An In-Depth Exploration of the Autoantibody Immune Profile in ME/CFS Using Novel Antigen Profiling Techniques
Abstract
1. Introduction
2. Results
2.1. Study Design and Cohort Characteristics
2.2. AAb Profiling Reveals No Significant Difference Between ME/CFS and Controls
2.3. Sex-Specific Trends in AAb Abundance: Lower Levels in Male ME/CFS Patients
2.4. ME/CFS Patients Lack Public Autoantigens Against the Human Exoproteome
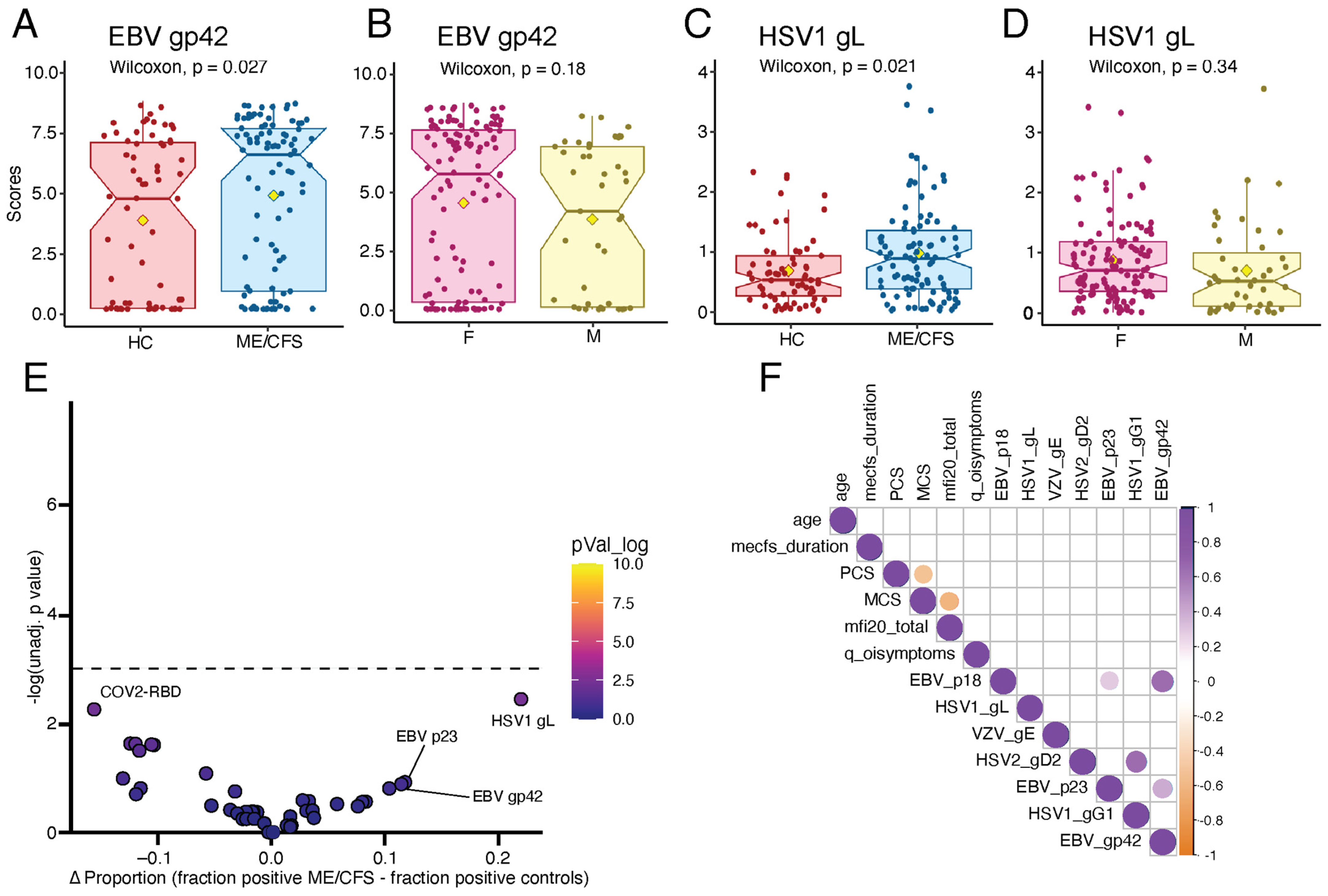
2.5. ME/CFS Patients Display Elevated Humoral Responses to Herpesviruses
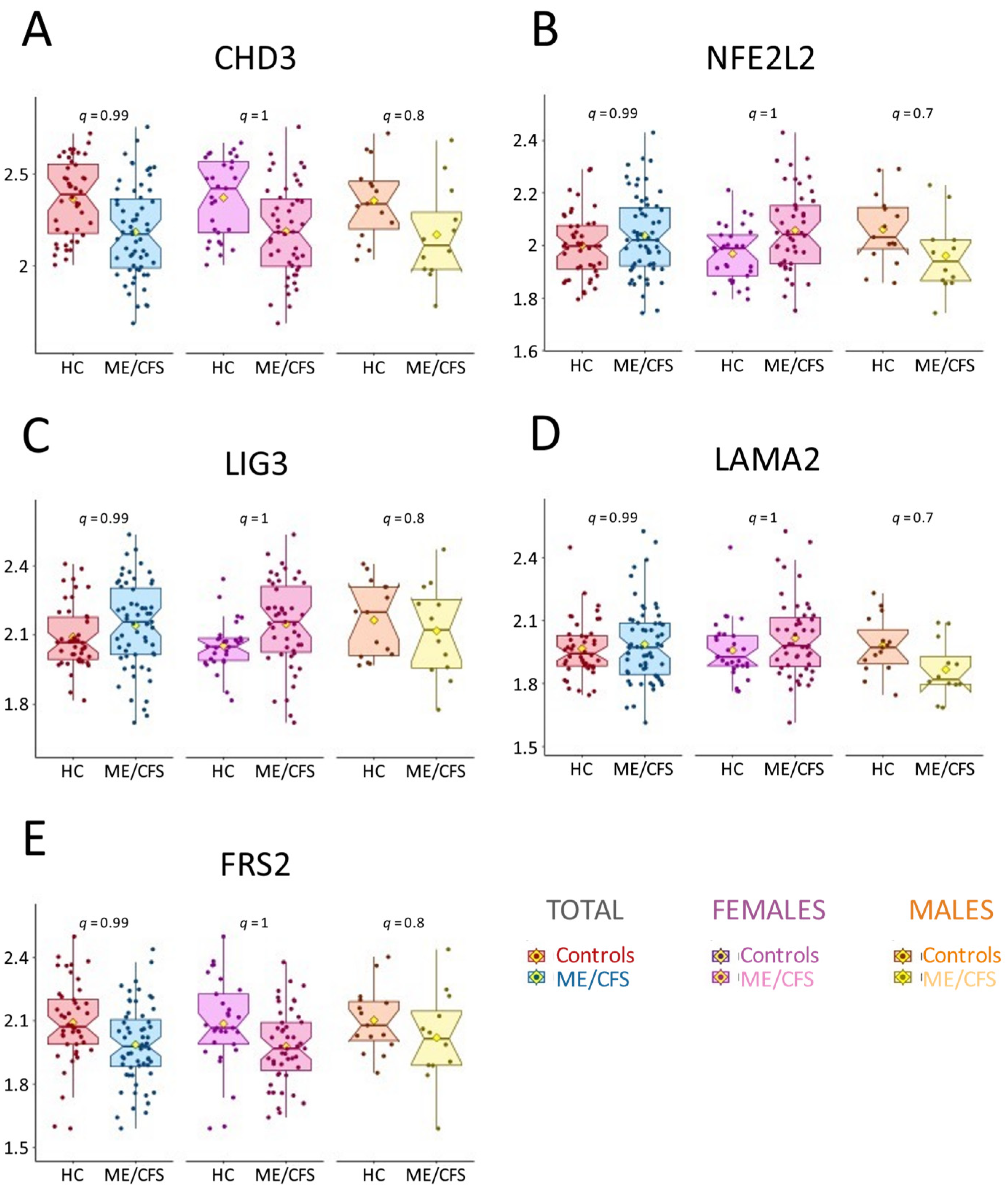
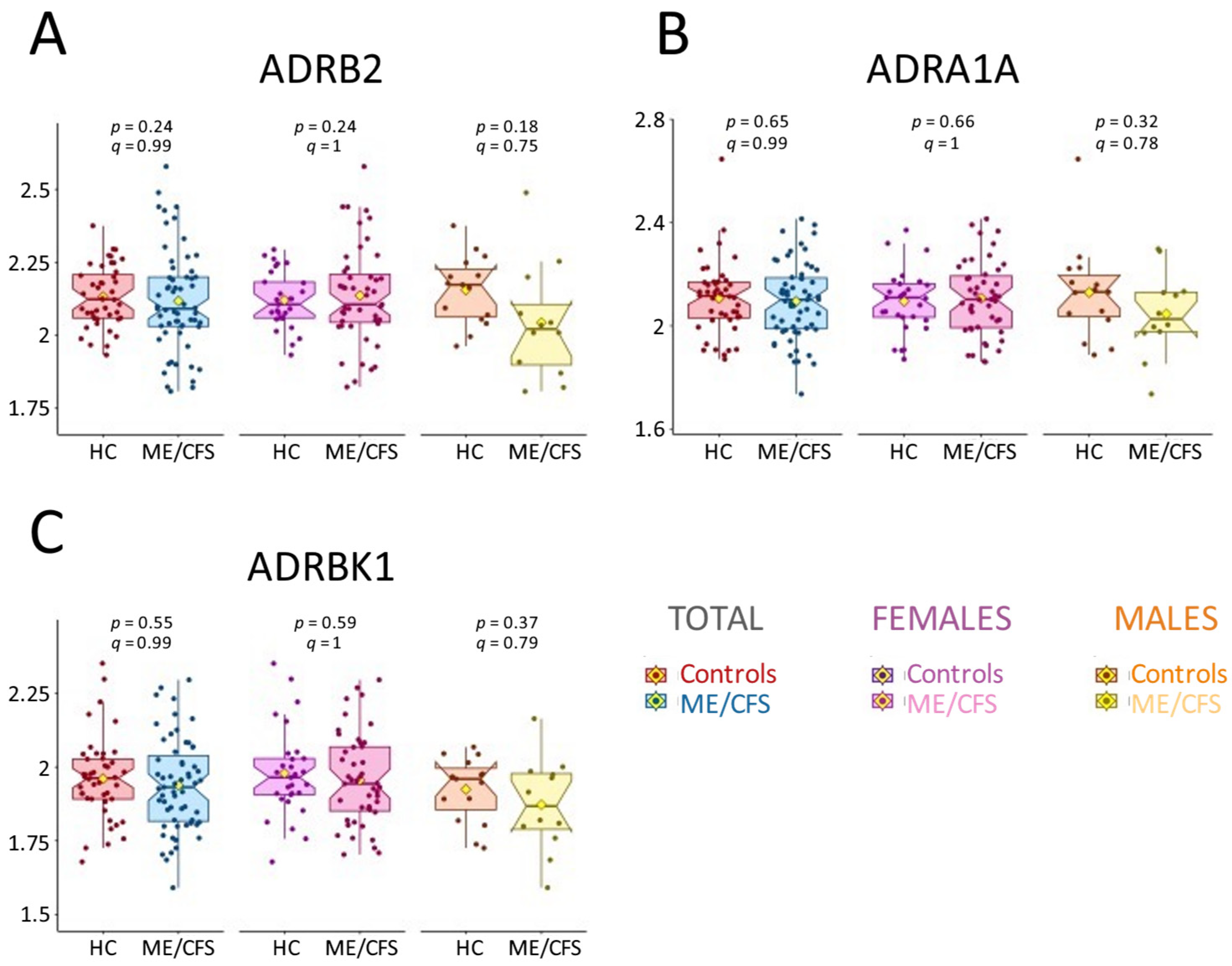
2.6. AAb Results Do Not Align with Previous Findings
3. Discussion
4. Materials and Methods
4.1. Blood Collection
4.2. Serological Testing by Augmenta Bioworks
4.3. Oncimmune SeroTag Profiling Overview
4.4. Oncimmune/Augmenta Data Analysis
4.5. REAP Library Expansion
4.6. REAP Protocol
4.7. REAP Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Institute of Medicine. Beyond Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Redefining an Illness; National Academies Press: Washington, DC, USA, 2015. [Google Scholar]
- Deumer, U.S.; Varesi, A.; Floris, V.; Savioli, G.; Mantovani, E.; Lopez-Carrasco, P.; Rosati, G.M.; Prasad, S.; Ricevuti, G. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (Me/Cfs): An Overview. J. Clin. Med. 2021, 10, 4786. [Google Scholar] [CrossRef] [PubMed]
- Gil, A.; Hoag, G.E.; Salerno, J.P.; Hornig, M.; Klimas, N.; Selin, L.K. Identification of Cd8 T-Cell Dysfunction Associated with Symptoms in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (Me/Cfs) and Long COVID and Treatment with a Nebulized Antioxidant/Anti-Pathogen Agent in a Retrospective Case Series. Brain Behav. Immun. Health 2024, 36, 100720. [Google Scholar] [CrossRef]
- Mandarano, A.H.; Maya, J.; Giloteaux, L.; Peterson, D.L.; Maynard, M.; Gottschalk, C.G.; Hanson, M.R. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Patients Exhibit Altered T Cell Metabolism and Cytokine Associations. J. Clin. Investig. 2020, 130, 1491–1505. [Google Scholar] [CrossRef]
- Maya, J. Surveying the Metabolic and Dysfunctional Profiles of T Cells and Nk Cells in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Int. J. Mol. Sci. 2023, 24, 11937. [Google Scholar] [CrossRef]
