Role of Adiponectin in Regulating Cytokines and Its Contribution to the Occurrence and Progression of Clinical Mastitis in Holstein Cows
Abstract
:1. Introduction
2. Results
2.1. Expression Pattern Analysis of TNF-α, IL-1β, IL-6, and IL-10 in Bovine Mammary Gland Tissues
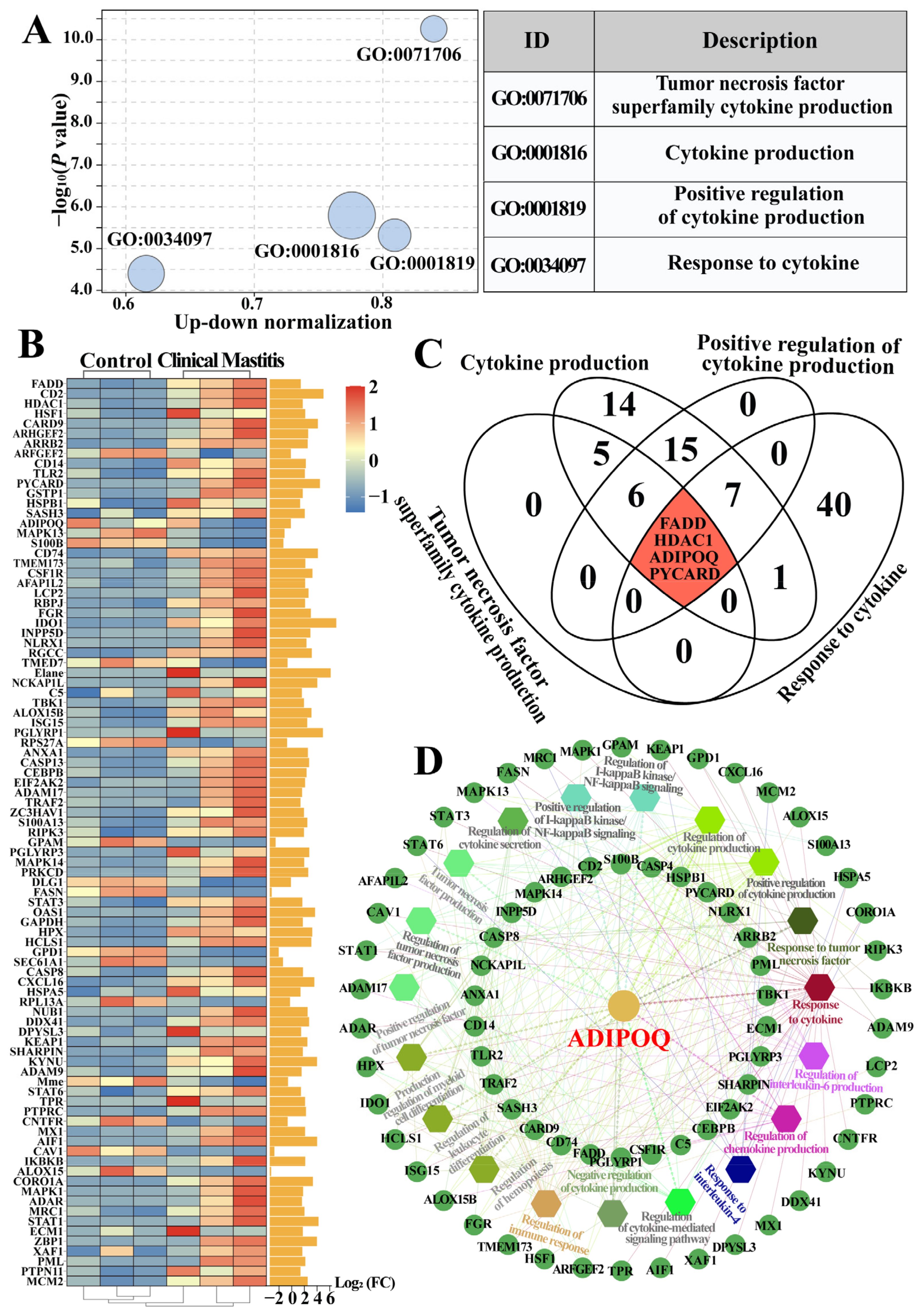
2.2. Identification of Candidate BP Terms and DEPs Related to Cytokines in the Bovine Mammary Gland Tissues
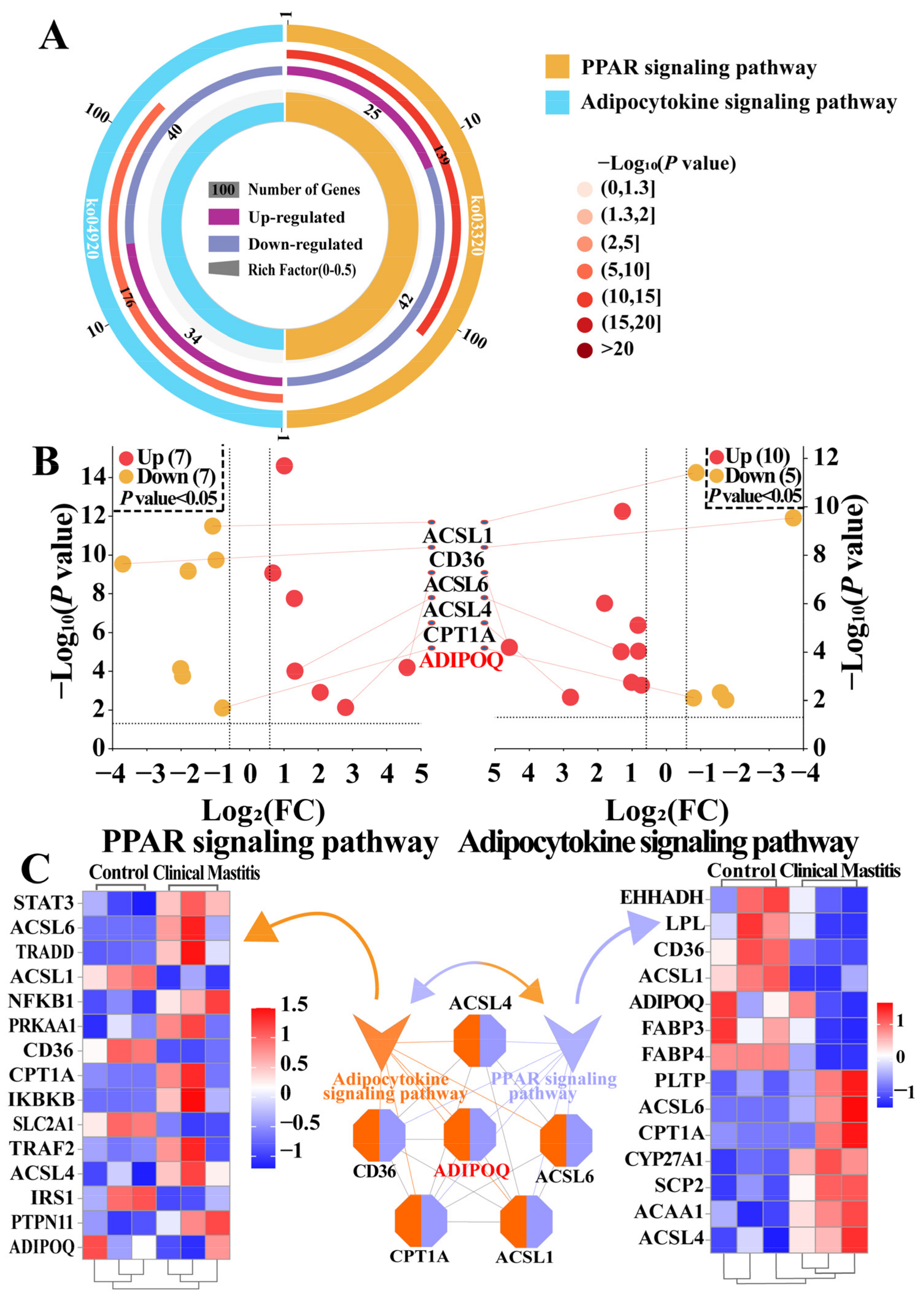
2.3. Identification of Pathways and DEPs Associated with ADIPOQ According to the DIA Data
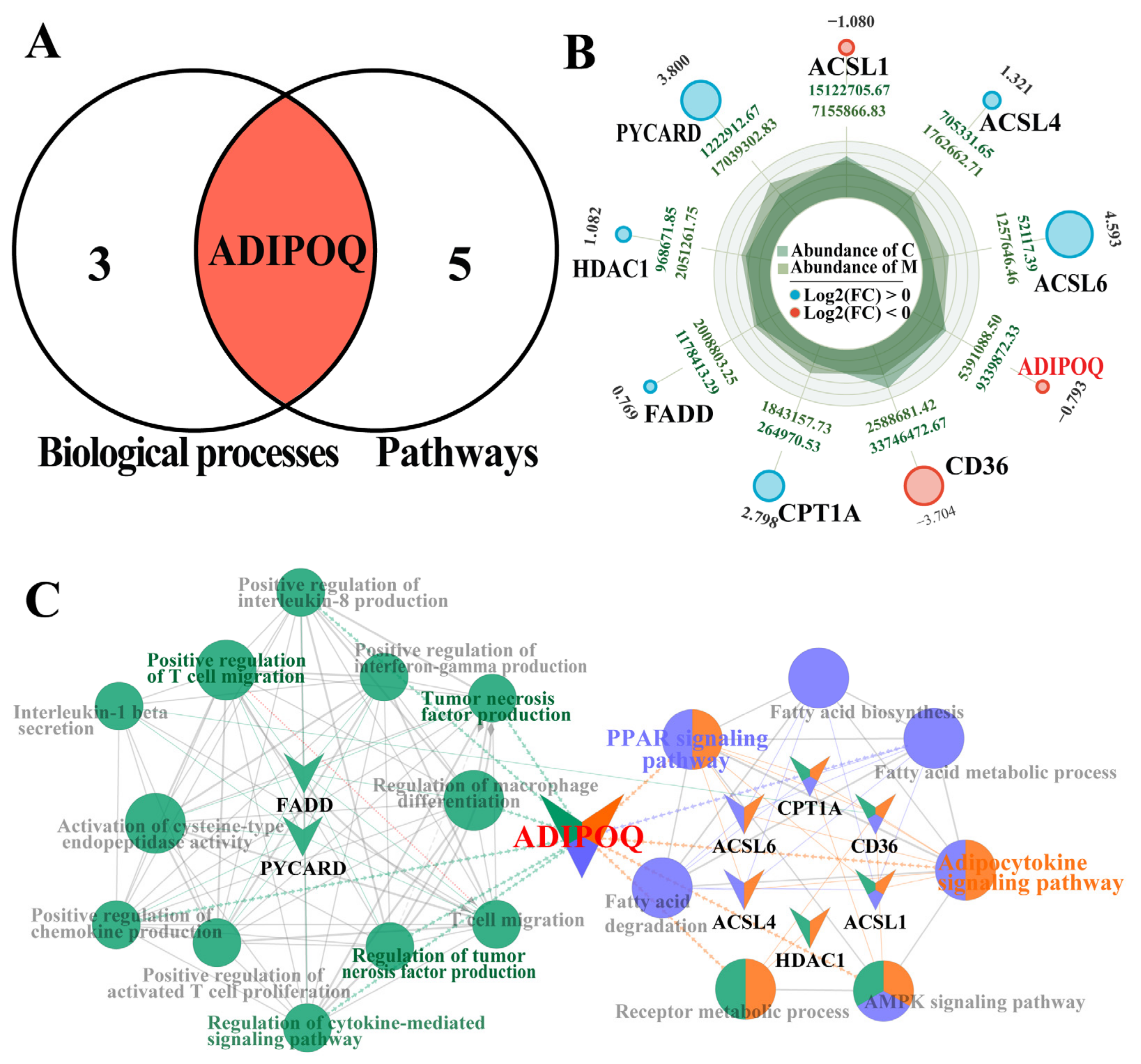
2.4. Combined Comparative Screening Based on BPs and Pathways
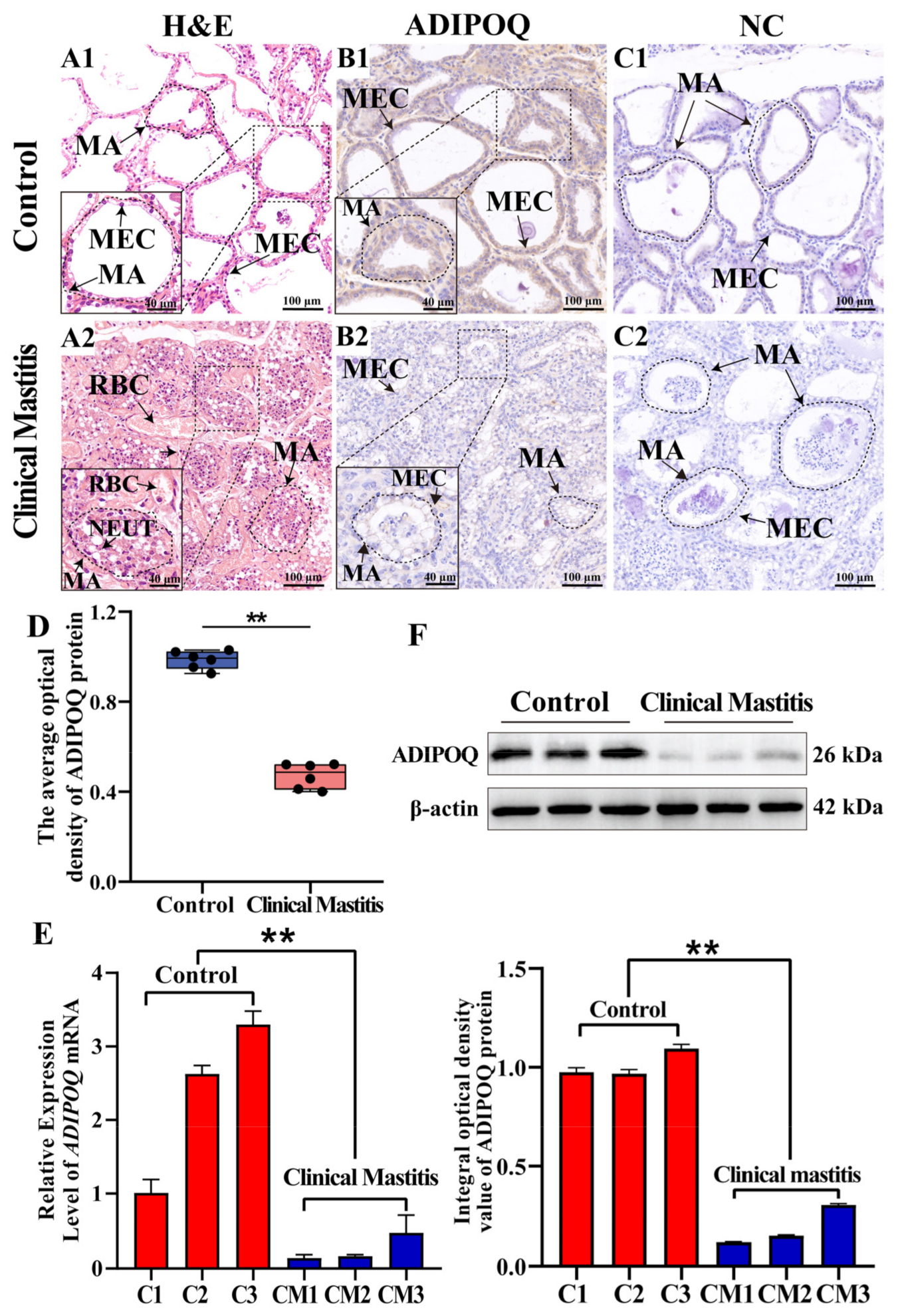
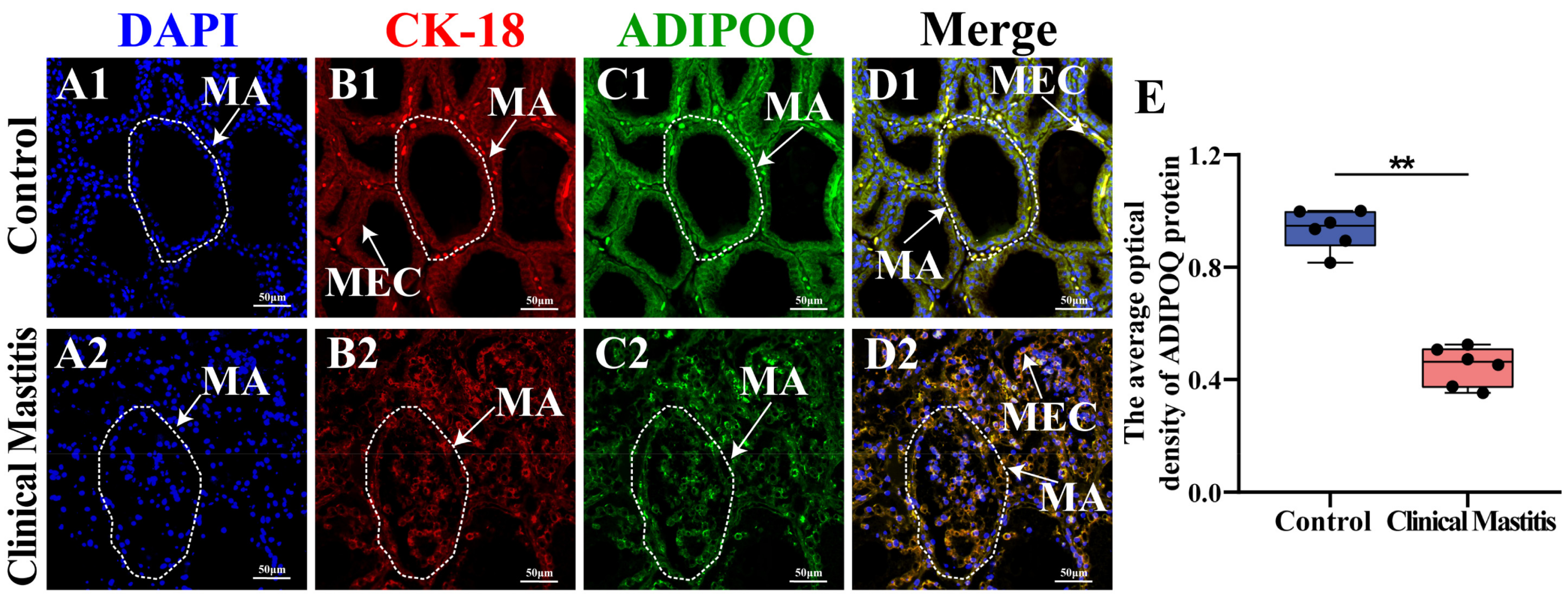
2.5. Localization and Expression Analyses of ADIPOQ in Bovine Mammary Gland Tissues
2.6. Subcellular Location Analysis of ADIPOQ Proteins in the MGs
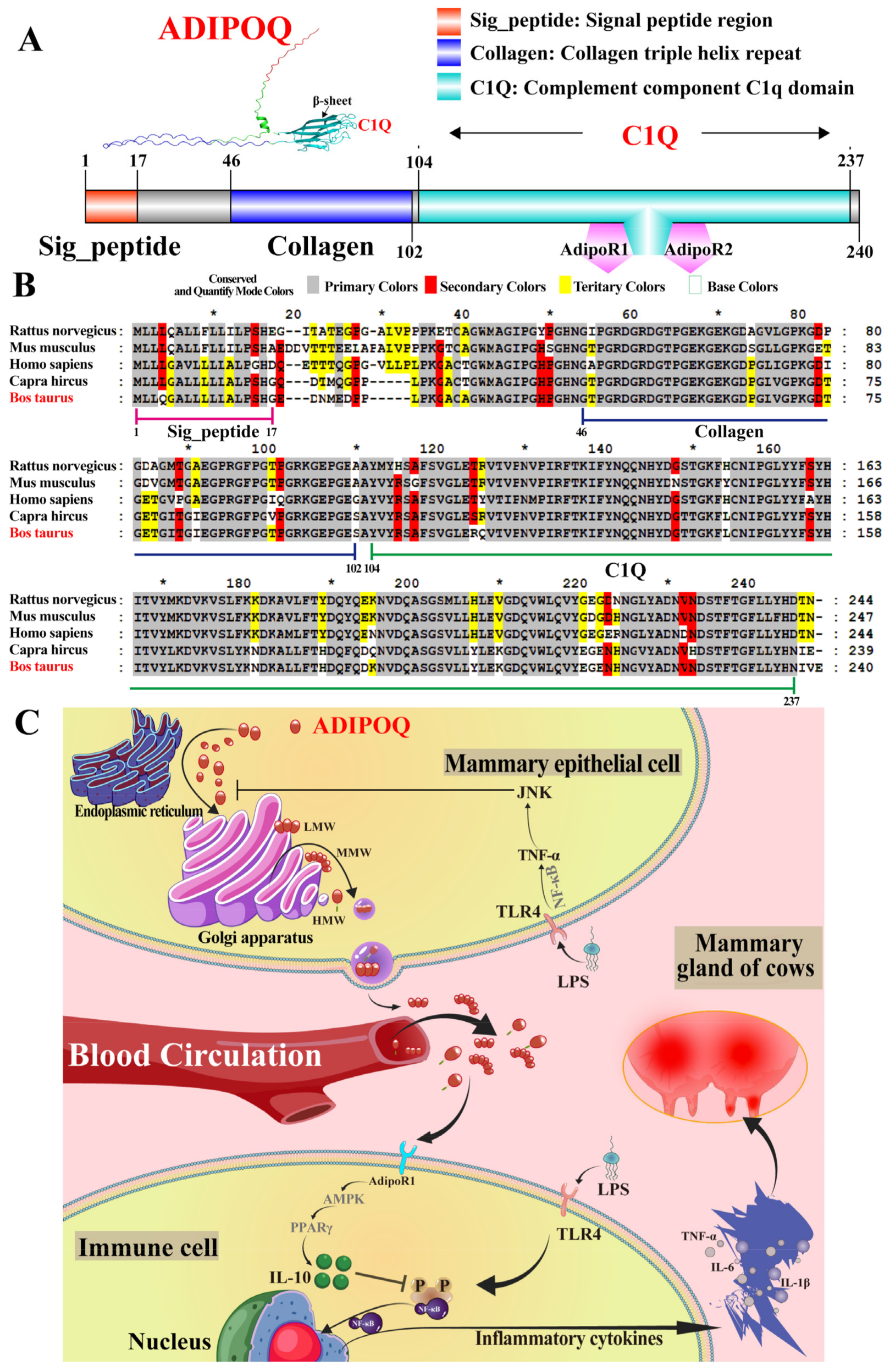
2.7. Multiple-Sequence Alignments, Tertiary Structure, and Potential Mechanism Prediction
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. RNA Extraction, cDNA Synthesis, and qRT-PCR
4.3. Western Blotting
4.4. Bioinformatic Analysis
4.5. Hematoxylin and Eosin Staining
4.6. Immunohistochemistry (IHC)
4.7. Immunofluorescence Staining (IF)
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Yatoo, M.I.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in Therapeutic and Managemental Approaches of Bovine Mastitis: A Comprehensive Review. Vet. Q. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, H.; Song, C.; Fan, S.; Fan, H.; Zhou, W.; Cao, J. Prevalence and Molecular Characterization of Multi-Resistant Escherichia coli Isolates from Clinical Bovine Mastitis in China. Anim. Biotechnol. 2024, 35, 2322541. