derived Neurotrophic Factor (BDNF) expression and monoamine levels in the amygdala—Implications for behavioral disorders. Balasubramanian et al., 2014. [61] | Ex vivo, Immunoassay, PCR. | To verify whether chronic low estrogen doses cause anxiety-like disorder by altering BDNF and monoamine levels in rats’ hippocampus and amygdala. | Chronic estrogen treatment decreased BDNF expression and protein levels in the central amygdala, which was accompanied by a reduction in dopamine levels. No changes were observed in the hippocampus. |
| 41 | Analyzing the influence of BDNF heterozygosity on spatial memory response to 17β-estrogen. Wu et al., 2015. [62] | Ex vivo, Immunofluorescence Western blot. | To test if disruption to the estrogen–parvalbumin pathway alters learning and memory and BDNF levels in mice. | Estrogen replacement prevented the reduction in BDNF and parvalbumin protein levels in the dorsal hippocampus and CA1. BDNF heterozygote mice showed either no response or an opposite response to estrogen treatment. |
| 42 | Aging-induced changes in sex-steroidogenic enzymes and sex-steroid receptors in the cortex, hypothalamus and cerebellum. Munetomo et al., 2015. [63] | Ex vivo, RT-PCR. | To examine age-induced changes in sex-steroidogenic enzymes and sex-steroid receptors in 3-, 12-, and 24-month-old male rats’ cerebral cortex, hypothalamus, and cerebellum. | BDNF expression decreased from 3 to 24 m in the cerebral cortex but increased in the hypothalamus and did not change in the cerebellum. The expression levels of AR, ERα, and ERβ were higher in the Hypothalamus than in the cerebral cortex and cerebellum. |
| 43 | ERα Signaling Is Required for TrkB-Mediated Hippocampal Neuroprotection in Female Neonatal Mice after Hypoxic–Ischemic Encephalopathy. Cikla et al., 2016. [64] | Ex vivo, Western blot, RT-PCR. | To investigate how hypoxia induces ERα expression in the female neonatal hippocampus. | TrkB phosphorylation post-trauma is greater in females than males after selective TrkB agonist therapy and depends on the presence of ERα. TrkB agonist therapy decreases c-caspase-3, but only in the presence of ERα. |
| 44 | Combined exercise ameliorates OVX-induced cognitive impairment by enhancing cell proliferation and suppressing apoptosis. Kim et al., 2016. [65] | Ex vivo, Immunohistochemistry, Western blot. | To evaluate the effects of exercise on memory deficits, cell proliferation, and apoptosis in the hippocampus of OVX rats. | The expression of BDNF and TrkB decreased in the DG, together with memory decrease in OVX rats. These expression levels increased in the exercise group. |
| 45 | Selective Oestrogen Receptor Agonists Rescued Hippocampus Parameters in Male Spontaneously Hypertensive Rats. Pietranera et al., 2016. [66] | Ex vivo, Immunocytochemistry, RT-PCR. | To examine which type of ER is involved in low BDNF expression in the hippocampus of hypertensive rats. | ERα agonist slightly increased BDNF expression but had no effect on the number of doublecortin progenitors. Treatment with ERβ agonist increased BDNF expression and doublecortin progenitors. |
| 46 | Regulation of endometrial cell proliferation by estrogen-induced BDNF signaling pathway. Dong et al., 2017. [67] | In vitro, Human endometrial cancer cells, Western blot. | To investigate the role of the BDNF Val66Met polymorphism in regulating proliferation in endometrial cells treated with estrogen. | BDNF signaling pathway activates with estrogen stimulation. BDNF production is induced by estrogen, and the BDNF Val66Met is a loss-of-function polymorphism in the regulation of endometrial cell proliferation. |
| 47 | Sex differences in the effect of chronic mild stress on mouse prefrontal cortical BDNF levels: A role of major ovarian hormones. Karisetty et al., 2017. [68] | Ex vivo, Westerns blot, RT-PCR. | To examine the effect of sex hormones on depression-like phenotypes in mice exposed to 21-day chronic stress. | There was a decrease in the BDNF protein levels in intact females but not in OVX or male mice. Estrogen treatment, and not PROG, increased BDNF expression in the prefrontal cortex of the stressed mice with OVX. |
