Protocols for Extraction of miRNA from Extracellular Vesicles of Lyophilized Human Saliva Samples
Abstract
1. Introduction
2. Results
2.1. Patients Information
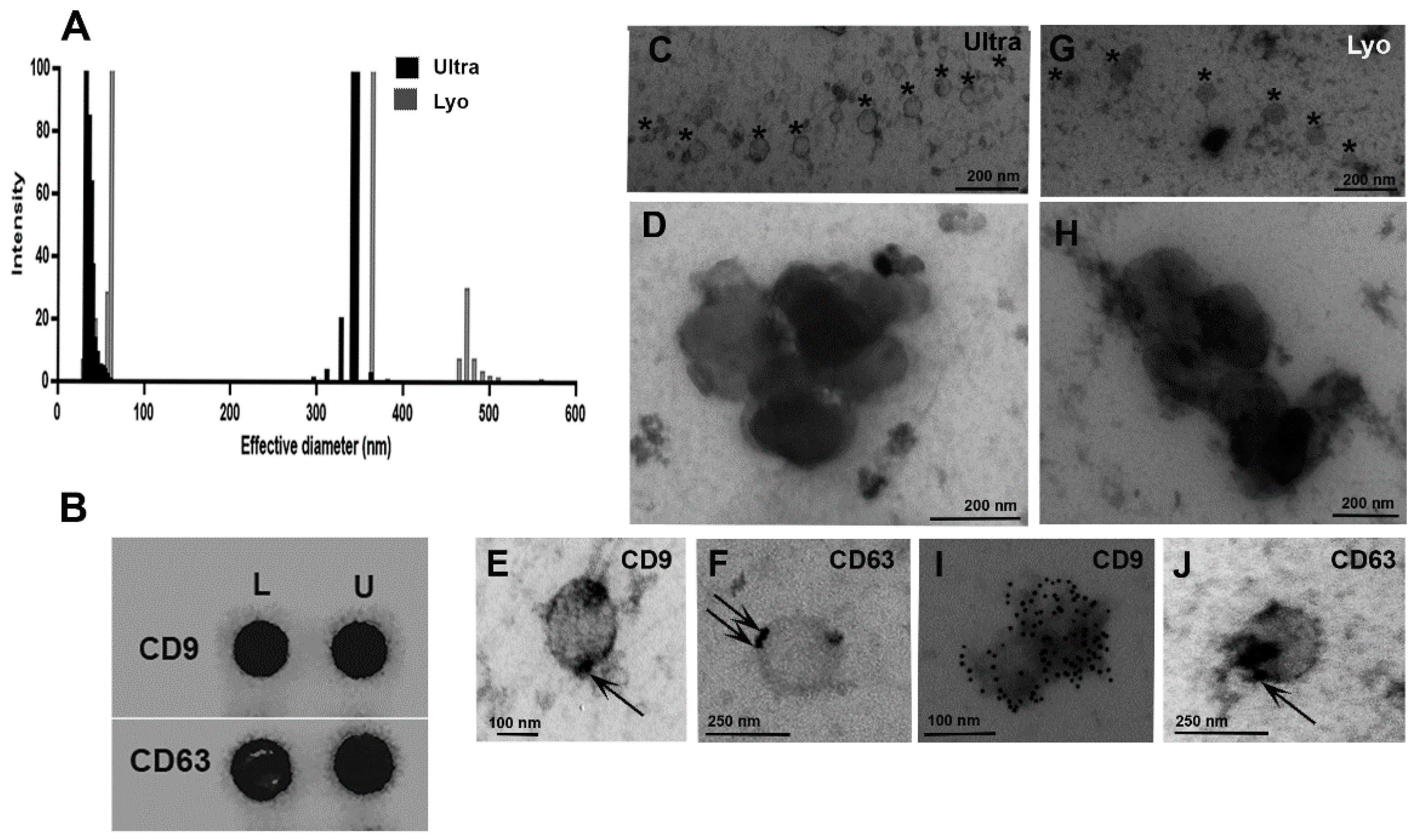
2.2. Extracellular Vesicles Characterization
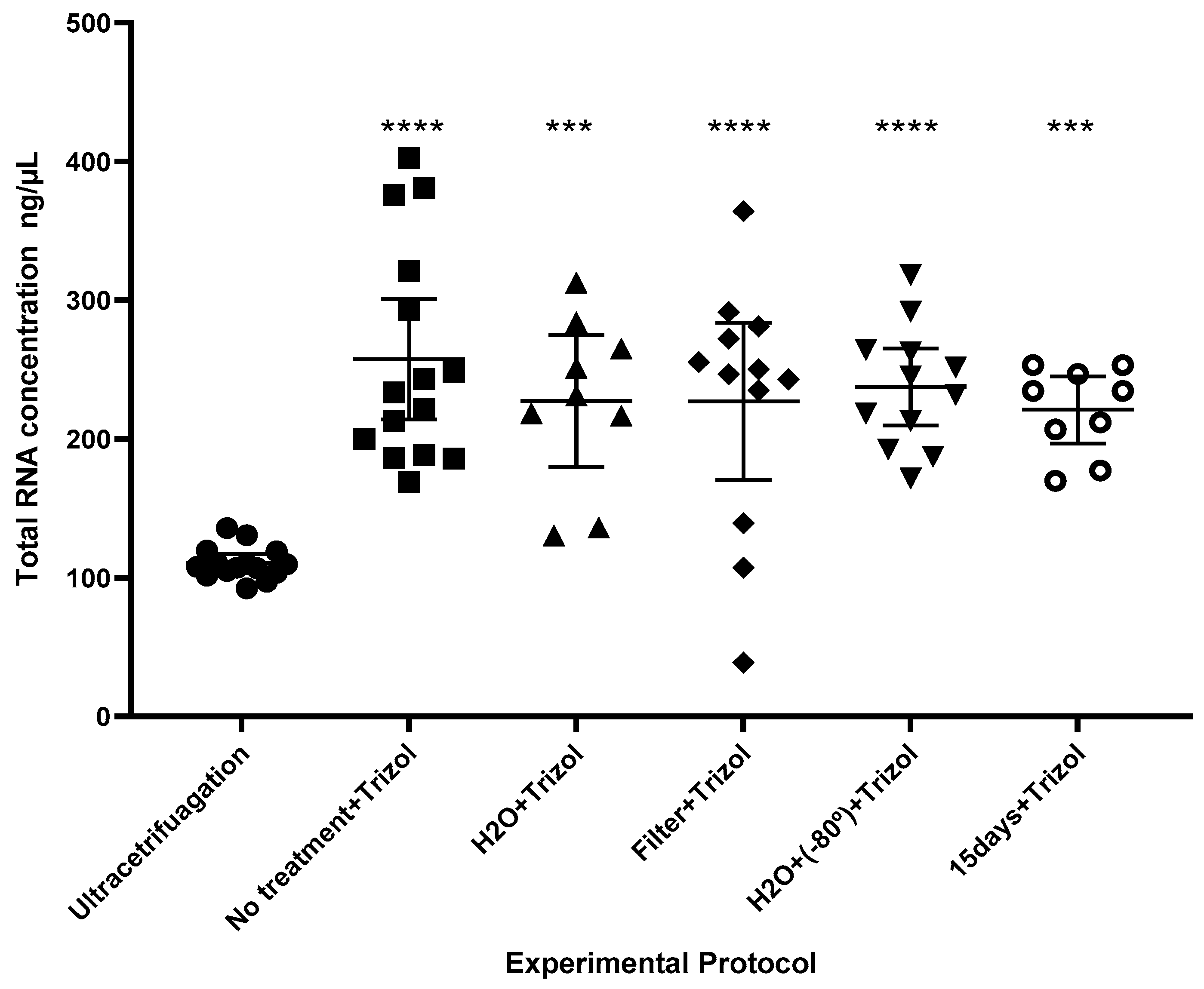
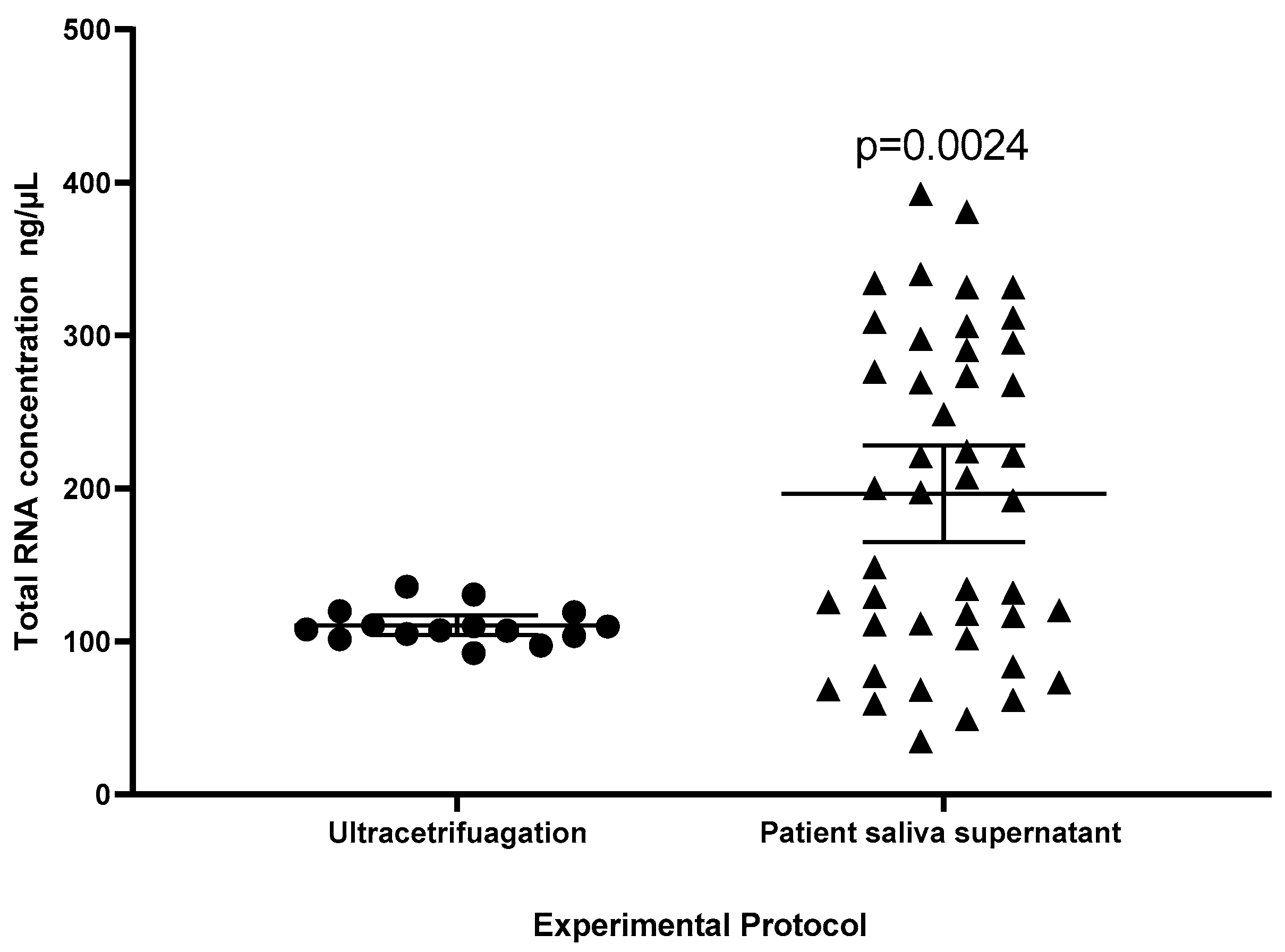
2.3. Comparison of Protocols
2.4. Bioinformatic Analysis
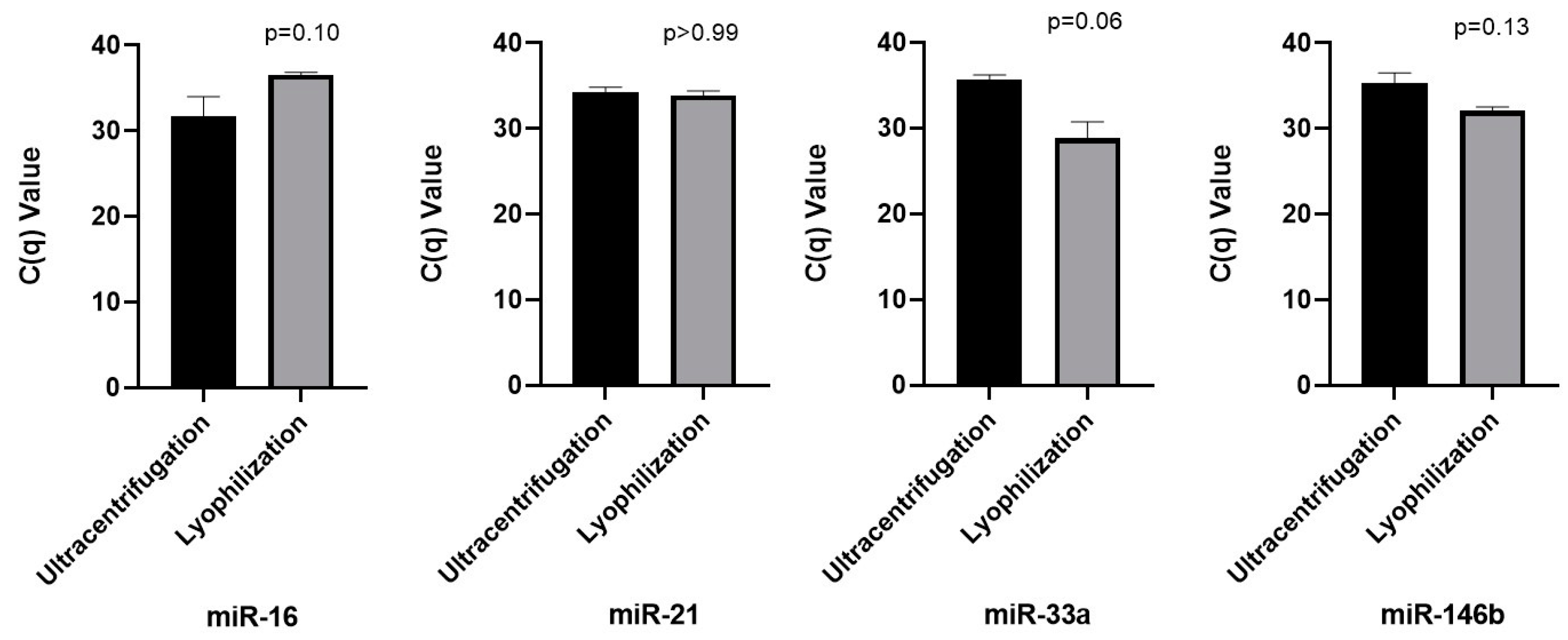
2.5. Analysis of microRNA in Lyophilized and Ultracentrifuged Saliva Supernatant Through Real-Time PCR
3. Discussion
4. Materials and Methods
4.1. Saliva Collection and Preparation
4.2. Isolation of Extracellular Vesicles
Clarification of Lyophilization Purpose
4.3. Extracellular Vesicle Size Measurements by Quasi-Elastic Light Scattering (QELS)
4.4. Electron Microscopy—Uranyl Acetate Stained Preparations
4.5. Dot Blotting Analysis
4.6. Sample Preparation Protocols
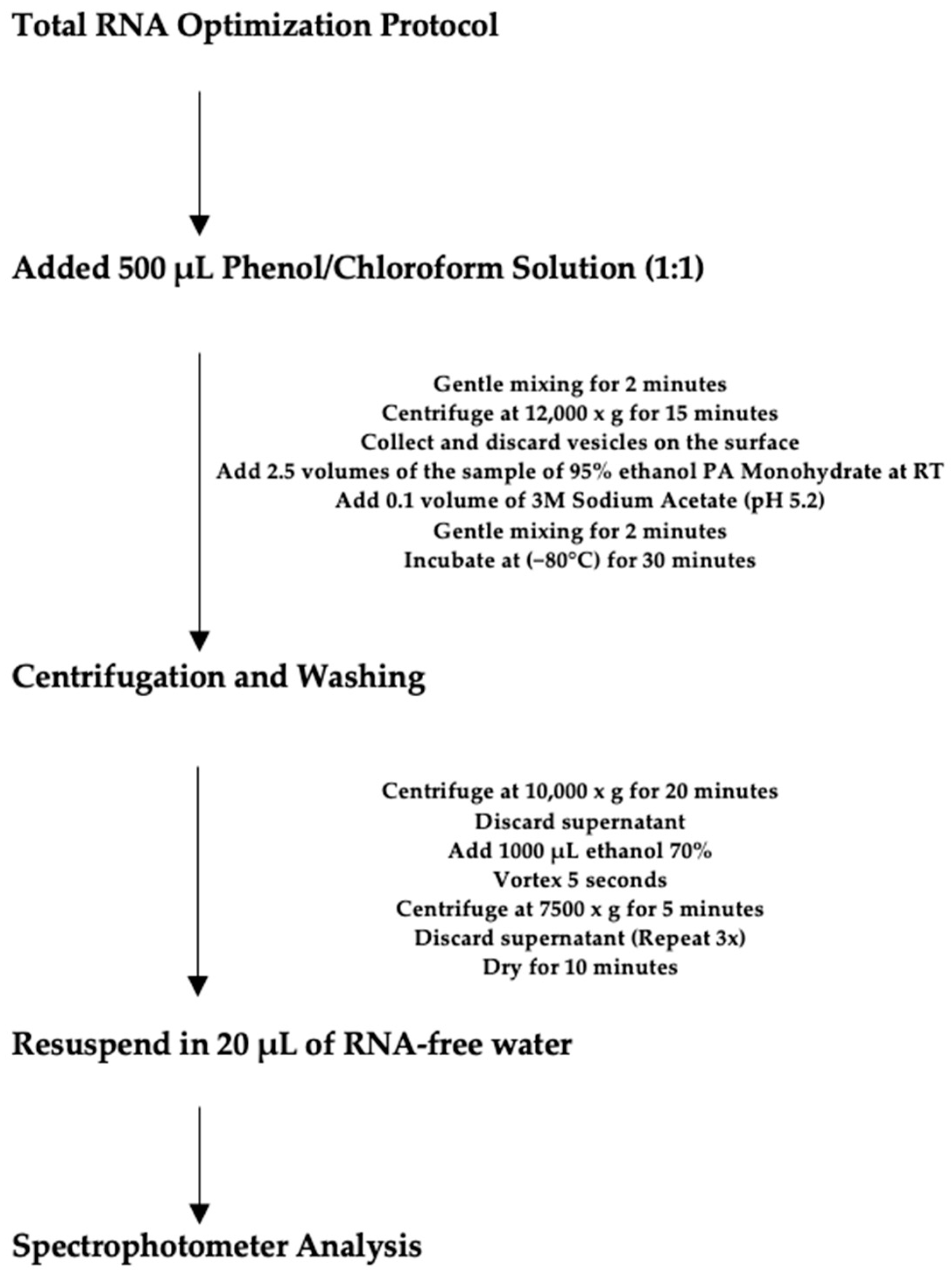
4.7. Total RNA Optimization Protocol
4.8. Patient Saliva Supernatant
4.9. MicroRNAs Selection
4.10. MicroRNA Analysis Using RT PCR
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abhange, K.; Makler, A.; Wen, Y.; Ramnauth, N.; Mao, W.; Asghar, W.; Wan, Y. Small Extracellular Vesicles in Cancer. Bioact. Mater. 2021, 6, 3705–3743. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-Y.; Papoutsakis, E.T. Extracellular Vesicles: Exosomes, Microparticles, Their Parts, and Their Targets to Enable Their Biomanufacturing and Clinical Applications. Curr. Opin. Biotechnol. 2019, 60, 89–98. [Google Scholar] [CrossRef]
- Liang, Y.; Lehrich, B.M.; Zheng, S.; Lu, M. Emerging Methods in Biomarker Identification for Extracellular Vesicle-based Liquid Biopsy. J. Extracell. Vesicle 2021, 10, e12090. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; How Huang, K.; Jen Lee, M.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Alevizos, I.; Alexander, S.; Turner, R.J.; Illei, G.G. MicroRNA Expression Profiles as Biomarkers of Minor Salivary Gland Inflammation and Dysfunction in Sjögren’s Syndrome. Arthritis Rheum. 2011, 63, 535–544. [Google Scholar] [CrossRef]
