The Probiotic Strain Clostridium butyricum TO-A Produces Butyrate by Utilizing Lactate and Acetate
Abstract
1. Introduction
2. Results
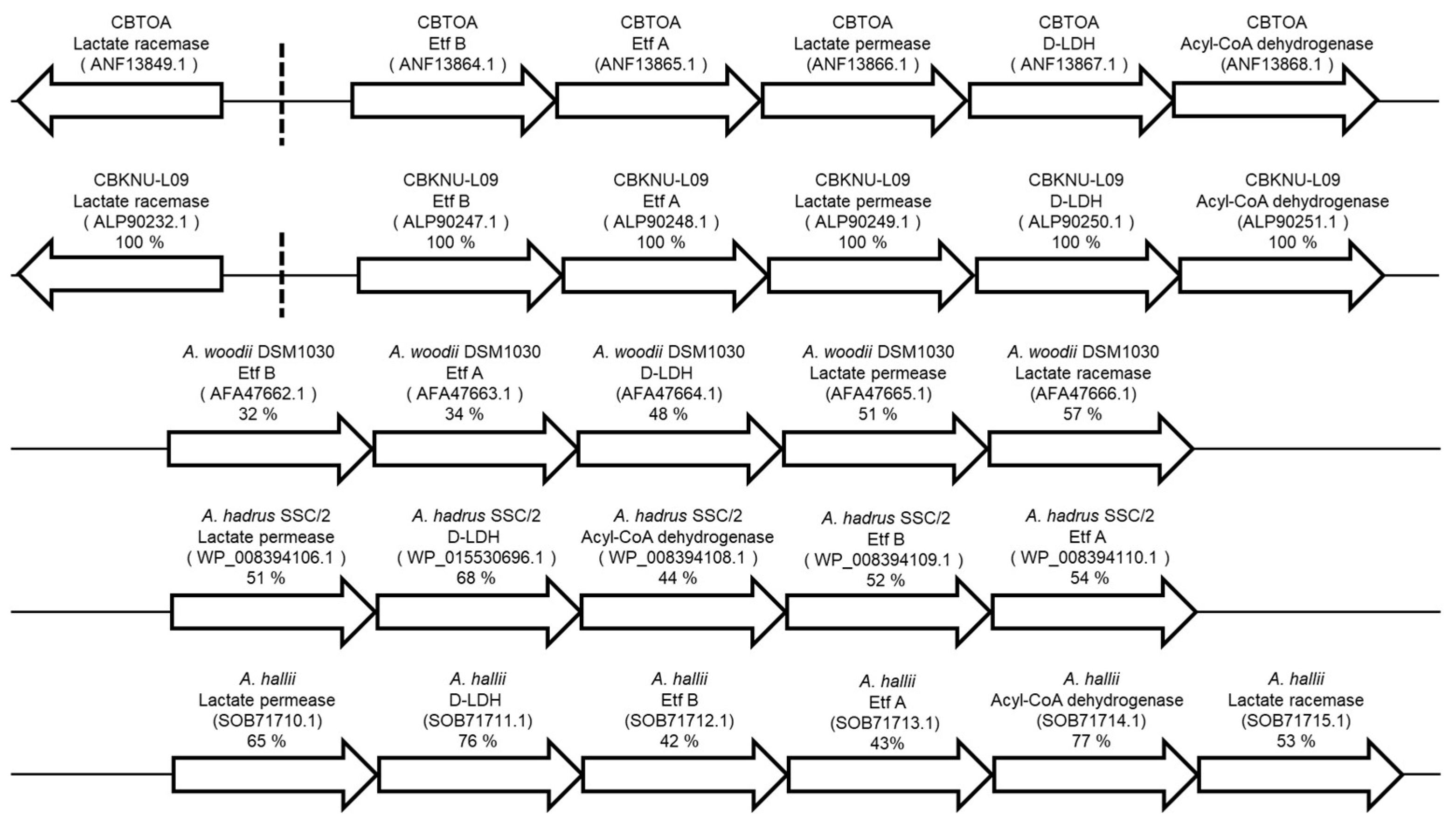
2.1. Sequence Search and Comparison of Putative Lactate Utilization-Related Genes of CBTOA
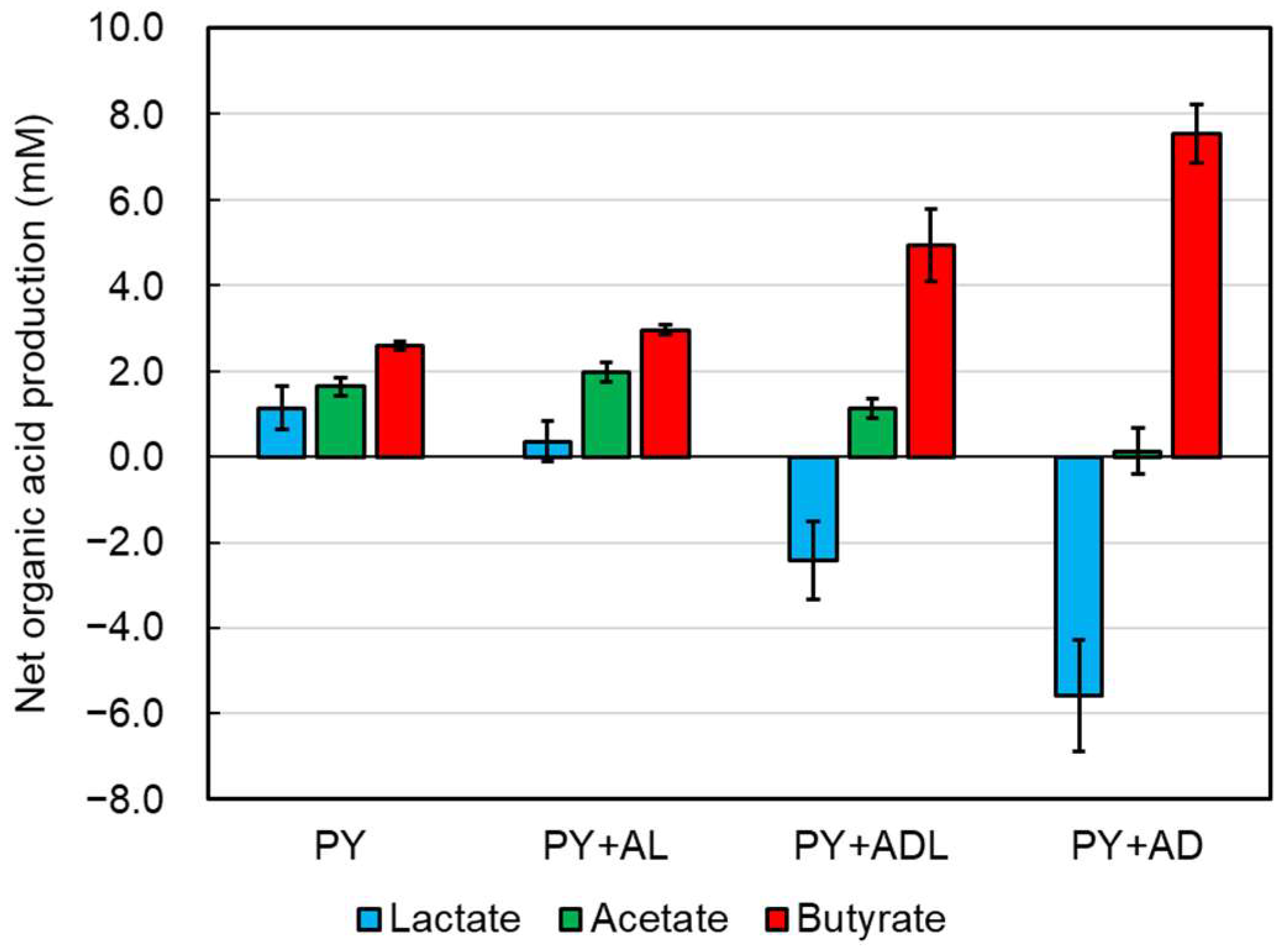
2.2. Evaluation of Lactate Utilization by CBTOA
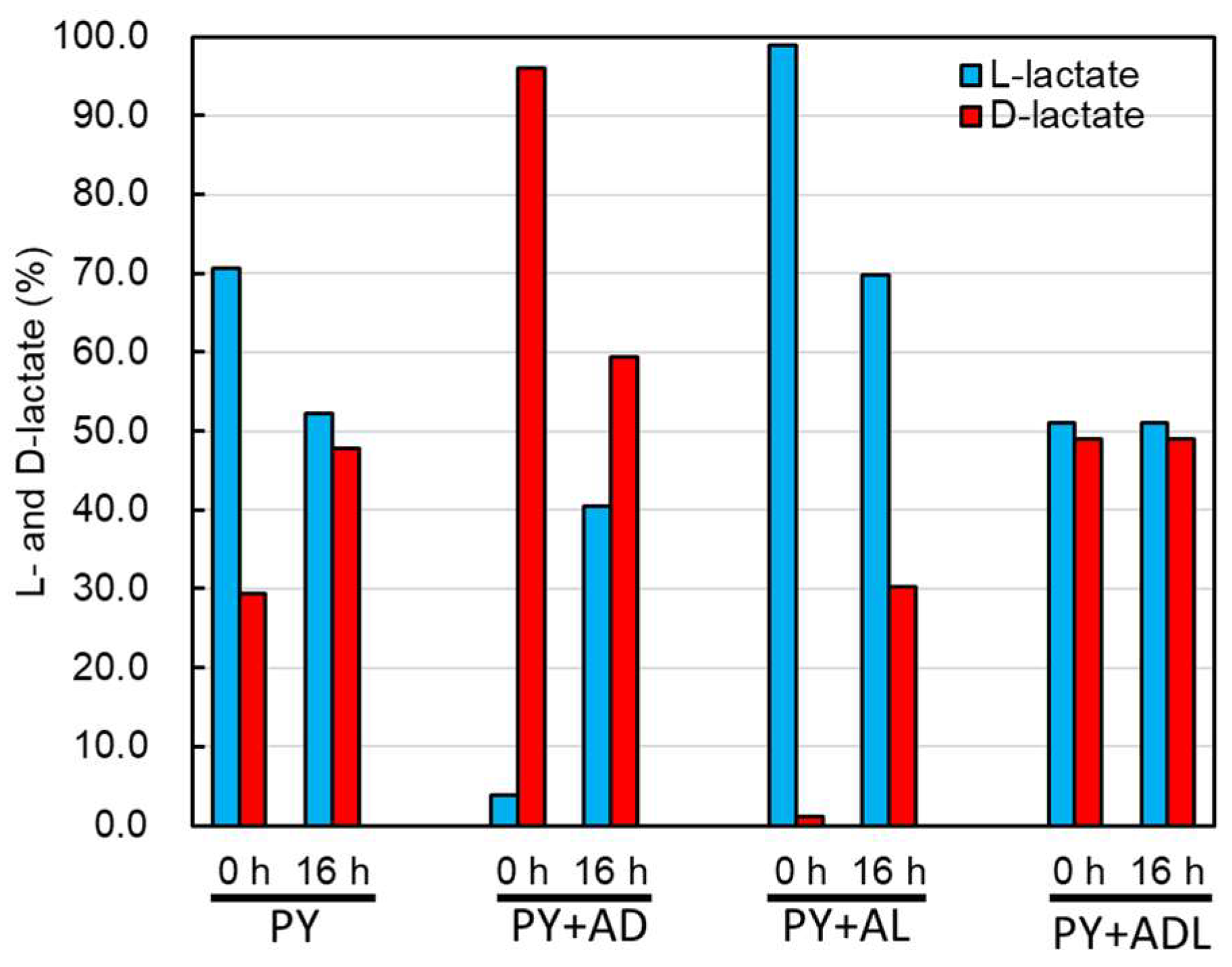
2.3. Effect of Lactate Enantiomers on Lactate Utilization by CBTOA
2.4. CBTOA Lactate Racemic Reaction
3. Discussion
4. Materials and Methods
4.1. Sequence Search and Comparison of Putative Lactate Utilization-Related Genes in CBTOA
4.2. Materials
4.2.1. Bacteria Strain
4.2.2. Basic Medium
4.3. Growth Conditions for CBTOA
4.4. Modified Medium
4.5. Evaluation of Lactate Utilization by CBTOA
4.6. Effect of Lactate Enantiomers on Lactate Utilization by CBTOA
4.7. Measurement of Total Viable Count and Spore Count
4.8. Measurement of Organic Acid Concentration in Culture Supernatant
4.9. Measurement of D-Lactate and L-Lactate Concentrations in Culture Supernatant
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergman, E.N. Energy contributions of volatile fatty acids from the gastrointestinal tract in various species. Physiol. Rev. 1990, 70, 567–590. [Google Scholar] [CrossRef] [PubMed]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Preston, T.; Frost, G.; Morrison, D.J. Role of gut microbiota-generated short-chain fatty acids in metabolic and cardiovascular health. Curr. Nutr. Rep. 2018, 7, 198–206. [Google Scholar] [PubMed]
- Maslowski, K.M.; Vieira, A.T.; Ng, A.; Kranich, J.; Sierro, F.; Yu, D.; Schilter, H.C.; Rolph, M.S.; Mackay, F.; Artis, D.; et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 2009, 461, 1282–1826. [Google Scholar]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar]
