Multiple Inhibitory Mechanisms of DS16570511 Targeting Mitochondrial Calcium Uptake: Insights from Biochemical Analysis of Rat Liver Mitochondria
Abstract
1. Introduction
2. Results
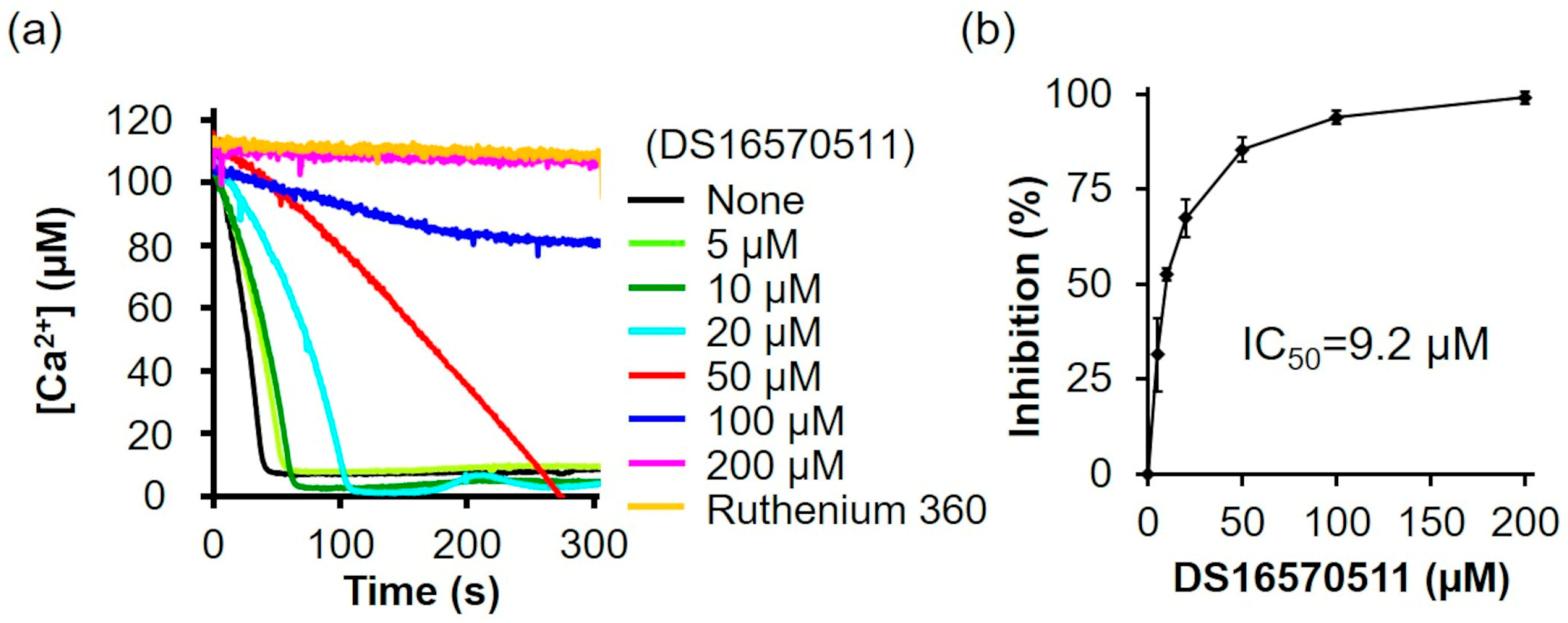
2.1. Inhibition of Mitochondrial Ca2+ Uptake by DS16570511
2.2. Effect of DS16570511 on Mitochondrial Membrane Potential Formation
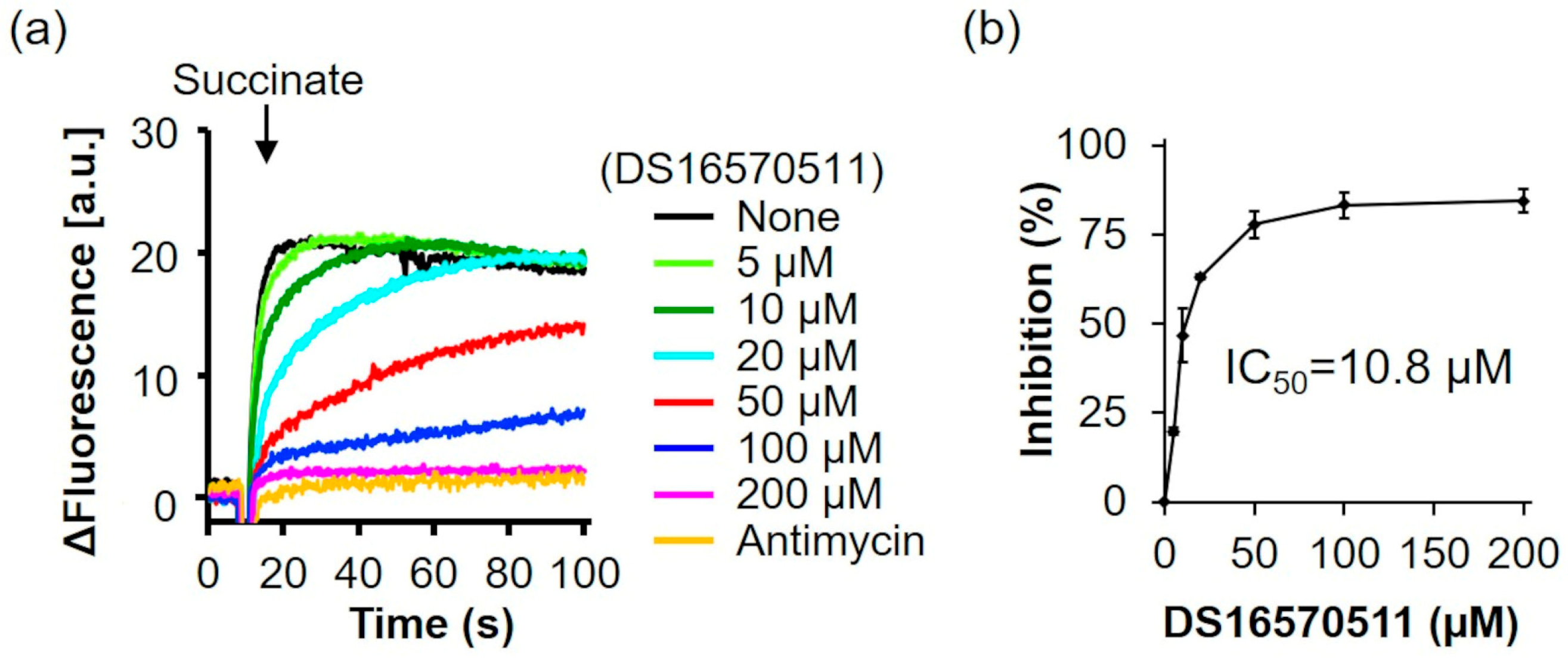
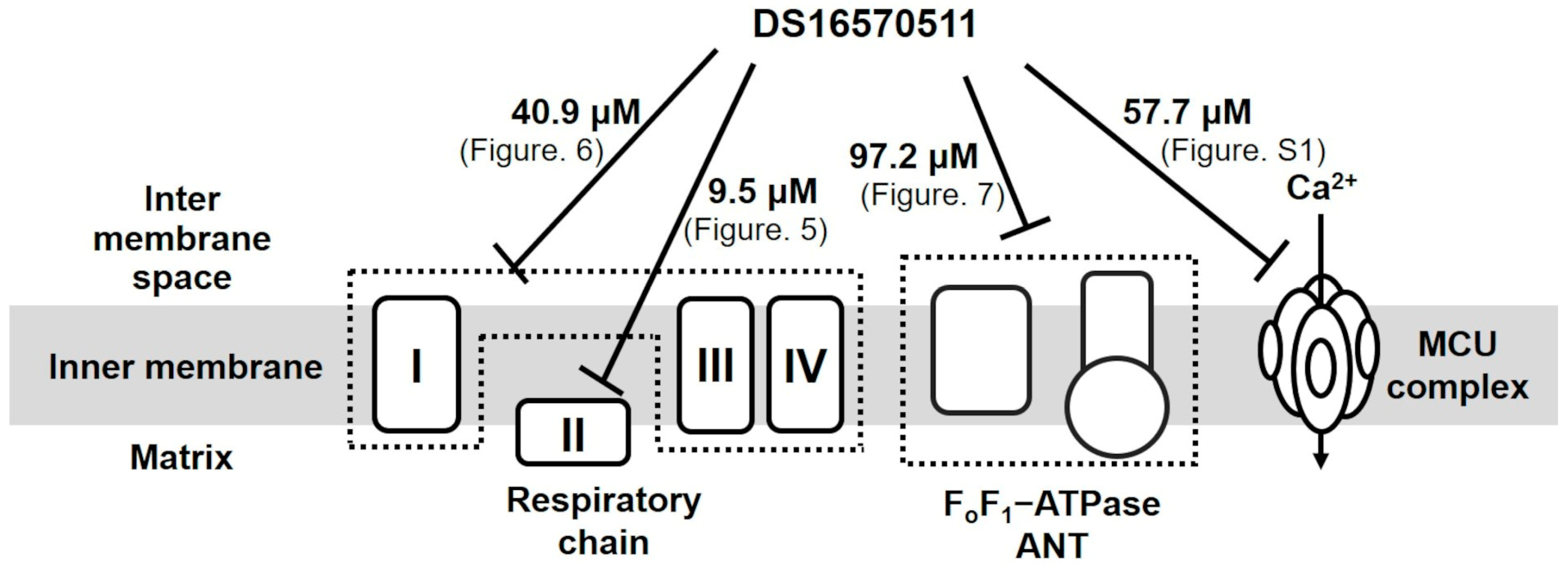
2.3. Effects of DS16570511 on the Activity of the Respiratory Chain Complex
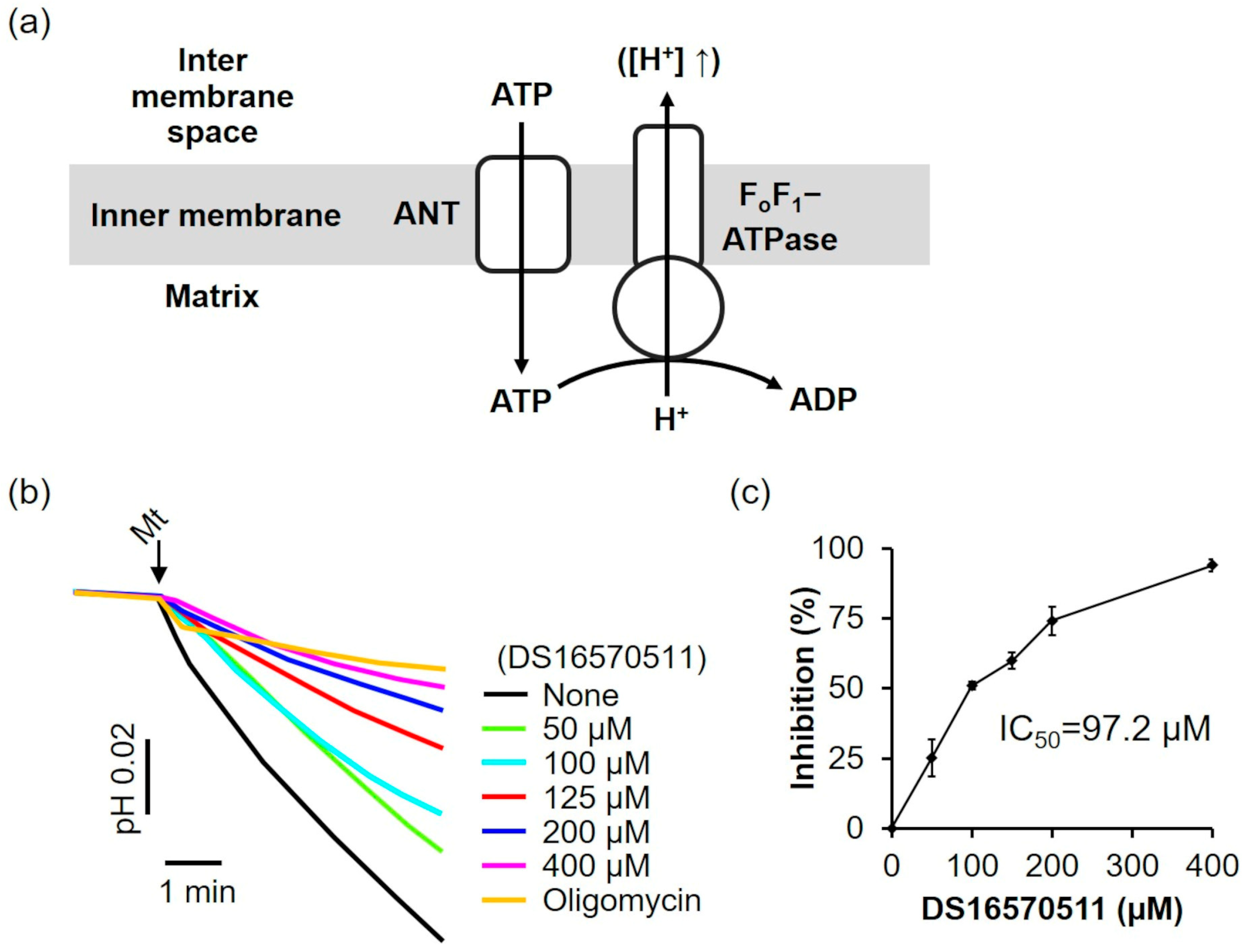
2.4. Effects of DS16570511 on Other Mitochondrial Functions
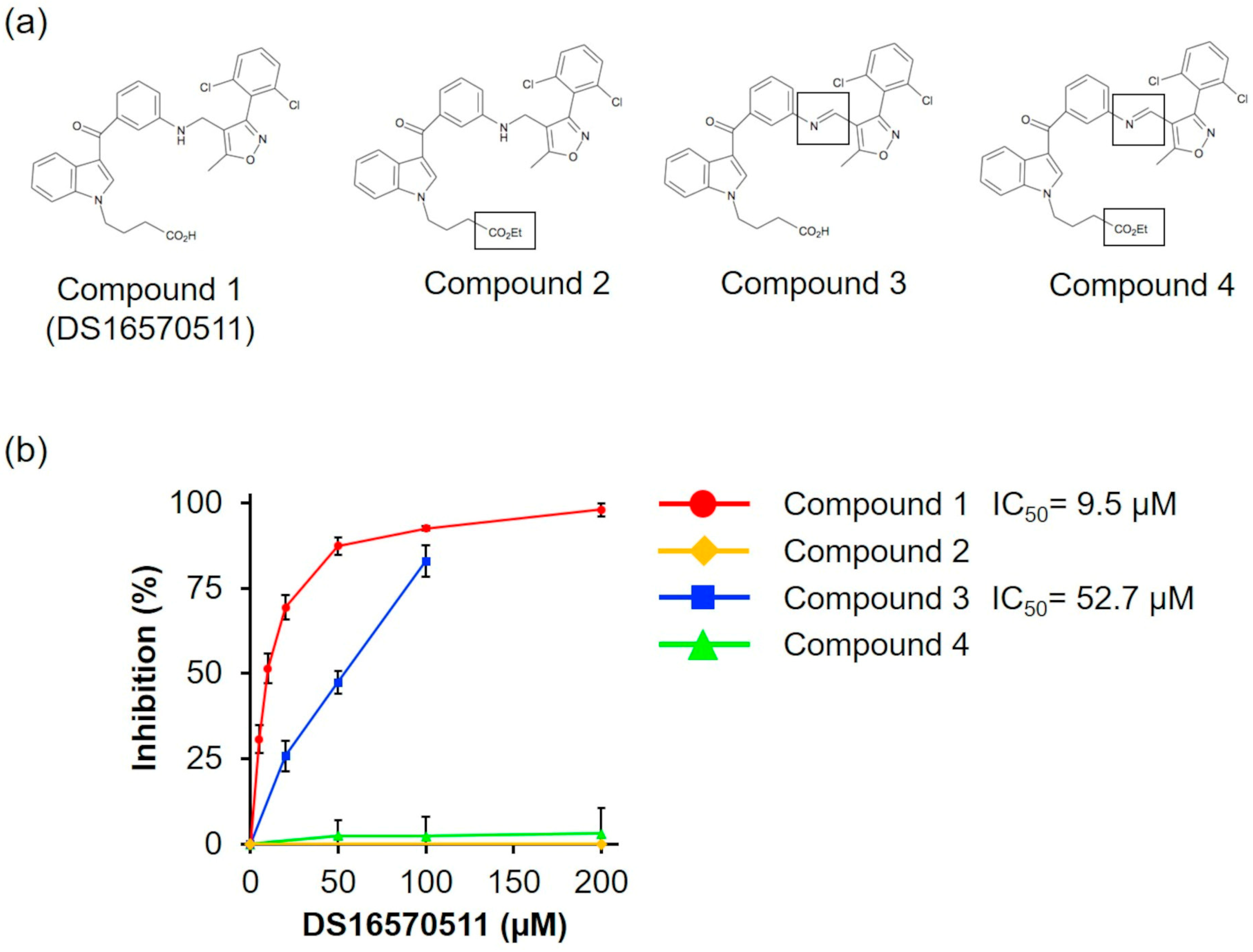
2.5. Structure–Activity Relationships of DS16570511
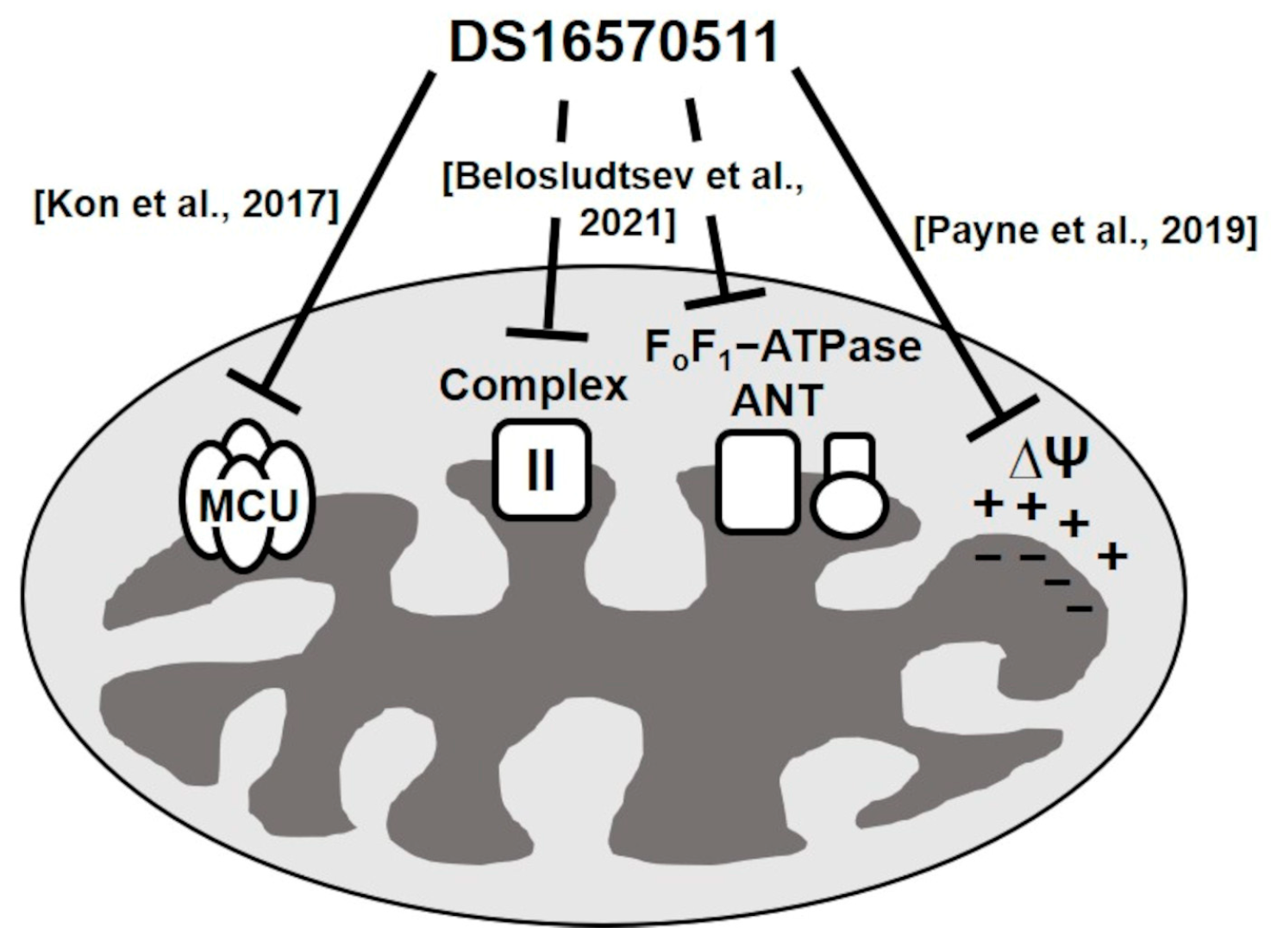
3. Discussion
4. Materials and Methods
4.1. Synthesis of DS16570511 and Its Analogs
4.2. Isolation of Rat Liver Mitochondria
4.3. Mitochondrial Ca2+ Uptake Assay
4.4. Measurement of Mitochondrial Membrane Potential
4.5. Measurement of the Rate of Mitochondrial Oxygen Consumption
4.6. Measurement of Mitochondrial FoF1-ATPase Activity
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANT | Adenine nucleotide translocator |
| ATP | Adenosine triphosphate |
| ADP | Adenosine diphosphate |
| EMRE | Essential MCU regulator |
| MCU | Mitochondrial calcium uniporter |
| MICU1 | Mitochondrial calcium uptake 1 |
References
