Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases
Abstract
:1. Introduction
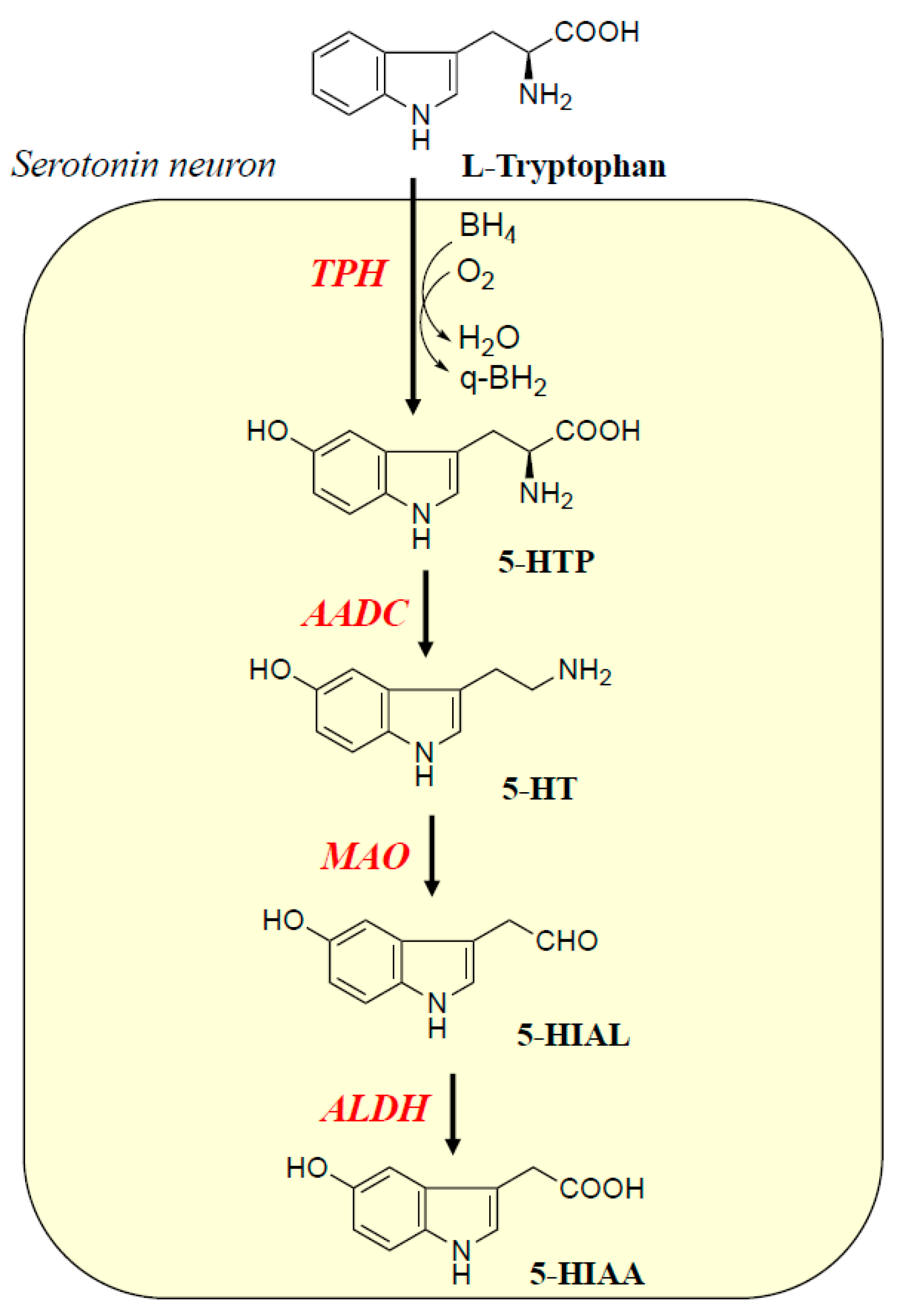
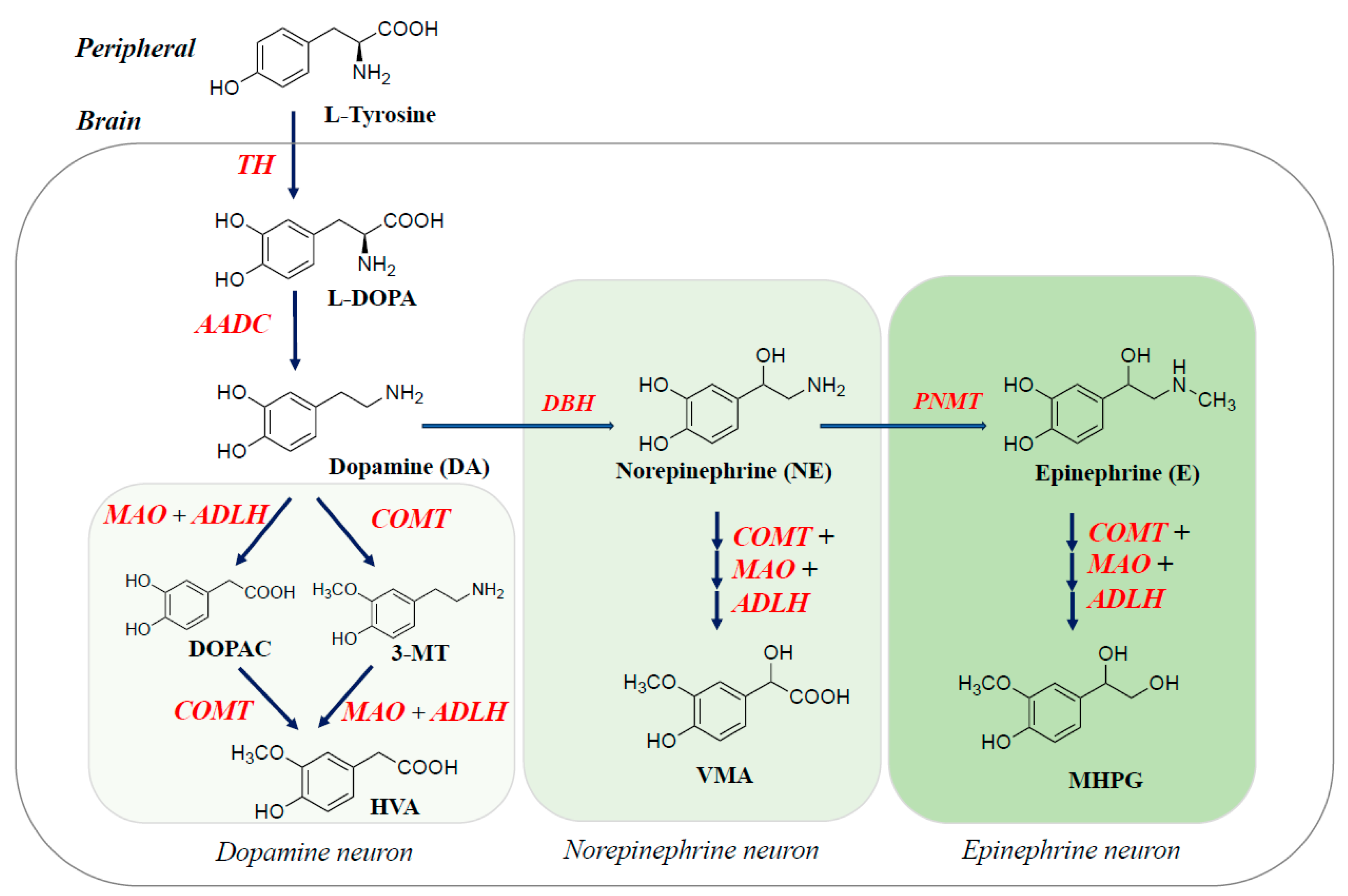
2. Biosynthesis, Metabolism, and Function of Monoamine Neurotransmitters and Related Enzymes in the Brain
3. Neuroprotective Effects of Phytochemicals in Brain Health
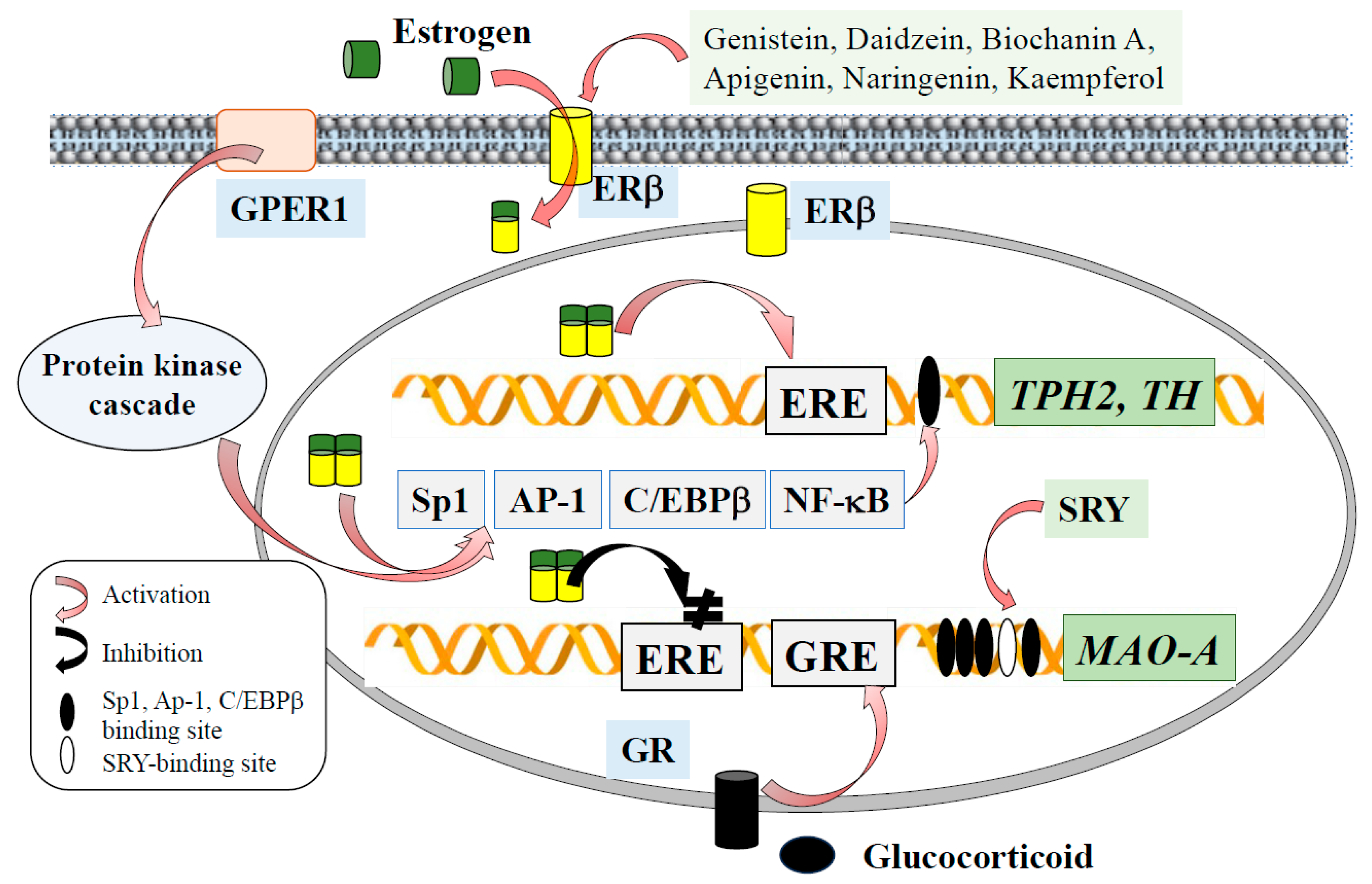
4. Sex- and Stress-Related Hormones Modulate Monoamine Neurotransmission in the Brain
5. Phytoestrogens Promote Expression and Activity of Tryptophan Hydroxylase and Tyrosine Hydroxylase
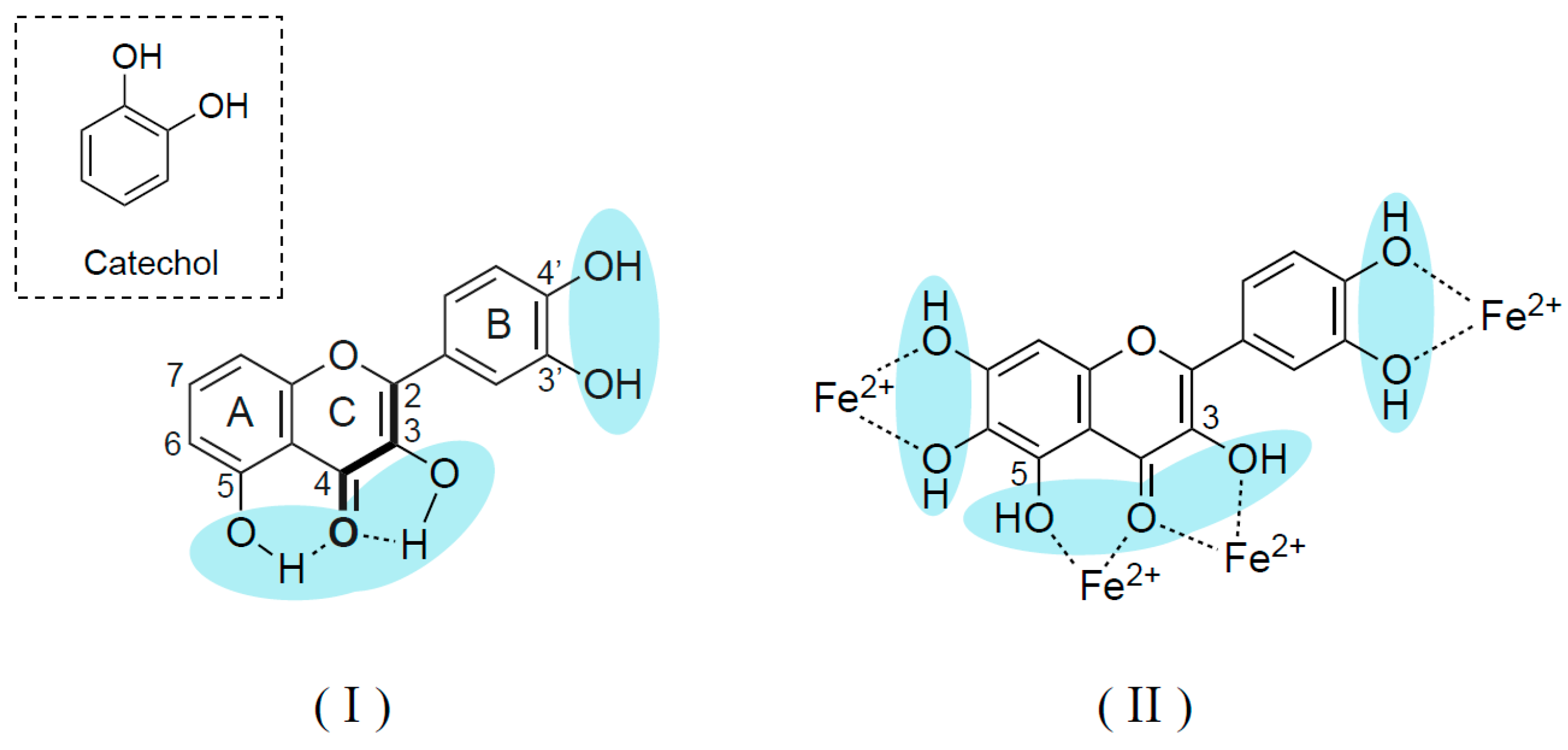
6. Phytochemicals Inhibit Expression and Activity of Monoamine Oxidase and Catechol-O-Methyltransferase
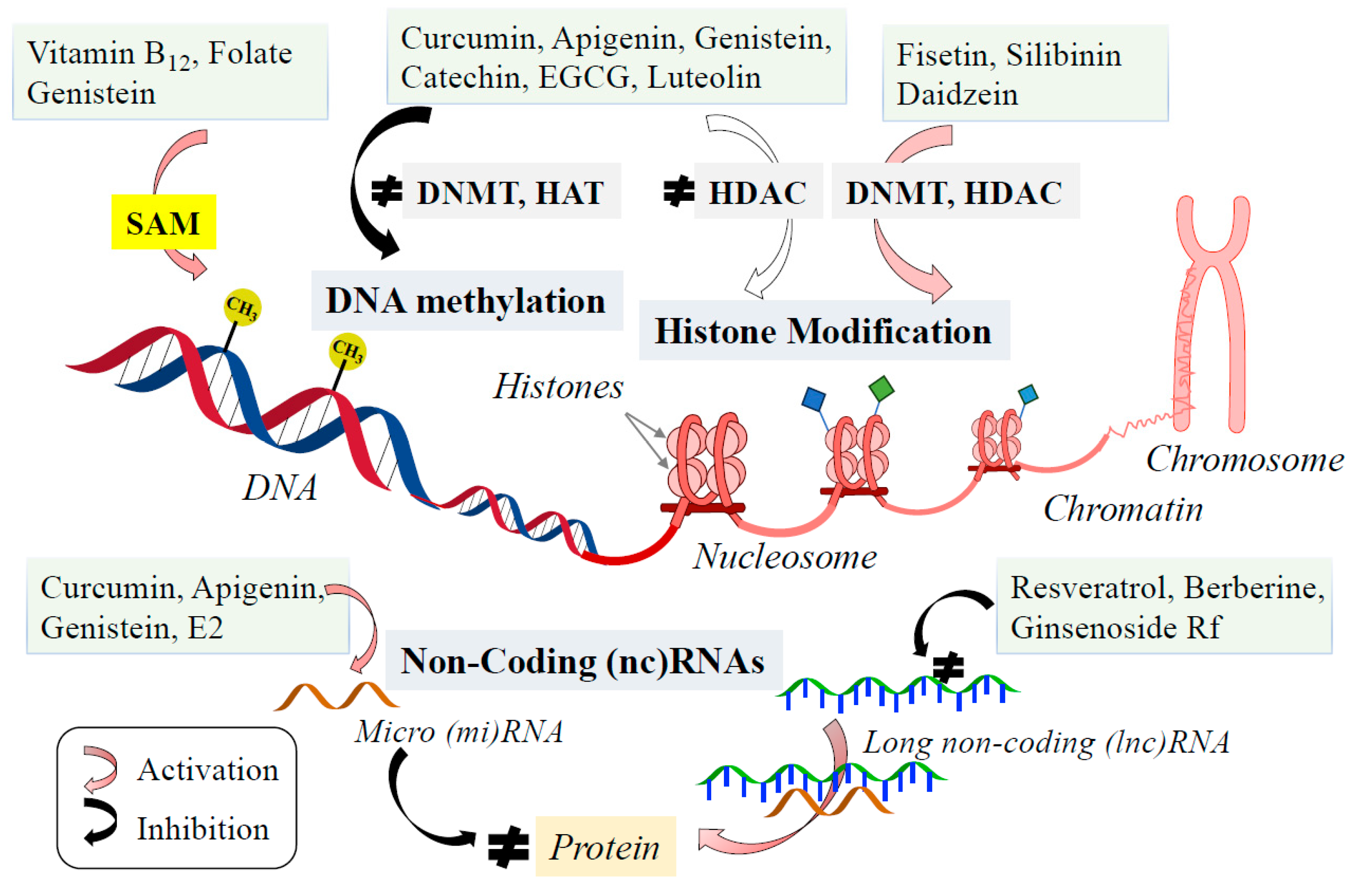
7. Epigenetic Phytochemicals Affect Monoamine Neurotransmission and Prevent and Treat Monoamine-Related Disorders
8. Clinical Applications of Phytochemical Treatment in Neuropsychiatric Disorders
9. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| AP-1 | activating protein-1 |
| BH4 | (6R)-L-tetrahydrobiopterin |
| CREB | CRE binding protein |
| ΔΨm | mitochondrial membrane potential |
| E2 | 17β-estradiol |
| ER | estrogen receptor |
| ERE | estrogen response element |
| GTPCH | guanidine triphosphate cyclohydrolase |
| mPTP | mitochondrial permeability transition pore |
| NTF | neurotrophic factor |
| Nrf2 | nuclear factor erythroid 3-related factor |
| PGC-1α | peroxisome proliferator-activated receptor γ coactivator α |
| SERM | ER type-specific ER modulators |
| SERT | 5-HT transporter |
| Sp1 | simian virus 40 promoter factor 1 |
| SRY | sex-determining region Y |
References
- Whitaker-Azmitia, P.M. Serotonin and brain development: Role in human developmental diseases. Brain Res. Bull. 2001, 56, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Lauder, J.M. Serotonin as a differentiation signal. Dev. Neurosci. 2016, 38, 239–240. [Google Scholar] [CrossRef] [PubMed]
- Lesch, K.P.; Waider, J. Serotonin in the modulation of neural plasticity and networks: Implications for neurodevelopmental disorders. Neuron 2012, 76, 175–191. [Google Scholar] [CrossRef]
- Klein, M.O.; Battagello, D.S.; Cardoso, A.R.; Hauser, D.N.; Bittencourt, J.C.; Correa, R.G. Dopamine: Functions, signaling, and association with neurological diseases. Cell. Mol. Neurobiol. 2019, 39, 31–59. [Google Scholar] [CrossRef] [PubMed]
- Burgoyne, R.D.; Morgan, A. Secretory granule exocytosis. Physiol. Rev. 2003, 83, 581–632. [Google Scholar] [CrossRef]
- Aurora, M.; Per, M.K.; Jan, H. A Structural Approach into Human Tryptophan Hydroxylase and its Implications for the Regulation of Serotonin Biosynthesis. Curr. Med. Chem. 2001, 8, 1077–1091. [Google Scholar] [CrossRef]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137 (Suppl. S1), 1539S–1547S. [Google Scholar] [CrossRef]
- Dunkley, P.R.; Bobrovskaya, L.; Graham, M.E.; von Nagy-Felsobuki, E.I.; Dickson, P.W. Tyrosine hydroxylase phosphorylation: Regulation and consequence. J. Neurochem. 2004, 91, 1025–1043. [Google Scholar] [CrossRef]
- Kumer, S.C.; Vrana, K.E. Intricate regulation of tyrosine hydroxylase activity and gene expression. J. Neurochem. 1996, 67, 443–462. [Google Scholar] [CrossRef]
- Chen, G.L.; Miller, G.M. Advances in tryptophan hydroxylase-2 gene expression regulation: New insights into serotonin-stress interaction and clinical implication. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012, 159B, 152–171. [Google Scholar] [CrossRef]
- Bendis, P.C.; Zimmerman, S.; Onisiforou, A.; Zanos, P.; Georgiou, P. The impact of estradiol on serotonin, glutamate, and dopamine systems. Front. Neurosci. 2024, 18, 1348551. [Google Scholar] [CrossRef]
- Ferrnstrom, J.D. Effects on the diet on brain neurotransmitters. Metabolism 1977, 26, 207–223. [Google Scholar] [CrossRef] [PubMed]
- Ki, M.R.; Youn, S.; Kim, D.H.; Pack, S.P. Natural compounds for preventing age-related diseases and cancers. Int. J. Mol. Sci. 2024, 25, 7530. [Google Scholar] [CrossRef] [PubMed]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, L. Neuroprotective polyphenols: A modulatory action on neurotransmission pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M. Disease-modifying treatment of Parkinson’s disease by phytochemicals: Targeting multiple pathogenic factors. J. Neural Transm. 2022, 129, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Noori, T.; Sureda, A.; Sobarzo-Sanchez, E.; Shirooie, S. The role of natural products in treatment of depressive disorder. Curr. Neuropharmacol. 2022, 20, 929–949. [Google Scholar] [CrossRef]
- Gorzkiewicz, J.; Bartosz, G.; Sadowska-Bartosz, I. The potential effects of phytoestrogens: The role in neuroprotection. Molecules 2021, 26, 2954. [Google Scholar] [CrossRef]
- Yanagihara, N.; Zhang, H.; Toyohira, Y.; Takahashi, R.; Ueno, S.; Tsutsui, M.; Takahashi, K. New insight into the pharmacological potential of plant flavonoids in the catecholamine system. J. Pharnacol. Sci. 2014, 124, 123–128. [Google Scholar] [CrossRef]
- Shah, R.; Courtiol, E.; Castellanos, F.X.; Teixeira, C.M. Abnormal serotonin levels during perinatal development lead to behavioral deficits in adulthood. Front. Behav. Neurosci. 2018, 12, 114. [Google Scholar] [CrossRef]
- Pandareesh, M.D.; Mythri, R.B.; Srinivas Bharath, M.M. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Inaba-Hasegawa, K.; Shamoto-Nagai, M.; Maruyama, W. Neurotrophic function of phytochemicals for neuroprotection in aging and neurodegenerative disorders: Modulation of intracellular signaling and gene expression. J. Neural Transm. 2017, 124, 1515–1527. [Google Scholar] [CrossRef]
- Naoi, M.; Wu, Y.; Shamoto-Nagai, M.; Maruyama, W. Mitochondrial in neuroprotection by phytochemicals: Bioactive polyphenols modulate mitochondrial apoptosis system, function and structure. Int. J. Mol. Sci. 2019, 20, 2451. [Google Scholar] [CrossRef]
- Wu, Y.; Shamoto-Nagai, M.; Maruyama, W.; Osawa, T.; Naoi, M. Phytochemicals prevent mitochondrial membrane permeabilization and protect SH-SY5Y cells against apoptosis induced by PK11195, a ligand for outer membrane translocator protein. J. Neural Transm. 2017, 124, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Asgharian, P.; Quispe, C.; Herrea-Bravo, J.; Sabernavaei, M.; Hosseini, K.; Forouhandeh, H.; Ebrahimi, T.; Sharafi-Badr, P.; Tarhriz, V.; Soofiyani, S.R.; et al. Pharmacological effects and therapeutic potential of natural compounds in neuropsychiatric disorders: An update. Front. Pharmacol. 2022, 13, 926607. [Google Scholar] [CrossRef]
- Fitzpatrick, P.F. Mechanism of aromatic amino acid hydroxylation. Biochemistry 2003, 42, 14083–14091. [Google Scholar] [CrossRef]
- Walther, D.J.; Baker, M. A unique central tryptophan hydroxylase isoform. Biochem. Pharmacol. 2003, 66, 1673–1680. [Google Scholar] [CrossRef]
- Murphy, K.L.; Zhang, X.; Gainetdinov, R.R.; Beaulieu, J.M.; Caron, M.G. A regulatory domain in the N terminus of tryptophan hydroxylase 2 controls enzyme expression. J. Biol. Chem. 2008, 283, 13216–13224. [Google Scholar] [CrossRef]
- Zill, P.; Baghai, T.C.; Zwanzger, P.; Schüle, C.; Eser, D.; Ruppercht, R.; Möller, H.J.; Bondy, B.; Ackenheil, M. SNP and haplotype analysis of a novel tryptophan isoform (TPH2) gene provide evidence for association with major depression. Mol. Psychiatry 2004, 9, 1030–1036. [Google Scholar] [CrossRef]
- Cichon, S.; Winge, I.; Mattheisen, M.; Georgi, A.; Karpushova, A.; Freudenberg, J.; Freudenberg-Hua, Y.; Babadjanova, G.; Van Den Bogaert, A.; Abramova, L.I.; et al. Brain-specific tryptophan hydroxylase 2 (TPH2): A functional Pro206Ser substitution and variation in the 5′-region are associated with bipolar affective disorder. Hum. Mol. Genet. 2008, 17, 87–97. [Google Scholar] [CrossRef]
