Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk
Abstract
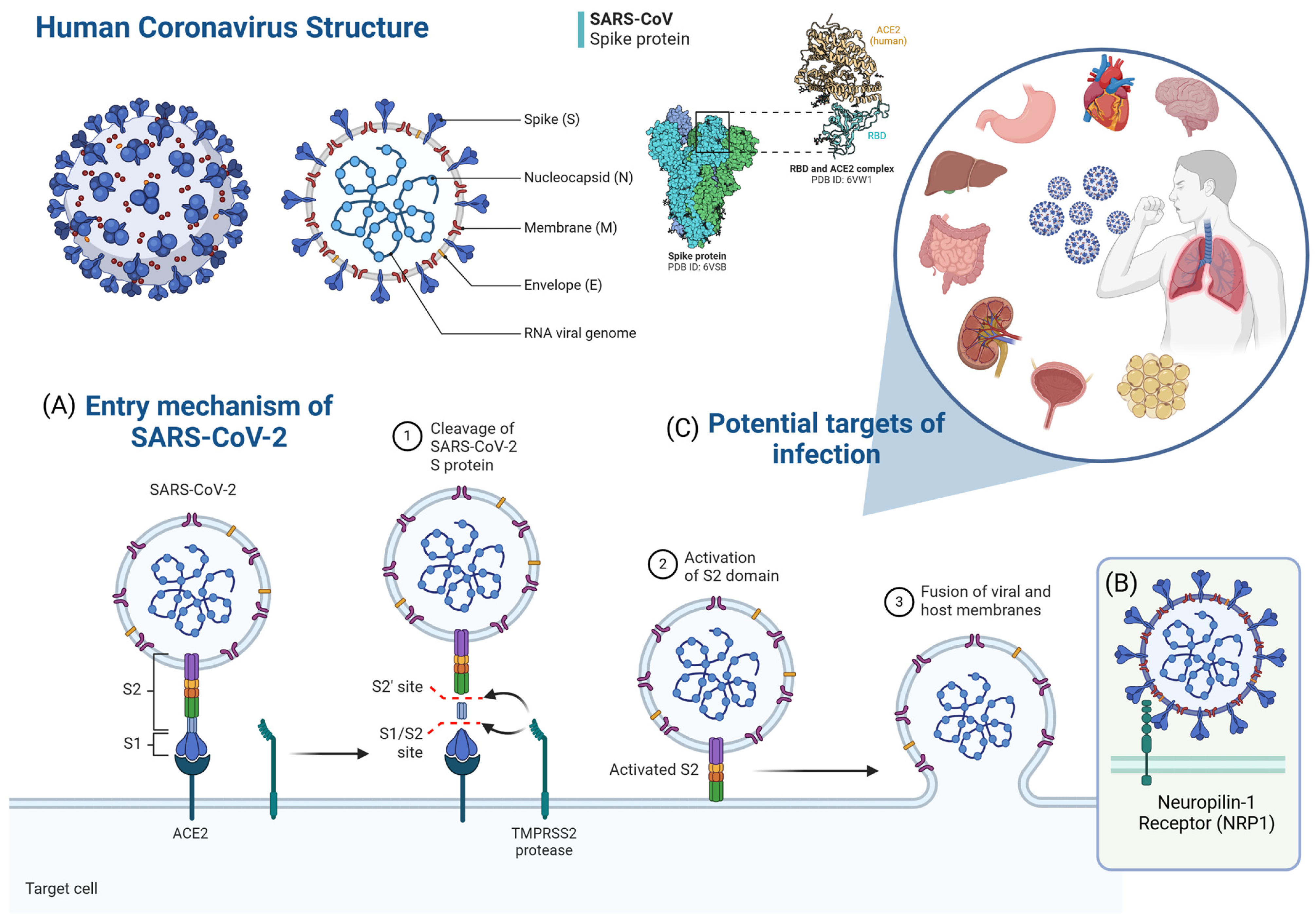
1. Introduction
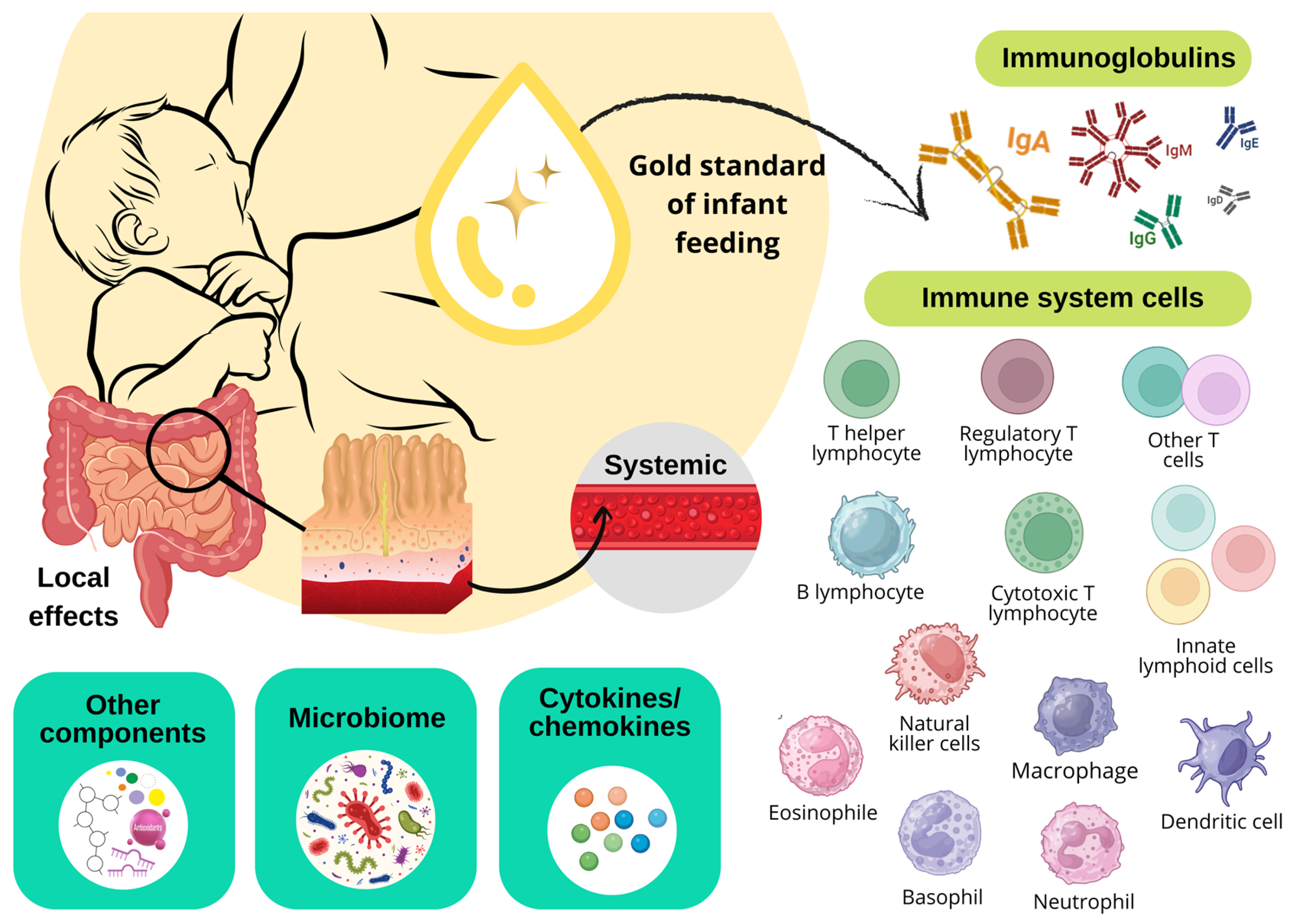
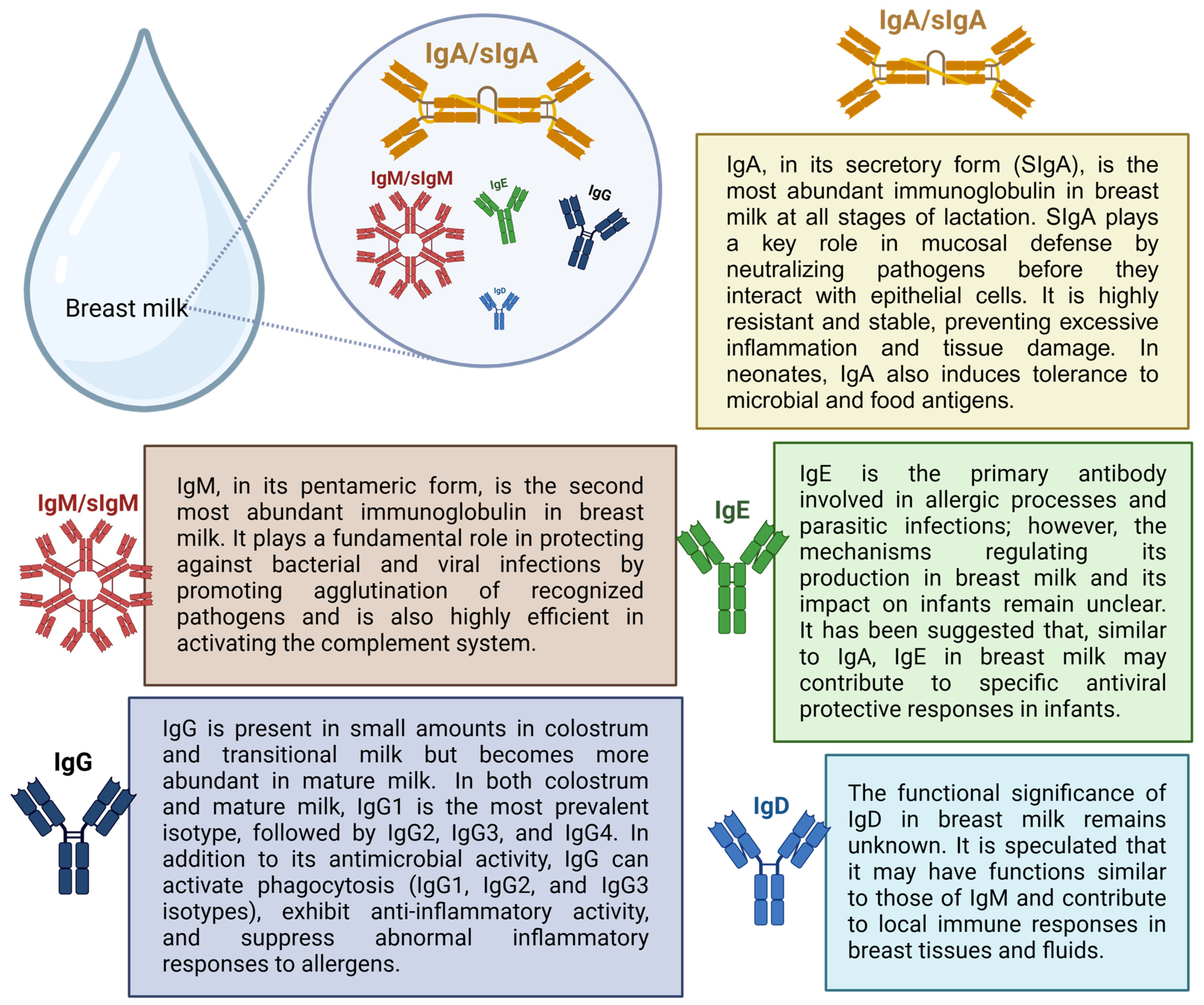
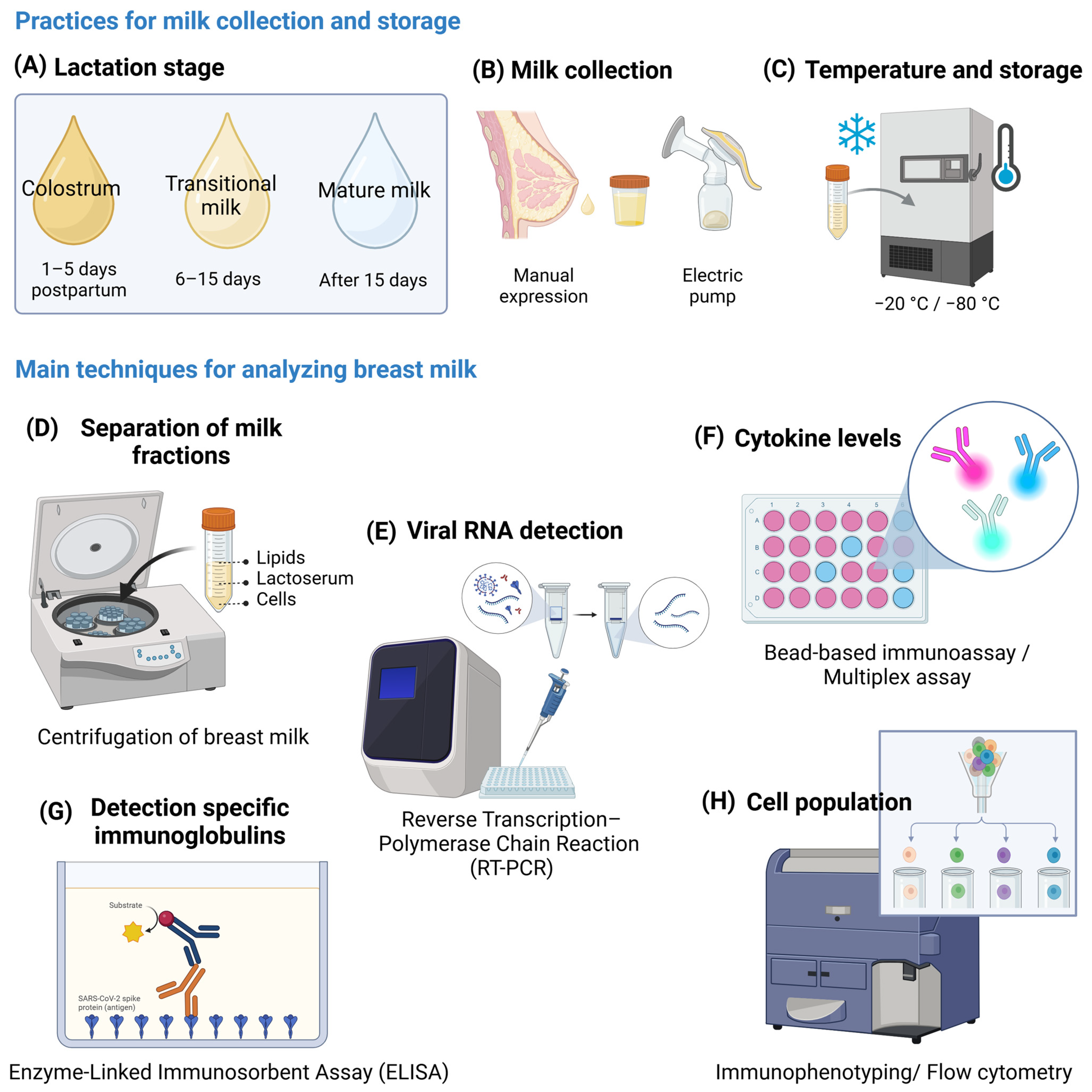
2. Presence of Viral RNA and Anti-SARS-CoV-2 Immunoglobulins in Breast Milk
3. Cytokines, Chemokines, Growth Factors, and Cellular Components in Breast Milk in the Context of COVID-19
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| COVID-19 | coronavirus disease 2019 |
| WHO | World Health Organization |
| ACE2 | Angiotensin-Converting Enzyme 2 |
| RBD | Receptor-Binding Domain |
| NRP1 | Neurolipin-1 |
| ARDS | acute respiratory distress syndrome |
| MIS-C | multisystem inflammatory syndrome in children |
| MIS-N | multisystem inflammatory syndrome in neonates |
| ILCs | innate lymphoid cells |
| NK | natural killer |
| Ig | immunoglobulin (IgA, IgM, IgG, IgE, and IgD) |
| N | nucleocapsid protein |
| S | spike protein |
| E | envelope protein |
| M | membrane protein |
| SIgA | secretory IgA |
| IFN | interferon |
| IL | interleukin (IL-1β, IL-2, IL-4, IL-6, IL-9, IL-10, IL-12, IL-13, IL-1Ra) |
| TGF | transforming growth factor |
| CSF | colony-stimulating factor |
| TNF | tumor necrosis factor |
| VEGF | vascular endothelial growth factor |
| HGF | hepatic growth factor |
| EGF | epidermal growth factor |
| RNA | ribonucleic acid |
| RT-PCR | reverse transcription–polymerase chain reaction |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| GALT | gut-associated lymphoid tissue |
| G-CSF | granulocyte–macrophage colony-stimulating factor |
| IP-10 | Interferon-Induced Protein-10 |
| MCP-1 | Monocyte Chemoattractant Protein-1 |
| MIG | Monokine-Induced Gamma Interferon |
| MIP-1α | Macrophage Inflammatory Protein-1 Alpha |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| PDGF-BB | platelet-derived growth factor BB |
| CDC | Centers for Disease Control and Prevention |
| OD | optical density |
| LOD | limits of detection |
| LOQ | limits of quantification |
| ROC | receiver operating characteristic |
| HIV-1 | human immunodeficiency virus 1 |
References
- World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. Available online: https://www.who.int/publications/i/item/clinical-management-of-covid-19/ (accessed on 10 October 2024).
