Phytochemical Composition and Wound Healing Properties of Echinacea angustifolia DC. Root Hydroalcoholic Extract
Abstract
:1. Introduction
2. Results
2.1. Phytochemical Composition
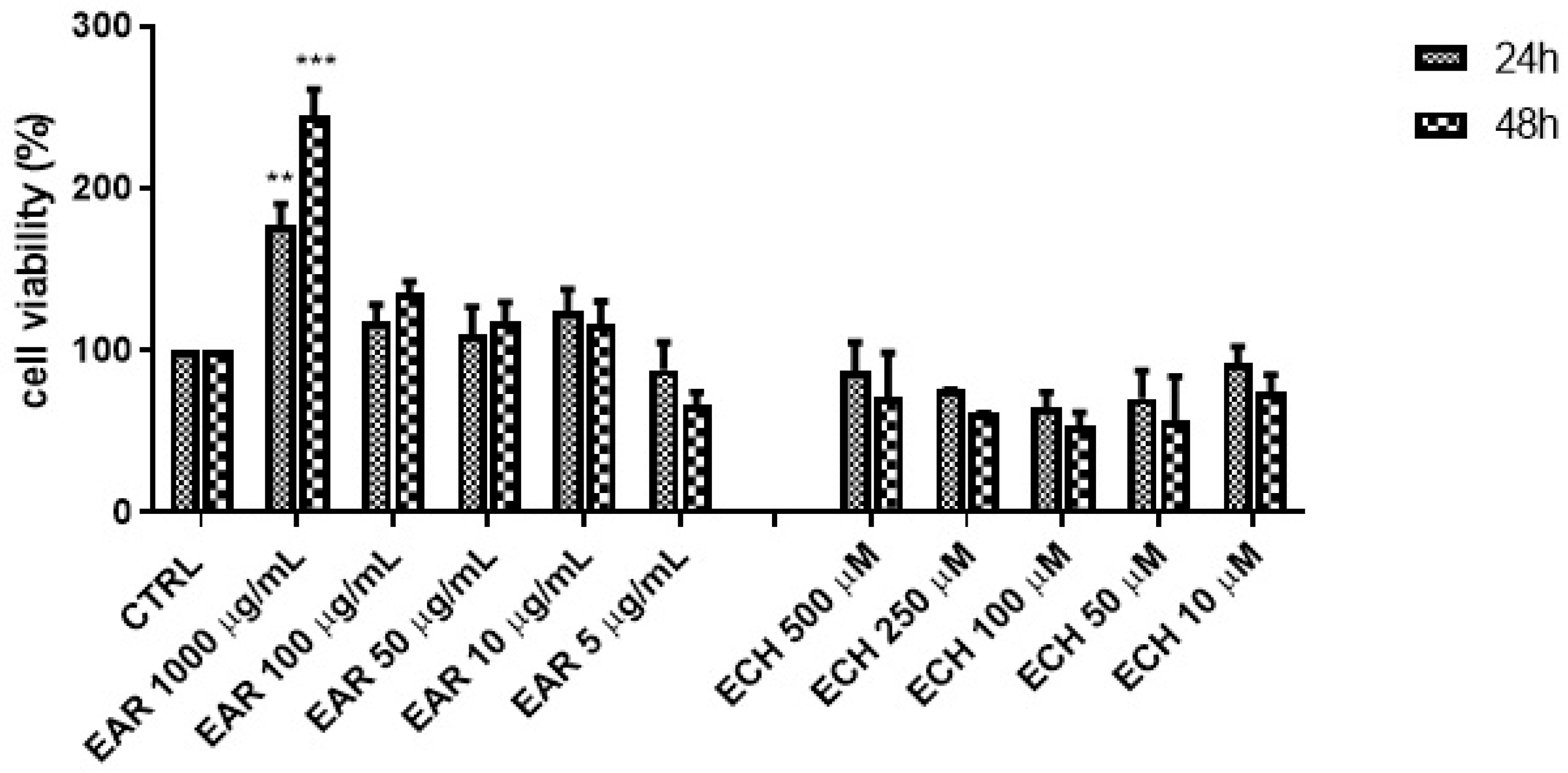
2.2. Cell Viability and Proliferation of 3T3 L1 Cells
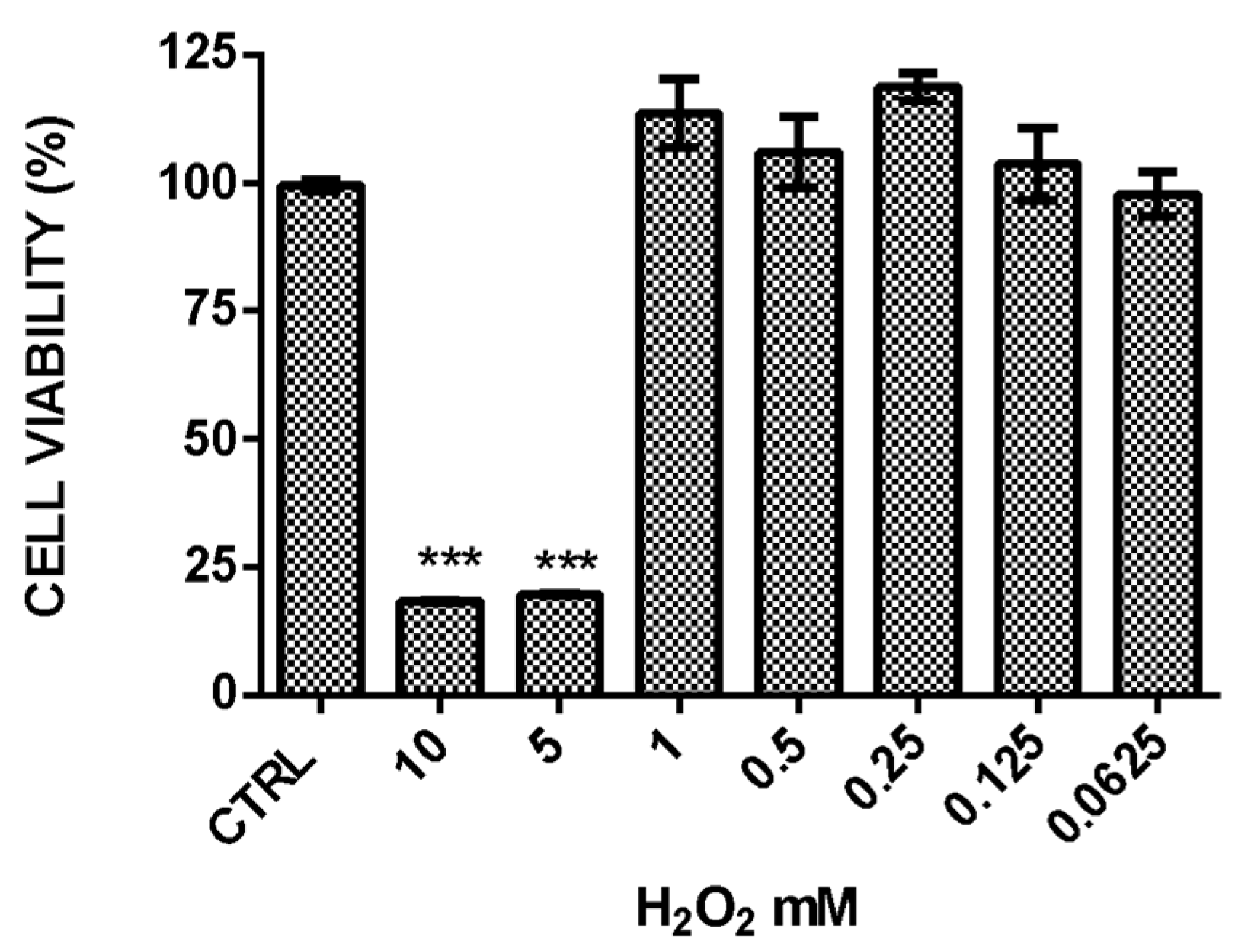
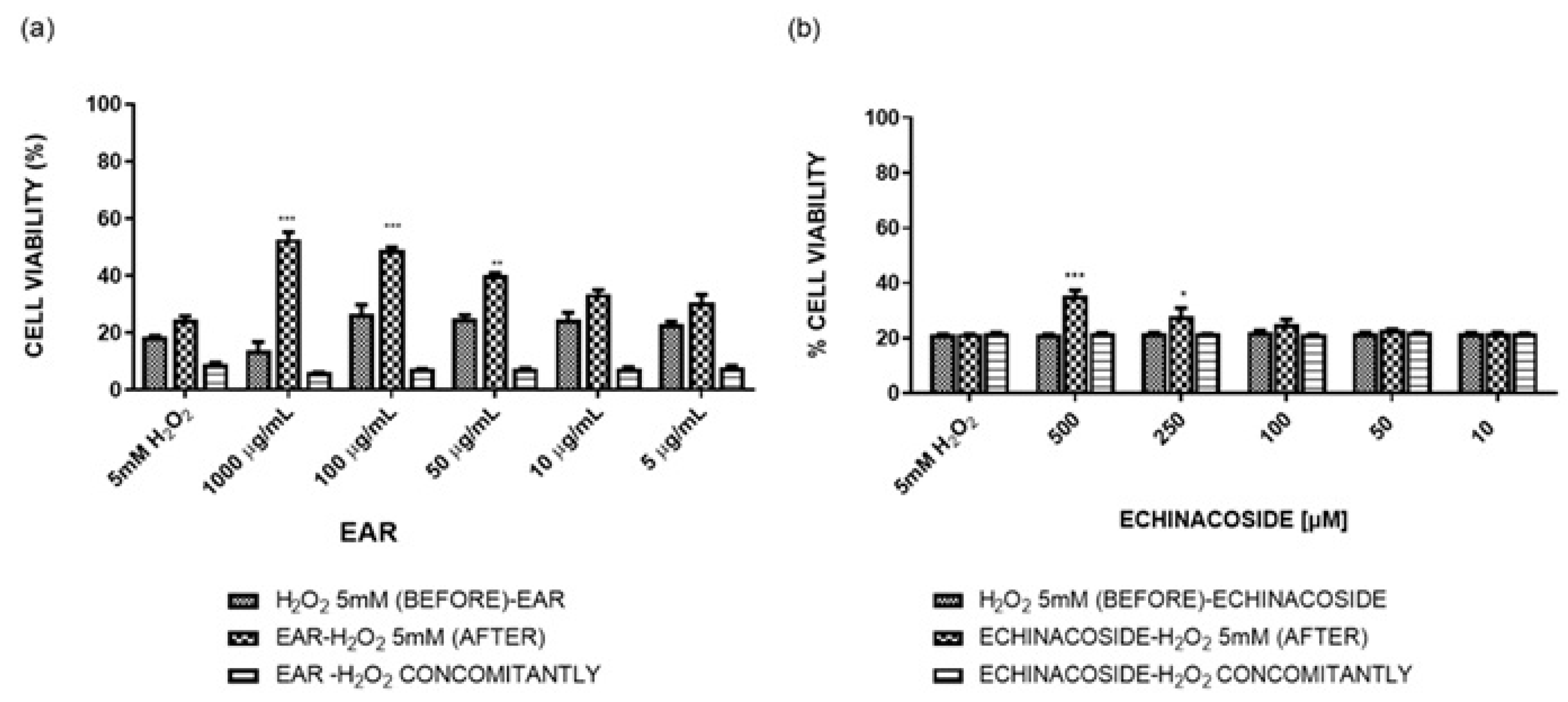
2.3. Hydrogen Peroxide-Induced Stress
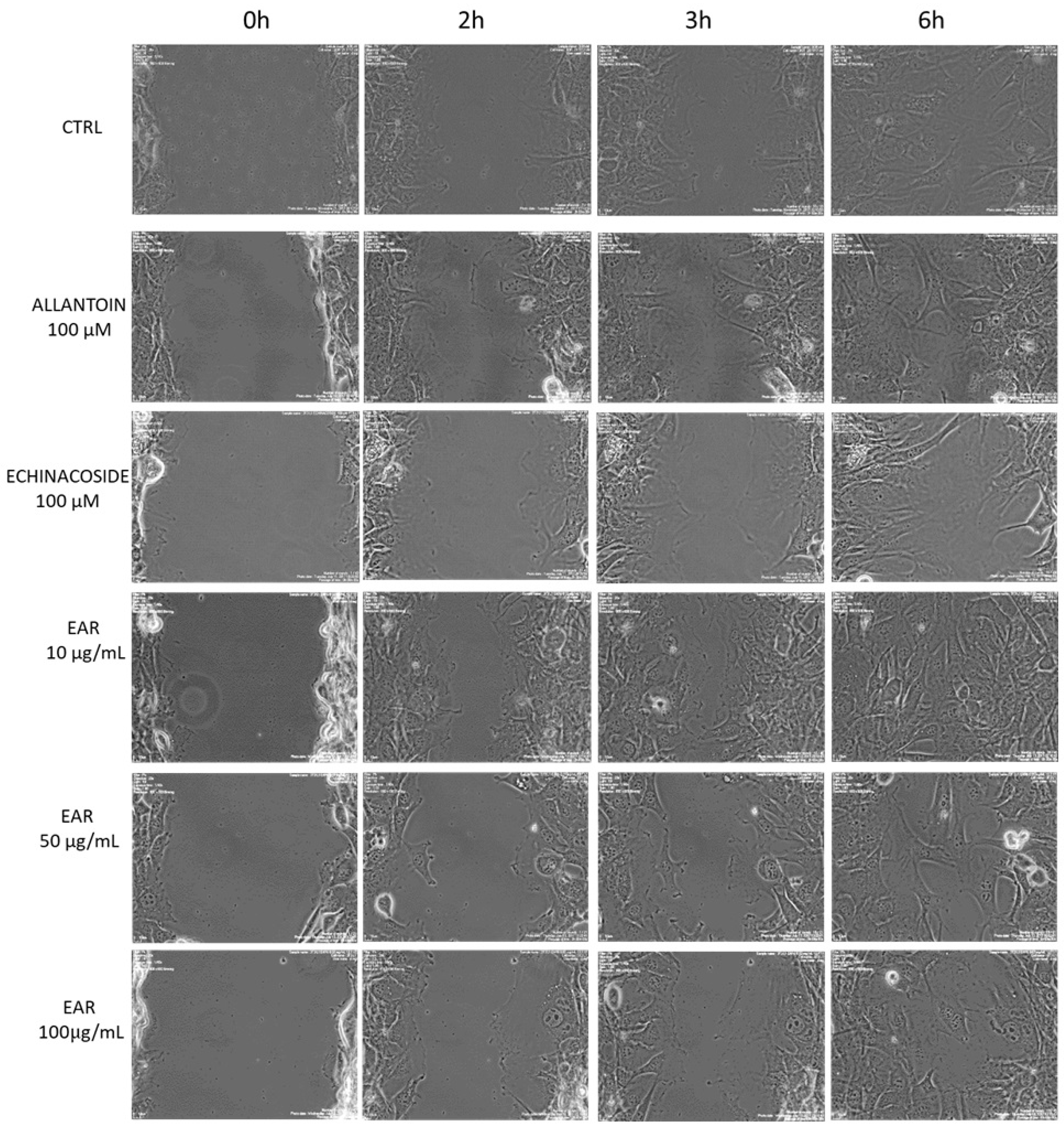
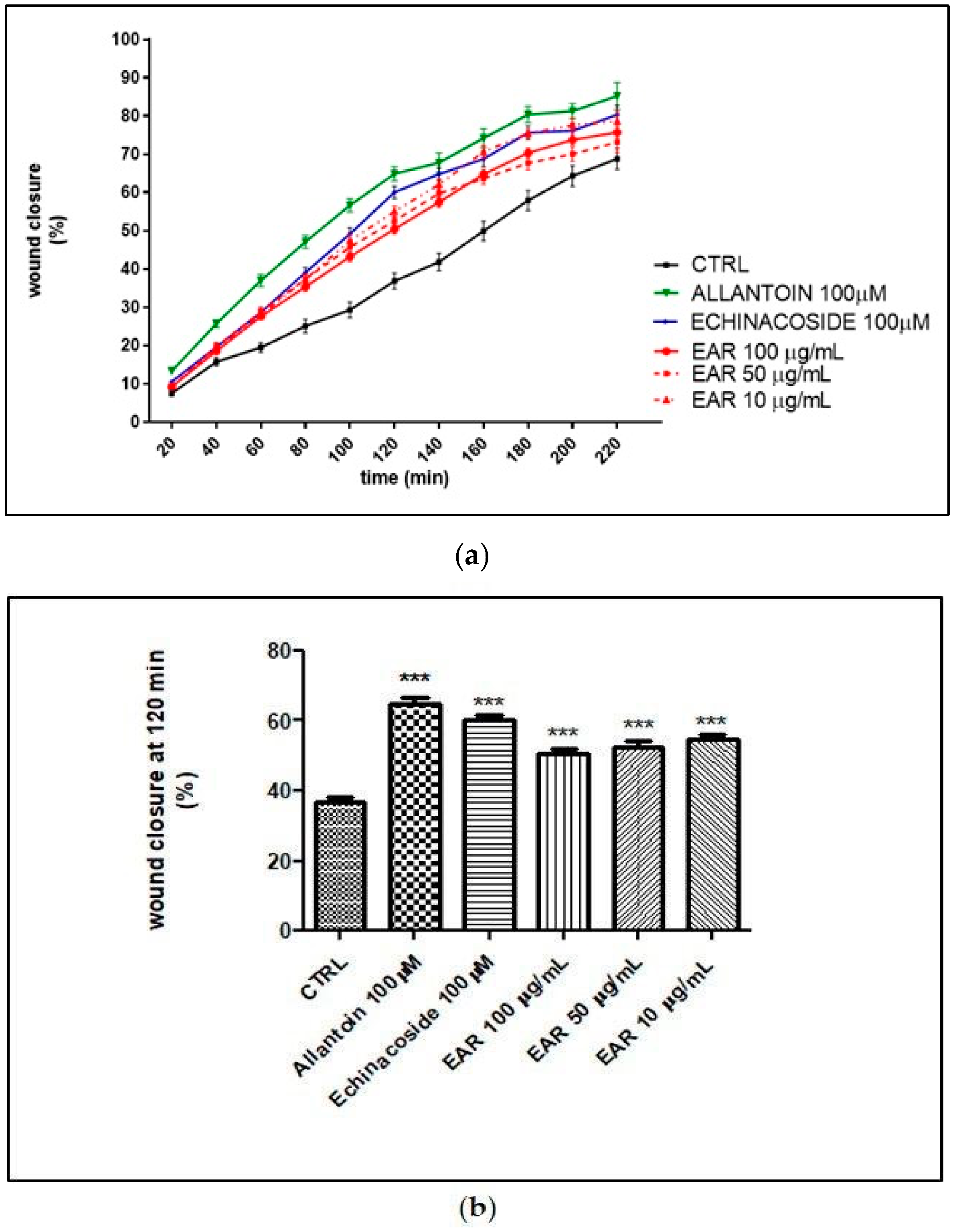
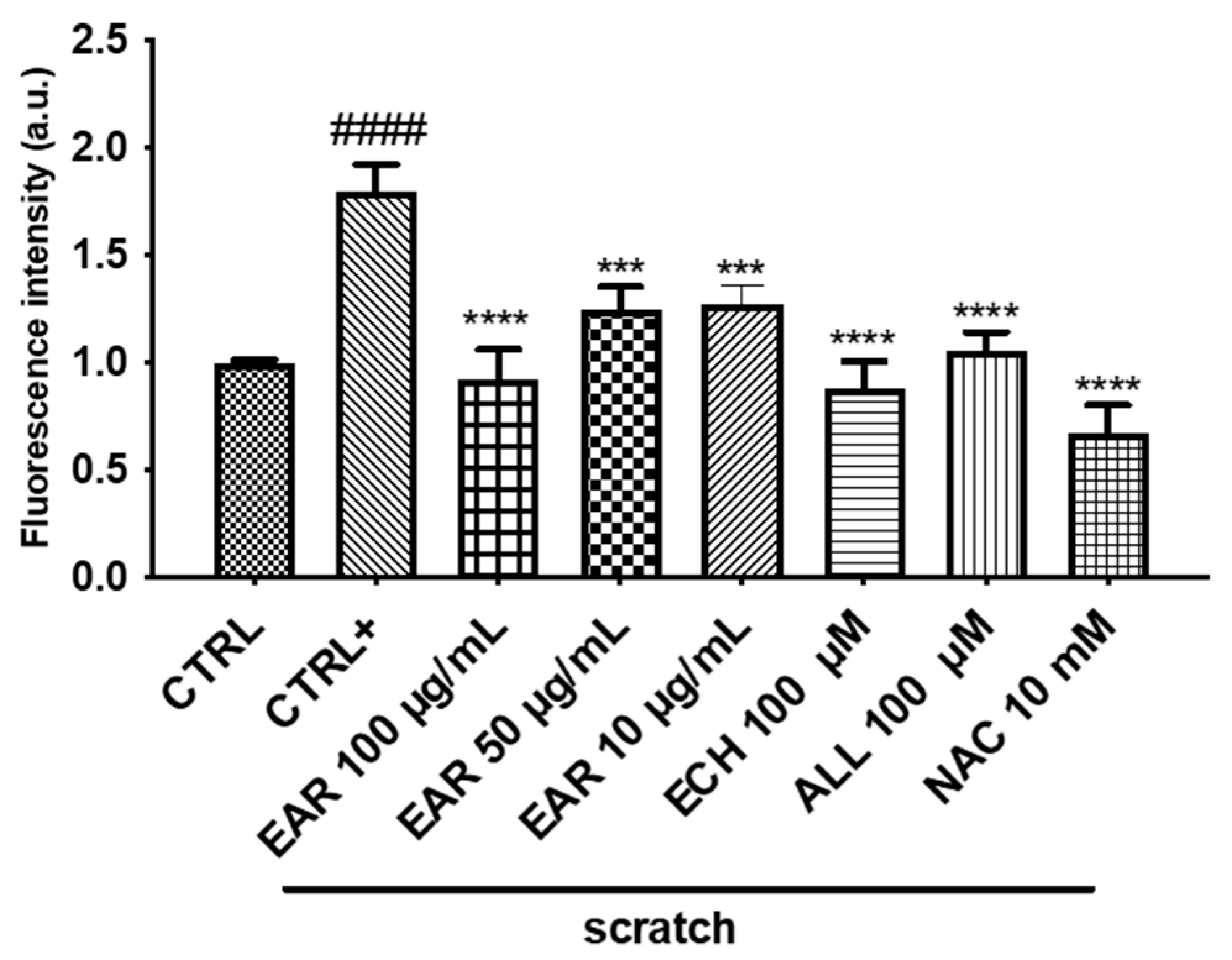
2.4. Antioxidant and Wound Healing Properties
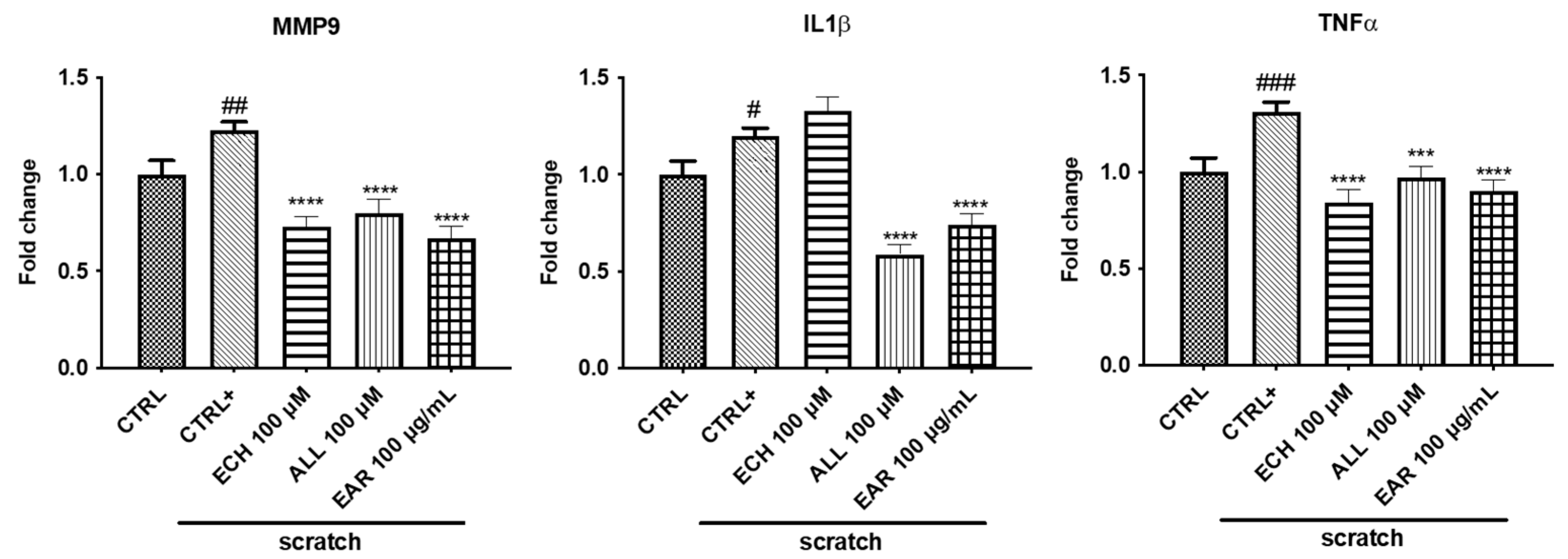
2.5. Anti-Inflammatory Gene and Protein Expression
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Extraction
4.3. Qualitative and Quantitative Analysis by HPLC-DAD
4.4. Qualitative Analysis by UHPLC-DAD-ESI-Orbitrap
4.5. Polysaccharide Content
4.6. Total Polyphenolic Content (TPC) and Antioxidant Activity
4.6.1. DPPH-Scavenging Activity
4.6.2. Lipid Peroxidation Inhibition
4.6.3. In Vitro Tyrosinase Inhibition Assay
4.7. Cell Culture Maintenance
4.8. Cell Proliferation and Viability Assay Using 3T3-L1 Cell Line
4.9. Hydrogen Peroxide-Induced Oxidative Stress Using 3T3-L1 Cell Line
4.10. Determination of Migration Using Wound Healing Assay
4.11. Assessment of Scratch Stress-Induced Intracellular ROS in 3T3-L1 Cells
4.12. Quantitative RT-PCR
4.13. Western Blotting
4.14. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Syarina, P.N.; Karthivashan, G.; Abas, F.; Arulselvan, P.; Fakurazi, S. Wound healing potential of Spirulina platensis extracts on human dermal fibroblast cells. EXCLI J. 2015, 14, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Rahayu, P.; Agustina, L.; Tjandrawinata, R.R. Tacorin, an extract from Ananas comosus stem, stimulates wound healing by modulating the expression of tumor necrosis factor (TNF)-α, transforming growth factor (TGF)-β and matrix metalloproteinases 2 (MMPs2). FEBS Open Bio 2017, 7, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Freiesleben, S.H.; Soelberg, J.; Nyberg, N.T.; Jager, A.K. Determination of the Wound Healing Potentials of Medicinal Plants Historically Used in Ghana. Evid. Based Complement. Altern. Med. 2017, 2017, 9480791. [Google Scholar] [CrossRef]
