Enhancing Febuxostat Solubility Through Cocrystal Formation: Role of Substrate Selection and Amide Coformers
Abstract
1. Introduction
2. Results and Discussion
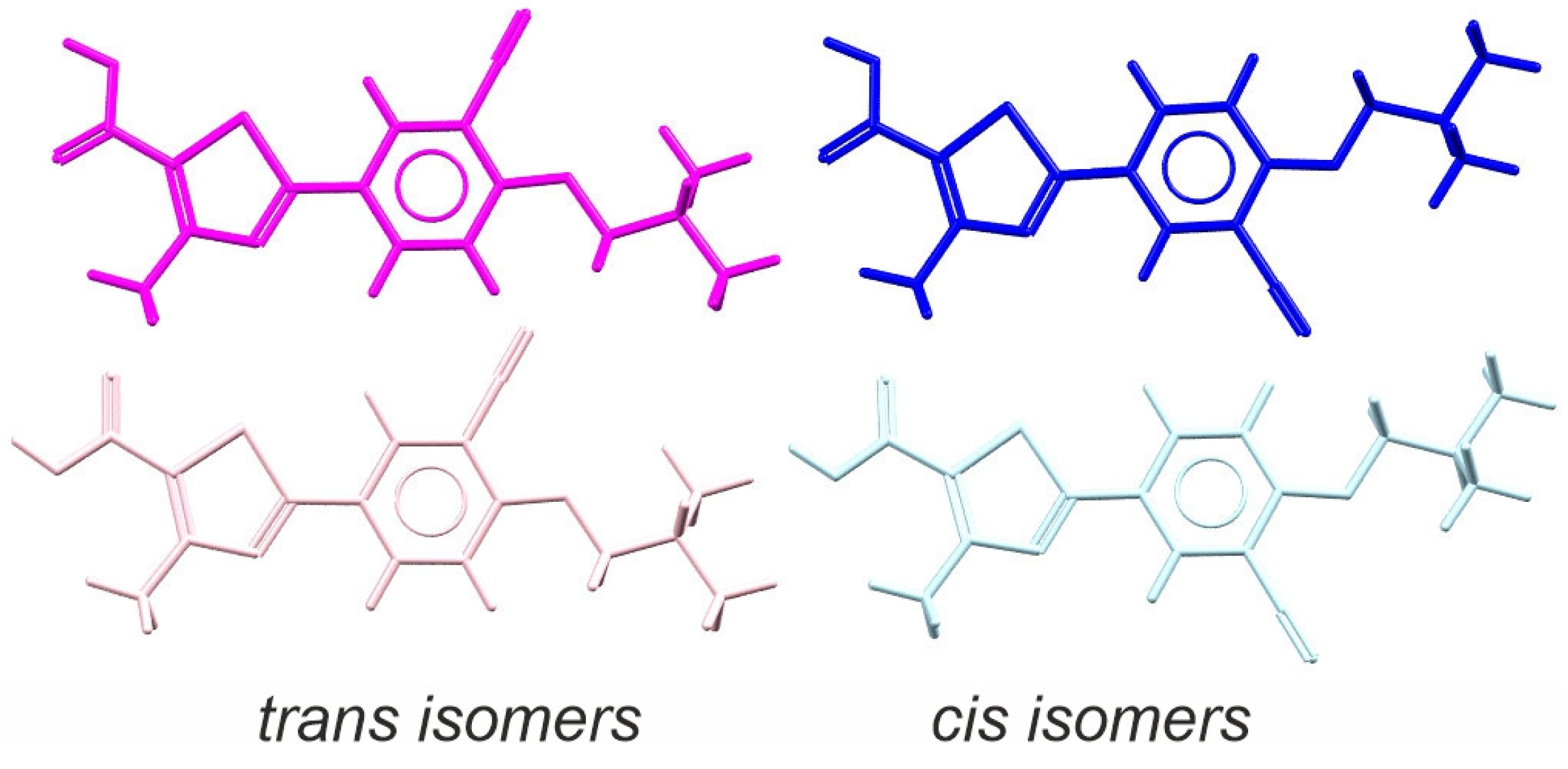
2.1. Choice of Substrate Crystalline Form
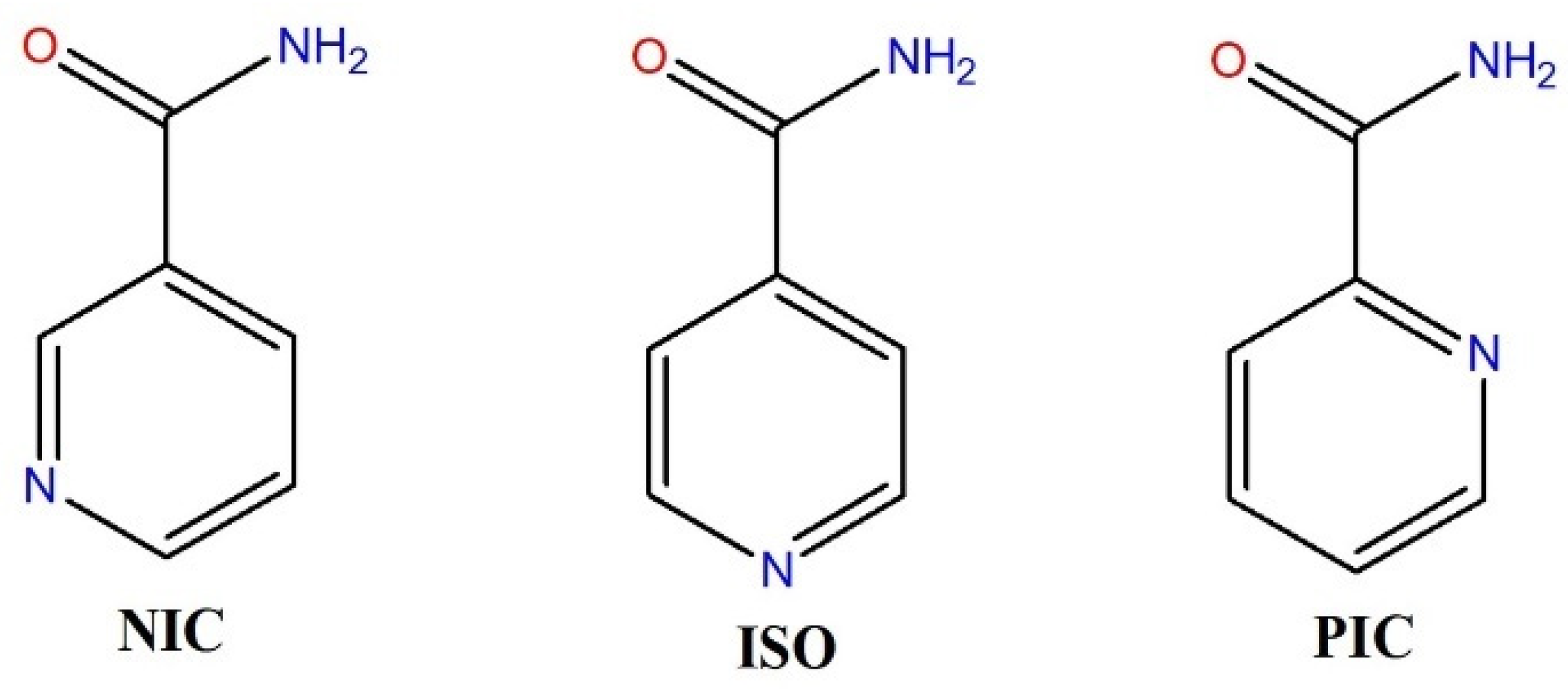
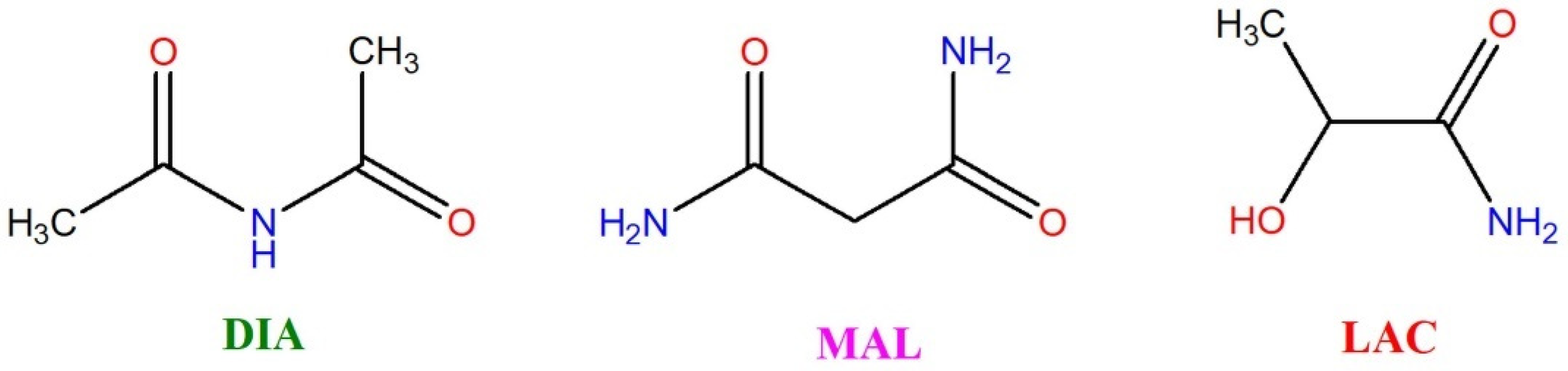
2.2. Rationale for Designing New Multicomponent Forms with Aliphatic Amides
2.3. Novel Cocrystals Synthesis and Characterization
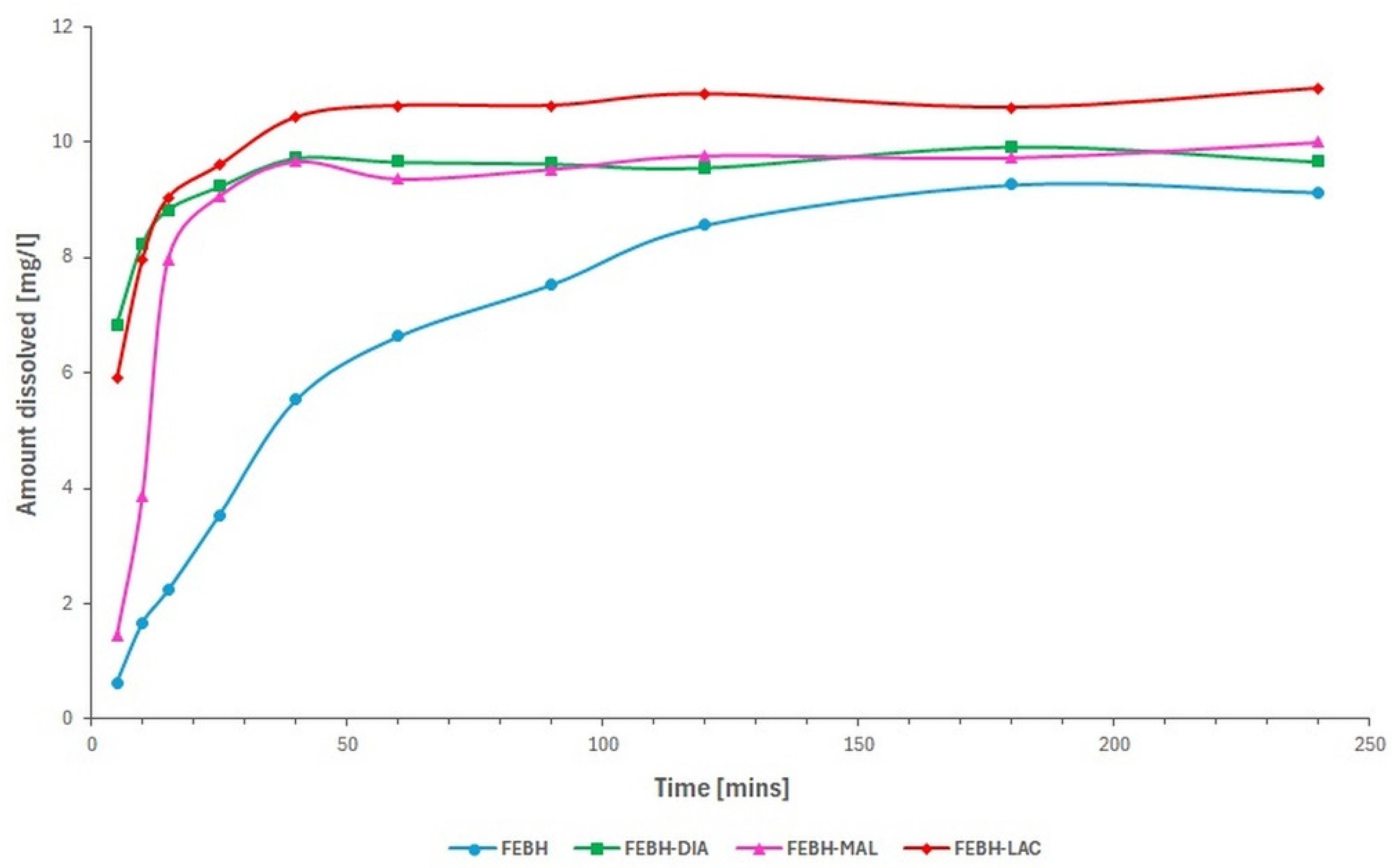
2.4. Solubility, Dissolution Rate and Stability Studies
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Infrared Spectroscopy (FTIR)
3.4. Powder X-ray Diffraction (PXRD)
3.5. Differential Scanning Calorimetry (DSC)
3.6. Thermogravimetric Analysis (TGA)
3.7. Equilibrium Solubility, Dissolution Rate and Stability Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Emami, S.; Siahi-Shadbad, M.; Adibkia, K.; Barzegar-Jalali, M. Recent Advances in Improving Oral Drug Bioavailability by Cocrystals. BioImpacts 2018, 8, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Hop, C.E.C.A.; Patilea-Vrana, G.; Gampa, G.; Seneviratne, H.K.; Unadkat, J.D.; Kenny, J.R.; Nagapudi, K.; Di, L.; Zhou, L.; et al. Drug Concentration Asymmetry in Tissues and Plasma for Small Molecule-Related Therapeutic Modalities. Drug Metab. Dispos. 2019, 47, 1122–1135. [Google Scholar] [CrossRef]
- Malaz, A.; Yousef, E.; Vangala, V.R. Pharmaceutical Cocrystals: Molecules, Crystals, Formulations, Medicines. Crys. Growth Des. 2019, 19, 7420–7438. [Google Scholar] [CrossRef]
- Friščić, T.; Jones, W. Benefits of Cocrystallisation in Pharmaceutical Materials Science: An Update. J. Pharm. Pharmacol. 2010, 62, 1547–1559. [Google Scholar] [CrossRef]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Bolla, G.; Sarma, B.; Nangia, A.K. Crystal Engineering of Pharmaceutical Cocrystals in the Discovery and Development of Improved Drugs. Chem. Rev. 2022, 122, 11514–11603. [Google Scholar] [CrossRef]