- Luo, Y.; Yin, K. Management of pregnant women infected with COVID-19. Lancet Infect. Dis. 2020, 20, 513–514. [Google Scholar] [CrossRef] [PubMed]
- Male, V. SARS-CoV-2 infection and COVID-19 vaccination in pregnancy. Nat. Rev. Immunol. 2022, 22, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Lamers, M.M.; Haagmans, B.L. SARS-CoV-2 pathogenesis. Nat. Rev. Microbiol. 2022, 20, 270–284. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A.; Dulebohn, S.C.; Di Napoli, R. Features, Evaluation, and Treatment of Coronavirus (COVID-19). In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Pérez-Bermejo, M.; Peris-Ochando, B.; Murillo-Llorente, M.T. COVID-19: Relationship and Impact on Breastfeeding—A Systematic Review. Nutrients 2021, 13, 2972. [Google Scholar] [CrossRef]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef]
- Han, Y.; Yang, L.; Lacko, L.A.; Chen, S. Human organoid models to study SARS-CoV-2 infection. Nat. Methods 2022, 19, 418–428. [Google Scholar] [CrossRef]
- Molloy, E.J.; Nakra, N.; Gale, C.; Dimitriades, V.R.; Lakshminrusimha, S. Multisystem inflammatory syndrome in children (MIS-C) and neonates (MIS-N) associated with COVID-19: Optimizing definition and management. Pediatr. Res. 2023, 93, 1499–1508. [Google Scholar] [CrossRef]
- Azkur, A.K.; Akdis, M.; Azkur, D.; Sokolowska, M.; van de Veen, W.; Brüggen, M.C.; O’Mahony, L.; Gao, Y.; Nadeau, K.; Akdis, C.A. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy 2020, 75, 1564–1581. [Google Scholar] [CrossRef]
- Diamond, M.S.; Kanneganti, T.D. Innate immunity: The first line of defense against SARS-CoV-2. Nat. Immunol. 2022, 23, 165–176. [Google Scholar] [CrossRef]
- Shen, J.; Fan, J.; Zhao, Y.; Jiang, D.; Niu, Z.; Zhang, Z.; Cao, G. Innate and adaptive immunity to SARS-CoV-2 and predisposing factors. Front. Immunol. 2023, 14, 1159326. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626, Erratum in Nat. Immunol. 2021, 22, 928. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Zhu, H.; Yang, L.; Cao, L.; Huang, X.; Dynes, M.; Narayan, A.; Xia, J.; Chen, Y.; Zhang, P.; et al. A study of breastfeeding practices, SARS-CoV-2 and its antibodies in the breast milk of mothers confirmed with COVID-19. Lancet Reg. Health West. Pac. 2020, 4, 100045. [Google Scholar] [CrossRef] [PubMed]
- Ganho-Ávila, A.; Guiomar, R.; Sobral, M.; Pacheco, F.; Caparros-Gonzalez, R.A.; Diaz-Louzao, C.; Motrico, E.; Domínguez-Salas, S.; Mesquita, A.; Costa, R.; et al. The impact of COVID-19 on breastfeeding rates: An international cross-sectional study. Midwifery 2023, 120, 103631. [Google Scholar] [CrossRef] [PubMed]
- Quigley, M.A.; Harrison, S.; Levene, I.; McLeish, J.; Buchanan, P.; Alderdice, F. Breastfeeding rates in England during the COVID-19 pandemic and the previous decade: Analysis of national surveys and routine data. PLoS ONE 2023, 18, e0291907. [Google Scholar] [CrossRef]
- Giuliani, F.; Oros, D.; Gunier, R.B.; Deantoni, S.; Rauch, S.; Casale, R.; Nieto, R.; Bertino, E.; Rego, A.; Menis, C.; et al. Effects of prenatal exposure to maternal COVID-19 and perinatal care on neonatal outcome: Results from the INTERCOVID Multinational Cohort Study. Am. J. Obstet. Gynecol. 2022, 227, 488.e1–488.e17. [Google Scholar] [CrossRef]
- Allotey, J.; Chatterjee, S.; Kew, T.; Gaetano, A.; Stallings, E.; Fernández-García, S.; Yap, M.; Sheikh, J.; Lawson, H.; Coomar, D.; et al. SARS-CoV-2 positivity in offspring and timing of mother-to-child transmission: Living systematic review and meta-analysis. BMJ 2022, 376, e067696. [Google Scholar] [CrossRef]
- Quitadamo, P.A.; Comegna, L.; Cristalli, P. Anti-Infective, anti-inflammatory, and immunomodulatory properties of breast milk factors for the protection of infants in the pandemic from COVID-19. Front. Public Health 2021, 8, 589736. [Google Scholar] [CrossRef]
- Verhasselt, V.; Tellier, J.; Carsetti, R.; Tepekule, B. Antibodies in breast milk: Pro-bodies designed for healthy newborn development. Immunol. Rev. 2024, 328, 192–204. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Agostoni, C.; Feketea, G.; Alberti, I.; Gianni, M.L.; Milani, G.P. The Role of Breastfeeding in Acute Respiratory Infections in Infancy. Pediatr. Infect. Dis. J. 2024, 43, 1090–1099. [Google Scholar] [CrossRef]
- Riskin, A.; Almog, M.; Peri, R.; Halasz, K.; Srugo, I.; Kessel, A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatr. Res. 2012, 71, 220–225. [Google Scholar] [CrossRef]
- Hassiotou, F.; Hepworth, A.R.; Metzger, P.; Tat Lai, C.; Trengove, N.; Hartmann, P.E.; Filgueira, L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl. Immunol. 2013, 2, e3. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Corrêa-Silva, S.; de Souza, E.C.; Maria Rodrigues, R.; da Fonseca, F.A.M.; Gilio, A.E.; Carneiro-Sampaio, M.; Palmeira, P. Macrophage profile and homing into breast milk in response to ongoing respiratory infections in the nursing infant. Cytokine 2020, 129, 155045. [Google Scholar] [CrossRef] [PubMed]
- Bosire, R.; Guthrie, B.L.; Lohman-Payne, B.; Mabuka, J.; Majiwa, M.; Wariua, G.; Mbori-Ngacha, D.; Richardson, B.; John-Stewart, G.; Farquhar, C. Longitudinal comparison of chemokines in breastmilk early postpartum among HIV-1-infected and uninfected Kenyan women. Breastfeed Med. 2007, 2, 129–138. [Google Scholar] [CrossRef]
- Farquhar, C.; Mbori-Ngacha, D.A.; Redman, M.W.; Bosire, R.K.; Lohman, B.L.; Piantadosi, A.L.; Goodman, R.B.; Ruzinski, J.T.; Emery, S.R.; Crudder, C.H.; et al. CC and CXC chemokines in breastmilk are associated with mother-to-child HIV-1 transmission. Curr. HIV Res. 2005, 3, 361–369. [Google Scholar] [CrossRef]
- Wu, X.; Jackson, R.T.; Khan, S.A.; Ahuja, J.; Pehrsson, P.R. Human Milk Nutrient Composition in the United States: Current Knowledge, Challenges, and Research Needs. Curr. Dev. Nutr. 2018, 2, nzy025. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Hatmal, M.M.; Al-Hatamleh, M.A.I.; Olaimat, A.N.; Alshaer, W.; Hasan, H.; Albakri, K.A.; Alkhafaji, E.; Issa, N.N.; Al-Holy, M.A.; Abderrahman, S.M.; et al. Immunomodulatory Properties of Human Breast Milk: MicroRNA Contents and Potential Epigenetic Effects. Biomedicines 2022, 10, 1219. [Google Scholar] [CrossRef]
- Czosnykowska-Łukacka, M.; Lis-Kuberka, J.; Królak-Olejnik, B.; Orczyk-Pawiłowicz, M. Changes in Human Milk Immunoglobulin Profile During Prolonged Lactation. Front. Pediatr. 2020, 8, 428. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Underwood, M.A.; Beverly, R.L.; Nielsen, S.D.; Dallas, D.C. Comparison of Human Milk Immunoglobulin Survival during Gastric Digestion between Preterm and Term Infants. Nutrients 2018, 10, 631. [Google Scholar] [CrossRef]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef]
- Kiełbasa, A.; Gadzała-Kopciuch, R.; Buszewski, B. Cytokines-Biogenesis and Their Role in Human Breast Milk and Determination. Int. J. Mol. Sci. 2021, 22, 6238. [Google Scholar] [CrossRef] [PubMed]
- Kobata, R.; Tsukahara, H.; Ohshima, Y.; Ohta, N.; Tokuriki, S.; Tamura, S.; Mayumi, M. High levels of growth factors in human breast milk. Early Hum. Dev. 2008, 84, 67–69. [Google Scholar] [CrossRef] [PubMed]
- Witkowska-Zimny, M.; Kaminska-El-Hassan, E. Cells of human breast milk. Cell. Mol. Biol. Lett. 2017, 22, 11. [Google Scholar] [CrossRef]