- Cussy-Poma, V.; Fernández, E.; Rondevaldova, J.; Foffová, H.; Russo, D. Ethnobotanical inventory of medicinal plants used in the Qampaya District, Bolivia. Bol. Latinoam. Caribe Plants Med. Aromat. 2017, 16, 68–77. [Google Scholar]
- Polat, R.; Cakılcıoglu, U. Ethnobotanical study on medicinal plants in Bing ol. Nature 2017, 1, 39–66. [Google Scholar] [CrossRef]
- Nicolaus, C.; Junghanns, S.; Hartmann, A.; Murillo, R.; Ganzera, M.; Merfort, I. In vitro studies to evaluate the wound healing properties of Calendula officinalis extracts. J. Ethnopharmacol. 2017, 196, 94–103. [Google Scholar] [CrossRef]
- Parkar, H.; Aiyegoro, O.A.; Steenkamp, P.; Steenkamp, V. Extracts of Terminalia sericea Enhance Cell Migratory Activity of Endothelial Hybrid and Fibroblast Cells In Vitro. Planta Med. 2017, 83, 1397–1404. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Madani, S.A.; Abediankenari, S. The Review on Properties of Aloe vera in Healing of Cutaneous Wounds. Biomed. Res. Int. 2015, 2015, 714216. [Google Scholar] [CrossRef]
- Jivad, N.; Bahmani, M.; Asadi-Samani, M. A review of the most important medicinal plants effective on wound healing on ethnobotany evidence of Iran. Pharm. Lett. 2016, 8, 353–357. [Google Scholar]
- Farzaei, M.H.; Abbasabadi, Z.; Shams-Ardekani, M.R.; Abdollahi, M.; Rahimi, R. A comprehensive review of plants and their active constituents with wound healing activity in traditional Iranian medicine. Wounds 2014, 26, 197–206. [Google Scholar]
- Parsons, J.L.; Liu, R.; Smith, M.L.; Harris, C.S. Echinacea fruit: Phytochemical localization and germination in four species of Echinacea. Botany 2018, 96, 461–470. [Google Scholar] [CrossRef]
- Blumenthal, M.; Ferrier, G.; Cavaliere, C. Total sales of herbal supplements in United States show steady growth. HerbalGram 2006, 71, 64–66. [Google Scholar]
