Photophysical Properties and Metal Ion Sensing of a Pyrene-Based Liquid Crystalline Dimer
Abstract
:1. Introduction
2. Results and Discussion
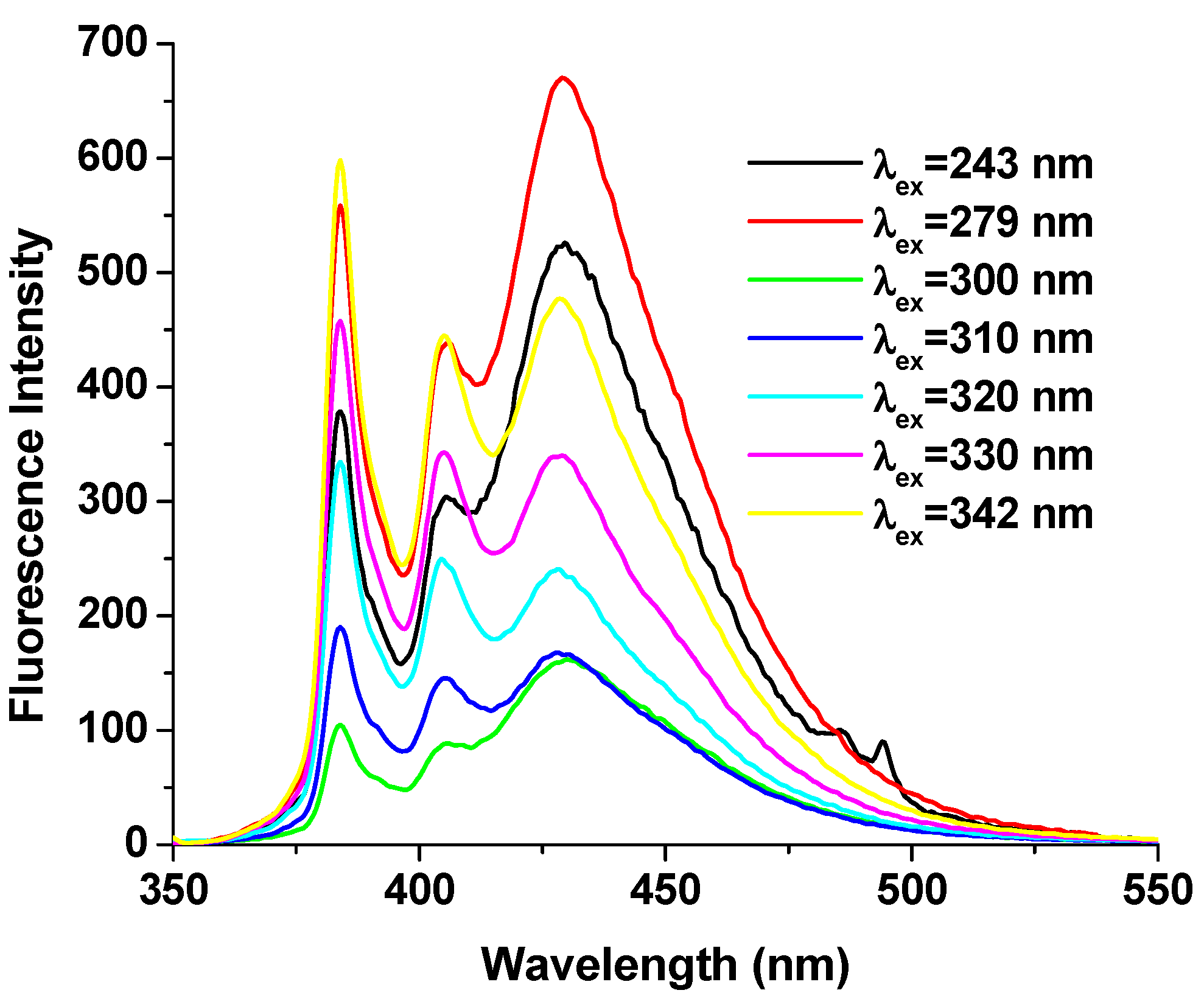
2.1. Photophysical Properties of the DPyH9
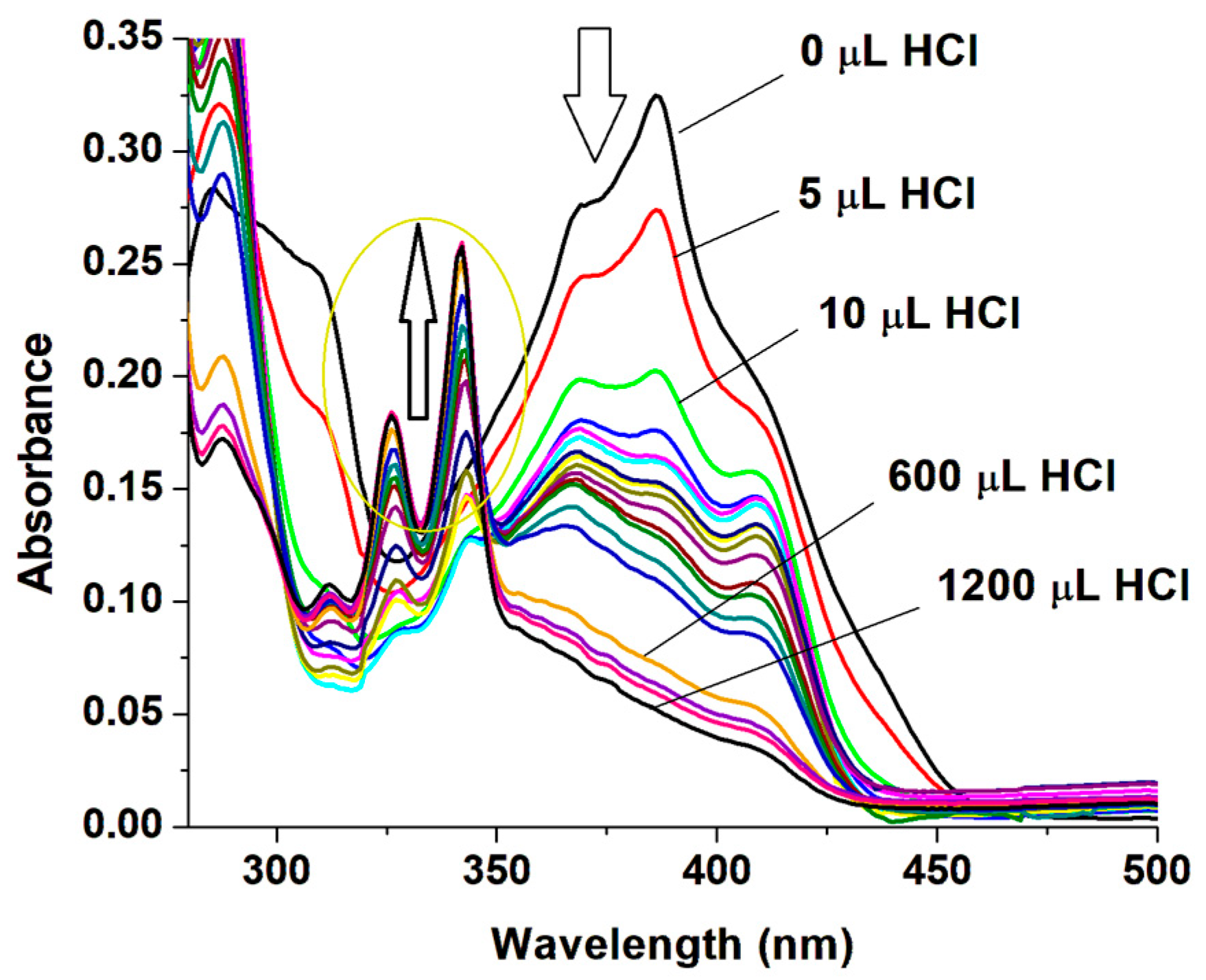
2.2. Acidochromic Behavior
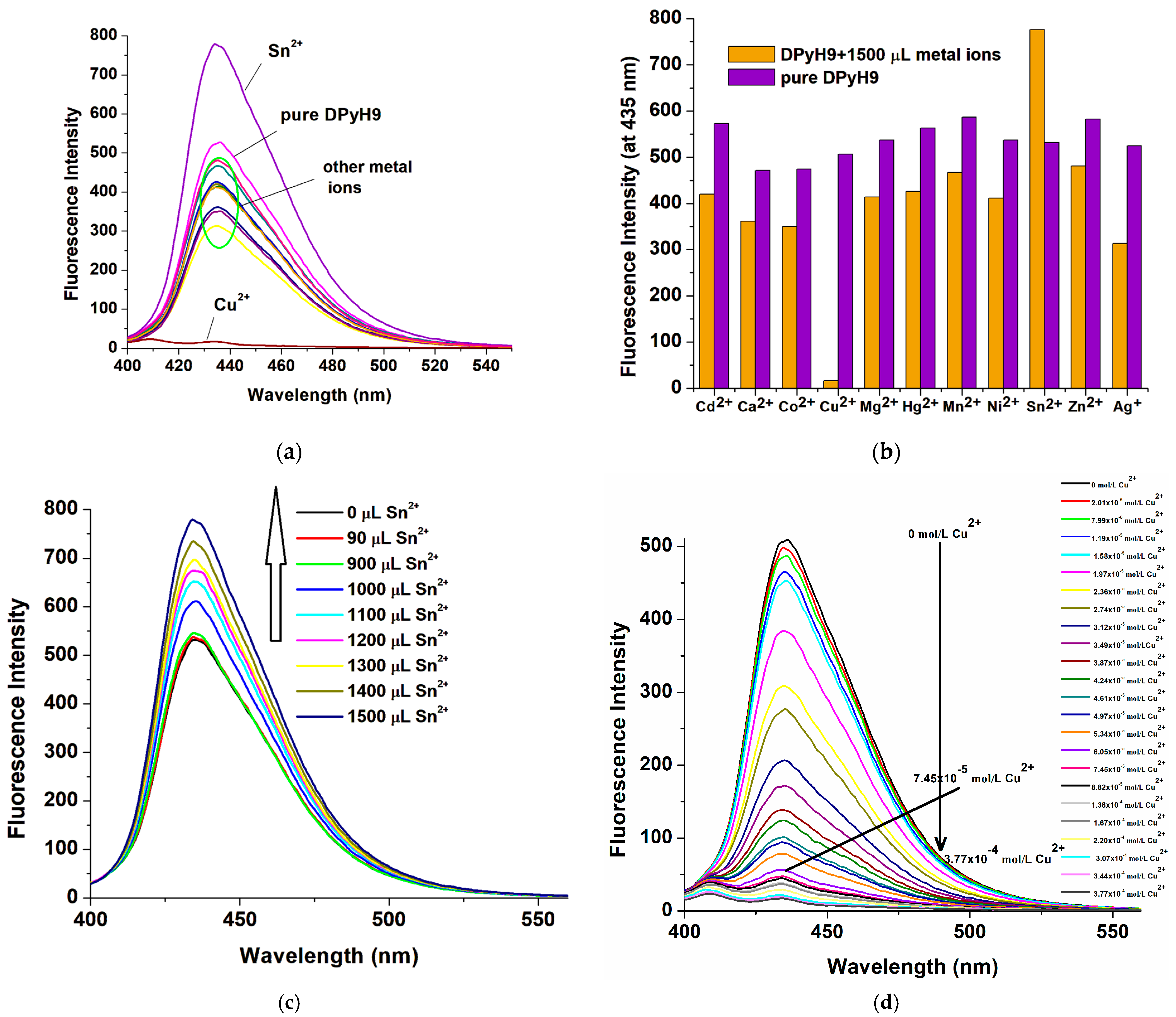
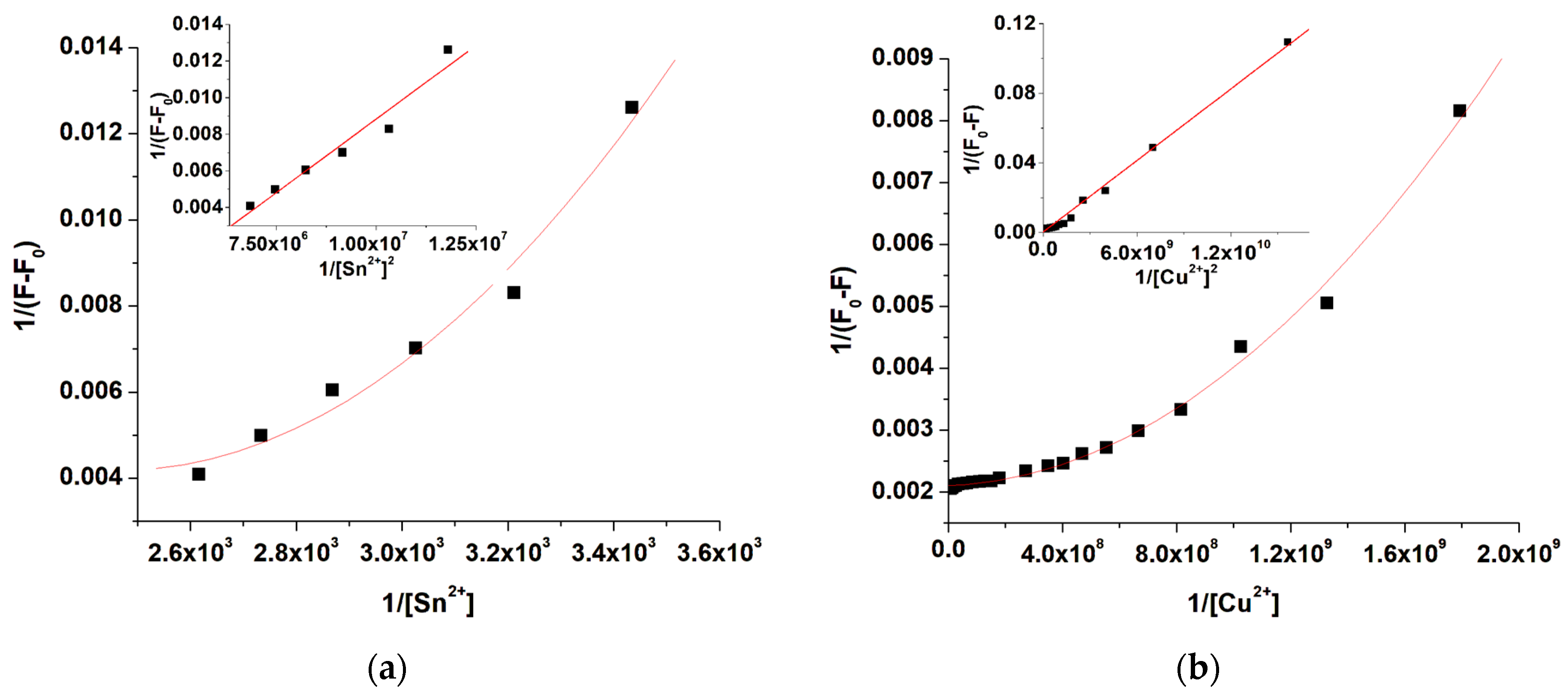
2.3. Metal Ion Sensing Behavior
3. Materials and Methods
3.1. Materials
3.2. Synthesis and Characterization of 1,9-bis[4-(Pyreneiminomethylidene)-phenoxy]-nonane [15]
3.3. Characterization Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crawford, S.E.; Ohodnicki, P.R., Jr.; Baltrus, J.P. Materials for the photoluminescent sensing of rare earth elements: Challenges and opportunities. J. Mater. Chem. C 2020, 8, 7975–8006. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.; Xu, Z.; Yoon, J. Recent progress on the development of chemosensors for gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef]
- Martínez-Denegri, G.; Soares, F.A.; Ślęczkowski, P. Wide color gamut and high sensitivity in luminescent thermal indicators from an organic energy donor-acceptor system with tunable molecular interactions. Adv. Opt. Mater. 2024, 31, 2403073. [Google Scholar] [CrossRef]
- Bhandari, P.; Ahmed, S.; Saha, R.; Mukherjee, P.S. Enhancing fluorescence in both solution and solid states induced by imine cage formation. Chem. Eur. J. 2024, 30, e202303101. [Google Scholar] [CrossRef]
- Joy, F.; Chaithra, K.P.; Nizam, A.; Deepti, A.; Chakrapani, P.S.B.; Das, A.K.; Vinod, T.P.; Nair, Y. A Multi-Stimuli responsive organic luminogen with aggregation induced emission for the selective detection of Zn2+ ions in solution and solid state. Chem. Eng. J. 2023, 453, 139798. [Google Scholar] [CrossRef]