- Center of Drug Evaluation and Research. Regulatory Classification of Pharmaceutical Co-crystals, Guidance for Industry; Food and Drug Administration, U.S. Department of Health and Human Services: Washington, DC, USA, 2018; pp. 1–4. [Google Scholar]
- Dai, X.L.; Wu, C.; Li, J.H.; Liu, L.C.; He, X.; Lu, T.B.; Chen, J.M. Modulating the Solubility and Pharmacokinetic Properties of 5-fluorouracil via Cocrystallization. CrystEngComm 2020, 22, 3670–3682. [Google Scholar] [CrossRef]
- Shan, N.; Zaworotko, M.J. The Role of Cocrystals in Pharmaceutical Science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef]
- Blagden, N.; Coles, S.; Berry, D. Pharmaceutical co-crystals–are we there yet? CrystEngComm 2014, 16, 5753–5761. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S.; Nanda, A. A review about regulatory status and recent patents of pharmaceutical co-crystals. Adv. Pharm. Bull. 2018, 8, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Kisicki, J.; Khosravan, R.; Wu, J.; Mulford, D.; Hunt, B.; MacDonald, P.; Joseph-Ridge, N. Febuxostat (TMX-67), a Novel, Non-Purine, Selective Inhibitor of Xanthine Oxidase, Is Safe and Decreases Serum Urate in Healthy Volunteers. Nucleosides Nucleotides Nucleic Acids 2004, 23, 1111–1116. [Google Scholar] [CrossRef]
- Piran, M.; Metsger, L. Crystalline Form of Febuxostat. U.S. Patent 2010/0317702 A1, 16 December 2010. [Google Scholar]
- Koichi, M.; Kenzo, W.; Toshiyuki, H.; Mitsutaka, K. Polymorphic Modifications of 2-(3-cyano-4-isobutyloxyphenyl)-4-methyl-5-thiazole-carboxylic Acid and Processes for the Preparation Thereof. EP 1020454 B1, 23 January 2013. [Google Scholar]
