Sustained Experimental Myopia Exacerbates the Effect of Eye Growth on Retinal Ganglion Cell Density and Function
Abstract
:1. Introduction
2. Results
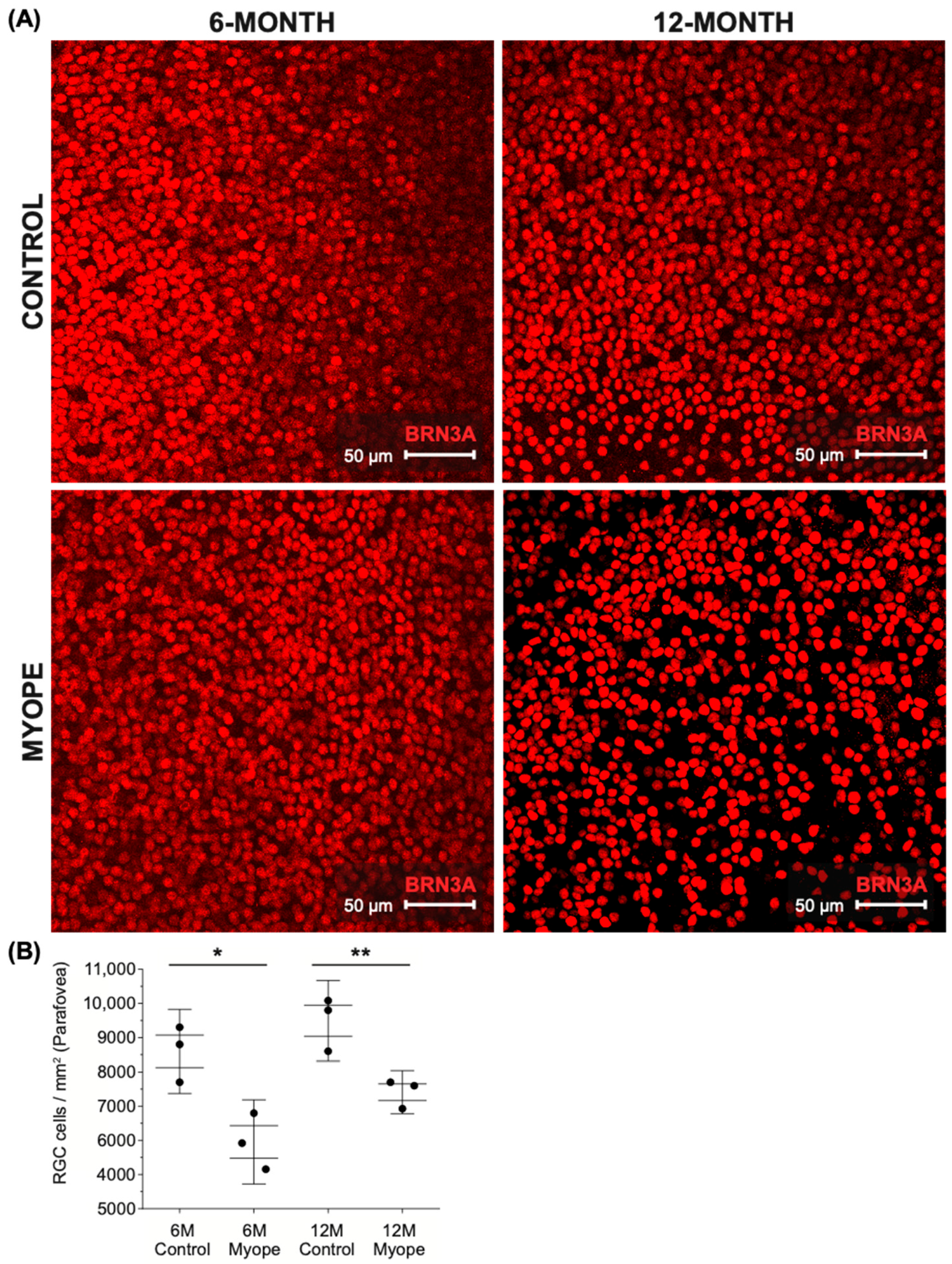
2.1. Parafoveal Ganglion Cell Density Is Lower in Marmosets Induced with Myopia for 4 and 10 Months Compared to Age-Matched Controls
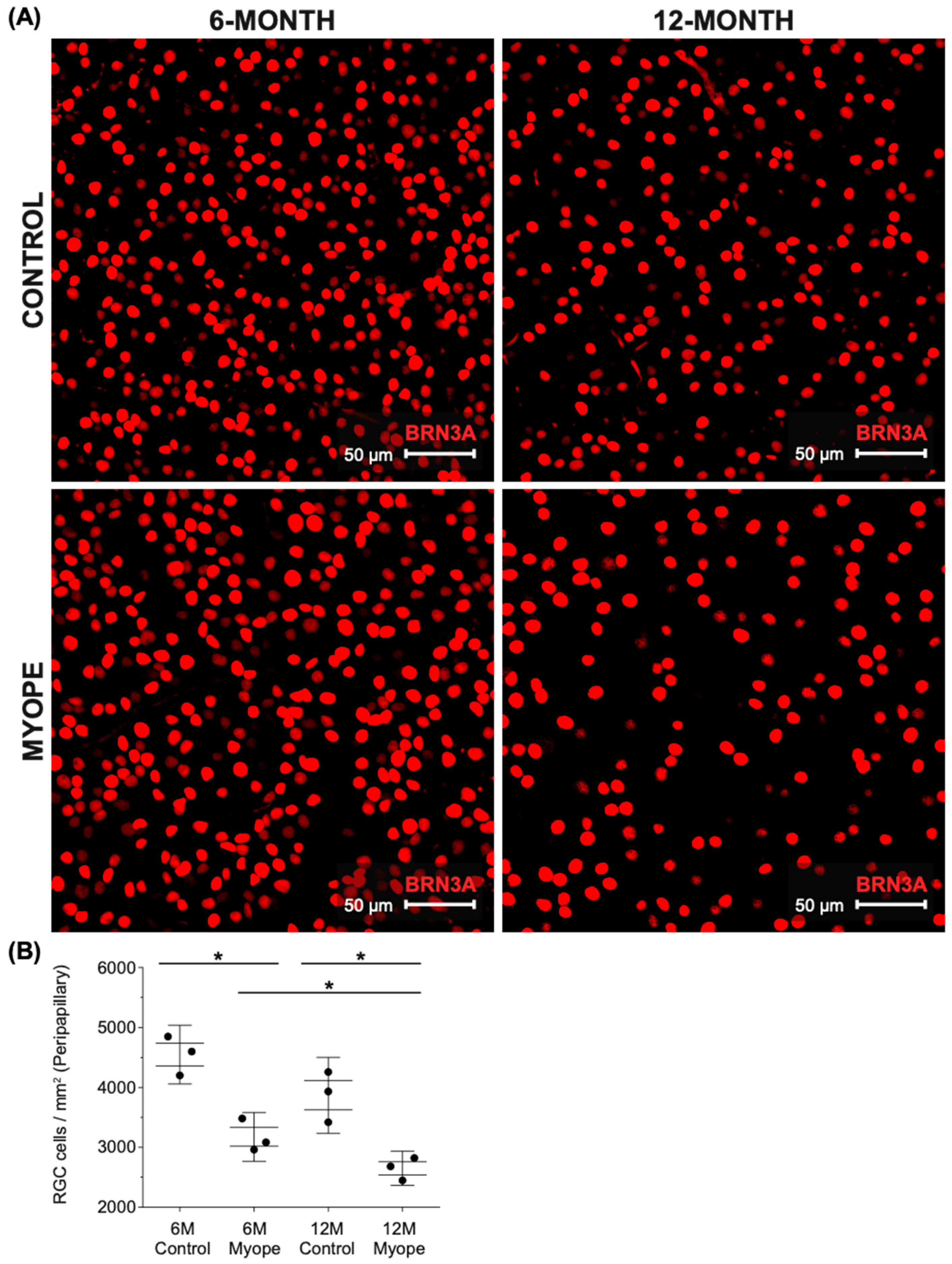
2.2. Peripapillary Ganglion Cell Density Is Lower in Marmosets Induced with Myopia for 10 Months vs. 4 Months
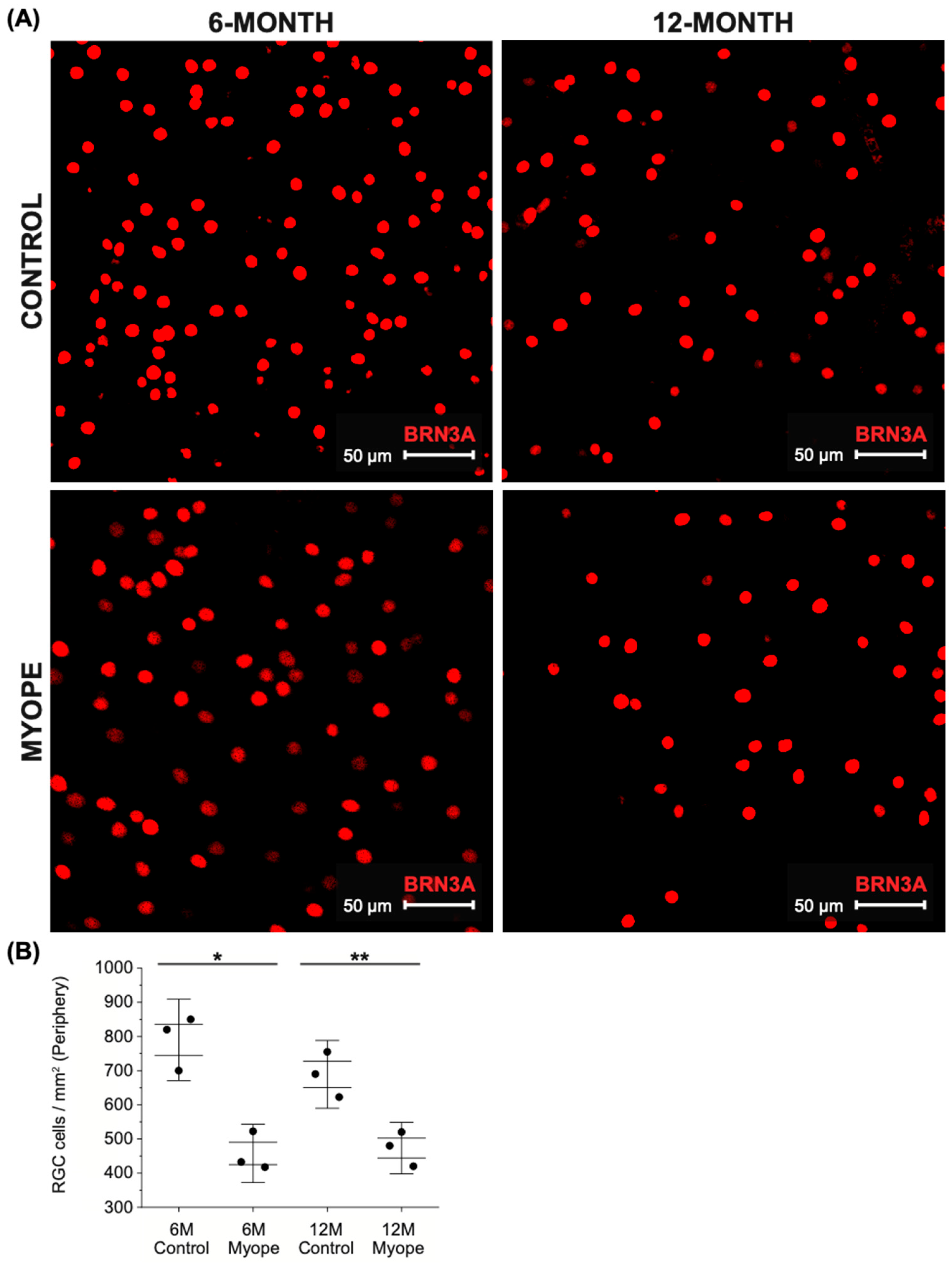
2.3. Peripheral Ganglion Cell Density Is Lower in Marmosets Induced with Myopia for 4 and 10 Months Compared to Age-Matched Controls
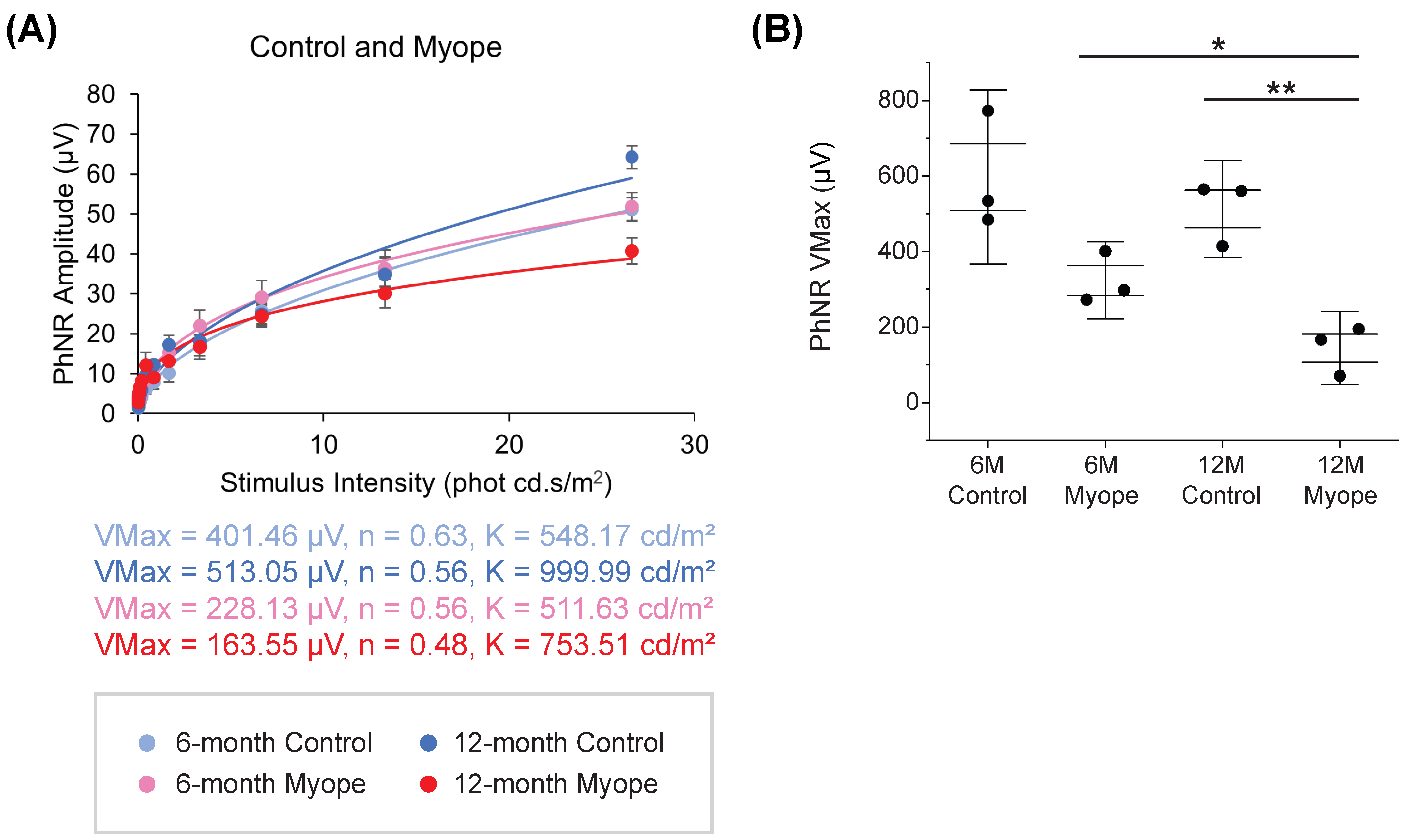
2.4. Dampening of PhNR Amplitude Occurs in Eyes Induced with Myopia for 10 but Not 4 Months
3. Discussion
3.1. Pan-Retinal Ganglion Cell Density Decreases in Marmoset Eyes Induced with Myopia for 4 and 10 Months
3.2. Photopic Negative Response (PhNR) Amplitude Is Significantly Dampened in Marmosets Induced with Myopia for 10 Months, but Not in Marmosets Treated for 4 Months
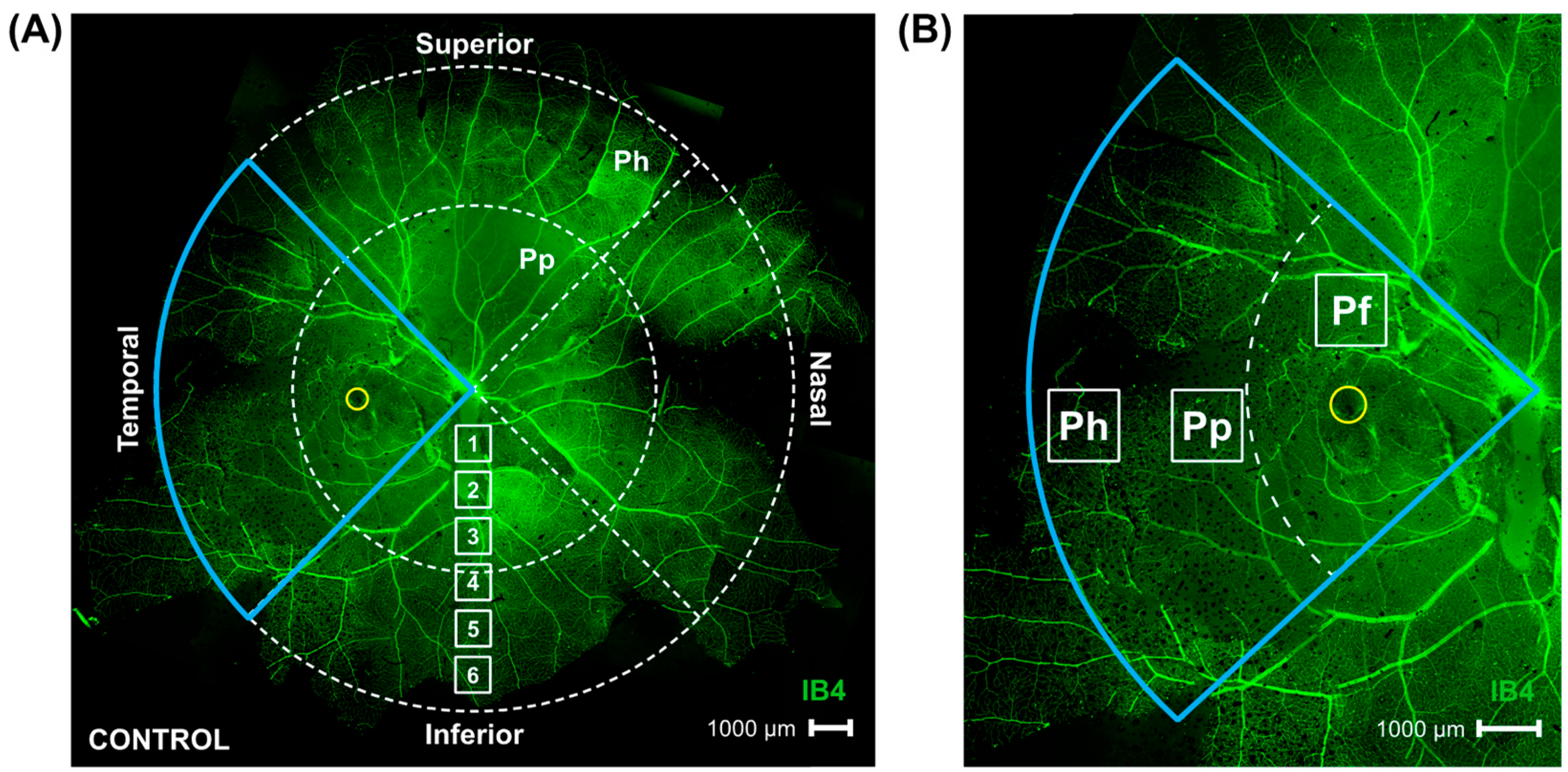
4. Materials and Methods
4.1. Marmoset Model of Myopia
4.2. Electroretinograms (ERGs)
4.3. Tissue Collection
4.4. Confocal Microscopy and Image Acquisition
4.5. Image and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saw, S.M.; Gazzard, G.; Shih-Yen, E.C.; Chua, W.H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005, 25, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Curtin, B.J. The Myopias: Basic Science and Clinical Management; Harper & Row: Philadelphia, PA, USA, 1985; p. xv. 495p. [Google Scholar]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef]
- Benavente-Perez, A.; Hosking, S.L.; Logan, N.S.; Broadway, D.C. Ocular blood flow measurements in healthy human myopic eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Abbott, C.J.; Grunert, U.; Pianta, M.J.; McBrien, N.A. Retinal thinning in tree shrews with induced high myopia: Optical coherence tomography and histological assessment. Vis. Res. 2011, 51, 376–385. [Google Scholar] [CrossRef]
- El-Shazly, A.A.; Farweez, Y.A.; ElSebaay, M.E.; El-Zawahry, W.M.A. Correlation between choroidal thickness and degree of myopia assessed with enhanced depth imaging optical coherence tomography. Eur. J. Ophthalmol. 2017, 27, 577–584. [Google Scholar] [CrossRef]
- Ismael, Z.F.; El-Shazly, A.A.E.; Farweez, Y.A.; Osman, M.M.M. Relationship between functional and structural retinal changes in myopic eyes. Clin. Exp. Optom. 2017, 100, 695–703. [Google Scholar] [CrossRef]
- McBrien, N.A.; Cornell, L.M.; Gentle, A. Structural and ultrastructural changes to the sclera in a mammalian model of high myopia. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2179–2187. [Google Scholar]
- Shaikh, A.W.; Siegwart, J.T., Jr.; Norton, T.T. Effect of interrupted lens wear on compensation for a minus lens in tree shrews. Optom. Vis. Sci. 1999, 76, 308–315. [Google Scholar] [CrossRef]
- Cheng, D.; Chen, Q.; Wu, Y.; Yu, X.; Shen, M.; Zhuang, X.; Tian, Z.; Yang, Y.; Wang, J.; Lu, F.; et al. Deep perifoveal vessel density as an indicator of capillary loss in high myopia. Eye 2019, 33, 1961–1968. [Google Scholar] [CrossRef]
- Fan, H.; Chen, H.Y.; Ma, H.J.; Chang, Z.; Yin, H.Q.; Ng, D.S.; Cheung, C.Y.; Hu, S.; Xiang, X.; Tang, S.B.; et al. Reduced Macular Vascular Density in Myopic Eyes. Chin. Med. J. 2017, 130, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, H.E.; Green, W.R. Pathologic findings in pathologic myopia. Retina 1992, 12, 127–133. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Q.; Yin, Y.; Zhou, H.; Fan, Y.; Zhu, J.; Zou, H.; Xu, X. Association between retinal microvasculature and optic disc alterations in high myopia. Eye 2019, 33, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Panda-Jonas, S.; Jonas, J.B.; Jonas, R.A. Photoreceptor density in relation to axial length and retinal location in human eyes. Sci. Rep. 2022, 12, 21371. [Google Scholar] [CrossRef]
- Su, L.; Ji, Y.S.; Tong, N.; Sarraf, D.; He, X.; Sun, X.; Xu, X.; Sadda, S.R. Quantitative assessment of the retinal microvasculature and choriocapillaris in myopic patients using swept-source optical coherence tomography angiography. Graefes Arch. Clin. Exp. Ophthalmol. 2020, 258, 1173–1180. [Google Scholar] [CrossRef]