- Zhang, X.; Beaulieu, J.M.; Gainetdinov, R.R.; Caron, M.G. Functional polymorphisms of the brain serotonin synthesizing enzyme tryptophan hydroxylase-2. Cell. Mol. Life Sci. 2006, 63, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Bademci, G.; Vance, J.M.; Wang, L. Tyrosine hydroxylase gene: Another piece of the genetic puzzle of Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2012, 11, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, A.J.; Fitzpatrick, P.F. Effects of phosphorylation of serine 40 of tyrosine hydroxylase on binding of catecholamines: Evidence of a novel regulatory mechanism. Biochemistry 1998, 37, 8980–8986. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakshima, A.; Ichinose, H.; Kobayashi, K. Juman tyrosine hydroxylase in Parkinson’s disease and in related disorders. J. Neural Transm. 2019, 126, 397–409. [Google Scholar] [CrossRef]
- Willemsen, M.A.; Verbeek, M.M.; Kamsteeg, E.J.; de Rijk-van Andel, J.F.; Aeby, A.; Blau, N.; Burlina, A.; Donati, M.A.; Geurtz, B.; Grattan-Smith, P.J.; et al. Tyrosine hydroxylase deficiency: A treatable disorder of brain catecholamine biosynthesis. Brain 2010, 1333 Pt 6, 1810–1822. [Google Scholar] [CrossRef]
- Nagatomo-Combs, K.; Piech, K.M.; Best, J.A.; Sun, B.; Tank, A.W. Tyrosine hydroxylase gene promoter activity is regulated by both cyclic AMP-responsive element and AP1 sites following calcium influx. J. Biol. Chem. 1997, 272, 6051–6058. [Google Scholar] [CrossRef]
- Sabban, E.L. Control of tyrosine hydroxylase gene expression in chromatin and PC12 cells. Semin. Cell Dev. Bilo. 1997, 8, 101–111. [Google Scholar] [CrossRef]
- Montioli, R.; Voltattorni, C.B. Aromatic amino acid decarboxylase deficiency: The added value of biochemistry. Int. J. Mol. Sci. 2021, 22, 3146. [Google Scholar] [CrossRef]
- Sumi-Ichinose, C.; Ichinose, H.; Takahashi, E.; Hori, T.; Nagatsu, T. Molecular cloning of genomic DNA and chromosomal assignment of the gene for human aromatic L-amino acid decarboxylase, the enzyme for catecholamine and serotonin synthesis. Biochemistry 1992, 31, 2229–2238. [Google Scholar] [CrossRef]
- Rizzi, S.; Spagnoli, C.; Frattini, D.; Pisani, F.; Fusco, C. Clinical features in aromatic L-amino acid decarboxylase (AADC) deficiency: A systematic review. Behav. Neurol. 2022, 2022, 2210555. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C. Monoamine oxidase isoenzymes: Genes, functions and targets for behavior and cancer therapy. J. Neural Transm. 2018, 125, 1553–1566. [Google Scholar] [CrossRef]
- Naoi, M.; Riederer, P.; Maruyama, W. Modulation of monoamine oxidase (MAO) expression in neuropsychiatric disorders: Genetic and environmental factors involved in type A MAO expression. J. Neural Transm. 2016, 123, 91–106. [Google Scholar] [CrossRef]
- Zhu, Q.; Shih, J.C. An extensive repeat structure down-regulates human monoamine oxidase A promoter activity independent of an initiator-like sequence. J. Neurochem. 1997, 69, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Contini, V.; Marques, F.Z.C.; Garcia, C.E.D.; Hutz, M.H.; Bau, C.H.D. MAOA-uVNTR polymorphism in a Brazilian sample: Further support for the association with impulsive behaviors and alcohol dependence. Am. J. Med. Gent. B. Neuropsychiatr. Genet. 2006, 141B, 305–308. [Google Scholar] [CrossRef]
- Fan, M.; Liu, B.; Jiang, T.; Jiang, X.; Zhao, H.; Zhang, J. Meta-analysis of the association between the monoamine oxidase-A gene and mood disorders. Psychiatr. Genet. 2010, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Brunner, H.G.; Nelen, M.; Breakfield, X.O.; Ropers, H.H.; van Oost, B.A. Abnormal behavior associated with a point mutation in the structural gene for monoamine oxidase A. Science 1991, 262, 578–580. [Google Scholar] [CrossRef]
- Lenders, J.W.; Eisenhofer, G.; Abeling, N.G.; Berger, W.; Murphy, D.L.; Konings, C.H.; Wagemakers, L.M.; Kopin, I.J.; Karoum, F.; van Gennip, A.H.; et al. Specific genetic deficiencies of the A and B isoenzymes of monoamine oxidase are characterized by distinct neurochemical and clinical phenotypes. J. Clin. Investig. 1996, 97, 1010–1019. [Google Scholar] [CrossRef]
- Matsumoto, M.; Shannon Weickert, C.; Akil, M.; Lipska, B.K.; Hyde, T.M.; Herman, M.M.; Kleinman, J.E.; Weinberger, D.R. Catechol O-methyltransferase mRNA expression in human and rat brain: Evidence for a role in cortical neuronal function. Neuroscience 2003, 116, 127–137. [Google Scholar] [CrossRef]
- Harrison, P.J.; Tunbridge, E.M. Catechol-O-methyltransferase: A gene contributing to sex differences in brain function, and to sexual dimorphism in the preposition to psychiatric disorders. Neuropsychopharmacology 2008, 33, 3037–3045. [Google Scholar] [CrossRef]
- Bastos, P.; Gomes, T.; Ribeiro, L. Catechol-O-methyltransferase: An update on its role in cancer, neurological and cardiovascular diseases. Rev. Physiol. Biochem. Pharmacol. 2017, 173, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Clelland, J.D.; Hesson, H.; Ramiah, K.; Anderson, J.; Thengampallil, A.; Girgis, R.R.; Chelland, C.L. The relationship between COMT, proline, and negative symptoms in clinical high risk and recent psychosis onset. Transl. Psychiatry 2024, 14, 409. [Google Scholar] [CrossRef] [PubMed]
- Babazadeh, A.; Vahed, F.M.; Liu, Q.; Siddiqui, S.A.; Khazazmi, M.S.; Jafari, S.M. Natural bioactive molecules as neuromedicines for the treatment/prevention of neurodegenerative diseases. ACS Omega 2023, 8, 3667–3683. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and human health: Prevention of disease and mechanisms of action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Cheynier, V. Polyphenols in foods are more complex than often thought. Am. J. Clin. Nutr. 2005, 81, 223S–229S. [Google Scholar] [CrossRef]
- Perron, N.R.; Brumahim, J.L. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell. Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Aspects. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Leonardo, C.C.; Dore, S. Dietary flavonoids are neuroprotective through Nrf2-coordinated induction of endogenous cytoprotective proteins. Nutr. Neurosci. 2011, 14, 226–236. [Google Scholar] [CrossRef]
- Habtemariam, S. The Nrf2/HO-1 axis as targets for flavanones: Neuroprotection by pinocembrin, naringenin, and eriodictyol. Oxid. Med. Cell Longev. 2019, 13, 4724920. [Google Scholar] [CrossRef]
- Williams, R.J.; Spencer, J.P.E.; Rice-Evans, C. Flavonoids: Antioxidants or signaling molecules? Free Rad. Biol. Med. 2004, 36, 838–849. [Google Scholar] [CrossRef]
- Spencer, J.P.E. The interactions of flavonoids within neuronal signaling pathways. Genes Nutr. 2007, 2, 257–273. [Google Scholar] [CrossRef] [PubMed]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuz, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Devel. Ther. 2015, 10, 23–42. [Google Scholar] [CrossRef]
- Venkatesan, R.; Ji, E.; Kim, S.Y. Phytochemicals that regulate neurodegenerative disease by targeting neurotrophins: A comprehensive review. Biomed. Res. Int. 2015, 2015, 814068. [Google Scholar] [CrossRef] [PubMed]
- Neshatdoust, S.; Saunders, C.; Castle, S.M.; Vauzour, D.; Williams, C.; Butler, L.; Lovegrove, J.A.; Spencer, J.P. High-flavonoid intake induced cognitive improvement linked to changes in serum brain-derived neurotrophic factor: Two randomized, controlled trials. Nutr. Healthy Aging 2016, 4, 81–93. [Google Scholar] [CrossRef]