- Zhou, X.; Tang, X.; Deng, J.; Ye, W.; Luo, J.; Zhang, D.; Fan, B. New Crystal Types of Febuxostat and Their Preparation Methods. WO 2008/067773 A1, 12 June 2008. [Google Scholar]
- Beijing Boshi Antai Technology Co., Ltd.; Shijiazhuang Pharmaceutical Group Ouyi Pharma Co., Ltd. Febuxostat Crystal Form and Preparation Method Thereof. CN101648926B, 16 November 2011. [Google Scholar]
- Khosravan, R.; Gabowski, B.; Wu, J.T.; Joseph-Ridge, N.; Vernillet, L. Effect of food or antacid on pharmacokinetics and pharmacodynamics of febuxostat in healthy subjects. Br. J. Clin. Pharmacol. 2007, 65, 355–363. [Google Scholar] [CrossRef]
- Chandu, B.R.; Kanala, K.; Hwisa, N.T.; Katakam, P.; Khagga, M. Bioequivalance and pharmacokinetic study of febuxostat in human plasma by using LC-MS/MS with liquid liquid extraction method. Springerplus 2013, 2, 194. [Google Scholar] [CrossRef] [PubMed]
- Healy, A.M.; Worku, Z.A.; Kumar, D.; Madi, A.M. Pharmaceutical solvates, hydrates and amorphous forms: A special emphasis on cocrystals. Adv. Drug Deliv. Rev. 2017, 117, 25–46. [Google Scholar] [CrossRef] [PubMed]
- Karki, S.; Friscić, T.; Jones, W.; Motherwell, W.D. Screening for Pharmaceutical Cocrystal Hydrates via Neat and Liquid-Assisted Grinding. Mol. Pharm. 2007, 4, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Maddileti, D.; Jayabun, S.K.; Nangia, A. Soluble Cocrystals of the Xanthine Oxidase Inhibitor Febuxostat. Cryst. Growth Des. 2013, 13, 3188–3196. [Google Scholar] [CrossRef]
- Kang, Y.; Gu, J.; Hu, X. Syntheses, structure characterization and dissolution of two novel cocrystals of febuxostat. J. Mol. Struct. 2017, 1130, 480–486. [Google Scholar] [CrossRef]
