Functional Characterization of miR-216a-5p and miR-125a-5p on Pancreatic Cancer Stem Cells
Abstract
1. Introduction
2. Results
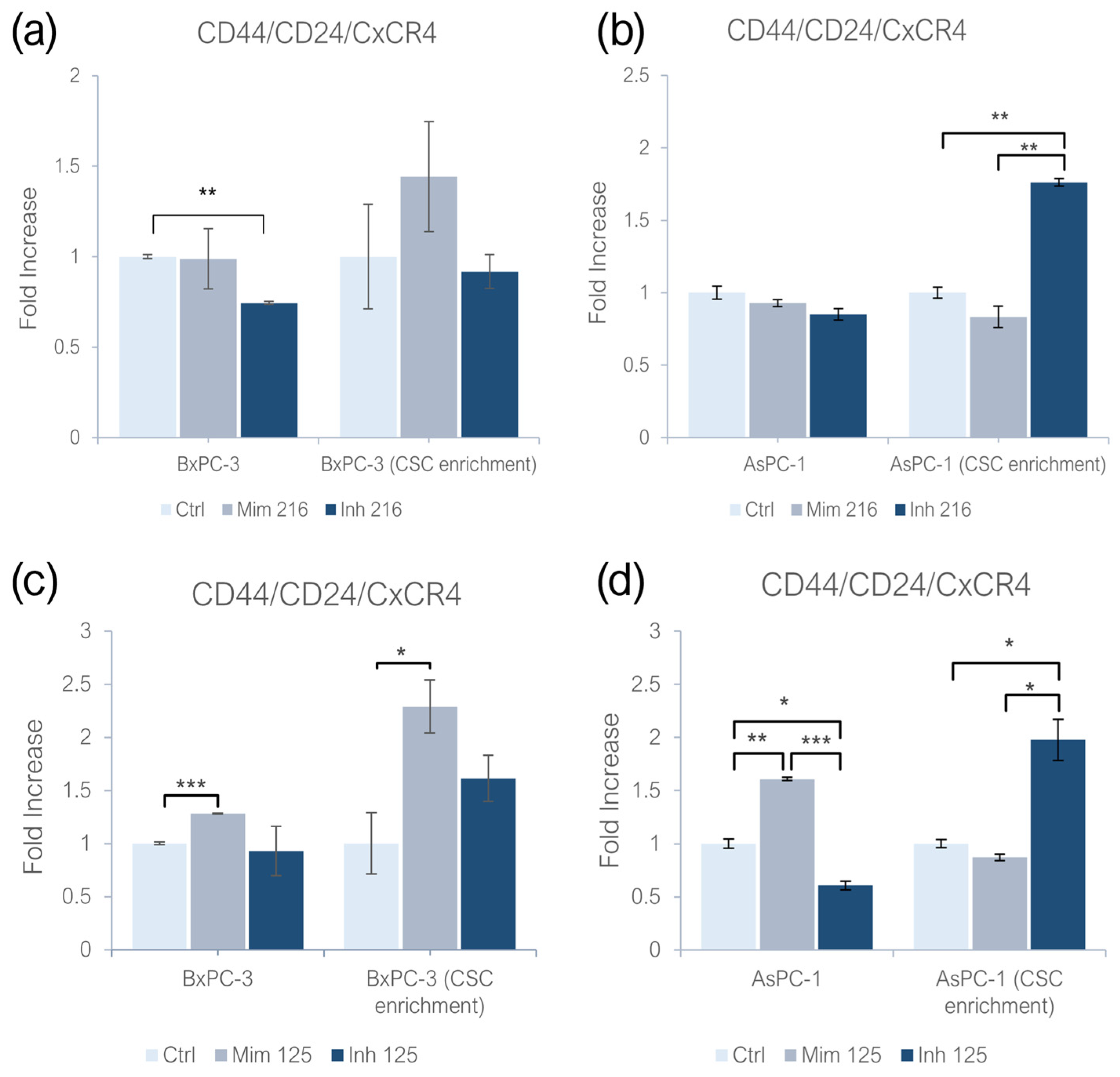
2.1. miR-216a-5p and miR-125a-5p Effect on CD44/CD24/CxCR4 Expression
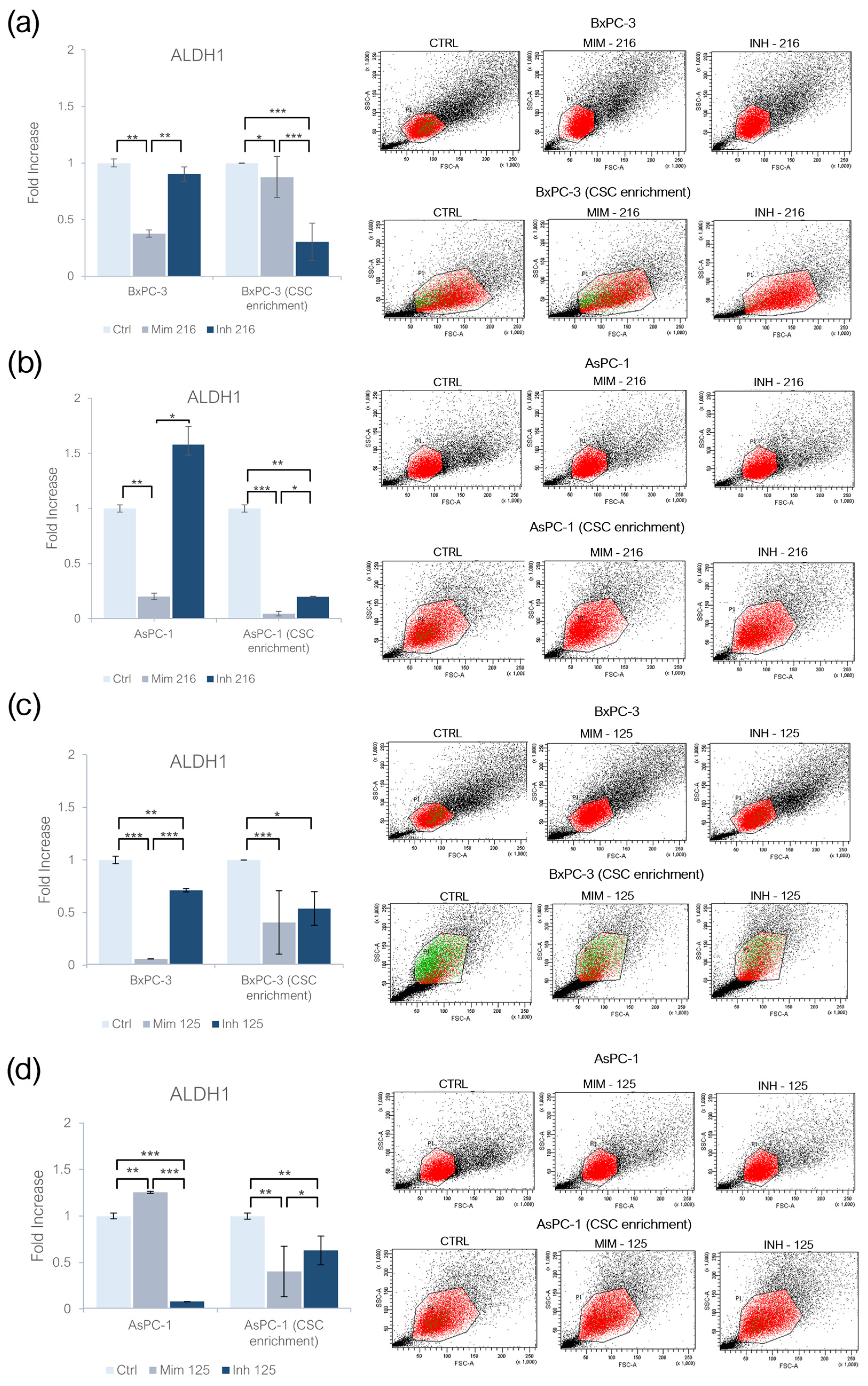
2.2. miR-216a-5p and miR-125a-5p Effect on ALDH1 Activity
2.3. miR-216a-5p Influence on Pluripotency-Related Genes
2.4. miR-125a-5p Influence on Pluripotency-Related Genes
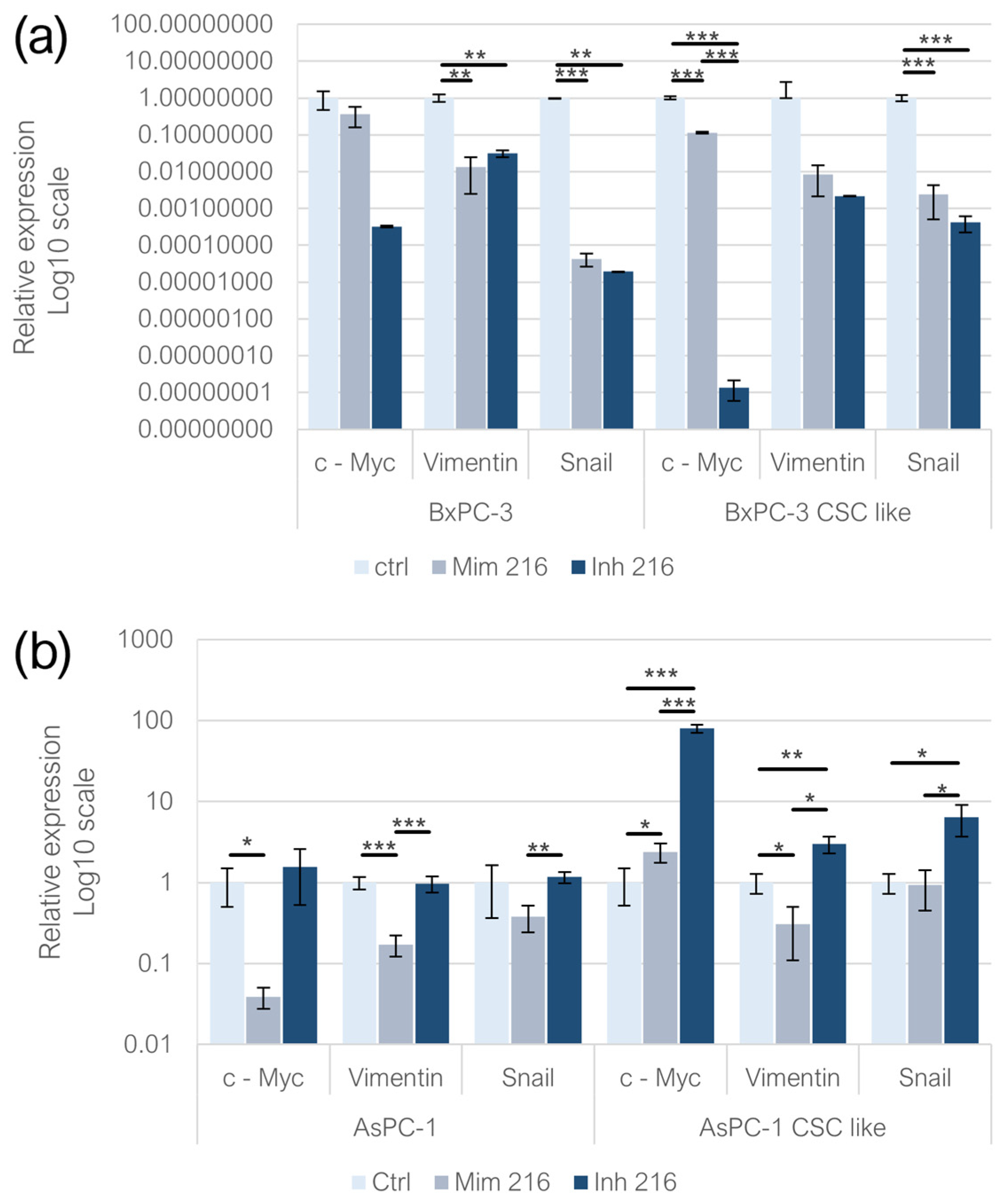
2.5. Effect of miR-216a-5p on EMT-Related Genes
2.6. Effect of miR-125a-5p on EMT-Related Genes
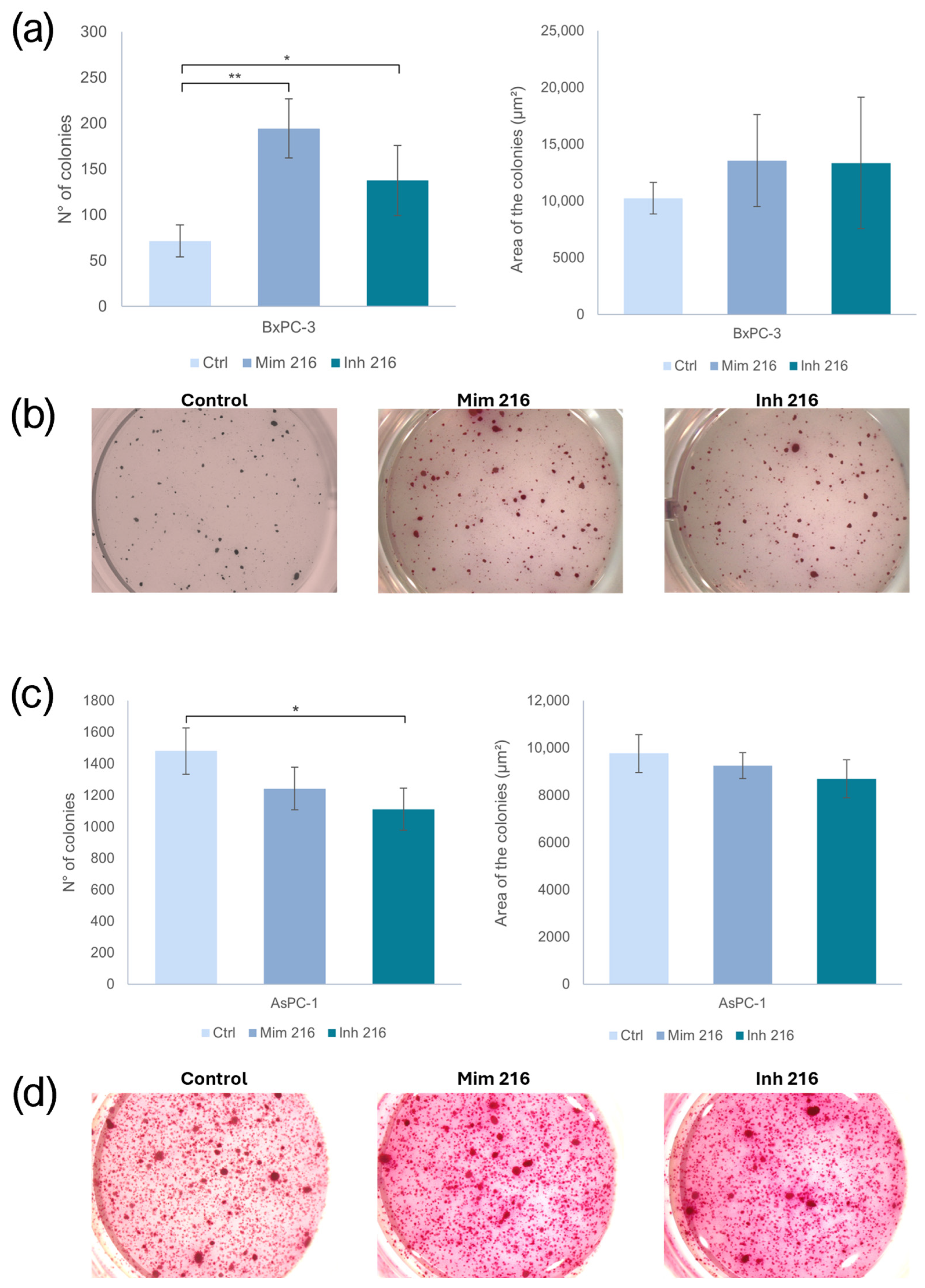
2.7. The Role of miR-216a-5p in Clonogenic Activity
2.8. The Role of miR-125a-5p in Clonogenic Activity
3. Discussion
4. Materials and Methods
4.1. Experimental Design
4.2. Cell Lines
4.3. CSC Enrichment
4.4. Transient Transfection with miR-216a-5p and miR-125a-5p
4.5. Cytofluorimetric Assays for PaCSC Markers
4.6. RNA Extraction and Retrotranscription
4.7. Real-Time PCR
4.8. Soft-Agar Colony Formation Assay
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| miRNA | microRNA |
| CSC | Cancer Stem Cell |
| PDAC | Pancreatic Ductal Adenocarcinoma |
| PaCSCs | Pancreatic Cancer Stem Cells |
| EMT | Epithelial–Mesenchymal Transition |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics. 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [PubMed]
- Wood, L.D.; Canto, M.I.; Jaffee, E.M.; Simeone, D.M. Pancreatic Cancer: Pathogenesis, Screening, Diagnosis, and Treatment. Gastroenterology 2022, 163, 386–402.e1. [Google Scholar] [CrossRef]
- Ishiwata, T.; Matsuda, Y.; Yoshimura, H.; Sasaki, N.; Ishiwata, S.; Ishikawa, N.; Takubo, K.; Arai, T.; Aida, J. Pancreatic cancer stem cells: Features and detection methods. Pathol. Oncol. Res. 2018, 24, 797–805. [Google Scholar] [CrossRef] [PubMed]