- Lokossou, G.A.G.; Kouakanou, L.; Schumacher, A.; Zenclussen, A.C. Human Breast Milk: From Food to Active Immune Response With Disease Protection in Infants and Mothers. Front. Immunol. 2022, 13, 849012. [Google Scholar] [CrossRef]
- Zimmermann, P.; Curtis, N. Breast milk microbiota: A review of the factors that influence composition. J. Infect. 2020, 81, 17–47. [Google Scholar] [CrossRef]
- Boix-Amorós, A.; Puente-Sánchez, F.; du Toit, E.; Linderborg, K.M.; Zhang, Y.; Yang, B.; Salminen, S.; Isolauri, E.; Tamames, J.; Mira, A.; et al. Mycobiome Profiles in Breast Milk from Healthy Women Depend on Mode of Delivery, Geographic Location, and Interaction with Bacteria. Appl. Environ. Microbiol. 2019, 85, e02994-18. [Google Scholar] [CrossRef]
- Camacho-Morales, A.; Caba, M.; García-Juárez, M.; Caba-Flores, M.D.; Viveros-Contreras, R.; Martínez-Valenzuela, C. Breastfeeding Contributes to Physiological Immune Programming in the Newborn. Front. Pediatr. 2021, 9, 744104. [Google Scholar] [CrossRef]
- Cabinian, A.; Sinsimer, D.; Tang, M.; Zumba, O.; Mehta, H.; Toma, A.; Sant’Angelo, D.; Laouar, Y.; Laouar, A. Transfer of Maternal Immune Cells by Breastfeeding: Maternal Cytotoxic T Lymphocytes Present in Breast Milk Localize in the Peyer’s Patches of the Nursed Infant. PLoS ONE 2016, 11, e0156762. [Google Scholar] [CrossRef]
- Ellis, J. Passive transfer of colostral leukocytes: A benefit/risk analysis. Can. Vet. J. 2021, 62, 233–239. [Google Scholar]
- Molès, J.P.; Tuaillon, E.; Kankasa, C.; Bedin, A.S.; Nagot, N.; Marchant, A.; McDermid, J.M.; Van de Perre, P. Breastmilk cell trafficking induces microchimerism-mediated immune system maturation in the infant. Pediatr. Allergy Immunol. 2018, 29, 133–143. [Google Scholar] [CrossRef]
- Reber, A.J.; Donovan, D.C.; Gabbard, J.; Galland, K.; Aceves-Avila, M.; Holbert, K.A.; Marshall, L.; Hurley, D.J. Transfer of maternal colostral leukocytes promotes development of the neonatal immune system Part II. Effects on neonatal lymphocytes. Vet. Immunol. Immunopathol. 2008, 123, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Tuboly, S.; Bernáth, S. Intestinal absorption of colostral lymphoid cells in newborn animals. Adv. Exp. Med. Biol. 2002, 503, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Yu, Y.; Xian, W.; Ye, F.; Ju, X.; Luo, Y.; Dong, H.; Zhou, Y.H.; Tan, W.; Zhuang, H.; et al. Identified human breast milk compositions effectively inhibit SARS-CoV-2 and variants infection and replication. Iscience 2022, 25, 104136. [Google Scholar] [CrossRef] [PubMed]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.A.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Link-Gelles, R.; Ciesla, A.A.; Rowley, E.A.K.; Klein, N.P.; Naleway, A.L.; Payne, A.B.; Kharbanda, A.; Natarajan, K.; DeSilva, M.B.; Dascomb, K.; et al. Effectiveness of Monovalent and Bivalent mRNA Vaccines in Preventing COVID-19-Associated Emergency Department and Urgent Care Encounters Among Children Aged 6 Months-5 Years—VISION Network, United States, July 2022–June 2023. MMWR Morb. Mortal. Wkly. Rep. 2023, 72, 886–892. [Google Scholar] [CrossRef]
- Demers-Mathieu, V. Editorial: Breast milk and passive immunity during the COVID-19 pandemic. Front. Nutr. 2023, 10, 1155901. [Google Scholar] [CrossRef]
- Razonable, R.R. Corrigendum: Protecting the vulnerable: Addressing the COVID-19 care needs of people with compromised immunity. Front. Immunol. 2024, 15, 1440571. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Weekly COVID-19 Vaccination Dashboard. 2025. Available online: https://www.cdc.gov/covidvaxview/weekly-dashboard/?CDC_AAref_Val=https://www.cdc.gov/vaccines/imz-managers/coverage/covidvaxview/interactive/vaccination-dashboard.html (accessed on 2 March 2025).
- Katherine, E.K.; Emma, G.; Hilda, R.; Tara, C.J.; Tami, H.S.; Sascha, R.E.; Carla, L.B. Flu, Tdap, and COVID-19 Vaccination Coverage Among Pregnant Women—United States, April 2024. Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/fluvaxview/coverage-by-season/pregnant-april-2024.html#cdc_report_pub_study_section_7-authors (accessed on 2 March 2025).
- Comparcini, D.; Tomietto, M.; Pastore, F.; Nichol, B.; Miniscalco, D.; Flacco, M.E.; Stefanizzi, P.; Tafuri, S.; Cicolini, G.; Simonetti, V. Factors Influencing COVID-19 Vaccine Hesitancy in Pregnant and Breastfeeding/Puerperium Women: A Cross-Sectional Study. Vaccines 2024, 12, 772. [Google Scholar] [CrossRef]
- Fox, A.; Marino, J.; Amanat, F.; Krammer, F.; Hahn-Holbrook, J.; Zolla-Pazner, S.; Powell, R.L. Robust and Specific Secretory IgA Against SARS-CoV-2 Detected in Human Milk. Iscience 2020, 23, 101735. [Google Scholar] [CrossRef]
- Pace, R.M.; Williams, J.E.; Järvinen, K.M.; Belfort, M.B.; Pace, C.D.W.; Lackey, K.A.; Gogel, A.C.; Nguyen-Contant, P.; Kanagaiah, P.; Fitzgerald, T.; et al. Characterization of SARS-CoV-2 RNA, Antibodies, and Neutralizing Capacity in Milk Produced by Women with COVID-19. mBio 2021, 12, e03192-20. [Google Scholar] [CrossRef]
- Fenizia, C.; Biasin, M.; Cetin, I.; Vergani, P.; Mileto, D.; Spinillo, A.; Gismondo, M.R.; Perotti, F.; Callegari, C.; Mancon, A.; et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat. Commun. 2020, 11, 5128. [Google Scholar] [CrossRef] [PubMed]
- Bastug, A.; Hanifehnezhad, A.; Tayman, C.; Ozkul, A.; Ozbay, O.; Kazancioglu, S.; Bodur, H. Virolactia in an Asymptomatic Mother with COVID-19. Breastfeed. Med. 2020, 15, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Groß, R.; Conzelmann, C.; Müller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Münch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef]
- Kilic, T.; Kilic, S.; Berber, N.K.; Gunduz, A.; Ersoy, Y. Investigation of SARS-CoV-2 RNA in milk produced by women with COVID-19 and follow-up of their infants: A preliminary study. Int. J. Clin. Pract. 2021, 75, e14175. [Google Scholar] [CrossRef]
- Dimitroglou, M.; Sokou, R.; Iacovidou, N.; Pouliakis, A.; Kafalidis, G.; Boutsikou, T.; Iliodromiti, Z. Anti-SARS-CoV-2 Immunoglobulins in Human Milk after Coronavirus Disease or Vaccination-Time Frame and Duration of Detection in Human Milk and Factors That Affect Their Titers: A Systematic Review. Nutrients 2023, 15, 1905. [Google Scholar] [CrossRef]
- Bäuerl, C.; Randazzo, W.; Sánchez, G.; Selma-Royo, M.; García Verdevio, E.; Martínez, L.; Parra-Llorca, A.; Lerin, C.; Fumadó, V.; Crovetto, F.; et al. SARS-CoV-2 RNA and antibody detection in breast milk from a prospective multicentre study in Spain. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 216–221. [Google Scholar] [CrossRef]
- Fernández-Buhigas, I.; Rayo, N.; Silos, J.C.; Serrano, B.; Ocón-Hernández, O.; Leung, B.W.; Delgado, J.L.; Fernández, D.S.; Valle, S.; De Miguel, L.; et al. Anti-SARS-CoV-2-specific antibodies in human breast milk following SARS-CoV-2 infection during pregnancy: A prospective cohort study. Int. Breastfeed. J. 2024, 19, 5. [Google Scholar] [CrossRef]
- He, Y.F.; Liu, J.Q.; Hu, X.D.; Li, H.M.; Wu, N.; Wang, J.; Jiang, Z.G. Breastfeeding vs. breast milk transmission during COVID-19 pandemic, which is more important? Front. Pediatr. 2023, 11, 1253333. [Google Scholar] [CrossRef]
- Jozsa, F.; Thistle, J. Anatomy, Colostrum. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025; Available online: https://www.ncbi.nlm.nih.gov/books/NBK513256/ (accessed on 22 January 2025).