- Lin, C.; Toychiev, A.; Ablordeppey, R.; Slavi, N.; Srinivas, M.; Benavente-Perez, A. Myopia Alters the Structural Organization of the Retinal Vasculature, GFAP-Positive Glia, and Ganglion Cell Layer Thickness. Int. J. Mol. Sci. 2022, 23, 6202. [Google Scholar] [CrossRef]
- Ablordeppey, R.K.; Lin, C.R.; Srinivas, M.; Benavente-Perez, A. Experimental Myopia Results in Peripapillary Ganglion Cell and Astrocyte Reorganization with No Functional Implications During Early Development. Int. J. Mol. Sci. 2024, 25, 13484. [Google Scholar] [CrossRef] [PubMed]
- Troilo, D.; Judge, S.J. Ocular development and visual deprivation myopia in the common marmoset (Callithrix jacchus). Vis. Res. 1993, 33, 1311–1324. [Google Scholar] [CrossRef]
- Lin, C.R.; Toychiev, A.; Ablordeppey, R.K.; Srinivas, M.; Benavente-Perez, A. Sustained Retinal Defocus Increases the Effect of Induced Myopia on the Retinal Astrocyte Template. Cells 2024, 13, 595. [Google Scholar] [CrossRef]
- Lin, C.R.; Toychiev, A.; Ablordeppey, R.K.; Srinivas, M.; Benavente-Perez, A. Age exacerbates the effect of myopia on retinal capillaries and string vessels. Front. Med. 2023, 10, 1112396. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, X.; Wen, Y.; Jin, L.; Zhang, L.; Zhu, H.; Zhang, D.; Xie, C.; Guo, D.; Tong, J.; et al. Functions of retinal astrocytes and Muller cells in mammalian myopia. BMC Ophthalmol. 2022, 22, 451. [Google Scholar] [CrossRef]
- Sanes, J.R.; Masland, R.H. The types of retinal ganglion cells: Current status and implications for neuronal classification. Annu. Rev. Neurosci. 2015, 38, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Sernagor, E.; Eglen, S.J.; Wong, R.O. Development of retinal ganglion cell structure and function. Prog. Retin. Eye Res. 2001, 20, 139–174. [Google Scholar] [CrossRef]
- Casson, R.J.; Chidlow, G.; Crowston, J.G.; Williams, P.A.; Wood, J.P.M. Retinal energy metabolism in health and glaucoma. Prog. Retin. Eye Res. 2021, 81, 100881. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Shiga, Y.; Villafranca-Baughman, D.; Cueva Vargas, J.L.; Vidal Paredes, I.A.; Quintero, H.; Fortune, B.; Danesh-Meyer, H.; Di Polo, A. Neurovascular dysfunction in glaucoma. Prog. Retin. Eye Res. 2023, 97, 101217. [Google Scholar] [CrossRef]
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Boal, A.M.; Risner, M.L.; Cooper, M.L.; Wareham, L.K.; Calkins, D.J. Astrocyte Networks as Therapeutic Targets in Glaucomatous Neurodegeneration. Cells 2021, 10, 1368. [Google Scholar] [CrossRef]
- Calkins, D.J. Critical pathogenic events underlying progression of neurodegeneration in glaucoma. Prog. Retin. Eye Res. 2012, 31, 702–719. [Google Scholar] [CrossRef]
- Flammer, J.; Haefliger, I.O.; Orgul, S.; Resink, T. Vascular dysregulation: A principal risk factor for glaucomatous damage? J. Glaucoma 1999, 8, 212–219. [Google Scholar]
- Mrugacz, M.; Bryl, A.; Zorena, K. Retinal Vascular Endothelial Cell Dysfunction and Neuroretinal Degeneration in Diabetic Patients. J. Clin. Med. 2021, 10, 458. [Google Scholar] [CrossRef]
- Tezel, G.; Chauhan, B.C.; LeBlanc, R.P.; Wax, M.B. Immunohistochemical assessment of the glial mitogen-activated protein kinase activation in glaucoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3025–3033. [Google Scholar] [CrossRef]
- Kashiwagi, K.; Iizuka, Y.; Araie, M.; Suzuki, Y.; Tsukahara, S. Effects of retinal glial cells on isolated rat retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2001, 42, 2686–2694. [Google Scholar]
- Coorey, N.J.; Shen, W.; Chung, S.H.; Zhu, L.; Gillies, M.C. The role of glia in retinal vascular disease. Clin. Exp. Optom. 2012, 95, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Kern, T.S.; Barber, A.J. Retinal ganglion cells in diabetes. J. Physiol. 2008, 586, 4401–4408. [Google Scholar] [CrossRef]
- Potilinski, M.C.; Lorenc, V.; Perisset, S.; Gallo, J.E. Mechanisms behind Retinal Ganglion Cell Loss in Diabetes and Therapeutic Approach. Int. J. Mol. Sci. 2020, 21, 2351. [Google Scholar] [CrossRef]
- Simo, R.; Stitt, A.W.; Gardner, T.W. Neurodegeneration in diabetic retinopathy: Does it really matter? Diabetologia 2018, 61, 1902–1912. [Google Scholar] [CrossRef]
- Buckley, C.; Hadoke, P.W.; Henry, E.; O’Brien, C. Systemic vascular endothelial cell dysfunction in normal pressure glaucoma. Br. J. Ophthalmol. 2002, 86, 227–232. [Google Scholar] [CrossRef]
- Flammer, J.; Orgul, S. Optic nerve blood-flow abnormalities in glaucoma. Prog. Retin. Eye Res. 1998, 17, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Bagnis, A.; Sacca, S.C. The role of oxidative stress in glaucoma. Mutat. Res. 2006, 612, 105–114. [Google Scholar] [CrossRef]