- Jagia, M.; Kale, D.P.; Bansal, A.K.; Patel, S. Novel Co-crystals and Eutectics of Febuxostat: Characterization, Mechanism of Formation, and Improved Dissolution. AAPS PharmSciTech 2022, 23, 43. [Google Scholar] [CrossRef]
- Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-adds-boxed-warning-increased-risk-death-gout-medicine-uloric-febuxostat (accessed on 10 February 2025).
- Nangia, A.K.; Desiraju, G.R. Crystal Engineering: An Outlook for the Future. Angew. Chem. 2019, 58, 4100–4107. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, 72, 171–179. [Google Scholar] [CrossRef]
- Yadav, J.A.; Khomane, K.S.; Modi, S.R.; Ugale, B.; Yadav, R.N.; Nagaraja, C.M.; Kumar, N.; Bansal, A.K. Correlating Single Crystal Structure, Nanomechanical, and Bulk Compaction Behavior of Febuxostat Polymorphs. Mol. Pharm. 2017, 14, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Ungur, D.T.; Lanza, A.; Stam, D.; Guguta, C.; Iordache, C.; Fruth, V.; Santiso-Quinones, G.; Pop, M.M. Crystal structure of febuxostat marketed polymorph determined by electron diffraction and reinforced by X-ray crystallography. CrystEngComm 2024, 26, 4295–4304. [Google Scholar] [CrossRef]
- Ilaveni, N.; Dama, N.; Kumar, Y.B.; Nanubolu, J.B. Synthon and conformational variations in the polymorphs and hemihydrate of Febuxostat. J. Mol. Struct. 2025, 1319, 139521. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, I.S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, X. Cocrystallization of Febuxostat with Pyridine Coformers: Crystal Structural and Physicochemical Properties Analysis. J. Chem. 2021, 3834368. [Google Scholar] [CrossRef]
- Modani, S.; Gunnam, A.; Yadav, B.; Nangia, A.K.; Shastri, N.R. Generation and Evaluation of Pharmacologically Relevant Drug–Drug Cocrystal for Gout Therapy. Cryst. Growth Des. 2020, 20, 3577–3583. [Google Scholar] [CrossRef]
