Lifestyle and Environmental Factors Affecting Male Fertility, Individual Predisposition, Prevention, and Intervention
Abstract
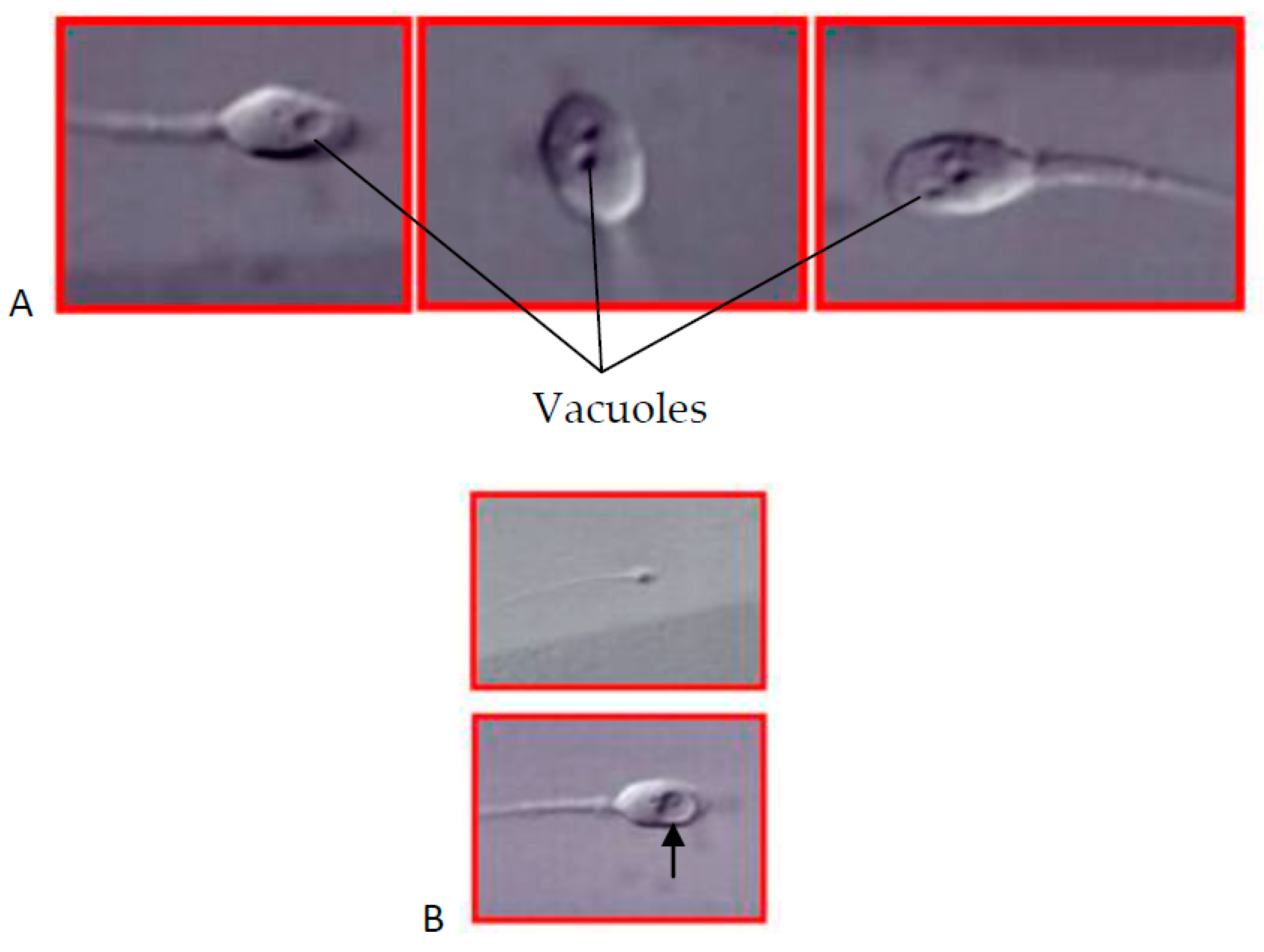
1. Introduction
2. Contemporary Lifestyle and Male Fertility
2.1. Obesity
2.2. Air Pollution
2.3. Harmful Chemicals (Other than Air Pollutants)
| Name | Sources | Effects | Mechanism of Action | References |
|---|---|---|---|---|
| Dioxins | Industrial accidents | 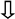 Sperm count Sperm count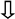 Sperm motility Sperm motility | Endocrine disruption | [41,42] |
| Bisphenols | Plastics | 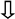 Spermatogenesis Spermatogenesis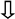 Sperm motility Sperm motility Sperm function Sperm function Sperm apoptosis Sperm apoptosis Sperm aneuploidy Sperm aneuploidy | Endocrine disruption Oxidative stress  MAPK MAPK Fas/FasL Fas/FasL Caspases 3, 9 Caspases 3, 9 Bax Bax DNA damage DNA damage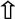 Mitochondrial damage Mitochondrial damage | [44,45,46,47] |
| Pesticides and herbicides | Agricultural products | 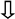 Spermatogenesis Spermatogenesis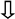 Sperm motility Sperm motility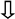 Sperm count Sperm count Sperm apoptosis Sperm apoptosis | Endocrine disruption Oxidative stress  DNA damage DNA damage Mitochondrial dysfunction Mitochondrial dysfunction | [48,49,50,51,52,53,54,55] |
| Phthalates | Various industrial goods |  Spermatogenesis Spermatogenesis Sperm motility Sperm motility Sperm count Sperm count Teratozoospermia Teratozoospermia |  Endocrine disruption Endocrine disruption Gene transcription Gene transcriptionAndrogene synthesis | [56,57,58,59,60,61,62,63,64] |
| Heavy metals | Various industrial goods |  Spermatogenesis Spermatogenesis Sperm motility Sperm motility Sperm count Sperm count Teratozoospermia Teratozoospermia Sperm viability Sperm viability | Endocrine disruption Oxidative stress  DNA damage DNA damage Androgene synthesis Androgene synthesis | [65,66] |
2.4. Heat
2.5. Cigarette Smoking
2.6. Alcohol Intake
2.7. Psychological Stress
2.8. Inadequate Physical Activity
2.9. Use of Mobile Telephones and Portable Computers
3. Individual Susceptibility to Negative Lifestyle and Environmental Factors
3.1. Genetic Predisposition
3.2. Epigenetic Factors
3.3. Systemic Disease and Medication
3.4. Local Affections of the Genitourinary System
| Conditions | Examples | Interactions | References |
|---|---|---|---|
| Genetic | GSTM1 mutation | Decreased air pollutant detoxification | [106,107] |
| XRCC1, XRCC1, CYP1A1 po1ymorphism | High DNA damage by air pollutants | [107] | |
| Mutations and deletions of mtDNA | Susceptibility to oxidative stress | [112,113,114] | |
| Epigenetic | H19 hypomethylation | Susceptibility to effects of smoking | [121,122,123] |
| Systemic disease and medication | Insulin resistance and diabetes | Potentiation of factors causing oxidative stress and inflammation | [125,126,127,128] |
| Infectious diseases | Potentiation of factors causing oxidative stress and inflammation | [136,137,138,139,140,141,142,143,144,145,146,147] | |
| Chemotherapeutic and antiepileptic drugs, paracetamol, aspirin, lansoprazole, marijuana | Potentiation of factors affecting spermatogenesis and sperm motility | [159,160,161,162,163,164] | |
| Local affections | Semen microbiome | Can exert both potentiating and protective action | [165,166,167,168,169,170,171,172,173,174] |
| Varicoceles, orchitis, and prostatitis | Potentiation of factors causing oxidative stress and inflammation | [175] |
4. Personalized Management
4.1. Prevention
4.2. Intervention
5. Conclusions
Funding
Conflicts of Interest
References
- Preventing Noncommunicable Diseases (NCDs) by Reducing Environmental Risk Factors; World Health Organization: Geneva, Switzerland, 2017; (WHO/FWC/EPE/17.1).
