Neuroprotection vs. Neurotoxicity: The Dual Impact of Brain Lipids in Depression
Abstract
1. Introduction
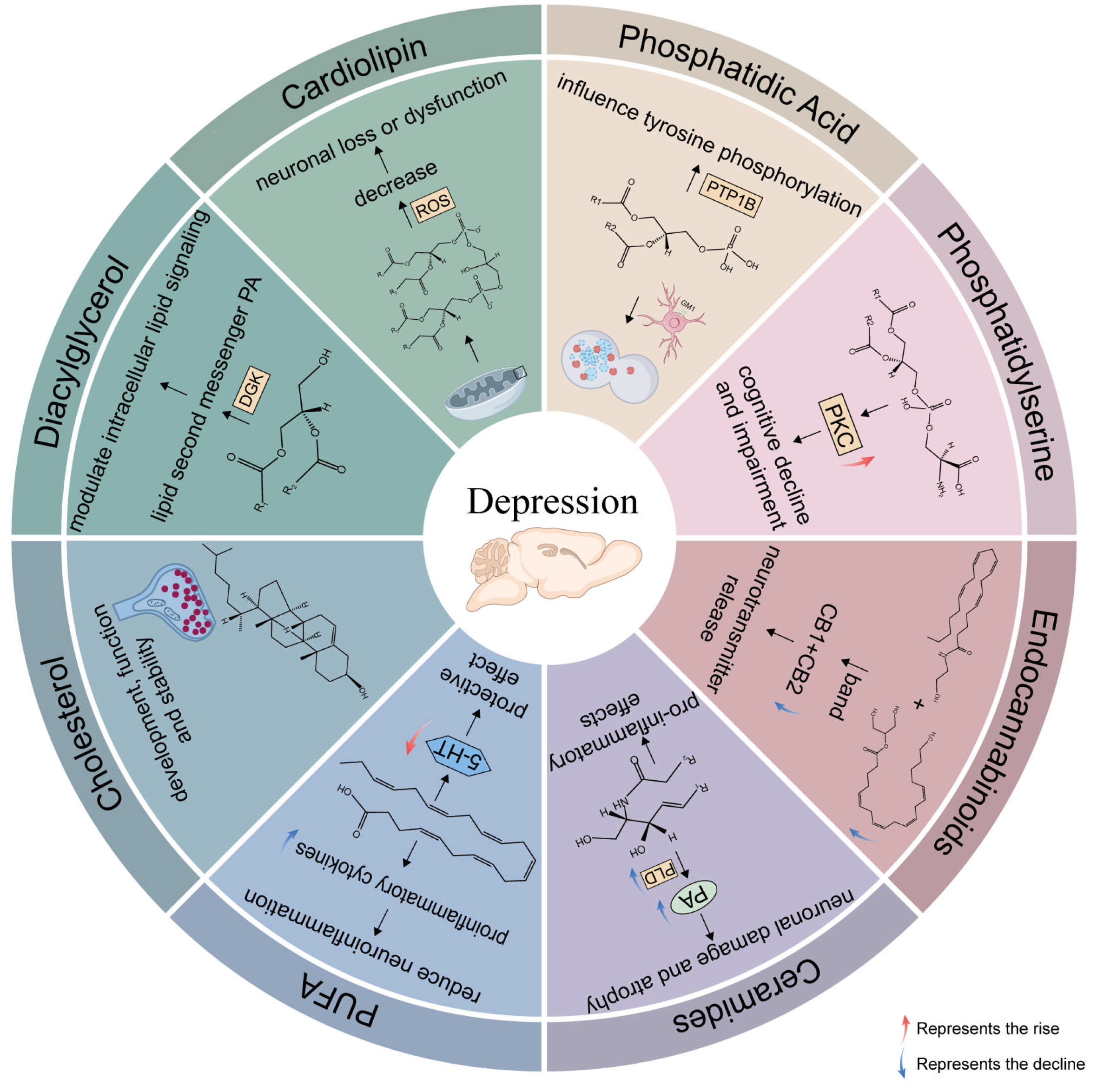
2. Application of Various Lipids in MDD
2.1. Sphingolipids, SPs
2.1.1. Neurotoxic (Cer)
2.1.2. Neuroprotective (SM)
2.1.3. Neuroprotective (Sphingosine)
2.2. Glycerophospholipids, GPs
2.2.1. Neurotoxic (PC, PE)
2.2.2. Neuroprotective (CL)
2.2.3. Neurotoxic (PI)
2.2.4. Neuroprotective (PA)
2.2.5. Neuroprotective (PS)
2.3. Fatty Acyls, FA
2.3.1. PUFA
2.3.2. Neuroprotective (eCBs)
2.4. Sterol Lipids, STs
2.4.1. Neuroprotective (Cholesterol)
2.4.2. BA
2.5. Glycerolipids, GLs
3. Conclusions and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Li, N.; Lee, B.; Liu, R.-J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.-Y.; Aghajanian, G.; Duman, R.S. mTOR-Dependent Synapse Formation Underlies the Rapid Antidepressant Effects of NMDA Antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Ramaker, M.J.; Dulawa, S.C. Identifying Fast-Onset Antidepressants Using Rodent Models. Mol. Psychiatry 2017, 22, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Shyu, H.; Ko, C.; Luo, Y.; Lin, H.; Wu, S.; Lan, S.; Cheng, T.; Hu, S.; Lee, M. Ketamine Increases Permeability and Alters Epithelial Phenotype of Renal Distal Tubular Cells via a GSK-3β-Dependent Mechanism. J. Cell. Biochem. 2016, 117, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Ding, Z.; Zhang, Y.; Shi, J.; Hashimoto, K.; Lu, L. Risks Associated with Misuse of Ketamine as a Rapid-Acting Antidepressant. Neurosci. Bull. 2016, 32, 557–564. [Google Scholar] [CrossRef]
- Gould, T.D.; Zarate, C.A.; Thompson, S.M. Molecular Pharmacology and Neurobiology of Rapid-Acting Antidepressants. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 213–236. [Google Scholar] [CrossRef]
- Müller, C.P.; Reichel, M.; Mühle, C.; Rhein, C.; Gulbins, E.; Kornhuber, J. Brain Membrane Lipids in Major Depression and Anxiety Disorders. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2015, 1851, 1052–1065. [Google Scholar] [CrossRef]
- Bekhbat, M.; Chu, K.; Le, N.-A.; Woolwine, B.J.; Haroon, E.; Miller, A.H.; Felger, J.C. Glucose and Lipid-Related Biomarkers and the Antidepressant Response to Infliximab in Patients with Treatment-Resistant Depression. Psychoneuroendocrinology 2018, 98, 222–229. [Google Scholar] [CrossRef]
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A Molecular and Cellular Theory of Depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. The Molecular Neurobiology of Depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef]
- Yang, D.; Wang, X.; Zhang, L.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Chen, S.; Ying, J.; Hua, F. Lipid Metabolism and Storage in Neuroglia: Role in Brain Development and Neurodegenerative Diseases. Cell Biosci. 2022, 12, 106. [Google Scholar] [CrossRef]
- Osetrova, M.; Tkachev, A.; Mair, W.; Guijarro Larraz, P.; Efimova, O.; Kurochkin, I.; Stekolshchikova, E.; Anikanov, N.; Foo, J.C.; Cazenave-Gassiot, A.; et al. Lipidome Atlas of the Adult Human Brain. Nat. Commun. 2024, 15, 4455. [Google Scholar] [CrossRef] [PubMed]
- Fahy, E.; Subramaniam, S.; Murphy, R.C.; Nishijima, M.; Raetz, C.R.H.; Shimizu, T.; Spener, F.; van Meer, G.; Wakelam, M.J.O.; Dennis, E.A. Update of the LIPID MAPS Comprehensive Classification System for Lipids. J. Lipid Res. 2009, 50, S9–S14. [Google Scholar] [CrossRef] [PubMed]
- Brunkhorst-Kanaan, N.; Klatt-Schreiner, K.; Hackel, J.; Schröter, K.; Trautmann, S.; Hahnefeld, L.; Wicker, S.; Reif, A.; Thomas, D.; Geisslinger, G.; et al. Targeted Lipidomics Reveal Derangement of Ceramides in Major Depression and Bipolar Disorder. Metabolism 2019, 95, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Palmada, M.; Reichel, M.; Lüth, A.; Böhmer, C.; Amato, D.; Müller, C.P.; Tischbirek, C.H.; Groemer, T.W.; Tabatabai, G.; et al. Acid Sphingomyelinase–Ceramide System Mediates Effects of Antidepressant Drugs. Nat. Med. 2013, 19, 934–938. [Google Scholar] [CrossRef]
