Spatial Metabolomics and Its Application in Plant Research
Abstract
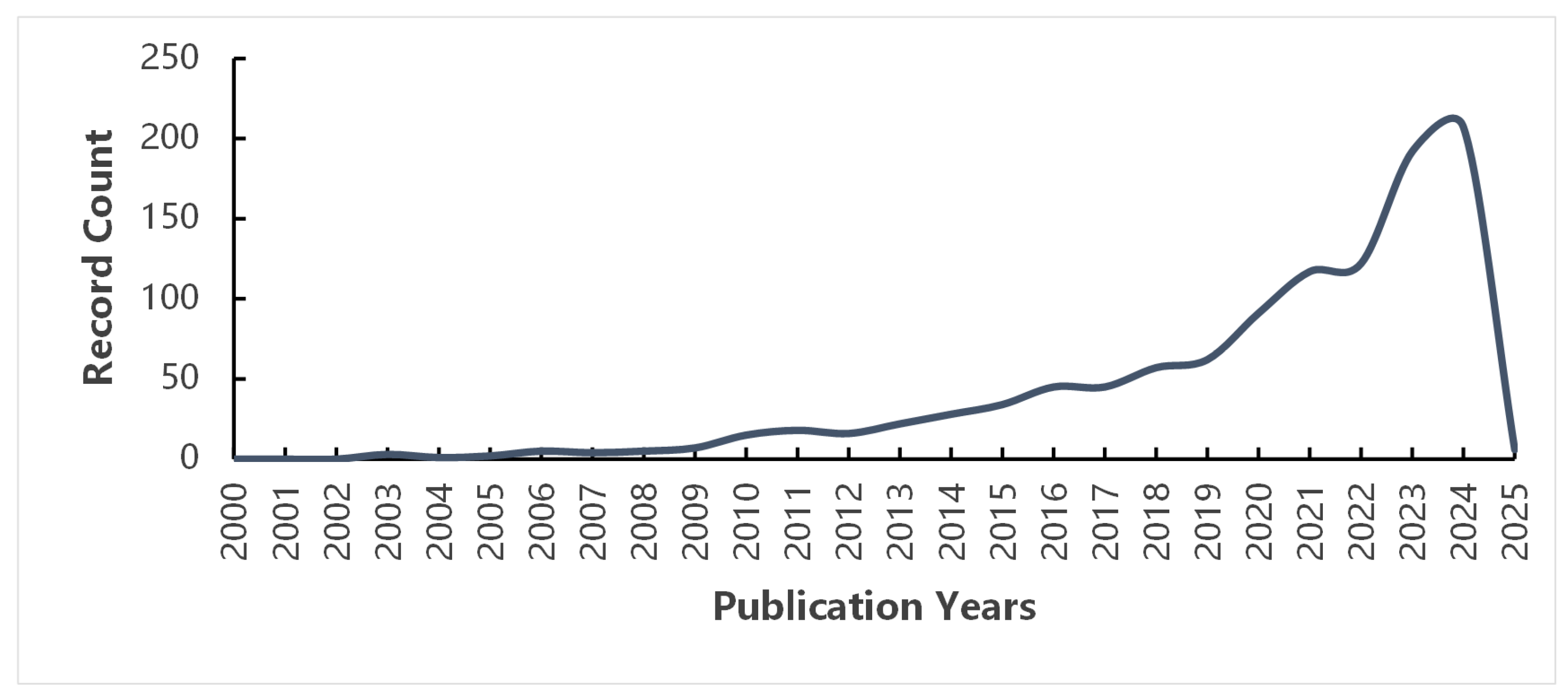
1. Introduction
2. Research Progress of Spatial Metabolomics
2.1. The Birth of Spatial Metabolomics
2.2. Spatial Metabolomics and Mass Spectrometry Imaging Technology
2.3. Advantages of Spatial Metabolomics
3. Application of Spatial Metabolomics in Plant Research
3.1. Application of Spatial Metabolomics in Medicinal Plants
3.1.1. Visual Analysis of Natural Ingredients
3.1.2. Quality Control of Medicinal Materials
3.2. Application of Spatial Metabolomics in Food Crops and Economic Crops
3.2.1. Assisting Specificity Identification
3.2.2. Analyzing Anabolic Pathways of Metabolites
3.2.3. Exploring the Regulation of Growth and Development
3.2.4. Revealing the Interaction Mechanism Between Crop and Environment
3.2.5. Verifying Gene Function
3.3. Advantages and Disadvantages of the Three Main MSI Technologies in Spatial Metabolomics
4. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Yin, Z.; Huang, W.; Wu, X.; Yan, S. Spatially resolved metabolomics: Progress and challenges. Biotechnol. Bull. 2021, 37, 32–51. [Google Scholar]
- Sun, J.; Hou, B.; Chen, X.; Tang, K.; Li, S.; Shang, Y.; Cui, G.; Duan, L.; Huang, S.; Qi, X. Important progress in plant metabolism in China since the establishment of the People’s Republic of China 70 years ago. Sci. Sin. Vitae 2019, 49, 1213–1226. [Google Scholar]
- Zhao, M. Study on Molecular Mechanisms of Anthocyanin Biosynthesis in Black Rice and Its Nutritional Value Evaluation. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2021. [Google Scholar]
- Li, Y. Enrichment of γ-Aminobutyric Acid and Preliminary Analysis of Its Anabolic Pathway in Rice Cultivar Jupeiheinuo 1. Master’s Thesis, Guangdong Ocean University, Zhanjiang, China, 2023. [Google Scholar]
- Jiang, H.; Song, Z.; Su, Q.W.; Wei, Z.H.; Li, W.C.; Jiang, Z.X.; Tian, P.; Wang, Z.H.; Yang, X.; Yang, M.Y.; et al. Transcriptomic and metabolomic reveals silicon enhances adaptation of rice under dry cultivation by improving flavonoid biosynthesis, osmoregulation, and photosynthesis. Front. Plant Sci. 2022, 13, 967537. [Google Scholar]
- Liu, X.; Liu, H.; Tian, B.; Shi, G.; Liu, C.; Guo, J.; Cao, G.; Wei, F. Metabolome and transcriptome analyses of anthocyanin biosynthesis reveal key metabolites and candidate genes in purple wheat (Triticum aestivum L.). Physiol. Plant 2023, 175, e13921. [Google Scholar]
- Wei, J. Dissection of Wheat Flavonoid Metabolic Pathway by Combining Linkage Mapping and Genome-Wide Association Study. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2022. [Google Scholar]
- Guo, Z.; Yuan, X.; Li, T.; Wang, S.; Yu, Y.; Liu, C.; Duan, C. Integrated transcriptomic and metabolomic analysis reveals the molecular regulatory mechanism of flavonoid biosynthesis in maize roots under lead stress. Int. J. Mol. Sci. 2024, 25, 6050. [Google Scholar] [CrossRef]
- Yang, X.; Ren, J.; Lin, X.; Yang, Z.; Deng, X.; Ke, Q. Melatonin alleviates chromium toxicity in maize by modulation of cell wall polysaccharides biosynthesis, glutathione metabolism, and antioxidant capacity. Int. J. Mol. Sci. 2023, 24, 3816. [Google Scholar] [CrossRef]
- Nicholson, J.K.; Lindon, J.C.; Holmes, E. ‘Metabonomics’: Understanding the metabolic responses of living systems to pathophysiological stimuli via multivariate statistical analysis of biological NMR spectroscopic data. Xenobiotica 1999, 29, 1181–1189. [Google Scholar]
- Fiehn, O.; Kopka, J.; Dörmann, P.; Altmann, T.; Trethewey, R.N.; Willmitzer, L. Metabolite profiling for plant functional genomics. Nat. Biotechnol. 2000, 18, 1157–1161. [Google Scholar]
- Mao, C.; He, J.; Wen, X.; Wu, C.; Yi, C.; Lian, J.; Guo, W. Advances in the application of metabolomics in the study of physiological and biochemical metabolism of tea plants. J. Tea Sci. 2023, 43, 607–620. [Google Scholar]
- German, J.B.; Bauman, D.E.; Burrin, D.G.; Failla, M.L.; Freake, H.C.; King, J.C.; Klein, S.; Milner, J.A.; Pelto, G.H.; Rasmussen, K.M.; et al. Metabolomics in the opening decade of the 21st century: Building the roads to individualized health. J. Nutr. 2004, 134, 2729–2732. [Google Scholar]