- Fanaei, H.; Khayat, S.; Kasaeian, A.; Jvadimehr, M. Effect of curcumin on serum brain-derived neurotrophic factor levels in women with premenstrual syndrome: A randomized, double-blind, placebo-controlled trial. Neuropeptides 2016, 56, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Kessas, K.; Chouari, Z.; Ghzaiel, I.; Zarrouk, A.; Ksila, M.; Ghrairi, T.; El Midaoui, A.; Lizard, G.; Kharoubi, O. Role of bioactive compounds in the regulation of mitochondrial dysfunctions in the brain and age-related neurodegenerative diseases. Cells 2022, 11, 257. [Google Scholar] [CrossRef]
- Lagouge, M.; Argann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- Nagley, P.; Higgins, G.C.; Atkin, J.D.; Beart, P.M. Multifaceted death orchestrated by mitochondria in neurons. Biochim. Biophys. Acta 2010, 1802, 167–185. [Google Scholar] [CrossRef]
- Wu, Y.; Kazumura, K.; Maruyama, W.; Osawa, T.; Naoi, M. Rasagiline and selegiline suppress calcium efflux from mitochondria by PK11195-induced opening of mitochondrial permeability transition pore: A novel anti-apoptotic function for neuroprotection. J. Neural Transm. 2015, 122, 1399–1407. [Google Scholar] [CrossRef]
- Wu, Y.; Shamoto-Nagai, M.; Maruyama, W.; Osawa, T.; Naoi, M. Rasagiline prevents cyclosporine A-sensitive superoxide flashes induced by PK11195, the initial signal of mitochondrial membrane permeabilization and apoptosis. J. Neural Transm. 2016, 123, 491–494. [Google Scholar] [CrossRef]
- Krestinina, O.; Baburina, Y.; Krestinin, R.; Odinokova, I.; Fedeeva, I.; Sotnikowa, L. Astaxanthin prevents mitochondrial impairment induced by isoproterenol in isolated rat heart mitochondria. Antioxidants 2020, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Xi, J.; Wang, H.; Müller, R.M.; Nirfleet, E.A.; Xu, Z. Mechanism for resveratrol-induced cardioprotection against reperfusion injury involves glycogen synthase kinas 3b and mitochondrial permeability transition pore. Eur. J. Pharmacol. 2009, 604, 111–116. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Liu, D.; Tang, L.; Yin, D.; Yin, S.; Yao, J.; He, M. Long-term oral resveratrol intake provides nutritional preconditioning against myocardial ischemia/reperfusion injury: Involvement of VDAC1 downregulation. Mol. Nutr. Food Res. 2015, 59, 454–464. [Google Scholar] [CrossRef]
- Tian, M.; Xie, Y.; Meng, Y.; Ma, W.; Tong, Z.; Yang, X.; Lai, S.; Zhou, Y.; He, M.; Liao, Z. Resveratrol protects cardiomyocytes against anoxia/reoxygenation via dephosphorylation of VDAC1 by Akt-GSK3 b pathway. Eur. J. Pharmcol. 2019, 843, 80–87. [Google Scholar] [CrossRef]
- Tewari, D.; Ahmed, T.; Chrirasani, V.R.; Singh, P.K.; Maji, S.M.; Senapati, S.; Bera, A.K. Modulation of the mitochondrial voltage dependent anion channel (VDAC) by curcumin. Biochim. Biophys. Acta. 2015, 1848, 151–158. [Google Scholar] [CrossRef]
- De Marchi, U.; Bissutto, L.; Garbisa, S.; Toninello, A.; Zoratti, M. Quercetin can act an inhibitor or an inducer of the mitochondrial permeability transition pore: A demonstration of the ambivalent redox character of polyphenols. Biochim. Biophys. Acta 2009, 1787, 1425–1432. [Google Scholar] [CrossRef]
- Naoi, M.; Maruyama, W.; Shamoto-Nagai, M.; Riederer, P. Type A monoamine oxidase; its unique role in mood, behavior and neurodegeneration. J. Neural Transm. 2024, in press. [CrossRef] [PubMed]
- Sabban, E.L.; Maharjan, S.; Nistramo, R.; Serova, L.I. Divergent effects of estradiol on gene expression of catecholamine biosynthetic enzymes. Physiol. Behav. 2010, 99, 163–168. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.T.; Martinez-Mota, L.; Herrera-Perez, J.; Jimenez-Rubio, G. Role of estradiol in the expression of genes involved in serotonin neurotransmission: Implications for female depression. Curr. Neuropharmacol. 2019, 17, 459–471. [Google Scholar] [CrossRef]
- De Vries, G.J. Minireview: Sex differences in adult and developing brains: Compensation, compensation, compensation. Endocrinology 2004, 145, 1063–1068. [Google Scholar] [CrossRef]
- Sumien, N.; Cunnungham, J.T.; Davis, D.L.; Engelland, R.; Fadeyibi, O.; Farmer, G.E.; Mabry, S.; Mensah-Kane, P.; Trinh, O.T.P.; Vann, P.H.; et al. Neurodegenerative disease: Role for sex, hormones, and oxidative stress. Endocrinology 2021, 162, bdab185. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.; Itoh, Y. The X factor in neurodegeneration. J. Exp. Med. 2022, 219, e20211488. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Li, B.; Ou-Yang, L. Role of estrogen receptors in health and disease. Front. Endocrinol. 2022, 13, 839005. [Google Scholar] [CrossRef]
- Hall, J.M.; Couse, J.F.; Korach, K.S. The multi faced mechanisms of estradiol and estrogen receptor signaling. J. Biol. Chem. 2001, 276, 36869–36872. [Google Scholar] [CrossRef]
- Nilsson, S.; Mäkelä, S.; Treuter, E.; Tujague, M.; Thomsen, J.; Andersson, G.; Enmark, E.; Pettersson, K.; Warner, M.; Gustafsson, J.A. Mechanisms of estrogen action. Physiol. Rev. 2001, 81, 1535–1565. [Google Scholar] [CrossRef]
- Wissink, S.; van der Burg, B.; Katzenellenbogen, B.S.; van der Saag, P.T. Synergistic activation of the serotonin-1A receptor by nuclear factor-kB and estrogen. Mol. Endocrinol. 2001, 15, 543–552. [Google Scholar] [CrossRef]
- Safe, S.; Kim, K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathway. J. Mol. Endocrinol. 2008, 41, 263–275. [Google Scholar] [CrossRef]
- Gundlah, C.; Lu, N.Z.; Mirkes, S.J.; Bethea, S.L. Estrogen receptor b (ERb) mRNA and protein in serotonin neurons of macaques. Brain Res. Mol. Brain Res. 2001, 91, 14–22. [Google Scholar] [CrossRef]
- Hudon Thibeault, A.A.T.; Sanderson, J.T.; Vaillamcourt, C. Serotonin-estrogen interactions: What can we learn from pregnancy? Biochimie 2019, 161, 88–108. [Google Scholar] [CrossRef]
- Hiroi, R.; Handa, R.J. Estrogen receptor-b regulates human tryptophan hydroxylase-2 through an estrogen response element in the 5′ untranslated region. J. Neurochem. 2013, 127, 487–495. [Google Scholar] [CrossRef]
- Sanchez, R.L.; Reddy, A.P.; Centeno, M.L.; Henderson, J.A.; Betha, C.L. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe regions of macaques. Brain Res. Mol. Brain Res. 2005, 135, 194–203. [Google Scholar] [CrossRef]
- Hiroi, R.; McDevitt, R.A.; Neumaler, J.F. Estrogen selectively increases tryptophan hydroxylase-2 mRNA expression in distinct subregions of rat midbrain raphe nucleus: Association between gene expression and anxiety behavior in the open field. Biol. Psychiatry 2006, 60, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Imwalle, D.B.; Gustafssom, J.A.; Rissman, E.F. Lack of functional estrogen receptor β influences anxiety behavior and serotonin content in female mice. Physiol. Behav. 2005, 84, 157–163. [Google Scholar] [CrossRef]
- Nomura, M.; Akama, K.T.; Alves, S.E.; Korach, K.S.; Gustafsson, J.A.; Pfaff, D.W.; Ogawa, S. Differential distribution of estrogen receptor (ER)-a and ER-b in the midbrain raphe nuclei and periaqueductal gray in male mouse: Predominant role of ER-b in the midbrain serotonergic system. Neuroscience 2005, 130, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Österlund, M.K. Underlying mechanisms mediating the antidepressant effects of estrogens. Biochem. Biophys. Acta 2010, 1800, 1136–1144. [Google Scholar] [CrossRef]
- McQueen, J.K.; Wilson, H.; Fink, G. Estradiol-17β increase serotonin transporter (SERT) mRNA levels and density of SERT-binding sites in female rat brain. Brain Res. Mol. Brain Res. 1997, 45, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.G.; Estrada-Camarena, E.; Belanger, N.; Morissette, M.; Di Paolo, T. Estradiol modulation of cortical, striatal and raphe nucleus 5-HT1A and 5-HT2A receptors of female hemiparkinsonian monkeys after long-term ovariectomy. Neuropharmacology 2011, 60, 642–652. [Google Scholar] [CrossRef]
- Sanchez, M.G.; Morissette, M.; Di Paolo, T. Osteradiol modulation of serotonin reuptake transporter and serotonin metabolism in the brain of monkeys. J. Neuroendocrinol. 2013, 25, 560–569. [Google Scholar] [CrossRef]