- McGuire, M.K.; Seppo, A.; Goga, A.; Buonsenso, D.; Collado, M.C.; Donovan, S.M.; Müller, J.A.; Ofman, G.; Monroy-Valle, M.; O’Connor, D.L.; et al. Best Practices for Human Milk Collection for COVID-19 Research. Breastfeed Med. 2021, 16, 29–38. [Google Scholar] [CrossRef]
- World Health Organization—W.H.O. Breastfeeding and COVID-19. 2020. Available online: https://www.who.int/publications/i/item/WHO-2019-nCoV-Sci_Brief-Breastfeeding-2020.1 (accessed on 11 September 2024).
- Dutta, D.; Naiyer, S.; Mansuri, S.; Soni, N.; Singh, V.; Bhat, K.H.; Singh, N.; Arora, G.; Mansuri, M.S. COVID-19 Diagnosis: A Comprehensive Review of the RT-qPCR Method for Detection of SARS-CoV-2. Diagnostics 2022, 12, 1503. [Google Scholar] [CrossRef]
- Gharibzahedi, S.M.T.; Altintas, Z. State-of-the-art sensor technologies for tracking SARS-CoV-2 in contaminated food and packaging: Towards the future techniques of food safety assurance. TrAC Trends Anal. Chem. 2024, 170, 117473. [Google Scholar] [CrossRef]
- Pace, R.M.; Williams, J.E.; Järvinen, K.M.; Meehan, C.L.; Martin, M.A.; Ley, S.H.; Barbosa-Leiker, C.; Andres, A.; Yeruva, L.; Belfort, M.B.; et al. Milk from Women Diagnosed with COVID-19 Does Not Contain SARS-CoV-2 RNA but Has Persistent Levels of SARS-CoV-2-Specific IgA Antibodies. Front. Immunol. 2021, 12, 801797. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Marino, J.; Amanat, F.; Oguntuyo, K.Y.; Hahn-Holbrook, J.; Lee, B.; Zolla-Pazner, S.; Powell, R.L. The IgA in milk induced by SARS-CoV-2 infection is comprised of mainly secretory antibody that is neutralizing and highly durable over time. PLoS ONE 2022, 17, e0249723. [Google Scholar] [CrossRef]
- Sinha, D.; Yaugel-Novoa, M.; Waeckel, L.; Paul, S.; Longet, S. Unmasking the potential of secretory IgA and its pivotal role in protection from respiratory viruses. Antiviral Res. 2024, 223, 105823. [Google Scholar] [CrossRef]
- Zheng, Y.; Correa-Silva, S.; Palmeira, P.; Carneiro-Sampaio, M. Maternal vaccination as an additional approach to improve the protection of the nursling: Anti-infective properties of breast milk. Clinics 2022, 77, 100093. [Google Scholar] [CrossRef]
- Conti, M.G.; Terreri, S.; Piano Mortari, E.; Albano, C.; Natale, F.; Boscarino, G.; Zacco, G.; Palomba, P.; Cascioli, S.; Corrente, F.; et al. Immune Response of Neonates Born to Mothers Infected With SARS-CoV-2. JAMA Netw. Open. 2021, 4, e2132563. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Akhter, H.; Aziz, F.; Ullah, F.R.; Ahsan, M.; Islam, S.N. Immunoglobulins content in colostrum, transitional and mature milk of Bangladeshi mothers: Influence of parity and sociodemographic characteristics. J. Mother. Child. 2021, 24, 8–15. [Google Scholar] [CrossRef]
- Oluka, G.K.; Namubiru, P.; Kato, L.; Ankunda, V.; Gombe, B.; Cotten, M.; COVID-19 Immunoprofiling Team; Musenero, M.; Kaleebu, P.; Fox, J.; et al. Optimisation and Validation of a conventional ELISA and cut-offs for detecting and quantifying anti-SARS-CoV-2 Spike, RBD, and Nucleoprotein IgG, IgM, and IgA antibodies in Uganda. Front. Immunol. 2023, 14, 1113194. [Google Scholar] [CrossRef]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef]
- Mattioli, I.A.; Hassan, A.; Oliveira, O.N., Jr.; Crespilho, F.N. On the Challenges for the Diagnosis of SARS-CoV-2 Based on a Review of Current Methodologies. ACS Sens. 2020, 5, 3655–3677. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Y.; Sun, Q.; Knopf, J.; Herrmann, M.; Lin, L.; Jiang, J.; Shao, C.; Li, P.; He, X.; et al. Immune response in COVID-19: What is next? Cell Death Differ. 2022, 29, 1107–1122. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Prahl, M.; Gaw, S.L.; Narasimhan, S.R.; Rai, D.S.; Huang, A.; Flores, C.V.; Lin, C.Y.; Jigmeddagva, U.; Wu, A.; et al. Passive and active immunity in infants born to mothers with SARS-CoV-2 infection during pregnancy: Prospective cohort study. BMJ Open 2021, 11, e053036. [Google Scholar] [CrossRef]
- Ryan, L.; Plötz, F.B.; van den Hoogen, A.; Latour, J.M.; Degtyareva, M.; Keuning, M.; Klingenberg, C.; Reiss, I.K.M.; Giannoni, E.; Roehr, C.; et al. Neonates and COVID-19: State of the art: Neonatal Sepsis series. Pediatr. Res. 2022, 91, 432–439. [Google Scholar] [CrossRef]
- Fich, L.; Christiansen, A.H.; Nilsson, A.C.; Lindman, J.; Juul-Larsen, H.G.; Hansen, C.B.; la Cour Freiesleben, N.; Khalil, M.R.; Nielsen, H.S. SARS-CoV-2 Antibodies in Breastmilk Three and Six Months Postpartum in Relation to the Trimester of Maternal SARS-CoV-2 Infection-An Exploratory Study. Int. J. Mol. Sci. 2024, 25, 3269. [Google Scholar] [CrossRef]
- Hochmayr, C.; Winkler, I.; Hammerl, M.; Höller, A.; Huber, E.; Urbanek, M.; Kiechl-Kohlendorfer, U.; Griesmaier, E.; Posod, A. Factors Influencing Breast Milk Antibody Titers during the Coronavirus Disease 2019 Pandemic: An Observational Study. Nutrients 2024, 16, 2320. [Google Scholar] [CrossRef]
- Wachman, E.M.; Snyder-Cappione, J.; Devera, J.; Boateng, J.; Dhole, Y.; Clarke, K.; Yuen, R.R.; Parker, S.E.; Hunnewell, J.; Ferraro, R.; et al. Maternal, Infant, and Breast Milk Antibody Response Following COVID-19 Infection in Early Versus Late Gestation. Pediatr. Infect. Dis. J. 2023, 42, e70–e76. [Google Scholar] [CrossRef]
- Bobik, T.V.; Kostin, N.N.; Skryabin, G.A.; Tsabai, P.N.; Simonova, M.A.; Knorre, V.D.; Mokrushina, Y.A.; Smirnov, I.V.; Kosolapova, J.A.; Vtorushina, V.V.; et al. Epitope-Specific Response of Human Milk Immunoglobulins in COVID-19 Recovered Women. Pathogens 2021, 10, 705. [Google Scholar] [CrossRef]
- Pullen, K.M.; Atyeo, C.; Collier, A.Y.; Gray, K.J.; Belfort, M.B.; Lauffenburger, D.A.; Edlow, A.G.; Alter, G. Selective functional antibody transfer into the breastmilk after SARS-CoV-2 infection. Cell Rep. 2021, 37, 109959. [Google Scholar] [CrossRef]
- Decenti, E.C.; Salvatore, M.A.; Mancon, A.; Portella, G.; Rocca, A.; Vocale, C.; Donati, S.; Alberi, I.; Anelli, G.M.; Baltaro, F.; et al. A large series of molecular and serological specimens to evaluate mother-to-child SARS-CoV-2 transmission: A prospective study from the Italian Obstetric Surveillance System. Int. J. Infect. Dis. 2023, 126, 1–9. [Google Scholar] [CrossRef]
- Forthal, D.N. Functions of Antibodies. Microbiol. Spectr. 2014, 2, 1–17. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheehan, J.; Andres, A.; Yeruva, L.; Ramsay, A.J. SARS-CoV-2 infection induces human milk antibodies capable of mediating multiple functional activities. Clin. Nutr. Open Sci. 2024, 58, 215–226. [Google Scholar] [CrossRef]
- Society for Maternal-Fetal Medicine. Management Considerations for Pregnant Patients with COVID-19. 2020. Available online: https://s3.amazonaws.com/cdn.smfm.org/media/2415/SMFM_COVID_Management_of_COVID_pos_preg_patients_7-2-20.PDF_.pdf (accessed on 12 September 2024).
