Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability
Abstract
1. Introduction
| Species | Order | Kingdom | Optimal Growth Temperature (°C) | Lipids (%) | References | |
|---|---|---|---|---|---|---|
| D | T | |||||
| N. piranensis (C) | Thaumarchaeota (Nitrosopumilales) | Proteoarchaeota | 25 | 8 | 68 | [32] |
| M. boonei | Stenosarchaea (Methanomicrobiales) | Euryarchaeota | 35 | 4 | 96 | [33] |
| M. concilii | Stenosarchaea (Methanosarcinales) | Euryarchaeota | 35 | 70 | ND | [34] |
| T. acidophilum | Thermoplasmatales | Euryarchaeota | 55–59 | 90 | 10 | [35] |
| M. Prunae | Crenarchaeota (Sulfolobales) | Proteoarchaeota | 75 | Traces | 70 | [36] |
| S. acidocaldarius | Crenarchaeota (Sulfolobales) | Proteoarchaeota | 80 | 25 | 75 | [37] |
| T. kodakarensis | Thermococcales | Euryarchaeota | 85 | 41 | 59 | [21] |
| T. barophilus MP | Thermococcales | Euryarchaeota | 85 | 55.1 | 44.3 | [38] |
| A. pernix | Desulfurococcales | Proteoarchaeota | 90–95 | 100 | 0 | [39] |
| I. aggregans | Thermoproteota (Desulfurococcales) | Proteoarchaeota | 93.5 | Traces | 61 | [18] |
| M. kandleri | Methanopyrales | Euryarchaeota | 98 | 100 | 0 | [25] |
| P. furiosus | Thermococcales | Euryarchaeota | 100 | 30 | 50 | [40] |
2. Results
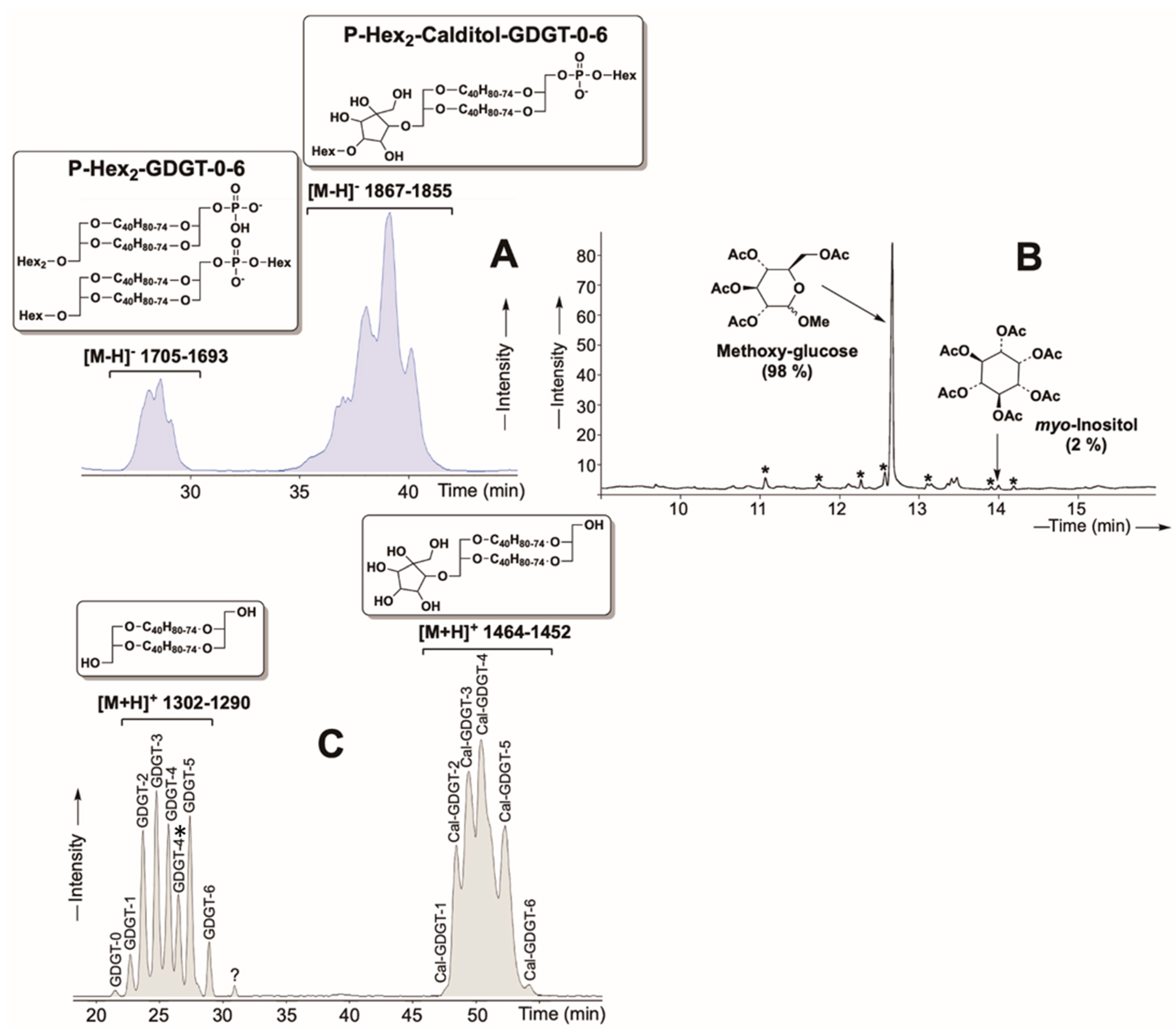
