TFRC Ablation Induces Insufficient Cartilage Development Through Mitochondrial p53 Translocation-Mediated Ferroptosis
Abstract
:1. Introduction
2. Results
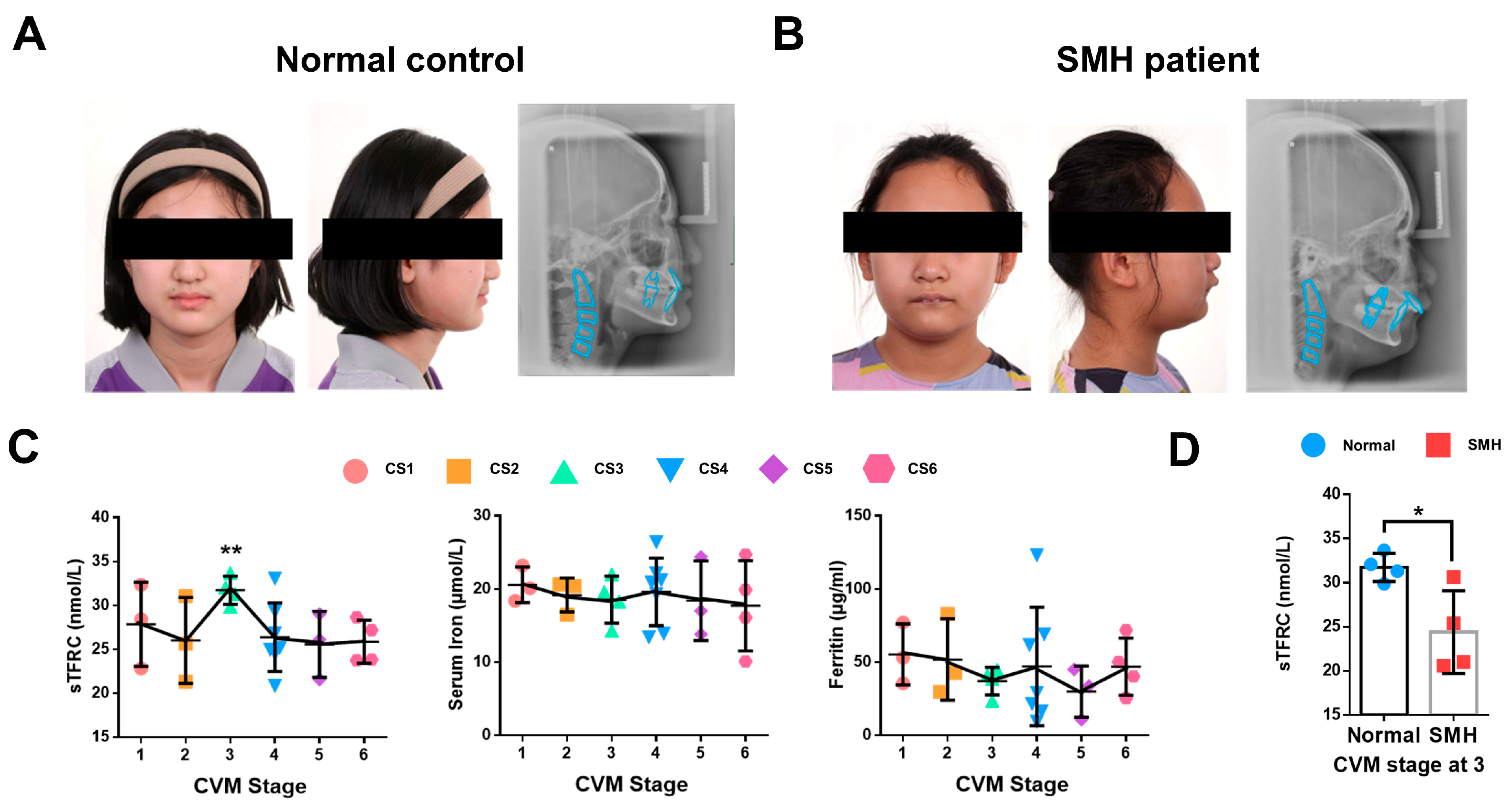
2.1. The Expression of sTFRC Decreases in SMH Patients at CVM Stage 3
2.2. The Relationship Among Condylar Cartilage Development and the Expression of TFRC and SL39A14
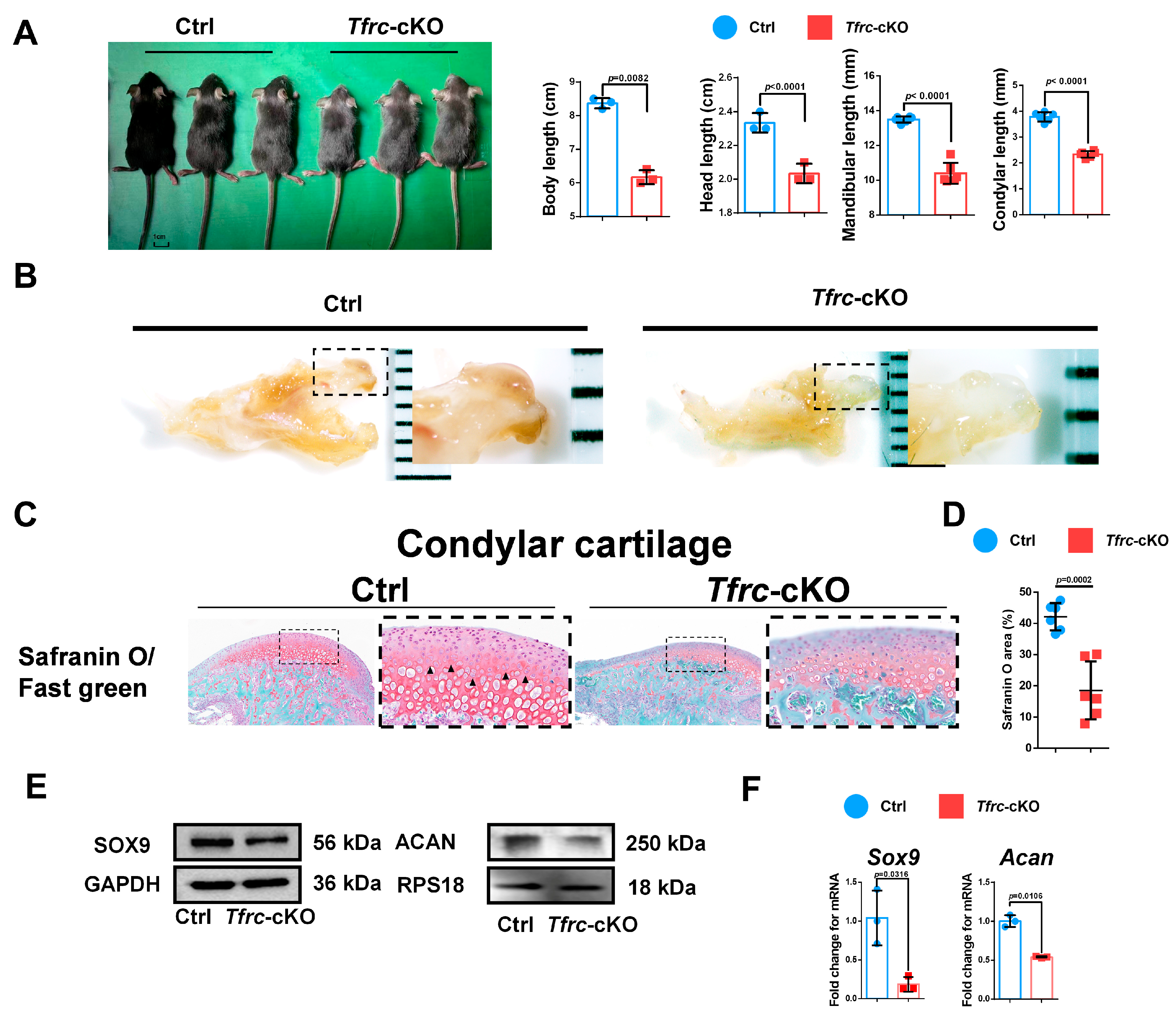
2.3. Generation of Chondrogenic Conditional Knockout Mice with Weaker Condylar Chondrogenic Differentiation Ability
2.4. Negative Correlation Expression Between TFRC and SLC39A14 in Condylar Cartilage of Tfrc-cKO Mice
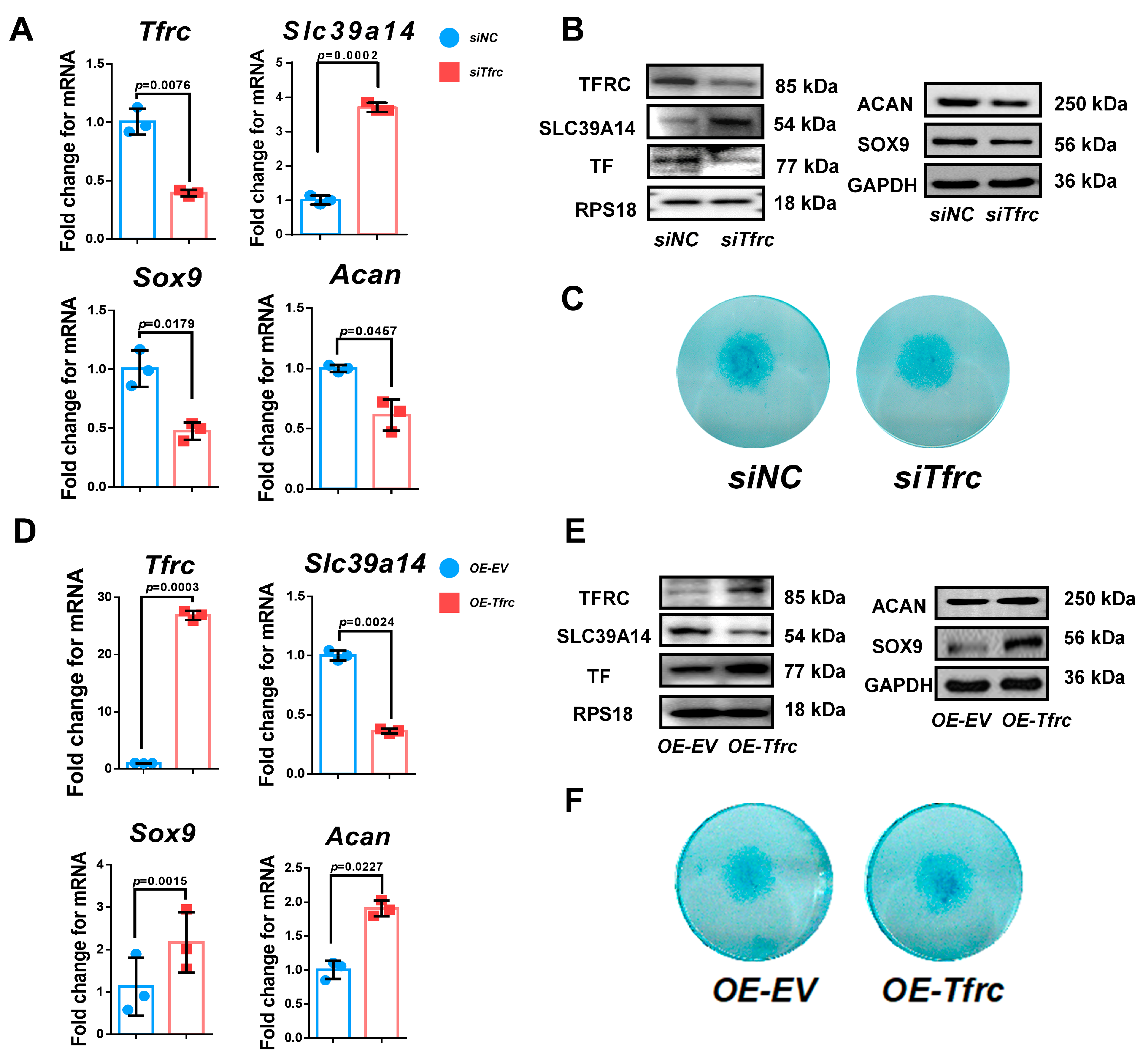
2.5. TFRC Promotes Chondrogenic Differentiation of ATDC5 Cells Through Negatively Regulating SLC39A14 Expression
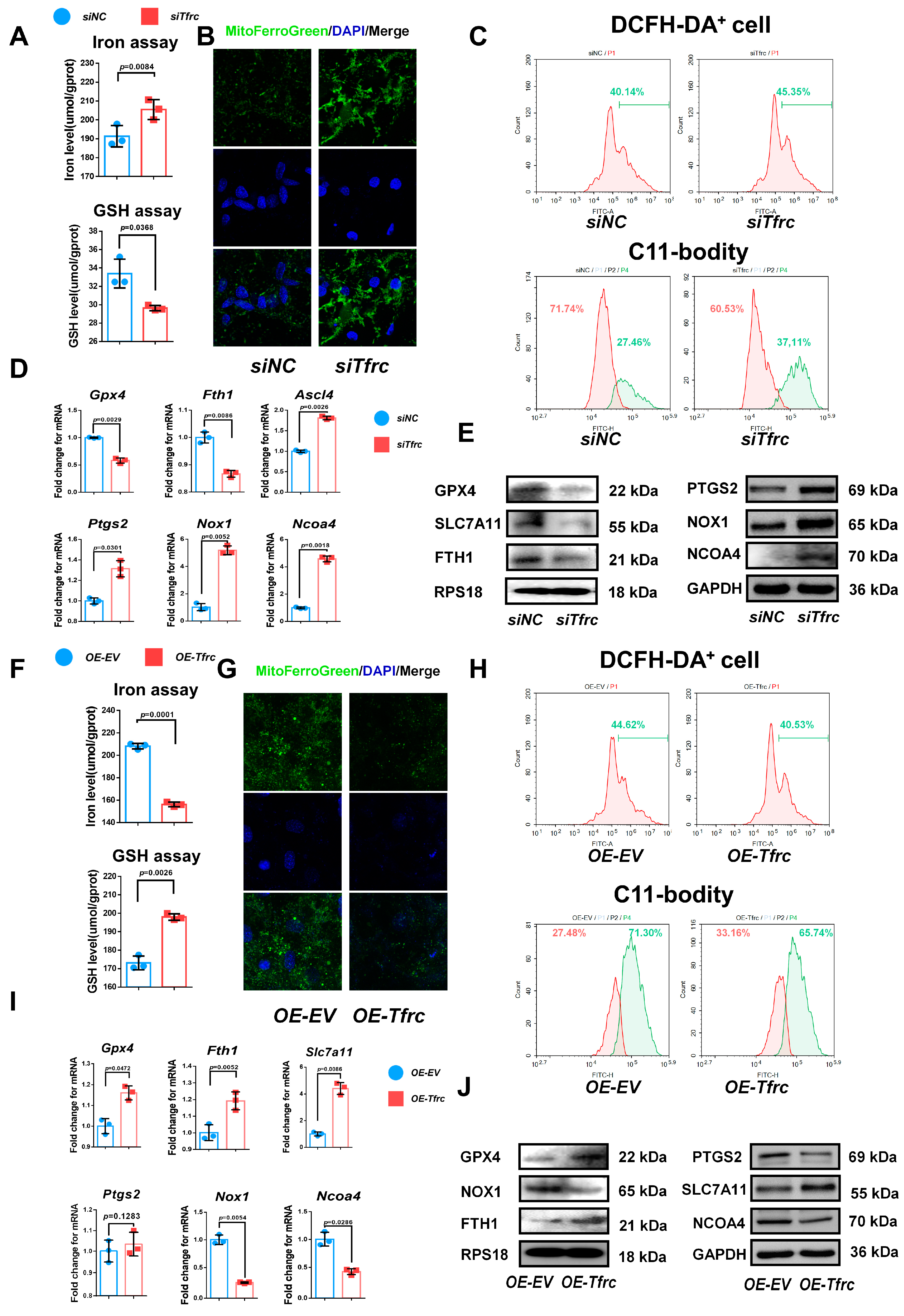
2.6. TFRC Promotes Chondrogenic Differentiation of ATDC5 Cells Through Regulating Ferroptosis
2.7. SLC39A14 Knockdown Promotes Chondrogenic Differentiation of ATDC5 Cells by Inhibiting Ferroptosis
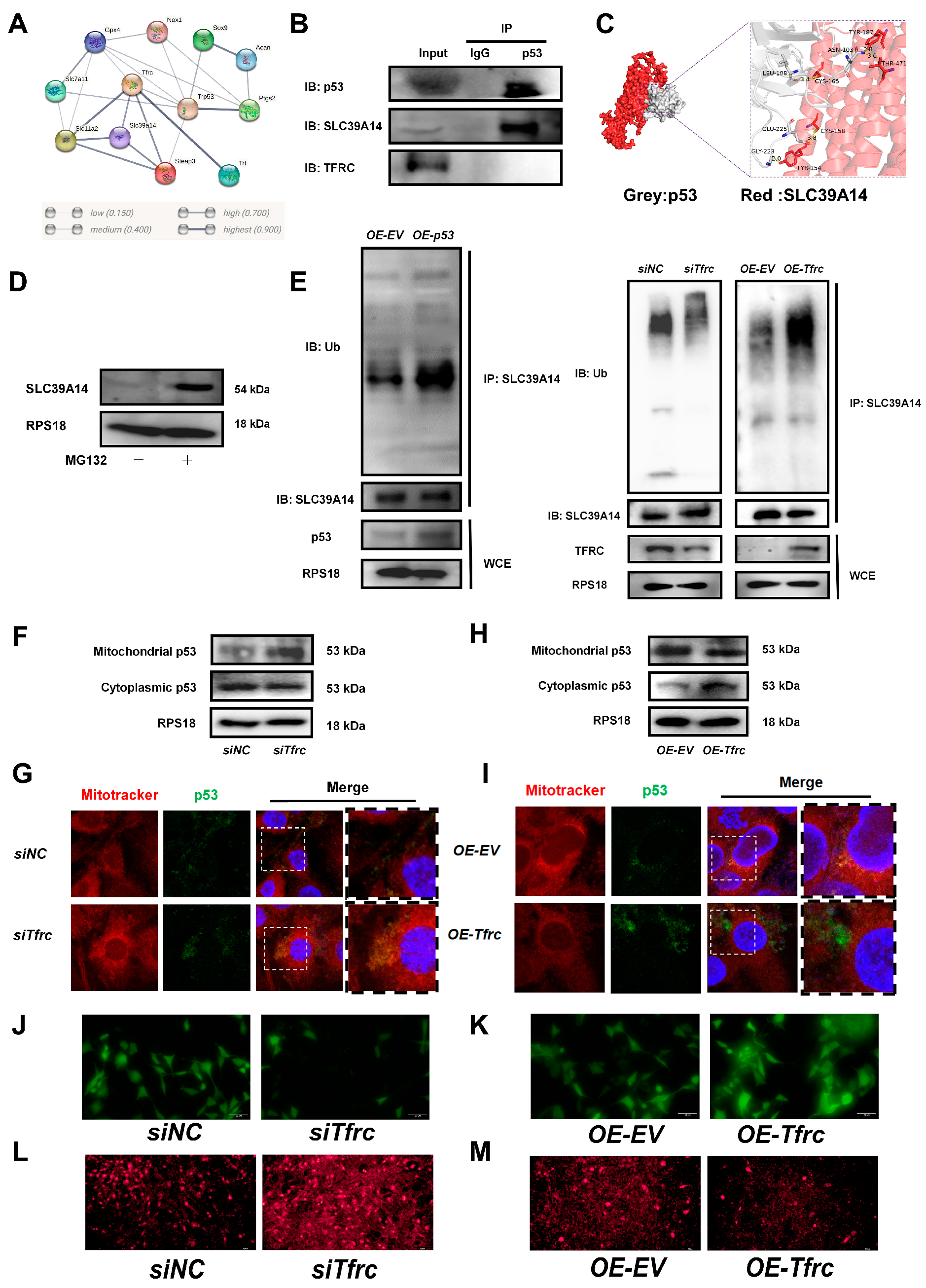
2.8. The Switch of TFRC–SLC39A14 Expression Regulates Ferroptosis Through Mitochondrial p53 Translocation in ATDC5 Cells
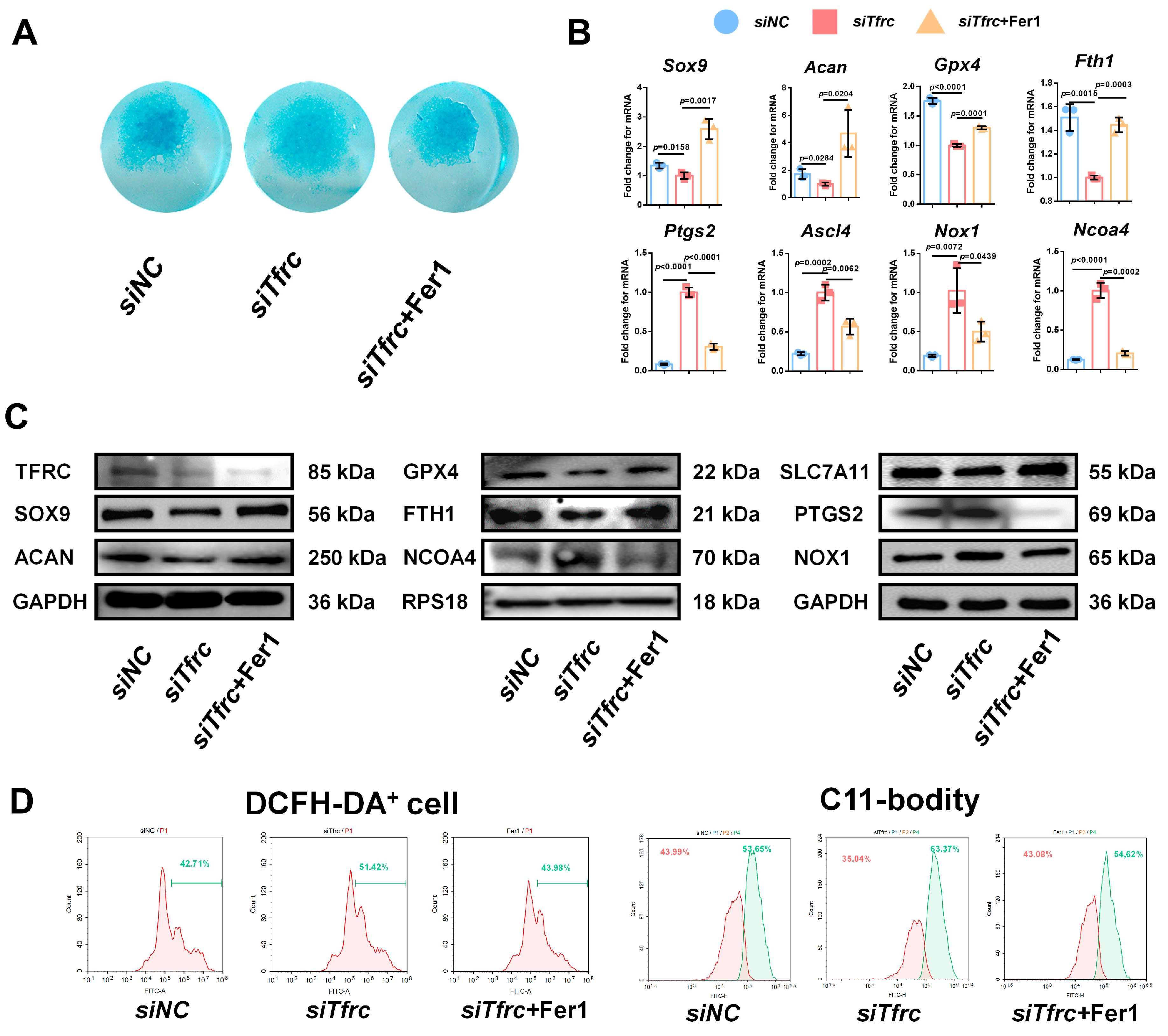
2.9. Ferroptosis Inhibitor Fer1 Restores Chondrogenic Differentiation of ATDC5 Cells After Tfrc Knockdown
2.10. ROS Scavenger Ac-Met-OH and Iron Chelating Agent DFP Restore Chondrogenic Differentiation of ATDC5 Cells After Tfrc Knockdown
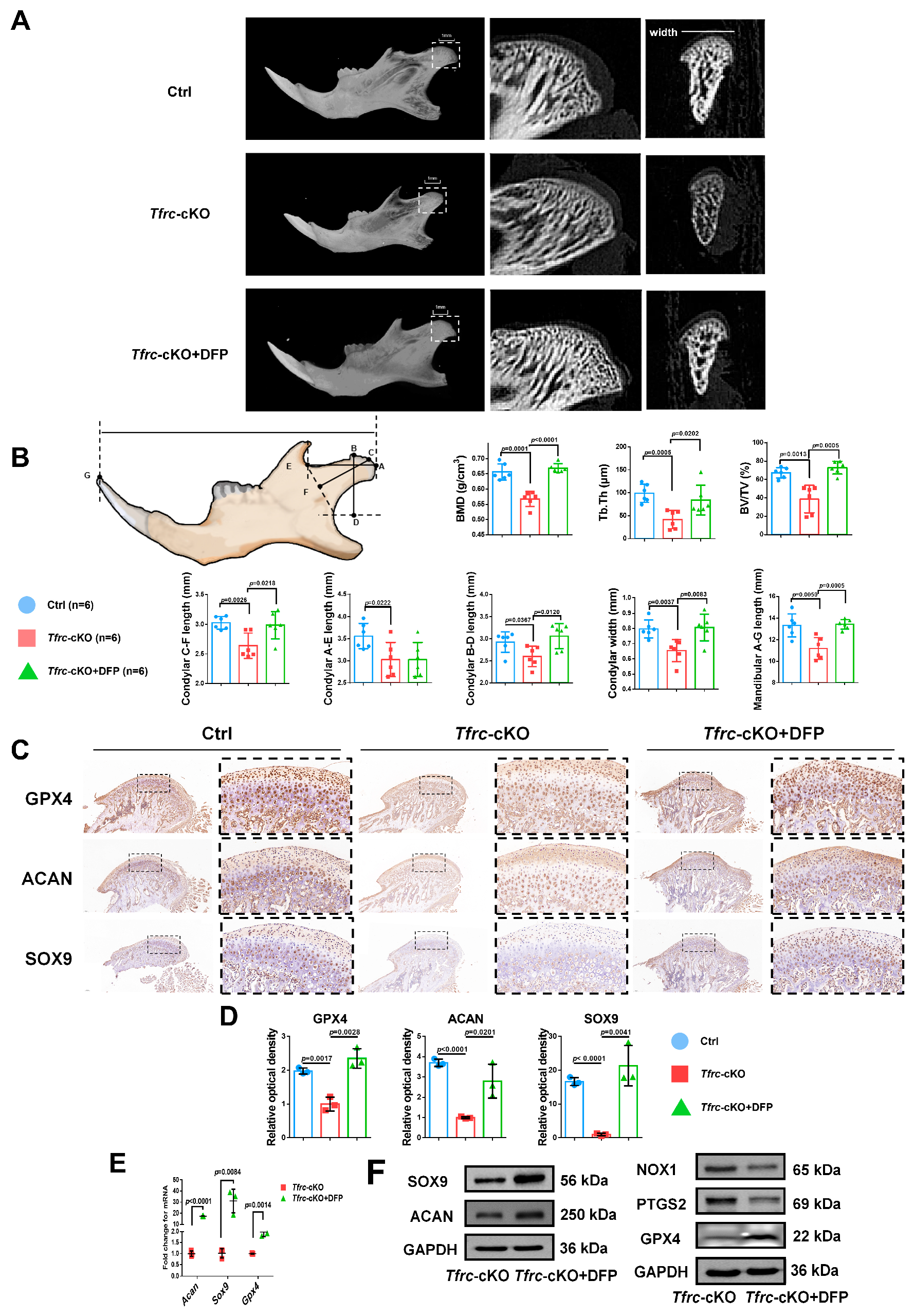
2.11. Iron Chelator DFP Rescues Mandibular (Condyle) Hypoplasia of Tfrc-cKO Mice
3. Discussion
4. Materials and Methods
4.1. Study Approval
4.2. Participants and Clinical Evaluations
4.3. Serum Analyses and Iron Metabolism Determination
4.4. Generation of Col2-CreERT; Tfrcfl/fl Mice and Treatments
4.5. Immunofluorescence (IF)
4.6. Immunohistochemistry (IHC) and Haematoxylin and Eosin (H&E) Staining
4.7. Saffron O and Fast Green Staining
4.8. Cell Culture and Micromass Culture
4.9. siRNA and Overexpression Plasmid Transfection
4.10. Chondrogenic Differentiation Induction
4.11. Alcian Blue Staining
4.12. Cell Proliferation and Cytotoxicity Assay