- Gaudier, E.; Jarry, A.; Blottière, H.M.; de Coppet, P.; Buisine, M.P.; Aubert, J.P.; Laboisse, C.; Cherbut, C.; Hoebler, C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G1168–G1174. [Google Scholar]
- Zhao, Y.; Chen, F.; Wu, W.; Sun, M.; Bilotta, A.J.; Yao, S.; Xiao, Y.; Huang, X.; Eaves-Pyles, T.D.; Golovko, G.; et al. GPR43 mediates microbiota metabolite SCFA regulation of antimicrobial peptide expression in intestinal epithelial cells via activation of mTOR and STAT3. Mucosal Immunol. 2018, 11, 752–762. [Google Scholar]
- Salvi, P.S.; Cowles, R.A. Butyrate and the intestinal epithelium: Modulation of proliferation and inflammation in homeostasis and disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Sun, Q.; Ji, Y.C.; Wang, Z.L.; She, X.; He, Y.; Ai, Q.; Li, L.Q. Sodium butyrate alleviates intestinal inflammation in mice with necrotizing enterocolitis. Mediators Inflamm. 2021, 2021, 6259381. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar]
- Duncan, S.H.; Louis, P.; Flint, H.J. Lactate-utilizing bacteria, isolated from human feces, that produce butyrate as a major fermentation product. Appl. Environ. Microbiol. 2004, 70, 5810–5817. [Google Scholar] [PubMed]
- Belenguer, A.; Duncan, S.H.; Calder, A.G.; Holtrop, G.; Louis, P.; Lobley, G.E.; Flint, H.J. Two routes of metabolic cross-feeding between Bifidobacterium adolescentis and butyrate-producing anaerobes from the human gut. Appl. Environ. Microbiol. 2006, 72, 3593–3599. [Google Scholar]
- Sheridan, P.O.; Louis, P.; Tsompanidou, E.; Shaw, S.; Harmsen, H.J.; Duncan, S.H.; Flint, H.J.; Walker, A.W. Distribution, organization and expression of genes concerned with anaerobic lactate utilization in human intestinal bacteria. Microb. Genom. 2022, 8, 000739. [Google Scholar]