- Aarland, R.C.; Banuelos-Hernandez, A.E.; Fragoso-Serrano, M.; Sierra-Palacios, E.D.; Diaz de Leon-Sanchez, F.; Perez-Flores, L.J.; Rivera-Cabrera, F.; Mendoza-Espinoza, J.A. Studies on phytochemical, antioxidant, anti-inflammatory, hypoglycaemic and antiproliferative activities of Echinacea purpurea and Echinacea angustifolia extracts. Pharm. Biol. 2017, 55, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Maggini, R.; Raffaelli, A.; Annaheim, K.E.; Tozzini, L.; Pacifici, S.; Guidi, L.; Pardossi, A. Effect of post-harvest handling and extraction on the content of echinacoside and cynarin in the root tissues of Echinacea angustifolia DC. J. Food Agric. Environ. 2010, 8, 266–271. [Google Scholar]
- Bauer, R.; Khan, I.A.; Wagner, H. TLC and HPLC analysis of Echinacea pallida and E. angustifolia roots. Planta Med. 1988, 54, 426–430. [Google Scholar] [CrossRef]
- Pellati, F.; Benvenuti, S.; Melegari, M.; Lasseigne, T. Variability in the composition of antioxidant compounds in Echinacea species by HPLC. Phytochem. Anal. 2005, 16, 77–85. [Google Scholar] [CrossRef]
- Bergeron, C.; Livesey, J.F.; Awang, D.V.; Arnason, J.T.; Rana, J.; Baum, B.R.; Letchamo, W. A quantitative HPLC method for the quality assurance of Echinacea products on the North American market. Phytochem. Anal. 2000, 11, 207–215. [Google Scholar] [CrossRef]
- Barnes, J.; Anderson, L.A.; Gibbons, S.; Phillipson, J.D. Echinacea species (Echinacea angustifolia (DC.) Hell., Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench): A review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 2005, 57, 929–954. [Google Scholar] [CrossRef]
- Kumar, K.; Ramaiah, S. Pharmacological importance of Echinacea purpurea. Int. J. Pharm. Sci. 2011, 2, 304–314. [Google Scholar]
- Borchers, A.T.; Keen, C.L.; Stern, J.S.; Gershwin, M.E. Inflammation and Native American medicine: The role of botanicals. Am. J. Clin. Nutr. 2000, 72, 339–347. [Google Scholar] [CrossRef]
- Zhai, Z.; Haney, D.M.; Wu, L.; Solco, A.K.; Murphy, P.A.; Wurtele, E.S.; Kohut, M.L.; Cunnick, J.E. Alcohol extract of Echinacea pallida reverses stress-delayed wound healing in mice. Phytomedicine 2009, 16, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Speroni, E.; Govoni, P.; Guizzardi, S.; Renzulli, C.; Guerra, M.C. Anti-inflammatory and cicatrizing activity of Echinacea pallida Nutt. root extract. J. Ethnopharmacol. 2002, 79, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Khoza, B.S.; Gbashi, S.; Steenkamp, P.A.; Njobeh, P.B.; Madala, N.E. Identification of hydroxylcinnamoyl tartaric acid esters in Bidens pilosa by UPLC-tandem mass spectrometry. S. Afr. J. Bot. 2016, 103, 95–100. [Google Scholar] [CrossRef]