- Ansari, D.; Gustafsson, A.; Andersson, R. Update on the management of pancreatic cancer: Surgery is not enough. World J. Gastroenterol. 2015, 21, 3157–3165. [Google Scholar] [CrossRef]
- Huang, J.; Lok, V.; Ngai, C.H.; Zhang, L.; Yuan, J.; Lao, X.Q.; Ng, K.; Chong, C.; Zheng, Z.-J.; Wong, M.C. Worldwide Burden of, Risk Factors for, and Trends in Pancreatic Cancer. Gastroenterology 2021, 160, 744–754. [Google Scholar] [CrossRef]
- Klein, A.P. Pancreatic cancer epidemiology: Understanding the role of lifestyle and inherited risk factors. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef]
- Ho, P.T.B.; Clark, I.M.; Le, L.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Rawat, M.; Kadian, K.; Gupta, Y.; Kumar, A.; Chain, P.S.; Kovbasnjuk, O.; Kumar, S.; Parasher, G. MicroRNA in Pancreatic Cancer: From Biology to Therapeutic Potential. Genes 2019, 10, 752. [Google Scholar] [CrossRef]
- Bartel, D.P. Review MicroRNAs: Genomics, Biogenesis, Mechanism, and Function. Cell 2004, 116, 281–297. [Google Scholar]
- Daoud, A.Z.; Mulholland, E.J.; Cole, G.; McCarthy, H.O. MicroRNAs in Pancreatic Cancer: Biomarkers, prognostic, and therapeutic modulators. BMC Cancer 2019, 19, 1130. [Google Scholar] [CrossRef]
- Ercan, G.; Karlitepe, A.; Ozpolat, B. Pancreatic Cancer Stem Cells and Therapeutic Approaches. Anticancer Res. 2017, 37, 2761–2775. [Google Scholar] [CrossRef]
- Woodward, W.A.; Hill, R.P. Cancer Stem Cells. Mol. Radio-Oncol. 2016, 198, 25–44. [Google Scholar]
- Nagaraju, G.P.; Farran, B.; Luong, T.; El-Rayes, B.F. Understanding the molecular mechanisms that regulate pancreatic cancer stem cell formation, stemness and chemoresistance: A brief overview. Semin. Cancer Biol. 2022, 88, 67–80. [Google Scholar] [CrossRef]
- Mortoglou, M.; Miralles, F.; Arisan, E.D.; Dart, A.; Jurcevic, S.; Lange, S.; Uysal-Onganer, P. microRNA-21 Regulates Stemness in Pancreatic Ductal Adenocarcinoma Cells. Int. J. Mol. Sci. 2022, 23, 1275. [Google Scholar] [CrossRef]