- Schumacher, F.; Edwards, M.J.; Mühle, C.; Carpinteiro, A.; Wilson, G.C.; Wilker, B.; Soddemann, M.; Keitsch, S.; Scherbaum, N.; Müller, B.W.; et al. Ceramide Levels in Blood Plasma Correlate with Major Depressive Disorder Severity and Its Neutralization Abrogates Depressive Behavior in Mice. J. Biol. Chem. 2022, 298, 102185. [Google Scholar] [CrossRef]
- Oliveira, T.G.; Chan, R.B.; Bravo, F.V.; Miranda, A.; Silva, R.R.; Zhou, B.; Marques, F.; Pinto, V.; Cerqueira, J.J.; Paolo, G.D.; et al. The Impact of Chronic Stress on the Rat Brain Lipidome. Mol. Psychiatry 2015, 21, 80. [Google Scholar] [CrossRef]
- Miranda, A.M.; Bravo, F.V.; Chan, R.B.; Sousa, N.; Paolo, G.D.; Oliveira, T.G. Differential Lipid Composition and Regulation along the Hippocampal Longitudinal Axis. Transl. Psychiatry 2019, 9, 144. [Google Scholar] [CrossRef]
- Zhou, C.; Zhao, X.; Ma, X.; Ma, H.; Li, R.; Hu, G.; Wang, H.; Peng, Z.; Cai, M. Effects of (S)-Ketamine on Depression-like Behaviors in a Chronic Variable Stress Model: A Role of Brain Lipidome. Front. Cell. Neurosci. 2023, 17, 1114914. [Google Scholar] [CrossRef]
- Xue, S.; Zhou, C.; Xue, F.; Liu, L.; Cai, Y.; Luo, J.; Wang, Y.; Tan, Q.; Wang, H.; Peng, Z. The Impact of Repetitive Transcranial Magnetic Stimulation and Fluoxetine on the Brain Lipidome in a Rat Model of Chronic Unpredictable Stress. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2020, 102, 109946. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Li, B.; Zhang, Y.; Gao, H.; Zhao, X.; Leng, K.; Song, Z. Krill Oil Treatment Ameliorates Lipid Metabolism Imbalance in Chronic Unpredicted Mild Stress-Induced Depression-like Behavior in Mice. Front. Cell Dev. Biol. 2023, 11, 1180483. [Google Scholar] [CrossRef]
- Zoicas, I.; Schumacher, F.; Kleuser, B.; Reichel, M.; Gulbins, E.; Fejtova, A.; Kornhuber, J.; Rhein, C. The Forebrain-Specific Overexpression of Acid Sphingomyelinase Induces Depressive-Like Symptoms in Mice. Cells 2020, 9, 1244. [Google Scholar] [CrossRef] [PubMed]
- Müller, C.P.; Kalinichenko, L.S.; Tiesel, J.; Witt, M.; Stöckl, T.; Sprenger, E.; Fuchser, J.; Beckmann, J.; Praetner, M.; Huber, S.E.; et al. Paradoxical Antidepressant Effects of Alcohol Are Related to Acid Sphingomyelinase and Its Control of Sphingolipid Homeostasis. Acta Neuropathol. 2016, 133, 463. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, A.A.; Michélsen, P.; Odham, G. Molecular Species of Sphingomyelin: Determination by High-Performance Liquid Chromatography/Mass Spectrometry with Electrospray and High-Performance Liquid Chromatography/Tandem Mass Spectrometry with Atmospheric Pressure Chemical Ionization. J. Mass Spectrom. 1998, 33, 1192–1198. [Google Scholar] [CrossRef]
- Faria, R.; Santana, M.M.; Aveleira, C.A.; Simões, C.; Maciel, E.; Melo, T.; Santinha, D.; Oliveira, M.M.; Peixoto, F.; Domingues, P.; et al. Alterations in Phospholipidomic Profile in the Brain of Mouse Model of Depression Induced by Chronic Unpredictable Stress. Neuroscience 2014, 273, 1–11. [Google Scholar] [CrossRef]
- Su, D.; Liao, Z.; Feng, B.; Wang, T.; Shan, B.; Zeng, Q.; Song, J.; Song, Y. Pulsatilla Chinensis Saponins Cause Liver Injury through Interfering Ceramide/Sphingomyelin Balance That Promotes Lipid Metabolism Dysregulation and Apoptosis. Phytomedicine 2020, 76, 153265. [Google Scholar] [CrossRef]
- Van Kruining, D.; Luo, Q.; van Echten-Deckert, G.; Mielke, M.M.; Bowman, A.; Ellis, S.; Oliveira, T.G.; Martinez-Martinez, P. Sphingolipids as Prognostic Biomarkers of Neurodegeneration, Neuroinflammation, and Psychiatric Diseases and Their Emerging Role in Lipidomic Investigation Methods. Adv. Drug Deliv. Rev. 2020, 159, 232. [Google Scholar] [CrossRef]