4.13. Reduced Glutathione (GSH) Assay and Iron Assay
4.14. Reactive Oxygen Species Assay and Lipid Peroxidation Detection
4.15. MitoFerroGreen Staining and Mitochondrial Staining
4.16. Mitochondrial Permeability Transition Pore (MPTP) and Mitochondrial Superoxide Detection
4.17. Quantitative Real-Time PCR (qPCR)
4.18. Western Blot Analysis
4.19. Co-Immunoprecipitation (Co-IP)
4.20. Ubiquitination Detection
4.21. Temporomandibular Joint Injection of DFP Rescues Mice In Vivo
4.22. Micro-CT Scanning and Analysis
4.23. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 3D | Three-dimensional |
| ASCL4 | Acylcoenzyme A synthetase long chain family member 4 |
| DFP | Deferiprone |
| EDTA | Ethylenediaminetetraacetic acid |
| FTH1 | Ferritin heavy chain 1 |
| GPX4 | Glutathione peroxidase 4 |
| H&E | Haematoxylin and eosin |
| IHC | Immunohistochemistry |
| ITS | Insulin-transferrin-selenium |
| NCOA4 | Nuclear receptor coactivator 4 |
| NOX1 | NADPH oxidase 1 |
| NTBI | Non-transferrin-bound iron |
| PBS | Phosphate-buffered saline |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 |
| ROIs | Regions of interests |
| ROS | Reactive oxygen species |
| SLC39A14 | Solute carrier family 39 member 14 |
| SLC7A11 | Solute carrier family 7 member 11 |
| SMH | Skeletal mandibular hypoplasia |
| TBI | Transferrin-bound iron |
| TF | Transferrin |
| TFRC | Transferrin receptor 1 |
| TMX | Tamoxifen |
Appendix A
| CS | Male | Female | Total | Age | sTFRC (nmol/L) | p-Value (vs. CS6) | Serum Iron (μmol/L) | p-Value (vs. CS6) | Ferritin (μg/mL) | p-Value (vs. CS6) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 1 | 3 | 8.67 ± 0.58 | 27.86 ± 4.79 | 0.4973 | 20.57 ± 2.43 | 0.4883 | 55.30 ± 20.89 | 0.6094 |
| 2 | 2 | 1 | 3 | 9.67 ± 1.53 | 26.02 ± 4.89 | 0.9592 | 19.17 ± 2.31 | 0.7166 | 51.87 ± 27.81 | 0.7924 |
| 3 | 3 | 1 | 4 | 10.25 ± 1.26 | 31.73 ± 1.58 | 0.0070 ** | 18.55 ± 3.22 | 0.8152 | 37.03 ± 9.35 | 0.3942 |
| 4 | 4 | 3 | 7 | 11.71 ± 0.49 | 26.39 ± 3.90 | 0.8172 | 19.60 ± 4.61 | 0.5731 | 47.15 ± 40.46 | 0.9929 |
| 5 | 1 | 2 | 3 | 14.00 ± 1.00 | 25.58 ± 3.75 | 0.9052 | 18.40 ± 5.44 | 0.8824 | 29.90 ± 17.48 | 0.2866 |