- McCormack, J.G.; Denton, R.M. The role of mitochondrial Ca2+ transport and matrix Ca2+ in signal transduction in mammalian tissues. BBA Bioenerg. 1990, 1018, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Pizzo, P.; Drago, I.; Filadi, R.; Pozzan, T. Mitochondrial Ca2+ homeostasis: Mechanism, role, and tissue specificities. Pflug. Arch. Eur. J. Physiol. 2012, 464, 3–17. [Google Scholar] [CrossRef]
- Pendin, D.; Greotti, E.; Pozzan, T. The elusive importance of being a mitochondrial Ca2+ uniporter. Cell Calcium 2014, 55, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, P. Mitochondrial transport of cations: Channels, exchangers, and permeability transition. Physiol. Rev. 1999, 79, 1127–1155. [Google Scholar] [CrossRef]
- Giorgio, V.; von Stockum, S.; Antoniel, M.; Fabbro, A.; Fogolari, F.; Forte, M.; Glick, G.D.; Petronilli, V.; Zoratti, M.; Szabó, I.; et al. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc. Natl. Acad. Sci. USA 2013, 110, 5887–5892. [Google Scholar] [CrossRef]
- Giorgio, V.; Burchell, V.; Schiavone, M.; Bassot, C.; Minervini, G.; Petronilli, V.; Argenton, F.; Forte, M.; Tosatto, S.; Lippe, G.; et al. Ca2+ binding to F-ATP synthase β subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017, 18, 1065–1076. [Google Scholar] [CrossRef]
- Baines, C.P.; Kaiser, R.A.; Purcell, N.H.; Blair, N.S.; Osinska, H.; Hambleton, M.A.; Brunskill, E.W.; Sayen, M.R.; Gottlieb, R.A.; Dorn, G.W.; et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature 2005, 434, 658–662. [Google Scholar] [CrossRef]