- Qin, J. Studies on the Mechanisms of Basic Fibroblast Growth Factor in the Treatment of Chemotherapy-Induced Alopecia. Ph.D. Thesis, China Medical University, Shenyang, China, 2021. [Google Scholar]
- Wang, S.; Yang, W.; Yin, F. Effects of fucoidan on rumen microflora and metabolomics of weaned lambs. Chin. J. Anim. Nutr. 2023, 35, 1827–1840. [Google Scholar]
- Xu, D. Toxicity Mechanism of Tributyltin Chloride Exposure Impacton Juvenile Takifugu obscurus, Based on Transcriptomics and Metabonomics. Ph.D. Thesis, Anhui Normal University, Wuhu, China, 2018. [Google Scholar]
- Huang, H.; Li, Y.; Gao, X.; Laba, Z.; Tian, P.; Nima, Y.; Chang, Z.; Liao, W. Broadly targeted metabolomics study of highland barley seeds of different qualities. Agric. Res. Arid Areas 2024, 42, 43–53+107. [Google Scholar]
- Zhao, J.; Feng, S. Application of spatial metabolomics in traditional Chinese medicine research. Chin. Tradit. Herb. Drugs 2023, 54, 6569–6579. [Google Scholar]
- Chen, J.; Zhou, Y.; Qin, X. Innovation and application of metabolomics technology for research in the biomedical field. Acta Pharm. Sin. 2023, 58, 2271–2282. [Google Scholar]
- Zeng, C.; Lin, H.; Liu, Z.; Liu, Z. Metabolomics analysis of Camellia sinensis with respect to harvesting time. Food Res. Int. 2020, 128, 108814. [Google Scholar]
- Pott, D.M.; de Abreu E Lima, F.; Soria, C.; Willmitzer, L.; Fernie, A.R.; Nikoloski, Z.; Osorio, S.; Vallarino, J.G. Metabolic reconfiguration of strawberry physiology in response to postharvest practices. Food Chem. 2020, 321, 126747. [Google Scholar]
- Zuo, J.; Grierson, D.; Courtney, L.T.; Wang, Y.; Gao, L.; Zhao, X.; Zhu, B.; Luo, Y.; Wang, Q.; Giovannoni, J.J. Relationships between genome methylation, levels of non-coding RNAs, mRNAs and metabolites in ripening tomato fruit. Plant J. 2020, 103, 980–994. [Google Scholar]
- Hong, J.; Yang, L.; Zhang, D.; Shi, J. Plant metabolomics: An indispensable system biology tool for plant science. Int. J. Mol. Sci. 2016, 17, 767. [Google Scholar] [CrossRef]
- Yang, Y.; Miao, S.; Li, W.; Ji, S. Research progress on plant metabolomics in root and rhizome traditional Chinese medicine. Chin. Tradit. Herb. Drugs 2023, 54, 6856–6865. [Google Scholar]
- Ma, L.; Cui, H.; Li, J. Analysis of patent trends for the spatial omics technology. China Biotechnol. 2023, 43, 111–117. [Google Scholar]
- Cui, H.; Jiang, Y.; Wang, Y.; Sun, Y.; Tang, C.; Zheng, J.; Xu, S.; Dai, Y.; Li, L.; Lin, H.; et al. Accurate determination of the meaning and implications of traditional Chinese medicine using panoramic spatiotemporal life atlas. Sci. Sin. Vitae 2024, 54, 1183–1196. [Google Scholar]
- Sun, C.; Li, T.; Song, X.; Huang, L.; Zang, Q.; Xu, J.; Bi, N.; Jiao, G.; Hao, Y.; Chen, Y.; et al. Spatially resolved metabolomics to discover tumor-associated metabolic alterations. Proc. Natl. Acad. Sci. USA 2019, 116, 52–57. [Google Scholar] [PubMed]
- Wang, X.; Tang, D.; Cai, W.; Yin, L.; Dai, Y. Research process in spatially resolved metabolomics. Clin. Med. Eng. 2021, 28, 36–40. [Google Scholar]
- Miao, Y.; Zhu, L.; Xu, W. Novel matrixes for mass spectrometry imaging and research progress of it in analyzing biological samples. Biotechnol. Bull. 2022, 38, 156–167. [Google Scholar]
- Dyar, K.A.; Eckel-Mahan, K.L. Circadian metabolomics in time and space. Front. Neurosci. 2017, 11, 369. [Google Scholar]
- Wu, H.; Su, H.; Zhu, C.; Wang, W.; Cui, S.; Wang, K.; Zhou, M. Application of spatial metabolomics in the research on meridian-viscera relationship. J. Anhui Univ. Chin. Med. 2022, 41, 85–89. [Google Scholar]
- Wu, C.; Dill, A.L.; Eberlin, L.S.; Cooks, R.G.; Ifa, D.R. Mass spectrometry imaging under ambient conditions. Mass Spectrom. Rev. 2013, 32, 218–243. [Google Scholar]
- Chughtai, K.; Heeren, R.M. Mass spectrometric imaging for biomedical tissue analysis. Chem. Rev. 2010, 110, 3237–3277. [Google Scholar]
- Fujimura, Y.; Miura, D. MALDI mass spectrometry imaging for visualizing in situ metabolism of endogenous metabolites and dietary phytochemicals. Metabolites 2014, 4, 319–346. [Google Scholar] [CrossRef]
