New Perspective on Aqueous Humor Circulation: Retina Takes the Lead
Abstract
:1. Introduction
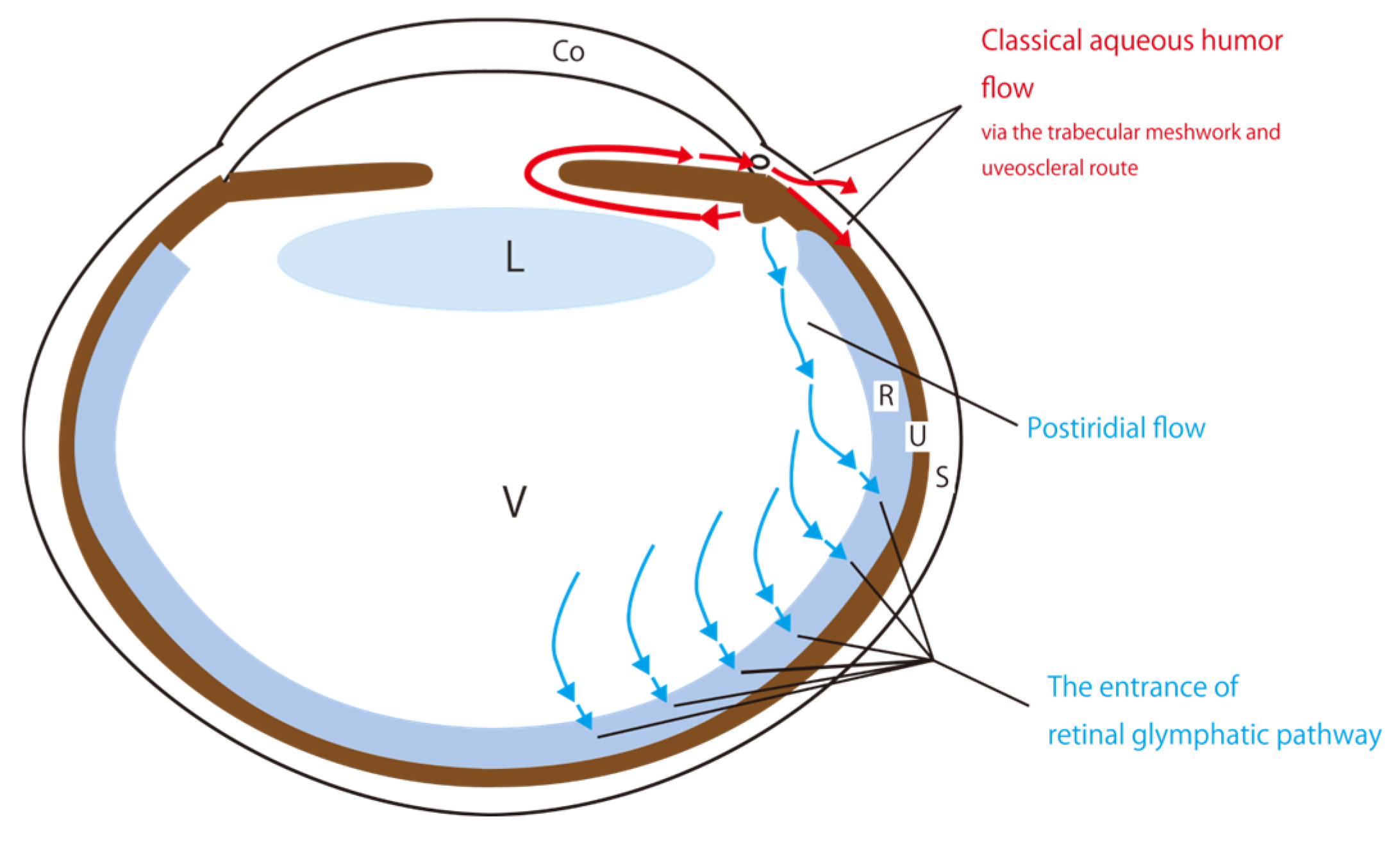
2. A New Perspective on Aqueous Humor Circulation
2.1. A Classical Perspective on Aqueous Humor Circulation
2.2. Proposal of the Retinal Glymphatic Pathway Analogy to the Cerebral Glymphatic Flow
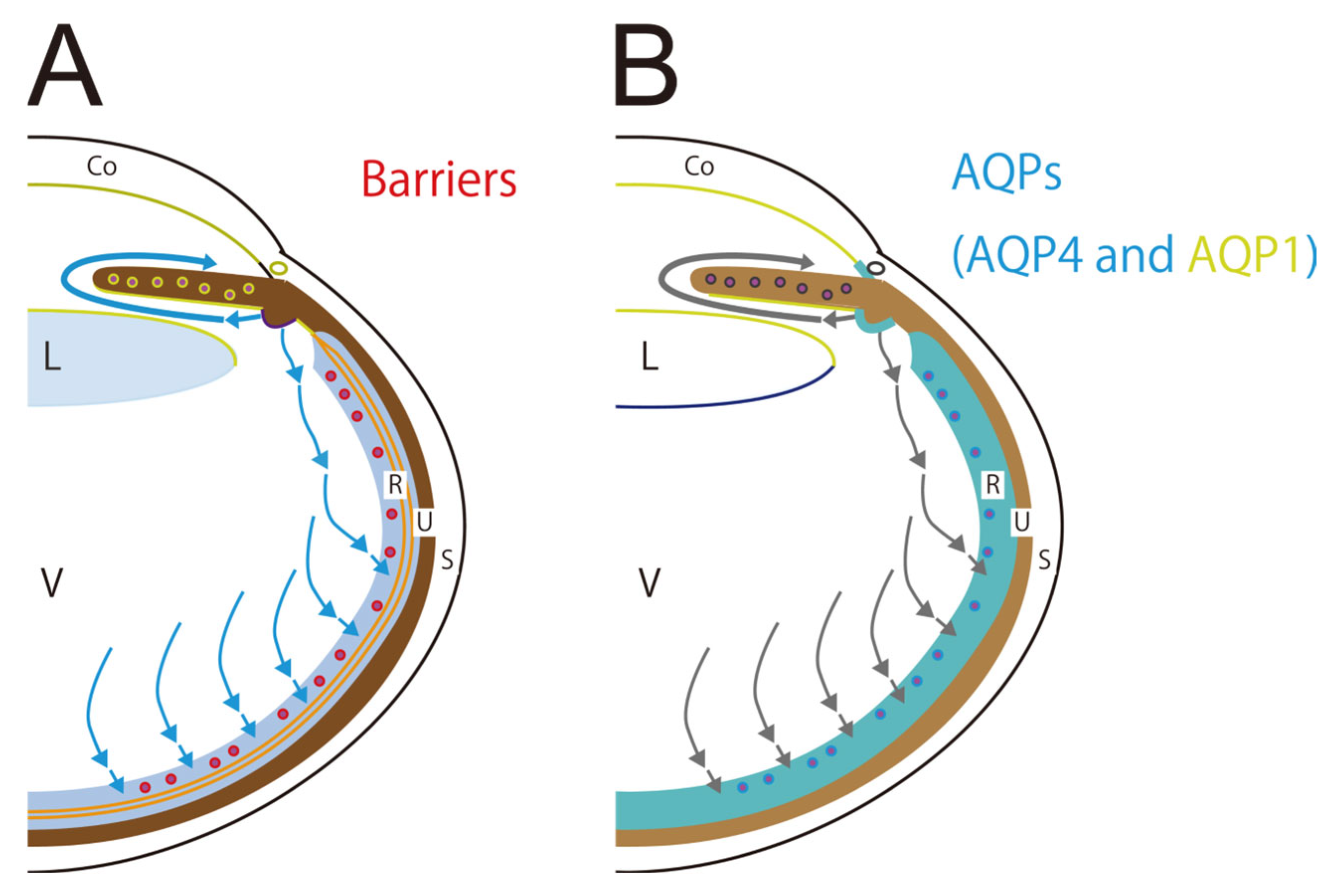
2.3. Aquaporins and Barriers Regulate Aqueous Humor and Cerebrospinal Fluid Circulations
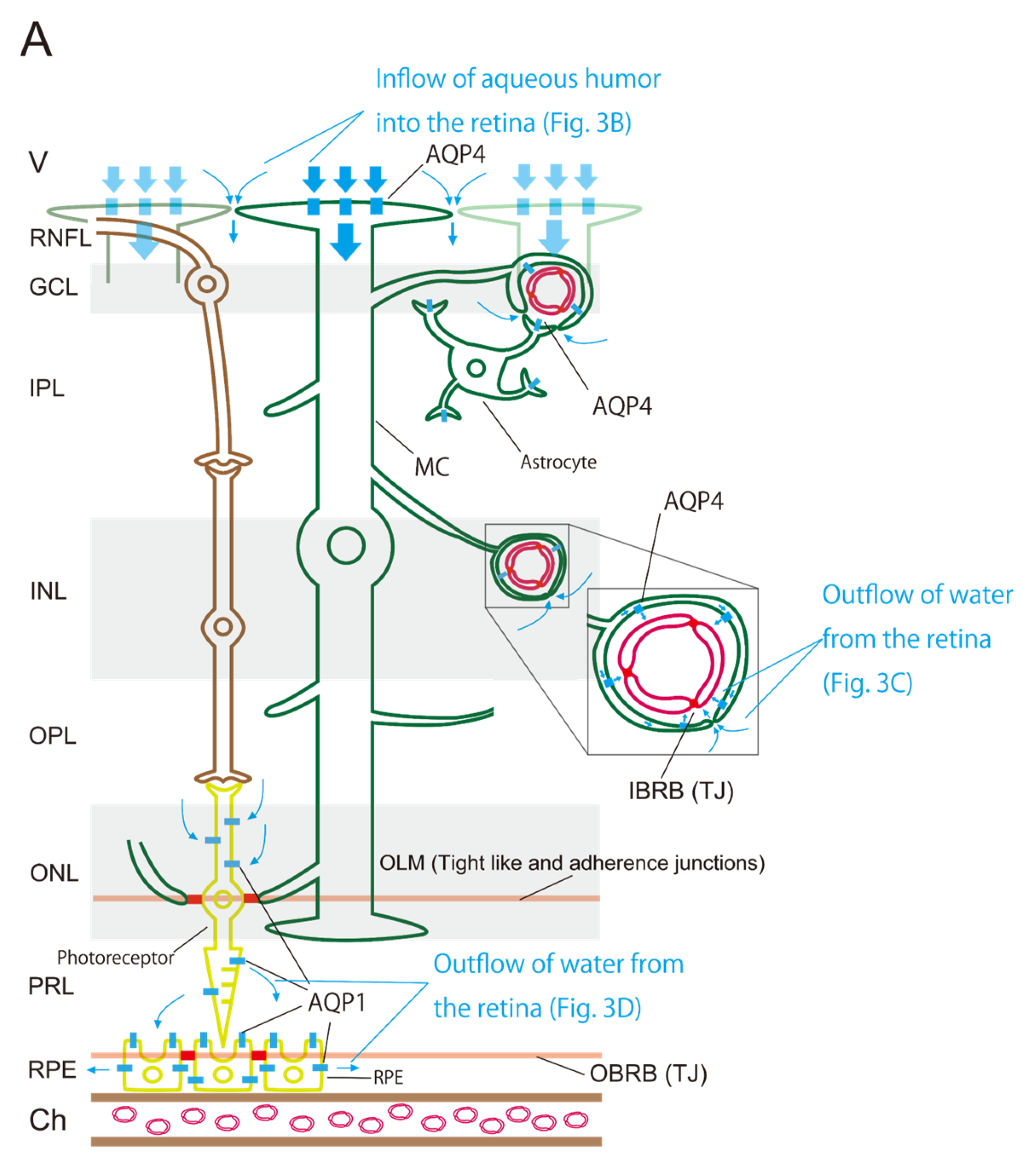
3. Water Dynamics in the Retina
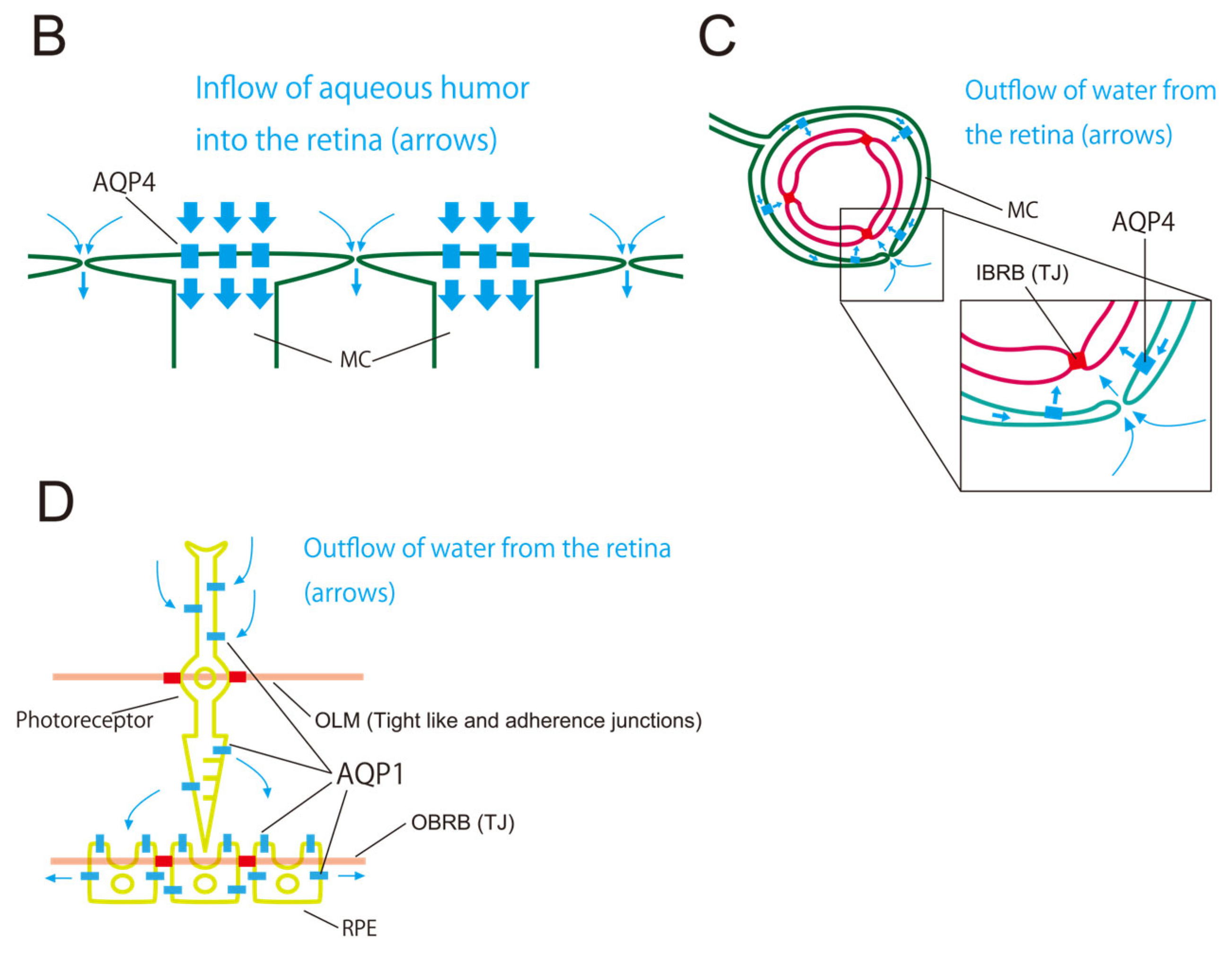
3.1. Three Barriers and Two AQPs
3.2. Retinal AQP-4 in the Pericapillary Space
3.3. Driving Force of the Retinal Glymphatic Pathway
3.4. Retinal AQP-1 in Photoreceptors and RPE
4. Role of the Retinal Glymphatic Pathway
4.1. Age-Dependent Changes in Water Inflow Regulation into the Vitreous Body
4.2. Age-Related Macular Degeneration and Glaucomatous Optic Neuropathy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AQP | Aquaporin |
| AQP1 | Aquaporin-1 |
| AQP4 | Aquaporin-4 |
| AMD | Age-related macular degeneration |
| CE | Ciliary Epithelium |
| D2O | Deuterium Oxide |
| GON | Glaucomatous optic neuropathy |
| IBRB | Inner Blood–Retinal Barrier |
| ILM | Inner Limiting Membrane |
| MCs | Müller Cells |
| MRI | Magnetic Resonance Imaging |
| OBRB | Outer Blood–Retinal Barrier |
| OLM | Outer Limiting Membrane |
| RPE | Retinal Pigment Epithelium |
| TJs | Tight Junctions |
| TM | Trabecular Meshwork |
| VRS | Virchow–Robin Space |
References
- Tsuboi, S. Measurement of the volume flow and hydraulic conductivity across the isolated dog retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1776–1782. [Google Scholar]
- Ueki, S.; Suzuki, Y.; Igarashi, H. Retinal aquaporin-4 and regulation of water inflow into the vitreous body. Investig. Ophthalmol. Vis. Sci. 2021, 62, 24. [Google Scholar] [CrossRef]
- Iliff, J.J.; Wang, M.; Liao, Y.; Plogg, B.A.; Peng, W.; Gundersen, G.A.; Benveniste, H.; Vates, G.E.; Deane, R.; Goldman, S.A.; et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012, 4, 147ra111. [Google Scholar] [CrossRef]