- Jang, S.; Kim, D.; Lee, Y.; Moon, S.; Oh, S. Modulation of Sphingosine 1-Phosphate and Tyrosine Hydroxylase in the Stress-Induced Anxiety. Neurochem. Res. 2011, 36, 258–267. [Google Scholar] [CrossRef]
- Li, B.; Yan, Y.; Zhang, T.; Xu, H.; Wu, X.; Yao, G.; Li, X.; Yan, C.; Wu, L.-L. Quercetin Reshapes Gut Microbiota Homeostasis and Modulates Brain Metabolic Profile to Regulate Depression-like Behaviors Induced by CUMS in Rats. Front. Pharmacol. 2024, 15, 1362464. [Google Scholar] [CrossRef]
- Guo, Y.; Gan, X.; Zhou, H.; Zhou, H.; Pu, S.; Long, X.; Ren, C.; Feng, T.; Tang, H. Fingolimod Suppressed the Chronic Unpredictable Mild Stress-Induced Depressive-like Behaviors via Affecting Microglial and NLRP3 Inflammasome Activation. Life Sci. 2020, 263, 118582. [Google Scholar] [CrossRef]
- Ye, R.; Zhang, M.; Zhang, S.; Bai, S.; Jiang, Z.; Cai, Q.; Cao, K.; Shen, C.; Shi, Y.; Zhang, R.; et al. Stress Causes Cognitive Impairment by Affecting Cholesterol Efflux and Reuptake Leading to Abnormalities in Lipid Metabolism of Rats. J. Integr. Neurosci. 2020, 19, 39–49. [Google Scholar] [CrossRef]
- Wu, X.; Xu, H.; Zeng, N.; Li, H.; Yao, G.; Liu, K.; Yan, C.; Wu, L. Luteolin Alleviates Depression-like Behavior by Modulating Glycerophospholipid Metabolism in the Hippocampus and Prefrontal Cortex of LOD Rats. CNS Neurosci. Ther. 2024, 30, e14455. [Google Scholar] [CrossRef] [PubMed]
- Geng, C.; Hao, G.; Yi, Q.; Guo, Y.; Chen, D.; Han, W.; Zhang, J.; Yang, M.; Jiang, P. The Impact of Dl-3-n-Butylphthalide on the Lipidomics of the Hippocampus in a Rat Model of Lipopolysaccharide-Induced Depression. Prostaglandins Other Lipid Mediat. 2020, 150, 106464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Chen, J.; Gong, L.; Liu, F.; Zhao, H.; Mu, J. Alteration of Glycerophospholipid Metabolism in Hippocampus of Post-Stroke Depression Rats. Neurochem. Res. 2022, 47, 2052–2063. [Google Scholar] [CrossRef] [PubMed]
- Olvera, R.L.; Caetano, S.C.; Stanley, J.A.; Chen, H.-H.; Nicoletti, M.; Hatch, J.P.; Fonseca, M.; Pliszka, S.R.; Soares, J.C. Reduced Medial Prefrontal N-Acetyl-Aspartate Levels in Pediatric Major Depressive Disorder: A Multi-Voxel in Vivo1H Spectroscopy Study. Psychiatry Res. Neuroimaging 2010, 184, 71–76. [Google Scholar] [CrossRef]
- Harper, D.G.; Jensen, J.E.; Ravichandran, C.; Sivrioglu, Y.; Silveri, M.; Iosifescu, D.V.; Renshaw, P.F.; Forester, B.P. Tissue-Specific Differences in Brain Phosphodiesters in Late-Life Major Depression. Am. J. Geriatr. Psychiatr. 2014, 22, 499–509. [Google Scholar] [CrossRef]
- Białek, W.; Hryniewicz-Jankowska, A.; Czechowicz, P.; Sławski, J.; Collawn, J.F.; Czogalla, A.; Bartoszewski, R. The Lipid Side of Unfolded Protein Response. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2024, 1869, 159515. [Google Scholar] [CrossRef]
- Vance, J.E.; Vance, D.E. Phospholipid Biosynthesis in Mammalian Cells. Biochem. Cell Biol. 2004, 82, 113–128. [Google Scholar] [CrossRef]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Mitochondrial Dysfunction in Brain Aging: Role of Oxidative Stress and Cardiolipin. Neurochem. Int. 2011, 58, 447–457. [Google Scholar] [CrossRef]
- Gonzalvez, F.; Gottlieb, E. Cardiolipin: Setting the Beat of Apoptosis. Apoptosis 2007, 12, 877–885. [Google Scholar] [CrossRef]
- Pope, S.; Land, J.M.; Heales, S.J.R. Oxidative Stress and Mitochondrial Dysfunction in Neurodegeneration; Cardiolipin a Critical Target? Biochim. Biophys. Acta (BBA)-Bioenerg. 2008, 1777, 794–799. [Google Scholar] [CrossRef]
