Placental Molecular Expression of Different Pathogenic Vaginal Infections
Abstract
1. Introduction
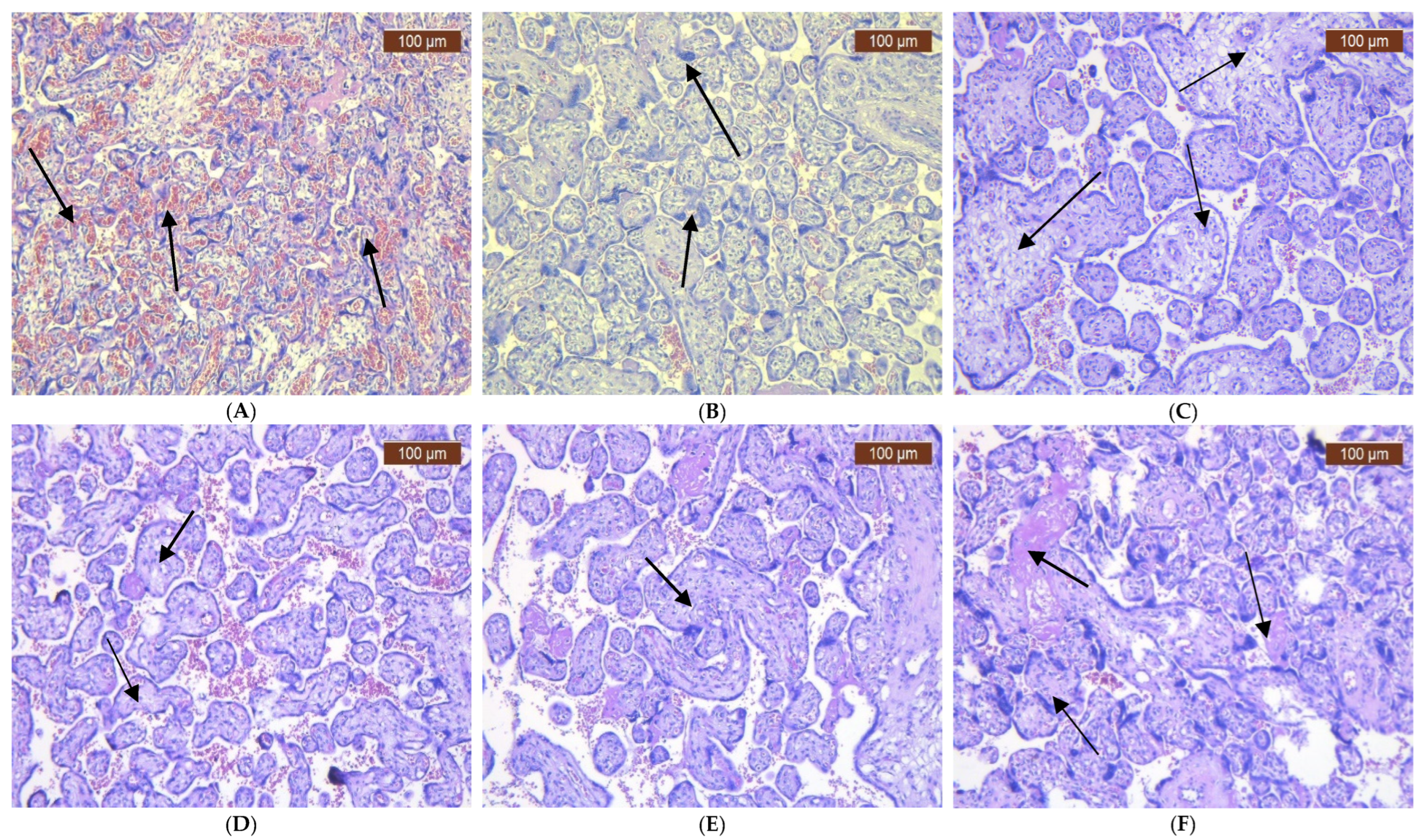
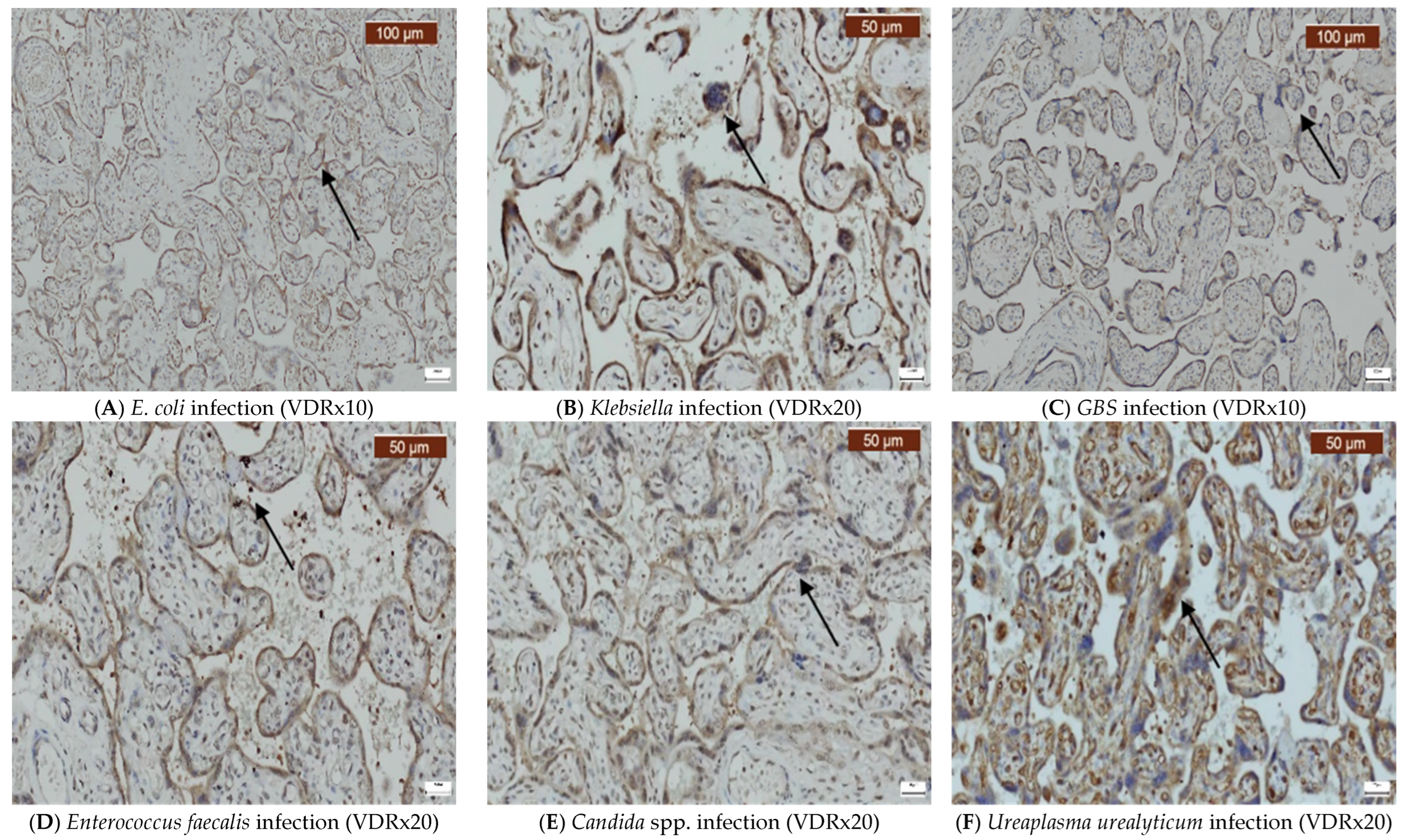
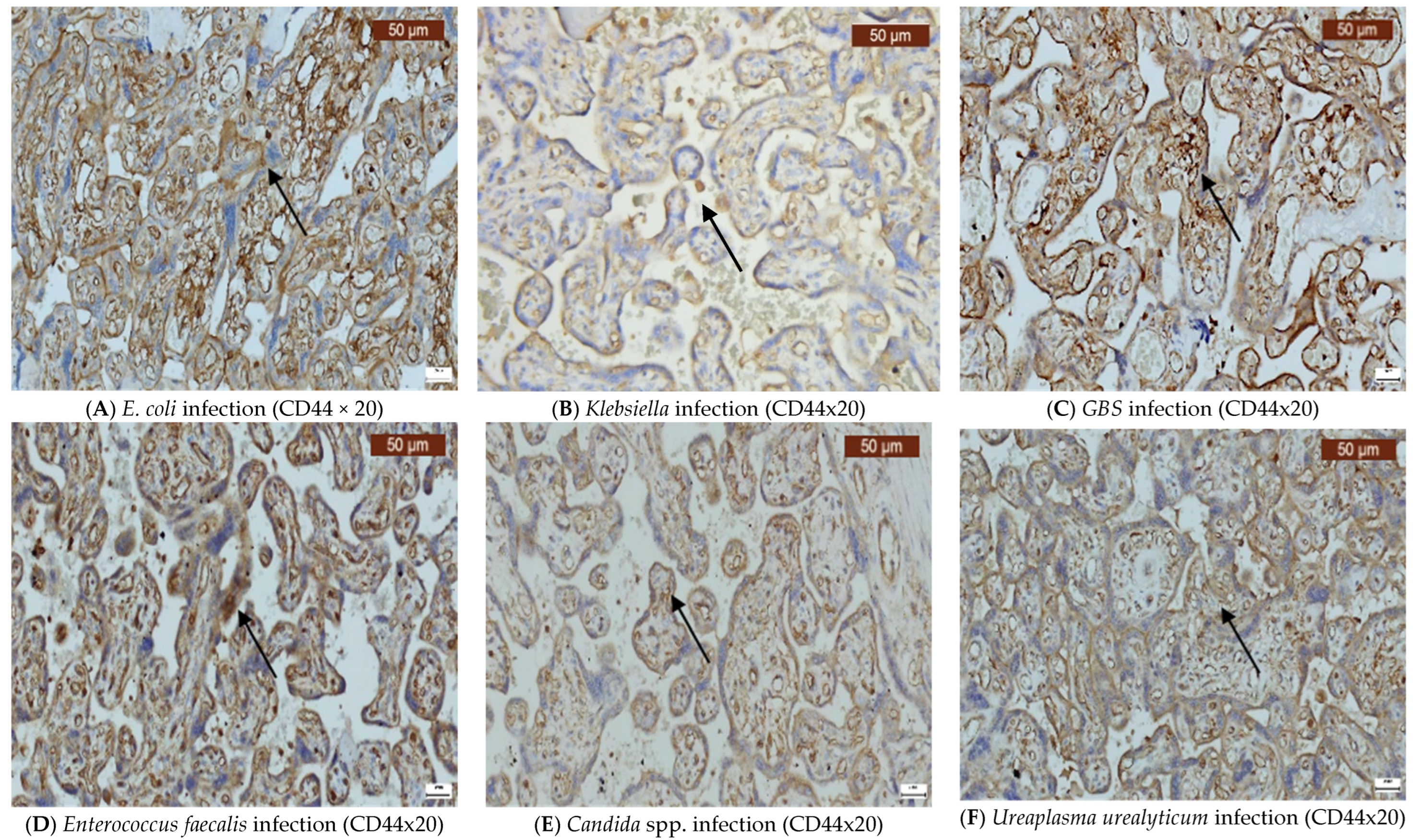
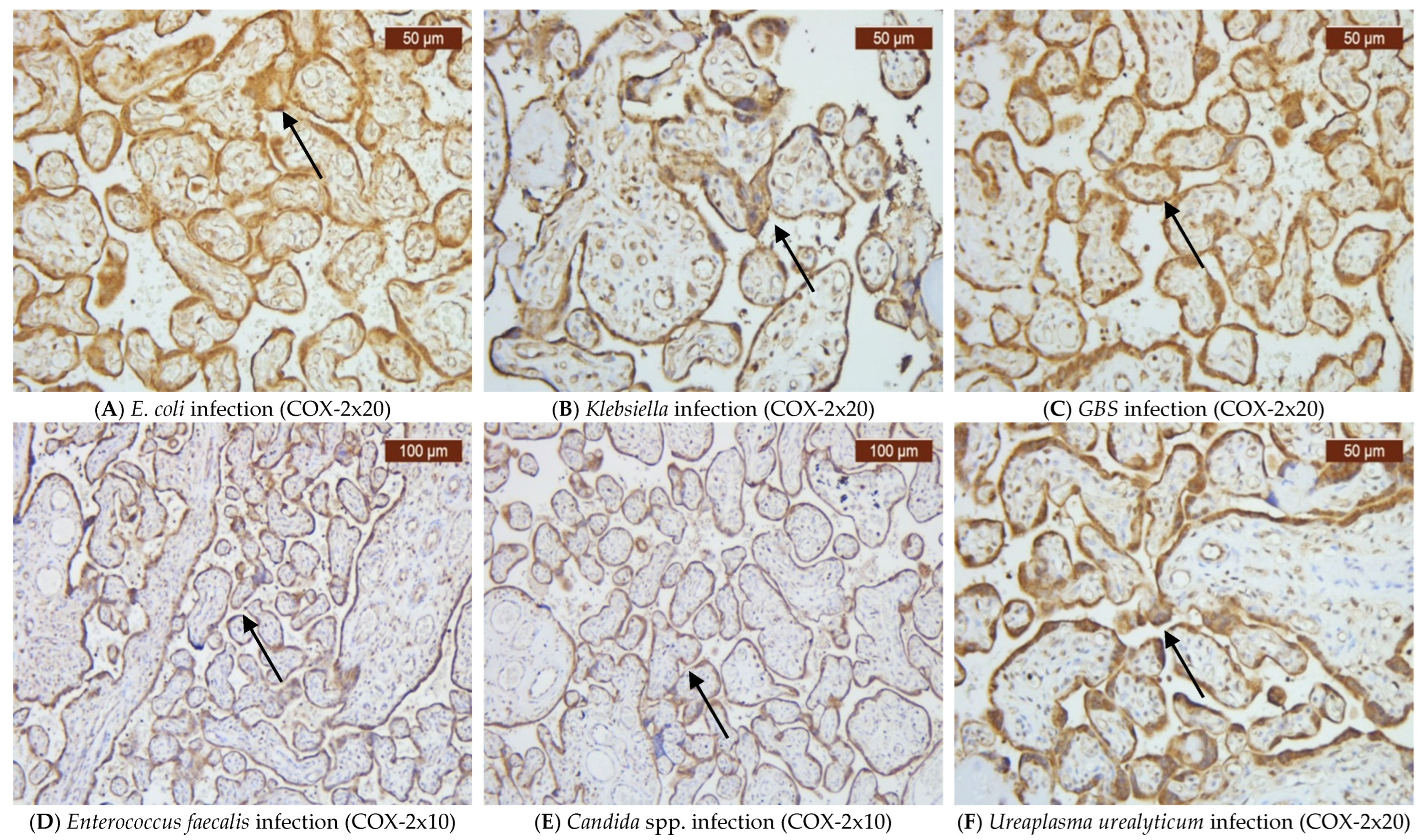
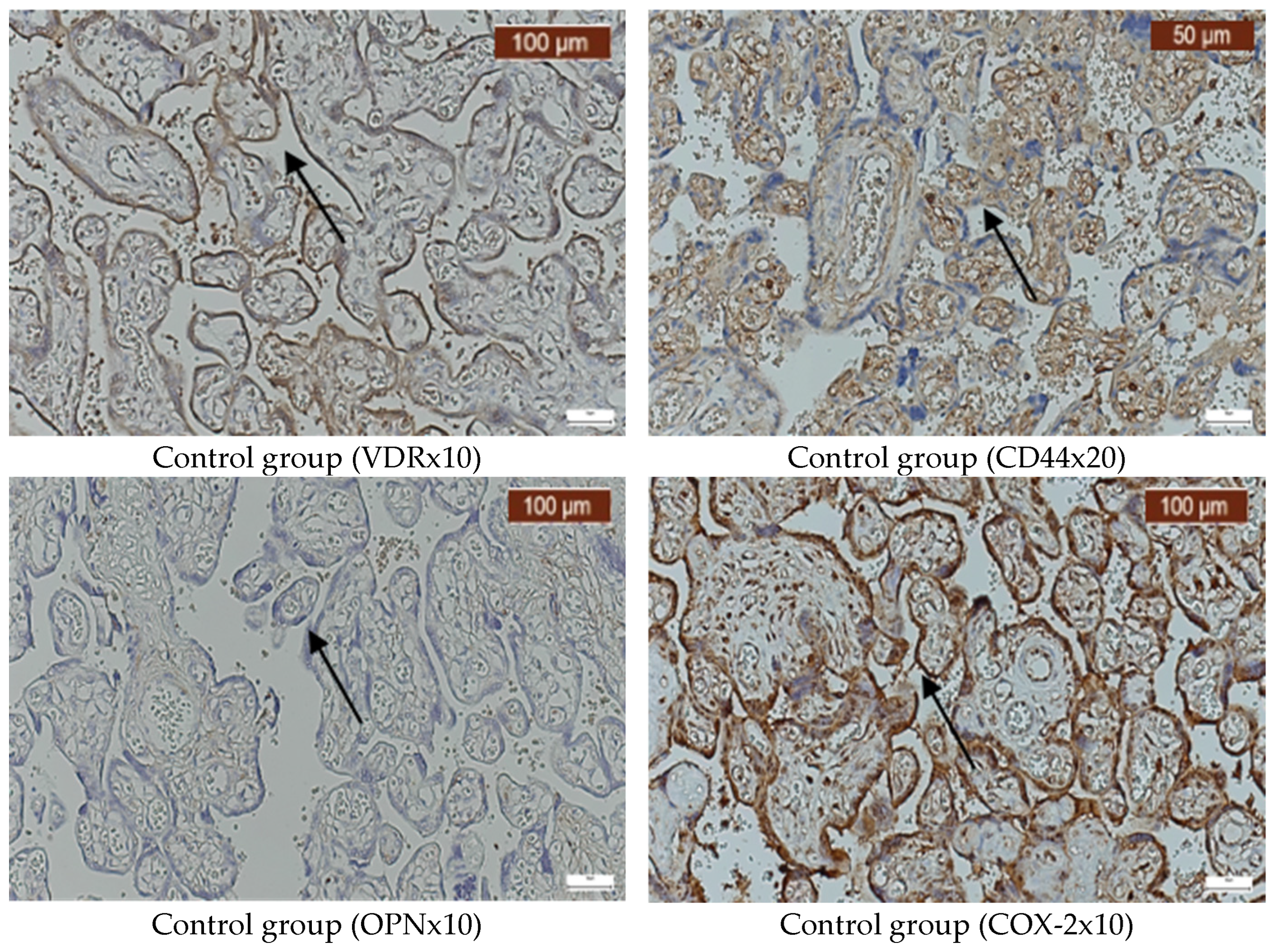
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Samples
4.1.1. Inclusion Criteria
4.1.2. Exclusion Criteria
4.2. Immunohistochemistry
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prasad, D.; Parween, S.; Kumari, K.; Singh, N. Prevalence, Etiology, and Associated Symptoms of Vaginal Discharge During Pregnancy in Women Seen in a Tertiary Care Hospital in Bihar. Cureus 2021, 13, e12700. [Google Scholar] [CrossRef]
- Daskalakis, G.; Psarris, A.; Koutras, A.; Fasoulakis, Z.; Prokopakis, I.; Varthaliti, A.; Karasmani, C.; Ntounis, T.; Domali, E.; Theodora, M.; et al. Maternal Infection and Preterm Birth: From Molecular Basis to Clinical Implications. Children 2023, 10, 907. [Google Scholar] [CrossRef]
- Tita, A.T.; Andrews, W.W. Diagnosis and management of clinical chorioamnionitis. Clin. Perinatol. 2010, 37, 339–354. [Google Scholar] [CrossRef]
- Williams, M.C.; O’Brien, W.F.; Nelson, R.N.; Spellacy, W.N. Histologic chorioamnionitis is associated with fetal growth restriction in term and preterm infants. Am. J. Obstet. Gynecol. 2000, 183, 1094–1099. [Google Scholar] [CrossRef]
- Romero, R.; Espinoza, J.; Gonçalves, L.F.; Kusanovic, J.P.; Friel, L.; Hassan, S. The role of inflammation and infection in preterm birth. Semin. Reprod. Med. 2007, 25, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Latino, M.A.; Botta, G.; Badino, C.; Maria, D.; Petrozziello, A.; Sensini, A.; Leli, C. Association between genital mycoplasmas, acute chorioamnionitis and fetal pneumonia in spontaneous abortions. J. Perinat. Med. 2018, 46, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.; Romero, R.; Yeo, L.; Diaz-Primera, R.; Marin-Concha, J.; Para, R.; Lopez, A.M.; Pacora, P.; Gomez-Lopez, N.; Yoon, B.H.; et al. The fetal inflammatory response syndrome: The origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin. Fetal Neonatal Med. 2020, 25, 101146. [Google Scholar] [CrossRef] [PubMed]
- Al-Adnani, M.; Sebire, N.J. The role of perinatal pathological examination in subclinical infection in obstetrics. Best. Pr. Res. Clin. Obs. Gynaecol. 2007, 21, 505–521. [Google Scholar] [CrossRef]
- Chalupska, M.; Kacerovsky, M.; Stranik, J.; Gregor, M.; Maly, J.; Jacobsson, B.; Musilova, I. Intra-Amniotic Infection and Sterile Intra-Amniotic Inflammation in Cervical Insufficiency with Prolapsed Fetal Membranes: Clinical Implications. Fetal Diagn. Ther. 2021, 48, 58–69. [Google Scholar] [CrossRef]
