The Protective Effects of Burdock Fructooligosaccharide on Preterm Labor Through Its Anti-Inflammatory Action
Abstract
:1. Introduction
2. Results
2.1. Purification of Fructooligosaccharides
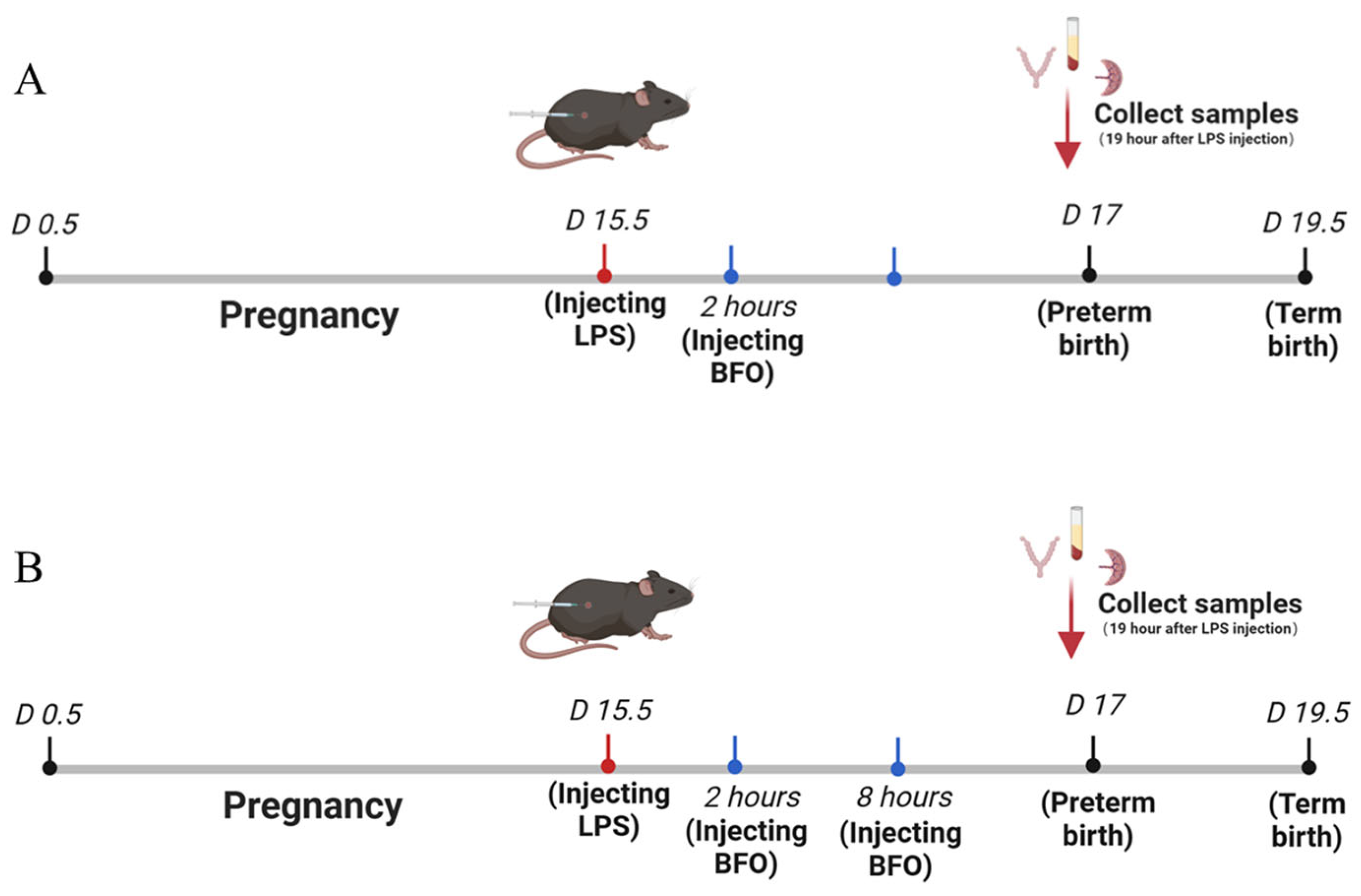
2.2. Single Injection of BFO Can Reduce the Occurrence of PTL
2.3. Repeated Injection of BFO Showed Extraordinary Efficacy in Preventing PTL
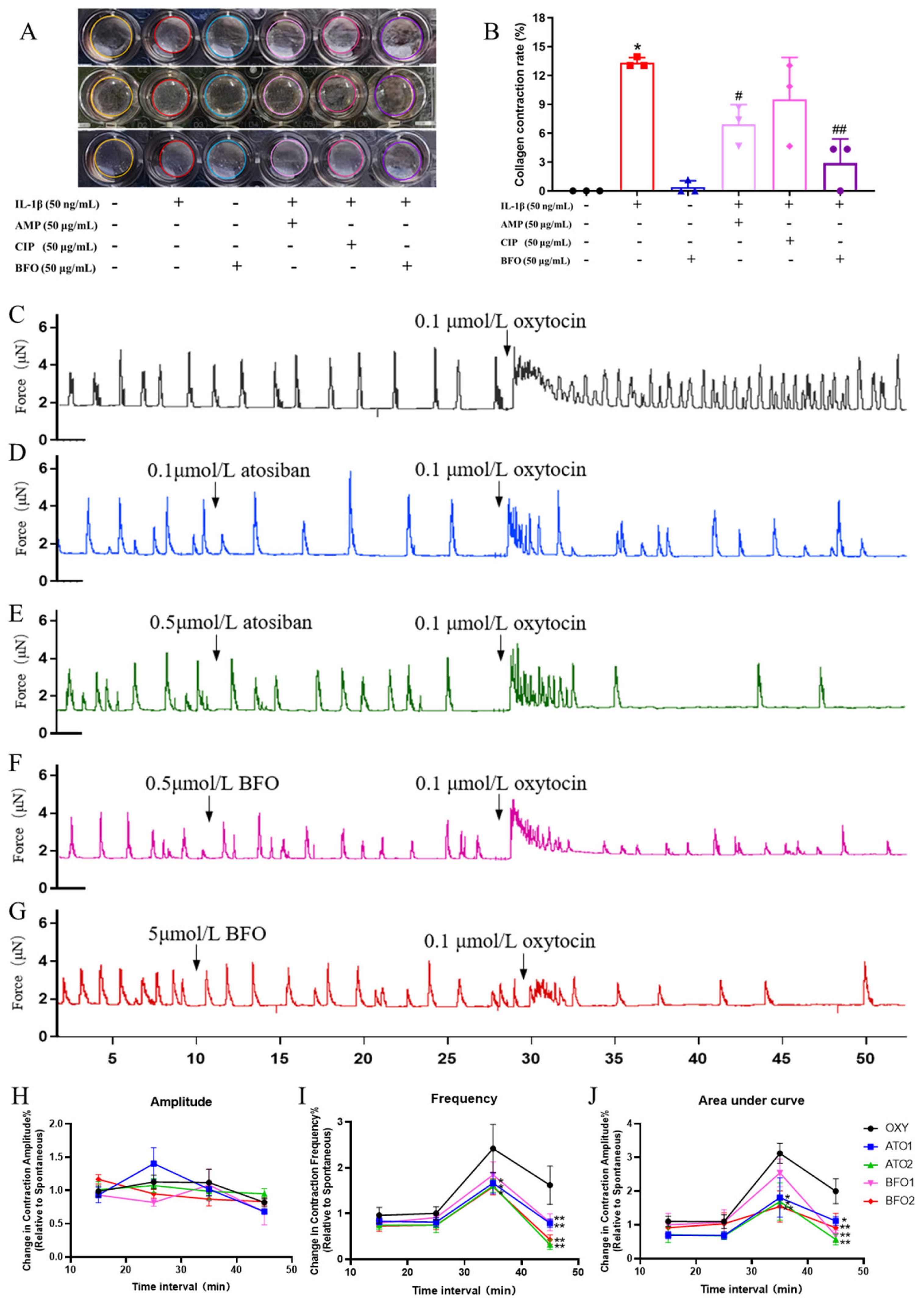
2.4. BFO Attenuates Inflammation-Induced USMC Contraction and Oxytocin-Induced Uterine Smooth Muscle Strip Contraction In Vitro
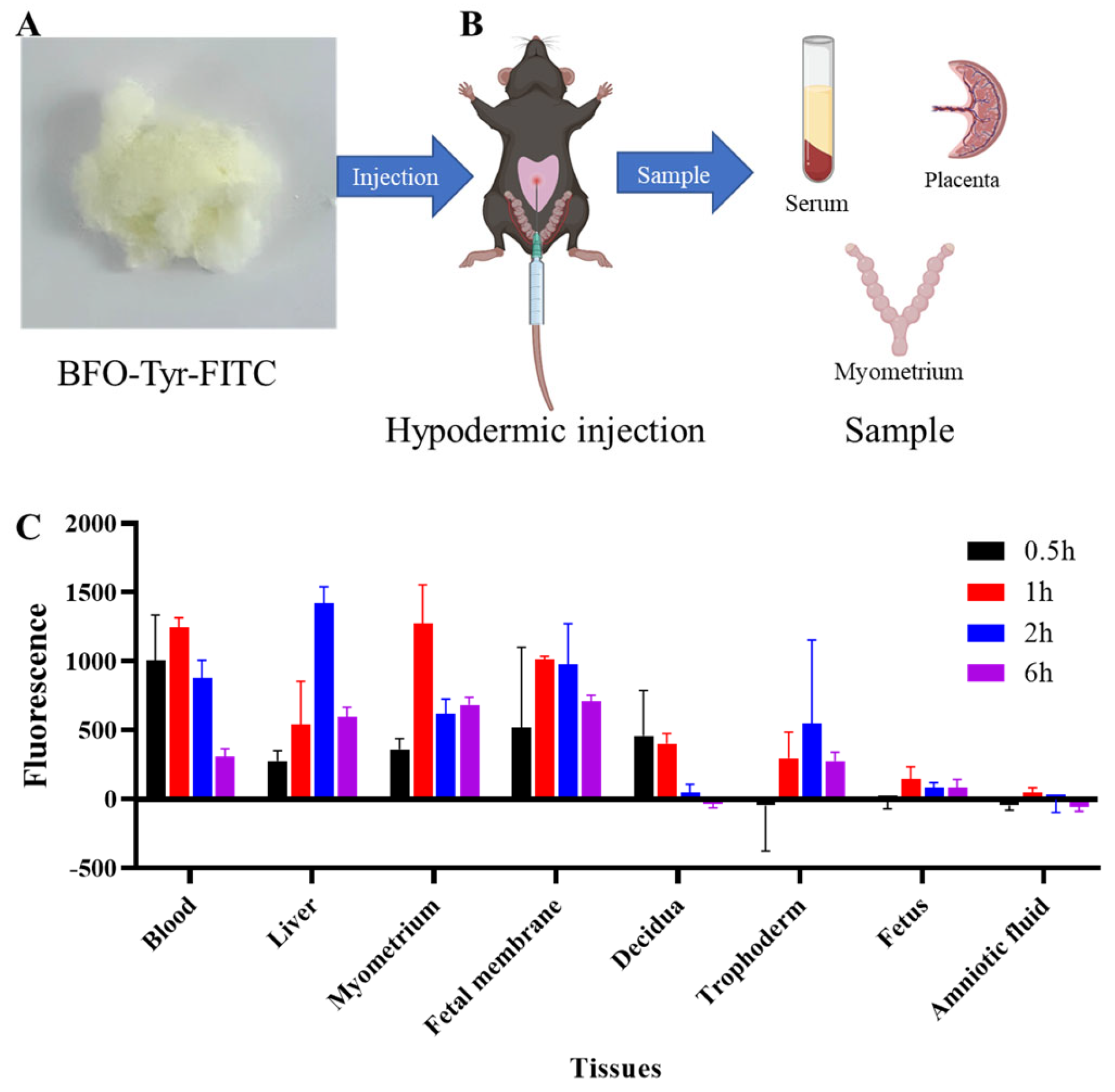
2.5. In Vivo BFO Is Highly Enriched in Myometrial Tissues but Not in Fetus
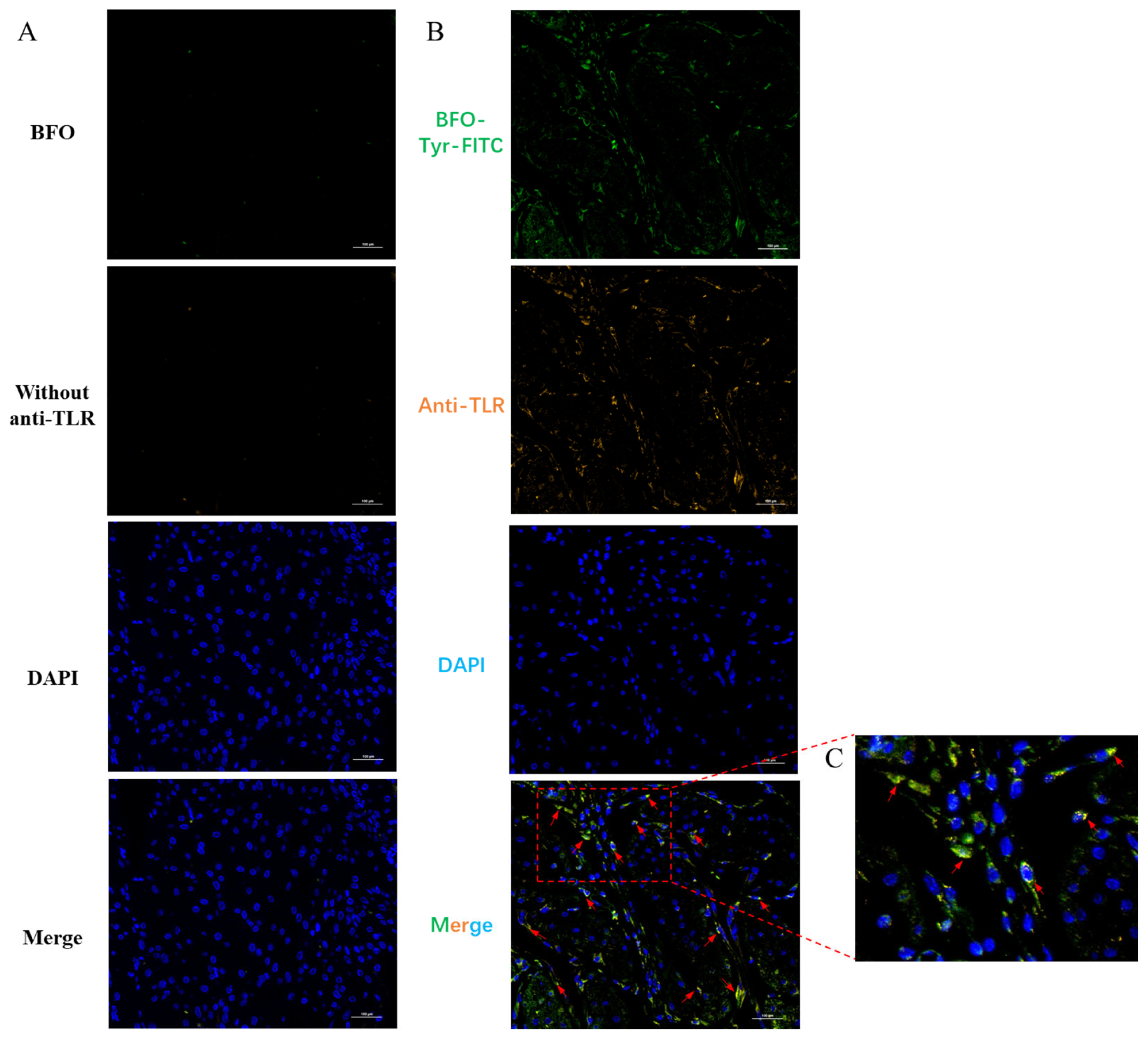
2.6. BFO Is Co-Localized with TLR4 on Uterine Smooth Muscle Cells
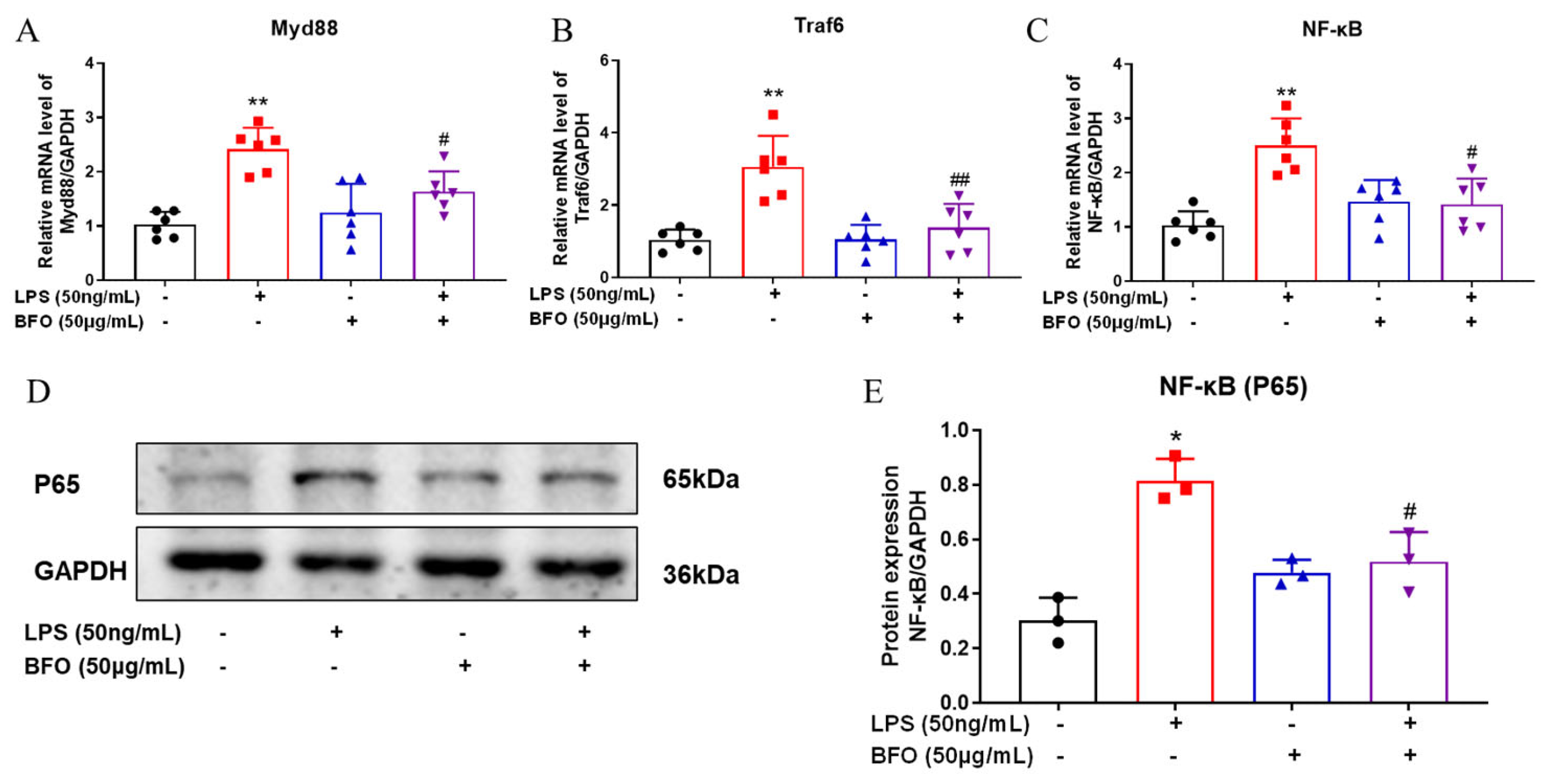
2.7. BFO Alleviates the Expression of TLR4-Downstream Genes
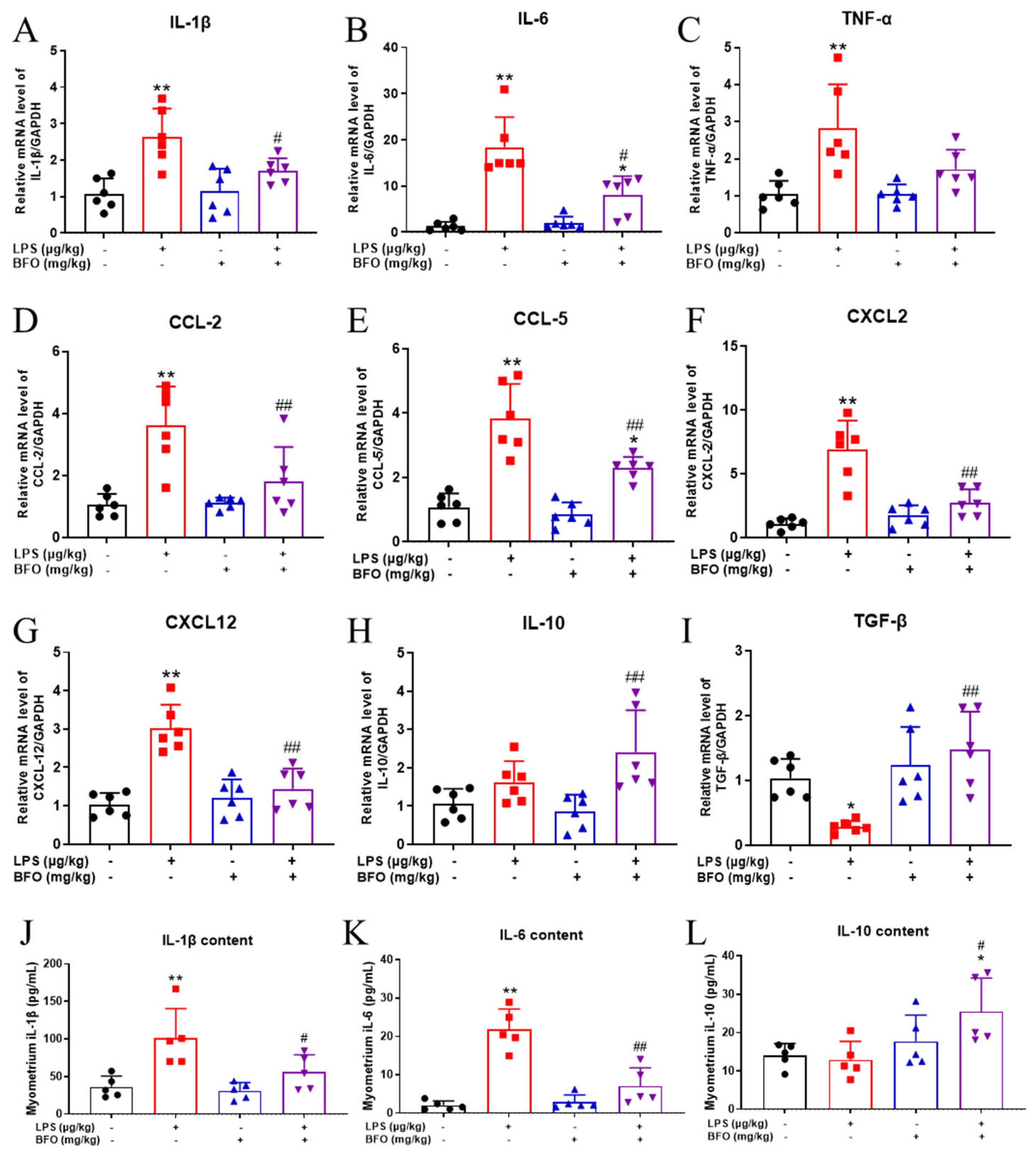
2.8. BFO Reduces the Elevation of Pro-Inflammatory Cytokines and Chemokines Induced by LPS in Maternal Blood
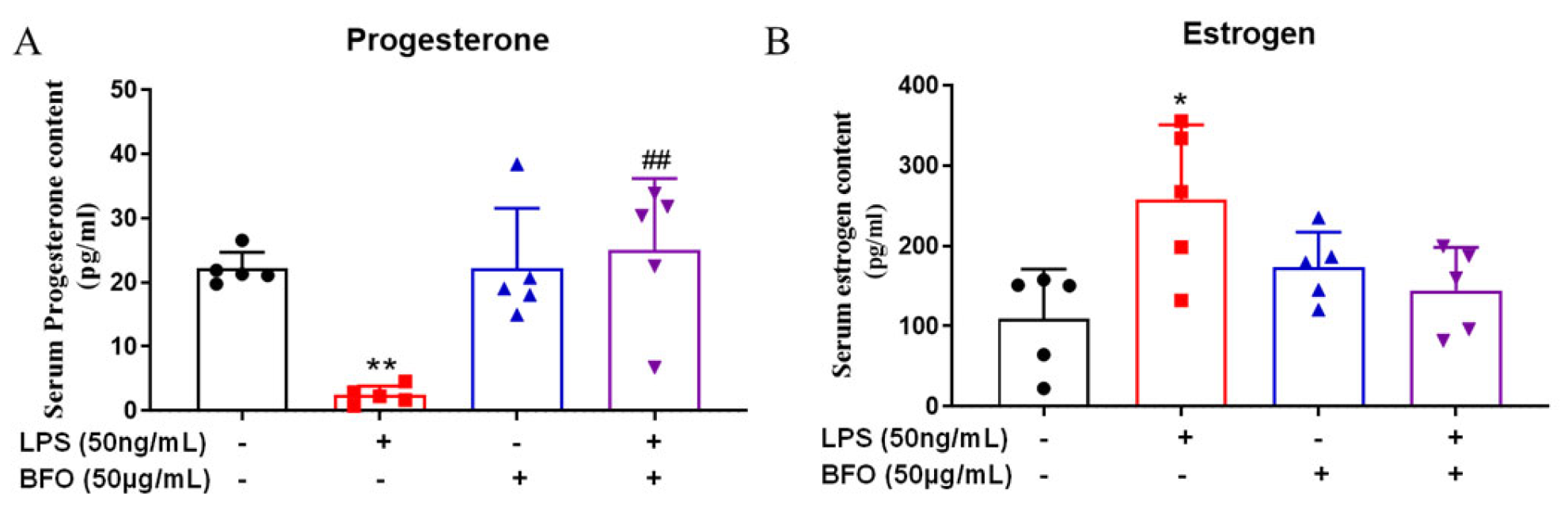
2.9. BFO Alleviates LPS-Induced Changes in Progesterone and Estradiol in Maternal Blood
2.10. BFO Reduces LPS-Induced Inflammatory Cytokine Expression in the Myometrium
2.11. BFO Reduces LPS-Induced Inflammatory Cells Infiltration into the Myometrium
3. Discussion
4. Materials and Methods
4.1. Isolation and Purification of Fructooligosaccharide
4.2. Identification of BFO
4.3. Preterm Birth Labor Animal Model
4.4. Administration of BFO to Preterm Labor Mice
4.5. Isolation and Culture of Primary Uterine Smooth Muscle Cells
4.6. Collagen Contraction Experiment of Uterine Smooth Muscle Cells
4.7. Detection of Contractility of Isolated Uterine Smooth Muscle Strips
4.8. Construction of Fluorescein-Labeled BFO
4.9. Tissue Distribution Analysis of BFO
4.10. Immunofluorescence Co-Localization of BFO and TLR4 on USMC
4.11. Expression of Key Genes Downstream of TLR4
4.12. RNA Extraction and Quantitative Real-Time PCR
4.13. Western Blot
4.14. Cytokine Assays in Serum and the Myometrium
4.15. Progesterone and Estradiol Assays in Serum
4.16. Infiltration Analysis of Inflammatory Cells
4.17. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTL | Preterm labor |
| BFO | Burdock fructooligosaccharide |
| CCBs | Calcium channel blockers |
| DCs | Dendritic Cells |
| AMP | Atractylodes Macrocephala polysaccharide |
| CPP | Codonopsis pilosula polysaccharide |
| LPS | Lipopolysaccharide |
