Chlorine Modulates Photosynthetic Efficiency, Chlorophyll Fluorescence in Tomato Leaves, and Carbohydrate Allocation in Developing Fruits
Abstract
:1. Introduction
2. Results
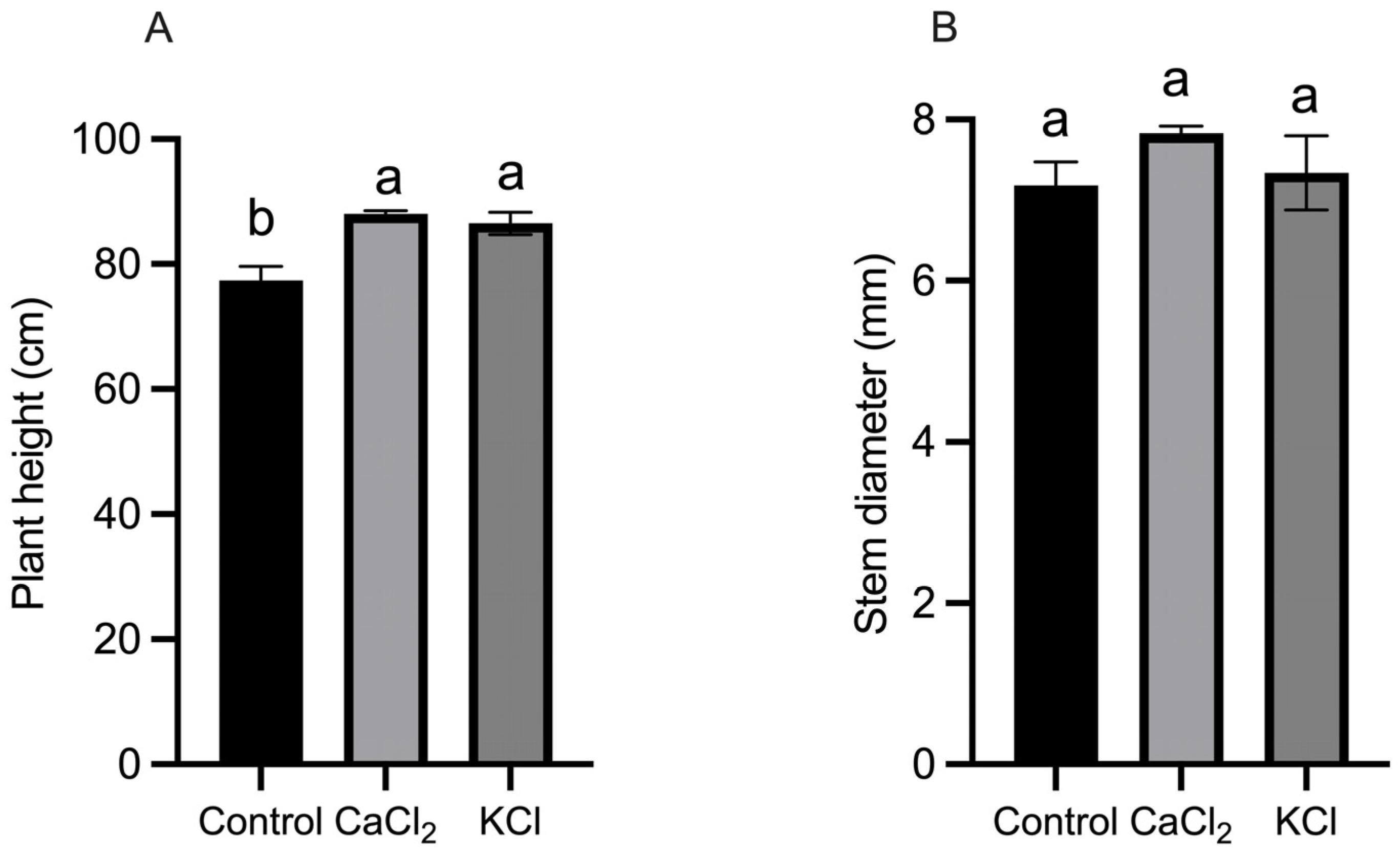
2.1. Effect of Exogenous Chlorine on Tomato Growth and Yield
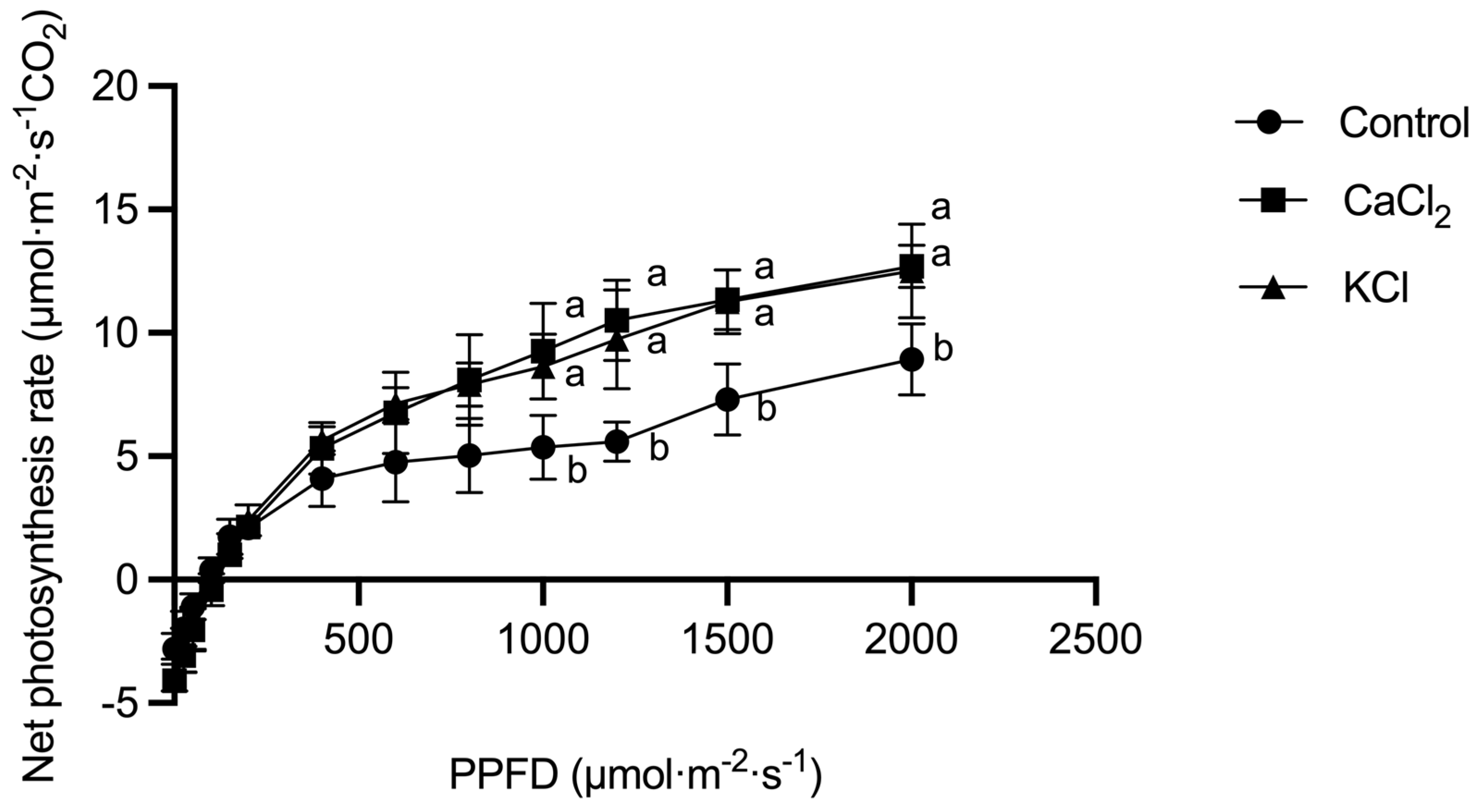
2.2. Effect of Exogenous Chlorine on Photosynthetic Performance
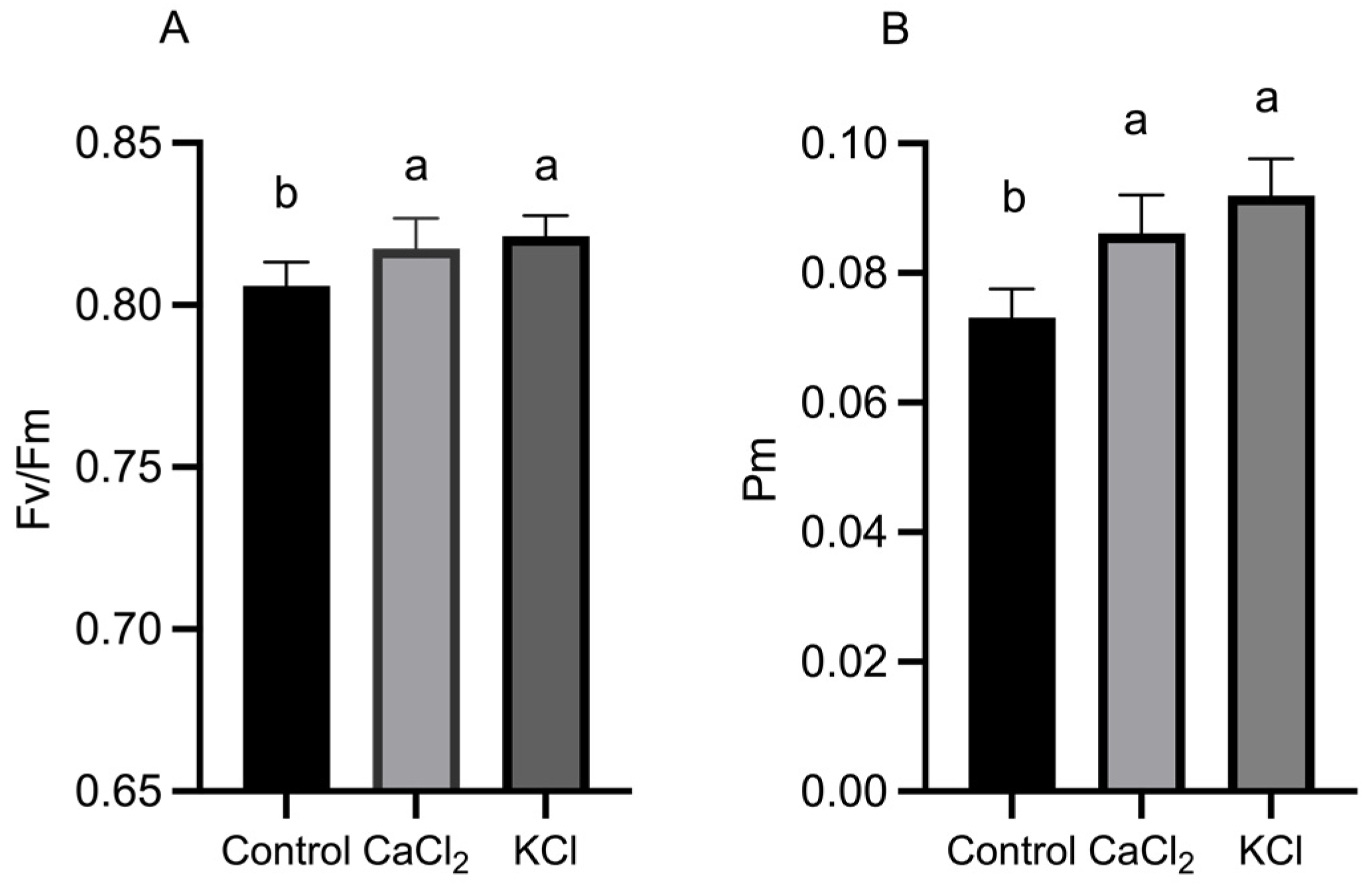
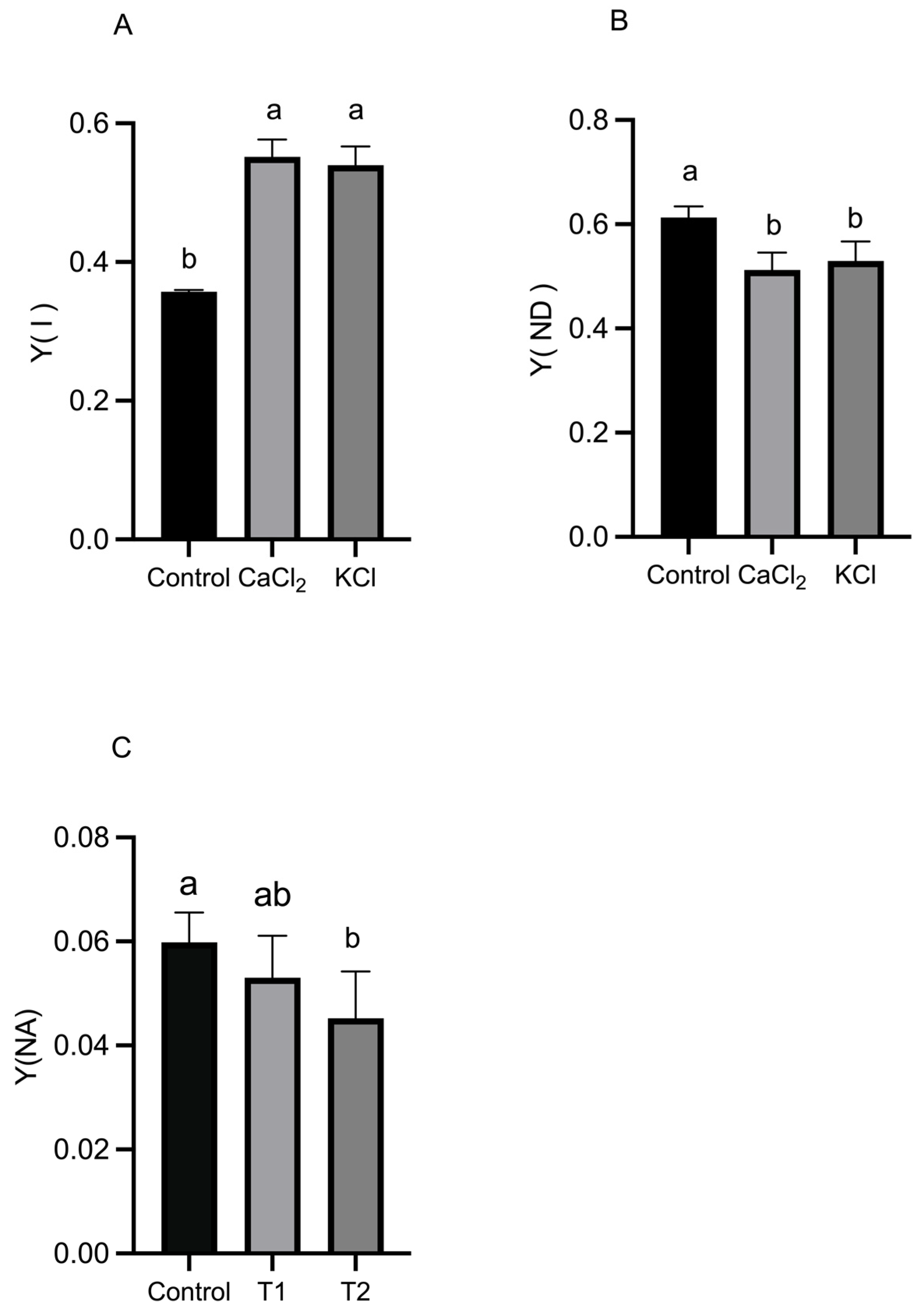
2.3. Effect of Exogenous Chlorine on Activity of Photosystems
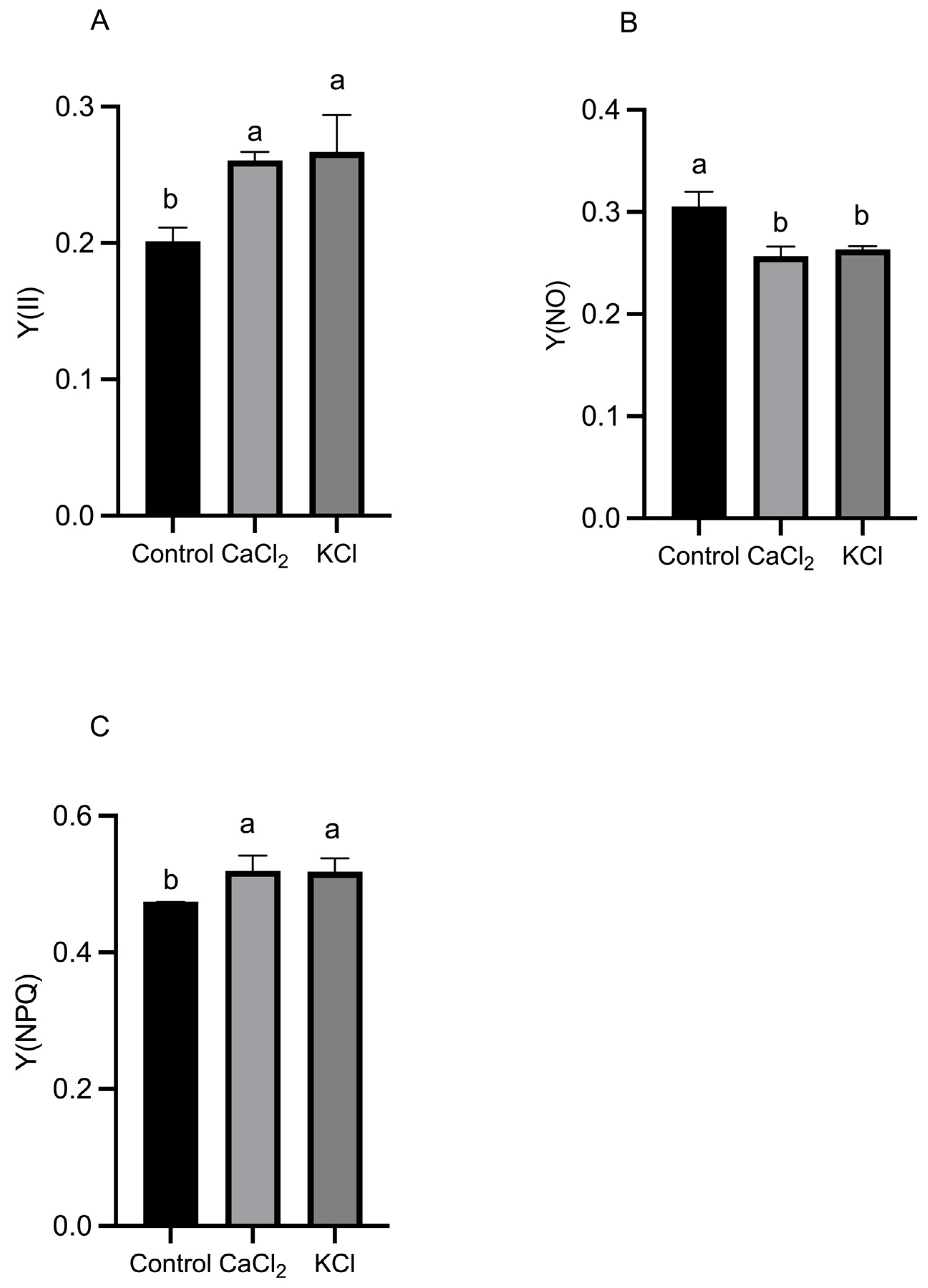
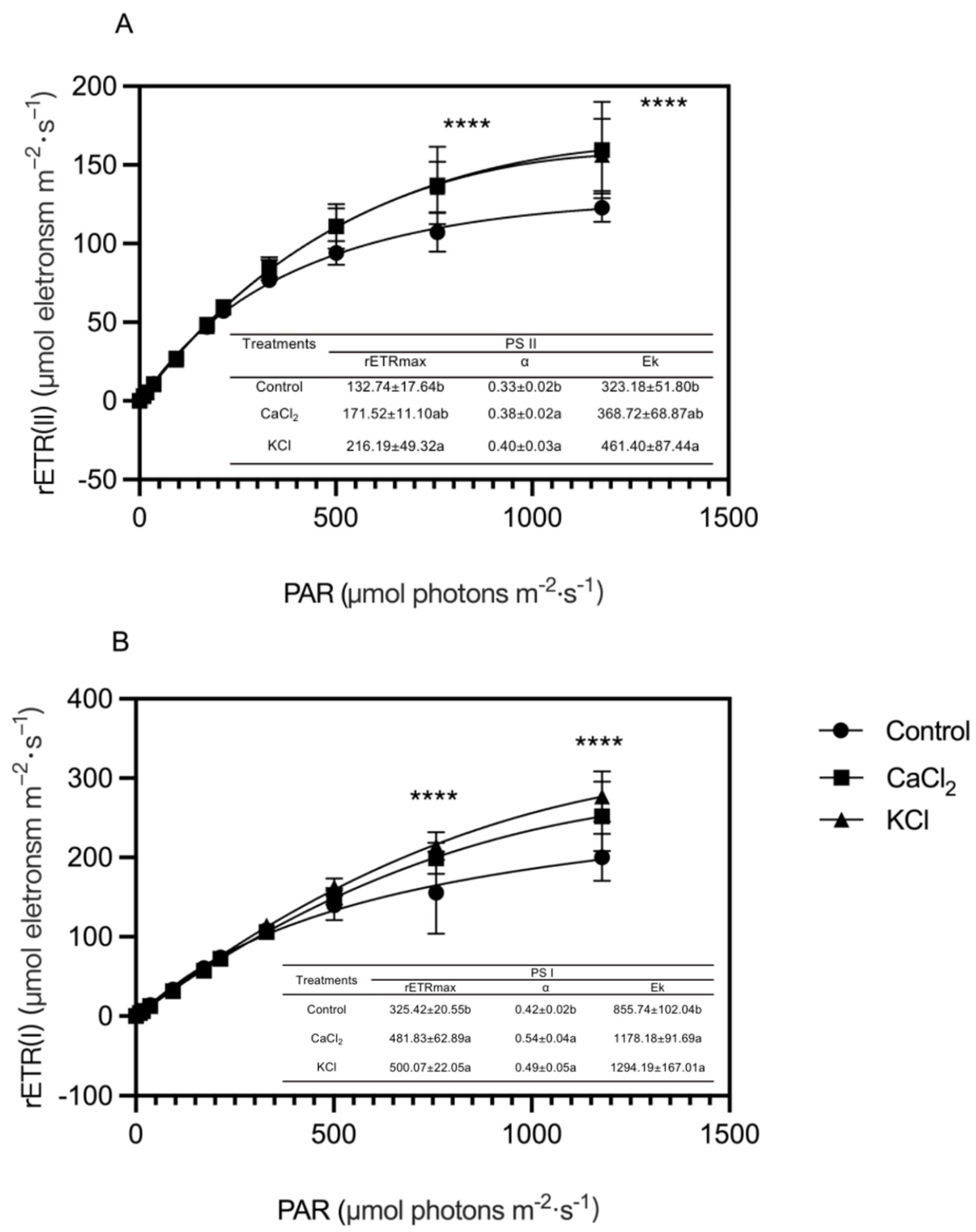
2.4. Effect of Exogenous Chlorine on Photosynthetic Electron Transport
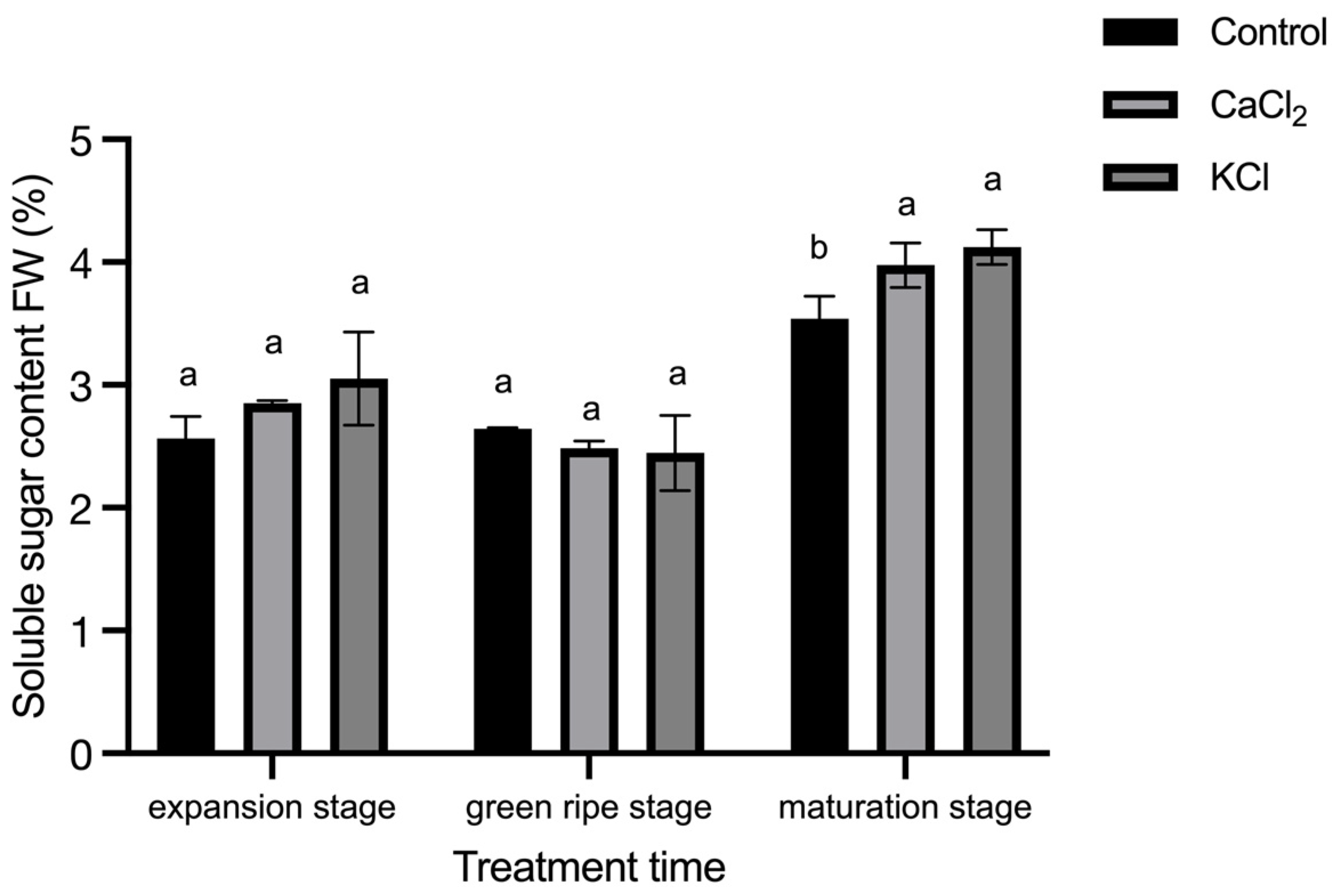
2.5. Effect of Exogenous Chlorine on Fruit Sugar Content and Sugar Ratio
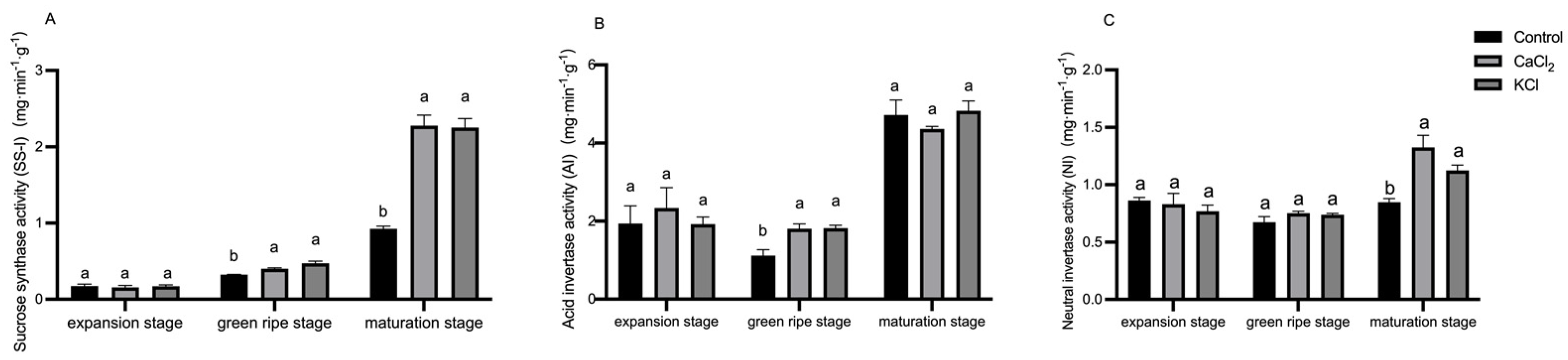
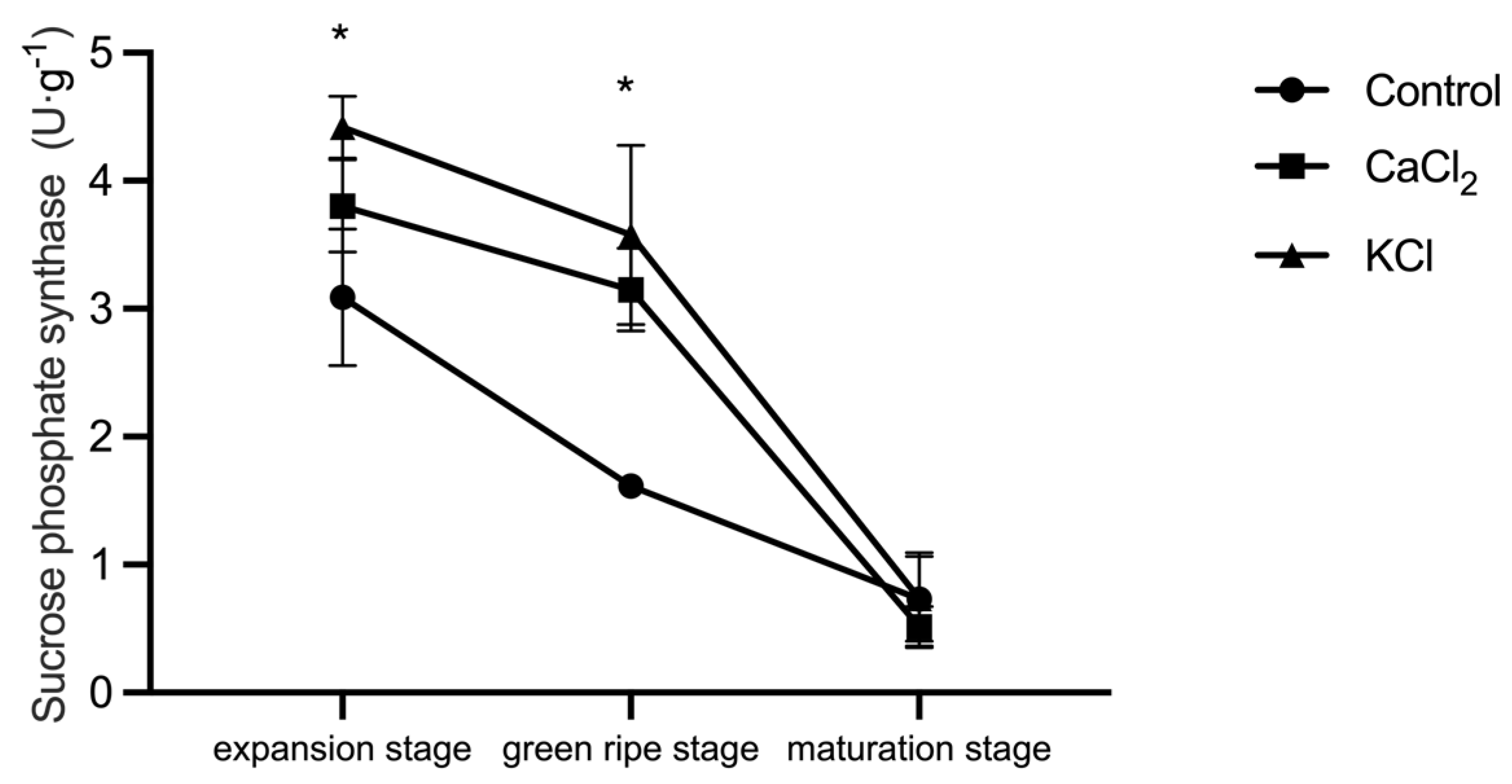
2.6. Effect of Exogenous Chlorine on Enzyme Activity Related to Sugar Metabolism
3. Discussion
3.1. Cl− Has a Positive Effect on the Growth of Tomato Plants
3.2. Cl− Promotes Photosynthesis in the Leaves of Tomato Plants and Improves the Photosynthetic Performance of the Leaves
3.3. Cl− Promotes Fruit Sugar Metabolism
4. Materials and Methods
4.1. Growth Conditions and Experimental Materials
4.2. Plant Growth Indicators
4.3. Gas Exchange and Chlorophyll Fluorescence Parameters
4.4. Sucrose Metabolism Enzyme Activity
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Acid invertase |
| AQE | Apparent quantum efficiency |
| Ci | Intercellular CO2 concentration |
| Cl | Chlorine |
| ETR(I) | PS I rate of electronic transmission |
| ETR(II) | PS II rate of electronic transmission |
| Fv/Fm | Maximal photochemical efficiency of PS II |
| Gs | Stomatal conductance |
| Ivr | Invertase |
| NI | Neutral invertase |
| Pm | PS I maximum oxidation state |
| Pn | Net photosynthesis rate |
| PS I | Photosystem I |
| PS II | Photosystem II |
| SPS | Sucrose phosphate synthase |
| SS-I | Sucrose synthase |
| Tr | Transpiration rate |
| Y(I) | Quantum efficiency of PS I photochemistry |
| Y(II) | Quantum efficiency of PS II photochemistry |
| Y(NA) | Acceptor-side limitation of PS I |
| Y(ND) | Donor-side limitation of PS I |
| Y(NO) | Yield of non-regulated energy dissipation |
| Y(NPQ) | Yield of regulated energy dissipation |
References
- Xu, G.; Magen, H.; Tarhitzkya, J. Advances in chloride nutrition of plants. Adv. Agron. 2000, 68, 97–110. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. ISBN 978-0-12-384905-2. [Google Scholar]
- Franco-Navarro, J.D.; Brumós, J.; Rosales, M.A.; Cubero-Font, P.; Talón, M.; Colmenero-Flores, J.M. Chloride Regulates Leaf Cell Size and Water Relations in Tobacco Plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef]
- Raven, J.A. Chloride: Essential Micronutrient and Multifunctional Beneficial Ion. J. Exp. Bot. 2016, 68, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Geilfus, C.-M. Review on the Significance of Chlorine for Crop Yield and Quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef]
- White, P. Chloride in Soils and Its Uptake and Movement Within the Plant: A Review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef]
- Broyer, T.C.; Carlton, A.B.; Johnson, C.M.; Stout, P.R. Chlorine—A Micronutrient Element for Higher Plants. Plant Physiol. 1954, 29, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl− Transport Contributing to Salt Tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar]
- Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. [Google Scholar] [CrossRef]
- Terry, N. Photosynthesis, Growth, and the Role of Chloride 12. Plant Physiol. 1977, 60, 69–75. [Google Scholar]
- Kawakami, K.; Umena, Y.; Kamiya, N.; Shen, J.-R. Location of Chloride and Its Possible Functions in Oxygen-Evolving Photosystem II Revealed by X-Ray Crystallography. Proc. Natl. Acad. Sci. USA 2009, 106, 8567–8572. [Google Scholar] [CrossRef]
- Pokhrel, R.; McConnell, I.L.; Brudvig, G.W. Chloride Regulation of Enzyme Turnover: Application to the Role of Chloride in Photosystem II. Biochemistry 2011, 50, 2725–2734. [Google Scholar] [CrossRef] [PubMed]
- Hänsch, R.; Mendel, R.R. Physiological Functions of Mineral Micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Wege, S.; Gilliham, M.; Henderson, S.W. Chloride: Not Simply a ‘Cheap Osmoticum’, but a Beneficial Plant Macronutrient. J. Exp. Bot. 2017, 68, 3057–3069. [Google Scholar] [CrossRef]
- Chen, Z.-H.; Hills, A.; Bätz, U.; Amtmann, A.; Lew, V.L.; Blatt, M.R. Systems Dynamic Modeling of the Stomatal Guard Cell Predicts Emergent Behaviors in Transport, Signaling, and Volume Control. Plant Physiol. 2012, 159, 1235–1251. [Google Scholar] [CrossRef]
- Schwenke, G.D.; Simpfendorfer, S.R.; Collard, B.C.Y. Confirmation of Chloride Deficiency as the Cause of Leaf Spotting in Durum Wheat Grown in the Australian Northern Grains Region. Crop Pasture Sci. 2015, 66, 122. [Google Scholar] [CrossRef]
- Saito, S.; Uozumi, N. Guard Cell Membrane Anion Transport Systems and Their Regulatory Components: An Elaborate Mechanism Controlling Stress-Induced Stomatal Closure. Plants 2019, 8, 9. [Google Scholar] [CrossRef]
- Hongqiao, L.; Suyama, A.; Mitani-Ueno, N.; Hell, R.; Maruyama-Nakashita, A. A Low Level of NaCl Stimulates Plant Growth by Improving Carbon and Sulfur Assimilation in Arabidopsis thaliana. Plants 2021, 10, 2138. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Liu, X.; Li, J.; Yue, L.; Yang, H.; Zou, H.; Wang, Z.; Xing, B. Copper Nanoclusters Promote Tomato (Solanum lycopersicum L.) Yield and Quality Through Improving Photosynthesis and Roots Growth. Environ. Pollut. 2021, 289, 117912. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Díaz-Rueda, P.; Rivero-Núñez, C.M.; Brumós, J.; Rubio-Casal, A.E.; De Cires, A.; Colmenero-Flores, J.M.; Rosales, M.A. Chloride Nutrition Improves Drought Resistance by Enhancing Water Deficit Avoidance and Tolerance Mechanisms. J. Exp. Bot. 2021, 72, 5246–5261. [Google Scholar] [CrossRef]
- Robinson, S.P.; Downton, W.J.S.; Millhouse, J.A. Photosynthesis and Ion Content of Leaves and Isolated Chloroplasts of Salt-Stressed Spinach. Plant Physiol. 1983, 73, 238–242. [Google Scholar] [CrossRef]
- Winter, K. Sodium, Potassium, Chloride and Proline Concentrations of Chloroplasts Isolated from a Halophyte, Mesembryanthemum crystmlinum L. Planta 1986, 168, 421–426. [Google Scholar]
- Petridis, A.; Van Der Kaay, J.; Chrysanthou, E.; McCallum, S.; Graham, J.; Hancock, R.D. Photosynthetic Limitation as a Factor Influencing Yield in Highbush Blueberries (Vaccinium corymbosum) Grown in a Northern European Environment. J. Exp. Bot. 2018, 69, 3069–3080. [Google Scholar] [CrossRef]