2.1. Purified Lipid Characterization
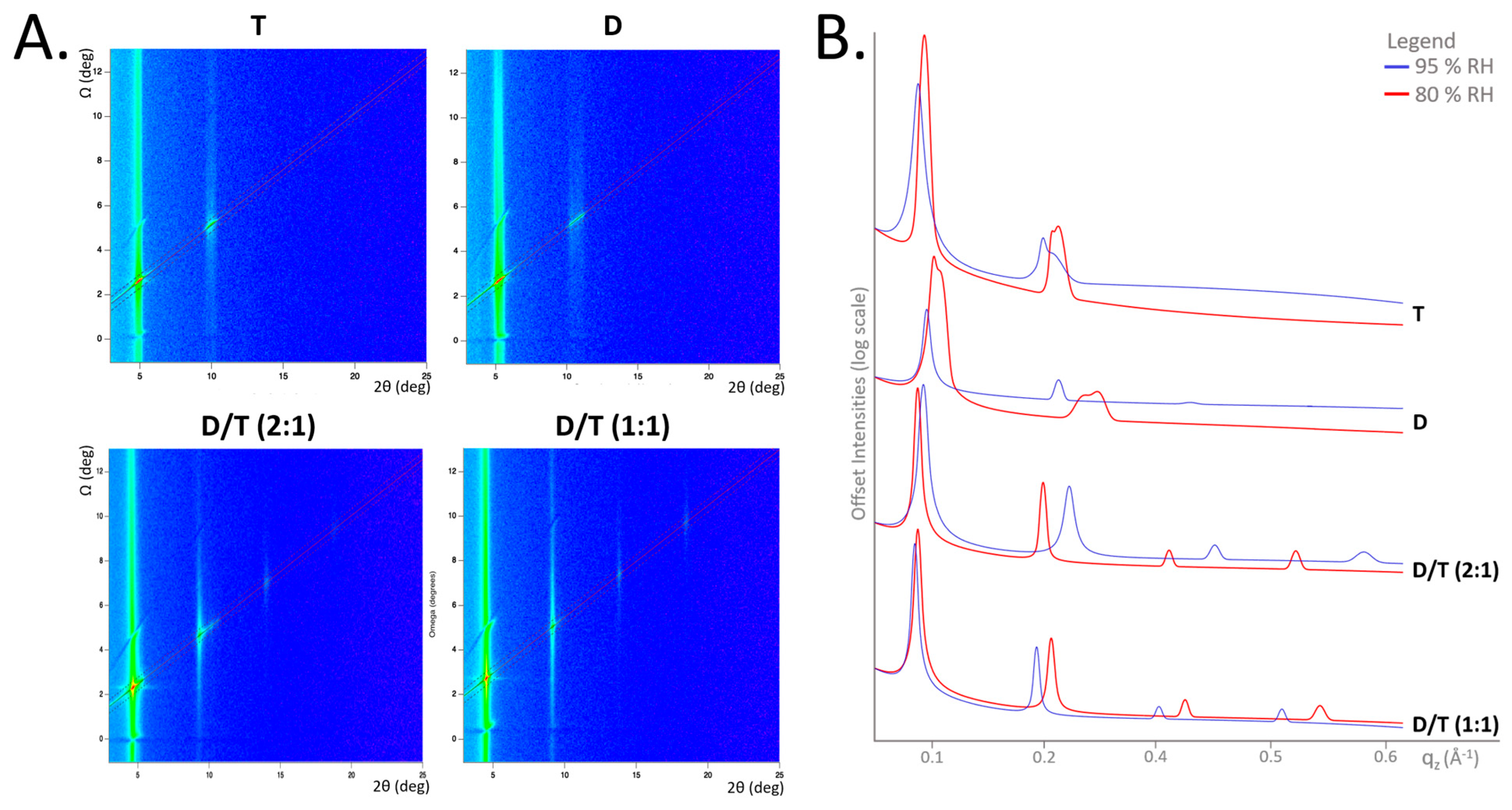
2.2. Membrane Ordering and Stability Across Experimental Conditions
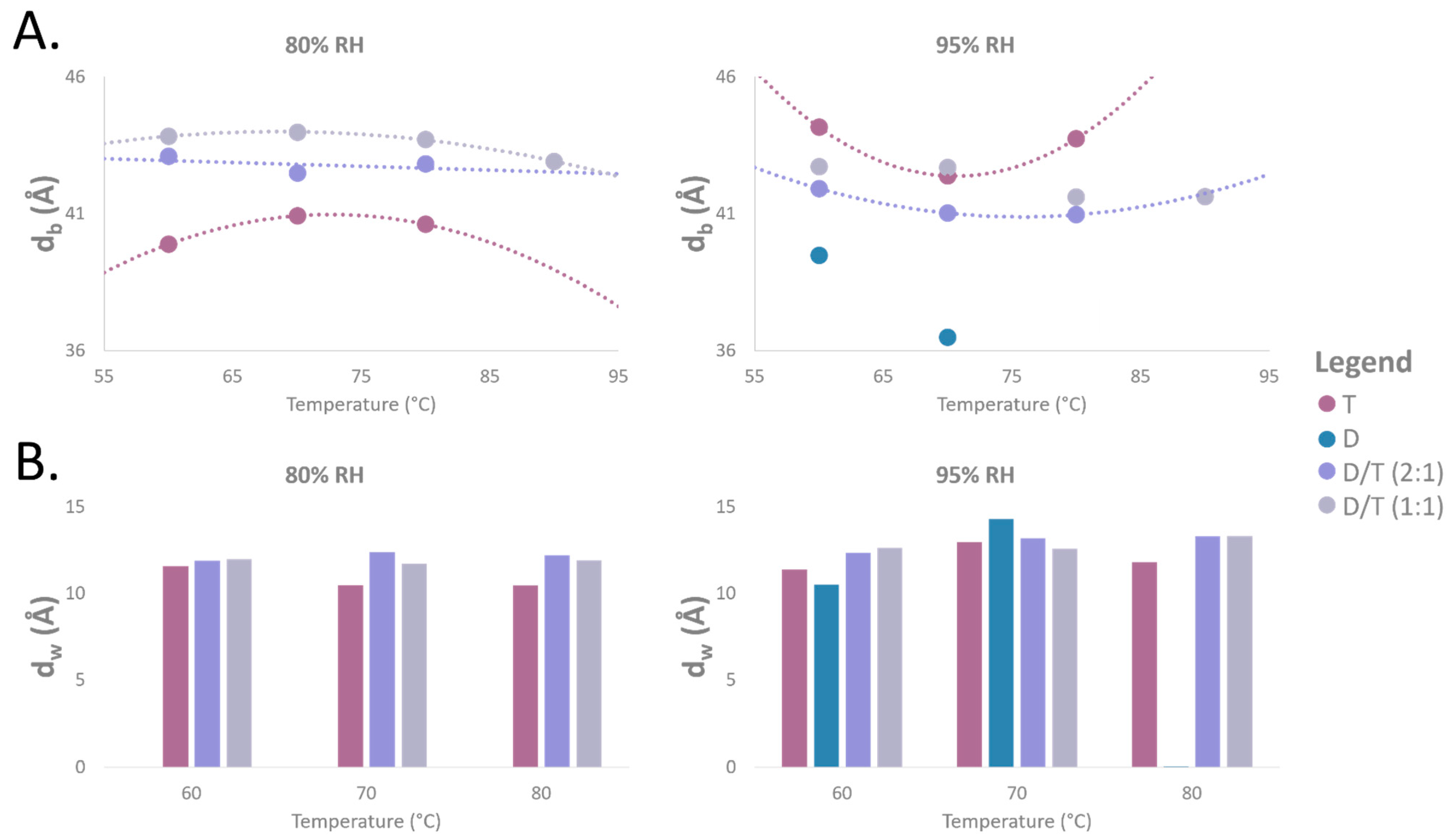
2.3. d-Spacing Fluctuation Across Temperature and Humidity
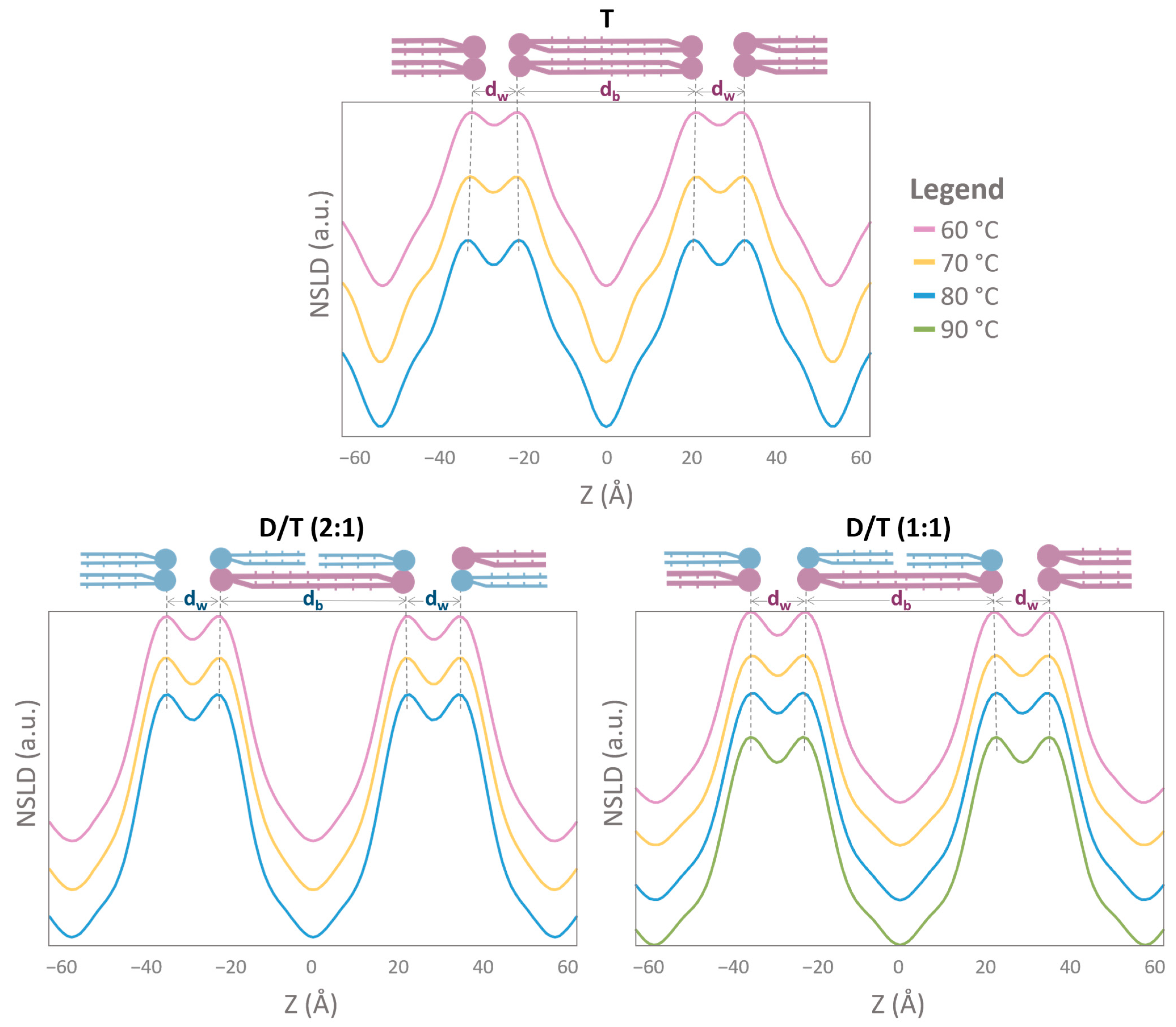
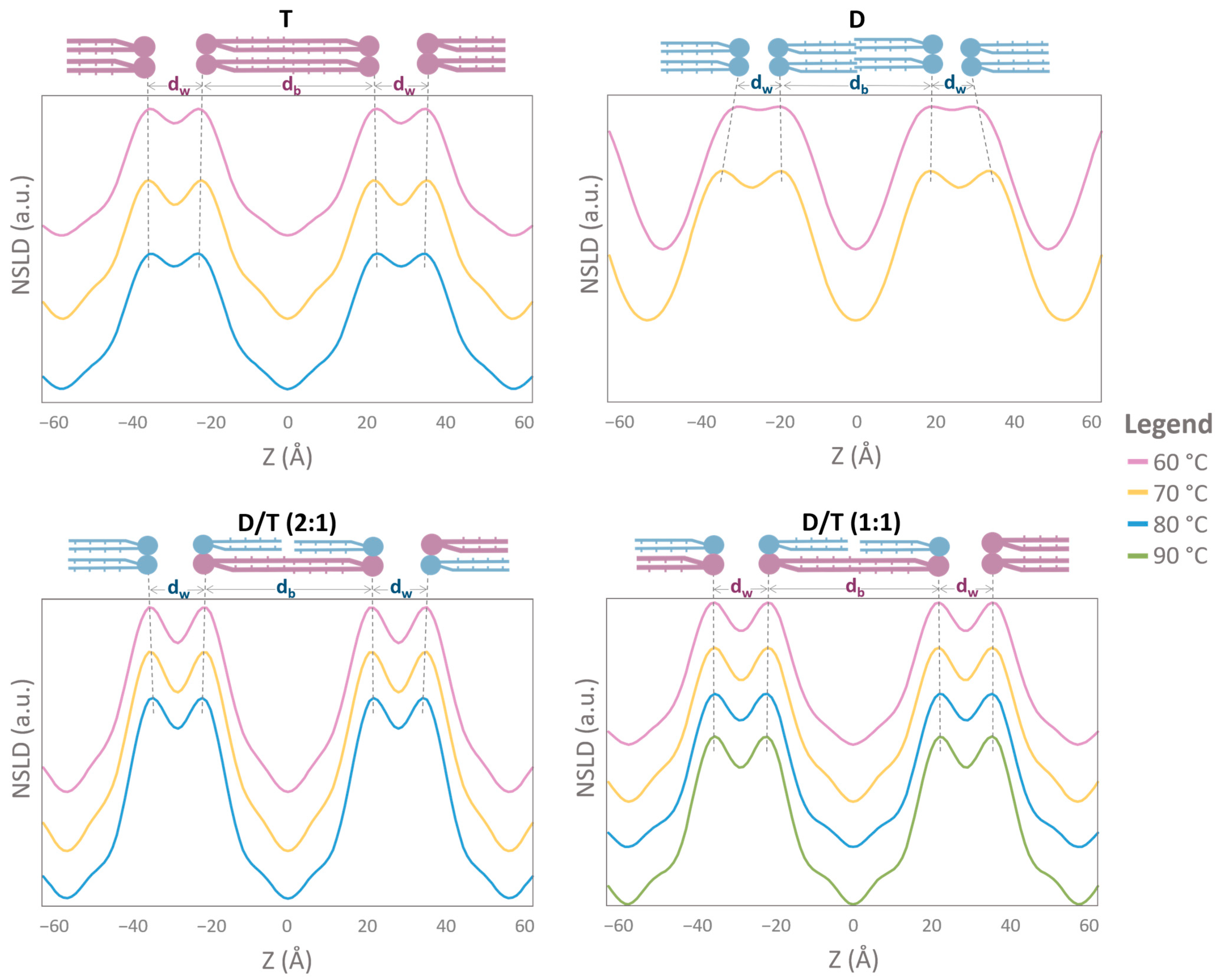
2.4. NLSD Profiles: Humidity-Induced Structural Modifications
2.5. The Specific Contribution of Molecular Groups to NLSD Profiles
2.6. Membrane Thickness and Water Layer Characteristics
3. Discussion
3.1. On the Importance of Natural Polar Headgroups on Membrane Stability
3.2. Impact of the Diether to Tetraether Ratio on Membrane Thickness
3.3. Impact of the Diether to Tetraether Ratio on Membrane Stability
3.4. Impact of the Cyclopentane Rings on Membrane Properties
3.5. Diether and Tetraether Lipids Form True Mixtures in the Reconstructed Membranes
3.6. Lipid Diversity as a Key to Membrane Stability and Adaptability
4. Materials and Methods
4.1. Experimental Design
4.2. Archaeal Lipids
4.3. Archaeal Membrane Reconstruction
4.4. d-Spacing Calculation
4.5. Neutron Scattering Length Density (NLSD) Profiles