- Pakholok, O. The Idea of Healthy Lifestyle and Its Transformation Into Health-Oriented Lifestyle in Contemporary Society. Sage Open. 2013, 3. [Google Scholar] [CrossRef]
- Kwon, H.-A.; Kim, S. Variation in the characteristics of everyday life and meaning of urban housing due to the transition of social structure: Focusing on articles published in lifestyle magazines. Sustainability 2017, 9, 1298. [Google Scholar] [CrossRef]
- Farhud, D.D. Impact of lifestyle on health. Iran. J. Public Health 2015, 44, 1442–1444. [Google Scholar]
- Tabish, S.A. Lifestyle diseases: Consequences, characteristics, causes and control. J. Cardiol. Curr. Res. 2017, 9, 00326. [Google Scholar] [CrossRef][Green Version]
- Balawender, K.; Orkisz, S. The impact of selected modifiable lifestyle factors on male fertility in the modern world. Cent. Eur. J. Urol. 2020, 73, 563–568. [Google Scholar] [CrossRef]
- Ilacqua, A.; Izzo, G.; Emerenziani, G.P.; Baldari, C.; Aversa, A. Lifestyle and fertility: The influence of stress and quality of life on male fertility. Reprod. Biol. Endocrinol. 2018, 16, 115. [Google Scholar] [CrossRef]
- Carlsen, E.; Giwercman, A.; Keiding, N.; Skakkebaek, N.E. Evidence for decreasing quality of semen during past 50 years. BMJ 1992, 305, 609–613. [Google Scholar] [CrossRef] [PubMed]
- Splingart, C.; Frapsauce, C.; Veau, S.; Barthélémy, C.; Royère, D.; Guérif, F. Semen variation in a population of fertile donors: Evaluation in a French centre over a 34-year period. Int. J. Androl. 2012, 35, 467–474. [Google Scholar] [CrossRef]
- Sengupta, P.; Dutta, S.; Krajewska-Kulak, E. The Ddisappearing sperms: Analysis of Reports Published Between 1980 and 2015. Am. J. Men’s Health. 2017, 11, 1279–1304. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Q.; Fan, Z.; Xu, R.; Deng, X.; Li, Y.; Liang, S.; Lv, Z.; Huang, S.; Duan, Y.G.; et al. Association between central obesity and semen quality: A cross-sectional study in 4513 Chinese sperm donation volunteers. Andrology 2024, 12, 316–326. [Google Scholar] [CrossRef]
- Bisht, S.; Faiq, M.; Tolahunase, M.; Dada, R. Oxidative stress and male infertility. Nat. Rev. Urol. 2017, 14, 470–485. [Google Scholar] [CrossRef] [PubMed]
- Alahmar, A.T. Role of oxidative stress in male infertility: An updated review. J. Hum. Reprod. Sci. 2019, 12, 4–18. [Google Scholar] [CrossRef]
- Takalani, N.B.; Monageng, E.M.; Mohlala, K.; Monsees, T.K.; Henkel, R.; Opuwari, C.S. Role of oxidative stress in male infertility. Reprod. Fertil. 2025, 4, e230024. [Google Scholar] [CrossRef]
- Evans, E.P.P.; Scholten, J.T.M.; Mzyk, A.; Reyes-San-Martin, C.; Llumbet, A.E.; Hamoh, T.; Arts, E.G.J.M.; Schirhagl, R.; Cantineau, A.E.P. Male subfertility and oxidative stress. Redox Biol. 2021, 46, 102071. [Google Scholar] [CrossRef]
- Mancini, A.; Oliva, A.; Vergani, E.; Festa, R.; Silvestrini, A. The Dual Role of Oxidants in Male (In)fertility: Every ROSe Has a Thorn. Int. J. Mol. Sci. 2023, 24, 4994. [Google Scholar] [CrossRef]
- Venigalla, G.; Ila, V.; Dornbush, J.; Bernstein, A.; Loloi, J.; Pozzi, E.; Miller, D.; Ramasamy, R. Male obesity: Associated effects on fertility and the outcomes of offspring. Andrology 2023, 13, 64–71. [Google Scholar] [CrossRef]
- Barbagallo, F.; Condorelli, R.A.; Mongioì, L.M.; Cannarella, R.; Cimino, L.; Magagnini, M.C.; Crafa, A.; La Vignera, S.; Calogero, A.E. Molecular mechanisms underlying the relationship between obesity and male infertility. Metabolites 2021, 11, 840. [Google Scholar] [CrossRef]
- Chavarro, J.E.; Toth, T.L.; Wright, D.L.; Meeker, J.D.; Hauser, R. Body mass index in relation to semen quality, sperm DNA integrity, and serum reproductive hormone levels among men attending an infertility clinic. Fertil. Steril. 2010, 93, 2222–2231. [Google Scholar] [CrossRef]
- Suganami, T.; Nishida, J.; Ogawa, Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: Role of free fatty acids and tumor necrosis factor alpha. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2062–2068. [Google Scholar] [CrossRef]
- Dupont, J.; Pollet-Villard, X.; Reverchon, M.; Mellouk, N.; Levy, R. Adipokines in human reproduction. Horm. Mol. Biol. Clin. Investig. 2015, 24, 11–24. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; Menafra, D.; Puliani, G.; Colao, A.; Savastano, S.; Obesity Programs of Nutrition, Education, Research and Assessment (OPERA) Group. How much does obesity affect the male reproductive function? Int. J. Obes. Suppl. 2019, 9, 50–64. [Google Scholar] [CrossRef]
- Agbaje, I.M.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef]
- Liu, Y.; Ding, Z. Obesity, a serious etiologic factor for male subfertility in modern society. Reproduction 2017, 154, R123–R131. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Law, C.; Lo Conte, R.; Power, C. Intergenerational influences on childhood body mass index: The effect of parental body mass index trajectories. Am. J. Clin. Nutr. 2009, 89, 551–557. [Google Scholar] [CrossRef]
- Huang, Q.; Ma, C.; Chen, L.; Luo, D.; Chen, R.; Liang, F. Mechanistic Insights into the Interaction Between Transcription Factors and Epigenetic Modifications and the Contribution to the Development of Obesity. Front. Endocrinol. 2018, 9, 370. [Google Scholar] [CrossRef]
- Keyhan, S.; Burke, E.; Schrott, R.; Huang, Z.; Grenier, C.; Price, T.; Raburn, D.; Corcoran, D.L.; Soubry, A.; Hoyo, C.; et al. Male obesity impacts DNA methylation reprogramming in sperm. Clin. Epigenet. 2021, 13, 17. [Google Scholar] [CrossRef]
- Soubry, A.; Guo, L.; Huang, Z.; Hoyo, C.; Romanus, S.; Price, T.; Murphy, S.K. Obesity-related DNA methylation at imprinted genes in human sperm: Results from the TIEGER study. Clin. Epigenetics 2016, 8, 51. [Google Scholar] [CrossRef]
- El Hajj, N.; Zechner, U.; Schneider, E.; Tresch, A.; Gromoll, J.; Hahn, T.; Schorsch, M.; Haaf, T. Methylation status of imprinted genes and repetitive elements in sperm DNA from infertile males. Sex Dev. 2011, 5, 60–69. [Google Scholar] [CrossRef]
- Tunc, O.; Bakos, H.W.; Tremellen, K. Impact of body mass index on seminal oxidative stress. Andrologia 2011, 43, 121–128. [Google Scholar] [CrossRef]
- Kumar, N.; Singh, A.K. Impact of environmental factors on human semen quality and male fertility: A narrative review. Environ. Sci. Eur. 2022, 34, 6. [Google Scholar] [CrossRef]