- Resch, H.; Garhofer, G.; Fuchsjager-Mayrl, G.; Hommer, A.; Schmetterer, L. Endothelial dysfunction in glaucoma. Acta Ophthalmol. 2009, 87, 4–12. [Google Scholar] [CrossRef]
- Alarcon-Martinez, L.; Villafranca-Baughman, D.; Quintero, H.; Kacerovsky, J.B.; Dotigny, F.; Murai, K.K.; Prat, A.; Drapeau, P.; Di Polo, A. Interpericyte tunnelling nanotubes regulate neurovascular coupling. Nature 2020, 585, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Dvoriantchikova, G.; Degterev, A.; Ivanov, D. Retinal ganglion cell (RGC) programmed necrosis contributes to ischemia-reperfusion-induced retinal damage. Exp. Eye Res. 2014, 123, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lafuente, M.P.; Villegas-Perez, M.P.; Selles-Navarro, I.; Mayor-Torroglosa, S.; Miralles de Imperial, J.; Vidal-Sanz, M. Retinal ganglion cell death after acute retinal ischemia is an ongoing process whose severity and duration depends on the duration of the insult. Neuroscience 2002, 109, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Selles-Navarro, I.; Villegas-Perez, M.P.; Salvador-Silva, M.; Ruiz-Gomez, J.M.; Vidal-Sanz, M. Retinal ganglion cell death after different transient periods of pressure-induced ischemia and survival intervals. A quantitative in vivo study. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2002–2014. [Google Scholar]
- Harman, A.; Abrahams, B.; Moore, S.; Hoskins, R. Neuronal density in the human retinal ganglion cell layer from 16–77 years. Anat. Rec. 2000, 260, 124–131. [Google Scholar] [CrossRef]
- Curcio, C.A.; Drucker, D.N. Retinal ganglion cells in Alzheimer’s disease and aging. Ann. Neurol. 1993, 33, 248–257. [Google Scholar] [CrossRef]
- Gao, H.; Hollyfield, J.G. Aging of the human retina. Differential loss of neurons and retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 1992, 33, 1–17. [Google Scholar]
- Eliasieh, K.; Liets, L.C.; Chalupa, L.M. Cellular reorganization in the human retina during normal aging. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2824–2830. [Google Scholar] [CrossRef]
- Ablordeppey, R.K.; Lin, C.; Benavente-Perez, A. The age-related pattern of inner retinal thickening is affected by myopia development and progression. Sci. Rep. 2022, 12, 22190. [Google Scholar] [CrossRef]
- Jonas, J.B.; Jonas, R.A.; Bikbov, M.M.; Wang, Y.X.; Panda-Jonas, S. Myopia: Histology, clinical features, and potential implications for the etiology of axial elongation. Prog. Retin. Eye Res. 2023, 96, 101156. [Google Scholar] [CrossRef]
- Jonas, J.B.; Xu, L. Histological changes of high axial myopia. Eye 2014, 28, 113–117. [Google Scholar] [CrossRef]
- McBrien, N.A.; Gentle, A. Role of the sclera in the development and pathological complications of myopia. Prog. Retin. Eye Res. 2003, 22, 307–338. [Google Scholar] [CrossRef]
- Al-Sheikh, M.; Phasukkijwatana, N.; Dolz-Marco, R.; Rahimi, M.; Iafe, N.A.; Freund, K.B.; Sadda, S.R.; Sarraf, D. Quantitative OCT Angiography of the Retinal Microvasculature and the Choriocapillaris in Myopic Eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2063–2069. [Google Scholar] [CrossRef] [PubMed]
- Leng, Y.; Tam, E.K.; Falavarjani, K.G.; Tsui, I. Effect of Age and Myopia on Retinal Microvasculature. Ophthalmic Surg. Lasers Imaging Retin. 2018, 49, 925–931. [Google Scholar] [CrossRef]
- Bringmann, A.; Unterlauft, J.D.; Barth, T.; Wiedemann, R.; Rehak, M.; Wiedemann, P. Muller cells and astrocytes in tractional macular disorders. Prog. Retin. Eye Res. 2022, 86, 100977. [Google Scholar] [CrossRef] [PubMed]
- Niyadurupola, N.; Luu, C.D.; Nguyen, D.Q.; Geddes, K.; Tan, G.X.; Wong, C.C.; Tran, T.; Coote, M.A.; Crowston, J.G. Intraocular pressure lowering is associated with an increase in the photopic negative response (PhNR) amplitude in glaucoma and ocular hypertensive eyes. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Colotto, A.; Falsini, B.; Salgarello, T.; Iarossi, G.; Galan, M.E.; Scullica, L. Photopic negative response of the human ERG: Losses associated with glaucomatous damage. Investig. Ophthalmol. Vis. Sci. 2000, 41, 2205–2211. [Google Scholar]
- Cvenkel, B.; Sustar, M.; Perovsek, D. Ganglion cell loss in early glaucoma, as assessed by photopic negative response, pattern electroretinogram, and spectral-domain optical coherence tomography. Doc. Ophthalmol. 2017, 135, 17–28. [Google Scholar] [CrossRef]