| USMC | Uterine smooth muscle cells |
| PSS | Physiological salt solution |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| AUC | Area under the curve |
| TLR4 | Toll-like receptor 4 |
| IF | Immunofluorescence |
| Icam-1 | Intercellular adhesion molecule-1 |
| Vcam-1 | Vascular cell adhesion molecule-1 |
| MCP-1 | Monocyte chemoattractant protein-1 |
References
- Sharrow, D.; Hug, L.; You, D.; Alkema, L.; Black, R.; Cousens, S.; Croft, T.; Gaigbe-Togbe, V.; Gerland, P.; Guillot, M.; et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: A systematic analysis by the UN Inter-agency Group for Child Mortality Estimation. Lancet Glob. Health 2022, 10, e195–e206. [Google Scholar] [CrossRef] [PubMed]
- Ward, V.C.; Lee, A.C.C.; Hawken, S.; Otieno, N.A.; Mujuru, H.A.; Chimhini, G.; Wilson, K.; Darmstadt, G.L. Overview of the Global and US Burden of Preterm Birth. Clin. Perinatol. 2024, 51, 301–311. [Google Scholar] [CrossRef]
- Gomez-Lopez, N.; Galaz, J.; Miller, D.; Farias-Jofre, M.; Liu, Z.; Arenas-Hernandez, M.; Garcia-Flores, V.; Shaffer, Z.; Greenberg, J.M.; Theis, K.R.; et al. The immunobiology of preterm labor and birth: Intra-amniotic inflammation or breakdown of maternal–fetal homeostasis. Reproduction 2022, 164, R11–R45. [Google Scholar] [CrossRef]
- Taylor, J.; Sharp, A.; Rannard, S.P.; Arrowsmith, S.; McDonald, T.O. Nanomedicine strategies to improve therapeutic agents for the prevention and treatment of preterm birth and future directions. Nanoscale Adv. 2023, 5, 1870–1889. [Google Scholar] [CrossRef]
- Miller, F.A.; Sacco, A.; David, A.L.; Boyle, A.K. Interventions for Infection and Inflammation-Induced Preterm Birth: A Preclinical Systematic Review. Reprod. Sci. 2023, 30, 361–379. [Google Scholar] [CrossRef] [PubMed]
- Habelrih, T.; Augustin, T.L.; Mauffette-Whyte, F.; Ferri, B.; Sawaya, K.; Côté, F.; Gallant, M.; Olson, D.M.; Chemtob, S. Inflammatory mechanisms of preterm labor and emerging anti-inflammatory interventions. Cytokine Growth Factor Rev. 2024, 78, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Keelan, J.A. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J. Reprod. Immunol. 2018, 125, 89–99. [Google Scholar] [CrossRef]
- Wilson, A.; Hodgetts-Morton, V.A.; Marson, E.J.; Markland, A.D.; Larkai, E.; Papadopoulou, A.; Coomarasamy, A.; Tobias, A.; Chou, D.; Oladapo, O.T.; et al. Tocolytics for delaying preterm birth: A network meta-analysis (0924). Cochrane Database Syst. Rev. 2022, 8, CD014978. [Google Scholar]