- Wymann, M.P.; Schneiter, R. Lipid Signalling in Disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Hicks, A.M.; DeLong, C.J.; Thomas, M.J.; Samuel, M.; Cui, Z. Unique Molecular Signatures of Glycerophospholipid Species in Different Rat Tissues Analyzed by Tandem Mass Spectrometry. Biochim. Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2006, 1761, 1022–1029. [Google Scholar] [CrossRef] [PubMed]
- Raghu, P.; Joseph, A.; Krishnan, H.; Singh, P.; Saha, S. Phosphoinositides: Regulators of Nervous System Function in Health and Disease. Front. Mol. Neurosci. 2019, 12, 208. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Whitten, R.J. Control of Neuronal Excitability by Cell Surface Receptor Density and Phosphoinositide Metabolism. Front. Pharmacol. 2021, 12, 663840. [Google Scholar] [CrossRef]
- Kale, M.B.; Wankhede, N.L.; Bishoyi, A.K.; Ballal, S.; Kalia, R.; Arya, R.; Kumar, S.; Khalid, M.; Gulati, M.; Umare, M.; et al. Emerging Biophysical Techniques for Probing Synaptic Transmission in Neurodegenerative Disorders. Neuroscience 2025, 565, 63–79. [Google Scholar] [CrossRef]
- Zegarlińska, J.; Piaścik, M.; Sikorski, A.F.; Czogalla, A. Phosphatidic Acid—A Simple Phospholipid with Multiple Faces. Acta Biochim. Pol. 2018, 65, 163–171. [Google Scholar] [CrossRef]
- Wu, J.; Chai, T.; Zhang, H.; Huang, Y.; Perry, S.W.; Li, Y.; Duan, J.; Tan, X.; Hu, X.; Liu, Y.; et al. Changes in Gut Viral and Bacterial Species Correlate with Altered 1,2-Diacylglyceride Levels and Structure in the Prefrontal Cortex in a Depression-like Non-Human Primate Model. Transl. Psychiatry 2022, 12, 74. [Google Scholar] [CrossRef]
- Schiller, M.; Wilson, G.C.; Keitsch, S.; Soddemann, M.; Wilker, B.; Edwards, M.J.; Scherbaum, N.; Gulbins, E. Phosphatidic Acid Is Involved in Regulation of Autophagy in Neurons in Vitro and in Vivo. Pflug. Arch. 2024, 476, 1881–1894. [Google Scholar] [CrossRef]
- Clementino, A.R.; Pellegrini, G.; Banella, S.; Colombo, G.; Cantù, L.; Sonvico, F.; Del Favero, E. Structure and Fate of Nanoparticles Designed for the Nasal Delivery of Poorly Soluble Drugs. Mol. Pharm. 2021, 18, 3132–3146. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Perrucci, F.; Morini, R.; Erreni, M.; Mahoney, M.; Witkowska, A.; Carey, A.; Faggiani, E.; Schuetz, L.T.; Mason, S.; et al. Local Externalization of Phosphatidylserine Mediates Developmental Synaptic Pruning by Microglia. EMBO J. 2020, 39, e105380. [Google Scholar] [CrossRef]
- Amaducci, L. Phosphatidylserine in the Treatment of Alzheimer’s Disease: Results of a Multicenter Study. Psychopharmacol. Bull. 1988, 24, 130–134. [Google Scholar] [PubMed]
- Nunzi, M.G.; Milan, F.; Guidolin, D.; Toffano, G. Dendritic Spine Loss in Hippocampus of Aged Rats. Effect of Brain Phosphatidylserine Administration. Neurobiol. Aging 1987, 8, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Müller, W.E. Age-Related Alterations of NMDA-Receptor Properties in the Mouse Forebrain: Partial Restoration by Chronic Phosphatidylserine Treatment. Brain Res. 1992, 584, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Layé, S.; Nadjar, A.; Joffre, C.; Bazinet, R.P. Anti-Inflammatory Effects of Omega-3 Fatty Acids in the Brain: Physiological Mechanisms and Relevance to Pharmacology. Pharmacol. Rev. 2018, 70, 12–38. [Google Scholar] [CrossRef]
- Yui, K.; Imataka, G.; Nakamura, H.; Ohara, N.; Naito, Y. Eicosanoids Derived from Arachidonic Acid and Their Family Prostaglandins and Cyclooxygenase in Psychiatric Disorders. Curr. Neuropharmacol. 2015, 13, 776. [Google Scholar] [CrossRef]
- Mocking, R.J.T.; Nap, T.S.; Westerink, A.M.; Assies, J.; Vaz, F.M.; Koeter, M.W.J.; Ruhé, H.G.; Schene, A.H. Biological Profiling of Prospective Antidepressant Response in Major Depressive Disorder: Associations with (Neuro)Inflammation, Fatty Acid Metabolism, and Amygdala-Reactivity. Psychoneuroendocrinology 2017, 79, 84–92. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Leonard, B.E.; Horrobin, D.F. Effects of Dietary N-3 or n-6 Fatty Acids on Interleukin-1beta-Induced Anxiety, Stress, and Inflammatory Responses in Rats. J. Lipid Res. 2003, 44, 1984–1991. [Google Scholar] [CrossRef]