- Müller, T.; Oradu, S.; Ifa, D.R.; Cooks, R.G.; Kräutler, B. Direct plant tissue analysis and imprint imaging by desorption electrospray ionization mass spectrometry. Anal. Chem. 2011, 83, 5754–5761. [Google Scholar]
- Xie, M.; Wang, X.; Wang, Q. Application of mass spectrometry imaging in metabolism of traditional Chinese medicine. Chin. J. Mod. Appl. Pharm. 2022, 39, 1382–1388. [Google Scholar]
- Taylor, M.J.; Lukowski, J.K.; Anderton, C.R. Spatially resolved mass spectrometry at the single cell: Recent innovations in proteomics and metabolomics. J. Am. Soc. Mass Spectrom. 2021, 32, 872–894. [Google Scholar] [PubMed]
- Huang, X.; Liu, H.; Mao, L.; Xiong, C.; Nie, Z. progress of mass spectrometry imaging in Neuroscience. Chin. J. Anal. Chem. 2019, 47, 1592–1600. [Google Scholar]
- Wang, X. Mass Spectrometry Imaging for Metabolites; University of Science and Technology of China: Hefei, China, 2021. [Google Scholar]
- Huang, L. A Molecular Pathological Method and Its Application in the Diagnosis of Thyroid Tumor Based on Mass Spectrometry Imaging Metabolomics. Master’s Thesis, Peking Union Medical College, Beijing, China, 2018. [Google Scholar]
- Chaurand, P.; Schwartz, S.A.; Reyzer, M.L.; Caprioli, R.M. Imaging mass spectrometry: Principles and potentials. Toxicol. Pathol. 2005, 33, 92–101. [Google Scholar]
- Seeley, E.H.; Caprioli, R.M. 3D imaging by mass spectrometry: A new frontier. Anal. Chem. 2012, 84, 2105–2110. [Google Scholar]
- Shen, Y.; Xu, X. Matrix-assisted laser desorption ionization mass spectrometry imaging and its application. J. Technol. 2022, 22, 106–114. [Google Scholar]
- Jin, Z.; Min, Q. Advances in nanomaterials facilitated mass spectrometry imaging. Chin. J. Anal. Chem. 2021, 49, 1176–1187. [Google Scholar]
- Zhou, S. The Distribution of Key Component in Coriandrum sativum L. by Desorption Electrospray Ionization Mass Spectrometry Imaging (DESI-MSI). Master’s Thesis, Harbin Institute of Technology, Harbin, China, 2022. [Google Scholar]
- Cooks, R.G.; Ouyang, Z.; Takats, Z.; Wiseman, J.M. Ambient mass spectrometry. Science 2006, 311, 1566–1570. [Google Scholar] [CrossRef]
- Jiao, Z.; Li, J. Application progress of spatial multi-omics technology in tumor research. J. Lanzhou Univ. Med. Sci. 2023, 49, 1–5. [Google Scholar]
- Zhang, Q.; Wang, Q.; Zhang, X.; Guo, S.; Li, A. Investigation on potential subtyping and progression biomarkers of nephrotic syndrome based on LC-MS metabolomics technology. Acta Pharm. Sin. 2024, 59, 1779–1786. [Google Scholar]
- Gao, S.Q.; Zhao, J.H.; Guan, Y.; Tang, Y.S.; Li, Y.; Liu, L.Y. Mass spectrometry imaging technology in metabolomics: A systematic review. Biomed. Chromatogr. 2023, 37, e5494. [Google Scholar]
- He, S.; Zeng, Z.; Li, B. Application progress of spatially resolved metabolomics in disease diagnosis research. Biotechnol. Bull. 2024, 40, 145–159. [Google Scholar]
- Fox, B.W.; Schroeder, F.C. Toward spatially resolved metabolomics. Nat. Chem. Biol. 2020, 16, 1039–1040. [Google Scholar] [PubMed]
- Li, P.; Qi, L.; Wen, X.; Sheng, L. Methods for the elucidation of bioactive components and quality control of traditional Chinese medicines. Chin. J. Nat. Med. 2007, 5, 1–9. [Google Scholar]
- Huang, L.; Nie, L.; Dong, J.; Yang, X.; Jia, X.; Yao, L.; He, F.; Dai, Z.; Ma, S. Recent application of mass spectrometry imaging in traditional Chinese medicine. Chin. J. Pharm. Anal. 2022, 42, 1675–1689. [Google Scholar]
- Zhao, H.; Gao, F. Study on methods of extraction of flavonoids in Radix Puerariae. Chin. Tradit. Pat. Med. 2000, 11, 10–12. [Google Scholar]
- Li, Y.; Guan, L.; Chen, L.; Zhao, M.; Ding, L.; Meng, C.; Gao, H.; Wang, Z. Qualitative and quantitative analysis of Paris polyphylla var. chinensis by UPLC-Q-TOF-MS/MS and HPLC. China J. Chin. Mater. Medica 2021, 46, 2900–2911. [Google Scholar]
- Zhang, G.; Liu, X.; Ma, C.; Li, W.; Wang, X. Spatial distribution characteristics of metabolities in rhizome of Paris polyphylla var. yunnanensis: Based on MALDI-MSI. China J. Chin. Mater. Medica 2022, 47, 1222–1229. [Google Scholar]