- Bohr, T.; Hjorth, P.G.; Holst, S.C.; Hrabětová, S.; Kiviniemi, V.; Lilius, T.; Lundgaard, I.; Mardal, K.-A.; Martens, E.A.; Mori, Y.; et al. The glymphatic system: Current understanding and modeling. iScience 2022, 25, 104987. [Google Scholar] [CrossRef]
- Dowling, J.E. The Retina an Approachable Part of the Brain, rev. ed.; The Belknap Press of Harvard University Press: Cambridge, MA, USA, 2012; pp. 285–304. [Google Scholar]
- Stamer, W.D.; Kaufman, P.L.; Delamore, N.A. Production and flow of aqueous humor. In Adler’s Physiology of the Eye, 12th ed.; Levin, L.A., Kaufman, P.L., Hartnett, M.E., Eds.; Elsevier: Philadelphia, PA, USA, 2024; pp. 245–283. [Google Scholar]
- Scott, J.E. The chemical morphology of the vitreous. Eye 1992, 6, 553–555. [Google Scholar] [CrossRef]
- Araie, M.; Sugiura, Y.; Sakurai, M.; Oshika, T. Effect of systemic acetazolamide on the fluid movement across the aqueous-vitreous interface. Exp. Eye Res. 1991, 53, 285–293. [Google Scholar] [CrossRef]
- Smith, D.W.; Lee, C.-J.; Gardiner, B.S. No flow through the vitreous humor: How strong is the evidence? Prog. Retin. Eye Res. 2020, 78, 100845. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Gardiner, B.S. Estimating outflow facility through pressure dependent pathways of the human eye. PLoS ONE 2017, 12, e0188769. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins. Curr. Biol. 2013, 23, R52–R55. [Google Scholar] [CrossRef]
- Ishibashi, K.; Morishita, Y.; Tanaka, Y. The evolutionary aspects of aquaporin family. Adv. Exp. Med. Biol. 2017, 969, 35–50. [Google Scholar]
- Verkman, A.S.; Ruiz-Ederra, J.; Levin, M.H. Functions of aquaporins in the eye. Prog. Retin. Eye Res. 2008, 27, 420–433. [Google Scholar] [CrossRef] [PubMed]
- Nagelhus, E.A.; Horio, Y.; Inanobe, A.; Fujita, A.; Haug, F.M.; Nielsen, S.; Kurachi, Y.; Ottersen, O.P. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia 1999, 26, 47–54. [Google Scholar] [CrossRef]