- Zadeh-Ardabili, P.M.; Rad, S.K.; Rad, S.K.; Movafagh, A. Antidepressant-like Effects of Fish, Krill Oils and Vit B12 against Exposure to Stress Environment in Mice Models: Current Status and Pilot Study. Sci. Rep. 2019, 9, 19953, Erratum in Sci. Rep. 2020, 10, 13797. https://doi.org/10.1038/s41598-020-70338-x. [Google Scholar] [CrossRef]
- Vancassel, S.; Leman, S.; Hanonick, L.; Denis, S.; Roger, J.; Nollet, M.; Bodard, S.; Kousignian, I.; Belzung, C.; Chalon, S. N-3 Polyunsaturated Fatty Acid Supplementation Reverses Stress-Induced Modifications on Brain Monoamine Levels in Mice. J. Lipid Res. 2008, 49, 340–348. [Google Scholar] [CrossRef]
- Arts, M.J.T.J.; Grun, C.; de Jong, R.L.; Voss, H.-P.; Bast, A.; Mueller, M.J.; Haenen, G.R.M.M. Oxidative Degradation of Lipids during Mashing. J. Agric. Food Chem. 2007, 55, 7010–7014. [Google Scholar] [CrossRef]
- Grosso, G.; Pajak, A.; Marventano, S.; Castellano, S.; Galvano, F.; Bucolo, C.; Drago, F.; Caraci, F. Role of Omega-3 Fatty Acids in the Treatment of Depressive Disorders: A Comprehensive Meta-Analysis of Randomized Clinical Trials. PLoS ONE 2014, 9, e96905. [Google Scholar] [CrossRef] [PubMed]
- Mocking, R.; Harmsen, I.; Assies, J.; Ruhe, H.; Koeter, M.; Schene, A. Meta-Analysis and Meta-Regression of Omega-3 Polyunsaturated Fatty Acid Supplementation for Major Depressive Disorder. Bipolar Disord. 2016, 18, 177. [Google Scholar] [CrossRef] [PubMed]
- Caughey, G.E.; Mantzioris, E.; Gibson, R.A.; Cleland, L.G.; James, M.J. The Effect on Human Tumor Necrosis Factor Alpha and Interleukin 1 Beta Production of Diets Enriched in N-3 Fatty Acids from Vegetable Oil or Fish Oil. Am. J. Clin. Nutr. 1996, 63, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xie, B.; Zhang, H.; He, Q.; Guo, L.; Subramaniapillai, M.; Fan, B.; Lu, C.; McIntyre, R.S. Efficacy of Omega-3 PUFAs in Depression: A Meta-Analysis. Transl. Psychiatry 2019, 9, 190, Erratum in Transl. Psychiatr. 2021, 11, 465. https://doi.org/10.1038/s41398-021-01582-6. [Google Scholar] [CrossRef]
- Chen, C.T.; Bazinet, R.P. β-Oxidation and Rapid Metabolism, but Not Uptake Regulate Brain Eicosapentaenoic Acid Levels. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 33–40. [Google Scholar] [CrossRef]
- Chen, C.T.; Liu, Z.; Bazinet, R.P. Rapid De-Esterification and Loss of Eicosapentaenoic Acid from Rat Brain Phospholipids: An Intracerebroventricular Study. J. Neurochem. 2011, 116, 363–373. [Google Scholar] [CrossRef]
- Rey, C.; Nadjar, A.; Joffre, F.; Amadieu, C.; Aubert, A.; Vaysse, C.; Pallet, V.; Laye, S.; Joffre, C. Maternal N-3 Polyunsaturated Fatty Acid Dietary Supply Modulates Microglia Lipid Content in the Offspring. Prostaglandins Leukot. Essent. Fat. Acids 2018, 133, 1–7. [Google Scholar] [CrossRef]
- Kreisel, T.; Frank, M.G.; Licht, T.; Reshef, R.; Ben-Menachem-Zidon, O.; Baratta, M.V.; Maier, S.F.; Yirmiya, R. Dynamic Microglial Alterations Underlie Stress-Induced Depressive-like Behavior and Suppressed Neurogenesis. Mol. Psychiatry 2014, 19, 699–709. [Google Scholar] [CrossRef]
- Lonergan, P.E.; Martin, D.S.D.; Horrobin, D.F.; Lynch, M.A. Neuroprotective Actions of Eicosapentaenoic Acid on Lipopolysaccharide-Induced Dysfunction in Rat Hippocampus. J. Neurochem. 2004, 91, 20–29. [Google Scholar] [CrossRef]
- Morris, G.; Walder, K.; Kloiber, S.; Amminger, P.; Berk, M.; Bortolasci, C.C.; Maes, M.; Puri, B.K.; Carvalho, A.F. The Endocannabinoidome in Neuropsychiatry: Opportunities and Potential Risks. Pharmacol. Res. 2021, 170, 105729. [Google Scholar] [CrossRef]