- Paul, S.; Barman, P.; Dey, N.; Watkinson, M. Recent developments in pyrene-based fluorescent recognition and imaging of Ag⁺ and Pb²⁺ Ions: Synthesis, applications, and challenges. Sens. Diagn. 2024, 3, 946–967. [Google Scholar] [CrossRef]
- Liang, J.; Ya, Y.; Ning, D.; Jiang, C.; Wang, Y.; Xie, L.; Huang, X.; Li, T.; Tang, L.; Yan, F. A pyrene-based “turn-on” fluorescent sensor for highly sensitive detection of Cu2⁺ and 3-nitropropionic acid. J. Food Compos. Anal. 2024, 130, 106174. [Google Scholar] [CrossRef]
- Yeldir, E.K.; Kaya, İ. Synthesis, characterization and investigation of fluorescent Sn2+ probe potential of pyrene-derived monomer and its oligo (azomethine) compound. Eur. Polym. J. 2022, 172, 111229. [Google Scholar] [CrossRef]
- Rana, V.S.; Anand, V.; Sarkar, S.S.; Sandhu, N.; Verma, M.; Naidu, S.; Singh, A.P. A novel pyrene-based aggregation induced enhanced emission active Schiff base fluorophore as a selective “turn-on” sensor for Sn2+ ions and its application in lung adenocarcinoma cells. J. Photochem. Photobiol. A Chem. 2023, 436, 114409. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Li, G.-S.; Yu, P.-J.; Ercan, E.; Chen, W.-C. Organic liquid crystals in optoelectronic device applications: Field-effect transistors, nonvolatile memory, and photovoltaics. J. Chin. Chem. Soc 2022, 69, 1289. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Zhao, Y.; Ye, F.; Shang, L. Liquid crystal materials for biomedical applications. Adv. Mater. 2023, 35, 2300220. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Majewska, M.; Pociecha, D.; Makal, A.; Storey, J.M.; Gorecka, E.; Imrie, C.T. Twist-bend nematic glasses: The synthesis and characterisation of pyrene-based nonsymmetric dimers. ChemPhysChem 2021, 22, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Figueira-Duarte, T.M.; Müllen, K. Pyrene-based materials for organic electronics. Chem. Rev. 2011, 111, 7260–7314. [Google Scholar] [CrossRef] [PubMed]
- Ayyavoo, K.; Velusamy, P. Pyrene based materials as fluorescent probes in chemical and biological fields. New J. Chem. 2021, 45, 10997–11017. [Google Scholar] [CrossRef]
- Perju, E.; Marin, L. Mesomorphic behavior of symmetric azomethine dimers containing different chromophore groups. Molecules 2021, 26, 2183. [Google Scholar] [CrossRef]
- Hoche, J.; Schmitt, H.C.; Humeniuk, A.; Fischer, I.; Mitrić, R.; Röhr, M.I. The Mechanism of Excimer Formation: An Experimental and Theoretical Study on the Pyrene Dimer. Phys. Chem. Chem. Phys. 2017, 19, 25002–25015. [Google Scholar] [CrossRef]
- Perju, E.; Cozan, V.; Timpu, D.; Bruma, M. The influence of methoxy side groups and halogen nature on the mesomorphic and optical properties of symmetrical azomethines. Liq. Cryst. 2017, 44, 798–808. [Google Scholar] [CrossRef]
- Sıdır, İ.; Gülseven Sıdır, Y.; Berber, H.; Demiray, F. Electronic structure and optical properties of Schiff base hydrazone derivatives by solution technique for optoelectronic devices: Synthesis, experiment and quantum chemical investigation. J. Mol. Struct. 2019, 1176, 31–46. [Google Scholar] [CrossRef]