- Li, B.; Bhandari, D.R.; Janfelt, C.; Römpp, A.; Spengler, B. Natural products in Glycyrrhiza glabra (licorice) rhizome imaged at the cellular level by atmospheric pressure matrix-assisted laser desorption/ionization tandem mass spectrometry imaging. Plant J. 2014, 80, 161–171. [Google Scholar]
- Li, B.; Bhandari, D.R.; Römpp, A.; Spengler, B. High-resolution MALDI mass spectrometry imaging of gallotannins and monoterpene glucosides in the root of Paeonia lactiflora. Sci. Rep. 2016, 6, 36074. [Google Scholar]
- Li, B.; Ge, J.; Liu, W.; Hu, D.; Li, P. Unveiling spatial metabolome of Paeonia suffruticosa and Paeonia lactiflora roots using MALDI MS imaging. New Phytol. 2021, 231, 892–902. [Google Scholar] [PubMed]
- Lange, B.M.; Fischedick, J.T.; Lange, M.F.; Srividya, N.; Šamec, D.; Poirier, B.C. Integrative approaches for the identification and localization of specialized metabolites in Tripterygium roots. Plant Physiol. 2017, 173, 456–469. [Google Scholar] [PubMed]
- Shimma, S.; Sagawa, T. Microscopy and mass spectrometry imaging reveals the distributions of curcumin species in dried turmeric root. J. Agric. Food Chem. 2019, 67, 9652–9657. [Google Scholar] [PubMed]
- Wang, S.; Bai, H.; Cai, Z.; Gao, D.; Jiang, Y.; Liu, J.; Liu, H. MALDI imaging for the localization of saponins in root tissues and rapid differentiation of three Panax herbs. Electrophoresis 2016, 37, 1956–1966. [Google Scholar]
- Bai, H.; Wang, S.; Liu, J.; Gao, D.; Jiang, Y.; Liu, H.; Cai, Z. Localization of ginsenosides in Panax ginseng with different age by matrix-assisted laser-desorption/ionization time-of-flight mass spectrometry imaging. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1026, 263–271. [Google Scholar]
- Sun, C.; Liu, W.; Ma, S.; Zhang, M.; Geng, Y.; Wang, X. Development of a high-coverage matrix-assisted laser desorption/ionization mass spectrometry imaging method for visualizing the spatial dynamics of functional metabolites in Salvia miltiorrhiza Bge. J. Chromatogr. A 2020, 1614, 460704. [Google Scholar]
- Li, S.; Zhu, N.; Tang, C.; Duan, H.; Wang, Y.; Zhao, G.; Liu, J.; Ye, Y. Differential distribution of characteristic constituents in root, stem and leaf tissues of Salvia miltiorrhiza using MALDI mass spectrometry imaging. Fitoterapia 2020, 146, 104679. [Google Scholar]
- Sun, C.; Ma, S.; Li, L.; Wang, D.; Liu, W.; Liu, F.; Guo, L.; Wang, X. Visualizing the distributions and spatiotemporal changes of metabolites in Panax notoginseng by MALDI mass spectrometry imaging. J. Ginseng Res. 2021, 45, 726–733. [Google Scholar]
- Li, Q.; Ji, D.; Gao, H. Spatial distribution of coumarins in Angelica pubescens fresh roots by MALDI-MSI. Chin. Tradit. Herb. Drugs 2023, 54, 3438–3445. [Google Scholar]
- Kuo, T.H.; Huang, H.C.; Hsu, C.C. Mass spectrometry imaging guided molecular networking to expedite discovery and structural analysis of agarwood natural products. Anal. Chim. Acta 2019, 1080, 95–103. [Google Scholar]
- Liu, X.; Pei, X.; Gong, C.; Xu, X. Matrix-assisted laser desorption ionization-mass spectrometry imaging of small molecules in mulberry leaf using ionic liquid as matrix. Chin. J. Anal. Chem. 2018, 46, 1923–1930. [Google Scholar]
- Li, B.; Neumann, E.K.; Ge, J.; Gao, W.; Yang, H.; Li, P.; Sweedler, J.V. Interrogation of spatial metabolome of Ginkgo biloba with high-resolution matrix-assisted laser desorption/ionization and laser desorption/ionization mass spectrometry imaging. Plant Cell Environ. 2018, 41, 2693–2703. [Google Scholar] [PubMed]
- Li, M.; Wang, X.; Han, L.; Jia, L.; Liu, E.; Li, Z.; Yu, H.; Wang, Y.; Gao, X.; Yang, W. Integration of multicomponent characterization, untargeted metabolomics and mass spectrometry imaging to unveil the holistic chemical transformations and key markers associated with wine steaming of Ligustri Lucidi Fructus. J. Chromatogr. A 2020, 1624, 461228. [Google Scholar]
- Liu, Q.; Huang, Y.; Linghu, C.; Xiao, J.; Gu, R. Metabolic profiling, in-situ spatial distribution, and biosynthetic pathway of functional metabolites in Dendrobium nobile stem revealed by combining UPLC-QTOF-MS with MALDI-TOF-MSI. Front. Plant Sci. 2023, 13, 1125872. [Google Scholar]