- Machida, S.; Gotoh, Y.; Tanaka, M.; Tazawa, Y. Predominant loss of the photopic negative response in central retinal artery occlusion. Am. J. Ophthalmol. 2004, 137, 938–940. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G.; Harwerth, R.S.; Smith, E.L., 3rd. The photopic negative response of the macaque electroretinogram: Reduction by experimental glaucoma. Investig. Ophthalmol. Vis. Sci. 1999, 40, 1124–1136. [Google Scholar]
- Kinoshita, J.; Takada, S.; Iwata, N.; Tani, Y. Comparison of photopic negative response (PhNR) between focal macular and full-field electroretinograms in monkeys. Doc. Ophthalmol. 2016, 132, 177–187. [Google Scholar] [CrossRef]
- Kim, H.D.; Park, J.Y.; Ohn, Y.H. Clinical applications of photopic negative response (PhNR) for the treatment of glaucoma and diabetic retinopathy. Korean J. Ophthalmol. 2010, 24, 89–95. [Google Scholar] [CrossRef] [PubMed]
- North, R.V.; Jones, A.L.; Drasdo, N.; Wild, J.M.; Morgan, J.E. Electrophysiological evidence of early functional damage in glaucoma and ocular hypertension. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1216–1222. [Google Scholar] [CrossRef]
- Prencipe, M.; Perossini, T.; Brancoli, G.; Perossini, M. The photopic negative response (PhNR): Measurement approaches and utility in glaucoma. Int. Ophthalmol. 2020, 40, 3565–3576. [Google Scholar]
- Kremers, J.; Jertila, M.; Link, B.; Pangeni, G.; Horn, F.K. Spectral characteristics of the PhNR in the full-field flash electroretinogram of normals and glaucoma patients. Doc. Ophthalmol. 2012, 124, 79–90. [Google Scholar] [CrossRef]
- Preiser, D.; Lagreze, W.A.; Bach, M.; Poloschek, C.M. Photopic negative response versus pattern electroretinogram in early glaucoma. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1182–1191. [Google Scholar] [CrossRef]
- Viswanathan, S.; Frishman, L.J.; Robson, J.G.; Walters, J.W. The photopic negative response of the flash electroretinogram in primary open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 514–522. [Google Scholar]
- Lee, M.W.; Nam, K.Y.; Park, H.J.; Lim, H.B.; Kim, J.Y. Longitudinal changes in the ganglion cell-inner plexiform layer thickness in high myopia: A prospective observational study. Br. J. Ophthalmol. 2020, 104, 604–609. [Google Scholar] [CrossRef]
- Seo, S.; Lee, C.E.; Jeong, J.H.; Park, K.H.; Kim, D.M.; Jeoung, J.W. Ganglion cell-inner plexiform layer and retinal nerve fiber layer thickness according to myopia and optic disc area: A quantitative and three-dimensional analysis. BMC Ophthalmol. 2017, 17, 22. [Google Scholar] [CrossRef]
- Swiatczak, B.; Feldkaemper, M.; Schraermeyer, U.; Schaeffel, F. Demyelination and shrinkage of axons in the retinal nerve fiber layer in chickens developing deprivation myopia. Exp. Eye Res. 2019, 188, 107783. [Google Scholar] [CrossRef]
- Alasil, T.; Wang, K.; Keane, P.A.; Lee, H.; Baniasadi, N.; de Boer, J.F.; Chen, T.C. Analysis of normal retinal nerve fiber layer thickness by age, sex, and race using spectral domain optical coherence tomography. J. Glaucoma 2013, 22, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Antwi-Boasiako, K.; Carter-Dawson, L.; Harwerth, R.; Gondo, M.; Patel, N. The Relationship Between Macula Retinal Ganglion Cell Density and Visual Function in the Nonhuman Primate. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Hu, F.; Wang, M.; Gao, F.; Xu, P.; Xing, C.; Sun, X.; Zhang, S.; Wu, J. Comparative analysis of retinal ganglion cell damage in three glaucomatous rat models. Exp. Eye Res. 2018, 172, 112–122. [Google Scholar] [CrossRef]
- Lee, S.; Han, S.X.; Young, M.; Beg, M.F.; Sarunic, M.V.; Mackenzie, P.J. Optic nerve head and peripapillary morphometrics in myopic glaucoma. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4378–4393. [Google Scholar] [CrossRef]
- Ho, W.C.; Kee, C.S.; Chan, H.H. Myopic children have central reduction in high contrast multifocal ERG response, while adults have paracentral reduction in low contrast response. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3695–3702. [Google Scholar] [CrossRef]
- Luu, C.D.; Lau, A.M.; Koh, A.H.; Tan, D. Multifocal electroretinogram in children on atropine treatment for myopia. Br. J. Ophthalmol. 2005, 89, 151–153. [Google Scholar] [CrossRef]
- Sachidanandam, R.; Ravi, P.; Sen, P. Effect of axial length on full-field and multifocal electroretinograms. Clin. Exp. Optom. 2017, 100, 668–675. [Google Scholar] [CrossRef]