- Katoozi, S.; Rao, S.B.; Skauli, N.; Froehner, S.C.; Ottersen, O.P.; Adams, M.E.; Amiry-Moghaddam, M. Functional specialization of retinal Müller cell endfeet depends on an interplay between two syntrophin isoforms. Mol. Brain 2020, 13, 40. [Google Scholar] [CrossRef]
- Ramírez, J.M.; Triviño, A.; Ramírez, A.I.; Salazar, J.J.; García-Sánchez, J. Structural specializations of human retinal glial cells. Vision Res. 1996, 36, 2029–2036. [Google Scholar] [CrossRef]
- Motulsky, E.; Koch, P.; Janssens, S.; Liénart, M.; Vanbellinghen, A.-M.; Bolaky, N.; Chan, C.-C.; Caspers, L.; Martin-Martinez, M.-D.; Xu, H.; et al. Aquaporin expression in blood-retinal barrier cells during experimental autoimmune uveitis. Mol. Vis. 2010, 16, 602–610. [Google Scholar] [CrossRef]
- Vogler, S.; Pannicke, T.; Hollborn, M.; Grosche, A.; Busch, S.; Hoffmann, S.; Wiedemann, P.; Reichenbach, A.; Hammes, H.-P.; Bringmann, A. Müller cell reactivity in response to photoreceptor degeneration in rats with defective polycystin-2. PLoS ONE 2014, 8, e61631. [Google Scholar] [CrossRef]
- Thiagarajah, J.R.; Verkman, A.S. Aquaporin deletion in mice reduces corneal water permeability and delays restoration of transparency after swelling. J. Biol. Chem. 2002, 277, 19139–19144. [Google Scholar] [CrossRef]
- Schey, K.L.; Gletten, R.B.; O’Neale, C.V.T.; Wang, Z.; Petrova, R.S.; Donaldson, P.J. Lens aquaporins in health and disease: Location is everything! Front. Physiol. 2022, 13, 882550. [Google Scholar] [CrossRef]
- Stamer, W.D.; Chan, D.W.H.; Conley, S.M.; Coons, S.; Ethier, C.R. Aquaporin-1 expression and conventional aqueous outflow in human eyes. Exp. Eye Res. 2008, 87, 349–355. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Watanabe, T.; Hirakata, A.; Hida, T. Localization and ontogeny of aquaporin-1 and -4 expression in iris and ciliary epithelial cells in rats. Cell Tissue Res. 2006, 325, 101–109. [Google Scholar] [CrossRef]
- Huang, O.S.; Seet, L.-F.; Ho, H.W.; Chu, S.W.; Narayanaswamy, A.; Perera, S.A.; Husain, R.; Aung, T.; Wong, T.T. Altered iris aquaporin expression and aqueous humor osmolality in glaucoma. Investig. Ophthalmol. Vis. Sci. 2021, 62, 34. [Google Scholar] [CrossRef] [PubMed]