- Palma, E.; Tiepolo, T.; Angelin, A.; Sabatelli, P.; Maraldi, N.M.; Basso, E.; Forte, M.A.; Bernardi, P.; Bonaldo, P. Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum. Mol. Genet. 2009, 18, 2024–2031. [Google Scholar] [CrossRef] [PubMed]
- Gunter, T.E.; Pfeiffer, D.R. Mechanisms by which mitochondria transport calcium. Am. J. Physiol. 1990, 258, C755–C786. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Santo-Domingo, J.; Demaurex, N. Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 907–912. [Google Scholar] [CrossRef] [PubMed]
- De Stefani, D.; Raffaello, A.; Teardo, E.; Szabó, I.; Rizzuto, R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 2011, 476, 336–340. [Google Scholar] [CrossRef]
- Baughman, J.M.; Perocchi, F.; Girgis, H.S.; Plovanich, M.; Belcher-Timme, C.A.; Sancak, Y.; Bao, X.R.; Strittmatter, L.; Goldberger, O.; Bogorad, R.L.; et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 2011, 476, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Perocchi, F.; Gohil, V.M.; Girgis, H.S.; Bao, X.R.; McCombs, J.E.; Palmer, A.E.; Mootha, V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature 2010, 467, 291–296. [Google Scholar] [CrossRef]
- Plovanich, M.; Bogorad, R.L.; Sancak, Y.; Kamer, K.J.; Strittmatter, L.; Li, A.A.; Girgis, H.S.; Kuchimanchi, S.; De Groot, J.; Speciner, L.; et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE 2013, 8, e55785. [Google Scholar] [CrossRef] [PubMed]