- Cangialosi, D. Physical aging and vitrification in polymers and other glasses: Complex behavior and size effects. J. Polym. Sci. 2024, 62, 1952. [Google Scholar] [CrossRef]
- Massalska-Arodź, M. On structural organisation and crystallisation/vitrification phenomena in low molecular weight glass-forming liquid crystals. Liq. Cryst. 2023, 51, 1073–1085. [Google Scholar] [CrossRef]
- Kinik, F.P.; Ortega-Guerrero, A.; Ongari, D.; Ireland, C.P.; Smit, B. Pyrene-based metal-organic frameworks: From synthesis to applications. Chem. Soc. Rev. 2021, 50, 3143–3177. [Google Scholar] [CrossRef] [PubMed]
- Garbovskiy, Y.A.; Gridyakina, A.V.; Klimusheva, G.V.; Tolochko, A.S.; Tokmenko, I.I.; Mirnaya, T.A. Tunable optical and nonlinear optical response of smectic glasses based on cobalt alkanoates. Liq. Cryst. 2010, 37, 1411–1418. [Google Scholar] [CrossRef]
- Dierking, I. Textures of Liquid Crystals; Wilez-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Baron, M. Definitions of basic terms relating to low-molar-mass and polymer liquid crystals (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 845–895. [Google Scholar] [CrossRef]
- Thoen, J.; Cordoyiannis, G.; Glorieux, C. Investigations of phase transitions in liquid crystals by means of adiabatic scanning calorimetry. Liq. Cryst. 2009, 36, 669–684. [Google Scholar] [CrossRef]
- Attard, G.S.; Imrie, C.T. Liquid-crystalline and glass-forming dimers derived from 1-aminopyrene. Liq. Cryst. 1992, 11, 785–789. [Google Scholar] [CrossRef]