- Ding, Y.; Ma, Q.; Liu, F.; Zhao, L.; Wei, W. The Potential Use of Salivary miRNAs as Promising Biomarkers for Detection of Cancer: A Meta-Analysis. PLoS ONE 2016, 11, e0166303. [Google Scholar] [CrossRef]
- Yap, T.; Vella, L.; Seers, C.; Nastri, A.; Reynolds, E.; Cirillo, N.; McCullough, M. Oral Swirl Samples—A Robust Source of microRNA Protected by Extracellular Vesicles. Oral Dis. 2017, 23, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Neupane, Y.R.; Lim, X.C.; Shekhani, R.; Czarny, B.; Wacker, M.G.; Pastorin, G.; Wang, J.-W. Extracellular Vesicles in Cardiovascular Disease. In Advances in Clinical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; Volume 103, pp. 47–95. ISBN 978-0-12-824616-0. [Google Scholar]
- Ho, P.T.B.; Clark, I.M.; Le, L.T.T. MicroRNA-Based Diagnosis and Therapy. Int. J. Mol. Sci. 2022, 23, 7167. [Google Scholar] [CrossRef]
- Gablo, N.A.; Prochazka, V.; Kala, Z.; Slaby, O.; Kiss, I. Cell-Free microRNAs as Non-Invasive Diagnostic and Prognostic Biomarkers in Pancreatic Cancer. Curr. Genom. 2020, 20, 569–580. [Google Scholar] [CrossRef]
- Gallo, A.; Tandon, M.; Alevizos, I.; Illei, G.G. The Majority of MicroRNAs Detectable in Serum and Saliva Is Concentrated in Exosomes. PLoS ONE 2012, 7, e30679. [Google Scholar] [CrossRef]
- Michael, A.; Bajracharya, S.; Yuen, P.; Zhou, H.; Star, R.; Illei, G.; Alevizos, I. Exosomes from Human Saliva as a Source of microRNA Biomarkers: microRNA Biomarkers in Salivary Exosomes. Oral Dis. 2010, 16, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Bjersing, J.L.; Lundborg, C.; Bokarewa, M.I.; Mannerkorpi, K. Profile of Cerebrospinal microRNAs in Fibromyalgia. PLoS ONE 2013, 8, e78762. [Google Scholar] [CrossRef]