- Kusari, S.; Sezgin, S.; Nigutova, K.; Cellarova, E.; Spiteller, M. Spatial chemo-profiling of hypericin and related phytochemicals in Hypericum species using MALDI-HRMS imaging. Anal. Bioanal. Chem. 2015, 407, 4779–4791. [Google Scholar]
- Nie, L.; Huang, L.; Qian, X.; Kang, S.; Yao, L.; Dai, Z.; Ma, S. Complementary mass spectrometry imaging and discovery of quality characters-related markers of Isatidis Radix based on AP-MALDI-IT-TOF/MS and DESI-Q-TOF/MS. Chin. Pharm. J. 2023, 58, 823–830. [Google Scholar]
- Feng, B.; Zhang, J.; Chang, C.; Li, L.; Li, M.; Xiong, X.; Guo, C.; Tang, F.; Bai, Y.; Liu, H. Ambient mass spectrometry imaging: Plasma assisted laser desorption ionization mass spectrometry imaging and its applications. Anal. Chem. 2014, 86, 4164–4169. [Google Scholar]
- Freitas, J.R.L.E.; Vendramini, P.H.; Melo, J.O.F.; Eberlin, M.N.; Augusti, R. Assessing the spatial distribution of key flavonoids in Mentha × piperita Leaves: An application of desorption electrospray ionization mass spectrometry imaging (DESI-MSI). J. Braz. Chem. Soc. 2019, 30, 1437–1446. [Google Scholar]
- Fowble, K.L.; Teramoto, K.; Cody, R.B.; Edwards, D.; Guarrera, D.; Musah, R.A. Development of “Laser ablation direct analysis in real time imaging” Mass spectrometry: Application to spatial distribution mapping of metabolites along the biosynthetic cascade leading to synthesis of atropine and scopolamine in plant tissue. Anal. Chem. 2017, 89, 3421–3429. [Google Scholar]
- Chen, W.; Zheng, Y.; Zhao, L.; Song, S.; Long, F.; Pei, Z.; Tang, C.; Xu, Z.; Lv, G. Distribution of bioactive compounds in different tissues of Paeonia lactiflora roots by DESI-MSI and UPLC. China J. Chin. Mater. Medica 2022, 47, 4333–4340. [Google Scholar]
- Liu, Q.B.; Lu, J.G.; Jiang, Z.H.; Zhang, W.; Li, W.J.; Qian, Z.M.; Bai, L.P. In situ chemical profiling and imaging of cultured and natural Cordyceps sinensis by TOF-SIMS. Front. Chem. 2022, 10, 862007. [Google Scholar]
- He, F.; Huang, Y.F.; Dai, W.; Qu, X.Y.; Lu, J.G.; Lao, C.C.; Luo, W.H.; Sun, D.M.; Wei, M.; Xiao, S.Y.; et al. The localization of the alkaloids in Coptis chinensis rhizome by time-of-flight secondary ion mass spectrometry. Front. Plant Sci. 2022, 13, 1092643. [Google Scholar]
- Lee, J.W.; Ji, S.H.; Lee, Y.S.; Choi, D.J.; Choi, B.R.; Kim, G.S.; Baek, N.I.; Lee, D.Y. Mass spectrometry based profiling and imaging of various ginsenosides from Panax ginseng roots at different ages. Int. J. Mol. Sci. 2017, 18, 1114. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.; Wang, L.; Yang, M.; Wu, C.; Li, J.; Shi, L.; Feng, S.; Li, F. Visualizing the spatial distribution of functional metabolites in Forsythia suspensa at different harvest stages by MALDI mass spectrometry imaging. Fitoterapia 2022, 162, 105285. [Google Scholar]
- Sun, C.; Cui, L.; Zhou, B.; Wang, X.; Guo, L.; Liu, W. Visualizing the spatial distribution and alteration of metabolites in continuously cropped Salvia miltiorrhiza Bge using MALDI-MSI. J. Pharm. Anal. 2022, 12, 719–724. [Google Scholar]
- Ma, T.; Sun, C.; Han, Y.; Guo, L.; Huang, L.; Wang, X. Matrix-assisted laser desorption/ionization mass spectrometry imaging reveals “Spatial-Temporal-Content” changes of parishins in Gastrodiae Rhizoma during the steaming process. Food Res. Int. 2022, 162, 112092. [Google Scholar]
- Liu, Y.; Yang, X.; Zhou, C.; Wang, Z.; Kuang, T.; Sun, J.; Xu, B.; Meng, X.; Zhang, Y.; Tang, C. Unveiling dynamic changes of chemical constituents in raw and processed fuzi with different steaming time points using desorption electrospray ionization mass spectrometry imaging combined with metabolomics. Front. Pharmacol. 2022, 13, 842890. [Google Scholar]
- Wang, Z.; He, B.; Liu, Y.; Huo, M.; Fu, W.; Yang, C.; Wei, J.; Abliz, Z. In situ metabolomics in nephrotoxicity of aristolochic acids based on air flow-assisted desorption electrospray ionization mass spectrometry imaging. Acta Pharm. Sin. B 2020, 10, 1083–1093. [Google Scholar]
- Woodfield, H.K.; Sturtevant, D.; Borisjuk, L.; Munz, E.; Guschina, I.A.; Chapman, K.; Harwood, J.L. Spatial and temporal mapping of key lipid species in Brassica napus seeds. Plant Physiol. 2017, 173, 1998–2009. [Google Scholar]