- Mallilankaraman, K.; Cárdenas, C.; Doonan, P.J.; Chandramoorthy, H.C.; Irrinki, K.M.; Golenár, T.; Csordás, G.; Madireddi, P.; Yang, J.; Müller, M.; et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 2012, 14, 1336–1343. [Google Scholar] [CrossRef]
- Raffaello, A.; De Stefani, D.; Sabbadin, D.; Teardo, E.; Merli, G.; Picard, A.; Checchetto, V.; Moro, S.; Szabò, I.; Rizzuto, R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013, 32, 2362–2376. [Google Scholar] [CrossRef]
- Sancak, Y.; Markhard, A.L.; Kitami, T.; Kovács-Bogdán, E.; Kamer, K.J.; Udeshi, N.D.; Carr, S.A.; Chaudhuri, D.; Clapham, D.E.; Li, A.A.; et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science 2013, 1979, 1379–1382. [Google Scholar] [CrossRef]
- Moore, C.L. Specific inhibition of mitochondrial Ca++ transport by ruthenium red. Biochem. Biophys. Res. Commun. 1971, 42, 298–305. [Google Scholar] [CrossRef]
- Ying, W.L.; Emerson, J.; Clarke, M.J.; Sanadi, D.R. Inhibition of mitochondrial calcium ion transport by an oxo-bridged dinuclear ruthenium ammine complex. Biochemistry 1991, 30, 4949–4952. [Google Scholar] [CrossRef]
- Matlib, M.A.; Zhou, Z.; Knight, S.; Ahmed, S.; Choi, K.M.; Krause-Bauer, J.; Phillips, R.; Altschuld, R.; Katsube, Y.; Sperelakis, N.; et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in Vitroand in situ in single cardiac myocytes. J. Biol. Chem. 1998, 273, 10223–10231. [Google Scholar] [CrossRef]
