Biofilm-Associated Amyloid Proteins Linked with the Progression of Neurodegenerative Diseases
Abstract
1. Introduction
2. Clinical Significance of Amyloid Proteins in Neurodegenerative Diseases
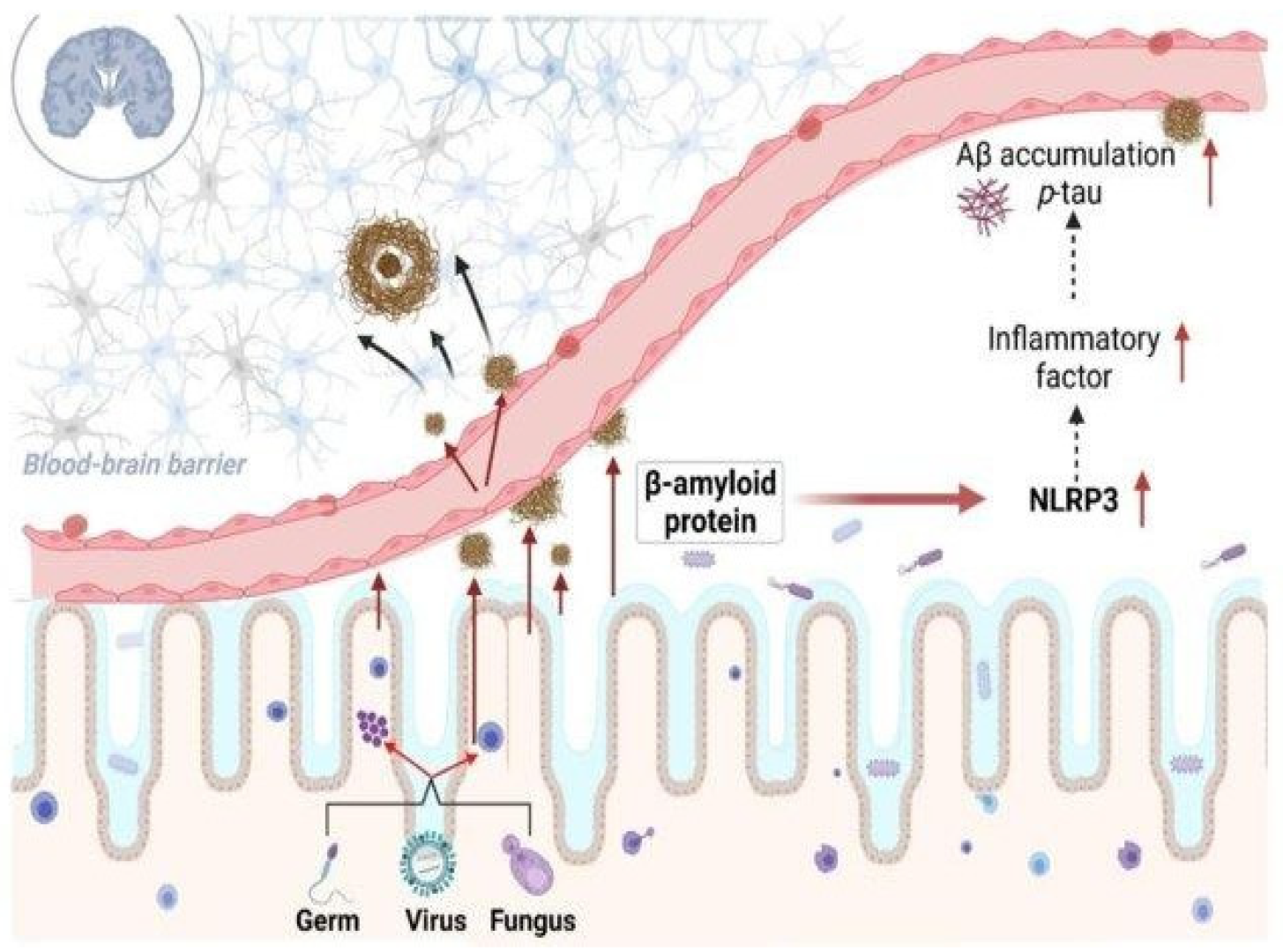
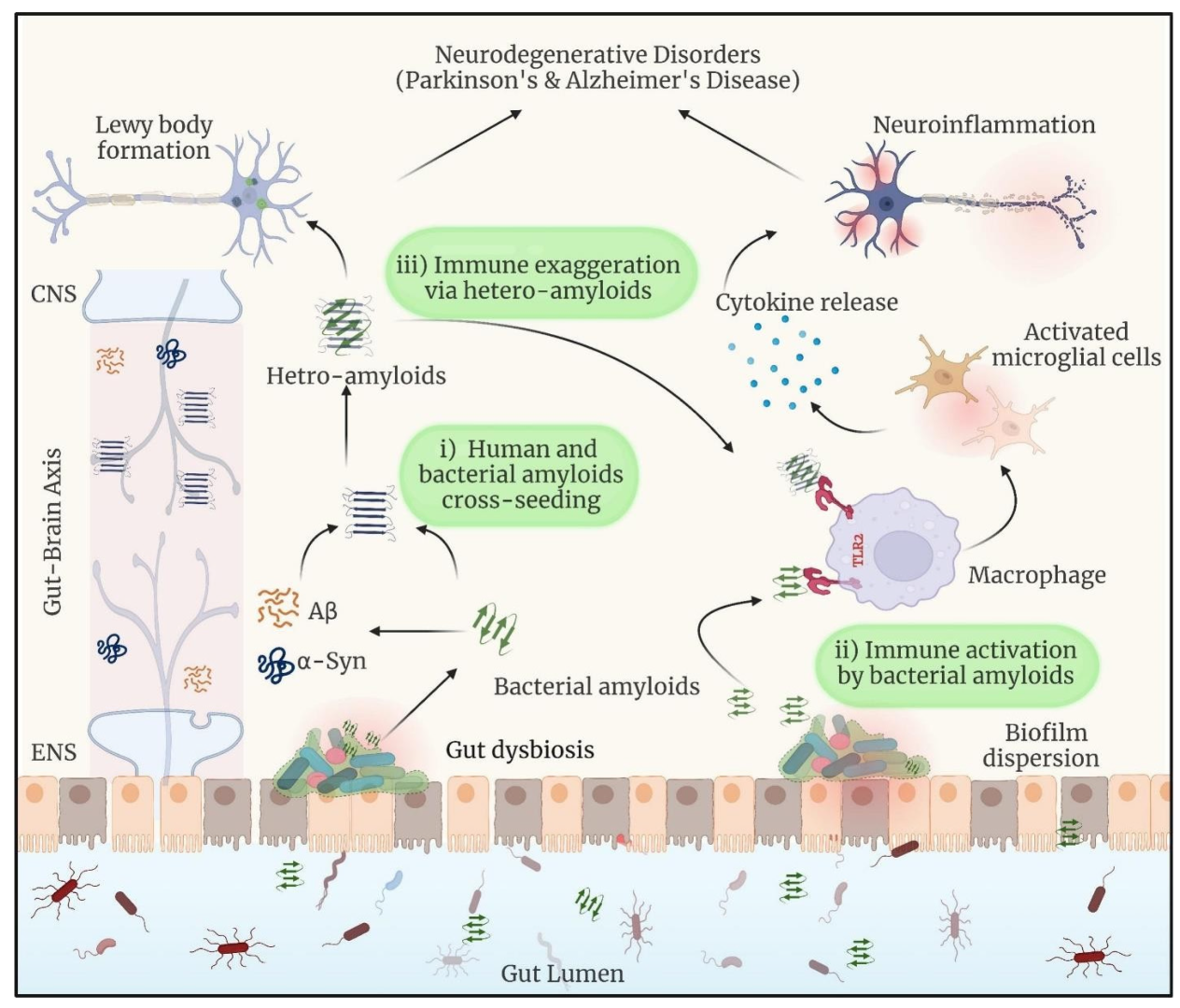
3. Intricate Connections Between Microbial Biofilms-Associated Proteins and Neurological Disorders
| Name of BAPs | Microbes | Role in Microbial Life | Experimental Evidence in the Involvement of Neurodegenerative Diseases | References |
|---|---|---|---|---|
| Phenol-Soluble Modulins (PSMs) | Staphylococcus aureus | I. Colony spread, biofilm formation, and immune cell death, II. Production of pro-inflammatory cytokines in human keratinocytes is caused by pore creation, membrane rupture, and, ultimately, cell death. | I. PSMα3, with its monomeric and oligomeric forms, controls Aβ40 aggregation and plays a complex role in AD. II. The oligomer promotes Aβ40 aggregation, while the monomer prevents it, demonstrating a dual effect on the disease’s molecular pathogenesis. | [67,68] |
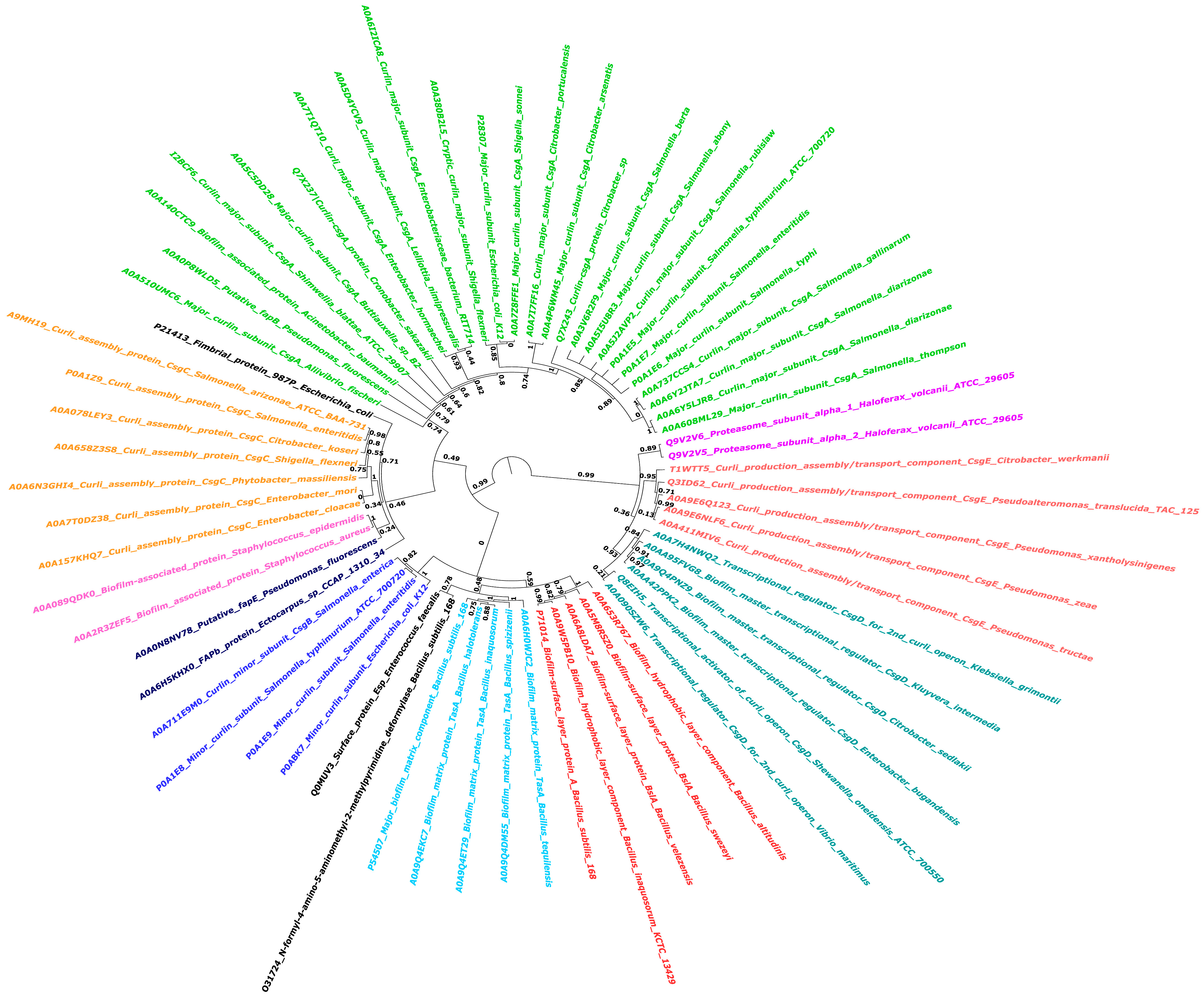
| Curli (CsgA and CsgB) | Escherichia coli | I. Curli formation, lipopolysaccharide (LPS) generation, and lysozyme inhibition. II. Adenosylcobalamin synthesis and oxidative stress response. | I. Curli exhibits cross-seeding and colocalization with α-syn in both C. elegans neurons and human neuroblastoma cells. II. Curli-induced α-syn aggregations inhibit mitochondrial gene expression, leading to energy failure in neurons. III. Curli may promote neuropathologies caused by many aggregation-prone proteins, including Aβ in Alzheimer’s disease, Huntingtin in Huntington’s disease, and SOD1 in amyotrophic lateral sclerosis. | [69] |
| FapC | Pseudomonas genus | I. FapC plays an important function in microbial life by encouraging biofilm development with its amyloidogenic characteristics. | I. FapC, especially its mutant version FapC ΔR1R2R3, inhibits the fibrillation of α-synuclein, which is a critical protein in Parkinson’s disease. II. The interaction is mediated by the production of disulfide bonds in FapC ΔR1R2R3. Bacterial amyloids may play a role in the onset and progression of Parkinson’s disease, as evidenced by the early presence of α-SN aggregates in GI tissues. III. FapC from gut bacteria like Pseudomonas may interact with α-SN in the GI tract, influencing its aggregation behavior. | [70] |
| TasA | Bacillus subtilis | I. Bacillus subtilis creates biofilms in which individual cells are kept together by an extracellular matrix. | I. TasA creates amyloid fibers. Amyloid fibers have been extensively researched in human neurodegenerative illnesses such as Alzheimer’s and Parkinson’s, as well as prion-induced spongiform encephalopathies. II. TasA restored wild-type biofilm morphology, demonstrating that the isolated protein preserved biological function. | [71] |
| Sup35 | S. cerevisiae | I. Confers a translation termination malfunction and expression level-dependent toxicity in its amyloid form. | I. Injection of Sup35 fibrils into the striatum of wild-type mice caused α-synopathy and PD-like motor impairment. II. In vitro and in vivo, Sup35-seeded α-syn fibrils outperformed pure α-syn fibrils in terms of seeding activity and neurotoxicity. These data suggest that the yeast prion protein Sup35 causes α-synopathy in PD. | [72,73] |
| Bap | Staphylococcus aureus | I. In the N-terminal region of these proteins, there are brief sections with amyloidogenic potential. II. After proteolytic cleavage, the N-terminal region adopts a β-sheet-rich shape and polymerizes in acidic conditions, forming fibrillar structures that promote biofilm formation. | I. The presence of specific BAP genes in the gut microbiome is associated with the occurrence of Parkinson’s disease (PD). II. In cultured dopaminergic neurons and Caenorhabditis elegans models, we found that BAP-derived amyloids cause α-synuclein aggregation. III. Research findings indicate that BAP amyloids interfere with chaperone-mediated autophagy. Indeed, introducing BAP fibrils into wild-type mice brains promotes critical degenerative hallmarks of Parkinson’s disease. | [19,74,75] |