- Wang, M.; Dong, C.; Gao, W. Evaluation of the Growth, Photosynthetic Characteristics, Antioxidant Capacity, Biomass Yield and Quality of Tomato Using Aeroponics, Hydroponics and Porous Tube-Vermiculite Systems in Bio-Regenerative Life Support Systems. Life Sci. Space Res. 2019, 22, 68–75. [Google Scholar] [CrossRef]
- Klee, H. Genetics and Control of Tomato Fruit Ripening and Quality Attributes. Annu. Rev. Genet. 2011, 45, 41–59. [Google Scholar] [CrossRef]
- Rosales, M.A.; Franco-Navarro, J.D.; Peinado-Torrubia, P.; Díaz-Rueda, P.; Álvarez, R.; Colmenero-Flores, J.M. Chloride Improves Nitrate Utilization and NUE in Plants. Front. Plant Sci. 2020, 11, 442. [Google Scholar] [CrossRef]
- Yuan, G.-F.; Jia, C.-G.; Li, Z.; Sun, B.; Zhang, L.-P.; Liu, N.; Wang, Q.-M. Effect of Brassinosteroids on Drought Resistance and Abscisic Acid Concentration in Tomato under Water Stress. Sci. Hortic. 2010, 126, 103–108. [Google Scholar] [CrossRef]
- Tanksley, S.D. The Genetic, Developmental, and Molecular Bases of Fruit Size and Shape Variation in Tomato. Plant Cell Online 2004, 16, S181–S189. [Google Scholar] [CrossRef]
- Bertin, N.; Génard, M. Tomato Quality as Influenced by Preharvest Factors. Sci. Hortic. 2018, 233, 264–276. [Google Scholar] [CrossRef]
- Yuan, Y.; Mei, L.; Wu, M.; Wei, W.; Shan, W.; Gong, Z.; Zhang, Q.; Yang, F.; Yan, F.; Zhang, Q.; et al. SlARF10, an Auxin Response Factor, Is Involved in Chlorophyll and Sugar Accumulation during Tomato Fruit Development. J. Exp. Bot. 2018, 69, 5507–5518. [Google Scholar] [CrossRef]
- Kimura, S.; Sinha, N. Tomato (Solanum lycopersicum): A Model Fruit-Bearing Crop. Cold Spring Harb. Protoc. 2008, 2008, pdb-emo105. [Google Scholar]
- Causse, M.M.; Friguet, C.; Coiret, C.; Lépicier, M.; Navez, B.; Lee, M.; Holthuysen, N.; Sinesio, F.; Moneta, E.; Grandillo, S. Consumer Preferences for Fresh Tomato at the European Scale: A Common Segmentation on Taste and Firmness. J. FoodSci. 2010, 75, S531–S541. [Google Scholar]
- Tieman, D.; Zhu, G.; Resende, M.F.R.; Lin, T.; Nguyen, C.; Bies, D.; Rambla, J.L.; Beltran, K.S.O.; Taylor, M.; Zhang, B.; et al. A Chemical Genetic Roadmap to Improved Tomato Flavor. Science 2017, 355, 391–394. [Google Scholar] [CrossRef]
- Lu, T.; Yu, H.; Li, Q.; Chai, L.; Jiang, W. Improving Plant Growth and Alleviating Photosynthetic Inhibition and Oxidative Stress From Low-Light Stress With Exogenous GR24 in Tomato (Solanum lycopersicum L.) Seedlings. Front. Plant Sci. 2019, 10, 490. [Google Scholar] [CrossRef]
- Matsukura, C. Sugar Accumulation in Tomato Fruit and Its Modification Using Molecular Breeding Techniques. In Functional Genomics and Biotechnology in Solanaceae and Cucurbitaceae Crops; Ezura, H., Ariizumi, T., Garcia-Mas, J., Rose, J., Eds.; Biotechnology in Agriculture and Forestry; Springer: Berlin/Heidelberg, Germany, 2016; Volume 70, pp. 141–154. ISBN 978-3-662-48533-0. [Google Scholar]
- Krapp, A. Plant Nitrogen Assimilation and Its Regulation: A Complex Puzzle with Missing Pieces. Curr. Opin. Plant Biol. 2015, 25, 115–122. [Google Scholar] [CrossRef]
- Guan, F.; Xia, M.; Tang, X.; Fan, S. Spatial Variability of Soil Nitrogen, Phosphorus and Potassium Contents in Moso Bamboo Forests in Yong’an City, China. Catena 2017, 150, 161–172. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Rosales, M.A.; Cubero-Font, P.; Calvo, P.; Álvarez, R.; Diaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as a Macronutrient Increases Water-Use Efficiency by Anatomically Driven Reduced Stomatal Conductance and Increased Mesophyll Diffusion to CO2. Plant J. 2019, 99, 815–831. [Google Scholar] [CrossRef]