- Deng, Z.; Chen, F.; Zhang, M.; Lan, L.; Qiao, Z.; Cui, Y.; An, J.; Wang, N.; Fan, Z.; Zhao, X.; et al. Association between air pollution and sperm quality: A systematic review and meta-analysis. Environ. Pollut. 2016, 208, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Giudice, L.C. Environmental toxicants: Hidden players on the reproductive stage. Fertil. Steril. 2016, 106, 791–794. [Google Scholar] [CrossRef]
- Chiang, C.; Mahalingam, S.; Flaws, J.A. Environmental Contaminants Affecting Fertility and Somatic Health. Semin. Reprod. Med. 2017, 35, 241–249. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, Z.; Ma, C.; Xiong, J.; Li, H. Impacts of Outdoor Air Pollution on Human Semen Quality: A Meta-Analysis and Systematic Review. Biomed. Res. Int. 2020, 2020, 7528901. [Google Scholar] [CrossRef] [PubMed]
- Jurewicz, J.; Dziewirska, E.; Radwan, M.; Hanke, W. Air pollution from natural and anthropic sources and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 109. [Google Scholar] [CrossRef]
- Calogero, A.E.; La Vignera, S.; Condorelli, R.A.; Perdichizzi, A.; Valenti, D.; Asero, P.; Carbone, U.; Boggia, B.; De Rosa, N.; Lombardi, G.; et al. Environmental car exhaust pollution damages human sperm chromatin and DNA. J. Endocrinol. Investig. 2011, 34, e139–e143. [Google Scholar] [CrossRef]
- Takeda, K.; Tsukue, N.; Yoshida, S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ. Sci. 2004, 11, 33–45. [Google Scholar]
- Carré, J.; Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R. Does air pollution play a role in infertility?: A systematic review. Environ. Health 2017, 16, 82. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Chang, S.S.; Santella, R.M.; Garte, S.; Pedotti, P.; Taioli, E. Polycyclic aromatic hydrocarbon-DNA adducts in human sperm as a marker of DNA damage and infertility. Mutat. Res. 2003, 535, 155–160. [Google Scholar] [CrossRef]
- Mocarelli, P.; Gerthoux, P.M.; Patterson, D.G., Jr.; Milani, S.; Limonta, G.; Bertona, M.; Signorini, S.; Tramacere, P.; Colombo, L.; Crespi, C.; et al. Dioxin exposure, from infancy through puberty, produces endocrine disruption and affects human semen quality. Environ. Health Perspect. 2008, 116, 70–77. [Google Scholar] [CrossRef]
- Schultz, R.; Suominen, J.; Värre, T.; Hakovirta, H.; Parvinen, M.; Toppari, J.; Pelto-Huikko, M. Expression of aryl hydrocarbon receptor and aryl hydrocarbon receptor nuclear translocator messenger ribonucleic acids and proteins in rat and human testis. Endocrinology 2003, 144, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Manfo, F.P.; Jubendradass, R.; Nantia, E.A.; Moundipa, P.F.; Mathur, P.P. Adverse effects of bisphenol A on male reproductive function. Rev. Environ. Contam. Toxicol. 2014, 228, 57–82. [Google Scholar] [CrossRef] [PubMed]
- Cariati, F.; D’Uonno, N.; Borrillo, F.; Iervolino, S.; Galdiero, G.; Tomaiuolo, R. Bisphenol a: An emerging threat to male fertility. Reprod. Biol. Endocrinol. 2019, 17, 6. [Google Scholar] [CrossRef]
- Rahman, M.S.; Kwon, W.S.; Lee, J.S.; Yoon, S.J.; Ryu, B.Y.; Pang, M.G. Bisphenol-A affects male fertility via fertility-related proteins in spermatozoa. Sci. Rep. 2015, 5, 9169. [Google Scholar] [CrossRef]
- Murata, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Barbonetti, A.; Castellini, C.; Di Giammarco, N.; Santilli, G.; Francavilla, S.; Francavilla, F. In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod. Toxicol. 2016, 66, 61–67. [Google Scholar] [CrossRef]
- Bretveld, R.; Brouwers, M.; Ebisch, I.; Roeleveld, N. Influence of pesticides on male fertility. Scand. J. Work. Environ. Health 2007, 33, 13–28. [Google Scholar] [CrossRef]
- Turusov, V.; Rakitsky, V.; Tomatis, L. Dichlorodiphenyltrichloroethane (DDT): Ubiquity, persistence, and risks. Environ. Health Perspect. 2002, 110, 125–128. [Google Scholar] [CrossRef]
- Bhatia, R.; Shiau, R.; Petreas, M.; Weintraub, J.M.; Farhang, L.; Eskenazi, B. Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ. Health Perspect. 2005, 113, 220–224. [Google Scholar] [CrossRef]
- Tavares, R.S.; Amaral, S.; Paiva, C.; Baptista, M.; Ramalho-Santos, J. In vitro exposure to the organochlorine p, p’-DDE affects functional human sperm parameters. Chemosphere 2015, 120, 443–446. [Google Scholar] [CrossRef]
- Pant, N.; Shukla, M.; Upadhyay, A.D.; Chaturvedi, P.K.; Saxena, D.K.; Gupta, Y.K. Association between environmental exposure to p, p’-DDE and lindane and semen quality. Environ. Sci. Pollut. Res. Int. 2014, 21, 11009–11016. [Google Scholar] [CrossRef]
- Longnecker, M.P. Invited Commentary: Why DDT matters now. Am. J. Epidemiol. 2005, 162, 726–728. [Google Scholar] [CrossRef] [PubMed]
- Anifandis, G.; Amiridis, G.; Dafopoulos, K.; Daponte, A.; Dovolou, E.; Gavriil, E.; Gorgogietas, V.; Kachpani, E.; Mamuris, Z.; Messini, C.I.; et al. The In Vitro Impact of the Herbicide Roundup on Human Sperm Motility and Sperm Mitochondria. Toxics 2017, 6, 2. [Google Scholar] [CrossRef] [PubMed]
- de Liz Oliveira Cavalli, V.L.; Cattani, D.; Heinz Rieg, C.E.; Pierozan, P.; Zanatta, L.; Benedetti Parisotto, E.; Wilhelm Filho, D.; Mena Barreto Silva, F.R.; Pessoa-Pureur, R.; Zamoner, A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radic. Biol. Med. 2013, 65, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Radke, E.G.; Braun, J.M.; Meeker, J.D.; Cooper, G.S. Phthalate exposure and male reproductive outcomes: A systematic review of the human epidemiological evidence. Environ. Int. 2018, 121, 764–793. [Google Scholar] [CrossRef]
- Broe, A.; Pottegård, A.; Hallas, J.; Ahern, T.P.; Fedder, J.; Damkier, P. Association between use of phthalate-containing medication and semen quality among men in couples referred for assisted reproduction. Hum. Reprod. 2018, 33, 503–511. [Google Scholar] [CrossRef]
- Cai, H.; Zheng, W.; Zheng, P.; Wang, S.; Tan, H.; He, G.; Qu, W. Human urinary/seminal phthalates or their metabolite levels and semen quality: A meta-analysis. Environ. Res. 2015, 142, 486–494. [Google Scholar] [CrossRef]
- Caporossi, L.; Alteri, A.; Campo, G.; Paci, E.; Tranfo, G.; Capanna, S.; Papaleo, E.; Pigini, D.; Viganò, P.; Papaleo, B. Cross Sectional Study on Exposure to BPA and Phthalates and Semen Parameters in Men Attending a Fertility Center. Int. J. Environ. Res. Public Health 2020, 17, 489. [Google Scholar] [CrossRef]
- Bloom, M.S.; Whitcomb, B.W.; Chen, Z.; Ye, A.; Kannan, K.; Buck Louis, G.M. Associations between urinary phthalate concentrations and semen quality parameters in a general population. Hum. Reprod. 2015, 30, 2645–2657. [Google Scholar] [CrossRef]