- Neilson, J.P.; West, H.M.; Dowswell, T. Betamimetics for inhibiting preterm labour. Cochrane Database Syst. Rev. 2014, 2014, CD004352. [Google Scholar] [CrossRef]
- Bhati, T.; Ray, A.; Arora, R.; Siraj, F.; Parvez, S.; Rastogi, S. Galectins are critical regulators of cytokine signalling at feto-maternal interface in infection-associated spontaneous preterm birth. Placenta 2023, 138, 10–19. [Google Scholar] [CrossRef]
- Griggs, K.M.; Hrelic, D.A.; Williams, N.; McEwen-Campbell, M.; Cypher, R. Preterm Labor and Birth: A Clinical Review. MCN Am. J. Matern./Child Nurs. 2020, 45, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, P.B.; Bland, C.M.; Griffin, B.; Stover, K.R.; Eiland, L.S.; McLaughlin, M. A Review of Antibiotic Use in Pregnancy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2015, 35, 1052–1062. [Google Scholar] [CrossRef]
- Ichihara, Y.; Suga, K.; Fukui, M.; Yonetani, N.; Shono, M.; Nakagawa, R.; Kagami, S. Serum biotin level during pregnancy is associated with fetal growth and preterm delivery. J. Med. Investig. 2020, 67, 170–173. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Yue, Z.; Bocheng, Y.; Zhaoyu, W.; Mingjing, L.; Wei, Z. Natural Polysaccharides with Immunomodulatory Activities. Mini-Rev. Med. Chem. 2020, 20, 96–106. [Google Scholar]
- Fang, D.-N.; Zheng, C.-W.; Ma, Y.-L. Effectiveness of Scutellaria baicalensis Georgi root in pregnancy-related diseases: A review. J. Integr. Med. 2023, 21, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Li, Q.; Wang, G.; Dong, F.; Zhou, H.; Zhang, J. Synthesis, characterization, and antifungal activity of novel inulin derivatives with chlorinated benzene. Carbohydr. Polym. 2014, 99, 469–473. [Google Scholar] [CrossRef]
- Versluys, M.; Porras-Domínguez, J.R.; Voet, A.; Struyf, T.; Van den Ende, W. Insights in inulin binding and inulin oligosaccharide formation by novel multi domain endo-inulinases from Botrytis cinerea. Carbohydr. Polym. 2024, 328, 121690. [Google Scholar] [CrossRef]
- Fernández-Lainez, C.; Akkerman, R.; Oerlemans, M.M.P.; Logtenberg, M.J.; Schols, H.A.; Silva-Lagos, L.A.; López-Velázquez, G.; de Vos, P. β(2→6)-Type fructans attenuate proinflammatory responses in a structure dependent fashion via Toll-like receptors. Carbohydr. Polym. 2022, 277, 118893. [Google Scholar] [CrossRef]