- Aguilera-Sigalat, J.; Sanchez-SanMartín, J.; Agudelo-Morales, C.E.; Zaballos, E.; Galian, R.E.; Pérez-Prieto, J. Further insight into the photostability of the pyrene fluorophore in halogenated solvents. ChemPhysChem 2012, 13, 835–844. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, X.; Liu, H.B. Highly sensitive pyrene–dansyl conjugate-based fluorescent sensor for discriminative detection of water in organic solvents. Dyes Pigments 2020, 182, 108685. [Google Scholar] [CrossRef]
- Juneja, S.; Pandey, S. Fluorescence of pyrene and its derivatives to reveal constituent and composition dependent solvation within hydrophobic deep eutectic solvents. Phys. Chem. Chem. Phys. 2023, 25, 11998–12012. [Google Scholar] [CrossRef]
- Batra, G.; Sharma, S.; Kaushik, K.; Rao, C.; Kumar, P.; Kumar, K.; Ghosh, S.; Jariwala, D.; Stach, E.; Yadav, A.; et al. Structural and spectroscopic characterization of pyrene derived carbon nanodots: A single-particle level analysis. Nanoscale 2022, 14, 3568–3578. [Google Scholar] [CrossRef]
- Babgi, B.A.; Alzahrani, A. Optical sensing properties of pyrene-Schiff bases toward different acids. J. Fluoresc. 2016, 26, 1415–1419. [Google Scholar] [CrossRef]
- Liu, L.N.; Tao, H.; Chen, G.; Chen, Y.; Cao, Q.Y. An amphiphilic pyrene-based probe for multiple channel sensing of mercury ions. J. Lumin. 2018, 203, 189–194. [Google Scholar] [CrossRef]
- Prabakaran, G.; David, C.I.; Nandhakumar, R. A review on pyrene based chemosensors for the specific detection on d-transition metal ions and their various applications. J. Environ. Chem. Eng. 2023, 11, 109701. [Google Scholar] [CrossRef]