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Y.; Zhang, W.; Chen, S.; Wen, X.; Ran, X.; Wang, H.; Zhao, J.; Qi, Y.; Xue, N. Prevalence of Subclinical Mastitis among Dairy Cattle and Associated Risks Factors in China during 2012–2021: A Systematic Review and Meta-Analysis. Res. Vet. Sci. 2022, 148, 65–73. [Google Scholar] [CrossRef]
- Rainard, P.; Gilbert, F.B.; Germon, P.; Foucras, G. Invited Review: A Critical Appraisal of Mastitis Vaccines for Dairy Cows. J. Dairy Sci. 2021, 104, 10427–10448. [Google Scholar] [CrossRef]
- Bishop, J.R.; Bodine, A.B.; Janzen, J.J. Sensitivities to Antibiotics and Seasonal Occurence of Mastitis Pathogens1. J. Dairy Sci. 1980, 63, 1134–1137. [Google Scholar] [CrossRef] [PubMed]
- Ruegg, P.L. A 100-Year Review: Mastitis Detection, Management, and Prevention. J. Dairy Sci. 2017, 100, 10381–10397. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Sanmamed, M.F.; Ochoa, M.C.; Etxeberria, I.; Aznar, M.A.; Pérez-Gracia, J.L.; Rodríguez-Ruiz, M.E.; Ponz-Sarvise, M.; Castañón, E.; Melero, I. Cytokines in Clinical Cancer Immunotherapy. Br. J. Cancer 2019, 120, 6–15. [Google Scholar] [CrossRef]
- Borish, L.C.; Steinke, J.W. 2. Cytokines and Chemokines. J. Allergy Clin. Immunol. 2003, 111, S460–S475. [Google Scholar] [CrossRef]
- Alluwaimi, A.M. The Cytokines of Bovine Mammary Gland: Prospects for Diagnosis and Therapy. Res. Vet. Sci. 2004, 77, 211–222. [Google Scholar] [CrossRef]
- Mohamed Ibrahim, H.M. Cytokine Response and Oxidative Stress Status in Dairy Cows with Acute Clinical Mastitis. J. Dairy Vet. Anim. Res. 2016, 3, 9–11. [Google Scholar] [CrossRef]