- Chan, H.L.; Mohidin, N. Variation of multifocal electroretinogram with axial length. Ophthalmic Physiol. Opt. 2003, 23, 133–140. [Google Scholar] [CrossRef]
- Ablordeppey, R.K.; Nieu, R.; Lin, C.R.; Benavente-Perez, A. Early Alterations in Inner-Retina Neural and Glial Saturated Responses in Lens-Induced Myopia. Transl. Vis. Sci. Technol. 2024, 13, 16. [Google Scholar] [CrossRef]
- Benavente-Perez, A.; Nour, A.; Troilo, D. Axial eye growth and refractive error development can be modified by exposing the peripheral retina to relative myopic or hyperopic defocus. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6765–6773. [Google Scholar] [CrossRef]
- Benavente-Perez, A.; Nour, A.; Troilo, D. The effect of simultaneous negative and positive defocus on eye growth and development of refractive state in marmosets. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6479–6487. [Google Scholar] [CrossRef]
- Benavente-Perez, A.; Nour, A.; Troilo, D. Short Interruptions of Imposed Hyperopic Defocus Earlier in Treatment are More Effective at Preventing Myopia Development. Sci. Rep. 2019, 9, 11459. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Jacobson, R.; Trauger, S.A.; Friedlander, J.; Siuzdak, G.; Friedlander, M. Maintaining retinal astrocytes normalizes revascularization and prevents vascular pathology associated with oxygen-induced retinopathy. Glia 2010, 58, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Varela, H.J.; Hernandez, M.R. Astrocyte responses in human optic nerve head with primary open-angle glaucoma. J. Glaucoma 1997, 6, 303–313. [Google Scholar] [PubMed]
- Ogden, T.E. Nerve fiber layer astrocytes of the primate retina: Morphology, distribution, and density. Investig. Ophthalmol. Vis. Sci. 1978, 17, 499–510. [Google Scholar]
- Ghosh, K.K.; Goodchild, A.K.; Sefton, A.E.; Martin, P.R. Morphology of retinal ganglion cells in a new world monkey, the marmoset Callithrix jacchus. J. Comp. Neurol. 1996, 366, 76–92. [Google Scholar] [CrossRef]
- Perry, V.H.; Cowey, A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience 1984, 12, 1125–1137. [Google Scholar] [CrossRef]
- Schiller, P.H.; Malpeli, J.G. Properties and tectal projections of monkey retinal ganglion cells. J. Neurophysiol. 1977, 40, 428–445. [Google Scholar] [CrossRef]
- Weber, A.J.; Kaufman, P.L.; Hubbard, W.C. Morphology of single ganglion cells in the glaucomatous primate retina. Investig. Ophthalmol. Vis. Sci. 1998, 39, 2304–2320. [Google Scholar]
- Baden, T.; Berens, P.; Franke, K.; Roman Roson, M.; Bethge, M.; Euler, T. The functional diversity of retinal ganglion cells in the mouse. Nature 2016, 529, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.L.; Crish, S.D.; Inman, D.M.; Horner, P.J.; Calkins, D.J. Early astrocyte redistribution in the optic nerve precedes axonopathy in the DBA/2J mouse model of glaucoma. Exp. Eye Res. 2016, 150, 22–33. [Google Scholar] [CrossRef]
- Pang, I.H.; Clark, A.F. Inducible rodent models of glaucoma. Prog. Retin. Eye Res. 2020, 75, 100799. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Li, N.; He, S. Large-scale morphological survey of mouse retinal ganglion cells. J. Comp. Neurol. 2002, 451, 115–126. [Google Scholar] [CrossRef]
- Williams, R.W.; Strom, R.C.; Rice, D.S.; Goldowitz, D. Genetic and environmental control of variation in retinal ganglion cell number in mice. J. Neurosci. 1996, 16, 7193–7205. [Google Scholar] [CrossRef] [PubMed]
- Bouhenni, R.A.; Dunmire, J.; Sewell, A.; Edward, D.P. Animal models of glaucoma. J. Biomed. Biotechnol. 2012, 2012, 692609. [Google Scholar] [CrossRef]
- Enroth-Cugell, C.; Goldstick, T.K.; Linsenmeier, R.A. The contrast sensitivity of cat retinal ganglion cells at reduced oxygen tensions. J. Physiol. 1980, 304, 59–81. [Google Scholar] [CrossRef]
- Rodieck, R.W.; Stone, J. Analysis of receptive fields of cat retinal ganglion cells. J. Neurophysiol. 1965, 28, 833–849. [Google Scholar] [CrossRef]
- Stone, J.; Fukuda, Y. Properties of cat retinal ganglion cells: A comparison of W-cells with X- and Y-cells. J. Neurophysiol. 1974, 37, 722–748. [Google Scholar] [CrossRef]
- Curcio, C.A.; Allen, K.A. Topography of ganglion cells in human retina. J. Comp. Neurol. 1990, 300, 5–25. [Google Scholar] [CrossRef]
- Reinhard, K.; Munch, T.A. Visual properties of human retinal ganglion cells. PLoS ONE 2021, 16, e0246952. [Google Scholar] [CrossRef] [PubMed]
- Mead, B.; Tomarev, S. Evaluating retinal ganglion cell loss and dysfunction. Exp. Eye Res. 2016, 151, 96–106. [Google Scholar] [CrossRef]