- Rao, C.V.; Mohammed, A. New insights into pancreatic cancer stem cells. World J. Stem Cells 2015, 7, 547–555. [Google Scholar] [CrossRef]
- Bubin, R.; Uljanovs, R.; Strumfa, I. Cancer Stem Cells in Pancreatic Ductal Adenocarcinoma. Int. J. Mol. Sci. 2023, 24, 7030. [Google Scholar] [CrossRef]
- Gzil, A.; Zarębska, I.; Bursiewicz, W.; Antosik, P.; Grzanka, D.; Szylberg, Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol. Biol. Rep. 2019, 46, 6629–6645. [Google Scholar] [CrossRef]
- Patil, K.; Khan, F.B.; Akhtar, S.; Ahmad, A.; Uddin, S. The plasticity of pancreatic cancer stem cells: Implications in therapeutic resistance. Cancer Metastasis Rev. 2021, 40, 691–720. [Google Scholar] [CrossRef]
- Barman, S.; Fatima, I.; Singh, A.B.; Dhawan, P. Pancreatic Cancer and Therapy: Role and Regulation of Cancer Stem Cells. Int. J. Mol. Sci. 2021, 22, 4765. [Google Scholar] [CrossRef]
- Zhan, H.; Xu, J.; Wu, D.; Zhang, T.P.; Hu, S.Y. Pancreatic Cancer Stem Cells: New Insight into a Stubborn Disease. Cancer Lett. 2015, 357, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Fenu, G.; Griñán-Lisón, C.; Pisano, A.; González-Titos, A.; Farace, C.; Fiorito, G.; Etzi, F.; Perra, T.; Sabalic, A.; Toledo, B.; et al. Unveiling the microRNA landscape in pancreatic ductal adenocarcinoma patients and cancer cell models. BMC Cancer 2024, 24, 1308. [Google Scholar] [CrossRef] [PubMed]
- Yonemori, K.; Seki, N.; Idichi, T.; Kurahara, H.; Osako, Y.; Koshizuka, K.; Arai, T.; Okato, A.; Kita, Y.; Arigami, T.; et al. The microRNA expression signature of pancreatic ductal adenocarcinoma by RNA sequencing: Anti-tumour functions of the microRNA-216 cluster. Oncotarget 2017, 8, 70097–70115. [Google Scholar] [CrossRef]