- Gelbrich, T.; Kahlenberg, V.; Adamer, V.; Nerdinger, S.; Griesser, U.J. Febuxostat ethanol monosolvate. Acta Crystallogr. Sect. E Cryst. Commun 2020, 76, 816–819. [Google Scholar] [CrossRef]
- Jiang, Q.Y.; Qian, J.J.; Gu, J.-M.; Tang, G.-P.; Hu, X.-R. Febuxostat methanol solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o1232. [Google Scholar] [CrossRef]
- Wu, M.; Hu, X.-R.; Gu, J.-M.; Tang, G.-P. Crystal structure of febuxostat–acetic acid (1/1). Acta Crystallogr. Sect. E Cryst. Commun. 2015, 71, o295. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Lu, T. 2-[3-Cyano-4-(2-methyl-prop-oxy)phen-yl]-4-methyl-thia-zole-5-carboxylic acid pyridine solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o2603. [Google Scholar] [CrossRef]
- Cerreia, V.P.; Chierotti, M.R.; Gobetto, R. Pharmaceutical aspects of salt and cocrystal forms of APIs and characterization challenges. Adv. Drug Deliv. Rev. 2017, 1, 86–110. [Google Scholar] [CrossRef]
- Saha, S.; Desiraju, G.R. Acid·Amide Supramolecular Synthon in Cocrystals: From Spectroscopic Detection to Property Engineering. J. Am. Chem. Soc. 2018, 140, 6361–6373. [Google Scholar] [CrossRef] [PubMed]
- Cheney, M.L.; Shan, N.; Healey, E.R.; Hanna, M.; Wojtas, L.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Effects of Crystal Form on Solubility and Pharmacokinetics: A Crystal Engineering Case Study of Lamotrigine. Cryst. Growth Des. 2010, 10, 394–405. [Google Scholar] [CrossRef]
- Glomme, A.; Marz, J.; Dressman, J.B.J. Comparison of a miniaturized shake-flask solubility method with automated potentiometric acid/base titrations and calculated solubilities. J. Pharm. Sci. 2005, 94, 1–16. [Google Scholar] [CrossRef]


| Drug/Coformer | mp of Coformer (°C) | Cocrystal | mp of Cocrystal (°C) |
