Seborrheic Dermatitis: Exploring the Complex Interplay with Malassezia
Abstract
:1. Introduction
- Seborrheic Dermatitis: Malassezia, Immunity, or Both?
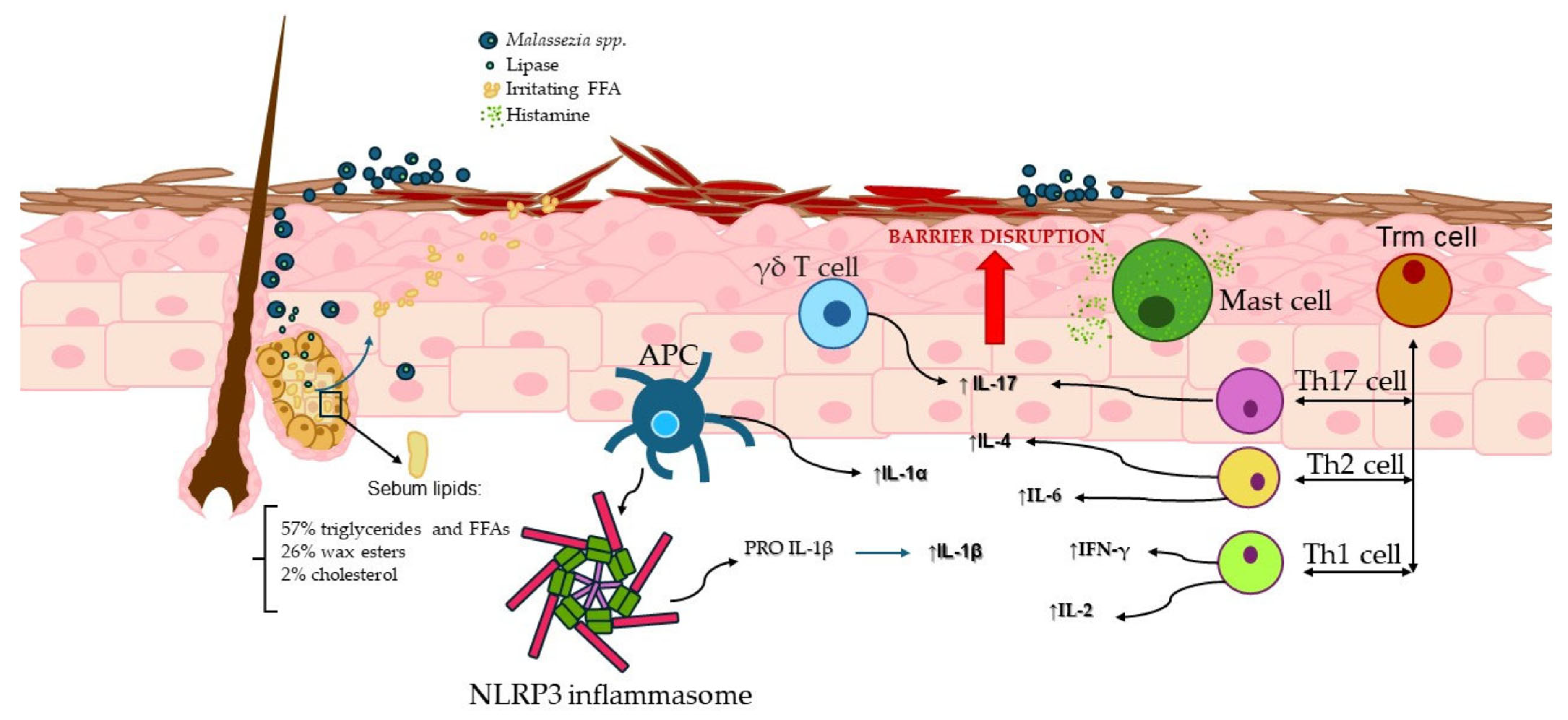
2. Skin Barrier
3. Sebaceous Glands
4. The Innate Immune System Involvement
4.1. γδ T Cells and the MPZL3 Gene
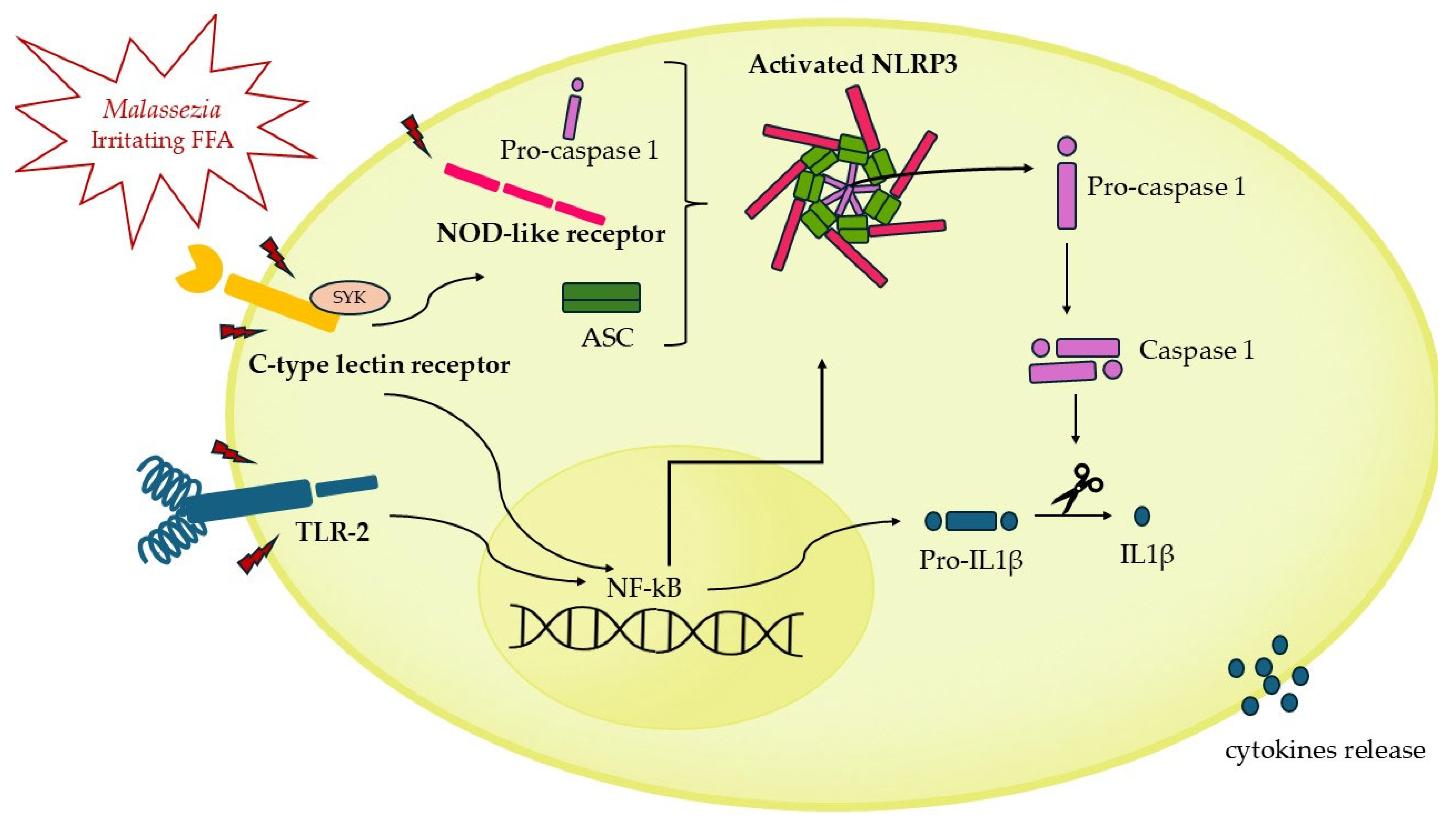
4.2. NLRP3 Inflammasome Activation
4.3. Mast Cells Potential Implication in Seborrheic Dermatitis
5. Adaptive Immunity
6. Th17 and IL-17: Potential Link to Seborrheic Dermatitis?
7. Treatment and Future Perspectives
7.1. Topical Antifungal Agents
7.2. Systemic Antifungal Agents
7.3. Anti-Inflammatory Agents
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APC | Antigen-presenting cell |
| FFA | Free fatty acid |
| FAS | Fatty acid synthase |
| HIV/AIDS | Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome |
| IFNg | Interferon-gamma |
| IL-17 | Interleukin 17 |
| MC | Mast cell |
| MRSD | Malassezia-related skin disease |
| PAMPs | Pathogen-associated molecular patterns |
| PRRs | Pattern recognition receptors |
| SCF | Stem cell factor |
| SD | Seborrheic dermatitis |
| SGs | Sebaceous glands |
| TLR2 | Toll-like receptor 2 |
| TEWL | Transepidermal water loss |
| ZNF750 | Zinc finger 750 |
References
- Dall’Oglio, F.; Nasca, M.R.; Gerbino, C.; Micali, G. An Overview of the Diagnosis and Management of Seborrheic Dermatitis. Clin. Cosmet. Investig. Dermatol. 2022, 15, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.K.; Bluhm, R. Seborrheic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 13–20. [Google Scholar] [CrossRef] [PubMed]
- De Avelar Breunig, J.; de Almeida, H.L., Jr.; Duquia, R.P.; Souza, P.R.M.; Staub, H.L. Scalp seborrheic dermatitis: Prevalence and associated factors in male adolescents. Int. J. Dermatol. 2012, 51, 46–49. [Google Scholar] [CrossRef] [PubMed]
- Dessinioti, C.; Katsambas, A. Seborrheic dermatitis: Etiology, risk factors, and treatments: Facts and controversies. Clin. Dermatol. 2013, 31, 343. [Google Scholar] [CrossRef]
- Laurence, M.; Benito-León, J.; Calon, F. Malassezia and Parkinson’s Disease. Front. Neurol. 2019, 10, 758. [Google Scholar] [CrossRef]
- Rietcheck, H.R.; Maghfour, J.; Rundle, C.W.; Husayn, S.S.; Presley, C.L.; Sillau, S.H.; Liu, Y.; Leehey, M.A.; Dunnick, C.A.; Dellavalle, R.P. A Review of the Current Evidence Connecting Seborrheic Dermatitis and Parkinson’s Disease and the Potential Role of Oral Cannabinoids. Dermatology 2021, 237, 872–877. [Google Scholar] [CrossRef]
- DeAngelis, Y.M.; Gemmer, C.M.; Kaczvinsky, J.R.; Kenneally, D.C.; Schwartz, J.R.; Dawson, T.L., Jr. Three etiologic facets of dandruff and seborrheic dermatitis: Malassezia fungi, sebaceous lipids, and individual sensitivity. J. Investig. Dermatol. Symp. Proc. 2005, 10, 295–297. [Google Scholar] [CrossRef]
- Sasikumar, J.; Ebrahim, R.A.; Das, S.P. Diverse Colonisation and Disease Associations of the Human Commensal Malassezia: Our Body’s Secret Tenant. Mycoses 2025, 68, e70014. [Google Scholar] [CrossRef]
- Hamdino, M.; Saudy, A.A.; El-Shahed, L.H.; Taha, M. Identification of Malassezia species isolated from some Malassezia associated skin diseases. J. Mycol. Med. 2022, 32, 101301. [Google Scholar] [CrossRef]