- Nakashima, A.; Ota, A.; Sabban, E.L. Interaction between Egr1 and AP1 factors in regulation of tyrosine hydroxylase transcription. Brain Res. Mol. Brain Res. 2003, 112, 61–69. [Google Scholar] [CrossRef]
- Lewis-Tuffin, L.J.; Quinn, P.G.; Chikaraishi, D.M. Tyrosine hydroxylase transcription depends primarily on cAMP response element activity, regardless of the type of inducing stimulus. Mol. Cell. Neurosci. 2004, 25, 536–547. [Google Scholar] [CrossRef]
- Maharjan, S.; Serova, L.; Sabban, E.L. Transcriptional regulation of tyrosine hydroxylase by estrogen: Opposite effects with estrogen receptor a and b and interactions with cyclic AMP. J. Neurochem. 2005, 93, 1503–1514. [Google Scholar] [CrossRef]
- Liaw, J.J.; He, J.R.; Hartman, R.D.; Barraclough, C.A. Changes in tyrosine hydroxylase mRNA levels in medullary A and A2 neurons and locus coeruleus following castration and estrogen replacement in rats. Brain Res. Mol. Brain Res. 1992, 13, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Serova, L.; Rivkin, M.; Nakashima, A.; Sabban, E.L. Estradiol stimulates gene expression of norepinephrine biosynthetic enzymes in rat locus coeruleus. Neuroendocrinology 2002, 75, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Serova, L.I.; Maharjan, S.; Huang, A.; Sun, D.; Kaley, G.; Sabban, E.L. Response of tyrosine hydroxylase and GTP cyclohydrolase I gene expression to estrogen in the brain catecholaminergic regions varies with mode of administration. Brain Res. 2004, 1015, 1–8. [Google Scholar] [CrossRef]
- Thanky, N.R.; Son, J.H.; Herbison, A.E. Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in locus coeruleus of TH9-LacZ transgenic mice. Brain Res. Mol. Brain Res. 2002, 104, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Maharjan, S.; Serova, L.I.; Sabban, E.L. Membrane-initiated estradiol signaling increases tyrosine hydroxylase promoter activity with ERα in PC12 cells. J. Neurochem. 2010, 112, 42–55. [Google Scholar] [CrossRef]
- Yanagihara, N.; Liu, M.; Toyohira, Y.; Tsutsui, M.; Ueno, S.; Shinohara, Y.; Takahashi, K.; Tanaka, K. Stimulation of catecholamine synthesis through unique estrogen receptors in the bovine adrenomedullary plasma membrane by 17β-estradiol. Biochem. Biophys. Res. Commun. 2006, 339, 548–553. [Google Scholar] [CrossRef]
- Inagaki, H.; Toyohira, Y.; Takahashi, K.; Ueno, S.; Kawagoe, T.; Tsutsui, M.; Hachisuga, T.; Yanagisawa, N. Effects of selective estrogen receptor modulators on plasma membrane estrogen receptors and catecholamine synthesis and secretion in cultured bovine adrenal medullary cells. J. Pharmacol. Sci. 2014, 124, 66–75. [Google Scholar] [CrossRef]
- Epperson, C.N.; Kim, D.R.; Bale, T.L. Estradiol modulation of monoamine metabolism one possible mechanism underlying sex differences in risk for depression and dementia. JAMA Psychiatry 2014, 71, 869–870. [Google Scholar] [CrossRef]
- Sacher, J.; Rabiner, E.A.; Clark, M.; Rusjan, P.; Soliman, A.; Boskovic, R.; Kish, S.J.; Wilson, A.A.; Houle, S.; Meyer, J.H. Dynamic, adaptive changes in MAO-A binding after alterations in substrate availability: An in vivo [11C]-harmine position emission tomography study. J. Cereb. Blood Flow. Metab. 2012, 32, 443–446. [Google Scholar] [CrossRef]
- Rekkas, P.V.; Wilson, A.A.; Lee, V.W.H.; Yogalingam, P.; Sacher, J.; Rusjan, P.; Houle, S.; Stewart, D.E.; Kolla, N.J.; Kish, S.; et al. Greater monoamine oxidase A binding in perimenopausal age as measured with carbon 11-labeled harmine position emission tomography. JAMA Psychiatry 2014, 17, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Holschneider, D.P.; Kumazawa, T.; Chen, K.; Shih, J.C. Tissue-specific effects of estrogen on monoamine oxidase A and B in the rat. Life Sci. 1998, 63, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Bethea, C.L.; Lu, N.Z.; Gundlah, C.; Streicher, J.M. Diverse actions of ovarian steroids in the serotonin neural system. Front. Neuroendocrinol. 2002, 23, 41–100. [Google Scholar] [CrossRef]
- Wu, J.B.; Chen, K.; Li, Y.; Lau, Y.F.C.; Shih, J.C. Regulation of monoamine oxidase A by the SRY gene on the Y chromosome. FASEB J. 2009, 23, 4029–4038. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.C.; Wu, J.; Chen, K. Transcriptional regulation and multiple functions of MAO genes. J. Neural Transm. 2011, 118, 979–986. [Google Scholar] [CrossRef]
- Ou, X.M.; Chen, K.; Shih, J.C. Glucocorticoid and androgen activation of monoamine oxidase A is regulated by R1 and Sp1. J. Biol. Chem. 2006, 281, 21512–21525. [Google Scholar] [CrossRef]
- Kranz, G.S.; Spies, M.; Vraka, C.; Kaufmann, U.; Klebermass, E.M.; Handschuh, P.A.; Ozenil, M.; Murgaš, M.; Pichler, V.; Rischka, L.; et al. High-dose testosterone treatment reduces monoamine oxidase A levels in human brain: A preliminary report. Psychoneuroendocrinology 2021, 133, 105381. [Google Scholar] [CrossRef]
- Xie, T.; Ho, S.L.; Ramsden, D. Characterization and implications of estrogenic down-regulation of human catechol-O-methyltransferase gene transcription. Mol. Pharmacol. 1999, 56, 31–38. [Google Scholar] [CrossRef]
- Jiang, H.; Xie, T.; Ramsden, D.B.; Ho, S.L. Human catechol-O-methyltransferase downregulation by estradiol. Neuropharmacology 2003, 45, 1011–1018. [Google Scholar] [CrossRef]
- Yao, J.; Li, Y.; Chang, M.; Wu, H.; Yang, X.; Goodman, J.E.; Liu, X.; Liu, H.; Mesecar, A.D.; Van Breemen, R.B.; et al. Catechol estrogen 4-hydroxyequilenin is a substrate and an inhibitor of catechol-O-methyltransferase. Chem. Res. Toxicol. 2003, 16, 668–675. [Google Scholar] [CrossRef]
- Patel, P.D.; Bochar, D.; Tumer, D.L.; Meng, F.; Moeller, H.M.; Pontrello, C.G. Regulation of tryptophan hydroxylase-2 gene expression by a bipartite RE-1 silencer of transcription/neuron-restrictive silencing factor (REST/NRSF) binding motif. J. Biol. Chem. 2007, 282, 26717–26724. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.A.; Flick, R.B.; Pai, L.Y.; Szalayova, I.; Key, S.; Conley, R.K.; Deutch, A.Y.; Hutson, P.H.; Mazey, E. Glucocorticoid modulation of tryptophan hydroxylase-2 protein in raphe nuclei and 6-hydroxytryptophan concentrations in frontal cortex of C56/B16 mice. Mol. Psychiatry 2008, 13, 498–506. [Google Scholar] [CrossRef]
- Malek, Z.S.; Sage, D.; Pevet, P.; Raison, S. Daily rhythm of tryptophan hydroxylase-2 messenger ribonucleic acid within raphe neurons is induced by corticoid daily surge and modulated by enhanced locomotor activity. Endocrinology 2007, 148, 5165–5172. [Google Scholar] [CrossRef]
- Sabban, E.; Nankova, B.B.; Serova, L.I.; Kvetnansky, R.; Liu, X. Molecular regulation of gene expression of catecholamine biosynthetic enzymes by stress: Sympathetic ganglia versus adrenal medulla. Ann. N. Y. Acad. Sci. 2004, 1018, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Rani, C.S.S.; Elango, N.; Wang, S.S.; Kobayashi, K.; Strong, R. Identification of an activator protein-1-like sequence as the glucocorticoid response element in the rat tyrosine hydroxylase. Mol. Pharmacol. 2009, 75, 589–598. [Google Scholar] [CrossRef]
- Grunewald, M.; Johnson, S.; Lu, D.; Wang, Z.; Lomberk, G.; Albert, P.R.; Stockmeier, C.A.; Meyer, J.H.; Urrutia, R.; Miczek, K.A.; et al. Mechanistic role for a novel glucocorticoid-KLF11(TIEG2) protein pathway in stress-induced monoamine oxidase A expression. J. Biol. Chem. 2012, 287, 24195–24205. [Google Scholar] [CrossRef]
- Walf, A.A.; Frye, C.A. Administration of estrogen receptor beta-specific selective estrogen receptor modulators to the hippocampus decrease anxiety and depressive behavior of ovariectomized rats. Pharmacol. Biochem. Behav. 2007, 86, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Rietjens, I.M.C.M.; Louisse, J.; Beekmann, K. The potential health effects of dietary phytoestrogens. Br. J. Pharmacol. 2017, 174, 1263–1280. [Google Scholar] [CrossRef]