- Lu, S.; Sturtevant, D.; Aziz, M.; Jin, C.; Li, Q.; Chapman, K.D.; Guo, L. Spatial analysis of lipid metabolites and expressed genes reveals tissue-specific heterogeneity of lipid metabolism in high- and low-oil Brassica napus L. seeds. Plant J. 2018, 94, 915–932. [Google Scholar]
- Han, X. Effect of Phytoplasma Invasion on Microrna Expression Profiles and Metabolome of Mulberry. Master’s Thesis, Shandong Agricultural University, Taian, China, 2013. [Google Scholar]
- Korenblum, E.; Dong, Y.; Szymanski, J.; Panda, S.; Jozwiak, A.; Massalha, H.; Meir, S.; Rogachev, I.; Aharoni, A. Rhizosphere microbiome mediates systemic root metabolite exudation by root-to-root signaling. Proc. Natl. Acad. Sci. USA 2020, 117, 3874–3883. [Google Scholar] [PubMed]
- Xiong, T.; Luo, S.; Pei, Z.; Tang, C. Visualization of tissue distribution of main chemical components in Glycyrrhizae Radix et Rhizoma decoction pieces based on mass spectrometry imaging. Asia-Pac. Tradit. Med. 2023, 19, 54–58. [Google Scholar]
- Qin, L.; Zhang, Y.; Liu, Y.; He, H.; Han, M.; Li, Y.; Zeng, M.; Wang, X. Recent advances in matrix-assisted laser desorption/ionisation mass spectrometry imaging (MALDI-MSI) for in situ analysis of endogenous molecules in plants. Phytochem. Anal. 2018, 29, 351–364. [Google Scholar] [PubMed]
- Alexander, L.; Grierson, D. Ethylene biosynthesis and action in tomato: A model for climacteric fruit ripening. J. Exp. Bot. 2002, 53, 2039–2055. [Google Scholar] [CrossRef]
- Nakamura, J.; Morikawa-Ichinose, T.; Fujimura, Y.; Hayakawa, E.; Takahashi, K.; Ishii, T.; Miura, D.; Wariishi, H. Spatially resolved metabolic distribution for unraveling the physiological change and responses in tomato fruit using matrix-assisted laser desorption/ionization-mass spectrometry imaging (MALDI-MSI). Anal. Bioanal. Chem. 2017, 409, 1697–1706. [Google Scholar]
- Zhao, W.H.; Zhang, Y.D.; Shi, Y.P. Visualizing the spatial distribution of endogenous molecules in wolfberry fruit at different development stages by matrix-assisted laser desorption/ionization mass spectrometry imaging. Talanta 2021, 234, 122687. [Google Scholar]
- Bhandari, D.R.; Wang, Q.; Friedt, W.; Spengler, B.; Gottwald, S.; Römpp, A. High resolution mass spectrometry imaging of plant tissues: Towards a plant metabolite atlas. Analyst 2015, 140, 7696–7709. [Google Scholar]
- O’Neill, K.C.; Lee, Y.J. Visualizing genotypic and developmental differences of free amino acids in maize roots with mass spectrometry imaging. Front. Plant Sci. 2020, 11, 639–650. [Google Scholar]
- Sugahara, K.; Kitao, K.; Watanabe, T.; Yamagaki, T. Imaging mass spectrometry analysis of flavonoids in Blue Viola petals and their enclosure effects on violanin during color expression. Anal. Chem. 2019, 91, 896–902. [Google Scholar]
- Wang, J.; Yang, E.; Chaurand, P.; Raghavan, V. Visualizing the distribution of strawberry plant metabolites at different maturity stages by MALDI-TOF imaging mass spectrometry. Food Chem. 2021, 345, 128838–128846. [Google Scholar]
- Barbosa, E.A.; Bonfim, M.F.; Bloch, C., Jr.; Engler, G.; Rocha, T.; de Almeida Engler, J. Imaging mass spectrometry of endogenous polypeptides and secondary metabolites from galls induced by root-knot nematodes in tomato roots. Mol. Plant Microbe Interact. 2018, 31, 1048–1059. [Google Scholar] [CrossRef] [PubMed]
- Schleyer, G.; Shahaf, N.; Ziv, C.; Dong, Y.; Meoded, R.A.; Helfrich, E.J.N.; Schatz, D.; Rosenwasser, S.; Rogachev, I.; Aharoni, A.; et al. In plaque-mass spectrometry imaging of a bloom-forming alga during viral infection reveals a metabolic shift towards odd-chain fatty acid lipids. Nat. Microbiol. 2019, 4, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Žukauskaitė, A.; Petřík, I.; Pěnčík, A.; Hönig, M.; Grúz, J.; Široká, J.; Novák, O.; DoleŽal, K. In situ characterisation of phytohormones from wounded Arabidopsis leaves using desorption electrospray ionisation mass spectrometry imaging. Analyst 2021, 146, 2653–2663. [Google Scholar] [CrossRef]