- Winters, A.D.; Romero, R.; Graffice, E.; Gomez-Lopez, N.; Jung, E.; Kanninen, T.; Theis, K.R. Optimization and validation of two multiplex qPCR assays for the rapid detection of microorganisms commonly invading the amniotic cavity. J. Reprod. Immunol. 2022, 149, 103460. [Google Scholar] [CrossRef]
- DiGiulio, D.B. Diversity of microbes in amniotic fluid. Semin. Fetal Neonatal Med. 2012, 17, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.W.; Shen, T.; Chung, P.; Buhimschi, I.A.; Buhimschi, C.S. Uncultivated bacteria as etiologic agents of intra-amniotic inflammation leading to preterm birth. J. Clin. Microbiol. 2009, 47, 38–47. [Google Scholar] [CrossRef] [PubMed]
- Bikle, D.D.; Feingold, K.R.; Anawalt, B.; Blackman, M.R.; Boyce, A.; Chrousos, G.; Corpas, E.; de Herder, W.W.; Dhatariya, K.; Dungan, K.; et al. Vitamin D: Production, Metabolism and Mechanisms of Action. In Endotext [Internet]; MDText.com Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Hsieh, J.C.; Nakajima, S.; Galligan, M.A.; Jurutka, P.W.; Haussler, C.A.; Whitfield, G.K.; Haussler, M.R. Receptor mediated genomic action of the 1,25(OH)2D3 hormone: Expression of the human vitamin D receptor in E. coli. J. Steroid Biochem. Mol. Biol. 1995, 53, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Jia, X.; Huang, Y.; Wang, J.; Lu, C.; Yuan, X.; Xu, J.; Zhu, H. Vitamin D stimulates miR-26b-5p to inhibit placental COX-2 expression in preeclampsia. Sci. Rep. 2021, 11, 11168. [Google Scholar] [CrossRef]
- Mahendroo, M. Cervical hyaluronan biology in pregnancy, parturition and preterm birth. Matrix Biol. 2019, 78–79, 24–31. [Google Scholar] [CrossRef]
- Ruscheinsky, M.; De la Motte, C.; Mahendroo, M. Hyaluronan and its binding proteins during cervical ripening and parturition: Dynamic changes in size, distribution and temporal sequence. Matrix Biol. 2008, 27, 487–497. [Google Scholar] [CrossRef]
- Gonzalez, J.M.; Xu, H.; Ofori, E.; Elovitz, M.A. Toll-like receptors in the uterus, cervix, and placenta: Is pregnancy an immunosuppressed state? Am. J. Obs. Gynecol. 2007, 197, 296.e1–296.e6. [Google Scholar] [CrossRef]
- Choi, C.H.; Roh, C.R.; Kim, T.J.; Choi, Y.L.; Lee, J.W.; Kim, B.G.; Lee, J.H.; Bae, D.S. Expression of CD44 adhesion molecules on human placentae. Eur. J. Obs. Gynecol. Reprod. Biol. 2006, 128, 243–247. [Google Scholar] [CrossRef]
- Wang, X.B.; Qi, Q.R.; Wu, K.L.; Xie, Q.Z. Role of osteopontin in decidualization and pregnancy success. Reproduction 2018, 155, 423–432. [Google Scholar] [CrossRef]