- Khan, S.; Yang, J.; Cobo, E.R.; Wang, Y.; Xu, M.; Wang, T.; Shi, Y.; Liu, G.; Han, B. Streptococcus Uberis Induced Expressions of Pro-Inflammatory IL-6, TNF-α, and IFN-γ in Bovine Mammary Epithelial Cells Associated with Inhibited Autophagy and Autophagy Flux Formation. Microb. Pathog. 2023, 183, 106270. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lacasse, P. Mammary Tissue Damage during Bovine Mastitis: Causes and Control. J. Anim. Sci. 2008, 86, 57–65. [Google Scholar] [CrossRef]
- Bannerman, D.D. Pathogen-Dependent Induction of Cytokines and Other Soluble Inflammatory Mediators during Intramammary Infection of Dairy Cows1. J. Anim. Sci. 2009, 87, 10–25. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical Review of Cytokines. Eur. J. Immunol. 2007, 37, S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, O.; Dong, X.; Roca, A.L.; Caroli, A.M.; Loor, J.J. Innate Immune Responses Induced by Lipopolysaccharide and Lipoteichoic Acid in Primary Goat Mammary Epithelial Cells. J. Anim. Sci. Biotechnol. 2017, 8, 29. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Lin, C.; Yan, R.; Li, Z.; Chen, Q.; Zhang, H.; Xu, H.; Chen, X.; Chen, Y.; et al. Regularity of Toll-Like Receptors in Bovine Mammary Epithelial Cells Induced by Mycoplasma Bovis. Front. Vet. Sci. 2022, 9, 846700. [Google Scholar] [CrossRef]
- Rainard, P.; Cunha, P.; Bougarn, S.; Fromageau, A.; Rossignol, C.; Gilbert, F.B.; Berthon, P. T Helper 17-Associated Cytokines Are Produced during Antigen-Specific Inflammation in the Mammary Gland. PLoS ONE 2013, 8, e63471. [Google Scholar] [CrossRef]
- Lin, T.; Bai, X.; Gao, Y.; Zhang, B.; Shi, J.; Yuan, B.; Chen, W.; Li, J.; Zhang, Y.; Zhang, Q.; et al. CTH/H2S Regulates LPS-Induced Inflammation through IL-8 Signaling in MAC-T Cells. Int. J. Mol. Sci. 2022, 23, 11822. [Google Scholar] [CrossRef]
- Torre, D.; Tambini, R.; Aristodemo, S.; Gavazzeni, G.; Goglio, A.; Cantamessa, C.; Pugliese, A.; Biondi, G. Anti-Inflammatory Response of IL-4, IL-10 and TGF-Beta in Patients with Systemic Inflammatory Response Syndrome. Mediators Inflamm. 2000, 9, 193–195. [Google Scholar] [CrossRef]