- Thurston, S.W.; Mendiola, J.; Bellamy, A.R.; Levine, H.; Wang, C.; Sparks, A.; Redmon, J.B.; Drobnis, E.Z.; Swan, S.H. Phthalate exposure and semen quality in fertile US men. Andrology 2016, 4, 632–638. [Google Scholar] [CrossRef]
- Lovekamp-Swan, T.; Jetten, A.M.; Davis, B.J. Dual activation of PPARalpha and PPARgamma by mono-(2-ethylhexyl) phthalate in rat ovarian granulosa cells. Mol. Cell Endocrinol. 2003, 201, 133–141. [Google Scholar] [CrossRef]
- Rehman, S.; Usman, Z.; Rehman, S.; AlDraihem, M.; Rehman, N.; Rehman, I.; Ahmad, G. Endocrine disrupting chemicals and impact on male reproductive health. Transl. Androl. Urol. 2018, 7, 490–503. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ao, H.; Chen, L.; Sottas, C.M.; Ge, R.S.; Li, L.; Zhang, Y. Mono-(2-ethylhexyl) phthalate affects the steroidogenesis in rat Leydig cells through provoking ROS perturbation. Toxicol. Vitr. 2012, 26, 950–955. [Google Scholar] [CrossRef]
- Sukhn, C.; Awwad, J.; Ghantous, A.; Zaatari, G. Associations of semen quality with non-essential heavy metals in blood and seminal fluid: Data from the Environment and Male Infertility (EMI) study in Lebanon. J. Assist. Reprod. Genet. 2018, 35, 1691–1701. [Google Scholar] [CrossRef] [PubMed]
- Jamalan, M.; Ghaffari, M.A.; Hoseinzadeh, P.; Hashemitabar, M.; Zeinali, M. Human Sperm Quality and Metal Toxicants: Protective Effects of some Flavonoids on Male Reproductive Function. Int. J. Fertil. Steril. 2016, 10, 215–223. [Google Scholar] [CrossRef]
- Ivell, R. Lifestyle impact and the biology of the human scrotum. Reprod. Biol. Endocrinol. 2007, 5, 15. [Google Scholar] [CrossRef] [PubMed]
- Garolla, A.; Torino, M.; Miola, P.; Caretta, N.; Pizzol, D.; Menegazzo, M.; Bertoldo, A.; Foresta, C. Twenty-four-hour monitoring of scrotal temperature in obese men and men with a varicocele as a mirror of spermatogenic function. Hum. Reprod. 2015, 30, 1006–1013. [Google Scholar] [CrossRef]
- Al-Otaibi, S.T. Male infertility among bakers associated with exposure to high environmental temperature at the workplace. J. Taibah Univ. Med. Sci. 2018, 13, 103–107. [Google Scholar] [CrossRef]
- Hamerezaee, M.; Dehghan, S.F.; Golbabaei, F.; Fathi, A.; Barzegar, L.; Heidarnejad, N. Assessment of semen quality among workers exposed to heat stress: A cross-sectional study in a steel industry. Saf. Health Work. 2018, 9, 232–235. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Gaskins, A.J.; Chiu, Y.H.; Messerlian, C.; Williams, P.L.; Ford, J.B.; Souter, I.; Hauser, R.; Chavarro, J.E. Type of underwear worn and markers of testicular function among men attending a fertility center. Hum. Reprod. 2018, 33, 1749–1756. [Google Scholar] [CrossRef]
- Hassun Filho, P.A. Re: Wet heat exposure: A potentially reversible cause of low semen quality in infertile men. Int. Braz. J. Urol. 2007, 33, 269–270. [Google Scholar] [CrossRef]
- Shefi, S.; Tarapore, P.E.; Walsh, T.J.; Croughan, M.; Turek, P.J. Wet heat exposure: A potentially reversible cause of low semen quality in infertile men. Int. Braz. J. Urol. 2007, 33, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Barazani, Y.; Katz, B.F.; Nagler, H.M.; Stember, D.S. Lifestyle, environment, and male reproductive health. Urol. Clin. N. Am. 2014, 41, 55–66. [Google Scholar] [CrossRef]
- Mao, H.; Feng, L.; Yang, W.X. Environmental factors contributed to circannual rhythm of semen quality. Chronobiol. Int. 2017, 34, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Levitas, E.; Lunenfeld, E.; Weisz, N.; Friger, M.; Har-Vardi, I. Seasonal variations of human sperm cells among 6455 semen samples: A plausible explanation of a seasonal birth pattern. Am. J. Obstet. Gynecol. 2013, 208, 406.e1-6. [Google Scholar] [CrossRef]
- Reyes, J.G.; Farias, J.G.; Henríquez-Olavarrieta, S.; Madrid, E.; Parraga, M.; Zepeda, A.B.; Moreno, R.D. The hypoxic testicle: Physiology and pathophysiology. Oxid. Med. Cell. Longev. 2012, 2012, 929285. [Google Scholar] [CrossRef] [PubMed]
- Vernet, P.; Aitken, R.J.; Drevet, J.R. Antioxidant strategies in the epididymis. Mol. Cell. Endocrinol. 2004, 216, 31–39. [Google Scholar] [CrossRef]
- Mahfouz, R.; Sharma, R.; Thiyagarajan, A.; Kale, V.; Gupta, S.; Sabanegh, E.; Agarwal, A. Semen characteristics and sperm DNA fragmentation in infertile men with low and high levels of seminal reactive oxygen species. Fertil. Steril. 2010, 94, 2141–2146. [Google Scholar] [CrossRef]
- Gong, Y.; Guo, H.; Zhang, Z.; Zhou, H.; Zhao, R.; He, B. Heat Stress Reduces Sperm Motility via Activation of Glycogen Synthase Kinase-3α and Inhibition of Mitochondrial Protein Import. Front. Physiol. 2017, 8, 718. [Google Scholar] [CrossRef]
- Rao, M.; Xia, W.; Yang, J.; Hu, L.X.; Hu, S.F.; Lei, H.; Wu, Y.Q.; Zhu, C.H. Transient scrotal hyperthermia affects human sperm DNA integrity, sperm apoptosis, and sperm protein expression. Andrology. 2016, 4, 1054–1063. [Google Scholar] [CrossRef]
- Talhout, R.; Schulz, T.; Florek, E.; van Benthem, J.; Wester, P.; Opperhuizen, A. Hazardous compounds in tobacco smoke. Int. J. Environ. Res. Public Health 2011, 8, 613–628. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Harlev, A.; Agarwal, A.; Esteves, S.C. Cigarette smoking and semen quality: A new meta-analysis examining the effect of the 2010 World Health Organization Laboratory Methods for the Examination of Human Semen. Eur. Urol. 2016, 70, 635–645. [Google Scholar] [CrossRef]
- Mostafa, R.M.; Nasrallah, Y.S.; Hassan, M.M.; Farrag, A.F.; Majzoub, A.; Agarwal, A. The effect of cigarette smoking on human seminal parameters, sperm chromatin structure and condensation. Andrologia. 2018, 50, e12910. [Google Scholar] [CrossRef]
- Osadchuk, L.; Kleshchev, M.; Osadchuk, A. Effects of cigarette smoking on semen quality, reproductive hormone levels, metabolic profile, zinc and sperm DNA fragmentation in men: Results from a population-based study. Front. Endocrinol. 2023, 14, 1255304. [Google Scholar] [CrossRef]
- Esakky, P.; Moley, K.H. Paternal smoking and germ cell death: A mechanistic link to the effects of cigarette smoke on spermatogenesis and possible long-term sequelae in offspring. Mol. Cell. Endocrinol. 2016, 435, 85–93. [Google Scholar] [CrossRef]
- Vivarelli, F.; Canistro, D.; Cirillo, S.; Cardenia, V.; Rodriguez-Estrada, M.T.; Paolini, M. Impairment of testicular function in electronic cigarette (e-cig, e-cigs) exposed rats under low-voltage and nicotine-free conditions. Life Sci. 2019, 228, 53–65. [Google Scholar] [CrossRef]