- Ding, M.; Li, Y.; Wang, H.; Lv, Y.; Liang, J.; Wang, J.; Li, C. Diagnostic value of urinary microRNAs as non-invasive biomarkers for bladder cancer: A meta-analysis. Int. J. Clin. Exp. Med. 2015, 15, 15432–15440. [Google Scholar]
- Humeau, M.; Vignolle-Vidoni, A.; Sicard, F.; Martins, F.; Bournet, B.; Buscail, L.; Torrisani, J.; Cordelier, P. Salivary MicroRNA in Pancreatic Cancer Patients. PLoS ONE 2015, 10, e0130996. [Google Scholar] [CrossRef] [PubMed]
- Izzotti, A.; Carozzo, S.C.; Pulliero, A.; Zhabayeva, D.; Ravetti, J.L.; Bersimbaev, R. Extracellular MicroRNA in Liquid Biopsy: Applicability in Cancer Diagnosis and Prevention. Am. J. Cancer Res. 2016, 6, 1461–1493. [Google Scholar]
- Nagata, M.; Muto, S.; Horie, S. Molecular Biomarkers in Bladder Cancer: Novel Potential Indicators of Prognosis and Treatment Outcomes. Dis. Markers 2016, 2016, 8205836. [Google Scholar] [CrossRef]
- Hicks, S.D.; Middleton, F.A. A Comparative Review of microRNA Expression Patterns in Autism Spectrum Disorder. Front. Psychiatry 2016, 7, 176. [Google Scholar] [CrossRef] [PubMed]
- Masotti, A.; Baldassarre, A.; Guzzo, M.P.; Iannuccelli, C.; Barbato, C.; Di Franco, M. Circulating microRNA Profiles as Liquid Biopsies for the Characterization and Diagnosis of Fibromyalgia Syndrome. Mol. Neurobiol. 2017, 54, 7129–7136. [Google Scholar] [CrossRef]
- Stanczyk, J.; Ospelt, C.; Karouzakis, E.; Filer, A.; Raza, K.; Kolling, C.; Gay, R.; Buckley, C.D.; Tak, P.P.; Gay, S.; et al. Altered Expression of microRNA-203 in Rheumatoid Arthritis Synovial Fibroblasts and Its Role in Fibroblast Activation. Arthritis Rheum. 2011, 63, 373–381. [Google Scholar] [CrossRef]
- Wiklund, E.D.; Gao, S.; Hulf, T.; Sibbritt, T.; Nair, S.; Costea, D.E.; Villadsen, S.B.; Bakholdt, V.; Bramsen, J.B.; Sørensen, J.A.; et al. MicroRNA Alterations and Associated Aberrant DNA Methylation Patterns across Multiple Sample Types in Oral Squamous Cell Carcinoma. PLoS ONE 2011, 6, e27840. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a Proinflammatory Regulator in Clinical and Experimental Arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- Bossel Ben-Moshe, N.; Avraham, R.; Kedmi, M.; Zeisel, A.; Yitzhaky, A.; Yarden, Y.; Domany, E. Context-Specific microRNA Analysis: Identification of Functional microRNAs and Their mRNA Targets. Nucleic Acids Res. 2012, 40, 10614–10627. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hurley, J.; Roberts, D.; Chakrabortty, S.K.; Enderle, D.; Noerholm, M.; Breakefield, X.O.; Skog, J.K. Exosome-Based Liquid Biopsies in Cancer: Opportunities and Challenges. Ann. Oncol. 2021, 32, 466–477. [Google Scholar] [CrossRef]
- Tsamou, M.; Kremers, F.A.C.; Samaritakis, K.A.; Roggen, E.L. Identifying microRNAs Possibly Implicated in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: A Review. Int. J. Mol. Sci. 2024, 25, 9551. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.Y.; Lee, C.K.; Huang, C.; Ou, Y.H.; Charles, C.J.; Richards, A.M.; Neupane, Y.R.; Pavon, M.V.; Zharkova, O.; Pastorin, G.; et al. Extracellular Vesicles in Cardiovascular Diseases: Alternative Biomarker Sources, Therapeutic Agents, and Drug Delivery Carriers. Int. J. Mol. Sci. 2019, 20, 3272. [Google Scholar] [CrossRef]
- Churov, A.V.; Oleinik, E.K.; Knip, M. MicroRNAs in Rheumatoid Arthritis: Altered Expression and Diagnostic Potential. Autoimmun. Rev. 2015, 14, 1029–1037. [Google Scholar] [CrossRef]
- Kwon, K.-C.; Verma, D.; Singh, N.D.; Herzog, R.; Daniell, H. Oral Delivery of Human Biopharmaceuticals, Autoantigens and Vaccine Antigens Bioencapsulated in Plant Cells. Adv. Drug Deliv. Rev. 2013, 65, 782–799. [Google Scholar] [CrossRef]
- Su, J.; Zhu, L.; Sherman, A.; Wang, X.; Lin, S.; Kamesh, A.; Norikane, J.H.; Streatfield, S.J.; Herzog, R.W.; Daniell, H. Low Cost Industrial Production of Coagulation Factor IX Bioencapsulated in Lettuce Cells for Oral Tolerance Induction in Hemophilia B. Biomaterials 2015, 70, 84–93. [Google Scholar] [CrossRef]