| 6 | 2 | 2 | 4 | 14.25 ± 0.96 | 25.87 ± 2.45 | / | 17.70 ± 6.17 | / | 46.95 ± 19.51 | / |
| Total | 14 | 10 | 24 | 11.54 ± 2.15 | 27.23 ± 3.88 | / | 19.03 ± 4.03 | / | 44.88 ± 26.13 | / |
| CVM Stage | Male | Female | Total | Age | sTFRC (nmol/L) | p-Value | |
|---|---|---|---|---|---|---|---|
| Normal | 3 | 3 | 1 | 4 | 10.25 ± 1.26 | 31.73 ± 1.58 | 0.0253 * |
| SMH | 3 | 0 | 4 | 4 | 11.50 ± 1.29 | 24.41 ± 4.69 |
| Gene | Sense Primer | Antisense Primer | Product Size (bp) |
|---|---|---|---|
| Rps18 | AGTTCCAGCACATTTTGCGAG | TCATCCTCCGTGAGTTCTCCA | 166 |
| Acan | AGGTGTCGCTCCCCAACTAT | CTTCACAGCGGTAGATCCCAG | 96 |
| Sox9 | AGTACCCGCATCTGCACAAC | ACGAAGGGTCTCTTCTCGCT | 88 |
| Tfrc | GTTTCTGCCAGCCCCTTATTAT | GCAAGGAAAGGATATGCAGCA | 152 |
| Slc39a14 | CCTCAGGACAATTACGTCTCCA | ATGGTGCTCGTTTTTCTGCTT | 111 |
| Gpx4 | TGTGCATCCCGCGATGATT | CCCTGTACTTATCCAGGCAGA | 97 |
| Ptgs2 | TTCCAATCCATGTCAAAACCGT | AGTCCGGGTACAGTCACACTT | 76 |
| Nox1 | CCTGATTCCTGTGTGTCGAAA | TTGGCTTCTTCTGTAGCGTTC | 190 |
| Ncoa4 | GAAAAGAGGCTATATCCAGGTGC | AAGAAGCCACTCACTCAGAGA | 79 |
| Ascl4 | TGAACGTATCCCTGGACTAGG | TCAGACAGTGTAAGGGGTGAA | 141 |
| Fth1 | TGCCTCCTACGTCTATCTGTC | GTCATCACGGTCTGGTTTCTTT | 190 |
| Slc7a11 | AGGGCATACTCCAGAACACG | GGACCAAAGACCTCCAGAATG | 160 |
Appendix B
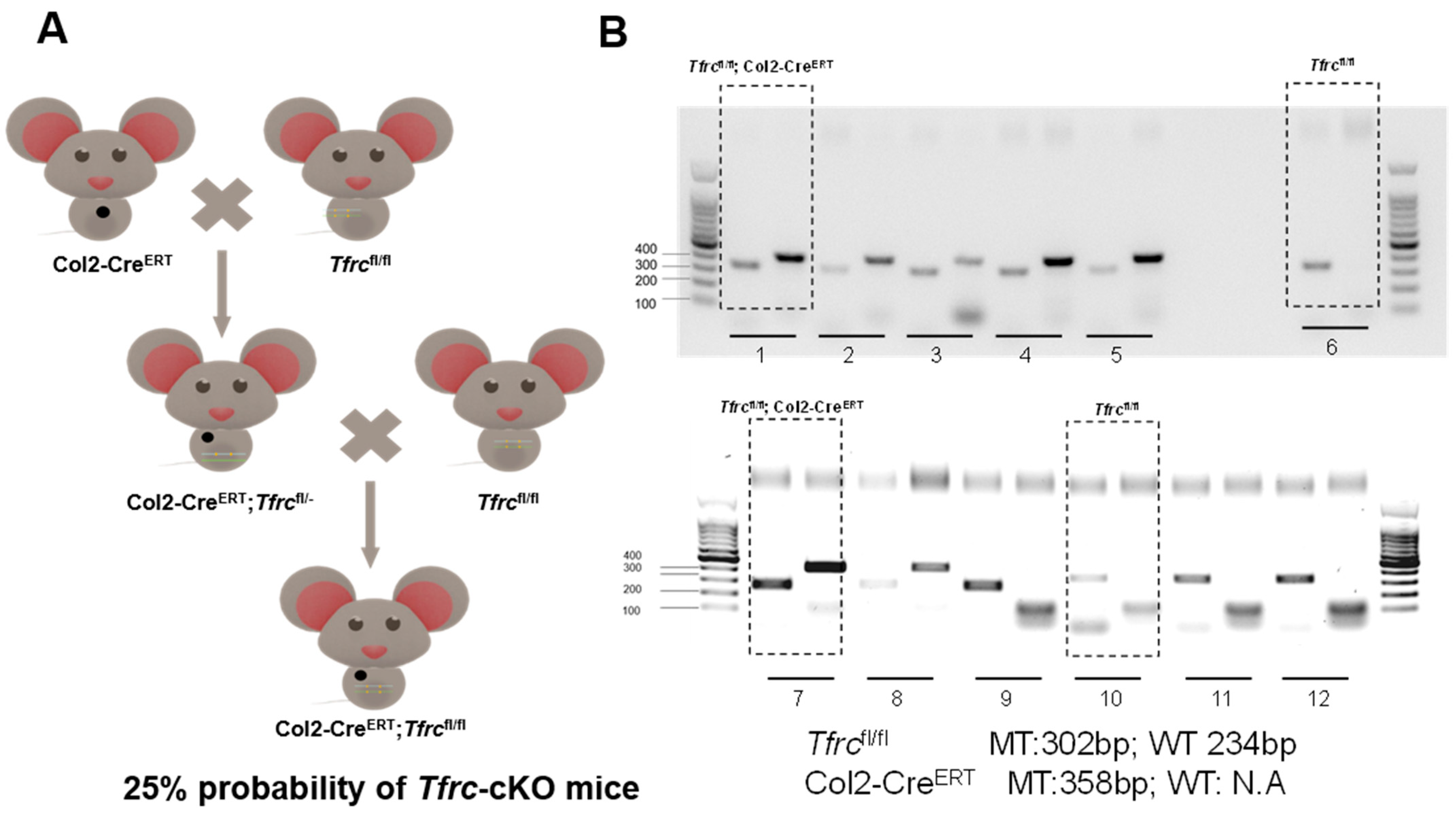
Appendix B.1. Schematic Diagram of Mice Reproduction and Genotyping
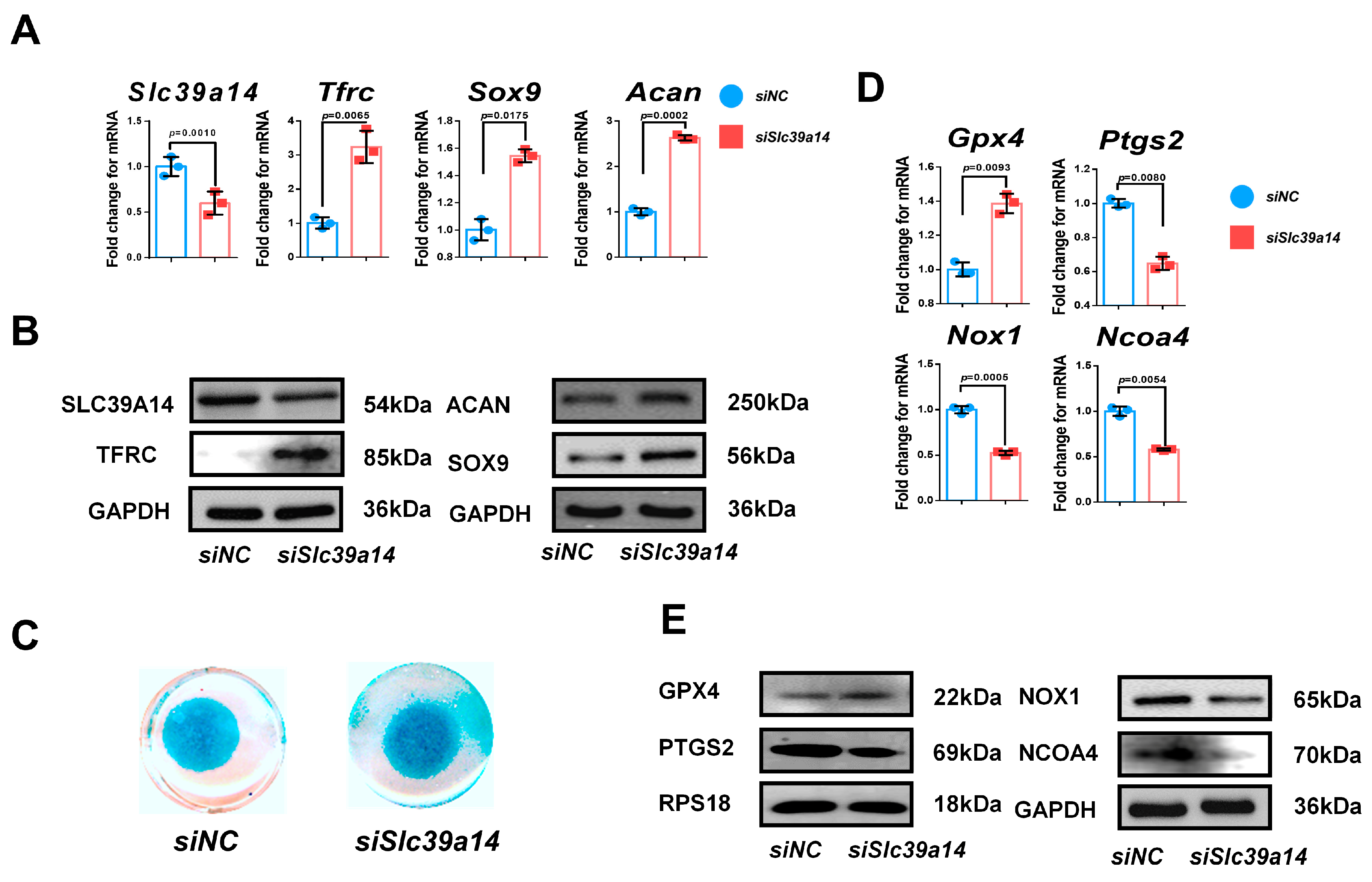
Appendix B.2. Specific Knockdown of Slc39a14 Promotes Chondrogenic Differentiation of siTfrc-Transfected ATDC5 Cell Line

Appendix B.3. Specific Knockdown of Slc39a14 Relieves Serum-Deprivation-Induced Ferroptosis in siTfrc-Transfected ATDC5 Cells
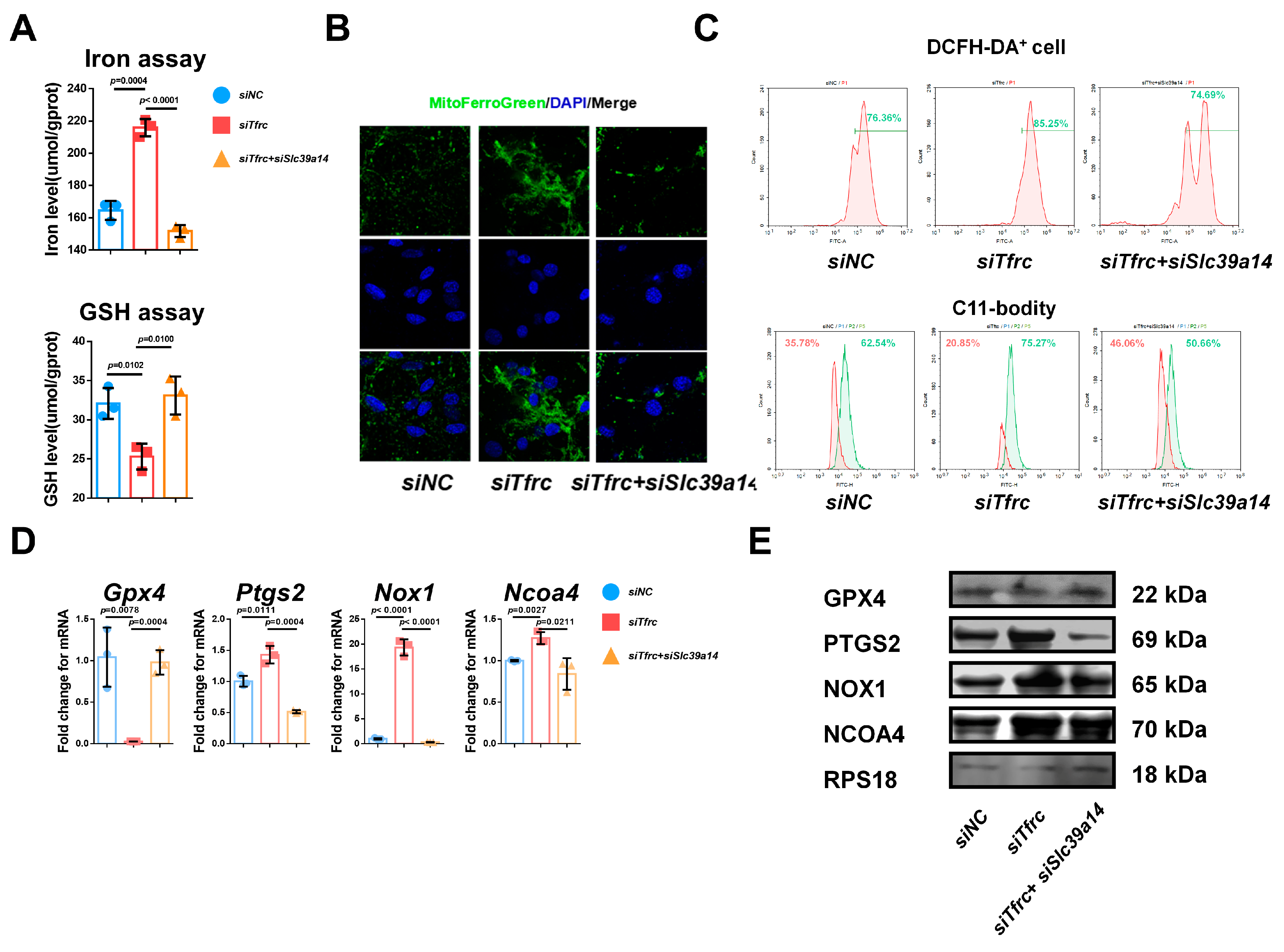
Appendix B.4. SLC39A14 Knockdown Promotes Chondrogenic Differentiation of ATDC5 Cells by Inhibiting Ferroptosis
Appendix B.5. Immunofluorescence Detection of the Co-Localization of p53 and SLC39A14

Appendix B.6. Immunofluorescence Detection of the Co-Localization of p53 and MitoFerroGreen

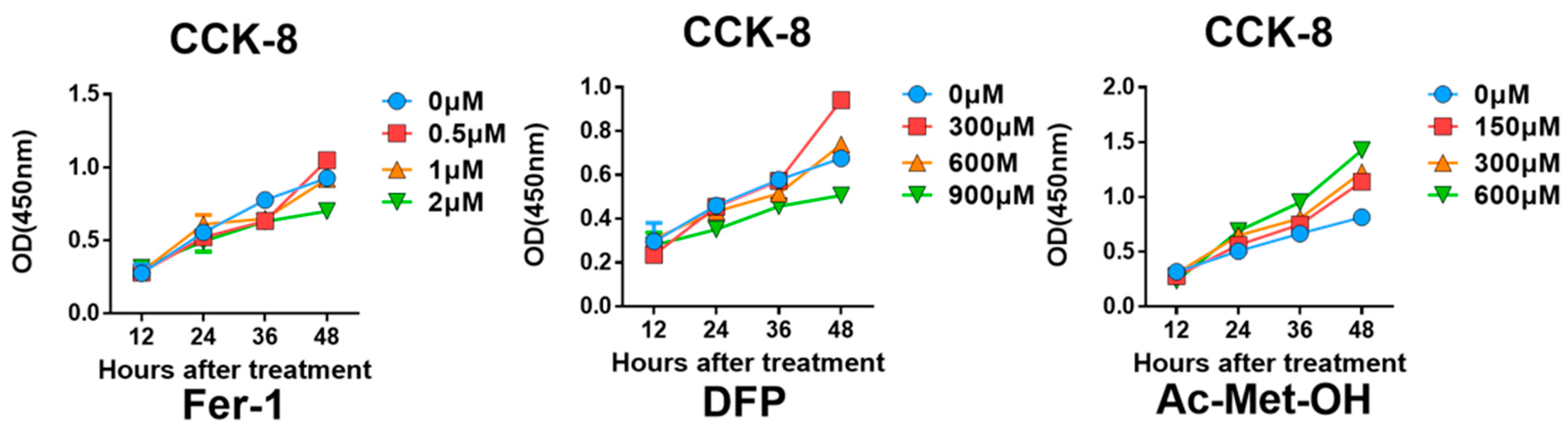
Appendix B.7. Optimal Inhibitor Concentration Finding for Rescue In Vitro
References