- Icer, M.A.; Gezmen-Karadag, M. The multiple functions and mechanisms of osteopontin. Clin. Biochem. 2018, 59, 17–24. [Google Scholar] [CrossRef]
- Baines, K.J.; Klausner, M.S.; Patterson, V.S.; Renaud, S.J. Interleukin-15 deficient rats have reduced osteopontin at the maternal-fetal interface. Front. Cell Dev. Biol. 2023, 11, 1079164. [Google Scholar] [CrossRef]
- Qi, Q.R.; Xie, Q.Z.; Liu, X.L.; Zhou, Y. Osteopontin is expressed in the mouse uterus during early pregnancy and promotes mouse blastocyst attachment and invasion in vitro. PLoS ONE 2014, 9, e104955. [Google Scholar] [CrossRef]
- Wu, L.Z.; Liu, X.L.; Xie, Q.Z. Osteopontin facilitates invasion in human trophoblastic cells via promoting matrix metalloproteinase-9 in vitro. Int. J. Clin. Exp. Pathol. 2015, 8, 14121–14130. [Google Scholar]
- Xia, J.; Qiao, F.; Su, F.; Liu, H. Implication of expression of osteopontin and its receptor integrin alphanubeta3 in the placenta in the development of preeclampsia. J. Huazhong Univ. Sci. Technol. Med. Sci. 2009, 29, 755–760. [Google Scholar] [CrossRef]
- Özer, A.; Yaylalı, A.; Koçarslan, S. The role of osteopontin in the pathogenesis of placenta percreta. Ginekol. Pol. 2018, 89, 437–441. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Chen, Y.; Fu, X.Q.; Yang, F.; Chen, Z.W.; Mo, G.L.; Lao, D.Y.; Li, M.J. Research on the expression of MRNA-518b in the pathogenesis of placenta accreta. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Wills, A.J.; Edwards, D.R.; Heath, J.K.; Hogan, B.L. Developmental expression of 2ar (osteopontin) and SPARC (osteonectin) RNA as revealed by in situ hybridization. J. Cell Biol. 1988, 106, 441–450. [Google Scholar] [CrossRef]
- Herington, J.L.; Bany, B.M. The conceptus increases secreted phosphoprotein 1 gene expression in the mouse uterus during the progression of decidualization mainly due to its effects on uterine natural killer cells. Reproduction 2007, 133, 1213–1221. [Google Scholar] [CrossRef]
- Kramer, A.C.; Erikson, D.W.; McLendon, B.A.; Seo, H.; Hayashi, K.; Spencer, T.E.; Bazer, F.W.; Burghardt, R.C.; Johnson, G.A. SPP1 expression in the mouse uterus and placenta: Implications for implantation†. Biol. Reprod. 2021, 105, 892–904. [Google Scholar] [CrossRef]
- Yi, Y.; Cheng, J.C.; Klausen, C.; Leung, P.C.K. TGF-β1 inhibits human trophoblast cell invasion by upregulating cyclooxygenase-2. Placenta 2018, 68, 44–51. [Google Scholar] [CrossRef]
- Wu, Z.; Liu, D.; Deng, F. The Role of Vitamin D in Immune System and Inflammatory Bowel Disease. J. Inflamm. Res. 2022, 15, 3167–3185. [Google Scholar] [CrossRef] [PubMed]