- Honnavar, P.; Chakrabarti, A.; Joseph, J.; Thakur, S.; Dogra, S.; Lakshmi, P.V.M.; Rudramurthy, S.M. Molecular epidemiology of seborrheic dermatitis/dandruff associated Malassezia species from northern India. Med. Mycol. 2024, 62, myae104. [Google Scholar] [CrossRef]
- Hiruma, J.; Nojo, H.; Hiruma, M.; Sugita, T.; Makimura, K.; Harada, K.; Kano, R. Malassezia polysorbatinonusus sp. nov., a Novel Isolate from a Japanese Patient with Seborrheic Dermatitis. Mycopathologia 2025, 190, 15. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Feng, Y.; Liu, C.; Yang, Z.; de Hoog, S.; Qu, Y.; Chen, B.; Li, D.; Xiong, H.; Shi, D. Presence of Malassezia Hyphae Is Correlated with Pathogenesis of Seborrheic Dermatitis. Microbiol. Spectr. 2022, 10, e0116921-21. [Google Scholar] [CrossRef] [PubMed]
- Jung, W.H. Alteration in skin mycobiome due to atopic dermatitis and seborrheic dermatitis. Biophys. Rev. 2023, 4, 011309. [Google Scholar] [CrossRef]
- Tao, R.; Li, R.; Wang, R. Skin microbiome alterations in seborrheic dermatitis and dandruff: A systematic review. Exp. Dermatol. 2021, 30, 1546–1553. [Google Scholar] [CrossRef]
- Chang, C.H.; Chovatiya, R. More yeast, more problems?: Reevaluating the role of Malassezia in seborrheic dermatitis. Arch. Dermatol. Res. 2024, 316, 100–107. [Google Scholar] [CrossRef]
- Gaitanis, G.; Magiatis, P.; Hantschke, M.; Bassukas, I.D.; Velegraki, A. The Malassezia genus in skin and systemic diseases. Clin. Microbiol. Rev. 2012, 25, 106–141. [Google Scholar] [CrossRef]
- Muñoz-Pérez, M.A.; Rodriguez-Pichardo, A.; Camacho, F.; Colmenero, M.A. Dermatological findings correlated with CD4 lymphocyte counts in a prospective 3 year study of 1161 patients with human immunodeficiency virus disease predominantly acquired through intravenous drug abuse. Br. J. Dermatol. 1998, 139, 33–39. [Google Scholar] [CrossRef]
- Chimbetete, T.; Buck, C.; Choshi, P.; Selim, R.; Pedretti, S.; Divito, S.J.; Phillips, E.J.; Lehloenya, R.; Peter, J. HIV-Associated Immune Dysregulation in the Skin: A Crucible for Exaggerated Inflammation and Hypersensitivity. J. Investig. Dermatol. 2023, 143, 362–373. [Google Scholar] [CrossRef]
- Proksch, E.; Brandner, J.M.; Jensen, J. The skin: An indispensable barrier. Exp. Dermatol. 2008, 17, 1063–1072. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Borda, L.J.; Miteva, M.; Paus, R. Seborrheic dermatitis—Looking beyond Malassezia. Exp. Dermatol. 2019, 28, 991. [Google Scholar] [CrossRef]
- Goh, J.P.; Ruchti, F.; Poh, S.E.; Koh, W.L.; Tan, K.Y.; Lim, Y.T.; Thng, S.T.; Sobota, R.M.; Hoon, S.S.; Liu, C.; et al. The human pathobiont Malassezia furfur secreted protease Mfsap1 regulates cell dispersal and exacerbates skin inflammation. Proc. Natl. Acad. Sci. USA 2022, 119, e2212533119. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Yu, B.S.; Heo, Y.M.; Kyung, S.; Lee, K.-E.; Kim, S.; Kang, S.; Han, K.; Kim, D.H. Characteristics of Malassezia furfur at various pH and effects of Malassezia lipids on skin cells. Appl. Microbiol. Biotechnol. 2024, 108, 455. [Google Scholar] [CrossRef] [PubMed]