- Sansone, A.; Di Dato, C.; de Angelis, C.; Menafra, D.; Pozza, C.; Pivonello, R.; Isidori, A.; Gianfrilli, D. Smoke, alcohol and drug addiction and male fertility. Reprod. Biol. Endocrinol. 2018, 16, 3. [Google Scholar] [CrossRef]
- Li, Y.; Lin, H.; Li, Y.; Cao, J. Association between socio-psycho-behavioral factors and male semen quality: Systematic review and meta-analyses. Fertil. Steril. 2011, 95, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; Calogero, A.E.; Vicari, E.; La Vignera, S. Chronic consumption of alcohol and sperm parameters: Our experience and the main evidences. Andrologia 2015, 47, 368–379. [Google Scholar] [CrossRef]
- Hassan, M.A.; Killick, S.R. Negative lifestyle is associated with a significant reduction in fecundity. Fertil. Steril. 2004, 81, 384–392. [Google Scholar] [CrossRef]
- Hansen, M.L.; Thulstrup, A.M.; Bonde, J.P.; Olsen, J.; Håkonsen, L.B.; Ramlau-Hansen, C.H. Does last week’s alcohol intake affect semen quality or reproductive hormones? A cross-sectional study among healthy young Danish men. Reprod. Toxicol. 2012, 34, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Aboulmaouahib, S.; Madkour, A.; Kaarouch, I.; Sefrioui, O.; Saadani, B.; Copin, H.; Benkhalifa, M.; Louanjli, N.; Cadi, R. Impact of alcohol and cigarette smoking consumption in male fertility potential: Looks at lipid peroxidation, enzymatic antioxidant activities and sperm DNA damage. Andrologia 2018, 50, e12926. [Google Scholar] [CrossRef]
- Szkodziak, F.; Krzyżanowski, J.; Szkodziak, P. Psychological aspects of infertility. A systematic review. J. Int. Med. Res. 2020, 48, 300060520932403. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.D.; Geraghty, A.C.; Ubuka, T.; Bentley, G.E.; Kaufer, D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proc. Natl. Acad. Sci. USA 2009, 106, 11324–11329. [Google Scholar] [CrossRef]
- Odetayo, A.F.; Akhigbe, R.E.; Bassey, G.E.; Hamed, M.A.; Olayaki, L.A. Impact of stress on male fertility: Role of gonadotropin inhibitory hormone. Front. Endocrinol. 2024, 14, 1329564. [Google Scholar] [CrossRef]
- Nargund, V.H. Effects of psychological stress on male fertility. Nat. Rev. Urol. 2015, 12, 373–382. [Google Scholar] [CrossRef]
- Belladelli, F.; Basran, S.; Eisenberg, M.L. Male Fertility and Physical Exercise. World J. Men’s Health 2023, 41, 482–488. [Google Scholar] [CrossRef]
- Hajizadeh Maleki, B.; Tartibian, B. Resistance exercise modulates male factor infertility through anti-inflammatory and antioxidative mechanisms in infertile men: A RCT. Life Sci. 2018, 203, 150–160. [Google Scholar] [CrossRef]
- Minas, A.; Fernandes, A.C.C.; Maciel Júnior, V.L.; Adami, L.; Intasqui, P.; Bertolla, R.P. Influence of physical activity on male fertility. Andrologia 2022, 54, e14433. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Singh, A.; Hamada, A.; Kesari, K. Cell phones and male infertility: A review of recent innovations in technology and consequences. Int. Braz. J. Urol. 2011, 37, 432–454. [Google Scholar] [CrossRef]
- Adams, J.A.; Galloway, T.S.; Mondal, D.; Esteves, S.C.; Mathews, F. Effect of mobile telephones on sperm quality: A systematic review and meta-analysis. Environ. Int. 2014, 70, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Li, Y.; Zhang, G.; Liu, J.; Cao, J.; Ao, L.; Zhang, S. Association between mobile phone use and semen quality: A systemic review and meta-analysis. Andrology 2014, 2, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Delli Muti, N.; Salvio, G.; Ciarloni, A.; Perrone, M.; Tossetta, G.; Lazzarini, R.; Bracci, M.; Balercia, G. Can extremely low frequency magnetic field affect human sperm parameters and male fertility? Tissue Cell 2023, 82, 102045. [Google Scholar] [CrossRef]
- Avendaño, C.; Mata, A.; Sanchez Sarmiento, C.A.; Doncel, G.F. Use of laptop computers connected to internet through Wi-Fi decreases human sperm motility and increases sperm DNA fragmentation. Fertil. Steril. 2012, 97, 39–45.e2. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Chen, M.; Wu, W.; Lu, J.; Zhao, D.; Pan, F.; Lu, C.; Xia, Y.; Hu, L.; Chen, D.; et al. Gene-gene and gene-environment interactions on risk of male infertility: Focus on the metabolites. Environ. Int. 2016, 91, 188–195. [Google Scholar] [CrossRef]
- Rubes, J.; Selevan, S.G.; Sram, R.J.; Evenson, D.P.; Perreault, S.D. GSTM1 genotype influences the susceptibility of men to sperm DNA damage associated with exposure to air pollution. Mutat. Res. 2007, 625, 20–28. [Google Scholar] [CrossRef]
- Gunes, S.; Esteves, S.C. Role of genetics and epigenetics in male infertility. Andrologia 2021, 53, e13586. [Google Scholar] [CrossRef]
- Laan, M.; Kasak, L.; Punab, M. Translational aspects of novel findings in genetics of male infertility-status quo 2021. Br. Med. Bull. 2021, 140, 5–22. [Google Scholar] [CrossRef]
- Montjean, D.; Beaumont, M.; Natiq, A.; Louanjli, N.; Hazout, A.; Miron, P.; Liehr, T.; Cabry, R.; Ratbi, I.; Benkhalifa, M. Genome and epigenome disorders and male infertility: Feedback from 15 years of clinical and research experience. Genes 2024, 15, 377. [Google Scholar] [CrossRef]
- Pereira, R.; Sousa, M. Morphological and molecular bases of male infertility: A closer look at sperm flagellum. Genes 2023, 14, 383. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Lapeña, A.C.; Díez-Sánchez, C.; Pérez-Martos, A.; Montoya, J.; Alvarez, E.; Díaz, M.; Urriés, A.; Montoro, L.; López-Pérez, M.J.; et al. Human mtDNA haplogroups associated with high or reduced spermatozoa motility. Am. J. Hum. Genet. 2000, 67, 682–696. [Google Scholar] [CrossRef] [PubMed]
- Selvi Rani, D.; Vanniarajan, A.; Gupta, N.J.; Chakravarty, B.; Singh, L.; Thangaraj, K. A novel missense mutation C11994T in the mitochondrial ND4 gene as a cause of low sperm motility in the Indian subcontinent. Fertil. Steril. 2006, 86, 1783–1785. [Google Scholar] [CrossRef]
- Baklouti-Gargouri, S.; Ghorbel, M.; Ben Mahmoud, A.; Mkaouar-Rebai, E.; Cherif, M.; Chakroun, N.; Sellami, A.; Fakhfakh, F.; Ammar-Keskes, L. Identification of a novel m.9588G > a missense mutation in the mitochondrial COIII gene in asthenozoospermic Tunisian infertile men. J. Assist. Reprod. Genet. 2014, 31, 595–600. [Google Scholar] [CrossRef]
- Kao, S.H.; Chao, H.T.; Liu, H.W.; Liao, T.L.; Wei, Y.H. Sperm mitochondrial DNA depletion in men with asthenospermia. Fertil. Steril. 2004, 82, 66–73. [Google Scholar] [CrossRef]