- Gollisch, T. Throwing a glance at the neural code: Rapid information transmission in the visual system. HFSP J. 2009, 3, 36–46. [Google Scholar] [CrossRef]
- Wassle, H.; Boycott, B.B. Functional architecture of the mammalian retina. Physiol. Rev. 1991, 71, 447–480. [Google Scholar] [CrossRef]
- Dacey, D.M. Primate retina: Cell types, circuits and color opponency. Prog. Retin. Eye Res. 1999, 18, 737–763. [Google Scholar] [CrossRef] [PubMed]
- Amthor, F.R.; Oyster, C.W.; Takahashi, E.S. Morphology of on-off direction-selective ganglion cells in the rabbit retina. Brain Res. 1984, 298, 187–190. [Google Scholar] [CrossRef]
- Amthor, F.R.; Takahashi, E.S.; Oyster, C.W. Morphologies of rabbit retinal ganglion cells with concentric receptive fields. J. Comp. Neurol. 1989, 280, 72–96. [Google Scholar] [CrossRef] [PubMed]
- Amthor, F.R.; Takahashi, E.S.; Oyster, C.W. Morphologies of rabbit retinal ganglion cells with complex receptive fields. J. Comp. Neurol. 1989, 280, 97–121. [Google Scholar] [CrossRef]
- Saito, H.A. Morphology of physiologically identified X-, Y-, and W-type retinal ganglion cells of the cat. J. Comp. Neurol. 1983, 221, 279–288. [Google Scholar] [CrossRef]
- Stanford, L.R.; Sherman, S.M. Structure/function relationships of retinal ganglion cells in the cat. Brain Res. 1984, 297, 381–386. [Google Scholar] [CrossRef]
- Jiang, S.M.; Zeng, L.P.; Zeng, J.H.; Tang, L.; Chen, X.M.; Wei, X. beta-III-Tubulin: A reliable marker for retinal ganglion cell labeling in experimental models of glaucoma. Int. J. Ophthalmol. 2015, 8, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Jimenez-Lopez, M.; Sobrado-Calvo, P.; Nieto-Lopez, L.; Canovas-Martinez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naive and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef]
- Kwong, J.M.; Caprioli, J.; Piri, N. RNA binding protein with multiple splicing: A new marker for retinal ganglion cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1052–1058. [Google Scholar] [CrossRef]
- Corral-Domenge, C.; de la Villa, P.; Mansilla, A.; Germain, F. Tools and Biomarkers for the Study of Retinal Ganglion Cell Degeneration. Int. J. Mol. Sci. 2022, 23, 4287. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Galindo-Romero, C.; Lucas-Ruiz, F.; Marsh-Amstrong, N.; Li, W.; Vidal-Sanz, M.; Agudo-Barriuso, M. Pan-retinal ganglion cell markers in mice, rats, and rhesus macaques. Zool. Res. 2023, 44, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Benavente-Perez, A.; Ablordeppey, R.; Lin, C.; Viswanathan, S.; Akopian, A.; Bloomfield, S.A. A Robust Microbead Occlusion Model of Glaucoma for the Common Marmoset. Transl. Vis. Sci. Technol. 2022, 11, 14. [Google Scholar] [CrossRef]
- Coudrillier, B.; Tian, J.; Alexander, S.; Myers, K.M.; Quigley, H.A.; Nguyen, T.D. Biomechanics of the human posterior sclera: Age- and glaucoma-related changes measured using inflation testing. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1714–1728. [Google Scholar] [CrossRef]
- Grytz, R.; Girkin, C.A.; Libertiaux, V.; Downs, J.C. Perspectives on biomechanical growth and remodeling mechanisms in glaucoma. Mech. Res. Commun. 2012, 42, 92–106. [Google Scholar] [CrossRef]
- Norman, R.E.; Flanagan, J.G.; Sigal, I.A.; Rausch, S.M.; Tertinegg, I.; Ethier, C.R. Finite element modeling of the human sclera: Influence on optic nerve head biomechanics and connections with glaucoma. Exp. Eye Res. 2011, 93, 4–12. [Google Scholar] [CrossRef]
- Jonas, J.B.; Berenshtein, E.; Holbach, L. Lamina cribrosa thickness and spatial relationships between intraocular space and cerebrospinal fluid space in highly myopic eyes. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2660–2665. [Google Scholar] [CrossRef]
- Wang, Y.X.; Panda-Jonas, S.; Jonas, J.B. Optic nerve head anatomy in myopia and glaucoma, including parapapillary zones alpha, beta, gamma and delta: Histology and clinical features. Prog. Retin. Eye Res. 2021, 83, 100933. [Google Scholar] [CrossRef] [PubMed]
- McMonnies, C.W. Glaucoma history and risk factors. J. Optom. 2017, 10, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, S.; Kuo, A.N.; Saw, S.M. An Update of Eye Shape and Myopia. Eye Contact Lens 2019, 45, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Nofziger, J.; Datta, R.; Gee, J.C.; Morgan, J.; Aguirre, G.K. The Influence of Axial Length Upon the Retinal Ganglion Cell Layer of the Human Eye. Transl. Vis. Sci. Technol. 2020, 9, 9. [Google Scholar] [CrossRef]