| 48 | Sex differences and estrogen regulation of BDNF gene expression, but not propeptide content, in the developing hippocampus. Kight and McCarthy, 2017. [69] | Ex vivo, RT-PCR, ELISA, Western blot. | To examine the downstream effects of estrogen on hippocampal cell proliferation and BDNF expression in male and female neonatal rats. | Estrogen had opposite effects on BDNF expression in different areas of the neonatal hippocampus. In the CA1, BDNF increased but decreased in DG. Blocking estrogen signaling decreased BDNF expression in the DG in males but not females. No differences were observed in pro-BDNF protein. |
| 49 | Estrogen receptor β deficiency impairs BDNF–5-HT2A signaling in the hippocampus of female brain: A possible mechanism for menopausal depression. Chhibber et al., 2017. [70] | Ex vivo, In vitro, Western blot. | To examine the ER subtypes in the regulation of BDNF and serotonin signaling in mice. | BDNF was downregulated in ERβ−/− mice in a brain-region-specific manner. There was a reduction in BDNF protein levels in the hippocampus of ERβ−/− mice and no changes in the cortex and hypothalamus. ERβ agonism enhanced BDNF/TrkB signaling pathways. |
| 50 | Additive antidepressant-like effects of fasting with β-Estrogen in mice. Wang et al., 2019. [71] | Ex vivo, Immunohistochemistry, Western blot. | To evaluate the antidepressant-like effects of acute fasting and estrogen treatment. | BDNF-TrkB signaling pathway was increased in the prefrontal cortex and hippocampus. Serum ghrelin and estrogen were increased by fasting plus estrogen. |
| 51 | Voluntary exercise and estrogen reverse OVX-induced spatial learning and memory deficits and reduction in hippocampal brain-derived neurotrophic factor in rats. Rashidy-Pour et al., 2019. [72] | Ex vivo, ELISA. | To investigate voluntary exercise and estrogen replacement in learning and memory deficits and hippocampal BDNF levels in OVX mice. | Either exercise and estrogen alone or their combination recovery the negative effects of OVX on learning and memory performance. Combined treatment does not potentiate the effect of either treatment alone. |
| 52 | Sex-dependent aberrant prefrontal cortex development in the adolescent offspring rats exposed to variable prenatal stress. Niu et al., 2020. [73] | Ex vivo, ELISA. | To examine neurochemical and behavioral changes in the offspring rats (adolescents) from rats treated with prenatal stress during the third week of gestation. | Prenatal stress increases BDNF levels in the prefrontal cortex of adolescent females. ER concentration increased with age in all animals. |
| 53 | Rapid effects of estrogen and its receptor agonists on object recognition and object placement in adult male zebrafish. Naderi et al., 2020. [74] | Ex vivo, Whole-brain RT-PCR. | To evaluate the effects of post-training estrogen in the consolidation of object recognition and object placement memory in adult male zebrafish. | Post-training estrogen treatment and selective ER agonist induced upregulation in the BDNF expression levels. BDNF expression increases with a high estrogen dose. |
| 54 | Reducing luteinizing hormone levels after OVX improves spatial memory: Possible role of brain-derived neurotrophic factor. Bohm-Levine et al., 2020. [75] | Ex vivo, Immunohistochemistry, Western blot. | To evaluate whether lowering luteinizing hormone increases BDNF expression levels. | Gonadotropin-releasing hormone receptor antagonists lower luteinizing hormone levels, and estrogen enhances spatial memory in OVX females, all of which are ineffective in the absence of TrKB. Both hormones increase BDNF expression in the hippocampus. |
| 55 | ERRγ ligand HPB2 upregulates BDNF-TrkB and enhances dopaminergic neuronal phenotype. Kim et al., 2021. [76] | In vitro, Immunohistochemistry, RT-PCR, Western blot. | To examine whether the ERRγ ligand regulates BDNF signaling and subsequent DAergic neuronal phenotype. | The ERRγ agonist increases BDNF expression and protein levels and TrkB expression in human neuroblastoma, differentiated Lund mesencephalic cells, and primary ventral mesencephalic neurons. Activation of ERK and phosphorylation of CREB induced BDNF upregulation in human neuroblastoma cells. |