- Jin, Y.; Wi, H.J.; Choi, M.-H.; Hong, S.-T.; Bae, Y.M. Regulation of Anti-Inflammatory Cytokines IL-10 and TGF-β in Mouse Dendritic Cells through Treatment with Clonorchis Sinensis Crude Antigen. Exp. Mol. Med. 2014, 46, e74. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, P.; Song, D.; Song, D.; Chen, Z.; Li, H. Growth Stimulation Expressed Gene 2 (ST2): Clinical Research and Application in the Cardiovascular Related Diseases. Front. Cardiovasc. Med. 2022, 9, 1007450. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Cheng, L.; Chen, T.; Liu, X.; Zhang, C.; Aji, A.; Guo, W.; Zhu, J.; Chu, Y.; Guo, D.; et al. Bergapten Ameliorates Psoriatic Skin Lesions and IL-17A-Induced Activation of the NF-κB Signaling Pathway via the Downregulation of CYP1B1. Phytother. Res. 2024, 39, 661–675. [Google Scholar] [CrossRef] [PubMed]
- Ban, T.; Sato, G.R.; Tamura, T. Regulation and Role of the Transcription Factor IRF5 in Innate Immune Responses and Systemic Lupus Erythematosus. Int. Immunol. 2018, 30, 529–536. [Google Scholar] [CrossRef]
- Lohova, E.; Pilmane, M.; Šerstņova, K.; Melderis, I.; Gontar, Ł.; Kochański, M.; Drutowska, A.; Maróti, G.; Prieto-Simón, B. Analysis of Inflammatory and Regulatory Cytokines in the Milk of Dairy Cows with Mastitis: A Comparative Study with Healthy Animals. Immunol. Invest. 2024, 53, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Choubey, M.; Bora, P.; Krishna, A. Adiponectin and Chemerin: Contrary Adipokines in Regulating Reproduction and Metabolic Disorders. Reprod. Sci. 2018, 25, 1462–1473. [Google Scholar] [CrossRef]
- Lim, J.-Y.; Templeton, S.P. Regulation of Lung Inflammation by Adiponectin. Front. Immunol. 2023, 14, 1244586. [Google Scholar] [CrossRef] [PubMed]