- Hinton, R.J.; Jing, Y.; Jing, J.; Feng, J.Q. Roles of Chondrocytes in Endochondral Bone Formation and Fracture Repair. J. Dent. Res. 2017, 96, 23–30. [Google Scholar] [CrossRef]
- Jing, Y.; Zhou, X.; Han, X.; Jing, J.; von der Mark, K.; Wang, J.; de Crombrugghe, B.; Hinton, R.J.; Feng, J.Q. Chondrocytes Directly Transform into Bone Cells in Mandibular Condyle Growth. J. Dent. Res. 2015, 94, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, P.; Mohan, S. The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Res. 2018, 6, 19. [Google Scholar] [CrossRef]
- Zhao, J.J.; Zhou, X.; Tang, Q.M.; Yu, R.; Yu, S.L.; Long, Y.L.; Cao, C.; Han, J.; Shi, A.B.; Mao, J.J.; et al. BMAL1 Deficiency Contributes to Mandibular Dysplasia by Upregulating MMP3. Stem Cell Rep. 2018, 10, 180–195. [Google Scholar] [CrossRef] [PubMed]
- Berraquero, R.; Palacios, J.; Rodriguez, J.I. The Role of the Condylar Cartilage in Mandibular Growth—A Study in Thanatophoric Dysplasia. Am. J. Orthod. Dentofac. Orthop. 1992, 102, 220–226. [Google Scholar] [CrossRef]
- Zimmerer, R.M.; Sander, A.K.; Schönfeld, A.; Lethaus, B.; Gellrich, N.C.; Neuhaus, M.T. Congenital Mandibular Hypoplasia: Patient-Specific Total Joint Replacement as a Line Extension in the Treatment of Complex Craniofacial Anomalies. J. Maxillofac. Oral Surg. 2023, 22, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Bibbins-Domingo, K.; Grossman, D.C.; Curry, S.J.; Davidson, K.W.; Epling, J.W.; García, F.A.R.; Herzstein, J.; Kemper, A.R.; Krist, A.H.; Kurth, A.E.; et al. Screening for Obstructive Sleep Apnea in Adults US Preventive Services Task Force Recommendation Statement. Jama-J. Am. Med. Assoc. 2017, 317, 407–414. [Google Scholar] [CrossRef]
- DelRosso, L.M. Epidemiology and Diagnosis of Pediatric Obstructive Sleep Apnea. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 2–6. [Google Scholar] [CrossRef]
- Cozza, P.; Baccetti, T.; Franchi, L.; De Toffol, L.; McNamara, J.A. Mandibular changes produced by functional appliances in Class II malocclusion: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 599.e1–599.e12. [Google Scholar] [CrossRef]