- Faraz, M.; Abbasi, A.; Naqvi, F.K.; Khare, N.; Prasad, R.; Barman, I.; Pandey, R. Polyindole/cadmium sulphide nanocomposite-based turn-on, multi-ion fluorescence sensor for detection of Cr3+, Fe3+, and Sn2+ ions. Sens. Actuators B Chem. 2018, 269, 195–202. [Google Scholar] [CrossRef]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Liu, Y.; Li, L.; Zhang, Y.; Sheng, M.; Wang, Y.; Xing, Z.; Yang, L.; Yue, M.; Fu, Y.; Ye, F. A novel functional fluorescent probe based on a pyrene derivative for the detection of multiple pollutants. J. Mol. Liq. 2023, 382, 121888. [Google Scholar] [CrossRef]
- Liu, Q.; Li, S.; Wang, Y.; Yang, L.; Yue, M.; Liu, Y.; Ye, F.; Fu, Y. Sensitive fluorescence assay for the detection of glyphosate with NACCu²⁺ complex. Sci. Total Environ. 2023, 882, 163548. [Google Scholar] [CrossRef]
- Pisanu, F.; Sykula, A.; Sciortino, G.; Maseras, F.; Lodyga-Chruscinska, E.; Garribba, E. Experimental and computational studies on the interaction of DNA with hesperetin Schiff Base Cu(II) complexes. Int. J. Mol. Sci. 2024, 25, 5283. [Google Scholar] [CrossRef]
- Shanmugapriya, R.; Kumar, P.S.; Poongodi, K.; Nandhini, C.; Elango, K.P. 3-Hydroxy-2-naphthoic hydrazide as a probe for fluorescent detection of cyanide and aluminium ions in organic and aquo-organic media and its application in food and pharmaceutical samples. Spectrochim. Acta Part A 2021, 249, 119315. [Google Scholar] [CrossRef]
- Shellaiah, M.; Venkatesan, P.; Thirumalaivasan, N.; Wu, S.P.; Sun, K.W. Pyrene-based fluorescent probe for “Off-on-Off” sequential detection of Cu2+ and CN− with HeLa cells imaging. Chemosensors 2023, 11, 115. [Google Scholar] [CrossRef]
- Tamrakar, A.; Nigam, K.K.; Maddeshiya, T.; Pandey, M.D. Pyrene functionalized luminescent phenylalanine for selective detection of copper (II) ions in aqueous media. J. Fluoresc. 2023, 33, 1175–1182. [Google Scholar] [CrossRef]