- Cui, Y.; Perez, S.; Venance, L. Endocannabinoid-LTP Mediated by CB1 and TRPV1 Receptors Encodes for Limited Occurrences of Coincident Activity in Neocortex. Front. Cell. Neurosci. 2018, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Lutjohann, D.; Brzezinka, A.; Barth, E.; Abramowski, D.; Staufenbiel, M.; von Bergmann, K.; Beyreuther, K.; Multhaup, G.; Bayer, T.A. Profile of Cholesterol-Related Sterols in Aged Amyloid Precursor Protein Transgenic Mouse Brain. J. Lipid Res. 2002, 43, 1078–1085. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A. Cholesterol Metabolism in the Myelin of Rat Brain. Experientia 1968, 24, 814–815. [Google Scholar] [CrossRef]
- Beasley, C.L.; Honer, W.G.; Bergmann, K.; Falkai, P.; Lutjohann, D.; Bayer, T.A. Reductions in Cholesterol and Synaptic Markers in Association Cortex in Mood Disorders. Bipolar Disord. 2005, 7, 449–455. [Google Scholar] [CrossRef]
- Li, X.; Qiu, W.; Li, N.; Da, X.; Ma, Q.; Hou, Y.; Wang, T.; Song, M.; Chen, J. Susceptibility to Hyperglycemia in Rats with Stress-Induced Depressive-Like Behavior: Involvement of IL-6 Mediated Glucose Homeostasis Signaling. Front. Psychiatry 2020, 11, 557. [Google Scholar] [CrossRef]
- Cotter, D.; Mackay, D.; Landau, S.; Kerwin, R.; Everall, I. Reduced Glial Cell Density and Neuronal Size in the Anterior Cingulate Cortex in Major Depressive Disorder. Arch. Gen. Psychiatry 2001, 58, 545–553. [Google Scholar] [CrossRef]
- Pfrieger, F.W. Role of Cholesterol in Synapse Formation and Function. Biochim. Biophys. Acta (BBA)-Biomembr. 2003, 1610, 271–280. [Google Scholar] [CrossRef]
- Wellington, C.L.; Walker, E.K.Y.; Suarez, A.; Kwok, A.; Bissada, N.; Singaraja, R.; Yang, Y.-Z.; Zhang, L.-H.; James, E.; Wilson, J.E.; et al. ABCA1 mRNA and Protein Distribution Patterns Predict Multiple Different Roles and Levels of Regulation. Lab. Investig. 2002, 82, 273–283. [Google Scholar] [CrossRef]
- Mauch, D.H.; Nägler, K.; Schumacher, S.; Göritz, C.; Müller, E.C.; Otto, A.; Pfrieger, F.W. CNS Synaptogenesis Promoted by Glia-Derived Cholesterol. Science 2001, 294, 1354–1357. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Corlianò, M.; Singaraja, R.R. Bile Acids: A Communication Channel in the Gut-Brain Axis. Neuromol. Med. 2021, 23, 99–117. [Google Scholar] [CrossRef]
- Kiriyama, Y.; Nochi, H. The Biosynthesis, Signaling, and Neurological Functions of Bile Acids. Biomolecules 2019, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; DeMorrow, S. Bile Acid Signaling in Neurodegenerative and Neurological Disorders. Int. J. Mol. Sci. 2020, 21, 5982. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.; McMillin, M.; Galindo, C.; Frampton, G.; Pae, H.Y.; DeMorrow, S. Bile Acids Permeabilize the Blood Brain Barrier after Bile Duct Ligation in Rats via Rac1-Dependent Mechanisms. Dig. Liver Dis. 2014, 46, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.; Li, H.; Jia, Y.; Xiao, Y.; Luo, S.; Zhang, D.; Han, L.; Dai, L.; Xiao, C.; Feng, L.; et al. Ganoderic Acid A Exerted Antidepressant-like Action through FXR Modulated NLRP3 Inflammasome and Synaptic Activity. Biochem. Pharmacol. 2021, 188, 114561. [Google Scholar] [CrossRef]
- McMillin, M.; Frampton, G.; Quinn, M.; Divan, A.; Grant, S.; Patel, N.; Newell-Rogers, K.; DeMorrow, S. Suppression of the HPA Axis During Cholestasis Can Be Attributed to Hypothalamic Bile Acid Signaling. Mol. Endocrinol. 2015, 29, 1720–1730. [Google Scholar] [CrossRef]
- Lirong, W.; Mingliang, Z.; Mengci, L.; Qihao, G.; Zhenxing, R.; Xiaojiao, Z.; Tianlu, C. The Clinical and Mechanistic Roles of Bile Acids in Depression, Alzheimer’s Disease, and Stroke. Proteomics 2022, 22, 2100324. [Google Scholar] [CrossRef]