- Begum, M.; Choubey, M.; Tirumalasetty, M.B.; Arbee, S.; Mohib, M.M.; Wahiduzzaman, M.; Mamun, M.A.; Uddin, M.B.; Mohiuddin, M.S. Adiponectin: A Promising Target for the Treatment of Diabetes and Its Complications. Life 2023, 13, 2213. [Google Scholar] [CrossRef]
- da Silva Rosa, S.C.; Liu, M.; Sweeney, G. Adiponectin Synthesis, Secretion and Extravasation from Circulation to Interstitial Space. Physiology 2021, 36, 134–149. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Salamon, H.; Nissim-Eliraz, E.; Ardronai, O.; Nissan, I.; Shpigel, N.Y. The Role of O-Polysaccharide Chain and Complement Resistance of Escherichia Coli in Mammary Virulence. Vet. Res. 2020, 51, 77. [Google Scholar] [CrossRef]
- Jian, M.; Kwan, J.S.-C.; Bunting, M.; Ng, R.C.-L.; Chan, K.H. Adiponectin Suppresses Amyloid-β Oligomer (AβO)-Induced Inflammatory Response of Microglia via AdipoR1-AMPK-NF-κB Signaling Pathway. J. Neuroinflammation 2019, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Li, X.; Weng, J.; Wei, C.; He, Z.; Doycheva, D.M.; Lenahan, C.; Tang, W.; Zhou, J.; Liu, Y.; et al. Adiponectin Ameliorates GMH-Induced Brain Injury by Regulating Microglia M1/M2 Polarization Via AdipoR1/APPL1/AMPK/PPARγ Signaling Pathway in Neonatal Rats. Front. Immunol. 2022, 13, 873382. [Google Scholar] [CrossRef]
- Hu, Q.; Cui, X.; Tao, L.; Xiu, L.; Wang, T.; Wang, X. Staphylococcus Aureus Induces Apoptosis in Primary Bovine Mammary Epithelial Cells through Fas-FADD Death Receptor-Linked Caspase-8 Signaling. DNA Cell Biol. 2014, 33, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lv, N.; Zhang, S.; Shui, G.; Qian, H.; Zhang, J.; Chen, Y.; Ye, J.; Xie, Y.; Shen, Y.; et al. Cidea Is an Essential Transcriptional Coactivator Regulating Mammary Gland Secretion of Milk Lipids. Nat. Med. 2012, 18, 235–243. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, M.-C.; Yang, J.; Wang, J.-F.; Zhu, Y.-H. Lactobacillus Rhamnosus GR-1 Ameliorates Escherichia Coli-Induced Inflammation and Cell Damage via Attenuation of ASC-Independent NLRP3 Inflammasome Activation. Appl. Environ. Microbiol. 2016, 82, 1173–1182. [Google Scholar] [CrossRef]
- Ajuwon, K.M.; Spurlock, M.E. Adiponectin Inhibits LPS-Induced NF-kappaB Activation and IL-6 Production and Increases PPARgamma2 Expression in Adipocytes. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1220–R1225. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, P.; Zhao, Y.; Wan, T.; Yuan, K.; Xiong, Y.; Wu, M.; Zha, M.; Li, Y.; Jiang, T.; et al. Endothelial Caveolin-1 Regulates Cerebral Thrombo-Inflammation in Acute Ischemia/Reperfusion Injury. EBioMedicine 2022, 84, 104275. [Google Scholar] [CrossRef]
- Ye, M.; Hu, C.; Chen, T.; Yu, P.; Chen, J.; Lu, F.; Xu, L.; Zhong, Y.; Yan, L.; Kan, J.; et al. FABP5 Suppresses Colorectal Cancer Progression via mTOR-Mediated Autophagy by Decreasing FASN Expression. Int. J. Biol. Sci. 2023, 19, 3115–3127. [Google Scholar] [CrossRef]
- Cohen, S.S.; Gammon, M.D.; North, K.E.; Millikan, R.C.; Lange, E.M.; Williams, S.M.; Zheng, W.; Cai, Q.; Long, J.; Smith, J.R.; et al. ADIPOQ, ADIPOR1, and ADIPOR2 Polymorphisms in Relation to Serum Adiponectin Levels and Body Mass Index in Black and White Women. Obesity 2011, 19, 2053–2062. [Google Scholar] [CrossRef]
- Mallardo, M.; Signoriello, E.; Lus, G.; Daniele, A.; Nigro, E. Adiponectin Alleviates Cell Injury Due to Cerebrospinal Fluid from Multiple Sclerosis Patients by Inhibiting Oxidative Stress and Proinflammatory Response. Biomedicines 2023, 11, 1692. [Google Scholar] [CrossRef]