- Bidjan, D.; Sallmann, R.; Eliades, T.; Papageorgiou, S.N. Orthopedic Treatment for Class II Malocclusion with Functional Appliances and Its Effect on Upper Airways: A Systematic Review with Meta-Analysis. J. Clin. Med. 2020, 9, 3806. [Google Scholar] [CrossRef]
- Carvalho, F.R.; Lentini-Oliveira, D.A.; Prado, L.B.F.; Prado, G.F.; Carvalho, L.B.C. Oral appliances and functional orthopaedic appliances for obstructive sleep apnoea in children. Cochrane Database Syst. Rev. 2016, 10, CD005520. [Google Scholar] [CrossRef] [PubMed]
- Gatter, K.C.; Brown, G.; Trowbridge, I.S.; Woolston, R.E.; Mason, D.Y. Transferrin Receptors in Human-Tissues—Their Distribution and Possible Clinical Relevance. J. Clin. Pathol. 1983, 36, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Kawabata, H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019, 133, 46–54. [Google Scholar] [CrossRef]
- Lebrón, J.A.; Bennett, M.J.; Vaughn, D.E.; Chirino, A.J.; Snow, P.M.; Mintier, G.A.; Feder, J.N.; Bjorkman, P.J. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell 1998, 93, 111–123. [Google Scholar] [CrossRef]
- Wen, X.; Franchi, L.; Chen, F.; Gu, Y. Proteomic analysis of gingival crevicular fluid for novel biomarkers of pubertal growth peak. Eur. J. Orthod. 2018, 40, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Zhang, K.; Liu, K.; Shao, X.; Ding, Z.; Wang, F.; Hong, Y.; Zhu, M.; Li, H.; Li, H. Transferrin receptor facilitates TGF-β and BMP signaling activation to control craniofacial morphogenesis. Cell Death Dis. 2016, 7, e2282. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Bayir, H.; Dixon, S.J.; Tyurina, Y.Y.; Kellum, J.A.; Kagan, V.E. Ferroptotic mechanisms and therapeutic targeting of iron metabolism and lipid peroxidation in the kidney. Nat. Rev. Nephrol. 2023, 19, 315–336. [Google Scholar] [CrossRef]
- Girijashanker, K.; He, L.; Soleimani, M.; Reed, J.M.; Li, H.; Liu, Z.; Wang, B.; Dalton, T.P.; Nebert, D.W. Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: Similarities to the ZIP8 transporter. Mol. Pharmacol. 2008, 73, 1413–1423. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Hiromatsu, M.; Toshida, K.; Itoh, S.; Harada, N.; Kohashi, K.; Oda, Y.; Yoshizumi, T. Transferrin Receptor is Associated with Sensitivity to Ferroptosis Inducers in Hepatocellular Carcinoma. Ann. Surg. Oncol. 