- Uche, L.E.; Gooris, G.S.; Bouwstra, J.A.; Beddoes, C.M. High concentration of the ester-linked ω-hydroxy ceramide increases the permeability in skin lipid model membranes. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183487. [Google Scholar] [CrossRef]
- Rousel, J.; Nădăban, A.; Saghari, M.; Pagan, L.; Zhuparris, A.; Theelen, B.; Gambrah, T.; van der Wall, H.E.C.; Vreeken, R.J.; Feiss, G.L.; et al. Lesional skin of seborrheic dermatitis patients is characterized by skin barrier dysfunction and correlating alterations in the stratum corneum ceramide composition. Exp. Dermatol. 2023, 33, e14952. [Google Scholar] [CrossRef]
- Desai, S.; McCormick, E.; Friedman, A. An Up-to-Date Approach to the Management of Seborrheic Dermatitis. J. Drugs Dermatol. 2022, 21, 1373–1374. [Google Scholar]
- Picardo, M.; Mastrofrancesco, A.; Bíró, T. Sebaceous gland-a major player in skin homoeostasis. Exp. Dermatol. 2015, 24, 485–486. [Google Scholar] [CrossRef]
- Cavallo, A.; Camera, E.; Bottillo, G.; Maiellaro, M.; Truglio, M.; Marini, F.; Chavagnac-Bonneville, M.; Fauger, A.; Perrier, E.; Pigliacelli, F.; et al. Biosignatures of defective sebaceous gland activity in sebum-rich and sebum-poor skin areas in adult atopic dermatitis. Exp. Dermatol. 2024, 33, e15066. [Google Scholar] [CrossRef]
- Okoro, O.E.; Adenle, A.; Ludovici, M.; Truglio, M.; Marini, F.; Camera, E. Lipidomics of facial sebum in the comparison between acne and non-acne adolescents with dark skin. Sci. Rep. 2021, 11, 16591. [Google Scholar] [CrossRef]
- Adalsteinsson, J.A.; Kaushik, S.; Muzumdar, S.; Guttman-Yassky, E.; Ungar, J. An update on the microbiology, immunology and genetics of seborrheic dermatitis. Exp. Dermatol. 2020, 29, 481. [Google Scholar] [CrossRef]
- Ro, B.I.; Dawson, T.L. The role of sebaceous gland activity and scalp microfloral metabolism in the etiology of seborrheic dermatitis and dandruff. J. Investig. Dermatol. Symp. Proc. 2005, 10, 194–197. [Google Scholar] [CrossRef]
- Picardo, M.; Ottaviani, M.; Camera, E.; Mastrofrancesco, A. Sebaceous gland lipids. Dermatoendocrinology 2009, 1, 68–71. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Valacchi, G. Surface lipids as multifunctional mediators of skin responses to environmental stimuli. Mediat. Inflamm. 2010, 2010, 321494. [Google Scholar] [CrossRef] [PubMed]
- DeAngelis, Y.M.; Saunders, C.W.; Johnstone, K.R.; Reeder, N.L.; Coleman, C.G.; Kaczvinsky, J.R.; Gale, C.; Walter, R.; Mekel, M.; Lacey, M.P.; et al. Isolation and Expression of a Malassezia globosa Lipase Gene, LIP1. J. Investig. Dermatol. 2007, 127, 2138–2146. [Google Scholar] [CrossRef] [PubMed]
- Shi, V.Y.; Leo, M.; Hassoun, L.; Chahal, D.S.; Maibach, H.I.; Sivamani, R.K. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 2015, 73, 856. [Google Scholar] [CrossRef]
- Jin, Z.; Song, Y.; He, L. A review of skin immune processes in acne. Front. Immunol. 2023, 14, 1324930. [Google Scholar] [CrossRef]
- Kawamura, T.; Ogawa, Y.; Aoki, R.; Shimada, S. Innate and intrinsic antiviral immunity in skin. J. Dermatol. Sci. 2014, 75, 159–166. [Google Scholar] [CrossRef]