- Kaur, B.; Maity, H.S.; Rakshit, M.; Srivastav, P.P.; Nag, A. Cryo-Ground Mango Kernel Powder: Characterization, LC-MS/MS Profiling, Purification of Antioxidant-Rich Gallic Acid, and Molecular Docking Study of Its Major Polyphenols as Potential Inhibitors against SARS-CoV-2 Mpro. ACS Food Sci. Technol. 2021, 1, 1776–1786. [Google Scholar] [CrossRef]
- Faraone, I.; Russo, D.; Chiummiento, L.; Fernandez, E.; Choudhary, A.; Monné, M.; Milella, L.; Rai, D.K. Phytochemicals of Minthostachys diffusa Epling and their health-promoting bioactivities. Foods 2020, 9, 144. [Google Scholar] [CrossRef]
- Song, Q.; Li, J.; Huo, H.; Cao, Y.; Wang, Y.; Song, Y.; Tu, P. Retention time and optimal collision energy advance structural annotation relied on LC–MS/MS: An application in metabolite identification of an antidementia agent namely echinacoside. Anal. Chem. 2019, 91, 15040–15048. [Google Scholar] [CrossRef]
- Guy, P.A.; Renouf, M.; Barron, D.; Cavin, C.; Dionisi, F.; Kochhar, S.; Rezzi, S.; Williamson, G.; Steiling, H. Quantitative analysis of plasma caffeic and ferulic acid equivalents by liquid chromatography tandem mass spectrometry. J. Chromatogr. B 2009, 877, 3965–3974. [Google Scholar] [CrossRef]
- Savarese, M.; De Marco, E.; Sacchi, R. Characterization of phenolic extracts from olives (Olea europaea cv. Pisciottana) by electrospray ionization mass spectrometry. Food Chem. 2007, 105, 761–770. [Google Scholar] [CrossRef]
- Firdous, S.M.; Sautya, D. Medicinal plants with wound healing potential. Bangladesh J. Pharmacol. 2018, 13, 41–52. [Google Scholar] [CrossRef]
- Pitz Hda, S.; Pereira, A.; Blasius, M.B.; Voytena, A.P.; Affonso, R.C.; Fanan, S.; Trevisan, A.C.; Ribeiro-do-Valle, R.M.; Maraschin, M. In Vitro Evaluation of the Antioxidant Activity and Wound Healing Properties of Jaboticaba (Plinia peruviana) Fruit Peel Hydroalcoholic Extract. Oxid. Med. Cell Longev. 2016, 2016, 3403586. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Faraone, I.; Labanca, F.; Sinisgalli, C.; Bartolo, M.; Andrade, P.B.; Valentao, P.; Milella, L. Comparison of different green-extraction techniques and determination of the phytochemical profile and antioxidant activity of Echinacea angustifolia L. extracts. Phytochem. Anal. 2019, 30, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, Z.; Barzegar, M.; Sahari, M.; Naghdi Badi, H. Antioxidant and antimicrobial potential of Echinacea purpurea extract and its effect on extension of cake shelf life. J. Med. Plants 2012, 3, 28–40. [Google Scholar]
- Bonomo, M.G.; Russo, D.; Cristiano, C.; Calabrone, L.; Di Tomaso, K.; Milella, L.; Salzano, G. Antimicrobial activity, antioxidant properties and phytochemical screening of Echinacea angustifolia, Fraxinus excelsior and Crataegus oxyacantha mother tinctures against food-borne bacteria. EC Microbiol. 2017, 7, 173–181. [Google Scholar]
- Chiou, S.-Y.; Sung, J.-M.; Huang, P.-W.; Lin, S.-D. Antioxidant, antidiabetic, and antihypertensive properties of Echinacea purpurea flower extract and caffeic acid derivatives using in vitro models. J. Med. Food 2017, 20, 171–179. [Google Scholar] [CrossRef]
- Liu, J.; Yang, L.; Dong, Y.; Zhang, B.; Ma, X. Echinacoside, an Inestimable Natural Product in Treatment of Neurological and other Disorders. Molecules 2018, 23, 1213. [Google Scholar] [CrossRef]
- Xiang, Z.; Ning, Z. Scavenging and antioxidant properties of compound derived from chlorogenic acid in South-China honeysuckle. LWT-Food Sci. Technol. 2008, 41, 1189–1203. [Google Scholar] [CrossRef]
- Villaño, D.; Fernández-Pachón, M.; Moyá, M.L.; Troncoso, A.; García-Parrilla, M. Radical scavenging ability of polyphenolic compounds towards DPPH free radical. Talanta 2007, 71, 230–235. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Vaid, B.; Chopra, B.S.; Raut, S.; Sagar, A.; Badmalia, M.D.; Ashish; Khatri, N. Antioxidant and Wound Healing Property of Gelsolin in 3T3-L1 Cells. Oxid. Med. Cell Longev. 2020, 2020, 4045365. [Google Scholar] [CrossRef]
- Lee, T.T.; Chen, C.L.; Shieh, Z.H.; Lin, J.C.; Yu, B. Study on antioxidant activity of Echinacea purpurea L. extracts and its impact on cell viability. Afr. J. Biotechnol. 2009, 8, 5097–5105. [Google Scholar]