- Zeng, F.; Li, Y.; Zhang, X.; Shen, L.; Zhao, X.; Beta, T.; Li, B.; Chen, R.; Huang, W. Immune regulation and inflammation inhibition of Arctium lappa L. polysaccharides by TLR4/NF-κB signaling pathway in cells. Int. J. Biol. Macromol. 2024, 254, 127700. [Google Scholar] [CrossRef]
- Meng, Y.; Ma, Q.; Xu, X.; Feng, L.; Chen, Q.; Chen, Y.; Li, Z.; Liu, C.; Chen, K. Burdock fructooligosaccharide ameliorates the hypercholesterolemia and vascular inflammation in mice by regulating cholesterol homeostasis and anti-inflammatory properties. J. Funct. Foods 2023, 107, 105678. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, X.; Xu, X.; Lu, Y.; Chen, Q.; Chen, Y.; Liu, C.; Chen, K. Long-term oral administration of burdock fructooligosaccharide alleviates DSS-induced colitis in mice by mediating anti-inflammatory effects and protection of intestinal barrier function. Immun. Inflamm. Dis. 2023, 11, e1092. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Rao, Z.; Xie, Z.; Qi, H.; Zeng, N. Isolation, structure and bioactivity of polysaccharides from Atractylodes macrocephala: A review. J. Ethnopharmacol. 2022, 296, 115506. [Google Scholar] [CrossRef]
- Ma, K.; Yi, X.; Yang, S.-T.; Zhu, H.; Liu, T.-Y.; Jia, S.-S.; Fan, J.-H.; Hu, D.-J.; Lv, G.-P.; Huang, H. Isolation, purification, and structural characterization of polysaccharides from Codonopsis pilosula and its therapeutic effects on non-alcoholic fatty liver disease in vitro and in vivo. Int. J. Biol. Macromol. 2024, 265, 130988. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Xu, J.; Cai, L.; Man, L.; Niu, L.; Hu, J.; Sun, T.; Zheng, X. Major depressive symptoms in breast cancer patients with ovarian function suppression: A cross-sectional study comparing ovarian ablation and gonadotropin-releasing hormone agonists. BMC Psychiatry 2021, 21, 624. [Google Scholar] [CrossRef]
- Meng, X.; Kuang, H.; Wang, Q.; Zhang, H.; Wang, D.; Kang, T. A polysaccharide from Codonopsis pilosula roots attenuates carbon tetrachloride-induced liver fibrosis via modulation of TLR4/NF-κB and TGF-β1/Smad3 signaling pathway. Int. Immunopharmacol. 2023, 119, 110180. [Google Scholar] [CrossRef]
- Wang, R.; Shan, H.; Zhang, G.; Li, Q.; Wang, J.; Yan, Q.; Li, E.; Diao, Y.; Wei, L. An inulin-type fructan (AMP1-1) from Atractylodes macrocephala with anti-weightlessness bone loss activity. Carbohydr. Polym. 2022, 294, 119742. [Google Scholar] [CrossRef]