- Parvizi, H.; Sepaskhah, A.R. Effect of Drip Irrigation and Fertilizer Regimes on Fruit Quality of a Pomegranate (Punica granatum (L.) Cv. Rabab) Orchard. Agric. Water Manag. 2015, 156, 70–78. [Google Scholar] [CrossRef]
- Koyama, K.; Ikeda, H.; Poudel, P.R.; Goto-Yamamoto, N. Light Quality Affects Flavonoid Biosynthesis in Young Berries of Cabernet Sauvignon Grape. Phytochemistry 2012, 78, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Huang, G.; Lu, X.; Gu, S.; Wen, W.; Wang, G.; Chang, W.; Guo, X.; Zhao, C. Prediction of Vertical Distribution of SPAD Values Within Maize Canopy Based on Unmanned Aerial Vehicles Multispectral Imagery. Front Plant Sci 2023, 14, 1253536. [Google Scholar] [CrossRef]
- D’Esposito, D.; Cappetta, E.; Andolfo, G.; Ferriello, F.; Borgonuovo, C.; Caruso, G.; De Natale, A.; Frusciante, L.; Ercolano, M.R. Deciphering the Biological Processes Underlying Tomato Biomass Production and Composition. Plant Physiol. Biochem. 2019, 143, 50–60. [Google Scholar] [CrossRef]
- Besada, C.; Gil, R.; Bonet, L.; Quiñones, A.; Intrigliolo, D.; Salvador, A. Chloride Stress Triggers Maturation and Negatively Affects the Postharvest Quality of Persimmon Fruit. Involvement of Calyx Ethylene Production. Plant Physiol. Biochem. 2016, 100, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.; Cheng, X.; Li, X.; Deng, X.; Dong, X.; Wang, S.; Pu, X. Effects of Silicon Application on Leaf Structure and Physiological Characteristics of Glycyrrhiza uralensis Fisch. and Glycyrrhiza inflata Bat. under Salt Treatment. BMC Plant Biol 2022, 22, 390. [Google Scholar] [CrossRef]
- Hanstein, S.M.; Felle, H.H. CO2-Triggered Chloride Release from Guard Cells in Intact Fava Bean Leaves. Kinetics of the Onset of Stomatal Closure. Plant Physiol. 2002, 130, 940–950. [Google Scholar] [PubMed]
- Abou Seeda, M.A.; Abou El-Nour EA, A.; Hammad, S.A.; Yassen, A.A. Chloride Ions as a Beneficial and Essential Micronutrient Multifunctional, Role and Regulation in Plant Physiology: A Review. Middle East J. Appl. Sci. 2021, 11, 76–125. [Google Scholar] [CrossRef]
- Goutam Kumar Dash, S.P.; Soumya Kumar Sahoo, M.B.; Mirza Jaynul Baig, R.K.P.; Swain, P. Photosystem II Photochemistry and Chlorophyll Intensity in Rice (Oryza sativa L.) under Drought at Flowering Stage. Int. J. Curr. Microbiol. App. Sci. 2021, 10, 746–756. [Google Scholar] [CrossRef]
- Anderson, J.M. Cytochrome B6f Complex: Dynamic Molecular Organization, Function and Acclimation. Photosynth. Res. 1992, 34, 341–357. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Saturation Pulse Method for Assessment of Energy Conversion in PS I. Planta 1994, 192, 261–268. [Google Scholar]
- Yamori, W.; Sakata, N.; Suzuki, Y.; Shikanai, T.; Makino, A. Cyclic Electron Flow Around Photosystem I via Chloroplast NAD(P)H Dehydrogenase (NDH) Complex Performs a Significant Physiological Role During Photosynthesis and Plant Growth at Low Temperature in Rice. Plant J. 2011, 68, 966–976. [Google Scholar] [CrossRef]
- Haddy, A.; Beravolu, S.; Johnston, J.; Kern, H.; McDaniel, M.; Ore, B.; Reed, R.; Tai, H. Exploring the Interdependence of Calcium and Chloride Activation of O2 Evolution in Photosystem II. Photosynth. Res. 2024, 162, 385–400. [Google Scholar]