- Miyachi, H.; Wakabayashi, S.; Sugihira, T.; Aoyama, R.; Saijo, S.; Koguchi-Yoshioka, H.; Fujimoto, M.; Núñez, G.; Matsue, H.; Nakamura, Y. Keratinocyte IL-36 Receptor/MyD88 Signaling Mediates Malassezia-Induced IL-17-Dependent Skin Inflammation. J. Infect. Dis. 2021, 223, 1753–1765. [Google Scholar] [CrossRef]
- Sparber, F.; LeibundGut-Landmann, S. Host Responses to Malassezia spp. in the Mammalian Skin. Front. Immunol. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Sato, Y.; Ogawa, E.; Okuyama, R. Role of Innate Immune Cells in Psoriasis. Int. J. Mol. Sci. 2020, 21, 6604. [Google Scholar] [CrossRef]
- Cai, Y.; Shen, X.; Ding, C.; Qi, C.; Li, K.; Li, X.; Jala, V.R.; Zhang, H.G.; Wang, T.; Zheng, J.; et al. Pivotal role of dermal IL-17-producing γδ T cells in skin inflammation. Immunity 2011, 35, 596–610. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Hirt, P.; Almastadi, M.; Mitchell, H.; Tomic-Canic, M.; Romero, L.; Garcia, D.; Strbo, N. Increased IL-17-expressing γδ T cells in seborrhoeic dermatitis-like lesions of the Mpzl3 knockout mice. Exp. Dermatol. 2018, 27, 1408. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Ruchti, F.; Tuor, M.; Mathew, L.; E McCarthy, N.; LeibundGut-Landmann, S. γδ T cells respond directly and selectively to the skin commensal yeast Malassezia for IL-17-dependent fungal control. PLoS Pathog. 2024, 20, e1011668. [Google Scholar] [CrossRef] [PubMed]
- Birnbaum, R.Y.; Zvulunov, A.; Hallel-Halevy, D.; Cagnano, E.; Finer, G.; Ofir, R.; Geiger, D.; Silberstein, E.; Feferman, Y.; Birk, O.S. Seborrhea-like dermatitis with psoriasiform elements caused by a mutation in ZNF750, encoding a putative C2H2 zinc finger protein. Nat. Genet. 2006, 38, 749–751. [Google Scholar] [CrossRef]
- Wikramanayake, T.C.; Nicu, C.; Gherardini, J.; Mello, A.C.; Chéret, J.; Paus, R. Mitochondrially Localized MPZL3 Functions as a Negative Regulator of Sebaceous Gland Size and Sebocyte Proliferation. J. Investig. Dermatol. 2022, 142, 2524–2527.e7. [Google Scholar] [CrossRef]
- Cohen, I.; Birnbaum, R.Y.; Leibson, K.; Taube, R.; Sivan, S.; Birk, O.S. ZNF750 is expressed in differentiated keratinocytes and regulates epidermal late differentiation genes. PLoS ONE 2012, 7, e42628. [Google Scholar] [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target. Ther. 2021, 6, 291. [Google Scholar] [CrossRef]
- Xu, J.; Núñez, G. The NLRP3 inflammasome: Activation and regulation. Trends Biochem. Sci. 2023, 48, 331–344. [Google Scholar] [CrossRef]
- Kistowska, M.; Fenini, G.; Jankovic, D.; Feldmeyer, L.; Kerl, K.; Bosshard, P.; Contassot, E.; French, L.E. Malassezia yeasts activate the NLRP3 inflammasome in antigen-presenting cells via Syk-kinase signalling. Exp. Dermatol. 2014, 23, 884–889. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805. [Google Scholar] [CrossRef]
- Park, H.R.; Oh, J.H.; Lee, Y.J.; Park, S.H.; Lee, Y.W.; Lee, S.; Kang, H.; Kim, J.E. Inflammasome-mediated Inflammation by Malassezia in human keratinocytes: A comparative analysis with different strains. Mycoses 2021, 64, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, D.D.; Baram, D.; Mekori, Y.A. Mast cells. Physiol. Rev. 1997, 77, 1033–1079. [Google Scholar] [CrossRef] [PubMed]
- Gurish, M.F.; Austen, K.F. Developmental origin and functional specialization of mast cell subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef]