- Kwon, K.-C.; Daniell, H. Oral Delivery of Protein Drugs Bioencapsulated in Plant Cells. Mol. Ther. 2016, 24, 1342–1350. [Google Scholar] [CrossRef]
- Flosdorf, E.W.; Mudd, S. Procedure and Apparatus for Preservation in “Lyophile” Form of Serum and Other Biological Substances. J. Immunol. 1935, 29, 389–425. [Google Scholar] [CrossRef]
- Butreddy, A.; Janga, K.Y.; Ajjarapu, S.; Sarabu, S.; Dudhipala, N. Instability of Therapeutic Proteins—An Overview of Stresses, Stabilization Mechanisms and Analytical Techniques Involved in Lyophilized Proteins. Int. J. Biol. Macromol. 2021, 167, 309–325. [Google Scholar] [CrossRef] [PubMed]
- Izutsu, K. Applications of Freezing and Freeze-Drying in Pharmaceutical Formulations. In Survival Strategies in Extreme Cold and Desiccation; Iwaya-Inoue, M., Sakurai, M., Uemura, M., Eds.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2018; Volume 1081, pp. 371–383. ISBN 9789811312434. [Google Scholar]
- Eshiet, E.R.; Zhu JinQiu, Z.J.; Smith, E.E. The Biologic Potential of Lyophilized Extracts of Brickellia Cavanillesii (Asteraceae): Apoptosis and Glut 2 Gene Expression. J. Food Process. Technol. 2016, 7, 541. [Google Scholar] [CrossRef]
- Beissner, M.; Phillips, R.O.; Battke, F.; Bauer, M.; Badziklou, K.; Sarfo, F.S.; Maman, I.; Rhomberg, A.; Piten, E.; Frimpong, M.; et al. Loop-Mediated Isothermal Amplification for Laboratory Confirmation of Buruli Ulcer Disease—Towards a Point-of-Care Test. PLoS Negl. Trop. Dis. 2015, 9, e0004219. [Google Scholar] [CrossRef]
- Neupane, Y.R.; Huang, C.; Wang, X.; Chng, W.H.; Venkatesan, G.; Zharkova, O.; Wacker, M.G.; Czarny, B.; Storm, G.; Wang, J.-W.; et al. Lyophilization Preserves the Intrinsic Cardioprotective Activity of Bioinspired Cell-Derived Nanovesicles. Pharmaceutics 2021, 13, 1052. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Kancelista, A.; Łaba, W.; Piegza, M.; Witkowska, D. The Effect of Lyophilization and Storage Time on the Survival Rate and Hydrolytic Activity of Trichoderma Strains. Folia Microbiol. 2018, 63, 433–441. [Google Scholar] [CrossRef]