| 56 | Previous oestradiol treatment during midlife maintains transcriptional regulation of memory-related proteins by ERα in the hippocampus in a rat model of menopause. Baumgartner et al., 2021. [77] | Ex vivo, RT-PCR, Western blot. | To examine whether previous estradiol treatment increases the levels of nuclear ERα, resulting in transcriptional regulation of proteins. | Continuous or previous estradiol treatments increase gene expression of BDNF. |
| 57 | 17β-Estrogen activation of dorsal hippocampal TrkB is independent of increased mature BDNF expression and is required for enhanced memory consolidation in female mice. Gross et al., 2021. [78] | Ex vivo, RT-PCR, Western blot. | To examine the effects of hippocampal TrkB signaling on estrogen-induced enhancement of memory consolidation in object placement and recognition tasks. | Dorsal hippocampal estrogen infusion increased levels of phospho-TrkB and BDNF. The estrogen-induced increase in hippocampal BDNF expression was not required for hippocampal TrkB activation and was not inhibited by TrkB antagonism. |
| 58 | ERRγ Ligand Regulates Adult Neurogenesis and Depression-like Behavior in a LRRK2-G2019S-associated Young Female Mouse Model of Parkinson’s Disease [79] | Ex vivo, Immunohistochemistry, RT-PRC Western blot. | To investigate whether the ERRγ ligand HPB2 could be a novel therapeutic agent for regulating depressive behavior in a PD mouse model. | HPB2 upregulated BDNF/TrkB signaling in the DG of young female LRRK2-G2019S mice. |
| 59 | Activation of GPER1 by G1 prevents PTSD-like behaviors in mice: Illustrating the mechanisms from BDNF/TrkB to mitochondria and synaptic connection [80] | Ex vivo Immunohistochemistry, Western blot. | To investigate the mechanism of G1 to improve PTSD from brain-derived neurotrophic factor (BDNF)/tyrosine kinase receptor B (TrkB) signaling. | G15 (GPER1 inhibitor) and ANA-12 (TrkB inhibitor) blocked the ameliorative effects of G1 on PTSD-like behaviors and the aberrant expression of hippocampal synaptic and mitochondrial proteins in mice. |
| Main Findings | Studies |
|---|---|
| BDNF gene expression is regulated via ERE and by the activation of nuclear steroid receptors, while the activation of some membrane-associate steroid receptors results in BDNF inhibition. | Frye, Rhodes, and Dudek 2005; Jodhka et al., 2009. [40,104] |
| While both BDNF and estrogen enhance BDNF expression and estrogen receptor activation, only the TrkB signal is able to inhibit ERE transcription and limit BDNF expression. | Cikla et al., 2016; Gross et al., 2021; Spencer-Segal et al., 2012; Wong, Woon, and Weickert 2011. [50,53,64,78] |
| Different sex steroid concentrations and combinations differently influence BDNF regulation, possibly due to the interactions of intracellular pathways. | Aguirre et al., 2010; Atif, Yousuf, and Stein 2016; Cekic et al., 2012; Coughlan, Gibson, and Murphy 2009. [97,105,106,110] |
| BDNF/TrkB signaling regulates the expression of androgen receptors. | Al-Shamma and Arnold 1997; Du et al., 2019; Halievski et al., 2015; Yang and Arnold 2000. [127,128,138,140] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Assis, G.G.; de Sousa, M.B.C.; Murawska-Ciałowicz, E. Sex Steroids and Brain-Derived Neurotrophic Factor Interactions in the Nervous System: A Comprehensive Review of Scientific Data. Int. J. Mol. Sci. 2025, 26, 2532. https://doi.org/10.3390/ijms26062532
de Assis GG, de Sousa MBC, Murawska-Ciałowicz E. Sex Steroids and Brain-Derived Neurotrophic Factor Interactions in the Nervous System: A Comprehensive Review of Scientific Data. International Journal of Molecular Sciences. 2025; 26(6):2532. https://doi.org/10.3390/ijms26062532
Chicago/Turabian Stylede Assis, Gilmara Gomes, Maria Bernardete Cordeiro de Sousa, and Eugenia Murawska-Ciałowicz. 2025. "Sex Steroids and Brain-Derived Neurotrophic Factor Interactions in the Nervous System: A Comprehensive Review of Scientific Data" International Journal of Molecular Sciences 26, no. 6: 2532. https://doi.org/10.3390/ijms26062532
APA Stylede Assis, G. G., de Sousa, M. B. C., & Murawska-Ciałowicz, E. (2025). Sex Steroids and Brain-Derived Neurotrophic Factor Interactions in the Nervous System: A Comprehensive Review of Scientific Data. International Journal of Molecular Sciences, 26(6), 2532. https://doi.org/10.3390/ijms26062532