|---|---|---|---|
| FEBH | 200.5 | ||
| DIA | 79.0 | FEBH-DIA | 173.7 |
| MAL | 169.8 | FEBH-MAL | 183.8 |
| LAC | 77.0 | FEBH-LAC | 169.0 |
| Compound | N-H [cm−1] | O-H [cm−1] | C=N [cm−1] | C=O [cm−1] | C≡N [cm−1] |
|---|---|---|---|---|---|
| FEBH | 3534 3459 2962 | 1511 | 1702 1682 | 2227 | |
| DIA | 3272 | 1747 | |||
| 3218 3159 3000 | 1700 | ||||
| MAL | 3387 3157 2791 | 1693 1668 | |||
| LAC | 3389 3303 | 2986 | 1651 | ||
| FEBH-DIA | 3303 3162 | 2952 | 1505 | 1702 1667 | 2239 |
| FEBH-MAL | 3237 3184 | 2952 | 1505 | 1745 1702 | 2239 |
| FEBH-LAC | 3387 3301 3196 | 2952 | 1505 | 1702 1652 | 2239 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pindelska, E.; Sarna, A.; Duszczyk, M.; Zep, A.; Madura, I.D. Enhancing Febuxostat Solubility Through Cocrystal Formation: Role of Substrate Selection and Amide Coformers. Int. J. Mol. Sci. 2025, 26, 3004. https://doi.org/10.3390/ijms26073004
Pindelska E, Sarna A, Duszczyk M, Zep A, Madura ID. Enhancing Febuxostat Solubility Through Cocrystal Formation: Role of Substrate Selection and Amide Coformers. International Journal of Molecular Sciences. 2025; 26(7):3004. https://doi.org/10.3390/ijms26073004
Chicago/Turabian StylePindelska, Edyta, Anita Sarna, Maciej Duszczyk, Anna Zep, and Izabela D. Madura. 2025. "Enhancing Febuxostat Solubility Through Cocrystal Formation: Role of Substrate Selection and Amide Coformers" International Journal of Molecular Sciences 26, no. 7: 3004. https://doi.org/10.3390/ijms26073004
APA StylePindelska, E., Sarna, A., Duszczyk, M., Zep, A., & Madura, I. D. (2025). Enhancing Febuxostat Solubility Through Cocrystal Formation: Role of Substrate Selection and Amide Coformers. International Journal of Molecular Sciences, 26(7), 3004. https://doi.org/10.3390/ijms26073004