- Senbanjo, L.T.; Chellaiah, M.A. CD44: A Multifunctional Cell Surface Adhesion Receptor Is a Regulator of Progression and Metastasis of Cancer Cells. Front. Cell Dev. Biol. 2017, 5, 18. [Google Scholar] [CrossRef] [PubMed]
- Pazhohan, A.; Amidi, F.; Akbari-Asbagh, F.; Seyedrezazadeh, E.; Aftabi, Y.; Abdolalizadeh, J.; Khodarahmian, M.; Khanlarkhani, N.; Sobhani, A. Expression and shedding of CD44 in the endometrium of women with endometriosis and modulating effects of vitamin D: A randomized exploratory trial. J. Steroid Biochem. Mol. Biol. 2018, 178, 150–158. [Google Scholar]
- Stout, M.J.; Conlon, B.; Landeau, M.; Lee, I.; Bower, C.; Zhao, Q.; Roehl, K.A.; Nelson, D.M.; Macones, G.A.; Mysorekar, I.U. Identification of intracellular bacteria in the basal plate of the human placenta in term and preterm gestations. Am. J. Obs. Gynecol. 2013, 208, 226.e1–226.e7. [Google Scholar] [CrossRef]
- Witt, R.G.; Blair, L.; Frascoli, M.; Rosen, M.J.; Nguyen, Q.H.; Bercovici, S.; Zompi, S.; Romero, R.; Mackenzie, T.C. Detection of microbial cell-free DNA in maternal and umbilical cord plasma in patients with chorioamnionitis using next generation sequencing. PLoS ONE 2020, 15, e0231239. [Google Scholar] [CrossRef]
- Ng, B.K.; Chuah, J.N.; Cheah, F.C.; Mohamed Ismail, N.A.; Tan, G.C.; Wong, K.K.; Lim, P.S. Maternal and fetal outcomes of pregnant women with bacterial vaginosis. Front. Surg. 2023, 10, 1084867. [Google Scholar] [CrossRef]
- McDonald, H.M.; Chambers, H.M. Intrauterine infection and spontaneous midgestation abortion: Is the spectrum of microorganisms similar to that in preterm labor? Infect. Dis. Obs. Gynecol. 2000, 5–6, 220–227. [Google Scholar] [CrossRef]
- Heerema-McKenney, A. Defense and infection of the human placenta. APMIS 2018, 126, 570–588. [Google Scholar] [CrossRef]
- He, J.R.; Tikellis, G.; Paltiel, O.; Klebanoff, M.; Magnus, P.; Northstone, K.; Golding, J.; Ward, M.H.; Linet, M.S.; Olsen, S.F.; et al. Association of common maternal infections with birth outcomes: A multinational cohort study. Infection 2024, 52, 1553–1561. [Google Scholar] [CrossRef]
- Li, H.; Dong, M.; Xie, W.; Qi, W.; Teng, F.; Li, H.; Yan, Y.; Wang, C.; Han, C.; Xue, F. Mixed Vaginitis in the Third Trimester of Pregnancy Is Associated with Adverse Pregnancy Outcomes: A Cross-Sectional Study. Front. Cell Infect. Microbiol. 2022, 12, 798738. [Google Scholar] [CrossRef]
- Holliday, M.; Uddipto, K.; Castillo, G.; Vera, L.E.; Quinlivan, J.A.; Mendz, G.L. Insights into the Genital Microbiota of Women Who Experienced Fetal Death in Utero. Microorganisms 2023, 11, 1877. [Google Scholar] [CrossRef]