- Troilo, D.; Xiong, M.; Crowley, J.C.; Finlay, B.L. Factors controlling the dendritic arborization of retinal ganglion cells. Vis. Neurosci. 1996, 13, 721–733. [Google Scholar] [CrossRef]
- Herculano-Houzel, S. Brains matter, bodies maybe not: The case for examining neuron numbers irrespective of body size. Ann. N. Y. Acad. Sci. 2011, 1225, 191–199. [Google Scholar] [CrossRef]
- Tower, D.B. Structural and functional organization of mammalian cerebral cortex: The correlation of neurone density with brain size. Cortical neurone density in the fin whale (Balaenoptera physalus L.) with a note on the cortical neurone density in the Indian elephant. J. Comp. Neurol. 1954, 101, 19–51. [Google Scholar] [CrossRef] [PubMed]
- Almasieh, M.; Levin, L.A. Neuroprotection in Glaucoma: Animal Models and Clinical Trials. Annu. Rev. Vis. Sci. 2017, 3, 91–120. [Google Scholar] [CrossRef]
- Farkas, R.H.; Grosskreutz, C.L. Apoptosis, neuroprotection, and retinal ganglion cell death: An overview. Int. Ophthalmol. Clin. 2001, 41, 111–130. [Google Scholar] [CrossRef]
- Garcia-Valenzuela, E.; Gorczyca, W.; Darzynkiewicz, Z.; Sharma, S.C. Apoptosis in adult retinal ganglion cells after axotomy. J. Neurobiol. 1994, 25, 431–438. [Google Scholar] [CrossRef]
- Kuehn, M.H.; Fingert, J.H.; Kwon, Y.H. Retinal ganglion cell death in glaucoma: Mechanisms and neuroprotective strategies. Ophthalmol. Clin. N. Am. 2005, 18, 383–395. [Google Scholar] [CrossRef]
- Morgan, J.E.; Uchida, H.; Caprioli, J. Retinal ganglion cell death in experimental glaucoma. Br. J. Ophthalmol. 2000, 84, 303–310. [Google Scholar] [CrossRef]
- Sigal, I.A.; Ethier, C.R. Biomechanics of the optic nerve head. Exp. Eye Res. 2009, 88, 799–807. [Google Scholar] [CrossRef]
- Ingber, D.E. Mechanobiology and diseases of mechanotransduction. Ann. Med. 2003, 35, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.C.; Kalapesi, F.B.; Coroneo, M.T. Mechanosensitivity and the eye: Cells coping with the pressure. Br. J. Ophthalmol. 2006, 90, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Harwerth, R.S.; Wheat, J.L. Modeling the effects of aging on retinal ganglion cell density and nerve fiber layer thickness. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Harwerth, R.S.; Smith, E.L., 3rd; DeSantis, L. Experimental glaucoma: Perimetric field defects and intraocular pressure. J. Glaucoma 1997, 6, 390–401. [Google Scholar]
- Danias, J.; Lee, K.C.; Zamora, M.F.; Chen, B.; Shen, F.; Filippopoulos, T.; Su, Y.; Goldblum, D.; Podos, S.M.; Mittag, T. Quantitative analysis of retinal ganglion cell (RGC) loss in aging DBA/2NNia glaucomatous mice: Comparison with RGC loss in aging C57/BL6 mice. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5151–5162. [Google Scholar] [CrossRef]
- Samuel, M.A.; Zhang, Y.; Meister, M.; Sanes, J.R. Age-related alterations in neurons of the mouse retina. J. Neurosci. 2011, 31, 16033–16044. [Google Scholar] [CrossRef]
- Kim, C.B.; Tom, B.W.; Spear, P.D. Effects of aging on the densities, numbers, and sizes of retinal ganglion cells in rhesus monkey. Neurobiol. Aging 1996, 17, 431–438. [Google Scholar] [CrossRef]
- Harman, A.M.; Moore, S. Number of neurons in the retinal ganglion cell layer of the quokka wallaby do not change throughout life. Anat. Rec. 1999, 256, 78–83. [Google Scholar] [CrossRef]
- Peters, A.; Josephson, K.; Vincent, S.L. Effects of aging on the neuroglial cells and pericytes within area 17 of the rhesus monkey cerebral cortex. Anat. Rec. 1991, 229, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Leahu, D.; Moss, M.B.; McNally, K.J. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb. Cortex 1994, 4, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Rosene, D.L.; Moss, M.B.; Kemper, T.L.; Abraham, C.R.; Tigges, J.; Albert, M.S. Neurobiological bases of age-related cognitive decline in the rhesus monkey. J. Neuropathol. Exp. Neurol. 1996, 55, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.; Sethares, C. Aging and the Meynert cells in rhesus monkey primary visual cortex. Anat. Rec. 1993, 236, 721–729. [Google Scholar] [CrossRef]
- Ramirez, A.I.; Fernandez-Albarral, J.A.; Hoz, R.; Lopez-Cuenca, I.; Salobrar-Garcia, E.; Rojas, P.; Valiente-Soriano, F.J.; Aviles-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; et al. Microglial changes in the early aging stage in a healthy retina and an experimental glaucoma model. Prog. Brain Res. 2020, 256, 125–149. [Google Scholar] [CrossRef]