2023, 30, 8675–8689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Kong, Y.; Ma, Y.; Ni, S.L.; Wikerholmen, T.; Xi, K.Y.; Zhao, F.H.; Zhao, Z.M.; Wang, J.P.; Huang, B.; et al. Loss of COPZ1 induces NCOA4 mediated autophagy and ferroptosis in glioblastoma cell lines. Oncogene 2021, 40, 1425–1439. [Google Scholar] [CrossRef]
- Mao, L.H.; Zhao, T.M.; Song, Y.; Lin, L.; Fan, X.F.; Cui, B.X.; Feng, H.J.; Wang, X.Y.; Yu, Q.X.; Zhang, J.; et al. The emerging role of ferroptosis in non-cancer liver diseases: Hype or increasing hope? Cell Death Dis. 2020, 11, 518. [Google Scholar] [CrossRef]
- Tang, Y.; Zhou, J.; Hooi, S.C.; Jiang, Y.M.; Lu, G.D. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-CoA synthetases. Oncol. Lett. 2018, 16, 1390–1396. [Google Scholar] [CrossRef]
- Zhu, J.H.; Xiong, Y.X.; Zhang, Y.X.; Wen, J.Y.; Cai, N.; Cheng, K.; Liang, H.F.; Zhang, W.G. The Molecular Mechanisms of Regulating Oxidative Stress-Induced Ferroptosis and Therapeutic Strategy in Tumors. Oxid. Med. Cell. Longev. 2020, 2020, 8810785. [Google Scholar] [CrossRef] [PubMed]
- Hao, R.L.; Ge, J.L.; Song, X.Y.; Li, F.; Sun-Waterhouse, D.; Li, D.P. Cadmium induces ferroptosis and apoptosis by modulating miR-34a-5p/Sirt1axis in PC12 cells. Environ. Toxicol. 2022, 37, 41–51. [Google Scholar] [CrossRef]
- Wen, X.; Wang, Y.X.; Gu, Y. Transferrin promotes chondrogenic differentiation in condylar growth through inducing autophagy via ULK1-ATG16L1 axis. Clin. Sci. 2023, 137, 1431–1449. [Google Scholar] [CrossRef]
- Perinetti, G.; Primozic, J.; Franchi, L.; Contardo, L. Treatment Effects of Removable Functional Appliances in Pre-Pubertal and Pubertal Class II Patients: A Systematic Review and Meta-Analysis of Controlled Studies. PLoS ONE 2015, 10, e0141198. [Google Scholar] [CrossRef]
- Suega, K.; Kandarini, Y.; Tubung, J. Role of Soluble Transferrin Receptor and Transferrin Receptor-Ferritin Index to Detect Iron Deficiency Anemia in Regular Hemodialysis Patients. Open Access Maced. J. Med. Sci. 2019, 7, 97–102. [Google Scholar] [CrossRef]