- Tesarik, J. Acquired sperm DNA modifications: Causes, consequences, and potential solutions. EMJ 2019, 4, 83–95. [Google Scholar] [CrossRef]
- Jenkins, T.G.; Aston, K.I.; James, E.R.; Carrell, D.T. Sperm epigenetics in the study of male fertility, offspring health, and potential clinical applications. Syst. Biol. Reprod. Med. 2017, 63, 69–76. [Google Scholar] [CrossRef]
- Santi, D.; De Vincentis, S.; Magnani, E.; Spaggiari, G. Impairment of sperm DNA methylation in male infertility: A meta-analytic study. Andrology 2017, 5, 695–703. [Google Scholar] [CrossRef]
- Pollard, C.A.; Jenkins, T.G. Epigenetic mechanisms within the sperm epigenome and their diagnostic potential. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101481. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Khalafiyan, A.; Zare, M.; Karimzadeh, H.; Bahrami, B.; Hammami, B.; Kazemi, M. Sperm epigenetics and male infertility: Unraveling the molecular puzzle. Hum. Genom. 2024, 18, 57. [Google Scholar] [CrossRef]
- Li, X.P.; Hao, C.L.; Wang, Q.; Yi, X.M.; Jiang, Z.S. H19 gene methylation status is associated with male infertility. Exp. Ther. Med. 2016, 12, 451–456. [Google Scholar] [CrossRef]
- Poplinski, A.; Tüttelmann, F.; Kanber, D.; Horsthemke, B.; Gromoll, J. Idiopathic male infertility is strongly associated with aberrant methylation of MEST and IGF2/H19 ICR1. Int. J. Androl. 2010, 33, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Wang, Y.; Zou, Z.; Chen, L.; Shen, C.; Xu, S.; Zhang, J.; Zhao, F.; Ge, S.; Gao, Q.; et al. Abnormal methylation of imprinted genes and cigarette smoking: Assessment of their association with the risk of male infertility. Reprod. Sci. 2017, 24, 114–123. [Google Scholar] [CrossRef]
- Cescon, M.; Chianese, R.; Tavares, R.S. Environmental impact on male (in)fertility via epigenetic route. J. Clin. Med. 2020, 9, 2520. [Google Scholar] [CrossRef]
- Santoleri, D.; Titchenell, P.M. Resolving the paradox of hepatic insulin resistance. Cell Mol. Gastroenterol. Hepatol. 2019, 7, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Zańko, A.; Martynowicz, I.; Citko, A.; Konopka, P.; Paszko, A.; Pawłowski, M.; Szczerbiński, Ł.; Siewko, K.; Krętowski, A.J.; Kuczyński, W.; et al. The influence of lifestyle on male fertility in the context of insulin resistance-identification of factors that influence semen quality. J. Clin. Med. 2024, 13, 2797. [Google Scholar] [CrossRef]
- Sasaki, N.; Ueno, Y.; Higashi, Y. Indicators of insulin resistance in clinical practice. Hypertens. Res. 2024, 47, 978–980. [Google Scholar] [CrossRef] [PubMed]
- Zańko, A.; Siewko, K.; Krętowski, A.J.; Milewski, R. Lifestyle, insulin resistance and semen quality as co-dependent factors of male infertility. Int. J. Environ. Res. Public Health 2022, 20, 732. [Google Scholar] [CrossRef] [PubMed]
- Asare-Anane, H.; Bannison, S.B.; Ofori, E.K.; Ateko, R.O.; Bawah, A.T.; Amanquah, S.D.; Oppong, S.Y.; Gandau, B.B.; Ziem, J.B. Tobacco smoking is associated with decreased semen quality. Reprod. Health 2016, 13, 90. [Google Scholar] [CrossRef]
- Du, C.Q.; Yang, Y.Y.; Chen, J.; Feng, L.; Lin, W.Q. Association between sleep quality and semen parameters and reproductive hormones: A cross-sectional study in Zhejiang, China. Nat. Sci. Sleep 2020, 12, 11–18. [Google Scholar] [CrossRef]
- Jóźków, P.; Rossato, M. The Impact of Intense Exercise on Semen Quality. Am. J. Men’s Health 2017, 11, 654–662. [Google Scholar] [CrossRef]
- Maresch, C.C.; Stute, D.C.; Fleming, T.; Lin, J.; Hammes, H.P.; Linn, T. Hyperglycemia induces spermatogenic disruption via major pathways of diabetes pathogenesis. Sci. Rep. 2019, 9, 13074. [Google Scholar] [CrossRef]
- Huang, R.; Chen, J.; Guo, B.; Jiang, C.; Sun, W. Diabetes-induced male infertility: Potential mechanisms and treatment options. Mol. Med. 2024, 30, 11. [Google Scholar] [CrossRef] [PubMed]
- Yaribeygi, H.; Sathyapalan, T.; Atkin, S.L.; Sahebkar, A. Molecular mechanisms linking oxidative stress and diabetes mellitus. Oxid. Med. Cell. Longev. 2020, 2020, 8609213. [Google Scholar] [CrossRef]
- Esposito, K.; Nappo, F.; Marfella, R.; Giugliano, G.; Giugliano, F.; Ciotola, M.; Quagliaro, L.; Ceriello, A.; Giugliano, D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: Role of oxidative stress. Circulation 2002, 106, 2067–2072. [Google Scholar] [CrossRef] [PubMed]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Corona, G.; Giorda, C.B.; Cucinotta, D.; Guida, P.; Nada, E.; Gruppo di studio SUBITO-DE. Sexual dysfunction at the onset of type 2 diabetes: The interplay of depression, hormonal and cardiovascular factors. J. Sex. Med. 2014, 11, 2065–2073. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Pizzol, D.; Cosco, T.; Thompson, T.; Carnaghi, M.; Bertoldo, A.; Solmi, M.; Stubbs, B.; Veronese, N. High prevalence of erectile dysfunction in diabetes: A systematic review and meta-analysis of 145 studies. Diabet. Med. 2017, 34, 1185–1192. [Google Scholar] [CrossRef]
- McLachlan, R.I. Basis, diagnosis and treatment of immunological infertility in men. J. Reprod. Immunol. 2002, 57, 35–45. [Google Scholar] [CrossRef]
- Cui, D.; Han, G.; Shang, Y.; Liu, C.; Xia, L.; Li, L.; Yi, S. Antisperm antibodies in infertile men and their effect on semen parameters: A systematic review and meta-analysis. Clin. Chim. Acta 2015, 444, 29–36. [Google Scholar] [CrossRef]
- Shibahara, H.; Shiraishi, Y.; Suzuki, M. Diagnosis and treatment of immunologically infertile males with antisperm antibodies. Reprod. Med. Biol. 2005, 4, 133–141. [Google Scholar] [CrossRef]
- Lai, Y.M.; Lee, J.F.; Huang, H.Y.; Soong, Y.K.; Yang, F.P.; Pao, C.C. The effect of human papillomavirus infection on sperm cell motility. Fertil. Steril. 1997, 67, 1152–1155. [Google Scholar] [CrossRef]
- Nasseri, S.; Monavari, S.H.; Keyvani, H.; Nikkhoo, B.; Vahabpour Roudsari, R.; Khazeni, M. The prevalence of Human Papilloma Virus (HPV) infection in the oligospermic and azoospermic men. Med. J. Islam. Repub. Iran 2015, 29, 272. [Google Scholar] [PubMed]
- Kurscheidt, F.A.; Damke, E.; Bento, J.C.; Balani, V.A.; Takeda, K.I.; Piva, S.; Piva, J.P.; Irie, M.M.T.; Gimenes, F.; Consolaro, M.E.L. Effects of Herpes Simplex Virus Infections on Seminal Parameters in Male Partners of Infertile Couples. Urology 2018, 113, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zou, Y.; Zhou, W.; Ruan, Z.; Kong, Y.; Zhou, Y.; Zhang, J.; Xie, X. Clonal diversity of Ureaplasma species and its relationship with oligozoospermia and semen quality in Chinese infertile males. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1957–1963. [Google Scholar] [CrossRef] [PubMed]