- Shroff, R.; Vergara, F.; Muck, A.; Svatos, A.; Gershenzon, J. Nonuniform distribution of glucosinolates in Arabidopsis thaliana leaves has important consequences for plant defense. Proc. Natl. Acad. Sci. USA 2008, 105, 6196–6201. [Google Scholar] [CrossRef]
- Montini, L.; Crocoll, C.; Gleadow, R.M.; Motawia, M.S.; Janfelt, C.; Bjarnholt, N. Matrix-assisted laser desorption/ionization-mass spectrometry imaging of metabolites during Sorghum Germination. Plant Physiol. 2020, 183, 925–942. [Google Scholar] [CrossRef]
- Dong, Y.; Sonawane, P.; Cohen, H.; Polturak, G.; Feldberg, L.; Avivi, S.H.; Rogachev, I.; Aharoni, A. High mass resolution, spatial metabolite mapping enhances the current plant gene and pathway discovery toolbox. New Phytol. 2020, 228, 1986–2002. [Google Scholar] [CrossRef]
- Wang, S.; Zou, Y.; Sun, S.; Yan, Z.; Tang, W.; Li, P.; Li, B. Recent advances in mass spectrometry imaging and its application in drug research. J. China Pharm. Univ. 2023, 54, 653–661. [Google Scholar]
- Li, X.; Wang, C.; Ding, W.; Mei, M.; Zhang, Y.; Lin, H.; Zhao, Y. Application and prospects of spatial metabolomics technology in plant research. Plant Sci. J. 2024, 42, 654–663. [Google Scholar]
| Technology | MALDI MSI | SIMS MSI | DESI MSI |
|---|---|---|---|
| Ionization type | Soft | Hard | Soft |
| Need matrix or not | Need | Not | Not |
| The type of substance that can be detected | Small-molecule metabolites, drugs, biological macromolecules such as peptides, proteins, nucleic acids, polysaccharides | Small-molecule metabolites, drugs, lipids, elements | Small-molecule metabolites, drugs, lipids, peptides |
| Mass range | 300–100,000 Da | <2000 Da | 100–2000 Da |
| Spatial resolution | 5–100 μm | 0.1–1 μm | 40–200 μm |
| Depth of the scanning | 0.1–20 μm | 0.5–10 μm | 1–50 μm |
| Advantages of Spatial Metabolomics | Limitations of Traditional Metabolomics | Improvement of Spatial Metabolomics |
|---|---|---|
| The sampling process of spatial metabolomics is relatively simple so that the test results have high accuracy. | Traditional metabolomics usually requires sample pretreatment such as tissue homogenate, metabolite extraction and purification and enrichment operations such as solid-phase extraction before detection, which will lead to differences between the sample to be tested and the original state [31]. | Spatial metabolomics does not require special treatment before sample detection. After the sample is simply cleaned or wiped clean, it can be embedded with reagents such as frozen section embedding agent (OCT), carboxymethyl cellulose (CMC) and gelatin [43], which maintain the original state of the metabolite to a greater extent to improve the accuracy of the test results. |
| Spatial metabolomics has a wider range of applications and can detect more abundant substances. | The detection sensitivity of traditional metabolomics is relatively low, and it is difficult to detect metabolites with large differences in high throughput at the same time [48]. | Spatial metabolomics can detect thousands of metabolites at the same time because of its high sensitivity, high coverage and high resolution, so it is easier to detect differential metabolites in some metabolic pathways [49]. |
| The spatial distribution information of spatial metabolomics is clear, and the analysis dimension is comprehensive. | Traditional metabolomics only detects the quality and quantity of metabolites, and lacks the spatial distribution information of metabolites in the original tissues and organs [50]. However, the spatial distribution information is very important for the study of the overall effects of physiological functions. | Spatial metabolomics is embedded in the original state of the sample, which can describe the specific location of changes in the micro-area directly related to the research target [51], and raise the metabolomics information from the two-dimensional level to the three-dimensional level, providing visual data information for plant research. |
| Plant Types | Application Purposes |
|---|---|
| Medicinal plants | Visual Analysis of Natural Ingredients |