- Strauss, L.; Santti, R.; Saarinen, N.; Streng, T.; Joshi, S.; Mäkelä, S. Dietary phytoestrogens and their role in hormonally dependent disease. Toxicol. Lett. 1998, 102–103, 349–354. [Google Scholar] [CrossRef]
- Tice, J.A.; Ettinger, B.; Ensrud, K.; Wallace, R.; Blackwell, T.; Cummings, S.R. Phytoestrogen supplements for the treatment of hot flashes: The isoflavone clover extract (ICE) study: A randomized controlled trial. JAMA 2003, 290, 207–214. [Google Scholar] [CrossRef]
- Chen, L.R.; Chen, K.H. Utilization of isoflavones in soybeans for women with menopausal syndrome: An overview. Int. J. Mol. Sci. 2021, 22, 3212. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, G.G.J.M.; Lemmen, J.B.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor-β. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef]
- Kostelac, D.; Rechkemmer, G.; Briviba, K. Phytoestrogens modulate binding response to estrogen receptors a and b to the estrogen response element. J. Agric. Food Chem. 2003, 51, 7632–7635. [Google Scholar] [CrossRef] [PubMed]
- Van Duursen, M.B.M. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol. Res. 2017, 6, 772–794. [Google Scholar] [CrossRef] [PubMed]
- De La Cruz, C.P.; Revilla, E.; Venero, J.L.; Ayala, A.; Cano, J.; Machado, A. Oxidative inactivation of tyrosine hydroxylase in substantia nigra of aged rat. Free Radic. Biol. Med. 1996, 20, 53–61. [Google Scholar] [CrossRef]
- Hussain, A.M.; Mitra, A.K. Effect of aging on tryptophan hydroxylase in rat brain: Implications on serotonin level. Drug Metab. Dispos. 2000, 28, 1038–1042. [Google Scholar] [CrossRef]
- Sarubbo, E.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic silymarin, quercetin and naringenin treatment increase monoamines synthesis and hippocampal Sirt1 levels improving cognition in aged rats. J. Neuroimmme. Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef]
- Ramis, M.R.; Sarubbo, F.; Tejada, S.; Jimenez, M.; Esteban, S.; Miralles, A.; Moranta, D. Chronic polyphenon-60 or catechin treatments increase brain monoamines syntheses and hippocampal SIRT1 levels improving cognition on aged rats. Nutrients 2020, 12, 326. [Google Scholar] [CrossRef]
- Sarubbo, E.; Ramis, M.R.; Aparicio, S.; Ruiz, L.; Esteban, S.; Miralles, A.; Moranta, D. Improving effects of chronic resveratrol treatment on central monoamine synthesis and cognition in aged rats. Age 2015, 37, 9777. [Google Scholar] [CrossRef]
- Lee, B.; Choi, G.M.; Sur, B. Silibinin prevents depression-like behaviors in a single prolonged stress rat model: The possible role of serotonin. BMC Complement. Med. Ther. 2020, 20, 70. [Google Scholar] [CrossRef]
- Lee, B.; Sur, B.; Lee, H.; Oh, S. Korean red ginseng posttraumatic stress disorder-triggered depression-like behaviors in rats via activation of the serotonergic system. J. Ginseng Res. 2020, 44, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Choi, G.M.; Sur, B. Anti-depressant-like effects of 142 in animal model of post-traumatic stress disorder. Clin. J. Interg. Med. 2021, 27, 39–46. [Google Scholar] [CrossRef]
- Vauzour, D.; Rendeiro, C.; D’Amatao, A.; Waffo-Téguo, P.; Richard, T.; Mérillon, J.M.; Pontifex, M.G.; Connell, E.; Müller, M.; Butler, L.T.; et al. Anthrocyanins promote learning through modulation of synaptic plasticity related proteins in animal model of ageing. Antioxidants 2021, 10, 1235. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, J.; Liu, S.; Wang, L.; Huang, J.; Liu, L.; Yang, J.; Zhang, G.; Guo, K.; Zhang, Z.; et al. Puerarin protects dopaminergic neurons in Parkinson’s disease models. Neuroscience 2014, 280, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Sonia Angeline, M.; Sarkar, A.; Anand, K.; Ambasta, R.K.; Kumar, P. Sesamol and naringenin reverse the effect of rotenone-induced PD rat model. Neuroscience 2013, 254, 379–394. [Google Scholar] [CrossRef]
- Singh, S.S.; Rai, S.N.; Birla, H.; Zahra, W.; Kumar, G.; Gedda, M.R.; Tiwari, N.; Patnaik, R.; Singh, R.K.; Singh, S.P. Effect of chlorogenic acid supplementation in MPTP-intoxicated mouse. Front. Pharmacol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Rose, K.; Parmar, M.; Cavanaugh, J.E. Dietary supplementation with resveratrol protects against striatal dopaminergic deficits produced by in utero LPS exposure. Brain Res. 2014, 1573, 37–43. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, G.; Liu, J. Phloretin attenuates behavior deficits and neuroinflammatory response in MPTP induced Parkinson’s disease in mice. Life Sci. 2019, 232, 116600. [Google Scholar] [CrossRef]
- Fatima, A.; Rahul; Siddique, Y.H. Role of tangeritin against cognitive impairments in transgenic Drosophila model of Parkinson’s disease. Neurosci. Lett. 2019, 705, 112–117. [Google Scholar] [CrossRef]
- Saied, N.M.; Georgy, G.S.; Hussien, R.M.; Hassan, W.A. Neuromodulatory effect of curcumin on catecholamine systems and inflammatory cytokines in ovariectomized female rats. Clin. Exp. Pharmacol. Physiol. 2021, 48, 337–346. [Google Scholar] [CrossRef]
- Hack, W.; Gladen-Kolarsky, N.; Chatterjee, S.; Liang, Q.; Maitra, U.; Giesla, L.; Gray, N.E. Gardenin A treatment attenuates inflammatory markers, synuclein pathology and deficits in tyrosine hydroxylase expression and improves cognitive and motor function in A53T-a-syn mice. Biomed. Pharmacother. 2024, 173, 116370. [Google Scholar] [CrossRef]
- Liu, M.; Yanagihara, N.; Toyohira, Y.; Tsutsui, M.; Ueno, S.; Shinohara, Y. Dual effects of daidzen, and soy isoflavone, on catecholamine synthesis in cultured bovine adrenal medullary cells. Endocrinology 2007, 148, 5348–5354. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yanagihara, N.; Toyohira, Y.; Takahashi, K.; Inagaki, H.; Satoh, N.; Li, X.; Goa, X.; Tsutsui, M.; Takahaishi, K. Stimulatory effect of nobiletin, a citrus polymethoxy flavone, on catecholamine synthesis through Ser19 and Ser40 phosphorylation of tyrosine hydroxylase in cultured bovine adrenal medullary cells. Naunyn-Schmiedebergs Arch. Pharmacol. 2014, 387, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Vina, D.; Serra, S.; Lamela, M.; Delogu, G. Herbal natural products as a source of monoamine oxidase inhibitors: A review. Curr. Top. Med. Chem. 2012, 12, 2131–2144. [Google Scholar] [CrossRef]
- Chimenti, F.; Cottiglia, F.; Bonsignore, L.; Casu, L.; Casu, M.; Floris, C.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; et al. Quercetin as the active principle of Hypericum hircinum exerts a selective inhibitory activity against MAO-A: Extraction, biological analysis, and computational study. J. Nat. Prod. 2006, 69, 945–949. [Google Scholar] [CrossRef]
- Carradori, S.; Gidaro, M.C.; Petzer, A.; Costa, G.; Guglielmi, P.; Chimenti, P.; Alcaro, S.; Petzer, J.P. Inhibition of human monoamine oxidase: Biological and molecular modeling studies on selected natural flavonoids. J. Agric. Food Chem. 2016, 64, 9004–9011. [Google Scholar] [CrossRef] [PubMed]
- Adeoluwa, O.A.; Eduviere, A.T.; Adeoluwa, G.O.; Otomewo, L.O.; Adeniyi, F.R. The monoaminergic pathways are involved in the antidepressant-like effect of quercetin. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 2497–2506. [Google Scholar] [CrossRef]
- Bandaruk, Y.; Mukai, R.; Kawamura, T.; Nemoto, H.; Terao, J. Evaluation of the inhibitory effects of quercetin-related flavonoids and tea catechins on the monoamine oxidase-A reaction in mouse brain mitochondria. J. Agric. Food Chem. 2012, 60, 10270–10277. [Google Scholar] [CrossRef]
- Lee, H.W.; Ryu, H.W.; Kang, M.G.; Park, D.; Oh, S.R.; Kim, H. Selective inhibition of monoamine oxidase A by purpurin, an anthraquinone. Bioorg. Med. Chem. Lett. 2017, 27, 1136–1140. [Google Scholar] [CrossRef]