4.6. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konings, W.N.; Albers, S.-V.; Koning, S.; Driessen, A.J.M. The Cell Membrane Plays a Crucial Role in Survival of Bacteria and Archaea in Extreme Environments. Antonie Van Leeuwenhoek 2002, 81, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Cai, M.; Zhou, L.; Wang, H. The Structure and Function of Cell Membranes Studied by Atomic Force Microscopy. Semin. Cell Dev. Biol. 2018, 73, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Hazel, J.R. Thermal Adaptation in Biological Membranes: Is Homeoviscous Adaptation the Explanation? Annu. Rev. Physiol. 1995, 57, 19–42. [Google Scholar] [CrossRef]
- Sinensky, M. Homeoviscous Adaptation—A Homeostatic Process That Regulates the Viscosity of Membrane Lipids in Escherichia coli. Proc. Natl. Acad. Sci. USA 1974, 71, 522–525. [Google Scholar] [CrossRef]
- Jebbar, M.; Franzetti, B.; Girard, E.; Oger, P. Microbial Diversity and Adaptation to High Hydrostatic Pressure in Deep-Sea Hydrothermal Vents Prokaryotes. Extremophiles 2015, 19, 721–740. [Google Scholar] [CrossRef]
- Siliakus, M.F.; van der Oost, J.; Kengen, S.W.M. Adaptations of Archaeal and Bacterial Membranes to Variations in Temperature, pH and Pressure. Extremophiles 2017, 21, 651–670. [Google Scholar] [CrossRef]