- Van Immerseel, F.; Ducatelle, R.; De Vos, M.; Boon, N.; Van De Wiele, T.; Verbeke, K.; Rutgeerts, P.; Sas, B.; Louis, P.; Flint, H.J. Butyric acid-producing anaerobic bacteria as a novel probiotic treatment approach for inflammatory bowel disease. J. Med. Microbiol. 2010, 59, 141–143. [Google Scholar] [PubMed]
- Weghoff, M.C.; Bertsch, J.; Müller, V.A. novel mode of lactate metabolism in strictly anaerobic bacteria. Environ. Microbiol. 2015, 17, 670–677. [Google Scholar]
- Shetty, S.A.; Boeren, S.; Bui, T.P.N.; Smidt, H.; de Vos, W.M. Unravelling lactate-acetate and sugar conversion into butyrate by intestinal Anaerobutyricum and Anaerostipes species by comparative proteogenomics. Environ. Microbiol. 2020, 22, 4863–4875. [Google Scholar]
- Detman, A.; Mielecki, D.; Chojnacka, A.; Salamon, A.; Błaszczyk, M.K.; Sikora, A. Cell factories converting lactate and acetate to butyrate: Clostridium butyricum and microbial communities from dark fermentation bioreactors. Microb. Cell Fact. 2019, 18, 36. [Google Scholar]
- Isono, A.; Katsuno, T.; Sato, T.; Nakagawa, T.; Kato, Y.; Sato, N.; Seo, G.; Suzuki, Y.; Saito, Y. Clostridium butyricum TO-A culture supernatant downregulates TLR4 in human colonic epithelial cells. Dig. Dis. Sci. 2007, 52, 2963–2971. [Google Scholar]
- Dürre, P. Physiology and sporulation in Clostridium. Microbiol. Spectr. 2014, 2, TBS-0010-2012. [Google Scholar]
- Rainey, F.A.; Hollen, B.J.; Small, A.; Genus, I. Clostridium Prazmowski, 1880. In Bergey’s Manual of Systematic Bacteriology; Vos, P.D., Garrity, G.M., Jones, D., Krieg, N.R., Ludwig, W., Rainey, F.A., Schleifer, K.H., Whitam, W.B., Eds.; Springer: New York, NY, USA, 2009; Volume 3, pp. 739–740. [Google Scholar]