- Tilg, H.; Wolf, A.M. Adiponectin: A Key Fat-Derived Molecule Regulating Inflammation. Expert Opin. Ther. Targets 2005, 9, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, B.; Peng, B.; Wang, J.; Hu, Y.; Wang, R.; Wang, S. Different Dose of Sucrose Consumption Divergently Influences Gut Microbiota and PPAR-γ/MAPK/NF-κB Pathway in DSS-Induced Colitis Mice. Nutrients 2022, 14, 2765. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agrawal, S.; Gollapudi, S. Increased Activation and Cytokine Secretion in B Cells Stimulated with Leptin in Aged Humans. Immun. Ageing 2013, 10, 3. [Google Scholar] [CrossRef]
- Su, Y.; Ren, J.; Zhang, J.; Zheng, J.; Zhang, Q.; Tian, Y.; Zhang, Y.; Jiang, Y.; Zhang, W. Lactobacillus Paracasei JY062 Alleviates Glucolipid Metabolism Disorders via the Adipoinsular Axis and Gut Microbiota. Nutrients 2024, 16, 267. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a Signaling Receptor and Fatty Acid Transporter That Regulates Immune Cell Metabolism and Fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef]
- Li, L.O.; Mashek, D.G.; An, J.; Doughman, S.D.; Newgard, C.B.; Coleman, R.A. Overexpression of Rat Long Chain Acyl-CoA Synthetase 1 Alters Fatty Acid Metabolism in Rat Primary Hepatocytes. J. Biol. Chem. 2006, 281, 37246–37255. [Google Scholar] [CrossRef]
- Miguel, V.; Rey-Serra, C.; Tituaña, J.; Sirera, B.; Alcalde-Estévez, E.; Herrero, J.I.; Ranz, I.; Fernández, L.; Castillo, C.; Sevilla, L.; et al. Enhanced Fatty Acid Oxidation through Metformin and Baicalin as Therapy for COVID-19 and Associated Inflammatory States in Lung and Kidney. Redox. Biol. 2023, 68, 102957. [Google Scholar] [CrossRef]