- Doster, R.S.; Sutton, J.A.; Rogers, L.M.; Aronoff, D.M.; Gaddy, J.A. Streptococcus agalactiae Induces Placental Macrophages to Release Extracellular Traps Loaded with Tissue Remodeling Enzymes via an Oxidative Burst-Dependent Mechanism. mBio 2018, 9, e02084-18. [Google Scholar] [CrossRef] [PubMed]
- Brokaw, A.; Furuta, A.; Dacanay, M.; Rajagopal, L.; Adams Waldorf, K.M. Bacterial and Host Determinants of Group B Streptococcal Vaginal Colonization and Ascending Infection in Pregnancy. Front. Cell Infect. Microbiol. 2021, 11, 720789. [Google Scholar] [CrossRef] [PubMed]
- Obut, M.; Oğlak, S.C. Expression of CD44 and IL-10 in normotensive and preeclamptic placental tissue. Ginekol. Pol. 2020, 91, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Diao, H.; Liu, X.; Chen, Y.; Xu, W.; Cao, H.; Kohanawa, M.; Li, L. Osteopontin expression and relation to streptococcal disease severity in mice. Scand J Infect Dis 2011, 43, 100–106. [Google Scholar] [CrossRef]
- Jordan, A.R.; Racine, R.R.; Hennig, M.J.; Lokeshwar, V.B. The Role of CD44 in Disease Pathophysiology and Targeted Treatment. Front. Immunol. 2015, 6, 182. [Google Scholar] [CrossRef]
- Castello, L.M.; Baldrighi, M.; Molinari, L.; Salmi, L.; Cantaluppi, V.; Vaschetto, R.; Zunino, G.; Quaglia, M.; Bellan, M.; Gavelli, F.; et al. The Role of Osteopontin as a Diagnostic and Prognostic Biomarker in Sepsis and Septic Shock. Cells 2019, 8, 174. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Chen, T. Vaginal microbiota: Potential targets for vulvovaginal candidiasis infection. Heliyon 2024, 10, e27239. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef]
- Gunduz, O.; Kucukozkan, T. Predictive Role of Osteopontin and Inflammation Markers in the Diagnosis and Monitoring of Premature Membrane Rupture. Adv. Res. Obs. Gynaecol. 2024, 2, 1–7. [Google Scholar] [CrossRef]
- Sweeney, E.L.; Dando, S.J.; Kallapur, S.G.; Knox, C.L. The Human Ureaplasma Species as Causative Agents of Chorioamnionitis. Clin. Microbiol. Rev. 2016, 30, 349–379. [Google Scholar] [CrossRef] [PubMed]
- Bespalova, O.; Bakleicheva, M.; Kovaleva, I.; Tolibova, G.; Tral, T.; Kogan, I. Expression of vitamin D and vitamin D receptor in chorionic villous in missed abortion. Gynecol. Endocrinol. 2019, 35, 49–55. [Google Scholar] [PubMed]
- Urrego, D.; Liwa, A.C.; Cole, W.C.; Wood, S.L.; Slater, D.M. Cyclooxygenase inhibitors for treating preterm labour: What is the molecular evidence? Can. J. Physiol. Pharmacol. 2019, 97, 222–231. [Google Scholar] [CrossRef]