- Gidaro, M.C.; Astorino, C.; Petzer, A.; Carradori, S.; Alcaro, F.; Costa, G.; Artese, A.; Rafele, G.; Russo, F.M.; Petzer, J.P.; et al. Kaempferol as selective MAO-A inhibitor: Analytical detection in Calabrian red wines, biological and molecular modeling studies. J. Agric. Food Chem. 2016, 64, 1394–1400. [Google Scholar] [CrossRef]
- Olayinka, J.N.; Akawa, O.B.; Ogbu, E.K.; Eduviere, A.T.; Ozolua, R.I.; Soliman, M. Apigenin attenuates depressive-like behavior via modulating monoamine oxidase A enzyme activity in chronically stressed mice. Curr. Res. Pharnacol. Drug Discov. 2023, 5, 100161. [Google Scholar] [CrossRef]
- Guo, B.; Zheng, C.; Cai, W.; Cheng, J.; Wang, H.; Li, H.; Sun, Y.; Cui, W.; Wang, Y.; Han, Y.; et al. Multifunction of chrysin in Parkinson’s model: Antineuronal apoptosis, neuroprotection via activation of MEF2D, and inhibition of monoamine oxidase-B. J. Agric. Food Chem. 2016, 64, 5324–5333. [Google Scholar] [CrossRef]
- Yanez, M.; Fraiz, N.; Cano, E.; Orallo, F. (-)-Trans-ε-viniferin, a polyphenol present in wines, is an inhibitor of noradrenaline and 5-hydroxytryptamine uptake and of monoamine oxidase activity. Eur. J. Pharmacol. 2006, 542, 54–60. [Google Scholar] [CrossRef]
- Carradori, S.; D’Ascenzio, M.; Chimenti, P.; Secchi, D.; Bolasco, A. Selective MAO-B inhibitors: A lesson from natural products. Mol. Divers. 2014, 18, 219–243. [Google Scholar] [CrossRef]
- Minders, C.; Petzer, J.P.; Petzer, A.; Lourens, A.C.U. Monoamine oxidase inhibitory activities of heterocyclic chalcones. Bioorg. Med. Chem. Lett. 2015, 25, 5270–5276. [Google Scholar] [CrossRef] [PubMed]
- Sashidhara, K.V.; Modukuri, R.K.; Singh, S.; Rao, K.B.; Teja, G.A.; Gupta, S.; Shukla, S. Design and synthesis of new series of coumarin-aminopyran derivatives possessing potential anti-depressant-like activity. Bioorg. Med. Chem. Lett. 2015, 25, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Bergström, M.; Westerberg, G.; Nemeth, G.; Traut, M.; Gross, G.; Greger, G.; Müller-Peltzer, H.; Safer, A.; Eckernäs, S.A.; Grahnér, A.; et al. MAO-A inhibition in brain after dosing with esuprone, moclobemide and placebo in healthy volunteers: In vivo studies with position emission tomography. Eur. J. Clin. Pharmacol. 1997, 52, 121–129. [Google Scholar] [CrossRef]
- Matos, M.J.; Teran, C.; Perez-Castillo, Y.; Uriarte, E.; Santana, L.; Vina, D. Synthesis and study of a series of 3-arylcoumarins as potent and selective monoamine oxidase B inhibitors. J. Med. Chem. 2011, 54, 7127–7137. [Google Scholar] [CrossRef]
- Abdelhafez, O.; Amin, K.M.; Ali, H.I.; Abdalla, M.; Batran, R.Z. Synthesis of new 7-oxycoumarin derivatives as potent and selective monoamine oxidase A inhibitors. J. Med. Chem. 2012, 55, 10424–10436. [Google Scholar] [CrossRef]
- Bindra, S.; Datta, A.; Yasin, H.K.A.; Hanttu, A.; Pollesello, P. Recent progress in synthetic and natural catechol-O-methyltransferase inhibitors for neurological disorders. ACS Omega 2024, 9, 44005–44018. [Google Scholar] [CrossRef]
- Hitge, R.; Smit, S.; Petzer, A.; Petzer, J. Evaluation of nitrocatechol chalcone and pyrazoline derivatives as inhibitors of catechol-O-methyltransferase and monoamine oxidase. Bioorg. Med. Chem. Lett. 2020, 30, 127188. [Google Scholar] [CrossRef] [PubMed]
- Engelbrecht, I.; Petzer, J.P.; Petzer, A. Evaluation of selective natural compounds as dual inhibitors of catechol-O-methyltransferase and monoamine oxidase. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, W.; Miyata, R.; Fuzinami, M.; Sato, Y.; Kumazawa, S. Catechol-O-methyltransferase inhibitors from Calendula officinalis leaf. Molecules 2023, 28, 1333. [Google Scholar] [CrossRef]
- Chen, D.; Wang, C.Y.; Lambert, J.D.; Ai, N.; Welsh, W.J.; Yang, C.S. Inhibition of human liver catechol-O-methyltransferase by tea catechins and their metabolites: Structure-activity relationship and molecular-modeling studies. Biochem. Pharmacol. 2005, 69, 1523–1531. [Google Scholar] [CrossRef]
- Tammen, S.A.; Friso, S.; Choi, S.W. Epigenetics: The link between nature and nurture. Mol. Aspect Med. 2013, 34, 753–764. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.L.; Hardy, T.M.; Tollefsbol, T.O. Medical chemistry of epigenetic diet and caloric restriction. Curr. Med. Chem. 2013, 20, 4050–4059. [Google Scholar] [CrossRef] [PubMed]
- Wachs, T.D.; Georgieff, M.; Cusick, S.; McEwen, B.S. Issues in the timing of integrated early interventions: Contributions from nutrition, neuroscience, and psychological research. Ann. N. Y. Acad. Sci. 2014, 1308, 89–106. [Google Scholar] [CrossRef]
- Tobi, E.W.; Goeman, J.J.; Monajemi, R.; Gu, H.; Putter, H.; Zhang, Y.; Slieker, R.C.; Stok, A.P.; Thijssen, P.E.; Müller, F.; et al. DNA methylation signatures link prenatal famine exposure to growth and metabolism. Nat. Commun. 2014, 5, 5592. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Garza-Cuellar, M.A.; Silva-Hernandez, I.A.; Cardenas-Perez, R.E.; Reyes-Castro, L.A.; Zambrano, E.; Gonzalez-Hernandez, B.; Garza-Ocañas, L.; Fuentes-Mera, L.; Camacho, A. Maternal flavonoids intake reverts depression-like behaviour in rat female offspring. Nutrients 2019, 11, 572. [Google Scholar] [CrossRef]
- Booij, L.; Tremblay, R.E.; Szyf, M.; Benkelfat, C. Genetic and early environmental influences on the serotonin system: Consequences for brain development and risk for psychopathology. J. Psychiatry Neurosci. 2015, 40, 5–18. [Google Scholar] [CrossRef]
- Lambe, E.K.; Krimer, L.S.; Goldman-Rakic, P.S. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J. Neurosci. 2000, 20, 8780–8787. [Google Scholar] [CrossRef]
- Suri, D.; Teixeira, C.M.; Cagliostro, M.K.C.; Mahadevia, D.; Ansorge, M.S. Monoamine-sensitive developmental periods impacting adult emotional and cognitive behaviors. Neuropsychopharmacology 2015, 40, 88–112. [Google Scholar] [CrossRef]
- Cheng, Z.; Su, J.; Zhang, K.; Jaing, H.; Li, B. Epigenetic mechanism of early life stress-induced depression: Focus on the neurotransmitter systems. Front. Cell Dev. Biol. 2022, 10, 929732. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, I.C.; Dhir, S.K.; Diorio, J.C.; Meaney, M.J. Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid-hormone-serotonin-NGFI-A signalling cascade. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 2495–2510. [Google Scholar] [CrossRef] [PubMed]
- Mazer, C.; Muneyyirci, J.; Taheny, K.; Raio, N.; Borella, A.; Whitaker-Azmitia, P. Serotonin depletion during synaptogenesis leads to decreased synaptic density and learning deficits in the adult rat: A possible model of neurodevelopmental disorders with cognitive deficits. Brain Res. 1997, 760, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Cases, O.; Vitalis, T.; Seif, I.; De Maeyer, E.; Sotelo, C.; Gasper, P. Lack of barrels in the somatosensory cortex of monoamine oxidase A-deficit mice: Role of a serotonin excess during the critical period. Neuron 1996, 16, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Cases, O.; Rebrin, I.; Wu, W.; Gallaher, T.K.; Shih, J.C. Forebrain-specific expression of monoamine oxidase A reduces neurotransmitter levels, restores the brain structure, and rescues aggressive behavior in monoamine oxidase A-deficit mice. J. Biol. Chem. 2007, 282, 115–123. [Google Scholar] [CrossRef]
- Srinivas, T.; Mathias, C.; Oliveira-Mateos, C.; Guil, S. Roles of lncRNAs in brain development and pathogenesis: Emerging therapeutic opportunities. Mol. Ther. 2023, 31, 1550–1561. [Google Scholar] [CrossRef]
- Jovcevska, I.; Paska, A.V. Neuroepigenetics of psychiatric disorders: Focus on lncRNA. Neurochem. Int. 2021, 149, 1054140. [Google Scholar] [CrossRef]