- Beguin, Y. Soluble transferrin receptor for the evaluation of erythropoiesis and iron status. Clin. Chim. Acta 2003, 329, 9–22. [Google Scholar] [CrossRef]
- Ding, H.R.; Chen, S.J.; Pan, X.H.; Dai, X.S.; Pan, G.H.; Li, Z.; Mai, X.D.; Tian, Y.; Zhang, S.S.; Liu, B.D.; et al. Transferrin receptor 1 ablation in satellite cells impedes skeletal muscle regeneration through activation of ferroptosis. J. Cachexia Sarcopenia Muscle 2021, 12, 746–768. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; An, P.; Xie, E.J.; Wu, Q.; Fang, X.X.; Gao, H.; Zhang, Z.Z.; Li, Y.Z.; Wang, X.D.; Zhang, J.Y.; et al. Characterization of Ferroptosis in Murine Models of Hemochromatosis. Hepatology 2017, 66, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.Y.; Jiang, L.; Wang, H.; Shen, Z.; Cheng, Q.; Zhang, P.; Wang, J.M.; Wu, Q.; Fang, X.X.; Duan, L.Y.; et al. Hepatic transferrin plays a role in systemic iron homeostasis and liver ferroptosis. Blood 2020, 136, 726–739. [Google Scholar] [CrossRef]
- Chen, B.Y.; Pathak, J.L.; Lin, H.Y.; Guo, W.; Chen, W.; Luo, G.; Wang, L.; Sun, X.; Ding, Y.; Li, J.; et al. Inflammation Triggers Chondrocyte Ferroptosis in TMJOA via HIF-1α/TFRC. J. Dent. Res. 2024, 103, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.N.; Zhang, A.S.; Wortham, A.M.; Jue, S.; Knutson, M.D.; Enns, C.A. The Tumor Suppressor, P53, Decreases the Metal Transporter, ZIP14. Nutrients 2017, 9, 1335. [Google Scholar] [CrossRef]
- Dai, C.Q.; Luo, T.T.; Luo, S.C.; Wang, J.Q.; Wang, S.M.; Bai, Y.H.; Yang, Y.L.; Wang, Y.Y. p53 and mitochondrial dysfunction: Novel insight of neurodegenerative diseases. J. Bioenerg. Biomembr. 2016, 48, 337–347. [Google Scholar] [CrossRef]
- Guo, Z.; Lin, J.M.; Sun, K.; Guo, J.Y.; Yao, X.D.; Wang, G.C.; Hou, L.C.; Xu, J.T.; Guo, J.C.; Guo, F.J. Deferoxamine Alleviates Osteoarthritis by Inhibiting Chondrocyte Ferroptosis and Activating the Nrf2 Pathway. Front. Pharmacol. 2022, 13, 791376. [Google Scholar] [CrossRef]