- Farr, T.; Wissner, J.L.; Hauer, B. A Simple and Efficient Method for Lyophilization of Recombinant E. Coli JM109 (DE3) Whole-Cells Harboring Active Rieske Non-Heme Iron Dioxygenases. MethodsX 2021, 8, 101323. [Google Scholar] [CrossRef]
- Molnar, A.; Lakat, T.; Hosszu, A.; Szebeni, B.; Balogh, A.; Orfi, L.; Szabo, A.J.; Fekete, A.; Hodrea, J. Lyophilization and Homogenization of Biological Samples Improves Reproducibility and Reduces Standard Deviation in Molecular Biology Techniques. Amino Acids 2021, 53, 917–928. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, M.; Zhang, Y.; Li, W.; Gao, Y.; Li, Z.; Wang, Z.; Lubec, G.; Zhang, C. Lyophilization Is Suitable for Storage and Shipment of Fresh Tissue Samples without Altering RNA and Protein Levels Stored at Room Temperature. Amino Acids 2012, 43, 1383–1388. [Google Scholar] [CrossRef]
- Mareninov, S.; De Jesus, J.; Sanchez, D.E.; Kay, A.B.; Wilson, R.W.; Babic, I.; Chen, W.; Telesca, D.; Lou, J.J.; Mirsadraei, L.; et al. Lyophilized Brain Tumor Specimens Can Be Used for Histologic, Nucleic Acid, and Protein Analyses after 1 Year of Room Temperature Storage. J. Neurooncol. 2013, 113, 365–373. [Google Scholar] [CrossRef]
- Kobayashi, T.; Tomofuji, T.; Machida, T.; Yoneda, T.; Ekuni, D.; Azuma, T.; Maruyama, T.; Hirose, A.; Morita, M. Expression of Salivary miR-203a-3p Was Related with Oral Health-Related Quality of Life in Healthy Volunteers. Int. J. Mol. Sci. 2017, 18, 1263. [Google Scholar] [CrossRef] [PubMed]
- Lane, R.E.; Korbie, D.; Trau, M.; Hill, M.M. Purification Protocols for Extracellular Vesicles. In Extracellular Vesicles; Kuo, W.P., Jia, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1660, pp. 111–130. ISBN 978-1-4939-7251-7. [Google Scholar]