- Anderson, S.M.; Thurman, A.R.; Chandra, N.; Jackson, S.S.; Asin, S.; Rollenhagen, C.; Ghosh, M.; Daniels, J.; Vann, N.C.; Clark, M.R.; et al. Vitamin D Status Impacts Genital Mucosal Immunity and Markers of HIV-1 Susceptibility in Women. Nutrients 2020, 12, 3176. [Google Scholar] [CrossRef]
- Bodnar, L.M.; Krohn, M.A.; Simhan, H.N. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J. Nutr. 2009, 139, 1157–1161. [Google Scholar] [CrossRef]
- Salvi, V.; Scutera, S.; Rossi, S.; Zucca, M.; Alessandria, M.; Greco, D.; Bosisio, D.; Sozzani, S.; Musso, T. Dual regulation of osteopontin production by TLR stimulation in dendritic cells. J. Leukoc. Biol. 2013, 94, 147–158. [Google Scholar] [CrossRef]
- Inoue, M.; Moriwaki, Y.; Arikawa, T.; Chen, Y.H.; Oh, Y.J.; Oliver, T.; Shinohara, M.L. Cutting edge: Critical role of intracellular osteopontin in antifungal innate immune responses. J. Immunol. 2011, 186, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Gigi, R.M.S.; Buitrago-Garcia, D.; Taghavi, K.; Dunaiski, C.M.; van de Wijgert, J.H.H.M.; Peters, R.P.H.; Low, N. Vulvovaginal yeast infections during pregnancy and perinatal outcomes: Systematic review and meta-analysis. BMC Womens Health 2023, 23, 116. [Google Scholar] [CrossRef]
- O’Brien, V.P.; Gilbert, N.M.; Lebratti, T.; Agarwal, K.; Foster, L.; Shin, H.; Lewis, A.L. Low-dose inoculation of Escherichia coli achieves robust vaginal colonization and results in ascending infection accompanied by severe uterine inflammation in mice. PLoS ONE 2019, 14, e0219941. [Google Scholar] [CrossRef]
- d’Enfert, C.; Kaune, A.K.; Alaban, L.R.; Chakraborty, S.; Cole, N.; Delavy, M.; Kosmala, D.; Marsaux, B.; Fróis-Martins, R.; Morelli, M.; et al. The impact of the Fungus-Host-Microbiota interplay upon Candida albicans infections: Current knowledge and new perspectives. FEMS Microbiol. Rev. 2021, 45, fuaa060. [Google Scholar] [CrossRef]
- Bengoechea, J.A.; Sa Pessoa, J. Klebsiella pneumoniae infection biology: Living to counteract host defences. FEMS Microbiol. Rev. 2019, 43, 123–144. [Google Scholar] [CrossRef] [PubMed]

| Mean | St. Dev. | 95.0% CI for Mean | Percentile 25 | Median | Percentile 75 | Min | Max | Mann– Whitney U p-Value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||||||||
| Age (years) | Control group | 27.8 | 4.3 | 26.8 | 28.8 | 27 | 29 | 31 | 19 | 34 | 0.13 |
| Vaginal infection group | 29.3 | 6.3 | 27.8 | 30.7 | 25 | 30 | 34 | 17 | 43 | ||
| Weeks of gestation | Control group | 39.7 | 1.4 | 39.4 | 40 | 38 | 40 | 41 | 37 | 41 | <0.01 |
| Vaginal infection group | 34.6 | 5.3 | 33.4 | 35.8 | 30 | 36 | 39 | 24 | 41 | ||
| Fetal birth weight (g) | Control group | 3503.1 | 313.9 | 3428.3 | 3578 | 3360 | 3530 | 3670 | 2800 | 4340 | <0.01 |