| enterococcal surface protein (Esp) | Enterococcus faecalis | I. The N-terminal region was not essential for biofilm development, but it dramatically fortified biofilms against mechanical or degradative disruption, hence enhancing Enterococcus retention within biofilms. II. Biofilm strengthening necessitated low pH, which caused Esp to unfold, aggregate, and form amyloid-like formations. The pH threshold for biofilm strengthening was determined by protein stability. | I. Research has been published showing a correlation between the function of Esp and a higher abundance of BAP genes in the context of neurological disorders, mainly Parkinson’s diseases. The binary logistic regression finding shows that there is a notably increased chance of the Esp gene being present in PD patients. II. Esp or Esp743, when produced from a plasmid in E. faecalis FA2-2, has been shown to result in enhanced biofilm development, as measured quantitatively by CV staining. | [19,75,76] |
4. Biofilm Bacteria and Amyloid-Beta-like Proteins in Neurodegenerative Diseases
5. Biofilm Bacteria and α-Synuclein-like Protein Production in Neurodegenerative Diseases
6. Biofilm Bacteria and TDP-43 Protein Production in Neurodegenerative Diseases
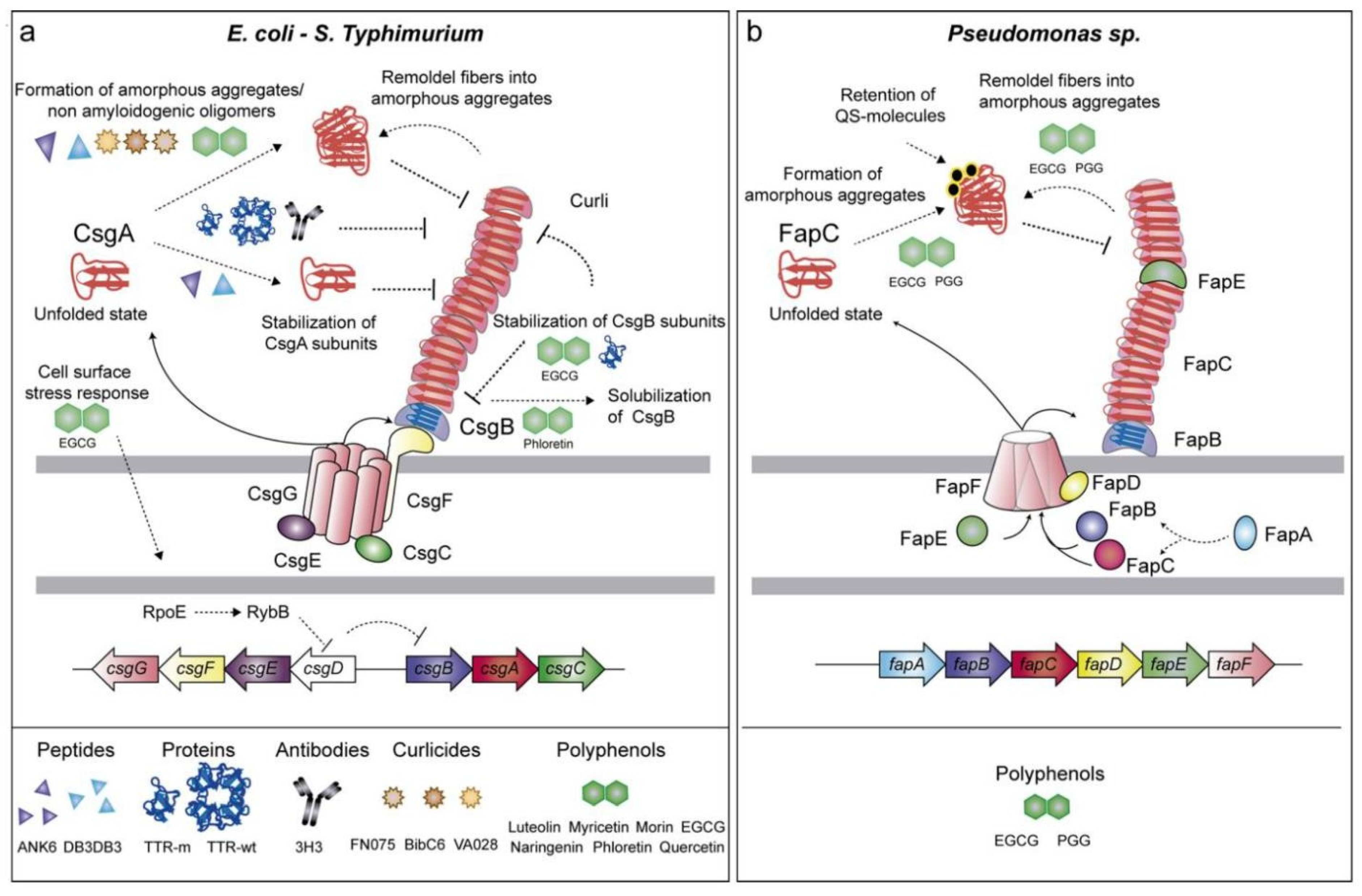
7. Mechanisms and Implications of Functional Amyloids in Biofilm-Forming Bacteria
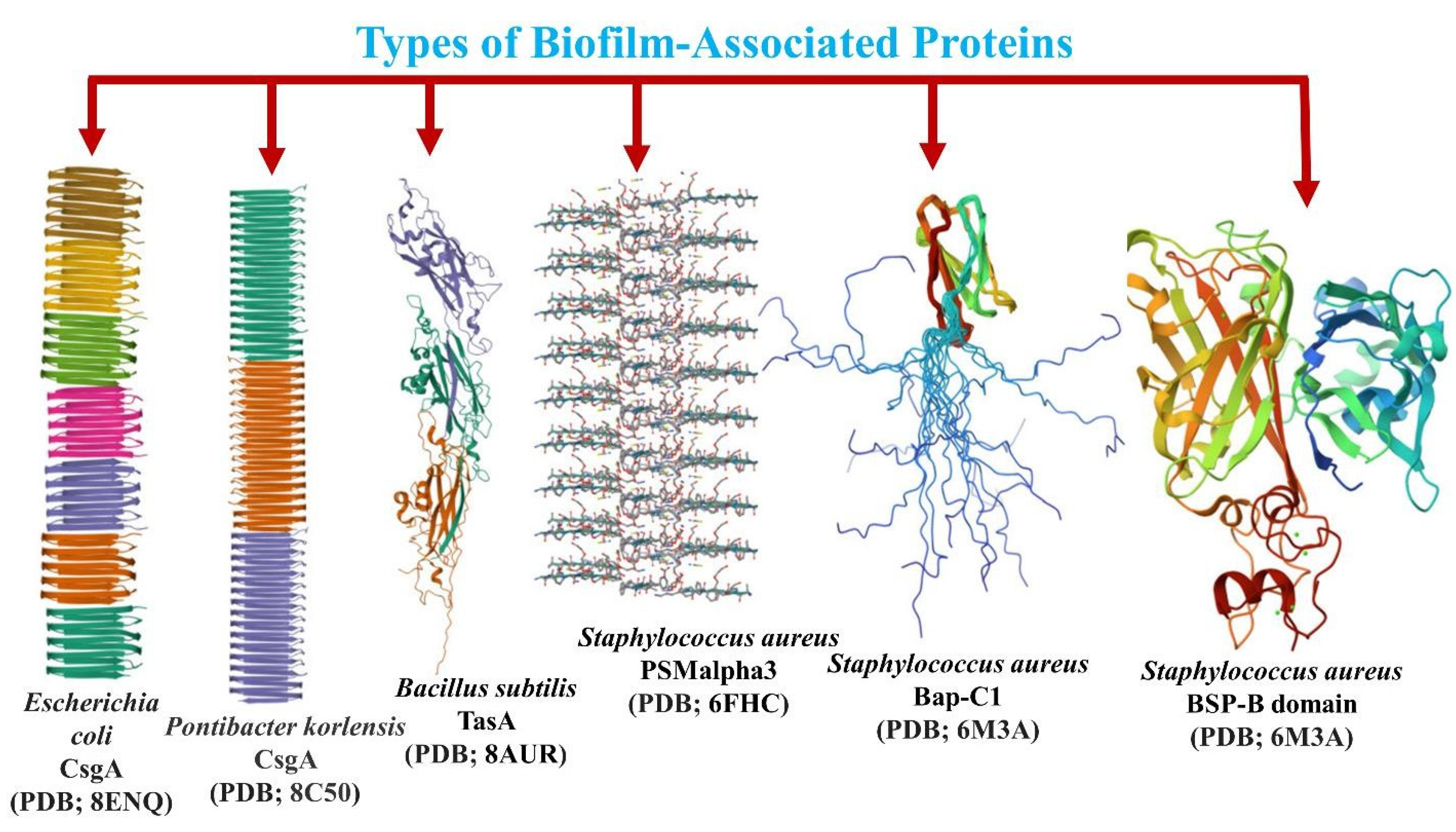
8. Diversity of Biofilm-Associated Proteins and Their Amyloid Fibril Formation Properties
9. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EPS | extracellular polymeric substances |
| ECM | extracellular matrix |
| PD | Parkinson’s disease |
| AD | Alzheimer’s disease |
| BAPs | bacterial-associated proteins |
| Aβ | amyloid beta |
| APP | amyloid precursor protein |
| PIGD | postural instability and gait difficulties |
| ALS | Amyotrophic lateral sclerosis |
| SOD1 | superoxide dismutase 1 |
| FUS | Fused in Sarcoma |
| p-tau | phosphorylated tau |
| TDP-43 | TAR DNA-binding protein 43 |
| P. gingivalis | Porphyromonas gingivalis |
| T. denticola | Treponema denticola |
| E. coli | Escherichia coli |
| B. subtilis | Bacillus subtilis |
| B. cereus | Bacillus cereus |
| APOE | apolipoprotein E |
| AβPP | amyloid-β protein precursor |
| PSM | phenol-soluble modulin |
References
- Li, B.; Mao, J.; Wu, J.; Mao, K.; Jia, Y.; Chen, F.; Liu, J. Nano–Bio Interactions: Biofilm-Targeted Antibacterial Nanomaterials. Small 2024, 20, 2306135. [Google Scholar] [CrossRef] [PubMed]
- Podobnik, M.; Marchioretto, M.; Zanetti, M.; Bavdek, A.; Kisovec, M.; Cajnko, M.M.; Lunelli, L.; Dalla Serra, M.; Anderluh, G. Plasticity of listeriolysin O pores and its regulation by pH and unique histidine. Sci. Rep. 2015, 5, 9623, Corrected in Sci. Rep. 2015, 5, 15690. [Google Scholar] [CrossRef] [PubMed]
- Bavdek, A.; Kostanjšek, R.; Antonini, V.; Lakey, J.H.; Dalla Serra, M.; Gilbert, R.J.; Anderluh, G. pH dependence of listeriolysin O aggregation and pore-forming ability. FEBS J. 2012, 279, 126–141. [Google Scholar] [CrossRef]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Stoodley, P. Evolving concepts in biofilm infections. Cell. Microbiol. 2009, 11, 1034–1043. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Cámara-Almirón, J.; Caro-Astorga, J.; de Vicente, A.; Romero, D. Beyond the expected: The structural and functional diversity of bacterial amyloids. Crit. Rev. Microbiol. 2018, 44, 653–666. [Google Scholar] [CrossRef]
- Ge, R.; Sun, X. The in vivo functions of a histidine-rich protein Hpn in Helicobacter pylori: Linking gastric and Alzheimer’s diseases together? Med. Hypotheses 2011, 77, 788–790. [Google Scholar] [CrossRef]
- Chuang, M.-H.; Wu, M.-S.; Lo, W.-L.; Lin, J.-T.; Wong, C.-H.; Chiou, S.-H. The antioxidant protein alkylhydroperoxide reductase of Helicobacter pylori switches from a peroxide reductase to a molecular chaperone function. Proc. Natl. Acad. Sci. USA 2006, 103, 2552–2557. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chuang, M.-H.; Wu, Y.-H.; Chuang, W.-C.; Jhuang, P.-J.; Chiou, S.-H. Characterization of site-specific mutants of alkylhydroperoxide reductase with dual functionality from Helicobacter pylori. J. Biochem. 2010, 147, 661–669. [Google Scholar] [CrossRef]
- Kierek-Pearson, K.; Karatan, E. Biofilm development in bacteria. Adv. Appl. Microbiol. 2005, 57, 79–111. [Google Scholar] [PubMed]
- Böhning, J.; Tarafder, A.K.; Bharat, T.A. The role of filamentous matrix molecules in shaping the architecture and emergent properties of bacterial biofilms. Biochem. J. 2024, 481, 245–263. [Google Scholar] [CrossRef]
- Miller, A.L.; Bessho, S.; Grando, K.; Tükel, Ç. Microbiome or infections: Amyloid-containing biofilms as a trigger for complex human diseases. Front. Immunol. 2021, 12, 638867. [Google Scholar] [CrossRef] [PubMed]
- Elkins, M.; Jain, N.; Tükel, Ç. The menace within: Bacterial amyloids as a trigger for autoimmune and neurodegenerative diseases. Curr. Opin. Microbiol. 2024, 79, 102473. [Google Scholar] [CrossRef]