- Meng, Y.; Xu, Y.; Chang, C.; Qiu, Z.; Hu, J.; Wu, Y.; Zhang, B.; Zheng, G. Extraction, characterization and anti-inflammatory activities of an inulin-type fructan from Codonopsis pilosula. Int. J. Biol. Macromol. 2020, 163, 1677–1686. [Google Scholar] [CrossRef]
- Zierden, H.C.; Shapiro, R.L.; DeLong, K.; Carter, D.M.; Ensign, L.M. Next generation strategies for preventing preterm birth. Adv. Drug Deliv. Rev. 2021, 174, 190–209. [Google Scholar] [CrossRef]
- Swarray-Deen, A.; Sepenu, P.; Mensah, T.E.; Osei-Agyapong, J.; Sefogah, P.E.; Appiah-Sakyi, K.; Ahmed, B.; Konje, J.C. Preterm birth in low-middle income Countries. Best Pract. Res. Clin. Obstet. Gynaecol. 2024, 95, 102518. [Google Scholar] [CrossRef]
- Yu, Y.; Shen, M.; Song, Q.; Xie, J. Biological activities and pharmaceutical applications of polysaccharide from natural resources: A review. Carbohydr. Polym. 2018, 183, 91–101. [Google Scholar] [CrossRef]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Dobrange, E.; Peshev, D.; Loedolff, B.; Van den Ende, W. Fructans as immunomodulatory and antiviral agents: The case of Echinacea. Biomolecules 2019, 9, 615. [Google Scholar] [CrossRef]
- Zhang, X.-J.; Liu, S.-F.; Lu, Y.; Wang, J.-Y.; Chen, K.-S. Immunomodulatory activity of a fructooligosaccharide isolated from burdock roots. RSC Adv. 2019, 9, 11092–11100. [Google Scholar] [CrossRef]
- Siricilla, S.; Hansen, C.J.; Rogers, J.H.; De, D.; Simpson, C.L.; Waterson, A.G.; Crockett, S.L.; Boatwright, N.; Reese, J. Arrest of mouse preterm labor until term delivery by combination therapy with atosiban and mundulone, a natural product with tocolytic efficacy. Pharmacol. Res. 2023, 195, 106876. [Google Scholar] [CrossRef] [PubMed]
- Murillo, C.; Migliorelli, F.; Nieto, C.; Martínez, H.; Rueda, C.; Bermejo, R.; Corrales, A.; Palacio, M. A multi-centre, open-label, prospective, observational study to assess the safety of a nifedipine oral solution in the treatment of preterm labor. Clínica E Investig. En Ginecol. Y Obstet. 2023, 50, 100883. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Control of adaptive immune responses by Toll-like receptors. Curr. Opin. Immunol. 2002, 14, 380–383. [Google Scholar] [CrossRef]
- Thornton, J.M.; Ramphul, M. Mechanisms and management of normal labour. Obstet. Gynaecol. Reprod. Med. 2023, 33, 160–167. [Google Scholar] [CrossRef]
- Green, E.S.; Arck, P.C. Pathogenesis of preterm birth: Bidirectional inflammation in mother and fetus. Semin. Immunopathol. 2020, 42, 413–429. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Lee, G.; Kim, S.G.; Choi, S. Toll-like receptor modulators: A patent review (2006-2010). Expert Opin. Ther. Pat. 2011, 21, 927–944. [Google Scholar] [CrossRef]