- Liu, H.; Tang, H.; Wang, R.; Xu, J. Adiponectin Antagonises LPS-regulated Secretion of Inflammatory Factors in Airway Epithelial Cells, and Its Expression Is Regulated by Many Factors. Cell Biochem. Funct. 2021, 39, 139–147. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, T.; Bai, X.; An, X.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X.; Zhang, Q. Sulfur Amino Acid Metabolism and the Role of Endogenous Cystathionine-γ-Lyase/H2S in Holstein Cows with Clinical Mastitis. Animals 2022, 12, 1451. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Chen, N.; Yang, Z.; Gao, Y.; Zhang, B.; Li, J.; Zhou, B.; Tang, Z.; Dong, W.; Zhao, X.; et al. Role of Adiponectin in Regulating Cytokines and Its Contribution to the Occurrence and Progression of Clinical Mastitis in Holstein Cows. Int. J. Mol. Sci. 2025, 26, 2898. https://doi.org/10.3390/ijms26072898
Zhang J, Chen N, Yang Z, Gao Y, Zhang B, Li J, Zhou B, Tang Z, Dong W, Zhao X, et al. Role of Adiponectin in Regulating Cytokines and Its Contribution to the Occurrence and Progression of Clinical Mastitis in Holstein Cows. International Journal of Molecular Sciences. 2025; 26(7):2898. https://doi.org/10.3390/ijms26072898
Chicago/Turabian StyleZhang, Junjun, Na Chen, Zhen Yang, Yumeng Gao, Bohao Zhang, Jianfu Li, Bin Zhou, Zhixiong Tang, Weitao Dong, Xingxu Zhao, and et al. 2025. "Role of Adiponectin in Regulating Cytokines and Its Contribution to the Occurrence and Progression of Clinical Mastitis in Holstein Cows" International Journal of Molecular Sciences 26, no. 7: 2898. https://doi.org/10.3390/ijms26072898
APA StyleZhang, J., Chen, N., Yang, Z., Gao, Y., Zhang, B., Li, J., Zhou, B., Tang, Z., Dong, W., Zhao, X., Zhang, Y., & Zhang, Q. (2025). Role of Adiponectin in Regulating Cytokines and Its Contribution to the Occurrence and Progression of Clinical Mastitis in Holstein Cows. International Journal of Molecular Sciences, 26(7), 2898. https://doi.org/10.3390/ijms26072898