- de Rosa, M.; de Rosa, S.; Gambacorta, A.; Bu’Lock, J.D. Lipid Structures in the Caldariella Group of Extreme Thermoacidophile Bacteria. J. Chem. Soc. Chem. Commun. 1977, 15, 514–515. [Google Scholar] [CrossRef]
- Zhang, D.L. Biosynthesis of Archaebacterial Membranes. Formation of Isoprene Ethers by a Prenyl Transfer Reaction. J. Am. Chem. Soc. 1990, 112, 1264–1265. [Google Scholar] [CrossRef]
- Nam, J.-W.; Jenkins, L.M.; Li, J.; Evans, B.S.; Jaworski, J.G.; Allen, D.K. A General Method for Quantification and Discovery of Acyl Groups Attached to Acyl Carrier Proteins in Fatty Acid Metabolism Using LC-MS/MS. Plant Cell 2020, 32, 820–832. [Google Scholar] [CrossRef]
- Kruczek, J.; Saunders, M.; Khosla, M.; Tu, Y.; Pandit, S.A. Molecular Dynamics Simulations of Ether- and Ester-Linked Phospholipids. Biochim. Biophys. Acta BBA-Biomembr. 2017, 1859, 2297–2307. [Google Scholar] [CrossRef]
- Mathai, J.C.; Sprott, G.D.; Zeidel, M.L. Molecular Mechanisms of Water and Solute Transport across Archaebacterial Lipid Membranes. J. Biol. Chem. 2001, 276, 27266–27271. [Google Scholar] [CrossRef] [PubMed]
- Komatsu, H.; Chong, P.L.-G. Low Permeability of Liposomal Membranes Composed of Bipolar Tetraether Lipids from Thermoacidophilic Archaebacterium Sulfolobus acidocaldarius. Biochemistry 1998, 37, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Polak, A.; Tarek, M.; Tomšič, M.; Valant, J.; Poklar