- Felix, T.F.; Lapa, R.M.L.; de Carvalho, M.; Bertoni, N.; Tokar, T.; Oliveira, R.A.; Rodrigues, M.A.M.; Hasimoto, C.N.; Oliveira, W.K.; Pelafsky, L.; et al. MicroRNA modulated networks of adaptive and innate immune response in pancreatic ductal adenocarcinoma. PLoS ONE 2019, 14, e0217421. [Google Scholar] [CrossRef]
- Guz, M.; Jeleniewicz, W.; Cybulski, M.; Kozicka, J.; Kurzepa, J.; Mądro, A. Serum miR-210-3p can be used to differentiate between patients with pancreatic ductal adenocarcinoma and chronic pancreatitis. Biomed. Rep. 2020, 14, 10. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, S.; Zhang, Y.; Yi, H.; Xu, M.; Xu, J.; Liu, H.; Ding, Z.; He, H.; Wang, H.; et al. Mir-216a-5p Inhibits Tumorigenesis in Pancreatic Cancer by Targeting Tpt1/Mtorc1 and Is Mediated by Linc01133. Int. J. Biol. Sci. 2020, 16, 2612–2627. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, E.; Rezaie, E.; Heiat, M.; Sefidi-Heris, Y. An Integrated Data Analysis of mRNA, miRNA and Signaling Pathways in Pancreatic Cancer. Biochem. Genet. 2021, 59, 1326–1358. [Google Scholar] [CrossRef]
- Erdos, Z.; E Barnum, J.; Wang, E.; DeMaula, C.; Dey, P.M.; Forest, T.; Bailey, W.J.; E Glaab, W. Evaluation of the Relative Performance of Pancreas-Specific MicroRNAs in Rat Plasma as Biomarkers of Pancreas Injury. Toxicol. Sci. 2020, 173, 5–18. [Google Scholar] [CrossRef]
- Kurashige, S.; Matsutani, N.; Aoki, T.; Kodama, T.; Otagiri, Y.; Togashi, Y. Evaluation of circulating miR-216a and miR-217 as biomarkers of pancreatic damage in the L-arginine-induced acute pancreatitis mouse model. J. Toxicol. Sci. 2023, 48, 527–534. [Google Scholar] [CrossRef]
- Bellone, G.; Smirne, C.; Mauri, F.A.; Tonel, E.; Carbone, A.; Buffolino, A.; Dughera, L.; Robecchi, A.; Pirisi, M.; Emanuelli, G. Cytokine expression profile in human pancreatic carcinoma cells and in surgical specimens: Implications for survival. Cancer Immunol. Immunother. 2005, 55, 684–698. [Google Scholar] [CrossRef]