- Nakada, T.; Kwee, I.L. Fluid dynamics inside the brain barrier: Current concept of interstitial flow, glymphatic flow, and cerebrospinal fluid circulation in the brain. Neuroscientist 2019, 25, 155–166. [Google Scholar] [CrossRef] [PubMed]
- Reeves, B.C.; Karimy, J.K.; Kundishora, A.J.; Mestre, H.; Cerci, H.M.; Matouk, C.; Alper, S.L.; Lundgaard, I.; Nedergaard, M.; Kahle, K.T. Glymphatic system impairment in Alzheimer’s disease and idiopathic normal pressure hydrocephalus. Trends Mol. Med. 2020, 26, 285–295. [Google Scholar] [CrossRef]
- Buccellato, F.R.; D’Anca, M.; Serpente, M.; Arighi, A.; Galimberti, D. The role of glymphatic system in Alzheimer’s and Parkinson’s disease pathogenesis. Biomedicines 2022, 10, 2261. [Google Scholar] [CrossRef]
- Igarashi, H.; Tsujita, M.; Kwee, I.L.; Nakada, T. Water influx into cerebrospinal fluid is primarily controlled by aquaporin-4, not by aquaporin-1: 17O JJVCPE MRI study in knockout mice. NeuroReport 2014, 25, 39–43. [Google Scholar] [CrossRef]
- Deng, S.; Huang, S.; Yang, A.; Muir, E.R. Imaging ocular water inflow in the mouse with deuterium oxide MRI. Magn. Reson. Imaging 2023, 101, 47–53. [Google Scholar] [CrossRef]
- Harilal, S.; Jose, J.; Parambi, D.G.T.; Kumar, R.; Unnikrishnan, M.K.; Uddin, M.S.; Mathew, G.E.; Pratap, R.; Marathakam, A.; Mathew, B. Revisiting the Blood-Brain Barrier: A Hard Nut to Crack in the Transportation of Drug Molecules. Brain Res. Bull. 2020, 160, 121–140. [Google Scholar] [CrossRef]
- Tsukita, S.; Furuse, M. Occludin and claudins in tight-junction strands: Leading or supporting players? Trends Cell Biol. 1999, 9, 268–273. [Google Scholar] [CrossRef]
- Daruich, A.; Matet, A.; Moulin, A.; Kowalczuk, L.; Nicolas, M.; Sellam, A.; Rothschild, P.-R.; Omri, S.; Gélizé, E.; Jonet, L.; et al. Mechanisms of macular edema: Beyond the surface. Prog. Retin. Eye Res. 2018, 63, 20–68. [Google Scholar] [CrossRef]
- Díaz-Coránguez, M.; Ramos, C.; Antonetti, D.A. The inner blood-retinal barrier: Cellular basis and development. Vision Res. 2017, 139, 123–137. [Google Scholar] [CrossRef]