| Vaginal infection group | 2557.3 | 1160 | 2295.7 | 2818.9 | 1350 | 2920 | 3520 | 570 | 4540 | ||
| APGAR score | Control group | 8.5 | 0.5 | 8.4 | 8.6 | 8 | 9 | 9 | 8 | 9 | 0.28 |
| Vaginal infection group | 8.1 | 1.5 | 7.7 | 8.4 | 7 | 8 | 9 | 4 | 10 | ||
| Antepartum hemoglobin level (milligrams/deciliter) | Control group | 12.3 | 1.1 | 12.0 | 12.6 | 11.8 | 12.1 | 13.6 | 10.0 | 13.7 | 0.34 |
| Vaginal infection group | 12.0 | 1.1 | 11.8 | 12.3 | 11.4 | 12.2 | 12.7 | 9.5 | 14.3 | ||
| Postpartum hemoglobin level (milligrams/deciliter) | Control group | 10.9 | .8 | 10.8 | 11.1 | 10.4 | 10.9 | 11.5 | 9.0 | 12.1 | 0.26 |
| Vaginal infection group | 11.1 | 3.2 | 10.4 | 11.8 | 10.0 | 10.8 | 11.4 | 9.0 | 37.3 | ||
| Antepartum hematocrit level (%) | Control group | 36.3 | 3.4 | 35.5 | 37.1 | 35.0 | 37.0 | 38.9 | 28.1 | 40.0 | 0.15 |
| Vaginal infection group | 35.2 | 5.2 | 34.0 | 36.4 | 34.3 | 36.1 | 38.3 | 12.2 | 42.1 | ||
| Postpartum hematocrit level (%) | Control group | 31.8 | 2.9 | 31.1 | 32.5 | 29.0 | 31.1 | 34.3 | 27.8 | 37.0 | 0.41 |
| Vaginal infection group | 31.3 | 3.3 | 30.5 | 32.0 | 28.5 | 30.4 | 33.6 | 25.6 | 38.5 | ||
| Leucocyte count (103/L) | Control group | 13,184 | 3874 | 12,311 | 14,058 | 10,260 | 12,970 | 16,110 | 7040 | 25,240 | 0.89 |
| Vaginal infection group | 25,920 | 41,820 | 15,949 | 35,892 | 10,350 | 11,485 | 15,430 | 9900 | 150,303 | ||
| Platelet count (106/L) | Control group | 220,171 | 78,308 | 201,500 | 238,843 | 147,000 | 217,500 | 278,000 | 132,000 | 355,000 | 0.46 |
| Vaginal infection group | 225,705 | 66,226 | 210,773 | 240,637 | 170,000 | 218,000 | 260,000 | 123,000 | 362,000 | ||
| C reactive protein value (milligrams/deciliter) | Control group | 3.12 | 1.52 | 2.76 | 3.49 | 2.00 | 2.75 | 4.10 | 1.00 | 10.00 | <0.01 |
| Vaginal infection group | 24.92 | 26.61 | 18.92 | 30.92 | 4.50 | 12.98 | 36.00 | 1.90 | 98.00 | ||
| VDR | CD44 | OPN | COX-2 | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | |||
| Candida spp. | Count | 2 | 10 | 10 | 2 | 5 | 7 | 1 | 11 | 12 |
| % within cultura | 16.7% | 83.3% | 83.3% | 16.7% | 41.7% | 58.3% | 8.3% | 91.7% | 100.0% | |
| Escherichia coli | Count | 3 | 13 | 0 | 16 | 16 | 0 | 0 | 16 | 16 |
| % within cultura | 18.8% | 81.3% | 0.0% | 100.0% | 100.0% | 0.0% | 0.0% | 100.0% | 100.0% | |
| Enterococcus faecalis | Count | 1 | 13 | 2 | 11 | 11 | 2 | 0 | 13 | 13 |
| % within cultura | 7.1% | 92.9% | 15.4% | 84.6% | 84.6% | 15.4% | 0.0% | 100.0% | 100.0% | |
| Klebsiella | Count | 9 | 1 | 9 | 1 | 8 | 2 | 0 | 10 | 10 |
| % within cultura | 90.0% | 10.0% | 90.0% | 10.0% | 80.0% | 20.0% | 0.0% | 100.0% | 100.0% | |