- Gorbe, E.; Calatayud, A. Applications of Chlorophyll Fluorescence Imaging Technique in Horticultural Research: A Review. Sci. Hortic. 2012, 138, 24–35. [Google Scholar] [CrossRef]
- Lu, T.; Song, Y.; Yu, H.; Li, Q.; Xu, J.; Qin, Y.; Zhang, G.; Liu, Y.; Jiang, W. Cold Stress Resistance of Tomato (Solanum lycopersicum) Seedlings Is Enhanced by Light Supplementation from Underneath the Canopy. Front. Plant Sci. 2022, 13, 831314. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Schideman, L.C.; Park, D.S.; Stirbet, A.; Govindjee; Rupassara, S.I.; Krehbiel, J.D.; Seufferheld, M.J. Characterization of a Chlamydomonas Reinhardtii Mutant Strain with Improved Biomass Production Under Low Light and Mixotrophic Conditions. Algal Res. 2015, 11, 134–147. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II Quantum Yields Calculated from Simple Fluorescence Parameters Measured by PAM Fluorometry and the Saturation Pulse Method. PAM Appl. Notes 2008, 1, 201–247. [Google Scholar]
- Tang, Y.; Wen, X.; Lu, Q.; Yang, Z.; Cheng, Z.; Lu, C. Heat Stress Induces an Aggregation of the Light-Harvesting Complex of Photosystem II in Spinach Plants. Plant Physiol. 2007, 143, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Kumar, S.; Cornish, K. Photosynthetic Response of In Vitro Guayule Plants in Low and High Lights and the Role of Non-Photochemical Quenching in Plant Acclimation. Ind. Crops Prod. 2014, 54, 266–271. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein Synthesis Is the Primary Target of Reactive Oxygen Species in the Photoinhibition of Photosystem II. Physiol. Plant. 2011, 142, 35–46. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, H.; Liu, H.; He, Y.; Yin, Z. Effects of OsRCA Overexpression on Rubisco Activation State and Photosynthesis in Maize. Plants 2023, 12, 1614. [Google Scholar] [CrossRef]
- Storti, M.; Puggioni, M.P.; Segalla, A.; Morosinotto, T.; Alboresi, A. The Activity of Chloroplast NADH Dehydrogenase-like Complex Influences the Photosynthetic Activity of the Moss Physcomitrella Patens. bioRxiv 2020. [Google Scholar] [CrossRef]
- Schumaker, M.F.; Kramer, D.M. Comparison of Monte Carlo Simulations of Cytochrome B6f with Experiment Using Latin Hypercube Sampling. Bull. Math. Biol. 2011, 73, 2152–2174. [Google Scholar] [CrossRef]
- Johnson, G.N. Physiology of PSI Cyclic Electron Transport in Higher Plants. Biochim. Biophys. Acta (BBA)-Bioenerg. 2011, 1807, 384–389. [Google Scholar]
- Herdean, A.; Teardo, E.; Nilsson, A.K.; Pfeil, B.E.; Johansson, O.N.; Ünnep, R.; Nagy, G.; Zsiros, O.; Dana, S.; Solymosi, K.; et al. A Voltage-Dependent Chloride Channel Fine-Tunes Photosynthesis in Plants. Nat. Commun. 2016, 7, 11654. [Google Scholar] [CrossRef] [PubMed]
- Duan, Z.; Kong, F.; Zhang, L.; Li, W.; Zhang, J.; Peng, L. A Bestrophin-like Protein Modulates the Proton Motive Force across the Thylakoid Membrane in Arabidopsis. J. Integr. Plant Biol. 2016, 58, 848–858. [Google Scholar] [CrossRef]
- Hagino, T.; Kato, T.; Kasuya, G.; Kobayashi, K.; Kusakizako, T.; Hamamoto, S.; Sobajima, T.; Fujiwara, Y.; Yamashita, K.; Kawasaki, H.; et al. Cryo-EM Structures of Thylakoid-Located Voltage-Dependent Chloride Channel VCCN1. Nat. Commun. 2022, 13, 2505. [Google Scholar] [CrossRef]
- Imaizumi, K.; Nishimura, T.; Nagao, R.; Saito, K.; Nakano, T.; Ishikita, H.; Noguchi, T.; Ifuku, K. D139N Mutation of PsbP Enhances the Oxygen-Evolving Activity of Photosystem II through Stabilized Binding of a Chloride Ion. PNAS Nexus 2022, 1, pgac136. [Google Scholar] [CrossRef] [PubMed]