Ulrih, N.; Jamnik, A.; Kramar, P.; Miklavčič, D. Structural Properties of Archaeal Lipid Bilayers: Small-Angle X-Ray Scattering and Molecular Dynamics Simulation Study. Langmuir 2014, 30, 8308–8315. [Google Scholar] [CrossRef] [PubMed]
- Boyd, E.; Hamilton, T.; Wang, J.; He, L.; Zhang, C. The Role of Tetraether Lipid Composition in the Adaptation of Thermophilic Archaea to Acidity. Front. Microbiol. 2013, 4, 62. [Google Scholar] [CrossRef]
- De Rosa, M.; Gambacorta, A.; Nicolaus, B. A New Type of Cell Membrane, in Thermophilic Archaebacteria, Based on Bipolar Ether Lipids. J. Membr. Sci. 1983, 16, 287–294. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Lee Gau Chong, P. Molecular Modeling of Archaebacterial Bipolar Tetraether Lipid Membranes. Chem. Phys. Lipids 2000, 105, 193–200. [Google Scholar] [CrossRef]
- Khan, T.K.; Chong, P.L.-G. Studies of Archaebacterial Bipolar Tetraether Liposomes by Perylene Fluorescence. Biophys. J. 2000, 78, 1390–1399. [Google Scholar] [CrossRef]
- Knappy, C.S.; Nunn, C.E.M.; Morgan, H.W.; Keely, B.J. The Major Lipid Cores of the Archaeon Ignisphaera Aggregans: Implications for the Phylogeny and Biosynthesis of Glycerol Monoalkyl Glycerol Tetraether Isoprenoid Lipids. Extremophiles 2011, 15, 517–528. [Google Scholar] [CrossRef]