- Woods, J.J.; Nemani, N.; Shanmughapriya, S.; Kumar, A.; Zhang, M.; Nathan, S.R.; Thomas, M.; Carvalho, E.; Ramachandran, K.; Srikantan, S.; et al. A selective and cell-permeable mitochondrial calcium uniporter (MCU) inhibitor preserves mitochondrial bioenergetics after hypoxia/reoxygenation injury. ACS Cent. Sci. 2019, 5, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Novorolsky, R.J.; Nichols, M.; Kim, J.S.; Pavlov, E.V.; J Woods, J.; Wilson, J.J.; Robertson, G.S. The cell-permeable mitochondrial calcium uniporter inhibitor Ru265 preserves cortical neuron respiration after lethal oxygen glucose deprivation and reduces hypoxic/ischemic brain injury. J. Cereb. Blood Flow Metab. 2020, 40, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Swain, S.; Novorolsky, R.J.; Garcia, E.; Huang, Z.; Snutch, T.P.; Wilson, J.J.; Robertson, G.S.; Renden, R.B. The mitochondrial calcium uniporter inhibitor Ru265 increases neuronal excitability and reduces neurotransmission via off-target effects. Br. J. Pharmacol. 2024, 181, 3503–3526. [Google Scholar] [CrossRef] [PubMed]
- Arduino, D.M.; Wettmarshausen, J.; Vais, H.; Navas-Navarro, P.; Cheng, Y.; Leimpek, A.; Ma, Z.; Delrio-Lorenzo, A.; Giordano, A.; Garcia-Perez, C.; et al. Systematic identification of MCU modulators by orthogonal interspecies chemical screening. Mol. Cell 2017, 67, 711–723.e7. [Google Scholar] [CrossRef]