- Centeno, D.C.; Osorio, S.; Nunes-Nesi, A.; Bertolo, A.L.F.; Carneiro, R.T.; Araújo, W.L.; Steinhauser, M.-C.; Michalska, J.; Rohrmann, J.; Geigenberger, P.; et al. Malate Plays a Crucial Role in Starch Metabolism, Ripening, and Soluble Solid Content of Tomato Fruit and Affects Postharvest Softening. Plant Cell 2011, 23, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Kang, S.; Du, T.; Guo, P.; Qiu, R.; Chen, R.; Gu, F. Modeling Relations of Tomato Yield and Fruit Quality with Water Deficit at Different Growth Stages Under Greenhouse Condition. Agric. Water Manag. 2014, 146, 131–148. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Tang, Z.; Yu, J.; Wu, Y.; Liu, Z.; Wang, J.; Wang, G.; Tian, Q. Application of Exogenous Melatonin Improves Tomato Fruit Quality by Promoting the Accumulation of Primary and Secondary Metabolites. Foods 2022, 11, 4097. [Google Scholar] [CrossRef]
- Grieco, M.; Tikkanen, M.; Paakkarinen, V.; Kangasjärvi, S.; Aro, E.-M. Steady-State Phosphorylation of Light-Harvesting Complex II Proteins Preserves Photosystem I Under Fluctuating White Light. Plant Physiol. 2012, 160, 1896–1910. [Google Scholar] [CrossRef]
- Ruan, Y.-L.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular Regulation of Seed and Fruit Set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Powell, A.L.T.; Nguyen, C.V.; Hill, T.; Cheng, K.L.; Figueroa-Balderas, R.; Aktas, H.; Ashrafi, H.; Pons, C.; Fernández-Muñoz, R.; Vicente, A.; et al. Uniform Ripening Encodes a Golden 2-like Transcription Factor Regulating Tomato Fruit Chloroplast Development. Science 2012, 336, 1711–1715. [Google Scholar] [CrossRef]
- Li, R.; Wang, J.; Yuan, H.; Niu, Y.; Sun, J.; Tian, Q.; Wu, Y.; Yu, J.; Tang, Z.; Xiao, X.; et al. Exogenous Application of ALA Enhanced Sugar, Acid and Aroma Qualities in Tomato Fruit. Front. Plant Sci. 2023, 14, 1323048. [Google Scholar] [CrossRef]
- Yamaki, S. Metabolism and Accumulation of Sugars Translocated to Fruit and Their Regulation. J. Japan. Soc. Hortic. Sci. 2010, 79, 1–15. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C. Effect of Salt Stress on the Growth and Fruit Quality of Tomato Plants. In Abiotic Stress Biology in Horticultural Plants; Kanayama, Y., Kochetov, A., Eds.; Springer: Tokyo, Japan, 2015; pp. 3–16. ISBN 978-4-431-55250-5. [Google Scholar]
- Luo, A.; Zhou, C.; Chen, J. The Associated with Carbon Conversion Rate and Source–Sink Enzyme Activity in Tomato Fruit Subjected to Water Stress and Potassium Application. Front. Plant Sci. 2021, 12, 681145. [Google Scholar] [CrossRef]
- Biais, B.; Bénard, C.; Beauvoit, B.; Colombié, S.; Prodhomme, D.; Ménard, G.; Bernillon, S.; Gehl, B.; Gautier, H.; Ballias, P.; et al. Remarkable Reproducibility of Enzyme Activity Profiles in Tomato Fruits Grown Under Contrasting Environments Provides a Roadmap for Studies of Fruit Metabolism. Plant Physiol. 2014, 164, 1204–1221. [Google Scholar] [CrossRef]
- Wang, B.; Li, N.; Huang, S.; Hu, J.; Wang, Q.; Tang, Y.; Yang, T.; Asmutola, P.; Wang, J.; Yu, Q. Enhanced Soluble Sugar Content in Tomato Fruit Using CRISPR/Cas9-Mediated SlINVINH1 and SlVPE5 Gene Editing. PeerJ 2021, 9, e12478. [Google Scholar] [CrossRef]