- Wernersson, S.; Pejler, G. Mast cell secretory granules: Armed for battle. Nat. Rev. Immunol. 2014, 14, 478. [Google Scholar] [CrossRef]
- John, A.L.S.; Abraham, S.N. Innate immunity and its regulation by mast cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef]
- Voss, M.; Kotrba, J.; Gaffal, E.; Katsoulis-Dimitriou, K.; Dudeck, A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int. J. Mol. Sci. 2021, 22, 4589. [Google Scholar] [CrossRef]
- Selander, C.; Engblom, C.; Nilsson, G.; Scheynius, A.; Andersson, C.L. TLR2/MyD88-dependent and -independent activation of mast cell IgE responses by the skin commensal yeast Malassezia sympodialis. J. Immunol. 2009, 182, 4208–4216. [Google Scholar] [CrossRef]
- Hiragun, T.; Ishii, K.; Hiragun, M.; Suzuki, H.; Kan, T.; Mihara, S.; Yanase, Y.; Bartels, J.; Schröder, J.-M.; Hide, M. Fungal protein MGL_1304 in sweat is an allergen for atopic dermatitis patients. J. Allergy Clin. Immunol. 2013, 132, 608–615.e4. [Google Scholar] [CrossRef]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Messenger, A.G.; Tosti, A.; Todd, G.; Hordinsky, M.; Hay, R.J.; Wang, X.; Zachariae, C.; Kerr, K.M.; Henry, J.P.; et al. A Comprehensive Pathophysiology of Dandruff and Seborrheic Dermatitis—Towards a More Precise Definition of Scalp Health. Acta Derm. Venerol. 2013, 93, 131. [Google Scholar] [CrossRef]
- Pedrosa, A.F.; Lisboa, C.; Rodrigues, A.G. Malassezia infections: A medical conundrum. J. Am. Acad. Dermatol. 2014, 71, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Bergbrant, I.M.; Andersson, B.; Faergemann, J. Cell-mediated immunity to Malassezia furfur in patients with seborrhoeic dermatitis and pityriasis versicolor. Clin. Exp. Dermatol. 1999, 24, 402–406. [Google Scholar] [CrossRef] [PubMed]
- Faergemann, J.; Bergbrant, I.-M.; Dohsé, M.; Scott, A.; Westgate, G. Seborrhoeic dermatitis and Pityrosporum (Malassezia) folliculitis: Characterization of inflammatory cells and mediators in the skin by immunohistochemistry. Br. J. Dermatol. 2001, 144, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Molinero, L.L.; Gruber, M.; Leoni, J.; Woscoff, A.; Zwirner, N.W. Up-regulated expression of MICA and proinflammatory cytokines in skin biopsies from patients with seborrhoeic dermatitis. Clin. Immunol. 2003, 106, 50–54. [Google Scholar] [CrossRef]
- Trznadel-Grodzka, E.; Błaszkowski, M.; Rotsztejn, H. Investigations of seborrheic dermatitis. Part, I. The role of selected cytokines in the pathogenesis of seborrheic dermatitis. Postepy Hig. Med. Dosw. 2012, 66, 843–847. [Google Scholar] [CrossRef]
- Corzo-León, D.E.; MacCallum, D.M.; Munro, C.A. Host Responses in an Ex Vivo Human Skin Model Challenged With Malassezia sympodialis. Front. Cell. Infect. Microbiol. 2021, 10, 561382. [Google Scholar] [CrossRef]
- Kawakami, Y.; Nakamura-Wakatsuki, T.; Yamamoto, T. Seborrheic dermatitis-like eruption following interleukin-2 administration. Dermatol. Online J. 2010, 16, 12. [Google Scholar] [CrossRef]
- Tuor, M.; Stappers, M.H.; Desgardin, A.; Ruchti, F.; Sparber, F.; Orr, S.J.; Gow, N.A.; LeibundGut-Landmann, S. Card9 and MyD88 differentially regulate Th17 immunity to the commensal yeast Malassezia in the murine skin. Mucosal Immunol. 2025, 18, 205–219. [Google Scholar] [CrossRef]