- Ramachandran, C.; Srinivas, S.P. Formation and disassembly of adherens and tight junctions in the corneal endothelium: Regulation by actomyosin contraction. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Zampighi, G.A.; Simon, S.A.; Hall, J.E. The specialized junctions of the lens. Int. Rev. Cytol. 1992, 136, 185–225. [Google Scholar] [PubMed]
- Campbell, M.; Cassidy, P.S.; O’Callaghan, J.; Crosbie, D.E.; Humphries, P. Manipulating ocular endothelial tight junctions: Applications in treatment of retinal disease pathology and ocular hypertension. Prog. Retin. Eye Res. 2018, 62, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Schlingemann, R.O.; Hofman, P.; Klooster, J.; Blaauwgeers, H.G.; Van der Gaag, R.; Vrensen, G.F. Ciliary muscle capillaries have blood-tissue barrier characteristics. Exp. Eye Res. 1998, 66, 747–754. [Google Scholar] [CrossRef]
- Hogan, M.J.; Alvarado, J.A.; Weddell, J.E. Chapter Six Iris and anterior chamber. In Histology of the Human Eye, 1st ed.; W. B. Saunders Company: Philadelphia, PA, USA, 1971; pp. 202–259. [Google Scholar]
- Hogan, M.J.; Alvarado, J.A.; Weddell, J.E. Chapter Seven Ciliary body and posterior chamber. In Histology of the Human Eye, 1st ed.; W. B. Saunders Company: Philadelphia, PA, USA, 1971; pp. 260–319. [Google Scholar]
- Noske, W.; Levarlet, B.; Kreusel, K.M.; Fromm, M.; Hirsch, M. Tight Junctions and Paracellular Permeability in Cultured Bovine Corneal Endothelial Cells. Graefes Arch. Clin. Exp. Ophthalmol. 1994, 232, 608–613. [Google Scholar] [CrossRef]
- Cassidy, P.S.; Kelly, R.A.; Reina-Torres, E.; Sherwood, J.M.; Humphries, M.M.; Kiang, A.-S.; Farrar, G.J.; O’Brien, C.; Campbell, M.; Stamer, W.D.; et al. siRNA Targeting Schlemm’s Canal Endothelial Tight Junctions Enhances Outflow Facility and Reduces IOP in a Steroid-Induced OHT Rodent Model. Mol. Ther.—Methods Clin. Dev. 2021, 20, 86–94. [Google Scholar] [CrossRef]
- Zhang, D.; Vetrivel, L.; Verkman, A.S. Aquaporin deletion in mice reduces intraocular pressure and aqueous fluid production. J. Gen. Physiol. 2002, 119, 561–569. [Google Scholar] [CrossRef]