- Yang, D.; Zhang, W.; Zhang, H.; Zhang, F.; Chen, L.; Ma, L.; Larcher, L.M.; Chen, S.; Liu, N.; Zhao, Q.; et al. Progress, Opportunity, and Perspective on Exosome Isolation—Efforts for Efficient Exosome-Based Theranostics. Theranostics 2020, 10, 3684–3707. [Google Scholar] [CrossRef] [PubMed]
- Vanbillemont, B.; Carpenter, J.F.; Probst, C.; De Beer, T. The Impact of Formulation Composition and Process Settings of Traditional Batch Versus Continuous Freeze-Drying On Protein Aggregation. J. Pharm. Sci. 2020, 109, 3308–3318. [Google Scholar] [CrossRef]
- Zocco, D.; Zarovni, N. Extraction and Analysis of Extracellular Vesicle-Associated miRNAs Following Antibody-Based Extracellular Vesicle Capture from Plasma Samples. In Extracellular Vesicles; Kuo, W.P., Jia, S., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2017; Volume 1660, pp. 269–285. ISBN 978-1-4939-7251-7. [Google Scholar]
- Byun, J.; Hong, S.; Choi, J.; Jung, J.; Lee, H. Diagnostic Profiling of Salivary Exosomal Micro RNA s in Oral Lichen Planus Patients. Oral Dis. 2015, 21, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Markowska, A.; Pendergrast, R.S.; Pendergrast, J.S.; Pendergrast, P.S. A Novel Method for the Isolation of Extracellular Vesicles and RNA from Urine. J. Circ. Biomark. 2017, 6, 184945441771266. [Google Scholar] [CrossRef]
- Wan, Y.; Cheng, G.; Liu, X.; Hao, S.-J.; Nisic, M.; Zhu, C.-D.; Xia, Y.-Q.; Li, W.-Q.; Wang, Z.-G.; Zhang, W.-L.; et al. Rapid Magnetic Isolation of Extracellular Vesicles via Lipid-Based Nanoprobes. Nat. Biomed. Eng. 2017, 1, 0058. [Google Scholar] [CrossRef]
- Lam, B.; Simkin, M.; Rghei, N.; Haj-Ahmad, Y. Revised Guidelines for RNA Quality Assessment for Diverse Biological Sample Input. Available online: https://norgenbiotek.com/sites/default/files/resources/App-Note-47-Revised-Guidelines-for-RNA-Quality_0.pdf?srsltid=AfmBOor67CUlns_BECWP3ojoe2Tqwj56oTCDuvUbSgIJNcmvgB5mOTFC (accessed on 19 September 2024).