- Fang, H.; Hou, Y.; Zhuang, H.; Wang, C. The effects of Malassezia in the activation of Interleukin (IL)-23/IL-17 axis in Psoriasis. New Microbiol. 2022, 45, 130–137. [Google Scholar]
- Jia, Q.; Hu, J.; Wang, X.; Deng, Y.; Zhang, J.; Li, H. Malassezia globosa Induces Differentiation of Pathogenic Th17 Cells by Inducing IL-23 Secretion by Keratinocytes. Mycopathologia 2024, 189, 85. [Google Scholar] [CrossRef]
- Andersen, P.L.; Jemec, G.B.; Erikstrup, C.; Didriksen, M.; Dinh, K.M.; Mikkelsen, S.; Sørensen, E.; DBDS Genetic Consortium; Nielsen, K.R.; Bruun, M.T.; et al. Human leukocyte antigen system associations in Malassezia-related skin diseases. Arch. Dermatol. Res. 2023, 315, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Baş, Y.; Seçkiïn, H.Y.; Kalkan, G.; Takci, Z.; Çitil, R.; Önder, Y.; Şahïn, Ş.; Demir, A.K. Prevalence and related factors of psoriasis and seborrheic dermatitis: A community-based study. Turk. J. Med. Sci. 2016, 46, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.; Hwang, M.; Kim, M.; Jo, S.J.; Je, M.; Jang, J.E.; Lee, D.H.; Hwang, J.Y. Smartphone-based multispectral imaging and machine-learning based analysis for discrimination between seborrheic dermatitis and psoriasis on the scalp. Biomed. Opt. Express 2019, 10, 879–891. [Google Scholar] [CrossRef] [PubMed]
- Sparber, F.; De Gregorio, C.; Steckholzer, S.; Ferreira, F.M.; Dolowschiak, T.; Ruchti, F.; Kirchner, F.R.; Mertens, S.; Prinz, I.; Joller, N.; et al. The Skin Commensal Yeast Malassezia Triggers a Type 17 Response that Coordinates Anti-fungal Immunity and Exacerbates Skin Inflammation. Cell Host Microbe 2019, 25, 389–403.e6. [Google Scholar] [CrossRef]
- Yu, R.; Lin, Q.; Zhai, Y.; Mao, Y.; Li, K.; Gao, Y.; Liu, Y.; Fu, L.; Fang, T.; Zhao, M.; et al. Recombinant human thymosin beta-4 (rhTβ4) improved scalp condition and microbiome homeostasis in seborrheic dermatitis. Microb. Biotechnol. 2021, 14, 2152–2163. [Google Scholar] [CrossRef]
- Truglio, M.; Sivori, F.; Cavallo, I.; Abril, E.; Licursi, V.; Fabrizio, G.; Cardinali, G.; Pignatti, M.; Toma, L.; Valensise, F.; et al. Modulating the skin mycobiome-bacteriome and treating seborrheic dermatitis with a probiotic-enriched oily suspension. Sci. Rep. 2024, 14, 2722. [Google Scholar] [CrossRef]
- Barak-Shinar, D.; Del Río, R.; Green, L.J. Treatment of Seborrheic Dermatitis Using a Novel Herbal-based Cream. J. Clin. Aesthet. Dermatol. 2017, 10, 17–23. [Google Scholar]
- Borda, L.J.; Perper, M.; Keri, J.E. Treatment of seborrheic dermatitis: A comprehensive review. J. Dermatolog Treat. 2019, 30, 158–169. [Google Scholar] [CrossRef]
- Choi, F.D.; Juhasz, M.L.; Mesinkovska, N.A. Topical ketoconazole: A systematic review of current dermatological applications and future developments. J. Dermatol. Treat. 2019, 30, 760–771. [Google Scholar] [CrossRef]
- Chowdhry, S.; Gupta, S.; D’Souza, P. Topical Antifungals used for Treatment of Seborrheic Dermatitis. J. Bacteriol. Mycol. Open Access 2017, 4, 76. [Google Scholar]