- Nichols, D.S.; Miller, M.R.; Davies, N.W.; Goodchild, A.; Raftery, M.; Cavicchioli, R. Cold Adaptation in the Antarctic Archaeon Methanococcoides Burtonii Involves Membrane Lipid Unsaturation. J. Bacteriol. 2004, 186, 8508–8515. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X.-L.; Farley, K.R.; Wei, J.H.; Metcalf, W.W.; Summons, R.E.; Welander, P.V. GDGT Cyclization Proteins Identify the Dominant Archaeal Sources of Tetraether Lipids in the Ocean. Proc. Natl. Acad. Sci. USA 2019, 116, 22505–22511. [Google Scholar] [CrossRef]
- Matsuno, Y.; Sugai, A.; Higashibata, H.; Fukuda, W.; Ueda, K.; Uda, I.; Sato, I.; Itoh, T.; Imanaka, T.; Fujiwara, S. Effect of Growth Temperature and Growth Phase on the Lipid Composition of the Archaeal Membrane from Thermococcus kodakaraensis. Biosci. Biotechnol. Biochem. 2009, 73, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Oger, P.M.; Cario, A. Adaptation of the Membrane in Archaea. Biophys. Chem. 2013, 183, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Cario, A.; Grossi, V.; Schaeffer, P.; Oger, P.M. Membrane Homeoviscous Adaptation in the Piezo-Hyperthermophilic Archaeon Thermococcus barophilus. Front. Microbiol. 2015, 6, 1152. [Google Scholar] [CrossRef]