- Lässer, C.; Eldh, M.; Lötvall, J. Isolation and Characterization of RNA-Containing Exosomes. JoVE 2012, 59, 3037. [Google Scholar] [CrossRef]
- Xie, Z.-J.; Chen, G.; Zhang, X.-C.; Li, D.-F.; Huang, J.; Li, Z.-J. Saliva Supernatant miR-21: A Novel Potential Biomarker for Esophageal Cancer Detection. Asian Pac. J. Cancer Prev. 2012, 13, 6145–6149. [Google Scholar] [CrossRef]
- Seelenfreund, E.; Robinson, W.A.; Amato, C.M.; Tan, A.-C.; Kim, J.; Robinson, S.E. Long Term Storage of Dry versus Frozen RNA for Next Generation Molecular Studies. PLoS ONE 2014, 9, e111827. [Google Scholar] [CrossRef]
- Momen-Heravi, F.; Trachtenberg, A.J.; Kuo, W.P.; Cheng, Y.S. Genomewide Study of Salivary MicroRNAs for Detection of Oral Cancer. J. Dent. Res. 2014, 93, 86S–93S. [Google Scholar] [CrossRef]
- Xie, Z.; Yin, X.; Gong, B.; Nie, W.; Wu, B.; Zhang, X.; Huang, J.; Zhang, P.; Zhou, Z.; Li, Z. Salivary microRNAs Show Potential as a Noninvasive Biomarker for Detecting Resectable Pancreatic Cancer. Cancer Prev. Res. 2015, 8, 165–173. [Google Scholar] [CrossRef]
- Pereira, M.G.; Visbal, G.; Costa, T.F.R.; Frases, S.; De Souza, W.; Atella, G.; Cunha-e-Silva, N. Trypanosoma Cruzi Epimastigotes Store Cholesteryl Esters in Lipid Droplets after Cholesterol Endocytosis. Mol. Biochem. Parasitol. 2018, 224, 6–16. [Google Scholar] [CrossRef] [PubMed]
- TRIzolTM Reagent User Guide 2023. Available online: https://assets.thermofisher.com/TFS-Assets/LSG/manuals/trizol_reagent.pdf (accessed on 19 September 2024).
- Xie, Z.; Chen, G.; Zhang, X.; Li, D.; Huang, J.; Yang, C.; Zhang, P.; Qin, Y.; Duan, Y.; Gong, B.; et al. Salivary MicroRNAs as Promising Biomarkers for Detection of Esophageal Cancer. PLoS ONE 2013, 8, e57502. [Google Scholar] [CrossRef]
| Method | Groups | Concentration ng/µL | 260/280 | 260/230 |
|---|---|---|---|---|
| Ultracentrifugation | Control | 110.50 (±11.65) | 1.73 (±0.01) | 2.18 (±0.10) |
| Lyophilization | No treatment + Trizol | 233.50 (±78.26) | 1.82 (±0.04) | 2.20 (±0.05) |
| H2O + Trizol | 227.50 (±61.63) | 1.82 (±0.05) | 2.22 (±0.05) | |
| Filter + Trizol | 227.13 (±89.16) | 1.83 (±0.02) | 2.24 (±0.02) | |
| H2O + (−80°) + Trizol | 237.47 (±43.64) | 1.84 (±0.01) | 2.13 (±0.10) | |
| 15 days + Trizol | 221.01 (±31.50) | 1.88 (±0.02) | 2.25 (±0.05) | |
| Patient | 196.5 (±103.9) | 1.80 (±0.15) | 2.18 (±0.19) |
| miRNA | Target Gene | Gene Name |
|---|---|---|
| hsa-miR-21 or hsa-miR-21–5p | TNFRSF11A or RANK | Tumor necrosis factor ligand superfamily member 11A or Receptor activator of nuclear factor κ B |
| STAT5A | Signal transducer and activator of transcription 5A | |
| has-miR-33a or has-miR-33a-5p | SATB2 | Special AT-rich sequence-binding protein 2 |
| has-miR-146b or has-miR-146b-5p | TRAF6 | TNF receptor-associated factor 6 |
| STAT3 | Signal transducer and activator of transcription 3 | |
| RUNX2 | Runt-related transcription factor 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quinelato, V.; Mourão, C.F.; Santos, T.A.B.; Cataldo de Felipe Cordeiro, P.; Ladeira Bonato, L.; Gomes Pereira, M.; Calasans-Maia, J.A.; Granjeiro, J.M.; Kawase, T.; Ladeira Casado, P. Protocols for Extraction of miRNA from Extracellular Vesicles of Lyophilized Human Saliva Samples. Int. J. Mol. Sci. 2025, 26, 2891. https://doi.org/10.3390/ijms26072891
Quinelato V, Mourão CF, Santos TAB, Cataldo de Felipe Cordeiro P, Ladeira Bonato L, Gomes Pereira M, Calasans-Maia JA, Granjeiro JM, Kawase T, Ladeira Casado P. Protocols for Extraction of miRNA from Extracellular Vesicles of Lyophilized Human Saliva Samples. International Journal of Molecular Sciences. 2025; 26(7):2891. https://doi.org/10.3390/ijms26072891
Chicago/Turabian StyleQuinelato, Valquiria, Carlos Fernando Mourão, Thalita Alves Barreto Santos, Patrícia Cataldo de Felipe Cordeiro, Leticia Ladeira Bonato, Miria Gomes Pereira, Jose Albuquerque Calasans-Maia, Jose Mauro Granjeiro, Tomoyuki Kawase, and Priscila Ladeira Casado. 2025. "Protocols for Extraction of miRNA from Extracellular Vesicles of Lyophilized Human Saliva Samples" International Journal of Molecular Sciences 26, no. 7: 2891. https://doi.org/10.3390/ijms26072891
APA StyleQuinelato, V., Mourão, C. F., Santos, T. A. B., Cataldo de Felipe Cordeiro, P., Ladeira Bonato, L., Gomes Pereira, M., Calasans-Maia, J. A., Granjeiro, J. M., Kawase, T., & Ladeira Casado, P. (2025). Protocols for Extraction of miRNA from Extracellular Vesicles of Lyophilized Human Saliva Samples. International Journal of Molecular Sciences, 26(7), 2891. https://doi.org/10.3390/ijms26072891