- Buechner, S.A. Multicenter, double-blind, parallel group study investigating the non-inferiority of efficacy and safety of a 2% miconazole nitrate shampoo in comparison with a 2% ketoconazole shampoo in the treatment of seborrhoeic dermatitis of the scalp. J. Dermatol. Treat. 2014, 25, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.Y.; Camp, W.L.; Elewski, B.E. Advances in topical and systemic antifungals. Dermatol. Clin. 2007, 25, 165–183. [Google Scholar] [CrossRef] [PubMed]
- Goldust, M.; Rezaee, E.; Raghifar, R.; Hemayat, S. Treatment of seborrheic dermatitis: The efficiency of sertaconazole 2% cream vs. tacrolimus 0.03% cream. Ann. Parasitol. 2013, 59, 73–77. [Google Scholar] [PubMed]
- Gary, G. Optimizing treatment approaches in seborrheic dermatitis. J. Clin. Aesthet. Dermatol. 2013, 6, 44–49. [Google Scholar]
- Vena, G.A.; Micali, G.; Santoianni, P.; Cassano, N.; Peruzzi, E. Oral terbinafine in the treatment of multi-site seborrhoic dermatitis: A multicenter, double-blind placebo-controlled study. Int. J. Immunopathol. Pharmacol. 2005, 18, 745–753. [Google Scholar] [CrossRef]
- Das, J.; Majumdar, M.; Chakraborty, U.; Majumdar, V.; Mazumdar, G.; Nath, J. Oral itraconazole for the treatment of severe seborrhoeic dermatitis. Indian J. Dermatol. 2011, 56, 515–516. [Google Scholar]
- Shemer, A.; Kaplan, B.; Nathansohn, N.; Grunwald, M.H.; Amichai, B.; Trau, H. Treatment of moderate to severe facial seborrheic dermatitis with itraconazole: An open non-comparative study. Isr. Med. Assoc. J. 2008, 10, 417–418. [Google Scholar]
- Kastarinen, H.; Oksanen, T.; O Okokon, E.; Kiviniemi, V.V.; Airola, K.; Jyrkkä, J.; Oravilahti, T.; Rannanheimo, P.K.; Verbeek, J.H. Topical anti-inflammatory agents for seborrhoeic dermatitis of the face or scalp. Cochrane Database Syst. Rev. 2014, 2017, CD009446. [Google Scholar] [CrossRef]
- Oglio, F.D.; Tedeschi, A.; Fusto, C.M.; Lacarrubba, F.; Dinotta, F.; Micali, G. A novel cosmetic antifungal/anti-inflammatory topical gel for the treatment of mild to moderate seborrheic dermatitis of the face: An open-label trial utilizing clinical evaluation and erythema-directed digital photography. G. Ital. Dermatol. Venereol. 2017, 152, 436–440. [Google Scholar]
- Turlier, V.; Viode, C.; Durbise, E.; Bacquey, A.; LeJeune, O.; Soares, R.O.; Lauze, C.; Villeneuve, C.; Rouquier, A.; Casas, C.; et al. Clinical and biochemical assessment of maintenance treatment in chronic recurrent seborrheic dermatitis: Randomized controlled study. Dermatol. Ther. 2014, 4, 43–59. [Google Scholar] [CrossRef]
- Youn, H.J.; Kim, S.Y.; Park, M.; Jung, W.H.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Efficacy and Safety of Cream Containing Climbazole/Piroctone Olamine for Facial Seborrheic Dermatitis: A Single-Center, Open-Label Split-Face Clinical Study. Ann. Dermatol. 2016, 28, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Braza, T.; DiCarlo, J.; Soon, S.; McCall, C. Tacrolimus 0.1% ointment for seborrhoeic dermatitis: An open-label pilot study. Br. J. Dermatol. 2003, 148, 1242–1244. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Mun, J.; Jwa, S.; Song, M.; Kim, H.; Ko, H.; Kim, M.; Song, K.; Lee, S.; Seo, J.; et al. Proactive treatment of adult facial seborrhoeic dermatitis with 0.1% tacrolimus ointment: Randomized, double-blind, vehicle-controlled, multi-centre trial. Acta Derm. Venereol. 2013, 93, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Koc, E.; Arca, E.; Kose, O.; Akar, A. An open, randomized, prospective, comparative study of topical pimecrolimus 1% cream and topical ketoconazole 2% cream in the treatment of seborrheic dermatitis. J. Dermatol. Treat. 2009, 20, 4–9. [Google Scholar] [CrossRef]