- Kon, N.; Murakoshi, M.; Isobe, A.; Kagechika, K.; Miyoshi, N.; Nagayama, T. DS16570511 is a small-molecule inhibitor of the mitochondrial calcium uniporter. Cell Death Discov. 2017, 3, 1–7. [Google Scholar] [CrossRef]
- Payne, R.; Li, C.; Fernandez-garcia, E.; Vais, H.; Foskett, K. The MCU inhibitor Ds16570511 has off-target effects on mitochondrial membrane potential. Biophys. J. 2019, 116, 270a. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Sharipov, R.R.; Boyarkin, D.P.; Belosludtseva, N.V.; Dubinin, M.V.; Krasilnikova, I.A.; Bakaeva, Z.V.; Zgodova, A.E.; Pinelis, V.G.; Surin, A.M. The effect of DS16570511, a new inhibitor of mitochondrial calcium uniporter, on calcium homeostasis, metabolism, and functional state of cultured cortical neurons and isolated brain mitochondria. Biochim. Biophys. Acta (BBA) Gen. Subj. 2021, 1865, 129847. [Google Scholar] [CrossRef]
- Marzo, I.; Brenner, C.; Zamzami, N.; Jürgensmeier, J.M.; Susin, S.A.; Vieira, H.L.; Prévost, M.C.; Xie, Z.; Matsuyama, S.; Reed, J.C.; et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 1998, 1979, 2027–2031. [Google Scholar] [CrossRef]
- Chevrollier, A.; Loiseau, D.; Reynier, P.; Stepien, G. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim. Biophys. Acta 2011, 1807, 562–567. [Google Scholar] [CrossRef]