- Chicca, A.; Adinolfi, B.; Martinotti, E.; Fogli, S.; Breschi, M.C.; Pellati, F.; Benvenuti, S.; Nieri, P. Cytotoxic effects of Echinacea root hexanic extracts on human cancer cell lines. J. Ethnopharmacol. 2007, 110, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.; Najafzadeh, H.; Poormahdi-Broojeni, M.; Mohammadian, B.; Heidari, M. Effects of Echinacea purpurea on wound healing after arsenic Induced skin necrosis. Zahedan J. Res. Med. Sci. 2013, 15, 19–23. [Google Scholar]
- Ritsu, M.; Kawakami, K.; Kanno, E.; Tanno, H.; Ishii, K.; Imai, Y.; Maruyama, R.; Tachi, M. Critical role of tumor necrosis factor-α in the early process of wound healing in skin. J. Dermatol. Surg. 2017, 21, 14–19. [Google Scholar] [CrossRef]
- Tardáguila-García, A.; García-Morales, E.; García-Alamino, J.M.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. Metalloproteinases in chronic and acute wounds: A systematic review and meta-analysis. Wound Repair. Regen. 2019, 27, 415–420. [Google Scholar] [CrossRef]
- Caley, M.P.; Martins, V.L.; O’Toole, E.A. Metalloproteinases and Wound Healing. Adv. Wound Care 2015, 4, 225–234. [Google Scholar] [CrossRef]
- Yen, Y.H.; Pu, C.M.; Liu, C.W.; Chen, Y.C.; Chen, Y.C.; Liang, C.J.; Hsieh, J.H.; Huang, H.F.; Chen, Y.L. Curcumin accelerates cutaneous wound healing via multiple biological actions: The involvement of TNF-alpha, MMP-9, alpha-SMA, and collagen. Int. Wound J. 2018, 15, 605–617. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Ding, D.; Wolter, W.R.; Pérez, R.L.; Champion, M.M.; Mahasenan, K.V.; Hesek, D.; Lee, M.; Schroeder, V.A.; Jones, J.I.; et al. Validation of matrix metalloproteinase-9 (MMP-9) as a novel target for treatment of diabetic foot ulcers in humans and discovery of a potent and selective small-molecule MMP-9 inhibitor that accelerates healing. J. Med. Chem. 2018, 61, 8825–8837. [Google Scholar] [CrossRef]
- Schultz, G.S.; Wysocki, A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009, 17, 153–162. [Google Scholar] [CrossRef]
- Salama, A.; Moustafa, P.E.; Elgohary, R. Exploring Echinacea purpurea’s effect on wound healing in rats: FOXO1/MIP2 pathway modulation. Comp. Clin. Path 2024, 33, 277–286. [Google Scholar] [CrossRef]