- Mitsunaga, S.; Ikeda, M.; Shimizu, S.; Ohno, I.; Furuse, J.; Inagaki, M.; Higashi, S.; Kato, H.; Terao, K.; Ochiai, A. Serum levels of IL-6 and IL-1β can predict the efficacy of gemcitabine in patients with advanced pancreatic cancer. Br. J. Cancer 2013, 108, 2063–2069. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, Y.; Chen, X.; Ge, P.; Wang, P.; Wu, B. Upregulation of miR-216a-5p by Lentinan Targeted Inhibition of JAK2/STAT3 Signaling Pathway to Reduce Lung Adenocarcinoma Cell Stemness, Promote Apoptosis, and Slow Down the Lung Adenocarcinoma Mechanisms. Front. Oncol. 2021, 11, 778096. [Google Scholar] [CrossRef]
- Shishavan, N.N.; Sasani, S.T.; Salehi, Z.; Azhang, M.R. Downregulation of miR-125a-5p Leads to STAT3 Increased Expression in Breast Cancer Patients. MicroRNA 2022, 11, 263–270. [Google Scholar]
- Babaei, G.; Aziz, G.G.S.; Jaghi, N.Z.Z. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother. 2021, 133, 110909. [Google Scholar] [CrossRef]
- Das, S.; Khan, T.H.; Sarkar, D. Comprehensive Review on the Effect of Stem Cells in Cancer Progression. Curr. Tiss. Microenv. Rep. 2024, 5, 39–59. [Google Scholar] [CrossRef]
- Lei, Z.N.; Teng, Q.-X.; Koya, J.; Liu, Y.; Chen, Z.; Zeng, L.; Chen, Z.-S.; Fang, S.; Wang, J.; Liu, Y.; et al. The correlation between cancer stem cells and epithelial-mesenchymal transition: Molecular mechanisms and significance in cancer theragnosis. Front. Immunol. 2024, 15, 1417201. [Google Scholar] [CrossRef]
- Zeng, X.; Liu, Y.; Zhu, H.; Chen, D.; Hu, W. Downregulation of miR-216a-5p by long noncoding RNA PVT1 suppresses colorectal cancer progression via modulation of YBX1 expression. Cancer Manag. Res. 2019, 11, 6981–6993. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, A.; Tashiro, K.; Dixit, A.; Soni, A.; Vogel, K.; Hall, B.; Shafqat, I.; Slaughter, J.; Param, N.; Le, A.; et al. Loss of HIF1A From Pancreatic Cancer Cells Increases Expression of PPP1R1B and Degradation of p53 to Promote Invasion and Metastasis. Gastroenterology 2020, 159, 1882–1897.e5. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, N.; Ergün, S.; Isenovic, E.R. Levels of MicroRNA Heterogeneity in Cancer Biology. Mol. Diagn. Ther. 2017, 21, 511–523. [Google Scholar] [CrossRef]
- Xiang, J.; Wu, J. Feud or Friend? The Role of the MiR-17-92 Cluster in Tumorigenesis. Curr. Genom. 2010, 11, 129–135. [Google Scholar]
- Li, Y.; Vecchiarelli-Federico, L.M.; Li, Y.J.; Egan, S.E.; Spaner, D.; Hough, M.R.; Ben-David, Y. The MiR-17-92 Cluster Expands Multipotent Hematopoietic Progenitors Whereas Imbalanced Expression of Its Individual Oncogenic MiRNAs Promotes Leukemia in Mice. Blood 2012, 119, 4486–4498. [Google Scholar] [CrossRef]
- Zekri, A.-R.N.; El-Sisi, E.R.; Youssef, A.S.E.-D.; Kamel, M.M.; Nassar, A.; Ahmed, O.S.; El Kassas, M.; Barakat, A.B.; El-Motaleb, A.I.A.; Bahnassy, A.A. MicroRNA Signatures for circulating CD133-positive cells in hepatocellular carcinoma with HCV infection. PLoS ONE 2018, 13, e0193709. [Google Scholar] [CrossRef]
- Guo, S.; Lu, J.; Schlanger, R.; Zhang, H.; Wang, J.Y.; Fox, M.C.; Purton, L.E.; Fleming, H.H.; Cobb, B.; Merkenschlager, M.; et al. MicroRNA miR-125a controls hematopoietic stem cell number. Proc. Natl. Acad. Sci. USA 2010, 107, 14229–14234. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.I. MicroRNA Control of TGF-β Signaling. Int. J. Mol. Sci. 2018, 19, 1901. [Google Scholar] [CrossRef]
- Shen, K.; Cao, Z.; Zhu, R.; You, L.; Zhang, T. The Dual Functional Role of MicroRNA-18a (MiR-18a) in Cancer Development. Clin. Transl. Med. 2019, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Zheng, X.M.; Liang, W.M.; Jiang, C.M.; Su, D.M.; Fu, B. Long Noncoding RNA MIR600HG Binds to MicroRNA-125a-5p to Prevent Pancreatic Cancer Progression