- Zhong, X.L.; Du, Y.; Chen, L.; Cheng, Y. The emerging role of long noncoding RNA in depression and its implications in diagnostics and therapeutic responses. J. Psychiatr. Res. 2023, 164, 251–258. [Google Scholar] [CrossRef]
- Scott, K.M.; McLaughlin, K.A.; Smith, D.A.R.; Ellis, P.M. Childhood maltreatment and DSM-IV adult mental disorders: Comparison of prospective and retrospective findings. Br. J. Psychiatry 2012, 200, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Torres-Berrio, A.; Issler, O.; Parise, E.M.; Nestler, E.J. Unveiling the epigenetic landscape of depression: Focus on early life stress. Dialogues Clin. Neurosci. 2019, 21, 341–357. [Google Scholar] [CrossRef]
- Konig, S.M.; Kessler, C.L.; Canli, T.; Duman, E.A.; Ada, E.K.; Zinbarg, R.; Craske, M.G.; Stephens, J.E.; Vrshek-Schallhorm, S. Early-life adversity severity, timing, and context type are associated with SLC6A4 methylation in emerging adults: Results from a prospective cohort study. Psychoneuroendocrinolgy 2024, 170, 107181. [Google Scholar] [CrossRef]
- Wang, D.; Szyf, M.; Benkelfat, C.; Provencal, N.; Turecki, G.; Caramaschi, D.; Cote, S.M.; Vitaro, F.; Tremblay, R.E.; Booij, L. Peripheral SLC6A4 DNA methylation is associated with in vivo measures of human brain serotonin synthesis and childhood physical aggression. PLoS ONE 2012, 7, e39501. [Google Scholar] [CrossRef] [PubMed]
- Carrard, A.; Salzmann, A.; Malafosse, A.; Karege, F. Increased DNA methylation status of the serotonin receptor 5HTR1A gene promoter in schizophrenia and bipolar disorder. J. Affect. Disord. 2011, 132, 450–453. [Google Scholar] [CrossRef]
- Quellet-Morin, I.; Wong, C.C.Y.; Danese, A.; Parieante, C.M.; Papadopoulos, A.S.; Mill, J.; Arsenault, L. Increased serotonin transporter gene (SERT) DNA methylation is associated with bullying victimization and blunt cortisol response to stress in childhood: A longitudinal study of discordant monozygotic twins. Psychol. Med. 2013, 13, 1813–1823. [Google Scholar] [CrossRef]
- Checknita, D.; Tihonen, J.; Hodgins, S.; Nilsson, K. Association of age, sex, sexual abuse, and gene type with monoamine oxidase gene a gene methylation. J. Neural Transm. 2021, 128, 1721–1739. [Google Scholar] [CrossRef] [PubMed]
- Rahman, I.; Chung, S. Dietary polyphenols, deacetylases and chromatin remodeling in inflammation. J. Nutrigenet. Neurogenomics 2010, 3, 220–230. [Google Scholar] [CrossRef]
- Schneider-Stock, R.; Ghantous, A.; Bajboul, K.; Saikali, M.; Darwiche, N. Epigenetic mechanisms of plant-derived anticancer drugs. Front. Biosci. 2012, 17, 129–173. [Google Scholar] [CrossRef]
- Remely, M.; Lovrecic, L.; de la Garza, A.L.; Magliore, L.; Peterlin, B.; Milagro, F.I.; Martinez, A.J.; Haslberger, A.G. Therapeutic perspectives of epigenetically active nutrients. Br. J. Pharmacol. 2015, 172, 2756–2768. [Google Scholar] [CrossRef]
- Choi, S.W.; Friso, S. Epigenetics: A new bridge between nutrition and health. Adv. Nutr. 2010, 1, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Ayissi, V.B.; Ebrahimi, A.; Schluesenner, H. Epigenetic effects of natural polyphenols: A focus on SIRT1-mediated mechanisms. Mol. Nutr. Food Res. 2014, 58, 22–32. [Google Scholar] [CrossRef] [PubMed]
- de Boer, V.C.; de Goffau, M.C.; Arts, I.C.W.; Hollman, P.C.H.; Keijer, J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.P.; Tran, N.; Geekiyanage, H.; Liu, L.; Chan, C. Curcumin-induced upregulation of the anti-tau cochaperone BAG2 in primary rat cortical neurons. Neurosci. Lett. 2013, 554, 121–125. [Google Scholar] [CrossRef]
- Taha, M.; Eldemerdash, O.M.; Elshaffei, I.M.; Yousef, E.M.; Soliman, A.S.; Senousy, M.A. Apigenin attenuates hippocampal microglial activation and restores cognitive function in methotrexate-treated rats: Targeting the miR-15a/Rock-1/ERK1/2 pathway. Mol. Neurobiol. 2023, 60, 3770–3787. [Google Scholar] [CrossRef]
- Habibi, P.; Babri, S.; Ahmadiasl, N.; Yousefi, H. Effects of genistein and swimming exercise on spatial memory and expression of microRNA 132, BDNF, and IGF-1 genes in the hippocampus of ovariectomized rats. Iran. J. Basic. Med. Sci. 2017, 20, 856–862. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, N.; Lv, C.; Li, X.; Li, W. lncRNA SNGH1 knockdown alleviates amyloid-β-induced neuronal injury by regulating ZNF27 vi sponging miR-361-3- in Alzheimer’s disease. J. Alzheimers Dis. 2020, 77, 85–98. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Sui, R.; Zhang, Z. Administration of resveratrol improved Parkinson’s disease-like phenotype by suppressing apoptosis of neurons via modulating the MALTAT1/miR-129/SNCA signaling pathway. J. Cell. Biochem. 2019, 120, 4942–4951. [Google Scholar] [CrossRef]
- Li, X.; Su, Y.; Li, N.; Zhang, F.R.; Zhang, N. Berberine attenuates MPP+-induced neuronal injury by regulating LINGC00943/miR-142-5p/KPNA4/NF-κB pathway in SK-N-SH cells. Neurochem. Res. 2021, 46, 3286–3300. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, H.; Wang, J.; Jin, W.; Yan, X.; Chen, X.; Wang, D.; Zhao, D.; Wang, Y.; Cong, D.; et al. Ginsenoside Rf inhibits human tau proteotoxicity and specific LncRNA, miRNA and mRNA expression changes in Caenorhabditis elegans model of tauopathy. Eur. J. Pharmacol. 2022, 922, 174887. [Google Scholar] [CrossRef]
- Hardy, T.M.; Tollefsbol, T. Epigenetic diet: Impact on the epigenome and cancer. Epigenomics 2011, 3, 503–518. [Google Scholar] [CrossRef]
- Prasanth, M.I.; Sivamaruthi, B.S.; Cheong, C.S.Y.; Verma, K.; Tencomnao, T.; Brimson, J.M.; Prasansuklab, A. Role of epigenetic modulation in neurodegenerative diseases: Implications of phytochemical intervention. Antioxidants 2024, 13, 606. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Zhu, W.; Shi, H.; Hewtt, J.E.; Rohlen, R.L.; MacDonald, R.S.; Rottinghaus, G.E.; Chen, Y.C.; Sauter, E.R. Soy isoflavones have an antiestrogenic effect and alter mammary promoter hypermethylation in healthy premenopausal women. Nutr. Cancer 2009, 61, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Mazzaferro, S.; Bitto, A.; Cannata, M.L.; D'Anna, R.; Squadrito, F.; Macrì, I.; Frisina, A.; Frisina, N.; Bagnato, G. Genistein effects on quality of life and depression symptoms in osteopenic postmenopausal women: A 2-year randomized, double-blind, controlled study. Osteoporos. Int. 2014, 25, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Behl, T.; Rana, T.; Sehgal, A.; Sharma, N.; Albarrati, A.; Albratty, M.; Makeen, H.A.; Najmi, A.; Verma, R.; Bungau, S.G. Exploring the multifocal role of phytoconstituents as antidepressants. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 123, 110693. [Google Scholar] [CrossRef]
- Bahramsoltani, R.; Farzaei, M.H.; Farahari, M.S.; Rahini, R. Phytochemical constituents as future antidepressants: A comprehensive review. Rev. Neurosci. 2015, 26, 699–719. [Google Scholar] [CrossRef]
- Al-Karawi, D.; Al Moori, D.A.; Tayyar, Y. The role of curcumin administration in patients with major depressive disorder: Mini metal-analysis of clinical trials. Phytother. Res. 2016, 30, 175–183. [Google Scholar] [CrossRef]
- Daubert, E.A.; Condron, B.G. Serotonin: A regulator of neuronal morphology and circuity. Trends Neurosci. 2010, 33, 424–434. [Google Scholar] [CrossRef]
- Lee, G.; Bae, H. Therapeutic effects of phytochemicals and medicinal herbs on depression. BioMed Res. Int. 2017, 2017, 6596241. [Google Scholar] [CrossRef]
- Phootha, N.; Yongparnichkul, N.; Fang, Z.X.; Gan, R.Y.; Zhang, P.Z. Plants and phytochemicals potentials in tackling anxiety: A systematic review. Phytomedicine Plus 2022, 2, 100375. [Google Scholar] [CrossRef]