- Zangerolamo, L.; Vettorazzi, J.F.; Rosa, L.R.O.; Carneiro, E.M.; Barbosa, H.C.L. The Bile Acid TUDCA and Neurodegenerative Disorders: An Overview. Life Sci. 2021, 272, 119252. [Google Scholar] [CrossRef]
- Cheng, L.; Huang, C.; Chen, Z. Tauroursodeoxycholic Acid Ameliorates Lipopolysaccharide-Induced Depression Like Behavior in Mice via the Inhibition of Neuroinflammation and Oxido-Nitrosative Stress. Pharmacology 2019, 103, 93–100. [Google Scholar] [CrossRef]
- Kawamata, Y.; Fujii, R.; Hosoya, M.; Harada, M.; Yoshida, H.; Miwa, M.; Fukusumi, S.; Habata, Y.; Itoh, T.; Shintani, Y.; et al. A G Protein-Coupled Receptor Responsive to Bile Acids. J. Biol. Chem. 2003, 278, 9435–9440. [Google Scholar] [CrossRef]
- Daruich, A.; Picard, E.; Boatright, J.H.; Behar-Cohen, F. Review: The Bile Acids Urso- and Tauroursodeoxycholic Acid as Neuroprotective Therapies in Retinal Disease. Mol. Vis. 2019, 25, 610–624. [Google Scholar]
- MahmoudianDehkordi, S.; Bhattacharyya, S.; Brydges, C.R.; Jia, W.; Fiehn, O.; Rush, A.J.; Dunlop, B.W.; Kaddurah-Daouk, R. Gut Microbiome-Linked Metabolites in the Pathobiology of Major Depression with or Without Anxiety—A Role for Bile Acids. Front. Neurosci. 2022, 16, 937906. [Google Scholar] [CrossRef] [PubMed]
- Topham, M.K.; Epand, R.M. Mammalian Diacylglycerol Kinases: Molecular Interactions and Biological Functions of Selected Isoforms. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2009, 1790, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.; Stone, N.J.; Ballantyne, C.; Bittner, V.; Criqui, M.H.; Ginsberg, H.N.; Goldberg, A.C.; Howard, W.J.; Jacobson, M.S.; Kris-Etherton, P.M.; et al. Triglycerides and Cardiovascular Disease. Circulation 2011, 123, 2292–2333. [Google Scholar] [CrossRef] [PubMed]
- Testai, L.; Martelli, A.; Flori, L.; Cicero, A.F.G.; Colletti, A. Coenzyme Q10: Clinical Applications beyond Cardiovascular Diseases. Nutrients 2021, 13, 1697. [Google Scholar] [CrossRef]
- Yan, L.; Han, P.; Man, J.; Tian, Y.; Wang, F.; Wang, J. Discovery of Lipid Profiles of Type 2 Diabetes Associated with Hyperlipidemia Using Untargeted UPLC Q-TOF/MS-Based Lipidomics Approach. Clin. Chim. Acta 2021, 520, 53–62. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Z.; Song, L.; Fu, W.; Liu, L. Anti-Alzheimer’s Natural Products Derived from Plant Endophytic Fungi. Molecules 2023, 28, 2259. [Google Scholar] [CrossRef]
- Qiao, P.-F.; Niu, G.-M. Resting-State fMRI Findings in Patients with First-Episode Idiopathic Epilepsy before and after Treatment. Neurosciences 2017, 22, 316–319. [Google Scholar] [CrossRef]
- Schuff, N.; Woerner, N.; Boreta, L.; Kornfield, T.; Shaw, L.M.; Trojanowski, J.Q.; Thompson, P.M.; Jack, C.R.; Weiner, M.W. MRI of Hippocampal Volume Loss in Early Alzheimers Disease in Relation to ApoE Genotype and Biomarkers. Brain 2009, 132, 1067–1077. [Google Scholar] [CrossRef]
- Andreasen, N.; Sjogren, M.; Blennow, K. CSF Markers for Alzheimer’s Disease: Total Tau, Phospho-Tau and Abeta42. World J. Biol. Psychiatry 2003, 4, 147–155. [Google Scholar] [CrossRef]
- Zwijnenburg, P.J.G.; van der Poll, T.; Roord, J.J.; van Furth, A.M. Chemotactic Factors in Cerebrospinal Fluid during Bacterial Meningitis. Infect. Immun. 2006, 74, 1445–1451. [Google Scholar] [CrossRef]

| Lipid | Hippocampus | PFC | AMY | Cerebellum | Other Regions | Directionality of the Changes | References | |
|---|---|---|---|---|---|---|---|---|
| SP | Cer | √ | √ | × | × | neurotoxic | [14,16,17,18,19,20,21,22] | |
| SM | √ | √ | / | / | neuroprotective | [14,16,17,18,20,24] | ||
| Sphingosine | √ | / | / | / | neuroprotective | [28] | ||
| GP | PC | √ | √ | / | / | anterior cingulate cortex, white matter | neurotoxic | [16,17,18,19,31,32,33,34,35] |
| PE | √ | √ | / | / | white matter | neurotoxic | [16,17,18,19,31,32,33,34,35,36] | |
| CL | √ | / | / | / | neuroprotective | [18,24,33] | ||