- Farias-Jofre, M.; Romero, R.; Galaz, J.; Xu, Y.; Miller, D.; Garcia-Flores, V.; Arenas-Hernandez, M.; Winters, A.D.; Berkowitz, B.A.; Podolsky, R.H.; et al. Blockade of IL-6R prevents preterm birth and adverse neonatal outcomes. eBioMedicine 2023, 98, 104865. [Google Scholar] [CrossRef]
- Saito, S.; Kasahara, T.; Kato, Y.; Ishihara, Y.; Ichijo, M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993, 5, 81–88. [Google Scholar] [CrossRef]
- McGoldrick, E.; Stewart, F.; Parker, R.; Dalziel, S.R. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 2020, 3, CD004454. [Google Scholar]
- Crowther, C.A.; Middleton, P.F.; Voysey, M.; Askie, L.; Pryde, P.G.; Marret, S.; Doyle, L.W. Assessing the neuroprotective benefits for babies of antenatal magnesium sulphate: An individual participant data meta-analysis. PLOS Med. 2017, 14, e1002398. [Google Scholar] [CrossRef] [PubMed]
- Condon, J.C.; Kyathanahalli, C.; Anamthathmakula, P.; Jeyasuria, P. Estrogen/estrogen receptor action and the pregnant myometrium. Curr. Opin. Physiol. 2020, 13, 135–140. [Google Scholar] [CrossRef]
- Brown, A.G.; Leite, R.S.; Strauss Iii, J.F. Mechanisms Underlying “Functional” Progesterone Withdrawal at Parturition. Ann. New York Acad. Sci. 2004, 1034, 36–49. [Google Scholar] [CrossRef]
- Nakaya, M.; Tachibana, H.; Yamada, K. Effect of Estrogens on the Interferon-γ Producing Cell Population of Mouse Splenocytes. Biosci. Biotechnol. Biochem. 2006, 70, 47–53. [Google Scholar] [CrossRef]
- Huang, Q.; Jin, X.; Li, P.; Zheng, Z.; Jiang, Y.; Liu, H. Elevated inflammatory mediators from the maternal-fetal interface to fetal circulation during labor. Cytokine 2021, 148, 155707. [Google Scholar] [CrossRef]
- Huang, Q.; Ye, A.; Li, P.; Bao, J.; Garfield, R.E.; Liu, H. Nicotine ameliorates inflammatory mediators in RU486 induced preterm labor model through activating cholinergic anti-inflammatory pathway. Cytokine 2022, 160, 156054. [Google Scholar] [CrossRef]
- Tong, M.; Abrahams, V.M. Neutrophils in preterm birth: Friend or foe? Placenta 2020, 102, 17–20. [Google Scholar] [CrossRef]