Via Mitochondrial Tumor Suppressor 1–Dependent Suppression of Extracellular Regulated Protein Kinases Signaling Pathway. Pancreas 2022, 51, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wei, J.; He, D.; He, J.; Liu, L.; Hou, H.; Shi, H.; Jin, S.; Li, J.; Shi, X.; et al. The MiRNA125a-5p and MiRNA125b-1-5p Cluster Induces Cell Invasion by down-Regulating DDB2-Reduced Epithelial-to-Mesenchymal Transition (EMT) in Colorectal Cancer. J. Gastrointest. Oncol. 2022, 13, 3112–3122. [Google Scholar] [CrossRef]
- Pan, G.; Liu, Y.; Shang, L.; Zhou, F.; Yang, S. EMT-associated microRNAs and their roles in cancer stemness and drug resistance. Cancer Commun. 2021, 41, 199–217. [Google Scholar] [CrossRef]
- Zha, Z.; Li, J. MicroRNA-125a-5p Regulates Liver Cancer Cell Growth, Migration and Invasion and EMT by Targeting HAX1. Int. J. Mol. Med. 2020, 46, 1849–1861. [Google Scholar] [CrossRef]
- Debnath, P.; Huirem, R.S.; Dutta, P.; Palchaudhuri, S. Epithelial–mesenchymal transition and its transcription factors. Biosci. Rep. 2021, 42, BSR20211754. [Google Scholar] [CrossRef]
- Etzi, F.; Griñán-Lisón, C.; Fenu, G.; González-Titos, A.; Pisano, A.; Farace, C.; Sabalic, A.; Picon-Ruiz, M.; Marchal, J.A.; Madeddu, R. The Role of miR-486-5p on CSCs Phenotypes in Colorectal Cancer. Cancers 2024, 16, 4237. [Google Scholar] [CrossRef] [PubMed]
- Pisano, A.; Griñan-Lison, C.; Farace, C.; Fiorito, G.; Fenu, G.; Jiménez, G.; Scognamillo, F.; Peña-Martin, J.; Naccarati, A.; Pröll, J.; et al. The Inhibitory Role of miR-486-5p on CSC Phenotype Has Diagnostic and Prognostic Potential in Colorectal Cancer. Cancers 2020, 12, 3432. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, G.; Hackenberg, M.; Catalina, P.; Boulaiz, H.; Griñán-Lisón, C.; García, M.