- Kumari, S.; Das, S. Functional amyloid fibrils of biofilm-forming marine bacterium Pseudomonas aeruginosa PFL-P1 interact spontaneously with pyrene and augment the biodegradation. Int. J. Biol. Macromol. 2024, 266, 131266. [Google Scholar] [CrossRef]
- Endres, K. Amyloidogenic peptides in human neuro-degenerative diseases and in microorganisms: A sorrow shared is a sorrow halved? Molecules 2020, 25, 925. [Google Scholar] [CrossRef]
- Chen, C.; Ahn, E.H.; Kang, S.S.; Liu, X.; Alam, A.; Ye, K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPβ/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020, 6, eaba0466. [Google Scholar] [CrossRef]
- Dodiya, H.B.; Lutz, H.L.; Weigle, I.Q.; Patel, P.; Michalkiewicz, J.; Roman-Santiago, C.J.; Zhang, C.M.; Liang, Y.; Srinath, A.; Zhang, X. Gut microbiota–driven brain Aβ amyloidosis in mice requires microglia. J. Exp. Med. 2022, 219, e20200895. [Google Scholar] [CrossRef]
- Fernández-Calvet, A.; Matilla-Cuenca, L.; Izco, M.; Navarro, S.; Serrano, M.; Ventura, S.; Blesa, J.; Herráiz, M.; Alkorta-Aranburu, G.; Galera, S. Gut microbiota produces biofilm-associated amyloids with potential for neurodegeneration. Nat. Commun. 2024, 15, 4150. [Google Scholar] [CrossRef]
- Jain, N. The molecular interplay between human and bacterial amyloids: Implications in neurodegenerative diseases. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2024, 1872, 141018. [Google Scholar] [CrossRef]
- Salahuddin, P.; Fatima, M.T.; Uversky, V.N.; Khan, R.H.; Islam, Z.; Furkan, M. The role of amyloids in Alzheimer’s and Parkinson’s diseases. Int. J. Biol. Macromol. 2021, 190, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Cuenca, L.; Taglialegna, A.; Gil, C.; Toledo-Arana, A.; Lasa, I.; Valle, J. Bacterial biofilm functionalization through Bap amyloid engineering. NPJ Biofilms Microbiomes 2022, 8, 62. [Google Scholar] [CrossRef] [PubMed]
- Subedi, S.; Sasidharan, S.; Nag, N.; Saudagar, P.; Tripathi, T. Amyloid cross-seeding: Mechanism, implication, and inhibition. Molecules 2022, 27, 1776. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Zhang, Y.; Zhang, M.; Liu, Y.; Zhang, D.; Gong, X.; Feng, Z.; Tang, J.; Chang, Y.; Zheng, J. Fundamentals of cross-seeding of amyloid proteins: An introduction. J. Mater. Chem. B 2019, 7, 7267–7282. [Google Scholar] [CrossRef]
- Yang, W.; Guan, F.; Yang, L.; Shou, G.; Zhu, F.; Xu, Y.; Meng, Y.; Li, M.; Dong, W. Highly sensitive blood-based biomarkers detection of beta-amyloid and phosphorylated-tau181 for Alzheimer’s disease. Front. Neurol. 2024, 15, 1445479. [Google Scholar] [CrossRef]
- Friedland, R.P.; Chapman, M.R. The role of microbial amyloid in neurodegeneration. PLoS Pathog. 2017, 13, e1006654. [Google Scholar] [CrossRef]
- Dueholm, M.S.; Nielsen, S.B.; Hein, K.L.; Nissen, P.; Chapman, M.; Christiansen, G.; Nielsen, P.H.; Otzen, D.E. Fibrillation of the major curli subunit CsgA under a wide range of conditions implies a robust design of aggregation. Biochemistry 2011, 50, 8281–8290. [Google Scholar] [CrossRef]
- Bashir, S.; Aiman, A.; Shahid, M.; Chaudhary, A.A.; Sami, N.; Basir, S.F.; Hassan, I.; Islam, A. Amyloid-induced neurodegeneration: A comprehensive review through aggregomics perception of proteins in health and pathology. Ageing Res. Rev. 2024, 96, 102276. [Google Scholar] [CrossRef]
- Anbarasu, K.; Sivakumar, J. Multidimensional significance of crystallin protein–protein interactions and their implications in various human diseases. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2016, 1860, 222–233. [Google Scholar] [CrossRef]
- Rukmangadachar, L.A.; Bollu, P.C. Amyloid beta peptide. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Honcharenko, D.; Juneja, A.; Roshan, F.; Maity, J.; Galan-Acosta, L.; Biverstal, H.; Hjorth, E.; Johansson, J.; Fisahn, A.; Nilsson, L. Amyloid-β peptide targeting peptidomimetics for prevention of neurotoxicity. ACS Chem. Neurosci. 2019, 10, 1462–1477. [Google Scholar] [CrossRef]
- Kowalski, K.; Mulak, A. Brain-gut-microbiota axis in Alzheimer’s disease. J. Neurogastroenterol. Motil. 2019, 25, 48. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Tanzi, R.E. Thirty years of Alzheimer’s disease genetics: The implications of systematic meta-analyses. Nat. Rev. Neurosci. 2008, 9, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.W.; Aarsland, D.; Ffytche, D.; Taddei, R.N.; van Wamelen, D.J.; Wan, Y.-M.; Tan, E.K.; Ray Chaudhuri, K. Amyloid-β and Parkinson’s disease. J. Neurol. 2019, 266, 2605–2619. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Jeon, G.S.; Shim, Y.-m.; Seong, S.-Y.; Lee, K.-W.; Sung, J.-J. Modulation of SOD1 subcellular localization by transfection with wild-or mutant-type SOD1 in primary neuron and astrocyte cultures from ALS mice. Exp. Neurobiol. 2015, 24, 226. [Google Scholar] [CrossRef]