- Kim, Y.; Kwak, C.; Park, J.B.; Nam, D.-H.; Rhee, H.-W.; Kim, S.S. The regulatory roles of mitochondrial metabolism dynamics and mitochondria calcium uniporter (MCU) in bevacizumab resistance of GBM. Adv. Ther. 2023, 6, 2300067. [Google Scholar] [CrossRef]
- Yamamoto, T.; Yamada, A.; Watanabe, M.; Yoshimura, Y.; Yamazaki, N.; Yoshimura, Y.; Yamauchi, T.; Kataoka, M.; Nagata, T.; Terada, H.; et al. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J. Proteome Res. 2006, 5, 3336–3344. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Okada, S.; Golden, P.; Kayakiri, N.; Sawada, Y.; Hashimoto, M.; Tanaka, H. 4-(1-Benzoylindol-3-yl) butyric acids and FK143: Novel nonsteroidal inhibitors of steroid 5α-reductase (II). Chem. Pharm. Bull. 1999, 47, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Epple, R.; Russo, R.; Azimioara, M.; Xie, Y. Isoxazole Compounds as PPAR Modulators, Their Preparation, Pharmaceutical Compositions, and Use in Therapy. U.S. Patent 016672, 1 December 2005. [Google Scholar]
- Yamada, A.; Yamamoto, T.; Yamazaki, N.; Yamashita, K.; Kataoka, M.; Nagata, T.; Terada, H.; Shinohara, Y. Differential permeabilization effects of Ca2+ and valinomycin on the inner and outer mitochondrial membranes as revealed by proteomics analysis of proteins released from mitochondria. Mol. Cell. Proteomics 2009, 8, 1265–1277. [Google Scholar] [CrossRef]