- Andrade, S.; Nunes, D.; Dabur, M.; Ramalho, M.J.; Pereira, M.C.; Loureiro, J.A. Therapeutic potential of natural compounds in neurodegenerative diseases: Insights from clinical Trials. Pharmaceutics 2023, 15, 212. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, alkaloids, and terpenoids against neurodegeneration: Evaluating the neuroprotective effects of phytocompounds through a comprehensive review of the current evidence. Metabolites 2025, 15, 124. [Google Scholar] [CrossRef]
- Crews, W.; Harrison, D.; Griffin, M.; Addison, K.; Yount, A.; Giovenco, M.; Hazell, J. A double-blinded, placebo-controlled, randomized trial of the neuropsychologic efficacy of cranberry juice in a sample of cognitively intact older adults: Pilot study findings. J. Altern. Complement. Med. 2005, 11, 305–309. [Google Scholar]
- Flanagan, E.; Cameron, D.; Sobhan, R.; Wong, C.C.; Pontifex, M.G.; Tosi, N.; Mena, P.; Del Rio, D.; Sami, S.A.; Narbad, A.; et al. Chronic consumption of cranberries (Vaccinium macrocarpon) for 12 weeks improves episodic memory and regional brain perfusion in healthy older adults: A randomised, placebo-controlled, parallel-groups feasibility study. Front. Nutr. 2022, 9, 849902. [Google Scholar] [CrossRef]
- Battaglia, S.; Avenanti, A.; Vécsei, L.; Tanaka, M. Neurodegeneration in cognitive impairment and mood disorders for experimental, clinical and translational neuropsychiatry. Biomedicines 2024, 12, 574. [Google Scholar] [CrossRef]
- Wang, Y.; Hernandez, G.M.D.; Mack, W.J.; Schneider, L.S.; Yin, F.; Brinton, R.D. Retrospective analysis of phytoSERM for management of menopause-associated vasomotor symptoms and cognitive decline: A pilot study on pharmacogenomic effects of mitochondrial haplogroup and APOE genotype on therapeutic efficacy. Menopause 2020, 27, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Colombage, R.L.; Holden, S.; Lamport, D.; Barfoot, K.L. The effects of flavonoid supplementation on the mental health of postpartum parents. Front. Glob. Women’s Health 2024, 5, 1345353. [Google Scholar] [CrossRef]
- Kabra, A.; Garg, R.; Brimson, J.; Živković, J.; Almawash, S.; Ayaz, M.; Nawaz, A.; Hassan, S.S.U.; Bungau, S. Mechanistic insights into the role of plant polyphenols and their nano-formulations in the management of depression. Front. Pharmacol. 2022, 13, 1046599. [Google Scholar] [CrossRef]
- Shariatgorji, M.; Nilsson, A.; Goowin, R.J.A.; Källback, P.; Schintu, N.; Zhang, X.; Crossman, A.R.; Bezard, E.; Svenningsson, P.; Andren, P.E. Direct targeted quantitative molecular imaging of neurotransmitters in brain tissue sections. Neuron 2014, 84, 697–707. [Google Scholar] [CrossRef]
- Makkonen, I.; Riikonen, R.; Kokki, H.; Airaksinen, M.M.; Kuikka, J.T. Serotonin and dopamine transporter binding in children with autism determined by SPECT. Dev. Med. Child. Neurol. 2008, 50, 593–597. [Google Scholar] [CrossRef]
- Fowler, J.S.; Logan, J.; Shumay, E.; Alia-Klein, N.; Wang, G.J.; Volkow, N.D. Monoamine oxidase: Radiotracer chemistry and human studies. J. Label. Compd. Radiopharm. 2015, 58, 51–64. [Google Scholar] [CrossRef]
- Sacher, J.; Houle, S.; Parkes, J.; Rusjan, P.; Sagrati, S.; Wilson, A.A.; Meter, J.H. Monoamine oxidase A inhibitor occupancy during treatment of major depressive episodes with moclobemide or St. John’s wort: An [11C]-harmine PET study. J. Psychiatry Neurosci. 2011, 36, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Manach, C.; Wikkiamson, G.; Morand, C.; Scalbert, A.; Remesy, C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005, 81 (Suppl. S1), 230S–242S. [Google Scholar] [CrossRef]
- Campisi, A.; Sposito, G.; Pellitteri, R.; Santonocito, D.; Bisicchia, J.; Raciti, G.; Russo, C.; Nardiello, P.; Pignatello, R.; Casamenti, F.; et al. Effect of unloaded and curcumin-loaded solid lipid nanoparticles on tissue transglutaminase isoforms expression levels in an experimental model of Alzheimer’s disease. Antioxidants 2022, 11, 1863. [Google Scholar] [CrossRef] [PubMed]
- Rassu, G.; Porcu, E.P.; Fancello, S.; Obinu, A.; Senes, N.; Galleri, G.; Migheli, R.; Gavini, E.; Giunchedi, P. Intranasal delivery of genistein-loaded nanoparticles as a potential preventive system against neurodegenerative disorders. Pharmaceutics 2019, 11, 8. [Google Scholar] [CrossRef]
- Bandiwadekar, A.; Jose, J.; Gopan, G.; Augustin, V.; Ashtekar, H.; Khot, K.B. Transdermal delivery of resveratrol loaded solid lipid nanoparticle as a microneedle patch: A novel approach for the treatment of Parkinson’s disease. Drug Deliv. Transl. Res. 2025, 15, 1043–1073. [Google Scholar] [CrossRef]
- Kisku, A.; Nishad, A.; Agrawal, S.; Paliwal, R.; Datusalia, A.K.; Gupta, G.; Singh, S.K.; Dua, K.; Sulakhiya, K. Recent developments in intranasal drug delivery of nanomedicines for the treatment of neuropsychiatric disorders. Front. Med. 2024, 11, 1463976. [Google Scholar] [CrossRef]
- Jithavech, P.; Suwattananuruk, P.; Hasriadi, M.C.; Thitikornpong, W.; Towiwat, P.; Vajragupta, O.; Rojsitthisak, P. Physicochemical investigation of a novel curcumin diethyl γ-aminobutyrate, a carbamate ester prodrug of curcumin with enhanced anti-neuroinflammatory activity. PLoS ONE 2022, 17, e0265689. [Google Scholar] [CrossRef]
- Ahmad, S.; Hafeez, A. Formulation and development of curcumin-piperine-loaded S-SNEDDS for the treatment of Alzheimer’s disease. Mol. Neurobiol. 2023, 60, 1067–1082. [Google Scholar] [CrossRef]
- Sree, N. Recent advances in drug delivery systems for targeting the blood-brain barrier: Review article. J. Pharma Insights Res. 2024, 2, 34–44. [Google Scholar] [CrossRef]
- Kitagawa, S. Inhibitory effects of polyphenols on p-glycoprotein-mediated transport. Biol. Pharm. Bull. 2006, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Campos-Bedolla, P.; Walter, F.R.; Vaszelka, S.; Deli, M.A. Role of the blood-brain barrier in the nutrition of the central nervous system. Arch. Med. Res. 2014, 45, 610–638. [Google Scholar] [CrossRef] [PubMed]
- Scheepens, A.; Tan, T.; Paxton, J.W. Improving the oral bioavailability of beneficial polyphenols through designed synergies. Genes. Nutr. 2010, 5, 75–87. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Yu, P.; Yang, C.; Xia, C.; Deng, J.; Yu, M.; Xiang, Z.; Gan, L.; Zhu, B.; et al. A Novel Quercetin Encapsulated Glucose Modified Liposome and Its Brain-Target Antioxidative Neuroprotection Effects. Molecules 2024, 29, 607. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Lopes, I.; Magalhães, L.; Sárria, M.P.; Machado, R.; Sousa, J.C.; Botelho, C.; Teixeira, J.; Gomes, A.C. Novel concept of exosome-like liposomes for the treatment of Alzheimer’s disease. J. Control. Release 2021, 336, 130–143. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Fu, B.M.; Zhang, Z.J. Borneol, a novel agent that improves central nervous system drug delivery by enhancing blood–brain barrier permeability. Drug Deliv. 2017, 24, 1037–1044. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naoi, M.; Wu, Y.; Maruyama, W.; Shamoto-Nagai, M. Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases. Int. J. Mol. Sci. 2025, 26, 2916. https://doi.org/10.3390/ijms26072916
Naoi M, Wu Y, Maruyama W, Shamoto-Nagai M. Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases. International Journal of Molecular Sciences. 2025; 26(7):2916. https://doi.org/10.3390/ijms26072916
Chicago/Turabian StyleNaoi, Makoto, Yuqiu Wu, Wakako Maruyama, and Masayo Shamoto-Nagai. 2025. "Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases" International Journal of Molecular Sciences 26, no. 7: 2916. https://doi.org/10.3390/ijms26072916
APA StyleNaoi, M., Wu, Y., Maruyama, W., & Shamoto-Nagai, M. (2025). Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases. International Journal of Molecular Sciences, 26(7), 2916. https://doi.org/10.3390/ijms26072916


_Kim.png)