- Luo, X.; Han, Z.; Kong, Q.; Wang, Y.; Mou, H.; Duan, X. Clostridium butyricum prevents dysbiosis and the rise in blood pressure in spontaneously hypertensive rats. Int. J. Mol. Sci. 2023, 24, 4955. [Google Scholar] [CrossRef]
- Inatomi, T.; Amatatsu, M.; Romero-Pérez, G.A.; Inoue, R.; Tsukahara, T. Dietary probiotic compound improves reproductive performance of porcine epidemic diarrhea virus-infected sows reared in a Japanese commercial swine farm under vaccine control condition. Front. Immunol. 2017, 8, 1877. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Kong, M.S.; Lai, M.W.; Chao, H.C.; Chang, K.W.; Chen, S.Y.; Huang, Y.C.; Chiu, C.H.; Li, W.C.; Lin, P.Y.; et al. Probiotics have clinical, microbiologic, and immunologic efficacy in acute infectious diarrhea. Pediatr. Infect. Dis. J. 2010, 29, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Inatomi, T.; Honma, M. Effects of probiotics on loperamide-induced constipation in rats. Sci. Rep. 2021, 11, 24098. [Google Scholar] [CrossRef]
- Tsuda, Y.; Yoshimatsu, Y.; Aoki, H.; Nakamura, K.; Irie, M.; Fukuda, K.; Hosoe, N.; Takada, N.; Shirai, K.; Suzuki, Y. Clinical effectiveness of probiotics therapy (BIO-THREE) in patients with ulcerative colitis refractory to conventional therapy. Scand. J. Gastroenterol. 2007, 42, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.F.; Liu, P.Y.; Chen, Y.Y.; Nong, B.R.; Huang, I.F.; Hsieh, K.S.; Chen, K.T. Three-combination probiotics therapy in children with Salmonella and rotavirus gastroenteritis. J. Clin. Gastroenterol. 2014, 48, 37–42. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.B.; Kim, H.W.; Lee, H.S.; Jee, S.R.; Lee, J.H.; Kim, T.O. Clinical efficacy of probiotic therapy on bowel-related symptoms in patients with ulcerative colitis during endoscopic remission: An observational study. Gastroenterol. Res. Pract. 2022, 2022, 9872230. [Google Scholar] [CrossRef]