- Aoshima, H.; Miyase, T.; Warashina, T. Caffeic acid oligomers with hyaluronidase inhibitory activity from Clinopodium gracile. Chem. Pharm. Bull. 2012, 60, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, B.; Tateya, I.; Lim, X.; Munoz-del-Rio, A.; Bless, D.M. Investigation of anti-hyaluronidase treatment on vocal fold wound healing. J. Voice 2006, 20, 443–451. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Hu, F.; Tai, Y.; Yang, X.; Yu, D.; Li, D.; Yuan, Y. Study on the extraction process and tyrosinase inhibition property of cichoric acid in Echinacea purpurea L. J. Med. Plant Res. 2012, 6, 5317–5321. [Google Scholar]
- Lamorte, D.; Faraone, I.; Laurenzana, I.; Milella, L.; Trino, S.; De Luca, L.; Del Vecchio, L.; Armentano, M.F.; Sinisgalli, C.; Chiummiento, L. Future in the past: Azorella glabra Wedd. as a source of new natural compounds with antiproliferative and cytotoxic activity on multiple myeloma cells. Int. J. Mol. Sci. 2018, 19, 3348. [Google Scholar] [CrossRef]
- Castronuovo, D.; Russo, D.; Libonati, R.; Faraone, I.; Candido, V.; Picuno, P.; Andrade, P.; Valentao, P.; Milella, L. Influence of shading treatment on yield, morphological traits and phenolic profile of sweet basil (Scientia horticulturae L.). Sci. Hortic. 2019, 254, 91–98. [Google Scholar] [CrossRef]
- Fidelis, Q.C.; Faraone, I.; Russo, D.; Aragao Catunda, F.E., Jr.; Vignola, L.; de Carvalho, M.G.; de Tommasi, N.; Milella, L. Chemical and Biological insights of Ouratea hexasperma (A. St.-Hil.) Baill.: A source of bioactive compounds with multifunctional properties. Nat. Prod. Res. 2019, 33, 1500–1503. [Google Scholar] [CrossRef]
- Russo, D.; Miglionico, R.; Carmosino, M.; Bisaccia, F.; Andrade, P.B.; Valentao, P.; Milella, L.; Armentano, M.F. A Comparative Study on Phytochemical Profiles and Biological Activities of Sclerocarya birrea (A.Rich.) Hochst Leaf and Bark Extracts. Int. J. Mol. Sci. 2018, 19, 186. [Google Scholar] [CrossRef]
- Labanca, F.; Faraone, I.; Nolè, M.R.; Hornedo-Ortega, R.; Russo, D.; García-Parrilla, M.C.; Chiummiento, L.; Bonomo, M.G.; Milella, L. New insights into the exploitation of Vitis vinifera L. cv. Aglianico leaf extracts for nutraceutical purposes. Antioxidants 2020, 9, 708. [Google Scholar] [CrossRef]