- Milivojevic, M.; Che, X.; Bateman, L.; Cheng, A.; Garcia, B.A.; Hornig, M.; Huber, M.; Klimas, N.G.; Lee, B.; Lee, H.; et al. Plasma Proteomic Profiling Suggests an Association between Antigen Driven Clonal B Cell Expansion and Me/Cfs. PLoS ONE 2020, 15, e0236148. [Google Scholar] [CrossRef]
- Rivas, J.L.; Palencia, T.; Fernandez, G.; Garcia, M. Association of T and Nk Cell Phenotype with the Diagnosis of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (Me/Cfs). Front. Immunol. 2018, 9, 1028. [Google Scholar] [CrossRef] [PubMed]
- Seton, K.A.; Espejo-Oltra, J.A.; Gimenez-Orenga, K.; Haagmans, R.; Ramadan, D.J.; Mehlsen, J.; European ME Research Group for Early Career Researchers (Young EMERG). Advancing Research and Treatment: An Overview of Clinical Trials in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (Me/Cfs) and Future Perspectives. J. Clin. Med. 2024, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.T.; Ahmed, F.; Zhu, H.; Iu, D.S.H.; Fogarty, E.A.; Kwak, Y.; Chen, W.; Franconi, C.J.; Munn, P.R.; Tate, A.E.; et al. Single-Cell Transcriptomics of the Immune System in Me/Cfs at Baseline and Following Symptom Provocation. Cell Rep. Med. 2024, 5, 101373. [Google Scholar] [CrossRef]
- Sotzny, F.; Blanco, J.; Capelli, E.; Castro-Marrero, J.; Steiner, S.; Murovska, M.; Scheibenbogen, C.; European Network on ME/CFS (EUROMENE). Myalgic Encephalomyelitis/Chronic Fatigue Syndrome-Evidence for an Autoimmune Disease. Autoimmun. Rev. 2018, 17, 601–609. [Google Scholar] [CrossRef]
- Fluge, O.; Bruland, O.; Risa, K.; Storstein, A.; Kristoffersen, E.K.; Sapkota, D.; Naess, H.; Dahl, O.; Nyland, H.; Mella, O. Benefit from B-Lymphocyte Depletion Using the Anti-Cd20 Antibody Rituximab in Chronic Fatigue Syndrome. A Double-Blind and Placebo-Controlled Study. PLoS ONE 2011, 6, e26358. [Google Scholar] [CrossRef]
- Fluge, O.; Risa, K.; Lunde, S.; Alme, K.; Rekeland, I.G.; Sapkota, D.; Kristoffersen, E.K.; Sorland, K.; Bruland, O.; Dahl, O.; et al. B-Lymphocyte Depletion in Myalgic Encephalopathy/Chronic Fatigue Syndrome. An Open-Label Phase Ii Study with Rituximab Maintenance Treatment. PLoS ONE 2015, 10, e0129898. [Google Scholar] [CrossRef] [PubMed]