- Beauvoit, B.P.; Colombié, S.; Monier, A.; Andrieu, M.-H.; Biais, B.; Bénard, C.; Chéniclet, C.; Dieuaide-Noubhani, M.; Nazaret, C.; Mazat, J.-P.; et al. Model-Assisted Analysis of Sugar Metabolism throughout Tomato Fruit Development Reveals Enzyme and Carrier Properties in Relation to Vacuole Expansion. Plant Cell 2014, 26, 3224–3242. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Quoc, B.; N’Tchobo, H.; Foyer, C.H.; Yelle, S. Overexpression of Sucrose Phosphate Synthase Increases Sucrose Unloading in Transformed Tomato Fruit. J. Exp. Bot. 1999, 50, 785–791. [Google Scholar] [CrossRef]
- Rojas-González, J.A.; Soto-Súarez, M.; García-Díaz, Á.; Romero-Puertas, M.C.; Sandalio, L.M.; Mérida, Á.; Thormählen, I.; Geigenberger, P.; Serrato, A.J.; Sahrawy, M. Disruption of Both Chloroplastic and Cytosolic FBPase Genes Results in a Dwarf Phenotype and Important Starch and Metabolite Changes in Arabidopsis thaliana. J. Exp. Bot. 2015, 66, 2673–2689. [Google Scholar] [CrossRef]
- Gerardeaux, E.; Jordan-Meille, L.; Constantin, J.; Pellerin, S.; Dingkuhn, M. Changes in Plant Morphology and Dry Matter Partitioning Caused by Potassium Deficiency in Gossypium hirsutum (L.). Environ. Exp. Bot. 2010, 67, 451–459. [Google Scholar] [CrossRef]
- Worrell, A.C.; Bruneau, J.-M.; Summerfelt, K.; Boersig, M.; Voelker, T.A. Expression of a Maize Sucrose Phosphate Synthase in Tomato Alters Leaf Carbohydrate Partitioning. Plant Cell 1991, 3, 1121–1130. [Google Scholar]
- Galtier, N.; Foyer, C.H.; Huber, J.; Voelker, T.A.; Huber, S.C. Effects of Elevated Sucrose-Phosphate Synthase Activity on Photosynthesis, Assimilate Partitioning, and Growth in Tomato (Lycopersicon esculentum Var UC82B). Plant Physiol. 1993, 101, 535–543. [Google Scholar] [CrossRef]
- Galtier, N.; Foyer, C.H.; Murchie, E.; Aired, R.; Quick, P.; Voelker, T.A.; Thépenier, C.; Lascève, G.; Betsche, T. Effects of Light and Atmospheric Carbon Dioxide Enrichment on Photosynthesis and Carbon Partitioning in the Leaves of Tomato (Lycopersicon esculentum L.) Plants over-Expressing Sucrose Phosphate Synthase. J. Exp. Bot. 1995, 46, 1335–1344. [Google Scholar] [CrossRef]
- Micallef, B.J.; Haskins, K.A.; Vanderveer, P.J.; Roh, K.-S.; Shewmaker, C.K.; Sharkey, T.D. Altered Photosynthesis, Flowering, and Fruiting in Transgenic Tomato Plants That Have an Increased Capacity for Sucrose Synthesis. Planta 1995, 196, 327–334. [Google Scholar] [CrossRef]
- Signora, L.; Galtier, N.; Skot, L.; Lucas, H.; Foyer, C.H. Over-Expression of Sucrose Phosphate Synthase in Arabidopsis thaliana Results in Increased Foliar Sucrose/Starch Ratios and Favours Decreased Foliar Carbohydrate Accumulation in Plants after Prolonged Growth with CO2 Enrichment. J. Exp. Bot. 1998, 49, 669–680. [Google Scholar] [CrossRef]
- Markússon, S.; Hjörleifsson, J.G.; Kursula, P.; Ásgeirsson, B. Structural Characterization of Functionally Important Chloride Binding Sites in the Marine Vibrio Alkaline Phosphatase. Biochemistry 2022, 61, 2248–2260. [Google Scholar]
- Amoah, J.N. Effect of Drought Acclimation on Sugar Metabolism in Millet. Protoplasma 2025, 262, 35–49. [Google Scholar] [CrossRef]
- Pottosin, I.I.; Muñiz, J. Higher plant vacuolar ionic transport in the cellular context. Acta Bot. Mex. 2002, 60, 37–77. [Google Scholar] [CrossRef]
- Mansour, M.M.F. Role of Vacuolar Membrane Transport Systems in Plant Salinity Tolerance. J. Plant Growth Regul. 2023, 42, 1364–1401. [Google Scholar] [CrossRef]