- Yang, X.; Fox, A.; DeCarlo, C.; Pineda, N.; Powell, R.L.R. The Secretory IgA Response in Human Milk Against the SARS-CoV-2 Spike Is Highly Durable and Neutralizing for At Least 1 Year of Lactation Postinfection. Breastfeed. Med. 2023, 18, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Murray, S.M.; Ansari, A.M.; Frater, J.; Klenerman, P.; Dunachie, S.; Barnes, E.; Ogbe, A. The impact of pre-existing cross-reactive immunity on SARS-CoV-2 infection and vaccine responses. Nat. Rev. Immunol. 2023, 23, 304–316. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; DaPra, C.; Mathijssen, G.; ASela, D.; MJarvinen, K.; Seppo, A.; Fels, S.; Medo, E. Human Milk Antibodies Against S1 and S2 Subunits from SARS-CoV-2, HCoV-OC43, and HCoV-229E in Mothers with A Confirmed COVID-19 PCR, Viral SYMPTOMS, and Unexposed Mothers. Int. J. Mol. Sci. 2021, 22, 1749. [Google Scholar] [CrossRef]
- Agrawal, B. Heterologous immunity: Role in natural and vaccine-induced resistance to infections. Front. Immunol. 2019, 10, 2631. [Google Scholar] [CrossRef]
- Selma-Royo, M.; Bäuerl, C.; Mena-Tudela, D.; Aguilar-Camprubí, L.; Pérez-Cano, F.J.; Parra-Llorca, A.; Lerin, C.; Martínez-Costa, C.; Collado, M.C. Anti-SARS-CoV-2 IgA and IgG in human milk after vaccination is dependent on vaccine type and previous SARS-CoV-2 exposure: A longitudinal study. Genome Med. 2022, 14, 42. [Google Scholar] [CrossRef]
- Young, B.E.; Seppo, A.E.; Diaz, N.; Rosen-Carole, C.; Nowak-Wegrzyn, A.; Cruz Vasquez, J.M.; Ferri-Huerta, R.; Nguyen-Contant, P.; Fitzgerald, T.; Sangster, M.Y.; et al. Association of Human Milk Antibody Induction, Persistence, and Neutralizing Capacity With SARS-CoV-2 Infection vs mRNA Vaccination. JAMA Pediatr. 2022, 176, 159–168. [Google Scholar] [CrossRef]
- Juncker, H.G.; Mulleners, S.J.; Ruhé, E.J.M.; Coenen, E.R.M.; Bakker, S.; van Doesburg, M.; Harinck, J.E.; Rood, R.D.; Bouhuijs, J.H.; Oomen, M.; et al. Comparing the human milk antibody response after vaccination with four COVID-19 vaccines: A prospective, longitudinal cohort study in the Netherlands. EClinicalMedicine 2022, 47, 101393. [Google Scholar] [CrossRef]
- Hendaus, M.A.; Jomha, F.A. mRNA Vaccines for COVID-19: A Simple Explanation. Qatar Med. J. 2021, 2021, 7. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
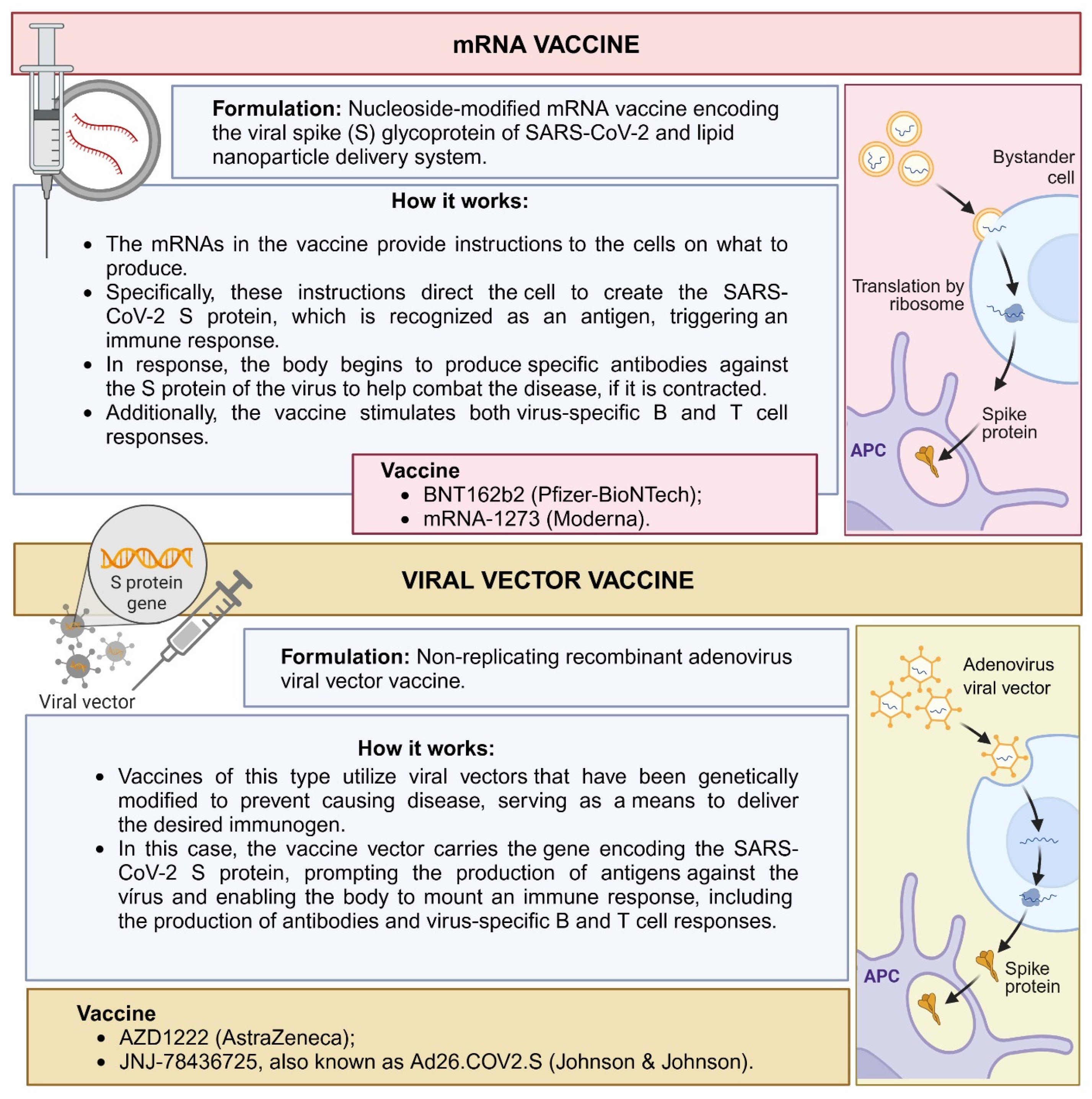
- Muhar, B.K.; Nehira, J.; Malhotra, A.; Kotchoni, S.O. The Race for COVID-19 Vaccines: The Various Types and Their Strengths and Weaknesses. J. Pharm. Pract. 2023, 36, 953–966. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hu, Y.; Lv, Y.; Obore, N.; Wang, Y.; Yu, H. SARS-CoV-2 Antibodies in Human Milk After mRNA and Adenovector-Based Vaccination: A Systematic Review and Meta-Analysis. J. Hum. Lact. 2024, 40, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hamouda, N.I.; Amin, A.M.; Hasan, M.T.; Baghagho, E. Persistence of COVID-19 Human Milk Antibodies After Maternal COVID-19 Vaccination: Systematic Review and Meta-Regression Analysis. Cureus 2024, 16, e59500. [Google Scholar] [CrossRef] [PubMed]
- Demers-Mathieu, V.; Huston, R.K.; Markell, A.M.; McCulley, E.A.; Martin, R.L.; Spooner, M.; Dallas, D.C. Differences in Maternal Immunoglobulins within Mother’s Own Breast Milk and Donor Breast Milk and across Digestion in Preterm Infants. Nutrients 2019, 11, 920. [Google Scholar] [CrossRef]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Prakash, S. Gastrointestinal digestion of dairy and soy proteins in infant formulas: An in vitro study. Food Res. Int. 2015, 76, 348–358. [Google Scholar] [CrossRef]
- Hurley, W.L.; Theil, P.K. Perspectives on immunoglobulins in colostrum and milk. Nutrients 2011, 3, 442–474. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pieri, M.; Maniori, M.A.; Shahabian, L.; Kanaan, E.; Paphiti-Demetriou, I.; Pipis, S.; Felekkis, K.; Nicolaidou, V.; Papaneophytou, C. Survival of Vaccine-Induced Human Milk SARS-CoV-2 IgG, IgA and SIgA Immunoglobulins across Simulated Human Infant Gastrointestinal Digestion. Nutrients 2022, 14, 3368. [Google Scholar] [CrossRef]
- Calvo-Lerma, J.; Bueno-Llamoga, P.; Bäuerl, C.; Cortés-Macias, E.; Selma-Royo, M.; Pérez-Cano, F.; Lerin, C.; Martínez-Costa, C.; Collado, M.C. Persistence of Anti SARS-CoV-2 Antibodies in Breast Milk from Infected and Vaccinated Women after In Vitro-Simulated Gastrointestinal Digestion. Nutrients 2022, 14, 2117. [Google Scholar] [CrossRef]
- Ménard, O.; Bourlieu, C.; De Oliveira, S.C.; Dellarosa, N.; Laghi, L.; Carrière, F.; Capozzi, F.; Dupont, D.; Deglaire, A. A first step towards a consensus static in vitro model for simulating full-term infant digestion. Food Chem. 2018, 240, 338–345. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). COVID-19 and Breastfeeding. 2024. Available online: https://www.cdc.gov/breastfeeding-special-circumstances/hcp/illnesses-conditions/covid-19.html#:~:text=Current%20evidence%20suggests%20that%20breast,of%20COVID%2D19%20vaccination%20status (accessed on 10 September 2024).
- Buszko, M.; Nita-Lazar, A.; Park, J.H.; Schwartzberg, P.L.; Verthelyi, D.; Young, H.A.; Rosenberg, A.S. Lessons learned: New insights on the role of cytokines in COVID-19. Nat. Immunol. 2021, 22, 404–411. [Google Scholar] [CrossRef]