- Chao, H.-H.; Li, L.; Gao, X.; Wang, C.; Yue, W. CXCL12 expression in aborted mouse uteri induced by IFN-γ: Potential anti-inflammatory effect involves in endometrial restoration after abortion in mice. Gene 2019, 700, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Flores, V.; Romero, R.; Peyvandipour, A.; Galaz, J.; Pusod, E.; Panaitescu, B.; Miller, D.; Xu, Y.; Tao, L.; Liu, Z.; et al. A single-cell atlas of murine reproductive tissues during preterm labor. Cell Rep. 2023, 42, 111846. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Li, L.; Yan, F.; Lei, Y.; Chen, J.; Wu, L.; Ye, J. Nile tilapia CXCR4, the receptor of chemokine CXCL12, is involved in host defense against bacterial infection and chemotactic activity. Dev. Comp. Immunol. 2021, 114, 103836. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, N.; Kan, J.; Zhang, X.; Wu, X.; Sun, R.; Liu, J.; Qian, C.; Jin, C. Structural characterization of water-soluble polysaccharide from Arctium lappa and its effects on colitis mice. Carbohydr. Polym. 2019, 213, 89–99. [Google Scholar] [CrossRef]
- Salminen, A.; Vuolteenaho, R.; Paananen, R.; Ojaniemi, M.; Hallman, M. Surfactant protein D modulates levels of IL-10 and TNF-α in intrauterine compartments during lipopolysaccharide-induced preterm birth. Cytokine 2012, 60, 423–430. [Google Scholar] [CrossRef]
- Li, K.; Li, S.; Du, Y.; Qin, X. Screening and structure study of active components of Astragalus polysaccharide for injection based on different molecular weights. J. Chromatogr. B 2020, 1152, 122255. [Google Scholar] [CrossRef]
- Xu, X.; Shao, T.; Meng, Y.; Liu, C.; Zhang, P.; Chen, K. Immunomodulatory mechanisms of an acidic polysaccharide from the fermented burdock residue by Rhizopus nigricans in RAW264.7 cells and cyclophosphamide-induced immunosuppressive mice. Int. J. Biol. Macromol. 2023, 252, 126462. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, W.; Zheng, Z.; Nie, G.; Zhan, Y.; Mu, X.; Liu, Y.; Wang, K. Metabolic degradation of polysaccharides from Lentinus edodes by Kupffer cells via the Dectin-1/Syk signaling pathway. Carbohydr. Polym. 2023, 317, 121108. [Google Scholar] [CrossRef]



| Groups | Preterm Birth % | Average Length from LPS Injection to Birth of the First Pup (h) | Average Length of Pregnancy/Day | Survival Rate of Pups% |
|---|---|---|---|---|
| PBS + PBS | 0 | 96.2 ± 3.1 | 19.50 ± 0.2 | 97.5 ± 5.6 |
| 50 μg/kg mouse LPS + PBS | 100 | 20.5 ± 0.9 * | 16.35 ± 0.1 * | / |
| 30 μg/g mouse BFO + PBS | 0 | 97.2 ± 2.6 ## | 19.55 ± 0.1 ## | 100 |
| 50 μg/kg mouse LPS + 15 mg/kg mouse BFO | 100 | 20.25 ± 1.1 | 16.34 ± 10.1 | / |