- Mathieu, E.; Gupta, N.; Ahari, A.; Zhou, X.; Hanna, J.; Yücel, Y.H. Evidence for cerebrospinal fluid entry into the optic nerve via a glymphatic pathway. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4784–4791. [Google Scholar] [CrossRef]
- Alleva, K.; Chara, O.; Amodeo, G. Aquaporins: Another piece in the osmotic puzzle. FEBS Lett. 2012, 586, 2991–2999. [Google Scholar] [CrossRef]
- Moseley, H.; Foulds, W.S.; Allan, D.; Kyle, P.M. Routes of clearance of radioactive water from the rabbit vitreous. Br. J. Ophthalmol. 1984, 68, 145–151. [Google Scholar] [CrossRef]
- Ueki, S.; Suzuki, Y.; Nakamura, Y.; Igarashi, H. Age-dependent changes in regulation of water inflow into the vitreous body. Investig. Ophthalmol. Vis. Sci. 2023, 64, 22. [Google Scholar] [CrossRef] [PubMed]
- Hokari, M.; Yokoseki, A.; Arakawa, M.; Saji, E.; Yanagawa, K.; Yanagimura, F.; Toyoshima, Y.; Okamoto, K.; Ueki, S.; Hatase, T.; et al. Clinicopathological features in anterior visual pathway in neuromyelitis optica. Ann. Neurol. 2016, 79, 605–624. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, D.M.; Simon, M.; Haswell, J.D.; D’Abreo, D.; Murchison, C.; Quinn, J.F.; Grafe, M.R.; Woltjer, R.L.; Kaye, J.; Iliff, J.J. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017, 74, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Nakamura, Y.; Igarashi, H. Blood cerebrospinal fluid barrier function disturbance can be followed by amyloid-β accumulation. J. Clin. Med. 2022, 11, 6118. [Google Scholar] [CrossRef]
- Iliff, J.J.; Chen, M.J.; Plog, B.A.; Zeppenfeld, D.M.; Soltero, M.; Yang, L.; Singh, I.; Deane, R.; Nedergaard, M. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J. Neurosci. 2014, 34, 16180–16193. [Google Scholar] [CrossRef]
- Parihar, M.S.; Brewer, G.J. Amyloid-β as a modulator of synaptic plasticity. J. Alzheimer’s Dis. 2010, 22, 741–763. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, R.; Sterling, K.; Song, W. Amyloid β-based therapy for Alzheimer’s disease: Challenges, successes and future. Signal Transduct. Target. Ther. 2023, 8, 248. [Google Scholar] [CrossRef]