- Montazersaheb, S.; Hosseiniyan Khatibi, S.M.; Hejazi, M.S.; Tarhriz, V.; Farjami, A.; Ghasemian Sorbeni, F.; Farahzadi, R.; Ghasemnejad, T. COVID-19 infection: An overview on cytokine storm and related interventions. Virol. J. 2022, 19, 92. [Google Scholar] [CrossRef] [PubMed]
- Hasanvand, A. COVID-19 and the role of cytokines in this disease. Inflammopharmacology 2022, 30, 789–798. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rubio, R.; Aguilar, R.; Bustamante, M.; Muñoz, E.; Vázquez-Santiago, M.; Santano, R.; Vidal, M.; Melero, N.R.; Parras, D.; Serra, P.; et al. Maternal and neonatal immune response to SARS-CoV-2, IgG transplacental transfer and cytokine profile. Front. Immunol. 2022, 13, 999136. [Google Scholar] [CrossRef]
- Sánchez García, L.; Gómez-Torres, N.; Cabañas, F.; González-Sánchez, R.; López-Azorín, M.; Moral-Pumarega, M.T.; Escuder-Vieco, D.; Cabañes-Alonso, E.; Castro, I.; Alba, C.; et al. Immediate Pre-Partum SARS-CoV-2 Status and Immune Profiling of Breastmilk: A Case-Control Study. Front. Immunol. 2021, 12, 720716. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Pentecost, B.; Alfandari, D.; Chin, E.; Minor, K.; Kastrinakis, A.; Lieberman, T.; Arcaro, K.F.; Leftwich, H. Humoral and Cell-Mediated Immune Response in Colostrum from Women Diagnosed Positive for SARS-CoV-2. Breastfeed. Med. 2021, 16, 987–994. [Google Scholar] [CrossRef]
- Erbağci, A.B.; Cekmen, M.B.; Balat, O.; Balat, A.; Aksoy, F.; Tarakçioğlu, M. Persistency of high proinflammatory cytokine levels from colostrum to mature milk in preeclampsia. Clin. Biochem. 2005, 38, 712–716. [Google Scholar] [CrossRef]
- Majeed, A.Y.; Zulkafli, N.E.S.; Ad’hiah, A.H. Serum profiles of pro-inflammatory and anti-inflammatory cytokines in non-hospitalized patients with mild/moderate COVID-19 infection. Immunol. Lett. 2023, 260, 24–34. [Google Scholar] [CrossRef]
- Dharra, R.; Kumar Sharma, A.; Datta, S. Emerging aspects of cytokine storm in COVID-19: The role of proinflammatory cytokines and therapeutic prospects. Cytokine 2023, 169, 156287. [Google Scholar] [CrossRef]
- Ramiro-Cortijo, D.; Herranz Carrillo, G.; Singh, P.; Rebollo-Hernanz, M.; Rodríguez-Rodríguez, P.; Ruvira, S.; Martín-Trueba, M.; Martin, C.R.; Arribas, S.M. Maternal and Neonatal Factors Modulating Breast Milk Cytokines in the First Month of Lactation. Antioxidants 2023, 12, 996. [Google Scholar] [CrossRef]
- Vass, R.A.; Kemeny, A.; Dergez, T.; Ertl, T.; Reglodi, D.; Jungling, A.; Tamas, A. Distribution of bioactive factors in human milk samples. Int. Breastfeed. J. 2019, 14, 9. [Google Scholar] [CrossRef]
- Trofin, F.; Dorneanu, O.S.; Constantinescu, D.; Nastase, E.V.; Luncă, C.; Iancu, L.S.; Andrioaie, I.M.; Duhaniuc, A.; Cianga, C.M.; Pavel-Tanasa, M.; et al. Cytokines and Chemokines in Breastmilk of SARS-CoV-2 Infected or COVID-19 Vaccinated Mothers. Vaccines 2022, 10, 2001. [Google Scholar] [CrossRef] [PubMed]
- Narayanaswamy, V.; Pentecost, B.T.; Schoen, C.N.; Alfandari, D.; Schneider, S.S.; Baker, R.; Arcaro, K.F. Neutralizing Antibodies and Cytokines in Breast Milk After Coronavirus Disease 2019 (COVID-19) mRNA Vaccination. Obs. Gynecol. 2022, 139, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Oberhardt, V.; Luxenburger, H.; Kemming, J.; Schulien, I.; Ciminski, K.; Giese, S.; Csernalabics, B.; Lang-Meli, J.; Janowska, I.; Staniek, J.; et al. Rapid and stable mobilization of CD8+ T cells by SARS-CoV-2 mRNA vaccine. Nature 2021, 597, 268–273. [Google Scholar] [CrossRef]
- Mateus, J.; Dan, J.M.; Zhang, Z.; Rydyznski Moderbacher, C.; Lammers, M.; Goodwin, B.; Sette, A.; Crotty, S.; Weiskopf, D. Low-dose mRNA-1273 COVID-19 vaccine generates durable memory enhanced by cross-reactive T cells. Science 2021, 374, eabj9853. [Google Scholar] [CrossRef]
- Cremoni, M.; Allouche, J.; Graça, D.; Zorzi, K.; Fernandez, C.; Teisseyre, M.; Benzaken, S.; Ruetsch-Chelli, C.; Esnault, V.L.M.; Dellamonica, J.; et al. Low baseline IFN-γ response could predict hospitalization in COVID-19 patients. Front. Immunol. 2022, 13, 953502. [Google Scholar] [CrossRef]
- Silva, B.J.A.; Krogstad, P.A.; Teles, R.M.B.; Andrade, P.R.; Rajfer, J.; Ferrini, M.G.; Yang, O.O.; Bloom, B.R.; Modlin, R.L. IFN-γ-mediated control of SARS-CoV-2 infection through nitric oxide. Front. Immunol. 2023, 14, 1284148. [Google Scholar] [CrossRef]
- Demers-Mathieu, V.; Lavangnananda, S.; Medo, E. Influence of Vitamin D3 Levels and T Cell-Related Cytokines in Human Milk on Coronavirus Disease 2019 Infection in Lactating Women. Breastfeed Med. 2021, 16, 995–1003. [Google Scholar] [CrossRef]
- Zhu, X.; Zhu, J. CD4 T Helper Cell Subsets and Related Human Immunological Disorders. Int. J. Mol. Sci. 2020, 21, 8011. [Google Scholar] [CrossRef]
- Yu, J.C.; Khodadadi, H.; Salles, E.L.; Pham, Q.; Patel, P.; Baban, B. High Levels of Interferon-Alpha Expressing Macrophages in Human Breast Milk During SARS-CoV-2 Infection: A Case Report. Breastfeed. Med. 2021, 16, 439–442. [Google Scholar] [CrossRef]