- Takaoka, S.; Ishii, T.; Umihara, Y.; Otani, R.; Akazawa, S.; Oda, T.; Ogino, Y.; Okino, Y.; Wang, D.-S.; Uchiumi, F. Effect of culture supernatant of Clostridium butyricum TO-A on human DNA-repair-factor-encoding gene promoters. Int. J. Mol. Sci. 2024, 25, 12151. [Google Scholar] [CrossRef]
- Takeshi, K.; Fujinaga, Y.; Inoue, K.; Nakajima, H.; Oguma, K.; Ueno, T.; Sunagawa, H.; Ohyama, T. Simple method for detection of Clostridium botulinum type A to F neurotoxin genes by ploymerase chain reaction. Microbiol. Immunol. 1996, 40, 5–11. [Google Scholar] [CrossRef]
- Inatomi, T.; Makita, K.; Otomaru, K. Effect of dietary supplementation with probiotics on fecal increase in butyric acid and immunoglobulin A in suckling Japanese black calves. J. Jpn. Vet. Med. Assoc. 2020, 73, 374–377. [Google Scholar] [CrossRef]
- Diez-Gonzalez, F.; Russell, J.B.; Hunter, J.B. The role of an NAD-independent lactate dehydrogenase and acetate in the utilization of lactate by Clostridium acetobutylicum strain P262. Arch. Microbiol. 1995, 164, 36–42. [Google Scholar] [CrossRef]
- Detman, A.; Laubitz, D.; Chojnacka, A.; Kiela, P.R.; Salamon, A.; Barberán, A.; Chen, Y.; Yang, F.; Błaszczyk, M.K.; Sikora, A. Dynamics of dark fermentation microbial communities in the light of lactate and butyrate production. Microbiome 2021, 9, 158. [Google Scholar]