- Kwiatkowski, J.L.; Hamdy, M.; El-Beshlawy, A.; Ebeid, F.S.E.; Badr, M.; Alshehri, A.; Kanter, J.; Inusa, B.; Adly, A.A.M.; Williams, S.; et al. Deferiprone vs deferoxamine for transfusional iron overload in SCD and other anemias: A randomized, open-label noninferiority study. Blood Adv. 2022, 6, 1243–1254. [Google Scholar] [CrossRef]
- Baccetti, T.; Franchi, L.; McNamara, J.A. An improved version of the cervical vertebral maturation (CVM) method for the assessment of mandibular growth. Angle Orthod. 2002, 72, 316–323. [Google Scholar]
- Jensen, E.C. Quantitative analysis of histological staining and fluorescence using ImageJ. Anat. Rec. 2013, 296, 378–381. [Google Scholar] [CrossRef]
- Kudva, A.K.; Dikina, A.D.; Luyten, F.P.; Alsberg, E.; Patterson, J. Gelatin microspheres releasing transforming growth factor drive chondrogenesis of human periosteum derived cells in micromass culture. Acta Biomater. 2019, 90, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Rayatpour, A.; Foolad, F.; Heibatollahi, M.; Khajeh, K.; Javan, M. Ferroptosis inhibition by deferiprone, attenuates myelin damage and promotes neuroprotection in demyelinated optic nerve. Sci. Rep. 2022, 12, 19630. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wen, X.; Guo, Y.; Wang, Y.; Gu, Y. TFRC Ablation Induces Insufficient Cartilage Development Through Mitochondrial p53 Translocation-Mediated Ferroptosis. Int. J. Mol. Sci. 2025, 26, 2724. https://doi.org/10.3390/ijms26062724
Wang Y, Wen X, Guo Y, Wang Y, Gu Y. TFRC Ablation Induces Insufficient Cartilage Development Through Mitochondrial p53 Translocation-Mediated Ferroptosis. International Journal of Molecular Sciences. 2025; 26(6):2724. https://doi.org/10.3390/ijms26062724
Chicago/Turabian StyleWang, Yidi, Xi Wen, Yutong Guo, Yixiang Wang, and Yan Gu. 2025. "TFRC Ablation Induces Insufficient Cartilage Development Through Mitochondrial p53 Translocation-Mediated Ferroptosis" International Journal of Molecular Sciences 26, no. 6: 2724. https://doi.org/10.3390/ijms26062724
APA StyleWang, Y., Wen, X., Guo, Y., Wang, Y., & Gu, Y. (2025). TFRC Ablation Induces Insufficient Cartilage Development Through Mitochondrial p53 Translocation-Mediated Ferroptosis. International Journal of Molecular Sciences, 26(6), 2724. https://doi.org/10.3390/ijms26062724