- Dentchev, T.; Milam, A.H.; Lee, V.M.-Y.; Trojanowski, J.Q.; Dunaief, J.L. Amyloid-Beta is found in drusen from some age-related macular degeneration retinas, but not in drusen from normal retinas. Mol. Vis. 2003, 9, 184–190. [Google Scholar]
- Christensen, J.; Yamakawa, G.R.; Shultz, S.R.; Mychasiuk, R. Is the glymphatic system the missing link between sleep impairments and neurological disorders? examining the implications and uncertainties. Prog. Neurobiol. 2021, 198, 101917. [Google Scholar] [CrossRef]
- Reddy, O.C.; van der Werf, Y.D. The sleeping brain: Harnessing the power of the glymphatic system through lifestyle choices. Brain Sci. 2020, 10, 868. [Google Scholar] [CrossRef]
- Lei, S.; Liu, Z.; Li, H. Sleep duration and age-related macular degeneration: A cross-sectional and Mendelian randomization study. Front. Aging Neurosci. 2023, 15, 1247413. [Google Scholar] [CrossRef] [PubMed]
- Furashova, O.; Engelmann, K. To peel or not to peel: Pars plana vitrectomy with macular membrane peel in eyes with abnormalities of vitreomacular interface and coexisting dry age-related macular degeneration. Clin. Ophthalmol. 2020, 14, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Liao, H.; Chen, H.; Deng, S.; Jia, Y.; Deng, C.; Lin, J.; Ge, J.; Zhuo, Y. Elevated intraocular pressure induces amyloid-β deposition and tauopathy in the lateral geniculate nucleus in a monkey model of glaucoma. Investig. Ophthalmol. Vis. Sci. 2017, 58, 5434–5443. [Google Scholar] [CrossRef]
- Ito, Y.; Shimazawa, M.; Tsuruma, K.; Mayama, C.; Ishii, K.; Onoe, H.; Aihara, M.; Araie, M.; Hara, H. Induction of amyloid-β(1-42) in the retina and optic nerve head of chronic ocular hypertensive monkeys. Mol. Vis. 2012, 18, 2647–2657. [Google Scholar]
- Cappelli, F.; Caudano, F.; Marenco, M.; Testa, V.; Masala, A.; Sindaco, D.; Macrì, A.; Traverso, C.E.; Iester, M.; Ricciarelli, R. Evaluating the correlation between Alzheimer’s amyloid-β peptides and glaucoma in human aqueous humor. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Mancino, R.; Martucci, A.; Cesareo, M.; Giannini, C.; Corasaniti, M.T.; Bagetta, G.; Nucci, C. Glaucoma and Alzheimer disease: One age-related neurodegenerative disease of the brain. Curr. Neuropharmacol. 2018, 16, 971–977. [Google Scholar] [CrossRef]