- Remund, B.; Yilmaz, B.; Sokollik, C. D-lactate: Implications for gastrointestinal diseases. Children 2023, 10, 945. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal acidosis in beef cattle: The current microbiological and nutritional outlook. J. Dairy Sci. 2007, 1, E17–E38. [Google Scholar]
- Desguin, B.; Zhang, T.; Soumillion, P.; Hols, P.; Hu, J.; Hausinger, R.P. Metalloproteins. A tethered niacin-derived pincer complex with a nickel-carbon bond in lactate racemase. Science 2015, 349, 66–69. [Google Scholar]
- Breuer, R.I.; Soergel, K.H.; Lashner, B.A.; Christ, M.L.; Hanauer, S.B.; Vanagunas, A.; Harig, J.M.; Keshavarzian, A.; Robinson, M.; Sellin, J.H.; et al. Short chain fatty acid rectal irrigation for left-sided ulcerative colitis: A randomised, placebo controlled trial. Gut 1997, 40, 485–491. [Google Scholar]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroenterology 1988, 95, 1564–1568. [Google Scholar]


| Medium | pH | OD600 | Total Viable Count (CFU mL−1) | Spore Count (CFU mL−1) | Sporulation Rate (%) |
|---|---|---|---|---|---|
| PY | 5.94 ± 0.05 | 0.57 ± 0.01 | 7.69 × 106 | 2.41 × 105 | 3.13 |
| PY + ADL | 6.24 ± 0.08 | 0.48 ± 0.05 | 1.32 × 107 | 4.36 × 105 | 3.31 |
| Medium | Organic Acid | Concentration (mM) | p-Value | Net Production (mM) | |
|---|---|---|---|---|---|
| 0 h | 16 h | ||||
| PY | Lactate | 2.78 ± 0.52 | 3.92 ± 0.08 | 0.059 | 1.14 ± 0.50 |
| Acetate | 0.94 ± 0.16 | 2.57 ± 0.08 | 0.006 | 1.63 ± 0.22 | |
| Butyrate | 0.10 ± 0.01 | 2.69 ± 0.10 | 0.0004 | 2.59 ± 0.10 | |
| PY + ADL | Lactate | 43.96 ± 0.62 | 41.55 ± 0.76 | 0.044 | −2.42 ± 0.91 |
| Acetate | 34.60 ± 0.61 | 35.73 ± 0.39 | 0.012 | 1.12 ± 0.22 | |
| Butyrate | 0.11 ± 0.02 | 5.05 ± 0.82 | 0.009 | 4.94 ± 0.83 | |
| Medium | pH | OD600 | Total Viable Count (CFU mL−1) | Spore Count (CFU mL−1) | Sporulation Rate (%) |
|---|---|---|---|---|---|