| Quality Control of Medicinal Materials | |
| Food Crops and Economic Crops | Assisting Specificity Identification |
| Analysising Anabolic Pathways of Metabolites | |
| Exploring the Regulation of Growth and Development | |
| Revealing the Interaction Mechanism between Crop and Environment | |
| Verifying Gene Function |
| Medicinal Plants | Tissue Sites | Natural Components | MSI Technology | Reference |
|---|---|---|---|---|
| Paris polyphylla Smith var. yunnanensis | Rhizome | Steroid saponin, amino acids, organic acids, sterols, ecdysterone, nucleosides, esters | MALDI | [56] |
| Glycyrrhiza glabra | Rhizome | Free flavonoids, flavonoid glycosides and saponins | MALDI | [57] |
| Paeonia lactiflora | Root | Gallotannins and monoterpene glucosides | MALDI | [58] |
| Paeonia lactiflora | Root | Monoterpene, paeonol glycosides, tannins, flavonoids, saccharides and lipids | MALDI | [59] |
| Tripterygium | Root | Triterpenoids and sesquiterpene alkaloids | MALDI | [60] |
| Curcuma longa | Root | Curcumin | MALDI | [61] |
| Panax | Root | Saponins | MALDI | [62] |
| Panax ginseng | Root | Ginsenosides | MALDI | [63] |
| Salvia miltiorrhiza | Root, stem | Amino acids, phenolic acids, fatty acids, oligosaccharides, cholines, polyamines, tanshinones and phospholipids | MALDI | [64] |
| Salvia miltiorrhiza | Root, stem, leaf | Salvianolic acids, tanshinones | MALDI | [65] |
| Panax notoginseng | Root | Notoginsenosides, ginsenosides, amino acids, dencichine, gluconic acid and low-molecular-weight organic acids | MALDI | [66] |
| Angelica pubescens | Root, velamen | Coumarins | MALDI | [67] |
| Aquilaria sinensis | Stem | (2-phenylethyl) chromones and their analogs | MALDI | [68] |
| Morus alba | Leaf | Protocatechuic acid, chlorogenic acid, monosaccharide, disaccharide, astragalin, rutin, isoquercetin, cyanidin-3-O-glucoside, quercetin-3-O-6″-O-acetyl-β-D-glucopyranoside and kaempferol-3-O-rutinoside | MALDI | [69] |
| Ginkgo biloba | Leaf | Flavonoids, ginkgolic acids, cardanols, saccharides, phospholipids, chlorophylls, ginkgolides | MALDI | [70] |
| Ligustri Lucidi Fructus | Fruit | 10-hydroxyoleoside dimethylester, 8-demethyl-7-ketoliganin, elenolic acid, salidroside, neonuezhenide/isomer, verbascoside/isomer, luteoline, nuzhenal A | MALDI | [71] |
| Dendrobium nobile | Stem | Alkaloids, sesquiterpenoids | MALDI | [72] |
| Hypericum | Flower, leaf | Hypericin | MALDI | [73] |
| Isatidis Radix | Root | 3-formylindole, epiprogoitrin/progoitrin, isatithioetherin C/isatithioetherin E, coniferin, syringing, clemastanin B, adenosine, adenine, uridine, arginine, malic acid, maleic acid/fumaric acid, citric acid, emodin-8-O-β-D-glucoside and isovitexin | MALDI/DESI | [74] |
| Radix Scutellariae | Root | Baicalein and wogonin | PALDI | [75] |
| Mentha piperita | Leaf | Flavonoids | DESI | [76] |
| Datura leichhardtii | Leaf | Alkaloids atropine, scopolamine | DESI | [77] |
| Paeonia lactiflora | Root | Paeonol glycosides, albiflorin | DESI | [78] |
| Cordyceps sinensis | Caterpillars | Fatty acids, glycerides, Glycerophospholipids, amino acids, nucleosides, monosaccharides, sphingolipids, sterols | SIMS | [79] |
| Coptis chinensis | Rhizome | Berberine, epiberberine, coptisine, palmatine, columbamine, jatrorrhizine, tetrahydricheilanthifolinium, oxyberberine | SIMS | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Wang, F.; Wang, J. Spatial Metabolomics and Its Application in Plant Research. Int. J. Mol. Sci. 2025, 26, 3043. https://doi.org/10.3390/ijms26073043
Li R, Wang F, Wang J. Spatial Metabolomics and Its Application in Plant Research. International Journal of Molecular Sciences. 2025; 26(7):3043. https://doi.org/10.3390/ijms26073043
Chicago/Turabian StyleLi, Rong, Fang Wang, and Jian Wang. 2025. "Spatial Metabolomics and Its Application in Plant Research" International Journal of Molecular Sciences 26, no. 7: 3043. https://doi.org/10.3390/ijms26073043
APA StyleLi, R., Wang, F., & Wang, J. (2025). Spatial Metabolomics and Its Application in Plant Research. International Journal of Molecular Sciences, 26(7), 3043. https://doi.org/10.3390/ijms26073043