- Yu, C.; Yang, M.; Cui, S.; Ji, Y.; Zhang, J. A ratiometric selective fluorescent probe derived from pyrene for Cu2+ detection. Chemosensors 2022, 10, 207. [Google Scholar] [CrossRef]
- Donahoe, H.B.; Benjamin, L.E.; Fennoy, L.V.; Greiff, D. Synthesis of potential rickettsiostatic agents. I. 4,4’-Dicarboxy-α,ι-diphenoxyalkanes. J. Org. Chem. 1961, 26, 474–476. [Google Scholar] [CrossRef]
- Marin, L.; Perju, E.; Damaceanu, M.D. Designing thermotropic liquid crystalline polyazomethines based on fluorene and/or oxadiazole chromophores. Eur. Polym. J. 2011, 47, 1284–1299. [Google Scholar] [CrossRef]
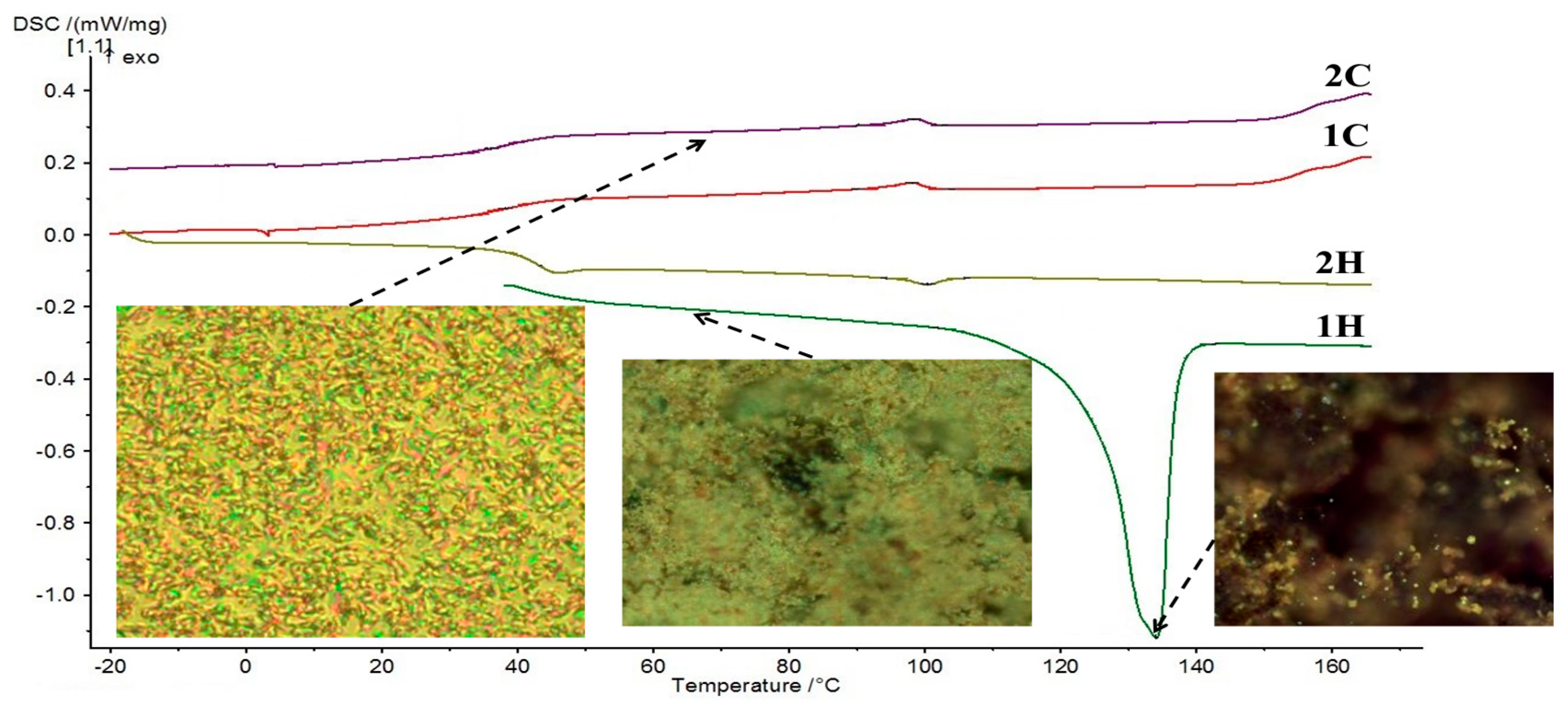
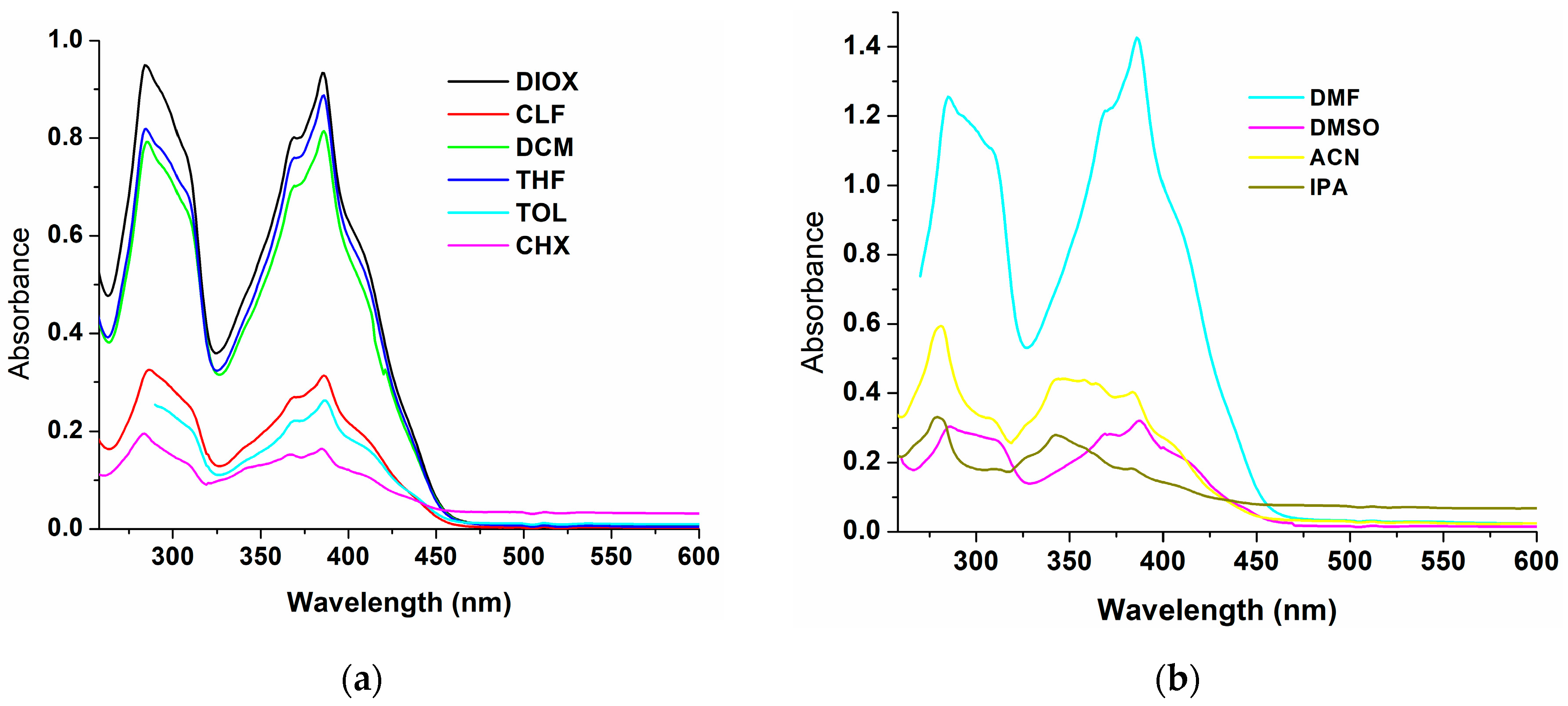

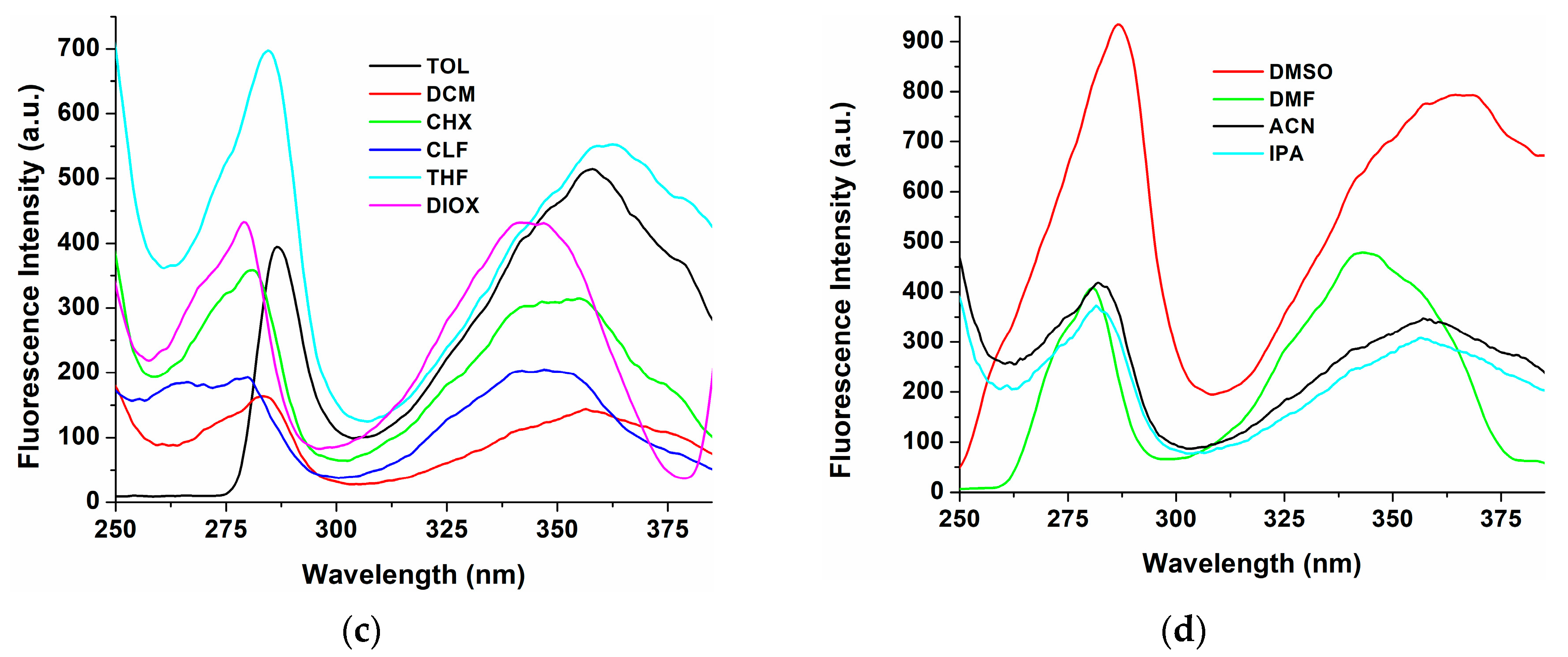
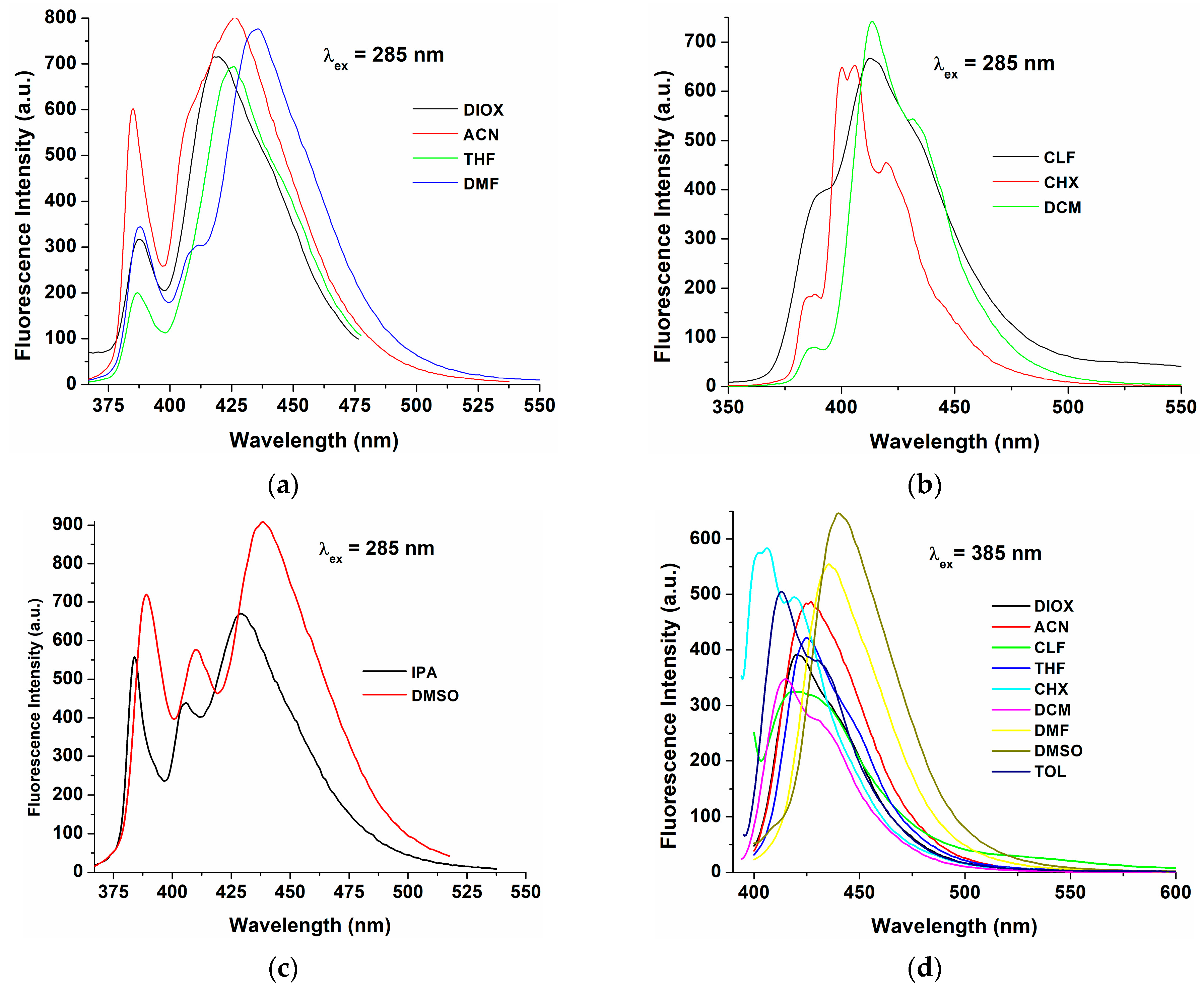

| PLM a | DSC b | |||
|---|---|---|---|---|
| 1st Scan | 2nd Scan | 1st Scan | 2nd Scan | |
| Heating | Cr 136 I | – 50 N 102 I | Cr 134 (49.77) d I | G 43 N 100 (0.50) I |
| Cooling | I 108 N – c | I 98 N – | I 98 (0.54) N 38 G | I 98 (0.55) N 36 G |
| Solvents | λabs,max (nm) | Δλ (a) (nm) | λem,max (nm) | |
|---|---|---|---|---|
| λex = 285 nm | λex = 385 nm | |||
| CHX | 284, 385 | 21 | 400, 406, 420 sh | 401 sh, 406, 419 sh |
| TOL | 311 sh, 386 | 27 | - | 413, 434 sh |
| DIOX | 285, 309 sh, 369 sh, 386 | 33 | 388, 419 | 420 |
| THF | 285, 309 sh, 369 sh, 386 | 41 | 387, 427 | 425 |
| CLF | 286, 309 sh, 369 sh, 386 | 27 | 390 sh, 413 | 421 |
| DCM | 285, 309 sh, 369 sh, 386 | 27 | 413, 431 sh | 415, 431 sh |
| DMF | 285, 309 sh, 368 sh, 386 | 49 | 388, 410 sh, 435 | 435 |