- Higelin, J.; Demestre, M.; Putz, S.; Delling, J.P.; Jacob, C.; Lutz, A.-K.; Bausinger, J.; Huber, A.-K.; Klingenstein, M.; Barbi, G. FUS mislocalization and vulnerability to DNA damage in ALS patients derived hiPSCs and aging motoneurons. Front. Cell. Neurosci. 2016, 10, 290. [Google Scholar] [CrossRef]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef]
- Geser, F.; Brandmeir, N.J.; Kwong, L.K.; Martinez-Lage, M.; Elman, L.; McCluskey, L.; Xie, S.X.; Lee, V.M.-Y.; Trojanowski, J.Q. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch. Neurol. 2008, 65, 636–641. [Google Scholar] [CrossRef]
- Barmada, S.J.; Skibinski, G.; Korb, E.; Rao, E.J.; Wu, J.Y.; Finkbeiner, S. Cytoplasmic mislocalization of TDP-43 is toxic to neurons and enhanced by a mutation associated with familial amyotrophic lateral sclerosis. J. Neurosci. 2010, 30, 639–649. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef]
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments in Alzheimer disease: An update. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520907397. [Google Scholar] [CrossRef]
- Kim, A.Y.; Al Jerdi, S.; MacDonald, R.; Triggle, C.R. Alzheimer’s disease and its treatment–yesterday, today, and tomorrow. Front. Pharmacol. 2024, 15, 1399121. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.A. Risk factors for Alzheimer’s disease. Folia Neuropathol. 2019, 57, 87–105. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B. A review on cholinesterase inhibitors for Alzheimer’s disease. Arch. Pharmacal Res. 2013, 36, 375–399. [Google Scholar] [CrossRef]
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020–2060). Alzheimer’s Dement. 2021, 17, 1966–1975. [Google Scholar] [CrossRef] [PubMed]
- Jack Jr, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J. NIA-AA research framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS–ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- Geng, T.; Li, Y.; Peng, Y.; Chen, X.; Xu, X.; Wang, J.; Sun, L.; Gao, X. Social isolation and the risk of Parkinson disease in the UK biobank study. NPJ Park. Dis. 2024, 10, 79. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Lewis, M.M.; Mailman, R.B.; Cheng, X.V.; Du, G.; Zhang, L.; Li, C.; De Jesus, S.; Tabbal, S.D.; Li, R.; Huang, X. Clinical progression of Parkinson’s disease in the early 21st century: Insights from the accelerating medicine partnership (AMP-PD) data. Park. Relat. Disord. 2025, 130, 107186. [Google Scholar] [CrossRef]
- Grotewold, N.; Albin, R.L. Update: Descriptive epidemiology of Parkinson disease. Park. Relat. Disord. 2024, 120, 106000. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Darweesh, S.; Llibre-Guerra, J.; Marras, C.; San Luciano, M.; Tanner, C. The epidemiology of Parkinson’s disease. Lancet 2024, 403, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Brundin, P.; Dave, K.D.; Kordower, J.H. Therapeutic approaches to target alpha-synuclein pathology. Exp. Neurol. 2017, 298, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Qiu, C.; Lu, Y.; Luo, B.; Jiang, X.; Chang, L.; Yan, J.; Sun, J.; Liu, W.; Zhang, L.; et al. Effect of deep brain stimulation compared with drug therapy alone on the progression of Parkinson’s disease. Front. Neurosci. 2023, 17, 1330752. [Google Scholar] [CrossRef] [PubMed]
- Stillman, N.H.; Joseph, J.A.; Ahmed, J.; Baysah, C.Z.; Dohoney, R.A.; Ball, T.D.; Thomas, A.G.; Fitch, T.C.; Donnelly, C.M.; Kumar, S. Protein mimetic 2D FAST rescues alpha synuclein aggregation mediated early and post disease Parkinson’s phenotypes. Nat. Commun. 2024, 15, 3658. [Google Scholar] [CrossRef]
- Matrone, C. The paradigm of amyloid precursor protein in amyotrophic lateral sclerosis: The potential role of the (682)YENPTY(687) motif. Comput. Struct. Biotechnol. J. 2023, 21, 923–930. [Google Scholar] [CrossRef]
- Wei, Y.; Zhong, S.; Yang, H.; Wang, X.; Lv, B.; Bian, Y.; Pei, Y.; Xu, C.; Zhao, Q.; Wu, Y.; et al. Current therapy in amyotrophic lateral sclerosis (ALS): A review on past and future therapeutic strategies. Eur. J. Med. Chem. 2024, 272, 116496. [Google Scholar] [CrossRef]
- Wang, H.; Guan, L.; Deng, M. Recent progress of the genetics of amyotrophic lateral sclerosis and challenges of gene therapy. Front. Neurosci. 2023, 17, 1170996. [Google Scholar] [CrossRef]
- Brown, R.H.; Al-Chalabi, A. Amyotrophic Lateral Sclerosis. N. Engl. J. Med. 2017, 377, 162–172. [Google Scholar] [CrossRef]
- Morgan, S.; Orrell, R.W. Pathogenesis of amyotrophic lateral sclerosis. Br. Med. Bull. 2016, 119, 87–98. [Google Scholar] [CrossRef]