- López-Hurtado, M.; Flores-Salazar, V.R.; Gutierréz-Trujillo, R.; Guerra-Infante, F.M. Prevalence, concordance and reproductive sequelae after Chlamydia trachomatis infection in Mexican infertile couples. Andrologia 2020, 52, e13772. [Google Scholar] [CrossRef]
- Li, H.; Xiao, X.; Zhang, J.; Zafar, M.I.; Wu, C.; Long, Y.; Lu, W.; Pan, F.; Meng, T.; Zhao, K.; et al. Impaired spermatogenesis in COVID-19 patients. EClinicalMedicine 2020, 28, 100604. [Google Scholar] [CrossRef]
- Apaydin, T.; Sahin, B.; Dashdamirova, S.; Dincer Yazan, C.; Elbasan, O.; Ilgin, C.; Bilgin, H.; Cam, H.K.; Bahramzada, G.; Kucuk, A.; et al. The association of free testosterone levels with coronavirus disease 2019. Andrology 2022, 10, 1038–1046. [Google Scholar] [CrossRef]
- Joguet, G.; Mansuy, J.M.; Matusali, G.; Hamdi, S.; Walschaerts, M.; Pavili, L.; Guyomard, S.; Prisant, N.; Lamarre, P.; Dejucq-Rainsford, N.; et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: A prospective observational study. Lancet Infect. Dis. 2017, 17, 1200–1208. [Google Scholar] [CrossRef]
- Erles, K.; Rohde, V.; Thaele, M.; Roth, S.; Edler, L.; Schlehofer, J.R. DNA of adeno-associated virus (AAV) in testicular tissue and in abnormal semen samples. Hum. Reprod. 2001, 16, 2333–2337. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Fang, Y.; Shen, Q.; Zhao, K.; Liu, C.; Zhang, H. Microbiology and immune mechanisms associated with male infertility. Front. Immunol. 2023, 14, 1139450. [Google Scholar] [CrossRef]
- Hussein, M.R.; Abou-Deif, E.S.; Bedaiwy, M.A.; Said, T.M.; Mustafa, M.G.; Nada, E.; Ezat, A.; Agarwal, A. Phenotypic characterization of the immune and mast cell infiltrates in the human testis shows normal and abnormal spermatogenesis. Fertil. Steril. 2005, 83, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Flanagan, B.; Vince, G.; Lewis-Jones, D.J. Detection of subpopulations of leucocytes in different subgroups of semen sample qualities. Andrologia 2012, 44 (Suppl. S1), 354–361. [Google Scholar] [CrossRef] [PubMed]
- Dcunha, R.; Hussein, R.S.; Ananda, H.; Kumari, S.; Adiga, S.K.; Kannan, N.; Zhao, Y.; Kalthur, G. Current insights and latest updates in sperm motility and associated applications in assisted reproduction. Reprod. Sci. 2022, 29, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Hendry, W.F.; Stedronska, J.; Jones, C.R.; Blackmore, C.A.; Barrett, A.; Peckham, M.J. Semen analysis in testicular cancer and Hodgkin’s disease: Pre- and post-treatment findings and implications for cryopreservation. Br. J. Urol. 1983, 55, 769–773. [Google Scholar] [CrossRef]
- Williams, D.H., IV; Karpman, E.; Sander, J.C.; Spiess, P.E.; Pisters, L.L.; Lipshultz, L.I. Pretreatment semen parameters in men with cancer. J. Urol. 2009, 181, 736–740. [Google Scholar] [CrossRef]
- Dias, T.R.; Agarwal, A.; Pushparaj, P.N.; Ahmad, G.; Sharma, R. Reduced semen quality in patients with testicular cancer seminoma is associated with alterations in the expression of sperm proteins. Asian J. Androl. 2020, 22, 88–93. [Google Scholar] [CrossRef]
- Drechsel, K.C.E.; Broer, S.L.; van Breda, H.M.K.; Stoutjesdijk, F.S.; van Dulmen-den Broeder, E.; Beishuizen, A.; Wallace, W.H.; Körholz, D.; Mauz-Körholz, C.; Hasenclever, D.; et al. Semen analysis and reproductive hormones in boys with classical Hodgkin lymphoma treated according to the EuroNet-PHL-C2 protocol. Hum. Reprod. 2024, 39, 2411–2422. [Google Scholar] [CrossRef]
- Levin, R.M.; Amsterdam, J.D.; Winokur, A.; Wein, A.J. Effects of psychotropic drugs on human sperm motility. Fertil. Steril. 1981, 36, 503–506. [Google Scholar] [CrossRef]
- Chen, S.S.; Shen, M.R.; Chen, T.J.; Lai, S.L. Effects of antiepileptic drugs on sperm motility of normal controls and epileptic patients with long-term therapy. Epilepsia 1992, 33, 149–153. [Google Scholar] [CrossRef]
- Banihani, S.A. Effect of paracetamol on semen quality. Andrologia 2018, 50, e12874. [Google Scholar] [CrossRef]
- Stutz, G.; Zamudio, J.; Santillán, M.E.; Vincenti, L.; de Cuneo, M.F.; Ruiz, R.D. The effect of alcohol, tobacco, and aspirin consumption on seminal quality among healthy young men. Arch. Environ. Health 2004, 59, 548–552. [Google Scholar] [CrossRef]
- Banihani, S.A.; Khasawneh, F.H. Effect of lansoprazole on human sperm motility, sperm viability, seminal nitric oxide production, and seminal calcium chelation. Res. Pharm. Sci. 2018, 13, 460–468. [Google Scholar] [CrossRef] [PubMed]
- du Plessis, S.S.; Agarwal, A.; Syriac, A. Marijuana, phytocannabinoids, the endocannabinoid system, and male fertility. J. Assist. Reprod. Genet. 2015, 32, 1575–1588. [Google Scholar] [CrossRef]
- Monteiro, C.; Marques, P.I.; Cavadas, B.; Damião, I.; Almeida, V.; Barros, N.; Barros, A.; Carvalho, F.; Gomes, S.; Seixas, S. Characterization of microbiota in male infertility cases uncovers differences in seminal hyperviscosity and oligoasthenoteratozoospermia possibly correlated with increased prevalence of infectious bacteria. Am. J. Reprod. Immunol. 2018, 79, e12838. [Google Scholar] [CrossRef]
- Altmäe, S.; Franasiak, J.M.; Mändar, R. The seminal microbiome in health and disease. Nat. Rev. Urol. 2019, 16, 703–721. [Google Scholar] [CrossRef]
- Farahani, L.; Tharakan, T.; Yap, T.; Ramsay, J.W.; Jayasena, C.N.; Minhas, S. The semen microbiome and its impact on sperm function and male fertility: A systematic review and meta-analysis. Andrology 2021, 9, 115–144. [Google Scholar] [CrossRef]
- Ma, Z.S.; Li, L. Semen Microbiome Biogeography: An Analysis Based on a Chinese Population Study. Front. Microbiol. 2019, 9, 3333. [Google Scholar] [CrossRef]
- Chatzokou, D.; Tsarna, E.; Davouti, E.; Siristatidis, C.S.; Christopoulou, S.; Spanakis, N.; Tsakris, A.; Christopoulos, P. Semen Microbiome, Male Infertility, and Reproductive Health. Int. J. Mol. Sci. 2025, 26, 1446. [Google Scholar] [CrossRef]
- Baud, D.; Pattaroni, C.; Vulliemoz, N.; Castella, V.; Marsland, B.J.; Stojanov, M. Sperm Microbiota and Its Impact on Semen Parameters. Front Microbiol. 2019, 10, 234. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, J.; Xue, Z.; Zhao, C.; Lei, L.; Wen, Y.; Dong, Y.; Yang, J.; Zhang, L. Potential Pathogenic Bacteria in Seminal Microbiota of Patients with Different Types of Dysspermatism. Sci. Rep. 2020, 10, 6876. [Google Scholar] [CrossRef]