- Fluge, O.; Rekeland, I.G.; Lien, K.; Thurmer, H.; Borchgrevink, P.C.; Schafer, C.; Sorland, K.; Assmus, J.; Ktoridou-Valen, I.; Herder, I.; et al. B-Lymphocyte Depletion in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: A Randomized, Double-Blind, Placebo-Controlled Trial. Ann. Intern. Med. 2019, 170, 585–593. [Google Scholar] [CrossRef]
- Rekeland, I.G.; Sorland, K.; Neteland, L.L.; Fossa, A.; Alme, K.; Risa, K.; Dahl, O.; Tronstad, K.J.; Mella, O.; Fluge, O. Six-Year Follow-up of Participants in Two Clinical Trials of Rituximab or Cyclophosphamide in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. PLoS ONE 2024, 19, e0307484. [Google Scholar] [CrossRef] [PubMed]
- Scheibenbogen, C.; Loebel, M.; Freitag, H.; Krueger, A.; Bauer, S.; Antelmann, M.; Doehner, W.; Scherbakov, N.; Heidecke, H.; Reinke, P.; et al. Immunoadsorption to Remove Ss2 Adrenergic Receptor Antibodies in Chronic Fatigue Syndrome Cfs/Me. PLoS ONE 2018, 13, e0193672. [Google Scholar] [CrossRef]
- Tolle, M.; Freitag, H.; Antelmann, M.; Hartwig, J.; Schuchardt, M.; van der Giet, M.; Eckardt, K.U.; Grabowski, P.; Scheibenbogen, C. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Efficacy of Repeat Immunoadsorption. J. Clin. Med. 2020, 9, 2443. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Kuratsune, H.; Hidaka, Y.; Hakariya, Y.; Tatsumi, K.I.; Takano, T.; Kanakura, Y.; Amino, N. Autoantibodies against Muscarinic Cholinergic Receptor in Chronic Fatigue Syndrome. Int. J. Mol. Med. 2003, 12, 225–230. [Google Scholar] [CrossRef]
- Loebel, M.; Grabowski, P.; Heidecke, H.; Bauer, S.; Hanitsch, L.G.; Wittke, K.; Meisel, C.; Reinke, P.; Volk, H.D.; Fluge, O.; et al. Antibodies to Beta Adrenergic and Muscarinic Cholinergic Receptors in Patients with Chronic Fatigue Syndrome. Brain Behav. Immun. 2016, 52, 32–39. [Google Scholar] [CrossRef]
- Bynke, A.; Julin, P.; Gottfries, C.G.; Heidecke, H.; Scheibenbogen, C.; Bergquist, J. Autoantibodies to Beta-Adrenergic and Muscarinic Cholinergic Receptors in Myalgic Encephalomyelitis (Me) Patients—A Validation Study in Plasma and Cerebrospinal Fluid from Two Swedish Cohorts. Brain Behav. Immun. Health 2020, 7, 100107. [Google Scholar] [CrossRef]