- Quinn, C.; Elman, L. Amyotrophic Lateral Sclerosis and Other Motor Neuron Diseases. Continuum 2020, 26, 1323–1347. [Google Scholar] [CrossRef]
- Tzeplaeff, L.; Wilfling, S.; Requardt, M.V.; Herdick, M. Current State and Future Directions in the Therapy of ALS. Cells 2023, 12, 1523. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, C.; Gauvin, D.E.; Ishola, F.; Oskoui, M. Global Prevalence and Incidence of Amyotrophic Lateral Sclerosis: A Systematic Review. Neurology 2023, 101, e613–e623. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Li, J.; Ma, R.X.; Cheng, X.Y.; Ma, R.Y.; Zhou, T.Y.; Wu, Z.Q.; Yao, Y.; Li, J. Gut Microbiota is an Impact Factor based on the Brain-Gut Axis to Alzheimer’s Disease: A Systematic Review. Aging Dis. 2023, 14, 964–1678. [Google Scholar] [CrossRef]
- Duncan, S.H.; Iyer, A.; Russell, W.R. Impact of protein on the composition and metabolism of the human gut microbiota and health. Proc. Nutr. Soc. 2021, 80, 173–185. [Google Scholar] [CrossRef]
- Scott, K.P.; Gratz, S.W.; Sheridan, P.O.; Flint, H.J.; Duncan, S.H. The influence of diet on the gut microbiota. Pharmacol. Res. 2013, 69, 52–60. [Google Scholar] [CrossRef]
- Chung, E.J.; Luo, C.H.; Thio, C.L.; Chang, Y.J. Immunomodulatory Role of Staphylococcus aureus in Atopic Dermatitis. Pathogens 2022, 11, 422. [Google Scholar] [CrossRef]
- Peng, B.; Xu, S.; Liang, Y.; Dong, X.; Sun, Y. Effect of Bacterial Amyloid Protein Phenol-Soluble Modulin Alpha 3 on the Aggregation of Amyloid Beta Protein Associated with Alzheimer’s Disease. Biomimetics 2023, 8, 459. [Google Scholar] [CrossRef]
- Wang, C.; Lau, C.Y.; Ma, F.; Zheng, C. Genome-wide screen identifies curli amyloid fibril as a bacterial component promoting host neurodegeneration. Proc. Natl. Acad. Sci. USA 2021, 118, e2106504118. [Google Scholar] [CrossRef]
- Christensen, L.F.B.; Jensen, K.F.; Nielsen, J.; Vad, B.S.; Christiansen, G.; Otzen, D.E. Reducing the Amyloidogenicity of Functional Amyloid Protein FapC Increases Its Ability To Inhibit α-Synuclein Fibrillation. ACS Omega 2019, 4, 4029–4039. [Google Scholar] [CrossRef]
- Romero, D.; Aguilar, C.; Losick, R.; Kolter, R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 2230–2234. [Google Scholar] [CrossRef]
- Meng, L.; Liu, C.; Li, Y.; Chen, G.; Xiong, M.; Yu, T.; Pan, L.; Zhang, X.; Zhou, L.; Guo, T.; et al. The yeast prion protein Sup35 initiates α-synuclein pathology in mouse models of Parkinson’s disease. Sci. Adv. 2023, 9, eadj1092. [Google Scholar] [CrossRef] [PubMed]
- Pezza, J.A.; Villali, J.; Sindi, S.S.; Serio, T.R. Amyloid-associated activity contributes to the severity and toxicity of a prion phenotype. Nat. Commun. 2014, 5, 4384. [Google Scholar] [CrossRef] [PubMed]
- Taglialegna, A.; Navarro, S.; Ventura, S.; Garnett, J.A.; Matthews, S.; Penades, J.R.; Lasa, I.; Valle, J. Staphylococcal Bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog. 2016, 12, e1005711. [Google Scholar] [CrossRef]
- Spiegelman, L.; Bahn-Suh, A.; Montaño, E.T.; Zhang, L.; Hura, G.L.; Patras, K.A.; Kumar, A.; Tezcan, F.A.; Nizet, V.; Tsutakawa, S.E. Strengthening of enterococcal biofilms by Esp. PLoS Pathog. 2022, 18, e1010829. [Google Scholar] [CrossRef] [PubMed]
- Tendolkar, P.M.; Baghdayan, A.S.; Shankar, N. The N-terminal domain of enterococcal surface protein, Esp, is sufficient for Esp-mediated biofilm enhancement in Enterococcus faecalis. J. Bacteriol. 2005, 187, 6213–6222. [Google Scholar] [CrossRef]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Nie, R.; Wu, Z.; Ni, J.; Zeng, F.; Yu, W.; Zhang, Y.; Kadowaki, T.; Kashiwazaki, H.; Teeling, J.L.; Zhou, Y. Porphyromonas gingivalis Infection Induces Amyloid-β Accumulation in Monocytes/Macrophages. J. Alzheimer’s Dis. 2019, 72, 479–494. [Google Scholar] [CrossRef]
- Kanagasingam, S.; Chukkapalli, S.S.; Welbury, R.; Singhrao, S.K. Porphyromonas gingivalis is a strong risk factor for Alzheimer’s disease. J. Alzheimer’s Dis. Rep. 2020, 4, 501–511. [Google Scholar] [CrossRef]
- Aguayo, S.; Schuh, C.M.A.P.; Vicente, B.; Aguayo, L.G. Association between Alzheimer’s disease and oral and gut microbiota: Are pore forming proteins the missing link? J. Alzheimer’s Dis. 2018, 65, 29–46. [Google Scholar] [CrossRef]
- HB, A.; Cusack, C.; Joshi, S. The Impact of Biofilms in Alzheimer’s disease compared to Other Diseases in Which They Play a Role. Int. J. Neurobiol. 2019, 1, 115. [Google Scholar]