| DMSO | 284, 312 sh, 369 sh, 388 | 51 | 390 sh, 410, 439 | 440 |
| ACN | 281, 347, 384 sh | 80 | 388, 427 | 427 |
| IPA | 280, 343 | 86 | 384, 405, 429 | 384, 405, 429 (b) |
| Sensors/Metal Ions | LOD | Association (Binding) Constant | Refs. |
|---|---|---|---|
| Pyrene-appended Schiff base/Cu2⁺ | 219 nM | 4.95 × 10−6 M−1 | [40] |
| 1(pyrene-1-ylmethyl)-L-phenylalanine/Cu2⁺ | 2 × 10−8 M | 5 × 104 M−1 | [41] |
| Fluorescent probe derived from Pyrene/Cu2+ | 0.16 μM | 6.5 × 105 M−1 | [42] |
| Schiff base PY-SB/Sn2+ | 5.4 µM | 2 × 104 M−1 | [9] |
| Pyrene-oligo(azomethine)/Sn2+ | 4.24 nM | 2.40 × 104 M−1 | [8] |
| DPyH9/Cu2+ | 4.73 × 10−5 M | 4.03 × 107 M−1 | this work |
| DPyH9/Sn2+ | 1.61 × 10−5 M | 4.51 × 106 M−1 | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Homocianu, M.; Perju, E. Photophysical Properties and Metal Ion Sensing of a Pyrene-Based Liquid Crystalline Dimer. Int. J. Mol. Sci. 2025, 26, 2566. https://doi.org/10.3390/ijms26062566
Homocianu M, Perju E. Photophysical Properties and Metal Ion Sensing of a Pyrene-Based Liquid Crystalline Dimer. International Journal of Molecular Sciences. 2025; 26(6):2566. https://doi.org/10.3390/ijms26062566
Chicago/Turabian StyleHomocianu, Mihaela, and Elena Perju. 2025. "Photophysical Properties and Metal Ion Sensing of a Pyrene-Based Liquid Crystalline Dimer" International Journal of Molecular Sciences 26, no. 6: 2566. https://doi.org/10.3390/ijms26062566
APA StyleHomocianu, M., & Perju, E. (2025). Photophysical Properties and Metal Ion Sensing of a Pyrene-Based Liquid Crystalline Dimer. International Journal of Molecular Sciences, 26(6), 2566. https://doi.org/10.3390/ijms26062566