- Ryabkova, V.; Gavrilova, N.Y.; Poletaeva, A.A.; Pukhalenko, A.I.; Koshkina, I.A.; Churilov, L.P.; Shoenfeld, Y. Autoantibody Correlation Signatures in Fibromyalgia and Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Association with Symptom Severity. Biomedicines 2023, 11, 257. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Marques, A.; Giil, L.M.; De Vito, R.; Rademacher, J.; Gunther, J.; Lange, T.; Humrich, J.Y.; Klapa, S.; Schinke, S.; et al. Gpcr-Specific Autoantibody Signatures Are Associated with Physiological and Pathological Immune Homeostasis. Nat. Commun. 2018, 9, 5224. [Google Scholar] [CrossRef]
- Cabral-Marques, O.; Halpert, G.; Schimke, L.F.; Ostrinski, Y.; Vojdani, A.; Baiocchi, G.C.; Freire, P.P.; Filgueiras, I.S.; Zyskind, I.; Lattin, M.T.; et al. Autoantibodies Targeting Gpcrs and Ras-Related Molecules Associate with COVID-19 Severity. Nat. Commun. 2022, 13, 1220. [Google Scholar] [CrossRef] [PubMed]
- Lutz, H.U.; Binder, C.J.; Kaveri, S. Naturally Occurring Auto-Antibodies in Homeostasis and Disease. Trends Immunol. 2009, 30, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Marques, O.; Riemekasten, G. Functional Autoantibodies Targeting G Protein-Coupled Receptors in Rheumatic Diseases. Nat. Rev. Rheumatol. 2017, 13, 648–656. [Google Scholar] [CrossRef]
- Nagele, E.P.; Han, M.; Acharya, N.K.; DeMarshall, C.; Kosciuk, M.C.; Nagele, R.G. Natural Igg Autoantibodies Are Abundant and Ubiquitous in Human Sera, and Their Number Is Influenced by Age, Gender, and Disease. PLoS ONE 2013, 8, e60726. [Google Scholar] [CrossRef]
- Amendt, T.; Allies, G.; Nicolo, A.; El Ayoubi, O.; Young, M.; Roszer, T.; Setz, C.S.; Warnatz, K.; Jumaa, H. Autoreactive Antibodies Control Blood Glucose by Regulating Insulin Homeostasis. Proc. Natl. Acad. Sci. USA 2022, 119, e2115695119. [Google Scholar] [CrossRef] [PubMed]
- Bradford, H.F.; Haljasmagi, L.; Menon, M.; McDonnell, T.C.R.; Sarekannu, K.; Vanker, M.; Peterson, P.; Wincup, C.; Abida, R.; Gonzalez, R.F.; et al. Inactive Disease in Patients with Lupus Is Linked to Autoantibodies to Type I Interferons That Normalize Blood Ifnalpha and B Cell Subsets. Cell Rep. Med. 2023, 4, 100894. [Google Scholar] [CrossRef]