- Ramirez, J.M.; Ramirez, A.I.; Salazar, J.J.; de Hoz, R.; Trivino, A. Changes of astrocytes in retinal ageing and age-related macular degeneration. Exp. Eye Res. 2001, 73, 601–615. [Google Scholar] [CrossRef]
- Harwerth, R.S.; Wheat, J.L.; Rangaswamy, N.V. Age-related losses of retinal ganglion cells and axons. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4437–4443. [Google Scholar] [CrossRef]
- Drasdo, N.; Aldebasi, Y.H.; Chiti, Z.; Mortlock, K.E.; Morgan, J.E.; North, R.V. The s-cone PHNR and pattern ERG in primary open angle glaucoma. Investig. Ophthalmol. Vis. Sci. 2001, 42, 1266–1272. [Google Scholar]
- Machida, S.; Gotoh, Y.; Toba, Y.; Ohtaki, A.; Kaneko, M.; Kurosaka, D. Correlation between photopic negative response and retinal nerve fiber layer thickness and optic disc topography in glaucomatous eyes. Investig. Ophthalmol. Vis. Sci. 2008, 49, 2201–2207. [Google Scholar] [CrossRef]
- Uchida, A. [Studies of electrical activities of the eye in high myopia (author’s transl)]. Nippon. Ganka Gakkai Zasshi 1977, 81, 1328–1350. [Google Scholar] [PubMed]
- Chen, J.C.; Brown, B.; Schmid, K.L. Delayed mfERG responses in myopia. Vis. Res. 2006, 46, 1221–1229. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.C.; Brown, B.; Schmid, K.L. Retinal adaptation responses revealed by global flash multifocal electroretinogram are dependent on the degree of myopic refractive error. Vis. Res. 2006, 46, 3413–3421. [Google Scholar] [CrossRef] [PubMed]
- Chui, T.Y.; Yap, M.K.; Chan, H.H.; Thibos, L.N. Retinal stretching limits peripheral visual acuity in myopia. Vis. Res. 2005, 45, 593–605. [Google Scholar] [CrossRef]
- Ablordeppey, R.K.; Lin, C.R.; Song, B.; Benavente-Perez, A. Choroidal Morphology and Photoreceptor Activity Are Related and Affected by Myopia Development. Investig. Ophthalmol. Vis. Sci. 2024, 65, 3. [Google Scholar] [CrossRef]
- Severns, M.L.; Johnson, M.A. The care and fitting of Naka-Rushton functions to electroretinographic intensity-response data. Doc. Ophthalmol. 1993, 85, 135–150. [Google Scholar] [CrossRef]
| 6 m Control ID, Eye | Eye Length (mm) | Refraction (D) | Gender | Age (Days) | 6 m Myope ID, Eye | Eye Length (mm) | Refraction (D) | Gender | Age (Days) |
|---|---|---|---|---|---|---|---|---|---|
| U19, Right | 9.83 | −1.73 | Male | 249 | U17, Right | 10.24 | −3.59 | Male | 220 |
| U19, Left | 9.79 | −2.24 | Male | 249 | E19, Right | 10.86 | −4.29 | Male | 221 |
| Z17, Left | 9.81 | −0.41 | Male | 214 | E19, Left | 10.80 | −3.64 | Male | 221 |
| AVG ± SD | 9.81 ± 0.0 | −1.46 ± 0.9 | 237.33 ± 20.2 | AVG ± SD | 10.63 ± 0.3 | −3.84 ± 0.4 | 220.67 ± 0.6 | ||
| p < 0.05 | p < 0.05 | p > 0.05 | |||||||
| 12 m Control ID, Eye | Eye Length (mm) | Refraction (D) | Gender | Age (Days) | 12 m Myope ID, Eye | Eye Length (mm) | Refraction (D) | Gender | Age (Days) |
| Trike, Right | 10.22 | −1.12 | Female | 382 | F20, Left | 11.12 | −7.86 | Male | 396 |
| R19, Left | 10.22 | −1.04 | Male | 381 | G20, Left | 11.33 | −5.715 | Female | 396 |
| P16, Right | 11.21 | +1.22 | Female | 396 | H20, Left | 11.05 | −11.70 | Female | 420 |
| AVG ± SD | 10.56 ± 0.5 | −0.31 ± 1.3 | 386.33 ± 8.4 | AVG ± SD | 11.17 ± 0.1 | −8.42 ± 3.0 | 404 ± 13.9 | ||
| p < 0.001 | p < 0.001 | p > 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.R.; Ablordeppey, R.K.; Benavente-Perez, A. Sustained Experimental Myopia Exacerbates the Effect of Eye Growth on Retinal Ganglion Cell Density and Function. Int. J. Mol. Sci. 2025, 26, 2824. https://doi.org/10.3390/ijms26062824
Lin CR, Ablordeppey RK, Benavente-Perez A. Sustained Experimental Myopia Exacerbates the Effect of Eye Growth on Retinal Ganglion Cell Density and Function. International Journal of Molecular Sciences. 2025; 26(6):2824. https://doi.org/10.3390/ijms26062824
Chicago/Turabian StyleLin, Carol Ren, Reynolds Kwame Ablordeppey, and Alexandra Benavente-Perez. 2025. "Sustained Experimental Myopia Exacerbates the Effect of Eye Growth on Retinal Ganglion Cell Density and Function" International Journal of Molecular Sciences 26, no. 6: 2824. https://doi.org/10.3390/ijms26062824
APA StyleLin, C. R., Ablordeppey, R. K., & Benavente-Perez, A. (2025). Sustained Experimental Myopia Exacerbates the Effect of Eye Growth on Retinal Ganglion Cell Density and Function. International Journal of Molecular Sciences, 26(6), 2824. https://doi.org/10.3390/ijms26062824