- Kim, B.-S.; Kim, S.-H.; Kim, M.-B.; Oh, C.-K.; Jang, H.-S.; Kwon, K.-S. Treatment of facial seborrheic dermatitis with pimecrolimus cream 1%: An open-label clinical study in Korean patients. J. Korean Med. Sci. 2007, 22, 868–872. [Google Scholar] [CrossRef]
- Rigopoulos, D.; Ioannides, D.; Kalogeromitros, D.; Gregoriou, S.; Katsambas, A. Pimecrolimus cream 1% vs. betamethasone 17-valerate 0.1% cream in the treatment of seborrhoeic dermatitis. A randomized open-label clinical trial. Br. J. Dermatol. 2004, 151, 1071–1075. [Google Scholar] [CrossRef]
- Ang-Tiu, C.U.; Meghrajani, C.F.; Maano, C.C. Pimecrolimus 1% cream for the treatment of seborrheic dermatitis: A systematic review of randomized controlled trials. Expert. Rev. Clin. Pharmacol. 2012, 5, 91–97. [Google Scholar] [CrossRef]
| Treatment Category | Therapy | Mechanism of Action | Medical Indications |
|---|---|---|---|
| Topical anti-fungal agents | Ketoconazole Miconazole Clottrimazole 1% Ciclopiroxolamine 1% | Inhibition of Ianosterol 14-a demethylase Depletion of ergosterol Toxic sterols in membrane | Mild to moderate SD |
| Systemic anti-fungal agents | Terbinafine Itraconazole | Inhibition of ergosterol synthesis Inhibition of squalene epoxidase | Mild to moderate SD |
| Inhibition of 5-lipoxygenase metabolites | Moderate to severe SD | ||
| Anti-inflammatory agents | Non-steroidal (ciclopirox7piroctonolamine) | Inhibition of P450-mediated reactions | Mild to moderate SD |
| Topical corticosteroids (betamethasone valerate, clobetasol propionate) | Anti-inflammatory Immunosuppressive Antiproliferative | Moderate to severe SD | |
| Immunomodulators | Tacrolimus Picrolimus | Inhibition of calcineurin | Mild to moderate SD (short-term treatment) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piacentini, F.; Camera, E.; Di Nardo, A.; Dell’Anna, M.L. Seborrheic Dermatitis: Exploring the Complex Interplay with Malassezia. Int. J. Mol. Sci. 2025, 26, 2650. https://doi.org/10.3390/ijms26062650
Piacentini F, Camera E, Di Nardo A, Dell’Anna ML. Seborrheic Dermatitis: Exploring the Complex Interplay with Malassezia. International Journal of Molecular Sciences. 2025; 26(6):2650. https://doi.org/10.3390/ijms26062650
Chicago/Turabian StylePiacentini, Francesca, Emanuela Camera, Anna Di Nardo, and Maria Lucia Dell’Anna. 2025. "Seborrheic Dermatitis: Exploring the Complex Interplay with Malassezia" International Journal of Molecular Sciences 26, no. 6: 2650. https://doi.org/10.3390/ijms26062650
APA StylePiacentini, F., Camera, E., Di Nardo, A., & Dell’Anna, M. L. (2025). Seborrheic Dermatitis: Exploring the Complex Interplay with Malassezia. International Journal of Molecular Sciences, 26(6), 2650. https://doi.org/10.3390/ijms26062650