- Okwelogu, S.I.; Ikechebelu, J.I.; Agbakoba, N.R.; Anukam, K.C. Microbiome Compositions From Infertile Couples Seeking In Vitro Fertilization, Using 16S rRNA Gene Sequencing Methods: Any Correlation to Clinical Outcomes? Front. Cell. Infect. Microbiol. 2021, 11, 709372. [Google Scholar] [CrossRef]
- Folliero, V.; Santonastaso, M.; Dell’Annunziata, F.; De Franciscis, P.; Boccia, G.; Colacurci, N.; De Filippis, A.; Galdiero, M.; Franci, G. Impact of Escherichia coli Outer Membrane Vesicles on Sperm Function. Pathogens 2022, 11, 782. [Google Scholar] [CrossRef]
- Molina, N.M.; Plaza-Díaz, J.; Vilchez-Vargas, R.; Sola-Leyva, A.; Vargas, E.; Mendoza-Tesarik, R.; Galán-Lázaro, M.; Mendoza-Ladrón de Guevara, N.; Tesarik, J.; Altmäe, S. Assessing the testicular sperm microbiome: A low-biomass site with abundant contamination. Reprod. Biomed. Online 2021, 43, 523–531. [Google Scholar] [CrossRef]
- Jensen, C.F.S.; Østergren, P.; Dupree, J.M.; Ohl, D.A.; Sønksen, J.; Fode, M. Varicocele and male infertility. Nat. Rev. Urol. 2017, 14, 523–533. [Google Scholar] [CrossRef]
- Alleyne, G.; Coll-Seck, A.M.; Frieden, T.R.; Tufton, C. Fourth time a charm?—How to make the UN High-Level Meeting on Noncommunicable Diseases effective. JAMA 2025. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; Bulló, M.; Salas-Salvadó, J. Dietary patterns, foods and nutrients in male fertility parameters and fecundability: A systematic review of observational studies. Hum. Reprod. Update 2017, 23, 371–389. [Google Scholar] [CrossRef]
- Agarwal, A.; Bui, A.D. Oxidation-reduction potential as a new marker for oxidative stress: Correlation to male infertility. Investig. Clin. Urol. 2017, 58, 385–399. [Google Scholar] [CrossRef]
- Agarwal, A.; Qiu, E.; Sharma, R. Laboratory assessment of oxidative stress in semen. Arab. J. Urol. 2018, 16, 77–86. [Google Scholar] [CrossRef]
- Katerji, M.; Filippova, M.; Duerksen-Hughes, P. Approaches and methods to measure oxidative stress in clinical samples: Research applications in the cancer field. Oxid. Med. Cell Longev. 2019, 2019, 1279250. [Google Scholar] [CrossRef]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Sumbalová, Z.; Rausová, Z.; Kucharská, J.; Šranko, P.; Harbulák, P.; Svitok, P.; López-Lluch, G.; Gvozdjáková, A. Platelet Mitochondrial Function and Endogenous Coenzyme Q10 Levels Could Be Used as Markers of Mitochondrial Health in Infertile Men: A Pilot Study. Int. J. Mol. Sci. 2024, 26, 268. [Google Scholar] [CrossRef]
- Kulaksiz, D.; Toprak, T.; Tokat, E.; Yilmaz, M.; Ramazanoglu, M.A.; Garayev, A.; Sulukaya, M.; Degirmentepe, R.B.; Allahverdiyev, E.; Gul, M.; et al. Sperm concentration and semen volume increase after smoking cessation in infertile men. Int. J. Impot. Res. 2022, 34, 614–619. [Google Scholar] [CrossRef]
- Morgante, G.; Tosti, C.; Orvieto, R.; Musacchio, M.C.; Piomboni, P.; De Leo, V. Metformin improves semen characteristics of oligo-terato-asthenozoospermic men with metabolic syndrome. Fertil. Steril. 2011, 95, 2150–2152. [Google Scholar] [CrossRef]
- Adel Domínguez, M.A.; Cardona Maya, W.D.; Mora Topete, A. Sperm DNA fragmentation: Focusing treatment on seminal transport fluid beyond sperm production. Arch. Ital. Urol. Androl. 2025, 30, 13128. [Google Scholar] [CrossRef]
- Greco, E.; Iacobelli, M.; Rienzi, L.; Ubaldi, F.; Ferrero, S.; Tesarik, J. Reduction of the incidence of sperm DNA fragmentation by oral antioxidant treatment. J. Androl. 2005, 26, 349–353. [Google Scholar] [CrossRef]
- Greco, E.; Romano, S.; Iacobelli, M.; Ferrero, S.; Baroni, E.; Minasi, M.G.; Ubaldi, F.; Rienzi, L.; Tesarik, J. ICSI in cases of sperm DNA damage: Beneficial effect of oral antioxidant treatment. Hum. Reprod. 2005, 20, 2590–2594. [Google Scholar] [CrossRef]
- Sharma, A.; Minhas, S.; Dhillo, W.S.; Jayasena, C.N. Male infertility due to testicular disorders. J. Clin. Endocrinol. Metab. 2021, 106, e442–e459. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Tesarik, J.; Greco, E.; Mendoza, C. Late, but not early, paternal effect on human embryo development is related to sperm DNA fragmentation. Hum. Reprod. 2004, 19, 611–615. [Google Scholar] [CrossRef]
- Hazout, A.; Dumont-Hassan, M.; Junca, A.M.; Cohen Bacrie, P.; Tesarik, J. High-magnification ICSI overcomes paternal effect resistant to conventional ICSI. Reprod. Biomed. Online 2006, 12, 19–25. [Google Scholar] [CrossRef]
- Bartoov, B.; Berkovitz, A.; Eltes, F. Selection of spermatozoa with normal nuclei to improve the pregnancy rate with intracytoplasmic sperm injection. N. Engl. J. Med. 2001, 345, 1067–1068. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza-Tesarik, R.; Mendoza, C. Sperm nuclear DNA damage: Update on the mechanism, diagnosis and treatment. Reprod. Biomed. Online 2006, 12, 715–721. [Google Scholar] [CrossRef]
- Tesarik, J.; Mendoza, C.; Testart, J. Viable embryos from injection of round spermatids into oocytes. N. Engl. J. Med. 1995, 333, 525. [Google Scholar] [CrossRef]
- Tanaka, A.; Suzuki, K.; Nagayoshi, M.; Tanaka, A.; Takemoto, Y.; Watanabe, S.; Takeda, S.; Irahara, M.; Kuji, N.; Yamagata, Z.; et al. Ninety babies born after round spermatid injection into oocytes: Survey of their development from fertilization to 2 years of age. Fertil. Steril. 2018, 110, 443–451. [Google Scholar] [CrossRef]
- Tesarik, J.; Bahceci, M.; Ozcan, C.; Greco, E.; Mendoza, C. Restoration of fertility by in-vitro spermatogenesis. Lancet 1999, 353, 555–556. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesarik, J. Lifestyle and Environmental Factors Affecting Male Fertility, Individual Predisposition, Prevention, and Intervention. Int. J. Mol. Sci. 2025, 26, 2797. https://doi.org/10.3390/ijms26062797
Tesarik J. Lifestyle and Environmental Factors Affecting Male Fertility, Individual Predisposition, Prevention, and Intervention. International Journal of Molecular Sciences. 2025; 26(6):2797. https://doi.org/10.3390/ijms26062797
Chicago/Turabian StyleTesarik, Jan. 2025. "Lifestyle and Environmental Factors Affecting Male Fertility, Individual Predisposition, Prevention, and Intervention" International Journal of Molecular Sciences 26, no. 6: 2797. https://doi.org/10.3390/ijms26062797
APA StyleTesarik, J. (2025). Lifestyle and Environmental Factors Affecting Male Fertility, Individual Predisposition, Prevention, and Intervention. International Journal of Molecular Sciences, 26(6), 2797. https://doi.org/10.3390/ijms26062797