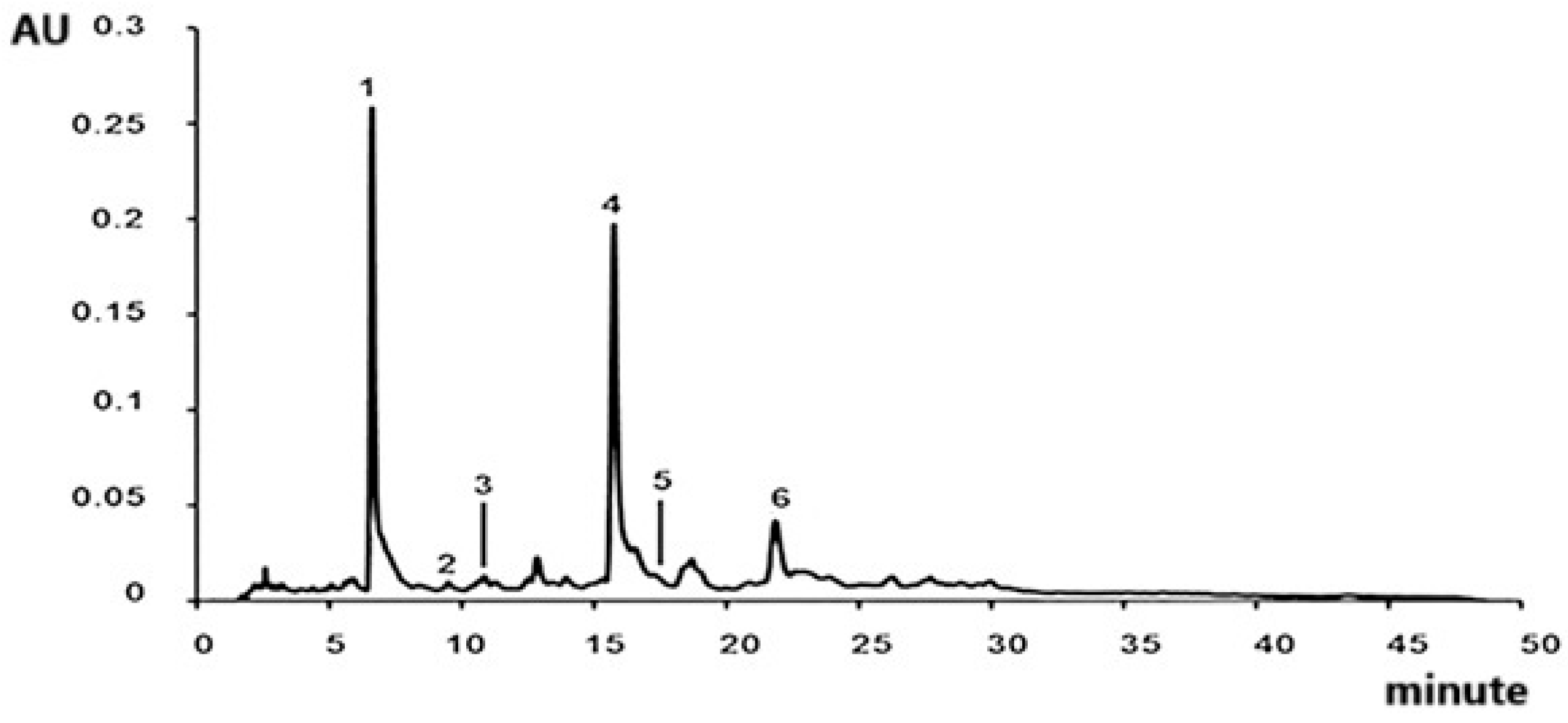
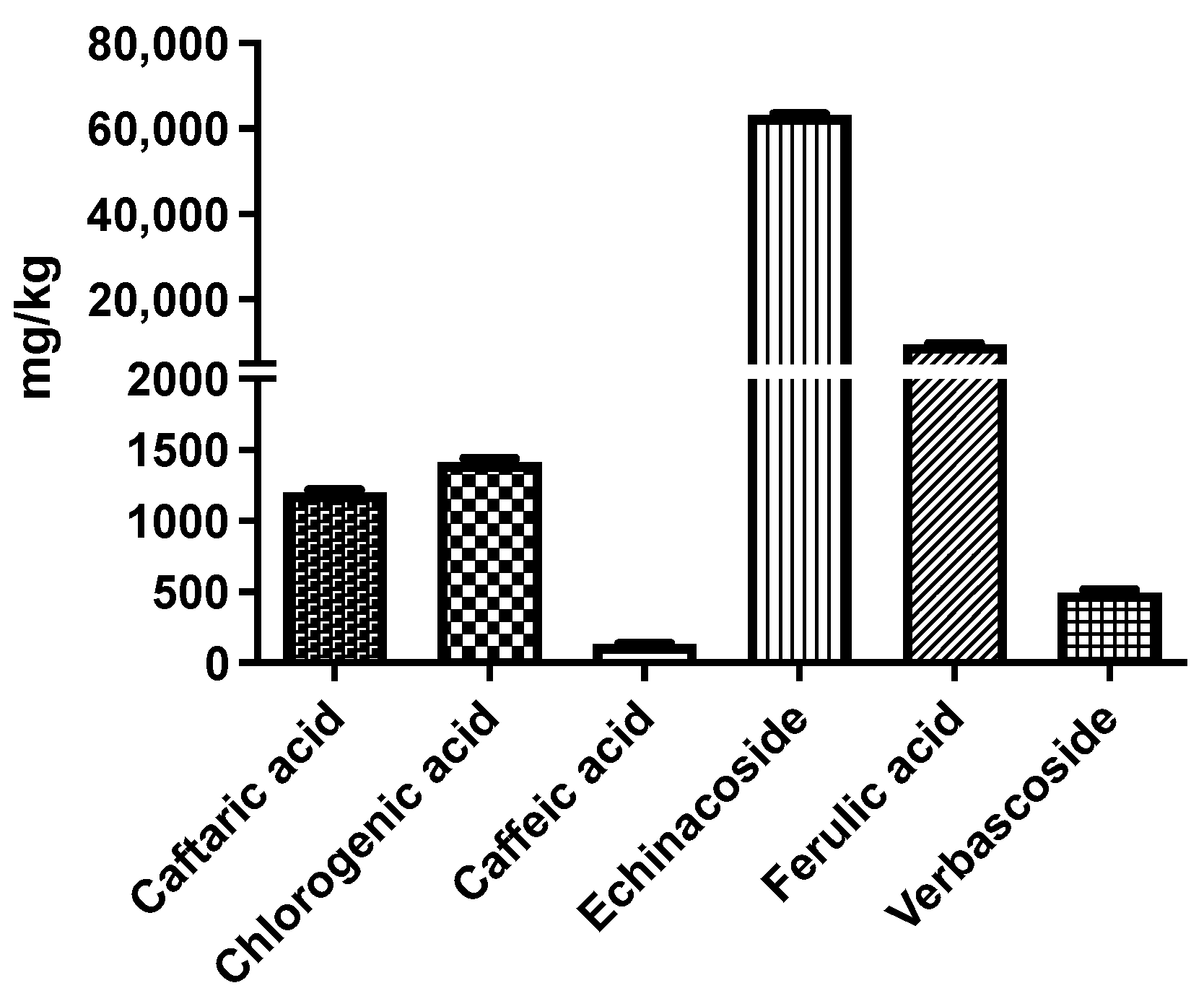

| Pk No. | RT (min) | [M-H]− m/z Calculated | [M-H]− m/z Observed | Predicted Molecular Formula | MS/MS m/z | Compound Identity | References |
|---|---|---|---|---|---|---|---|
| 1 | 9.58 | 311.0409 | 311.0407 | C13H12O9 | 179, 149 (100), 135 | Caftaric acid | [24] |
| 2 | 10.66 | 353.0878 | 353.0874 | C16H18O9 | 191 (100) | Chlorogenic acid | [25] |
| 3 | 11.04 | 179.0350 | 179.0349 | C9H8O4 | 135 (100) | Caffeic acid | [26] |
| 4 | 11.46 | 785.2510 | 785.2498 | C35H46O20 | 623, 161 (100) | Echinacoside | [27] |
| 5 | 12.72 | 193.0506 | 193.0507 | C10H10O4 | 178, 134 (100) | Ferulic acid | [28] |
| 6 | 12.91 | 623.1981 | 623.1980 | C29H36O15 | 461, 161 (100) | Verbascoside | [29] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Russo, D.; Lela, L.; Benedetto, N.; Faraone, I.; Paternoster, G.; Valentão, P.; Milella, L.; Carmosino, M. Phytochemical Composition and Wound Healing Properties of Echinacea angustifolia DC. Root Hydroalcoholic Extract. Int. J. Mol. Sci. 2025, 26, 2562. https://doi.org/10.3390/ijms26062562
Russo D, Lela L, Benedetto N, Faraone I, Paternoster G, Valentão P, Milella L, Carmosino M. Phytochemical Composition and Wound Healing Properties of Echinacea angustifolia DC. Root Hydroalcoholic Extract. International Journal of Molecular Sciences. 2025; 26(6):2562. https://doi.org/10.3390/ijms26062562
Chicago/Turabian StyleRusso, Daniela, Ludovica Lela, Nadia Benedetto, Immacolata Faraone, Gianluca Paternoster, Patricia Valentão, Luigi Milella, and Monica Carmosino. 2025. "Phytochemical Composition and Wound Healing Properties of Echinacea angustifolia DC. Root Hydroalcoholic Extract" International Journal of Molecular Sciences 26, no. 6: 2562. https://doi.org/10.3390/ijms26062562
APA StyleRusso, D., Lela, L., Benedetto, N., Faraone, I., Paternoster, G., Valentão, P., Milella, L., & Carmosino, M. (2025). Phytochemical Composition and Wound Healing Properties of Echinacea angustifolia DC. Root Hydroalcoholic Extract. International Journal of Molecular Sciences, 26(6), 2562. https://doi.org/10.3390/ijms26062562