| PY + AD | 6.43 ± 0.03 | 0.56 ± 0.02 | 1.72 × 107 | 9.34 × 105 | 5.44 |
| PY + AL | 6.04 ± 0.08 | 0.43 ± 0.04 | 4.37 × 106 | 2.68 × 105 | 6.13 |
| Medium | Organic Acid | Concentration (mM) | p-Value | Net Production (mM) | |
|---|---|---|---|---|---|
| 0 h | 16 h | ||||
| PY + AD | Lactate | 44.50 ± 0.95 | 38.93 ± 0.37 | 0.018 | −5.57 ± 1.31 |
| Acetate | 34.22 ± 0.48 | 34.35 ± 0.07 | 0.719 | 0.13 ± 0.54 | |
| Butyrate | 0.10 ± 0.00 | 7.64 ± 0.67 | 0.003 | 7.54 ± 0.67 | |
| PY + AL | Lactate | 44.05 ± 1.29 | 44.40 ± 1.38 | 0.327 | 0.36 ± 0.48 |
| Acetate | 34.82 ± 1.47 | 36.80 ± 1.25 | 0.004 | 1.97 ± 0.23 | |
| Butyrate | 0.11 ± 0.01 | 3.07 ± 0.10 | 0.0004 | 2.96 ± 0.10 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honda, S.; Eguchi, H.; Okino, Y.; Wang, D.-S. The Probiotic Strain Clostridium butyricum TO-A Produces Butyrate by Utilizing Lactate and Acetate. Int. J. Mol. Sci. 2025, 26, 2951. https://doi.org/10.3390/ijms26072951
Honda S, Eguchi H, Okino Y, Wang D-S. The Probiotic Strain Clostridium butyricum TO-A Produces Butyrate by Utilizing Lactate and Acetate. International Journal of Molecular Sciences. 2025; 26(7):2951. https://doi.org/10.3390/ijms26072951
Chicago/Turabian StyleHonda, Shotaro, Hiromichi Eguchi, Yoichi Okino, and Dian-Sheng Wang. 2025. "The Probiotic Strain Clostridium butyricum TO-A Produces Butyrate by Utilizing Lactate and Acetate" International Journal of Molecular Sciences 26, no. 7: 2951. https://doi.org/10.3390/ijms26072951
APA StyleHonda, S., Eguchi, H., Okino, Y., & Wang, D.-S. (2025). The Probiotic Strain Clostridium butyricum TO-A Produces Butyrate by Utilizing Lactate and Acetate. International Journal of Molecular Sciences, 26(7), 2951. https://doi.org/10.3390/ijms26072951