Á.; Perán, M.; López-Ruiz, E.; Ramírez, A.; Morata-Tarifa, C.; et al. Mesenchymal stem cell’s secretome promotes selective enrichment of cancer stem-like cells with specific cytogenetic profile. Cancer Lett. 2018, 429, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Farace, C.; Pisano, A.; Griñan-Lison, C.; Solinas, G.; Jiménez, G.; Serra, M.; Carrillo, E.; Scognamillo, F.; Attene, F.; Montella, A.; et al. Deregulation of cancer-stem-cell-associated miRNAs in tissues and sera of colorectal cancer patients. Oncotarget 2020, 11, 116–130. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fenu, G.; Griñán-Lisón, C.; Etzi, F.; González-Titos, A.; Pisano, A.; Toledo, B.; Farace, C.; Sabalic, A.; Carrillo, E.; Marchal, J.A.; et al. Functional Characterization of miR-216a-5p and miR-125a-5p on Pancreatic Cancer Stem Cells. Int. J. Mol. Sci. 2025, 26, 2830. https://doi.org/10.3390/ijms26072830
Fenu G, Griñán-Lisón C, Etzi F, González-Titos A, Pisano A, Toledo B, Farace C, Sabalic A, Carrillo E, Marchal JA, et al. Functional Characterization of miR-216a-5p and miR-125a-5p on Pancreatic Cancer Stem Cells. International Journal of Molecular Sciences. 2025; 26(7):2830. https://doi.org/10.3390/ijms26072830
Chicago/Turabian StyleFenu, Grazia, Carmen Griñán-Lisón, Federica Etzi, Aitor González-Titos, Andrea Pisano, Belén Toledo, Cristiano Farace, Angela Sabalic, Esmeralda Carrillo, Juan Antonio Marchal, and et al. 2025. "Functional Characterization of miR-216a-5p and miR-125a-5p on Pancreatic Cancer Stem Cells" International Journal of Molecular Sciences 26, no. 7: 2830. https://doi.org/10.3390/ijms26072830
APA StyleFenu, G., Griñán-Lisón, C., Etzi, F., González-Titos, A., Pisano, A., Toledo, B., Farace, C., Sabalic, A., Carrillo, E., Marchal, J. A., & Madeddu, R. (2025). Functional Characterization of miR-216a-5p and miR-125a-5p on Pancreatic Cancer Stem Cells. International Journal of Molecular Sciences, 26(7), 2830. https://doi.org/10.3390/ijms26072830