- Nishimura, M.; Ito, T.; Chance, B. Studies on bacterial photophosphorylation. III. A sensitive and rapid method of determination of photophosphorylation. Biochim. Biophys. Acta 1962, 59, 177–182. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, A.; Watanabe, A.; Nara, A.; Inokuma, T.; Asano, M.; Shinohara, Y.; Yamamoto, T. Multiple Inhibitory Mechanisms of DS16570511 Targeting Mitochondrial Calcium Uptake: Insights from Biochemical Analysis of Rat Liver Mitochondria. Int. J. Mol. Sci. 2025, 26, 2670. https://doi.org/10.3390/ijms26062670
Yamada A, Watanabe A, Nara A, Inokuma T, Asano M, Shinohara Y, Yamamoto T. Multiple Inhibitory Mechanisms of DS16570511 Targeting Mitochondrial Calcium Uptake: Insights from Biochemical Analysis of Rat Liver Mitochondria. International Journal of Molecular Sciences. 2025; 26(6):2670. https://doi.org/10.3390/ijms26062670
Chicago/Turabian StyleYamada, Akiko, Akira Watanabe, Atsushi Nara, Tsubasa Inokuma, Masatake Asano, Yasuo Shinohara, and Takenori Yamamoto. 2025. "Multiple Inhibitory Mechanisms of DS16570511 Targeting Mitochondrial Calcium Uptake: Insights from Biochemical Analysis of Rat Liver Mitochondria" International Journal of Molecular Sciences 26, no. 6: 2670. https://doi.org/10.3390/ijms26062670
APA StyleYamada, A., Watanabe, A., Nara, A., Inokuma, T., Asano, M., Shinohara, Y., & Yamamoto, T. (2025). Multiple Inhibitory Mechanisms of DS16570511 Targeting Mitochondrial Calcium Uptake: Insights from Biochemical Analysis of Rat Liver Mitochondria. International Journal of Molecular Sciences, 26(6), 2670. https://doi.org/10.3390/ijms26062670