- Wang, X.; Lou, N.; Eberhardt, A.; Yang, Y.; Kusk, P.; Xu, Q.; Förstera, B.; Peng, S.; Shi, M.; Ladrón-de-Guevara, A.; et al. An ocular glymphatic clearance system removes β-amyloid from the rodent eye. Sci. Transl. Med. 2020, 12, eaaw3210. [Google Scholar] [CrossRef]
- Wang, X.; Delle, C.; Peng, W.; Plá, V.; Giannetto, M.; Kusk, P.; Sigurdsson, B.; Sakurai, S.; Sweeney, A.; Sun, Q.; et al. Age- and glaucoma-induced changes to the ocular glymphatic system. Neurobiol. Dis. 2023, 188, 106322. [Google Scholar] [CrossRef]
| AMD | Amyloid-β is a component of intraretinal drusen [52]. |
| Sleep promotes cerebral glymphatic flow [53,54]. | |
| A link between AMD and sleep disorders [55]. | |
| Inner limiting membrane peeling exacerbates choroidal neovascularization [56]. | |
| GON | In intraocular pressure elevation models, amyloid-β is detected in the retina, optic nerve, and lateral geniculate body [57,58]. |
| The total protein levels in the anterior chamber are higher in patients with GON [59]. | |
| GON is more prevalent in patients with Alzheimer’s disease [60]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueki, S.; Suzuki, Y. New Perspective on Aqueous Humor Circulation: Retina Takes the Lead. Int. J. Mol. Sci. 2025, 26, 2645. https://doi.org/10.3390/ijms26062645
Ueki S, Suzuki Y. New Perspective on Aqueous Humor Circulation: Retina Takes the Lead. International Journal of Molecular Sciences. 2025; 26(6):2645. https://doi.org/10.3390/ijms26062645
Chicago/Turabian StyleUeki, Satoshi, and Yuji Suzuki. 2025. "New Perspective on Aqueous Humor Circulation: Retina Takes the Lead" International Journal of Molecular Sciences 26, no. 6: 2645. https://doi.org/10.3390/ijms26062645
APA StyleUeki, S., & Suzuki, Y. (2025). New Perspective on Aqueous Humor Circulation: Retina Takes the Lead. International Journal of Molecular Sciences, 26(6), 2645. https://doi.org/10.3390/ijms26062645