- Walker, L.C.; Jucker, M. Amyloid by default. Nat. Neurosci. 2011, 14, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Paumier, K.L.; Luk, K.C.; Manfredsson, F.P.; Kanaan, N.M.; Lipton, J.W.; Collier, T.J.; Steece-Collier, K.; Kemp, C.J.; Celano, S.; Schulz, E. Intrastriatal injection of pre-formed mouse α-synuclein fibrils into rats triggers α-synuclein pathology and bilateral nigrostriatal degeneration. Neurobiol. Dis. 2015, 82, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Betensky, R.A.; Hyman, B.T.; Serrano-Pozo, A. Association of APOE genotype with heterogeneity of cognitive decline rate in Alzheimer disease. Neurology 2021, 96, e2414–e2428. [Google Scholar] [CrossRef]
- Serrano-Pozo, A.; Li, Z.; Noori, A.; Nguyen, H.N.; Mezlini, A.; Li, L.; Hudry, E.; Jackson, R.J.; Hyman, B.T.; Das, S. Effect of APOE alleles on the glial transcriptome in normal aging and Alzheimer’s disease. Nat. Aging 2021, 1, 919–931. [Google Scholar] [CrossRef]
- Werner, T.; Horvath, I.; Wittung-Stafshede, P. Crosstalk between alpha-synuclein and other human and non-human amyloidogenic proteins: Consequences for amyloid formation in Parkinson’s disease. J. Park. Dis. 2020, 10, 819–830. [Google Scholar] [CrossRef]
- Phillips, R.J.; Walter, G.C.; Ringer, B.E.; Higgs, K.M.; Powley, T.L. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp. Neurol. 2009, 220, 109–119. [Google Scholar] [CrossRef]
- Baker, P.J.; Dixon, M.; Roopenian, D.C. Genetic control of susceptibility to Porphyromonas gingivalis-induced alveolar bone loss in mice. Infect. Immun. 2000, 68, 5864–5868. [Google Scholar] [CrossRef]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef]
- Nagaraj, M.; Najarzadeh, Z.; Pansieri, J.; Biverstål, H.; Musteikyte, G.; Smirnovas, V.; Matthews, S.; Emanuelsson, C.; Johansson, J.; Buxbaum, J.N. Chaperones mainly suppress primary nucleation during formation of functional amyloid required for bacterial biofilm formation. Chem. Sci. 2022, 13, 536–553. [Google Scholar] [CrossRef]
- Toyama, B.H.; Weissman, J.S. Amyloid structure: Conformational diversity and consequences. Annu. Rev. Biochem. 2011, 80, 557–585. [Google Scholar] [CrossRef] [PubMed]
- Blanco, L.P.; Evans, M.L.; Smith, D.R.; Badtke, M.P.; Chapman, M.R. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012, 20, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Sleutel, M.; Pradhan, B.; Volkov, A.N.; Remaut, H. Structural analysis and architectural principles of the bacterial amyloid curli. Nat. Commun. 2023, 14, 2822. [Google Scholar] [CrossRef] [PubMed]
- Sønderby, T.V.; Najarzadeh, Z.; Otzen, D.E. Functional bacterial amyloids: Understanding fibrillation, regulating biofilm fibril formation and organizing surface assemblies. Molecules 2022, 27, 4080. [Google Scholar] [CrossRef]
- Meisl, G.; Xu, C.K.; Taylor, J.D.; Michaels, T.C.; Levin, A.; Otzen, D.; Klenerman, D.; Matthews, S.; Linse, S.; Andreasen, M. Uncovering the universality of self-replication in protein aggregation and its link to disease. Sci. Adv. 2022, 8, eabn6831. [Google Scholar] [CrossRef]
- Sønderby, T.V.; Rasmussen, H.Ø.; Frank, S.A.; Pedersen, J.S.; Otzen, D.E. Folding steps in the fibrillation of functional amyloid: Denaturant sensitivity reveals common features in nucleation and elongation. J. Mol. Biol. 2022, 434, 167337. [Google Scholar] [CrossRef]
- Daniels, M.J.; Nourse Jr, J.B.; Kim, H.; Sainati, V.; Schiavina, M.; Murrali, M.G.; Pan, B.; Ferrie, J.J.; Haney, C.M.; Moons, R. Cyclized NDGA modifies dynamic α-synuclein monomers preventing aggregation and toxicity. Sci. Rep. 2019, 9, 2937. [Google Scholar] [CrossRef]
- Chen, K.S.; Menezes, K.; Rodgers, J.B.; O’Hara, D.M.; Tran, N.; Fujisawa, K.; Ishikura, S.; Khodaei, S.; Chau, H.; Cranston, A. Small molecule inhibitors of α-synuclein oligomers identified by targeting early dopamine-mediated motor impairment in C. elegans. Mol. Neurodegener. 2021, 16, 77. [Google Scholar] [CrossRef]
- Sampson, T.R.; Challis, C.; Jain, N.; Moiseyenko, A.; Ladinsky, M.S.; Shastri, G.G.; Thron, T.; Needham, B.D.; Horvath, I.; Debelius, J.W. A gut bacterial amyloid promotes α-synuclein aggregation and motor impairment in mice. elife 2020, 9, e53111. [Google Scholar] [CrossRef]
- Iqbal, K.; Alonso, A.d.C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.-X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2005, 1739, 198–210. [Google Scholar] [CrossRef]
- Tabaton, M.; Cammarata, S.; Mancardi, G.; Manetto, V.; Autilio-Gambetti, L.; Perry, G.; Gambetti, P. Ultrastructural localization of beta-amyloid, tau, and ubiquitin epitopes in extracellular neurofibrillary tangles. Proc. Natl. Acad. Sci. USA 1991, 88, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.D.; Landreth, G.E. The role of microglia in amyloid clearance from the AD brain. J. Neural Transm. 2010, 117, 949–960. [Google Scholar] [CrossRef]