- Acharya, D.; Liu, G.; Gack, M.U. Dysregulation of type I interferon responses in COVID-19. Nat. Rev. Immunol. 2020, 20, 397–398. [Google Scholar] [CrossRef]
- Samuel, C.E. Interferon at the crossroads of SARS-CoV-2 infection and COVID-19 disease. J. Biol. Chem. 2023, 299, 104960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Narayanaswamy, V.; Pentecost, B.T.; Telfer, J.C.; Burnside, A.S.; Schneider, S.S.; Alfandari, D.; Baker, R.L.; Saiju, A.; Nodiff, S.; Arcaro, K.F. Durable antibody and effector memory T cell responses in breastmilk from women with SARS-CoV-2. Front. Immunol. 2022, 13, 985226. [Google Scholar] [CrossRef] [PubMed]
- Sabbaj, S.; Ghosh, M.K.; Edwards, B.H.; Leeth, R.; Decker, W.D.; Goepfert, P.A.; Aldrovandi, G.M. Breast milk-derived antigen-specific CD8+ T cells: An extralymphoid effector memory cell population in humans. J. Immunol. 2005, 174, 2951–2956. [Google Scholar] [CrossRef] [PubMed]
- Mittrücker, H.W.; Visekruna, A.; Huber, M. Heterogeneity in the differentiation and function of CD8+ T cells. Arch. Immunol. Ther. Exp. 2014, 62, 449–458. [Google Scholar] [CrossRef]
- Ciardelli, L.; Garofoli, F.; Stronati, M.; Mazzucchelli, I.; Avanzini, M.A.; Figar, T.; Gasparoni, A.; De Silvestri, A.; Sabatino, G.; Chirico, G. Human colostrum T lymphocytes and their effector cytokines actively aid the development of the newborn immune system. Int. J. Immunopathol. Pharmacol. 2008, 21, 781–786. [Google Scholar] [CrossRef]
- Graciliano, N.G.; Tenório, M.C.S.; Fragoso, M.B.T.; Moura, F.A.; Botelho, R.M.; Tanabe, E.L.L.; Borbely, K.S.C.; Borbely, A.U.; Oliveira, A.C.M.; Goulart, M.O.F. The impact on colostrum oxidative stress, cytokines, and immune cells composition after SARS-CoV-2 infection during pregnancy. Front. Immunol. 2022, 13, 1031248. [Google Scholar] [CrossRef]
- Chang, J.T.; Wherry, E.J.; Goldrath, A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014, 15, 1104–1115. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef]
- Di Vito, C.; Calcaterra, F.; Coianiz, N.; Terzoli, S.; Voza, A.; Mikulak, J.; Della Bella, S.; Mavilio, D. Natural Killer Cells in SARS-CoV-2 Infection: Pathophysiology and Therapeutic Implications. Front. Immunol. 2022, 13, 888248. [Google Scholar] [CrossRef]
- Kalimuddin, S.; Tham, C.Y.L.; Qui, M.; de Alwis, R.; Sim, J.X.Y.; Lim, J.M.E.; Tan, H.C.; Syenina, A.; Zhang, S.L.; Le Bert, N.; et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med 2021, 2, 682–688.e4. [Google Scholar] [CrossRef]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.; Juliano, A.M.; Charepe, N.; Alenquer, M.; Athayde, D.; Ferreira, F.; Archer, M.; Amorim, M.J.; Serrano, F.; Soares, H. Secretory IgA and T cells targeting SARS-CoV-2 spike protein are transferred to the breastmilk upon mRNA vaccination. Cell Rep. Med. 2021, 2, 100468. [Google Scholar] [CrossRef]
- Li, C.; Liu, Y.; Jiang, Y.; Xu, N.; Lei, J. Immunomodulatory constituents of human breast milk and immunity from bronchiolitis. Ital. J. Pediatr. 2017, 43, 8. [Google Scholar] [CrossRef] [PubMed]
- Tuaillon, E.; Valea, D.; Becquart, P.; Al Tabaa, Y.; Meda, N.; Bollore, K.; Van de Perre, P.; Vendrell, J.P. Human milk-derived B cells: A highly activated switched memory cell population primed to secrete antibodies. J. Immunol. 2009, 182, 7155–7162. [Google Scholar] [CrossRef] [PubMed]
- Niimi, K.; Usami, K.; Fujita, Y.; Abe, M.; Furukawa, M.; Suyama, Y.; Sakai, Y.; Kamioka, M.; Shibata, N.; Park, E.J.; et al. Development of immune and microbial environments is independently regulated in the mammary gland. Mucosal Immunol. 2018, 11, 643–653. [Google Scholar] [CrossRef]
- Armistead, B.; Jiang, Y.; Carlson, M.; Ford, E.S.; Jani, S.; Houck, J.; Wu, X.; Jing, L.; Pecor, T.; Kachikis, A.; et al. Spike-specific T cells are enriched in breastmilk following SARS-CoV-2 mRNA vaccination. Mucosal Immunol. 2023, 16, 39–49. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. COVID-19 Vaccination for Women Who Are Pregnant or Breastfeeding. 2024. Available online: https://www.cdc.gov/covid/vaccines/pregnant-or-breastfeeding.html#:~:text=Everyone%20ages%206%20months%20and,pregnancy%20is%20safe%20and%20effective (accessed on 2 March 2025).


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graciliano, N.G.; Goulart, M.O.F.; de Oliveira, A.C.M. Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk. Int. J. Mol. Sci. 2025, 26, 2600. https://doi.org/10.3390/ijms26062600
Graciliano NG, Goulart MOF, de Oliveira ACM. Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk. International Journal of Molecular Sciences. 2025; 26(6):2600. https://doi.org/10.3390/ijms26062600
Chicago/Turabian StyleGraciliano, Nayara Gomes, Marília Oliveira Fonseca Goulart, and Alane Cabral Menezes de Oliveira. 2025. "Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk" International Journal of Molecular Sciences 26, no. 6: 2600. https://doi.org/10.3390/ijms26062600
APA StyleGraciliano, N. G., Goulart, M. O. F., & de Oliveira, A. C. M. (2025). Impact of Maternal Exposure to SARS-CoV-2 on Immunological Components of Breast Milk. International Journal of Molecular Sciences, 26(6), 2600. https://doi.org/10.3390/ijms26062600