| Streptococcus beta hemolitic | Count | 1 | 13 | 1 | 14 | 2 | 13 | 0 | 15 | 15 |
| % within cultura | 7.1% | 92.9% | 6.7% | 93.3% | 13.3% | 86.7% | 0.0% | 100.0% | 100.0% | |
| Ureaplasma urealyticum | Count | 1 | 11 | 1 | 11 | 3 | 9 | 0 | 12 | 12 |
| % within cultura | 8.3% | 91.7% | 8.3% | 91.7% | 25.0% | 75.0% | 0.0% | 100.0% | 100.0% | |
| Total | Count | 17 | 61 | 23 | 55 | 45 | 33 | 1 | 77 | 78 |
| % within cultura | 24.6% | 75.4% | 29.5% | 70.5% | 57.7% | 42.3% | 1.3% | 98.7% | 100% | |
| VDR | CD44 | OPN | COX-2 | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | ||||
| Group | Vaginal infections | Count | 16 | 62 | 23 | 55 | 45 | 33 | 1 | 77 | 78 |
| %within lot | 20.51% | 79.49% | 29.5% | 70.5% | 57.7% | 42.3% | 1.3% | 98.7% | 100% | ||
| No vaginal infections | Count | 14 | 56 | 14 | 56 | 56 | 14 | 0 | 70 | 70 | |
| %within lot | 20% | 80% | 20% | 80% | 80% | 20% | 0% | 100% | 100% | ||
| Total | Count | 30 | 118 | 37 | 111 | 101 | 47 | 1 | 147 | 148 | |
| %within lot | 20.27% | 79.7% | 25% | 75% | 68.2% | 31.8% | 0.7% | 99.3% | 100% | ||
| Analyzed Markers | VDR | CD44 | OPN | COX-2 |
|---|---|---|---|---|
| Sig. | 0.907 | 0.185 | 0.004 | Not computed |
| Exp (B) | 0.953 | 0.598 | 2.933 | |
| Lower | 0.427 | 0.279 | 1.402 | |
| Upper | 2.129 | 1.280 | 6.136 | |
| OR | 1.102 | 1.67 | 2.933 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matasariu, D.R.; Condac, C.; Bîrluțiu, V.; Lozneanu, L.; Bujor, I.E.; Boiculese, V.L.; Sava, M.; Ursache, A. Placental Molecular Expression of Different Pathogenic Vaginal Infections. Int. J. Mol. Sci. 2025, 26, 2863. https://doi.org/10.3390/ijms26072863
Matasariu DR, Condac C, Bîrluțiu V, Lozneanu L, Bujor IE, Boiculese VL, Sava M, Ursache A. Placental Molecular Expression of Different Pathogenic Vaginal Infections. International Journal of Molecular Sciences. 2025; 26(7):2863. https://doi.org/10.3390/ijms26072863
Chicago/Turabian StyleMatasariu, Daniela Roxana, Constantin Condac, Victoria Bîrluțiu, Ludmila Lozneanu, Iuliana Elena Bujor, Vasile Lucian Boiculese, Mihai Sava, and Alexandra Ursache. 2025. "Placental Molecular Expression of Different Pathogenic Vaginal Infections" International Journal of Molecular Sciences 26, no. 7: 2863. https://doi.org/10.3390/ijms26072863
APA StyleMatasariu, D. R., Condac, C., Bîrluțiu, V., Lozneanu, L., Bujor, I. E., Boiculese, V. L., Sava, M., & Ursache, A. (2025). Placental Molecular Expression of Different Pathogenic Vaginal Infections. International Journal of Molecular Sciences, 26(7), 2863. https://doi.org/10.3390/ijms26072863