- Buchwald, D.; Cheney, P.R.; Peterson, D.L.; Henry, B.; Wormsley, S.B.; Geiger, A.; Ablashi, D.V.; Salahuddin, S.Z.; Saxinger, C.; Biddle, R.; et al. A Chronic Illness Characterized by Fatigue, Neurologic and Immunologic Disorders, and Active Human Herpesvirus Type 6 Infection. Ann. Intern. Med. 1992, 116, 103–113. [Google Scholar] [CrossRef]
- Lee, J.S.; Lacerda, E.M.; Nacul, L.; Kingdon, C.C.; Norris, J.; O’Boyle, S.; Roberts, C.H.; Palla, L.; Riley, E.M.; Cliff, J.M. Salivary DNA Loads for Human Herpesviruses 6 and 7 Are Correlated with Disease Phenotype in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Front. Med. 2021, 8, 656692. [Google Scholar] [CrossRef]
- Shafiee, A.; Athar, M.M.T.; Amini, M.J.; Hajishah, H.; Siahvoshi, S.; Jalali, M.; Jahanbakhshi, B.; Mozhgani, S.H. Reactivation of Herpesviruses During COVID-19: A Systematic Review and Meta-Analysis. Rev. Med. Virol. 2023, 33, e2437. [Google Scholar] [CrossRef]
- Carruthers, B.M.; Jain, A.K.; De Meirleir, K.L.; Peterson, D.L.; Klimas, N.G.; Lerner, A.M.; Bested, A.C.; Flor-Henry, P.; Joshi, P.; Powles, A.P.; et al. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome: Clinical Working Case Definition, Diagnostic and Treatment Protocols. J. Chronic Fatigue Syndr. 2003, 11, 7–115. [Google Scholar] [CrossRef]
- Quandt, D.; Zucht, H.D.; Amann, A.; Wulf-Goldenberg, A.; Borrebaeck, C.; Cannarile, M.; Lambrechts, D.; Oberacher, H.; Garrett, J.; Nayak, T.; et al. Implementing Liquid Biopsies into Clinical Decision Making for Cancer Immunotherapy. Oncotarget 2017, 8, 48507. [Google Scholar] [CrossRef] [PubMed]
- Welberry, C.; Macdonald, I.; McElveen, J.; Parsy-Kowalska, C.; Allen, J.; Healey, G.; Irving, W.; Murray, A.; Chapman, C. Tumor-Associated Autoantibodies in Combination with Alpha-Fetoprotein for Detection of Early Stage Hepatocellular Carcinoma. PLoS ONE 2020, 15, e0232247. [Google Scholar] [CrossRef]
- Schulz-Knappe, P.; Budde, P.; Zucht, H.-D.; Konings, S.; Steeg, L.; Friedrich, E.; Gutjahr, C.; Steil, R.; Bhandari, S.; Tuschen, M. Thu0042 Cancer Immunotherapy Array: A Novel Screening Tool for Immune System Profiling in Cancer Immunotherapy Bridging Autoimmunity and Cancer. Ann. Rheum. Dis. 2018, 77, 247. [Google Scholar] [CrossRef]