| PI | √ | √ | / | / | neurotoxic | [16,17,18,19,24,32] | ||
| PS | √ | / | / | / | neuroprotective | [17,19,20,32] | ||
| PA | √ | √ | √ | / | neuroprotective | [16,17,46] | ||
| FA | DHA | √ | √ | √ | √ | pituitary gland | [54,55,56,57,58] | |
| EPA | √ | √ | / | / | [54,55,56,57,58] | |||
| eCBs | / | / | √ | / | dentate gyrus, striatum, nucleus accumbens | neuroprotective | [69,70] | |
| ST | Cholesterol | √ | √ | / | / | visual association cortex | neuroprotective | [71,72,73] |
| BA | √ | √ | / | / | [84,85,86,87,88,89,90] | |||
| GL | √ | √ | √ | / | [7,17,18,19,20,32,33,46,74] | |||
| Lipid | Mechanisms Influencing Depression | |
|---|---|---|
| SP | Cer | acid sphingomyelinase–ceramide pathway; impair neuronal proliferation, maturation, and survival; enhance oxidative stress; pro-inflammatory; mediate the pro-inflammatory cytokines |
| SM | acid sphingomyelinase–ceramide pathway, neuroplasticity, inflammatory processes, cell membrane stability, affect the normal function of cells | |
| Sphingosine | protect rat hippocampal neurons against oxidative and inflammatory damage by inhibiting the NLRP3 inflammasome in the resident microglia | |
| GP | PC | higher brain levels of PC cause a decrease in acetylcholine release, may lead to the impairment of the brain synapses plasticity |
| PE | influence the topology of cell membranes, thereby promoting membrane activation | |
| CL | an increase in catalase activity in brain mitochondrial membrane associated with neuronal loss or dysfunction | |
| PI | phosphorylated with formation of phosphorylated phosphoinositides, trigger signaling cascades, play a main role in cell metabolism and apoptotic cellular events, modulate neurotransmitter levels | |
| PS | activation protein kinase C, maintenance of the amount of the neurons that produce neurotransmitters, improve neurotransmission by glutamic acid | |
| PA | acid sphingomyelinase–ceramide pathway, regulation of autophagy by regulating ganglioside (GM1) levels, bind to and control the activity of PTP1B | |
| PUFA | DHA, EPA | maintaining membrane fluidity, lipid peroxidation, eicosanoid synthesis, receptor and channel functioning, and gene expression; neuroprotective; anti-inflammatory |
| ARA | correlated with neuroinflammation, cause an increase in the secretion of corticosterone | |
| lipid raft | reduce neurotransmitter signaling, lead to aberrant G-protein coupled receptor signaling | |
| ST | Cholesterol | support synapse generation, maintain synaptic connections, affect synaptic plasticity |
| BA | affect the permeability of the BBB by disrupting tight junctions of RBMECs, mediate neurological activity and inflammation | |
| GL | affect cerebral hemodynamic changes, neurotransmitters, neuroendocrine, neuroimmune regulation, and neuroplasticity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Zhang, Y.; Liu, M.; Li, L.; Zheng, Y. Neuroprotection vs. Neurotoxicity: The Dual Impact of Brain Lipids in Depression. Int. J. Mol. Sci. 2025, 26, 2722. https://doi.org/10.3390/ijms26062722
Yan Y, Zhang Y, Liu M, Li L, Zheng Y. Neuroprotection vs. Neurotoxicity: The Dual Impact of Brain Lipids in Depression. International Journal of Molecular Sciences. 2025; 26(6):2722. https://doi.org/10.3390/ijms26062722
Chicago/Turabian StyleYan, Yuting, Yan Zhang, Mengting Liu, Lingjie Li, and Yanrong Zheng. 2025. "Neuroprotection vs. Neurotoxicity: The Dual Impact of Brain Lipids in Depression" International Journal of Molecular Sciences 26, no. 6: 2722. https://doi.org/10.3390/ijms26062722
APA StyleYan, Y., Zhang, Y., Liu, M., Li, L., & Zheng, Y. (2025). Neuroprotection vs. Neurotoxicity: The Dual Impact of Brain Lipids in Depression. International Journal of Molecular Sciences, 26(6), 2722. https://doi.org/10.3390/ijms26062722