- Valdivieso, Á.G. The Chloride Anion as a Signalling Effector. Biol. Rev. 2019, 94, 1839–1856. [Google Scholar] [CrossRef]
- Zhang, X.; Franzisky, B.L.; Eigner, L.; Geilfus, C.; Zörb, C. Antagonism of Chloride and Nitrate Inhibits Nitrate Reductase Activity in Chloride-Stressed Maize. Plant Growth Regul. 2021, 93, 279–289. [Google Scholar] [CrossRef]
- Xu, Y.; Fu, X.; Sharkey, T.D.; Shachar-Hill, Y.; Walker, B.J. The Metabolic Origins of Non-Photorespiratory CO2 Release during Photosynthesis: A Metabolic Flux Analysis. Plant Physiol. 2021, 186, 297–314. [Google Scholar] [PubMed]
- White, A.C.; Rogers, A.; Rees, M.; Osborne, C.P. How Can We Make Plants Grow Faster? A Source–Sink Perspective on Growth Rate. J. Exp. Bot. 2016, 67, 31–45. [Google Scholar] [PubMed]
- Duran, C. Chloride Channels: Often Enigmatic, Rarely Predictable. Annu. Rev. Physiol. 2010, 72, 95–121. [Google Scholar] [CrossRef]
- Xin, W.; Zhang, L.; Zhang, W.; Gao, J.; Yi, J.; Zhen, X.; Li, Z.; Zhao, Y.; Peng, C.; Zhao, C. An Integrated Analysis of the Rice Transcriptome and Metabolome Reveals Differential Regulation of Carbon and Nitrogen Metabolism in Response to Nitrogen Availability. Int. J. Mol.Sci. 2019, 20, 2349. [Google Scholar] [PubMed]
- Pietrzykowska, M.; Suorsa, M.; Semchonok, D.A.; Tikkanen, M.; Boekema, E.J.; Aro, E.-M.; Jansson, S. The Light-Harvesting Chlorophyll a/b Binding Proteins Lhcb1 and Lhcb2 Play Complementary Roles during State Transitions in Arabidopsis. Plant Cell 2014, 26, 3646–3660. [Google Scholar] [CrossRef]
- Pružinská, A.; Shindo, T.; Niessen, S.; Kaschani, F.; Tóth, R.; Millar, A.H.; van der Hoorn, R.A.L. Major Cys Protease Activities Are Not Essential for Senescence in Individually Darkened Arabidopsis Leaves. BMC Plant Biol. 2017, 17, 4. [Google Scholar] [CrossRef]

| Treatments | Pn (μmol·m−2·s−1) | Ci (μmol·mol−1) | Tr (mmol·m−2·s−1) | Gs (mmol·m−2·s−1) |
|---|---|---|---|---|
| Control | 14.1 ± 1.31 b | 264.83 ± 25.38 a | 7.28 ± 0.38 b | 336.00 ± 28.46 b |
| CaCl2 | 17.7 ± 0.71 a | 269.50 ± 9.54 a | 9.68 ± 1.03 a | 646.17 ± 191.57 a |
| KCl | 18.1 ± 1.30 a | 284.67 ± 0.87 a | 10.05 ± 0.32 a | 676.83 ± 38.68 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, L.; Lu, T.; Li, Q.; Li, Y.; Wan, X.; Jiang, W.; Yu, H. Chlorine Modulates Photosynthetic Efficiency, Chlorophyll Fluorescence in Tomato Leaves, and Carbohydrate Allocation in Developing Fruits. Int. J. Mol. Sci. 2025, 26, 2922. https://doi.org/10.3390/ijms26072922
Su L, Lu T, Li Q, Li Y, Wan X, Jiang W, Yu H. Chlorine Modulates Photosynthetic Efficiency, Chlorophyll Fluorescence in Tomato Leaves, and Carbohydrate Allocation in Developing Fruits. International Journal of Molecular Sciences. 2025; 26(7):2922. https://doi.org/10.3390/ijms26072922
Chicago/Turabian StyleSu, Longpu, Tao Lu, Qiang Li, Yang Li, Xiaoyang Wan, Weijie Jiang, and Hongjun Yu. 2025. "Chlorine Modulates Photosynthetic Efficiency, Chlorophyll Fluorescence in Tomato Leaves, and Carbohydrate Allocation in Developing Fruits" International Journal of Molecular Sciences 26, no. 7: 2922. https://doi.org/10.3390/ijms26072922
APA StyleSu, L., Lu, T., Li, Q., Li, Y., Wan, X., Jiang, W., & Yu, H. (2025). Chlorine Modulates Photosynthetic Efficiency, Chlorophyll Fluorescence in Tomato Leaves, and Carbohydrate Allocation in Developing Fruits. International Journal of Molecular Sciences, 26(7), 2922. https://doi.org/10.3390/ijms26072922