- Vojtechova, I.; Machacek, T.; Kristofikova, Z.; Stuchlik, A.; Petrasek, T. Infectious origin of Alzheimer’s disease: Amyloid beta as a component of brain antimicrobial immunity. PLoS Pathog. 2022, 18, e1010929. [Google Scholar] [CrossRef]
- Wei, W.; Wang, S.; Xu, C.; Zhou, X.; Lian, X.; He, L.; Li, K. Gut microbiota, pathogenic proteins and neurodegenerative diseases. Front. Microbiol. 2022, 13, 959856. [Google Scholar] [CrossRef]
- Jo, M.; Lee, S.; Jeon, Y.-M.; Kim, S.; Kwon, Y.; Kim, H.-J. The role of TDP-43 propagation in neurodegenerative diseases: Integrating insights from clinical and experimental studies. Exp. Mol. Med. 2020, 52, 1652–1662. [Google Scholar] [CrossRef]
- Josephs, K.A.; Murray, M.E.; Whitwell, J.L.; Parisi, J.E.; Petrucelli, L.; Jack, C.R.; Petersen, R.C.; Dickson, D.W. Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol. 2014, 127, 441–450. [Google Scholar] [CrossRef]
- Amador-Ortiz, C.; Lin, W.L.; Ahmed, Z.; Personett, D.; Davies, P.; Duara, R.; Graff-Radford, N.R.; Hutton, M.L.; Dickson, D.W. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2007, 61, 435–445. [Google Scholar] [CrossRef]
- Uryu, K.; Nakashima-Yasuda, H.; Forman, M.S.; Kwong, L.K.; Clark, C.M.; Grossman, M.; Miller, B.L.; Kretzschmar, H.A.; Lee, V.M.-Y.; Trojanowski, J.Q. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J. Neuropathol. Exp. Neurol. 2008, 67, 555–564. [Google Scholar] [CrossRef]
- Wang, J.; Farr, G.W.; Hall, D.H.; Li, F.; Furtak, K.; Dreier, L.; Horwich, A.L. An ALS-linked mutant SOD1 produces a locomotor defect associated with aggregation and synaptic dysfunction when expressed in neurons of Caenorhabditis elegans. PLoS Genet. 2009, 5, e1000350. [Google Scholar] [CrossRef]
- Baba, T.; Ara, T.; Hasegawa, M.; Takai, Y.; Okumura, Y.; Baba, M.; Datsenko, K.A.; Tomita, M.; Wanner, B.L.; Mori, H. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: The Keio collection. Mol. Syst. Biol. 2006, 2, 2006.0008. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.T.A.; Zhu, X.; Jamsranjav, A.; Lee, L.P.; Cho, H. Escherichia coli K1-colibactin meningitis induces microglial NLRP3/IL-18 exacerbating H3K4me3-synucleinopathy in the human inflammatory gut-brain axis. Commun. Biol. 2025, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- French, R.L.; Grese, Z.R.; Aligireddy, H.; Dhavale, D.D.; Reeb, A.N.; Kedia, N.; Kotzbauer, P.T.; Bieschke, J.; Ayala, Y.M. Detection of TAR DNA-binding protein 43 (TDP-43) oligomers as initial intermediate species during aggregate formation. J. Biol. Chem. 2019, 294, 6696–6709. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Das, S.; Mohite, G.M.; Rout, S.K.; Halder, S.; Jha, N.N.; Ray, S.; Mehra, S.; Agarwal, V.; Maji, S.K. Cytotoxic oligomers and fibrils trapped in a gel-like state of α-synuclein assemblies. Angew. Chem. Int. Ed. 2018, 57, 5262–5266. [Google Scholar] [CrossRef]
- Dueholm, M.S.; Petersen, S.V.; Sønderkær, M.; Larsen, P.; Christiansen, G.; Hein, K.L.; Enghild, J.J.; Nielsen, J.L.; Nielsen, K.L.; Nielsen, P.H. Functional amyloid in Pseudomonas. Mol. Microbiol. 2010, 77, 1009–1020. [Google Scholar] [CrossRef]
- Dueholm, M.S.; Otzen, D.; Nielsen, P.H. Evolutionary insight into the functional amyloids of the pseudomonads. PLoS ONE 2013, 8, e76630. [Google Scholar] [CrossRef]
- Barnhart, M.M.; Chapman, M.R. Curli biogenesis and function. Annu. Rev. Microbiol. 2006, 60, 131–147. [Google Scholar] [CrossRef]
- Zeng, G.; Vad, B.S.; Dueholm, M.S.; Christiansen, G.; Nilsson, M.; Tolker-Nielsen, T.; Nielsen, P.H.; Meyer, R.L.; Otzen, D.E. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 2015, 6, 1099. [Google Scholar] [CrossRef]
- Voth, S.; Gwin, M.; Francis, C.M.; Balczon, R.; Frank, D.W.; Pittet, J.F.; Wagener, B.M.; Moser, S.A.; Alexeyev, M.; Housley, N.; et al. Virulent Pseudomonas aeruginosa infection converts antimicrobial amyloids into cytotoxic prions. FASEB J. 2020, 34, 9156–9179. [Google Scholar] [CrossRef]
- Bleem, A.; Christiansen, G.; Madsen, D.J.; Maric, H.; Strømgaard, K.; Bryers, J.D.; Daggett, V.; Meyer, R.L.; Otzen, D.E. Protein engineering reveals mechanisms of functional amyloid formation in Pseudomonas aeruginosa biofilms. J. Mol. Biol. 2018, 430, 3751–3763. [Google Scholar] [CrossRef]
- Nagaraj, M.; Ahmed, M.; Lyngsø, J.; Vad, B.S.; Bøggild, A.; Fillipsen, A.; Pedersen, J.S.; Otzen, D.E.; Akbey, Ü. Predicted Loop Regions Promote Aggregation: A Study of Amyloidogenic Domains in the Functional Amyloid FapC. J. Mol. Biol. 2020, 432, 2232–2252. [Google Scholar] [CrossRef] [PubMed]