- Liu, Y.; Ebinger, J.E.; Mostafa, R.; Budde, P.; Gajewski, J.; Walker, B.; Joung, S.; Wu, M.; Brautigam, M.; Hesping, F.; et al. Paradoxical Sex-Specific Patterns of Autoantibody Response to Sars-Cov-2 Infection. J. Transl. Med. 2021, 19, 524. [Google Scholar] [CrossRef]
- The RA-MAP Consortium. Ra-Map, Molecular Immunological Landscapes in Early Rheumatoid Arthritis and Healthy Vaccine Recipients. Sci. Data 2022, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Mahler, M.; Hudson, M.; Bentow, C.; Roup, F.; Beretta, L.; Simeón, C.P.; Guillén-Del-Castillo, A.; Casas, S.; Fritzler, M.J. Autoantibodies to Stratify Systemic Sclerosis Patients into Clinically Actionable Subsets. Autoimmun. Rev. 2020, 19, 102583. [Google Scholar] [CrossRef]
- Bucukovski, J.; Carter, J.A.; Striemer, C.C.; Mullner, S.; Schulte-Pelkum, J.; Schulz-Knappe, P.; Miller, B.L. Label-Free Microarray-Based Detection of Autoantibodies in Human Serum. J. Immunol. Methods 2018, 459, 44–49. [Google Scholar] [CrossRef]
- O’Neal, A.J.; Glass, K.A.; Emig, C.J.; Vitug, A.A.; Henry, S.J.; Shungu, D.C.; Mao, X.; Levine, S.M.; Hanson, M.R. Survey of Anti-Pathogen Antibody Levels in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. Proteomes 2022, 10, 21. [Google Scholar] [CrossRef]
- Penela, P.; Rivas, V.; Salcedo, A.; Mayor, F., Jr. G Protein-Coupled Receptor Kinase 2 (Grk2) Modulation and Cell Cycle Progression. Proc. Natl. Acad. Sci. USA 2010, 107, 1118–1123. [Google Scholar] [CrossRef]
- Gold, S.M.; Willing, A.; Leypoldt, F.; Paul, F.; Friese, M.A. Sex Differences in Autoimmune Disorders of the Central Nervous System. Semin. Immunopathol. 2019, 41, 177–188. [Google Scholar] [CrossRef]
- Fagan, T.F.; Faustman, D.L. Sex Differences in Autoimmunity. In Advances in Molecular and Cell Biology; Elsevier: Amsterdam, The Netherlands, 2004; pp. 295–306. [Google Scholar]
- Edwards, M.; Dai, R.; Ahmed, S.A. Our Environment Shapes Us: The Importance of Environment and Sex Differences in Regulation of Autoantibody Production. Front. Immunol. 2018, 9, 478. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.P.; Wang, C.G.; Liu, X.; Huang, Y.Q.; Guo, D.L.; Jing, X.Z.; Yuan, C.G.; Yang, S.; Liu, J.M.; Han, M.S.; et al. The Prevalence of Antinuclear Antibodies in the General Population of China: A Cross-Sectional Study. Curr. Ther. Res. Clin. Exp. 2014, 76, 116–119. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Quintela, A.; Alende, R.; Gude, F.; Campos, J.; Rey, J.; Meijide, L.M.; Fernandez-Merino, C.; Vidal, C. Serum Levels of Immunoglobulins (Igg, Iga, Igm) in a General Adult Population and Their Relationship with Alcohol Consumption, Smoking and Common Metabolic Abnormalities. Clin. Exp. Immunol. 2008, 151, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Freitag, H.; Szklarski, M.; Lorenz, S.; Sotzny, F.; Bauer, S.; Philippe, A.; Kedor, C.; Grabowski, P.; Lange, T.; Riemekasten, G.; et al. Autoantibodies to Vasoregulative G-Protein-Coupled Receptors Correlate with Symptom Severity, Autonomic Dysfunction and Disability in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Clin. Med. 2021, 10, 3675. [Google Scholar] [CrossRef]
- Sotzny, F.; Filgueiras, I.S.; Kedor, C.; Freitag, H.; Wittke, K.; Bauer, S.; Sepulveda, N.; da Fonseca, D.L.M.; Baiocchi, G.C.; Marques, A.H.C.; et al. Dysregulated Autoantibodies Targeting Vaso- and Immunoregulatory Receptors in Post Covid Syndrome Correlate with Symptom Severity. Front. Immunol. 2022, 13, 981532. [Google Scholar] [CrossRef]
- Mozhgani, S.H.; Rajabi, F.; Qurbani, M.; Erfani, Y.; Yaslianifard, S.; Moosavi, A.; Pourrostami, K.; Bagheri, A.B.; Soleimani, A.; Behzadian, F.; et al. Human Herpesvirus 6 Infection and Risk of Chronic Fatigue Syndrome: A Systematic Review and Meta-Analysis. Intervirology 2022, 65, 49–57. [Google Scholar] [CrossRef]
- Ruiz-Pablos, M.; Paiva, B.; Montero-Mateo, R.; Garcia, N.; Zabaleta, A. Epstein-Barr Virus and the Origin of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome. Front. Immunol. 2021, 12, 656797. [Google Scholar] [CrossRef]
- Klein, J.; Wood, J.; Jaycox, J.R.; Dhodapkar, R.M.; Lu, P.; Gehlhausen, J.R.; Tabachnikova, A.; Greene, K.; Tabacof, L.; Malik, A.A.; et al. Distinguishing Features of Long Covid Identified through Immune Profiling. Nature 2023, 623, 139–148. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Wang, E.Y.; Mao, T.; Klein, J.; Dai, Y.; Huck, J.D.; Jaycox, J.R.; Liu, F.; Zhou, T.; Israelow, B.; Wong, P.; et al. Diverse Functional Autoantibodies in Patients with COVID-19. Nature 2021, 595, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.Y.; Dai, Y.; Rosen, C.E.; Schmitt, M.M.; Dong, M.X.; Ferre, E.M.N.; Liu, F.; Yang, Y.; Gonzalez-Hernandez, J.A.; Meffre, E.; et al. High-Throughput Identification of Autoantibodies That Target the Human Exoproteome. Cell Rep. Methods 2022, 2, 100172. [Google Scholar] [CrossRef] [PubMed]


| Oncimmune/Augmenta | REAP | |||||
|---|---|---|---|---|---|---|
| ME/CFS | Controls | p-Value | ME/CFS | Controls | p-Value | |
| Cohort (n) | 59 | 44 | NA | 95 | 69 | NA |
| Females/Males (n) | 47/12 | 29/15 | NA | 71/24 | 50/19 | NA |
| Age (years) | 45.9/44.8 | 42.3/41.8 | 0.15 | 46.6/44.4 | 42.8/42.5 | 0.14 |
| BMI (kg·m−2) | 26.1/27.2 | 28.2/26.3 | 0.25 | 26.2/27.7 | 28/26.5 | 0.25 |
| ME/CFS duration (years) | 11.3/13.7 | NA | NA | 12.4/10.8 | NA | NA |
| Bell disability scale | 40.9/32.9 | 97.3/94.3 | 2 × 10−16 * | 39.3/33.3 | 96.8/94.7 | 2 × 10−16 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germain, A.; Jaycox, J.R.; Emig, C.J.; Ring, A.M.; Hanson, M.R. An In-Depth Exploration of the Autoantibody Immune Profile in ME/CFS Using Novel Antigen Profiling Techniques. Int. J. Mol. Sci. 2025, 26, 2799. https://doi.org/10.3390/ijms26062799
Germain A, Jaycox JR, Emig CJ, Ring AM, Hanson MR. An In-Depth Exploration of the Autoantibody Immune Profile in ME/CFS Using Novel Antigen Profiling Techniques. International Journal of Molecular Sciences. 2025; 26(6):2799. https://doi.org/10.3390/ijms26062799
Chicago/Turabian StyleGermain, Arnaud, Jillian R. Jaycox, Christopher J. Emig, Aaron M. Ring, and Maureen R. Hanson. 2025. "An In-Depth Exploration of the Autoantibody Immune Profile in ME/CFS Using Novel Antigen Profiling Techniques" International Journal of Molecular Sciences 26, no. 6: 2799. https://doi.org/10.3390/ijms26062799
APA StyleGermain, A., Jaycox, J. R., Emig, C. J., Ring, A. M., & Hanson, M. R. (2025). An In-Depth Exploration of the Autoantibody Immune Profile in ME/CFS Using Novel Antigen Profiling Techniques. International Journal of Molecular Sciences, 26(6), 2799. https://doi.org/10.3390/ijms26062799