| 50 μg/kg mouse LPS + 30 mg/kg mouse BFO | 42.86 | 20.03 ± 1.1 | 16.35 ± 6.5 | 87.83 ± 11.3 * |
| 102.2 ± 7.5 ## | 19.76 ± 0.3 ## | |||
| 50 μg/kg mouse LPS + 60 mg/kg mouse BFO | 28.57 | 28.2 ± 7.4 # | 16.67 ± 6.8 | 93.30 ± 7.8 |
| 105.5 ± 6.9 ## | 19.89 ± 0.3 ## | |||
| 50 μg/kg mouse LPS + 60 mg/kg mouse AMP | 71.43 | 23.62 ± 2.9 | 16.48 ± 6.2 | 96.67 ± 9.1 |
| 102.8 ± 6.0 ## | 19.78 ± 0.2 ## | |||
| 50 μg/kg mouse LPS + 60 mg/kg mouse CPP | 100 | 21.18 ± 1.2 | 16.38 ± 0.1 | / |
| Groups | Preterm Birth % | Average Length from LPS Injection to Birth of the First Pup/h | Average Length of Pregnancy/Day | Survival Rate of Pups % |
|---|---|---|---|---|
| PBS + PBS + PBS | 0 | 96.2 ± 3.1 | 19.50 ± 0.2 | 96.17 ± 6.6 |
| 50 μg/kg mouse LPS + PBS + PBS | 100 | 20.24 ± 0.7 ** | 16.34 ± 0.1 ** | / |
| PBS 30 mg/kg mouse BFO + 30 mg/kg mouse BFO | 0 | 97.92 ± 5.2 ## | 19.77 ± 0.3 ## | 95.14 ± 7.6 |
| 50 μg/kg mouse LPS + 15 mg/kg mouse BFO + 15 mg/kg mouse BFO | 90.91 | 20.41 ± 0.9 | 16.35 ± 0.1 | / |
| 96.00 | ||||
| 50 μg/kg mouse LPS + 30 mg/kg mouse BFO + 30 mg/kg mouse BFO | 9.09 | 19.50 | 19.88 ± 0.3 ## | 93.98 ± 6.6 |
| 102.5 ± 8.5 ## | ||||
| 50 μg/kg mouse LPS + 60 mg/kg mouse BFO + 60 mg/kg mouse BFO | 9.09 | 20.00 | 20.2 ± 0.4 ## | 94.40 ± 6.2 |
| 113.5 ± 7.8 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Du, R.; Long, P.; Sun, K.; Wang, Y.; Yang, Y.; Shen, X.; Gao, L. The Protective Effects of Burdock Fructooligosaccharide on Preterm Labor Through Its Anti-Inflammatory Action. Int. J. Mol. Sci. 2025, 26, 2659. https://doi.org/10.3390/ijms26062659
Ma Q, Du R, Long P, Sun K, Wang Y, Yang Y, Shen X, Gao L. The Protective Effects of Burdock Fructooligosaccharide on Preterm Labor Through Its Anti-Inflammatory Action. International Journal of Molecular Sciences. 2025; 26(6):2659. https://doi.org/10.3390/ijms26062659
Chicago/Turabian StyleMa, Qunfei, Ruoheng Du, Peihua Long, Kaiyi Sun, Youxia Wang, Ye Yang, Xinyu Shen, and Lu Gao. 2025. "The Protective Effects of Burdock Fructooligosaccharide on Preterm Labor Through Its Anti-Inflammatory Action" International Journal of Molecular Sciences 26, no. 6: 2659. https://doi.org/10.3390/ijms26062659
APA StyleMa, Q., Du, R., Long, P., Sun, K., Wang, Y., Yang, Y., Shen, X., & Gao, L. (2025). The Protective Effects of Burdock Fructooligosaccharide on Preterm Labor Through Its Anti-Inflammatory Action. International Journal of Molecular Sciences, 26(6), 2659. https://doi.org/10.3390/ijms26062659