- Rouse, S.L.; Hawthorne, W.J.; Berry, J.L.; Chorev, D.S.; Ionescu, S.A.; Lambert, S.; Stylianou, F.; Ewert, W.; Mackie, U.; Morgan, R.M.L.; et al. A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis. Nat. Commun. 2017, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Cuenca, L.; Toledo-Arana, A.; Valle, J. Anti-Biofilm Molecules Targeting Functional Amyloids. Antibiotics 2021, 10, 795. [Google Scholar] [CrossRef] [PubMed]
- Branda, S.S.; Chu, F.; Kearns, D.B.; Losick, R.; Kolter, R. A major protein component of the Bacillus subtilis biofilm matrix. Mol. Microbiol. 2006, 59, 1229–1238. [Google Scholar] [CrossRef]
- El Mammeri, N.; Hierrezuelo, J.; Tolchard, J.; Cámara-Almirón, J.; Caro-Astorga, J.; Álvarez-Mena, A.; Dutour, A.; Berbon, M.; Shenoy, J.; Morvan, E.; et al. Molecular architecture of bacterial amyloids in Bacillus biofilms. FASEB J. 2019, 33, 12146–12163. [Google Scholar] [CrossRef]
- Azulay, D.N.; Ghrayeb, M.; Ktorza, I.B.; Nir, I.; Nasser, R.; Harel, Y.S.; Chai, L. Colloidal-like aggregation of a functional amyloid protein. Phys. Chem. Chem. Phys. 2020, 22, 23286–23294. [Google Scholar] [CrossRef]
- Ghrayeb, M.; Hayet, S.; Lester-Zer, N.; Levi-Kalisman, Y.; Chai, L. Fibrilar Polymorphism of the Bacterial Extracellular Matrix Protein TasA. Microorganisms 2021, 9, 529. [Google Scholar] [CrossRef]
- Böhning, J.; Ghrayeb, M.; Pedebos, C.; Abbas, D.K.; Khalid, S.; Chai, L.; Bharat, T.A.M. Donor-strand exchange drives assembly of the TasA scaffold in Bacillus subtilis biofilms. Nat. Commun. 2022, 13, 7082. [Google Scholar] [CrossRef]
- Romero, D. Bacterial determinants of the social behavior of Bacillus subtilis. Res. Microbiol. 2013, 164, 788–798. [Google Scholar] [CrossRef]
- Yan, F.; Yu, Y.; Gozzi, K.; Chen, Y.; Guo, J.H.; Chai, Y. Genome-Wide Investigation of Biofilm Formation in Bacillus cereus. Appl. Env. Microbiol. 2017, 83, e00561-17. [Google Scholar] [CrossRef]
- Caro-Astorga, J.; Pérez-García, A.; de Vicente, A.; Romero, D. A genomic region involved in the formation of adhesin fibers in Bacillus cereus biofilms. Front. Microbiol. 2014, 5, 745. [Google Scholar] [CrossRef] [PubMed]
- Stöver, A.G.; Driks, A. Secretion, localization, and antibacterial activity of TasA, a Bacillus subtilis spore-associated protein. J. Bacteriol. 1999, 181, 1664–1672. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2--approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Taylor, J.D.; Zhou, Y.; Salgado, P.S.; Patwardhan, A.; McGuffie, M.; Pape, T.; Grabe, G.; Ashman, E.; Constable, S.C.; Simpson, P.J.; et al. Atomic resolution insights into curli fiber biogenesis. Structure 2011, 19, 1307–1316. [Google Scholar] [CrossRef]
- Trunk, T.; Khalil, H.S.; Leo, J.C. Bacterial autoaggregation. AIMS Microbiol. 2018, 4, 140–164. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Desvaux, M.; Hébraud, M.; Møretrø, T.; Langsrud, S.; Doulgeraki, A.; Nychas, G.J.; Kačániová, M.; Czaczyk, K.; et al. Intra- and inter-species interactions within biofilms of important foodborne bacterial pathogens. Front. Microbiol. 2015, 6, 841. [Google Scholar] [CrossRef]
- Evans, M.L.; Chapman, M.R. Curli biogenesis: Order out of disorder. Biochim. Biophys. Acta 2014, 1843, 1551–1558. [Google Scholar] [CrossRef]
- Bu, F.; Dee, D.R.; Liu, B. Structural insight into Escherichia coli CsgA amyloid fibril assembly. mBio 2024, 15, e0041924. [Google Scholar] [CrossRef]
- Irvine, G.B.; El-Agnaf, O.M.; Shankar, G.M.; Walsh, D.M. Protein aggregation in the brain: The molecular basis for Alzheimer’s and Parkinson’s diseases. Mol. Med. 2008, 14, 451–464. [Google Scholar] [CrossRef]
- Diehl, A.; Roske, Y.; Ball, L.; Chowdhury, A.; Hiller, M.; Molière, N.; Kramer, R.; Stöppler, D.; Worth, C.L.; Schlegel, B.; et al. Structural changes of TasA in biofilm formation of Bacillus subtilis. Proc. Natl. Acad. Sci. USA 2018, 115, 3237–3242. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.A.; Khan, F.; Song, M. Biofilm-Associated Amyloid Proteins Linked with the Progression of Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 2695. https://doi.org/10.3390/ijms26062695
Singh AA, Khan F, Song M. Biofilm-Associated Amyloid Proteins Linked with the Progression of Neurodegenerative Diseases. International Journal of Molecular Sciences. 2025; 26(6):2695. https://doi.org/10.3390/ijms26062695
Chicago/Turabian StyleSingh, Alka Ashok, Fazlurrahman Khan, and Minseok Song. 2025. "Biofilm-Associated Amyloid Proteins Linked with the Progression of Neurodegenerative Diseases" International Journal of Molecular Sciences 26, no. 6: 2695. https://doi.org/10.3390/ijms26062695
APA StyleSingh, A. A., Khan, F., & Song, M. (2025). Biofilm-Associated Amyloid Proteins Linked with the Progression of Neurodegenerative Diseases. International Journal of Molecular Sciences, 26(6), 2695. https://doi.org/10.3390/ijms26062695