- Sprott, G.D.; Meloche, M.; Richards, J.C. Proportions of Diether, Macrocyclic Diether, and Tetraether Lipids in Methanococcus jannaschii Grown at Different Temperatures. J. Bacteriol. 1991, 173, 3907–3910. [Google Scholar] [CrossRef]
- Hafenbradl, D.; Keller, M.; Stetter, K.O. Lipid Analysis of Methanopyrus Kandleri. FEMS Microbiol. Lett. 1996, 136, 199–202. [Google Scholar] [CrossRef]
- Ulrih, N.P.; Gmajner, D.; Raspor, P. Structural and Physicochemical Properties of Polar Lipids from Thermophilic Archaea. Appl. Microbiol. Biotechnol. 2009, 84, 249–260. [Google Scholar] [CrossRef]
- Liman, G.L.S.; Garcia, A.A.; Fluke, K.A.; Anderson, H.R.; Davidson, S.C.; Welander, P.V.; Santangelo, T.J. Tetraether Archaeal Lipids Promote Long-Term Survival in Extreme Conditions. Mol. Microbiol. 2024, 121, 882–894. [Google Scholar] [CrossRef]
- Bagatolli, L.; Gratton, E.; Khan, T.K.; Chong, P.L.-G. Two-Photon Fluorescence Microscopy Studies of Bipolar Tetraether Giant Liposomes from Thermoacidophilic Archaebacteria Sulfolobus acidocaldarius. Biophys. J. 2000, 79, 416–425. [Google Scholar] [CrossRef]
- Bagatolli, L.A.; Gratton, E. Direct Observation of Lipid Domains in Free-Standing Bilayers Using Two-Photon Excitation Fluorescence Microscopy. J. Fluoresc. 2001, 11, 141–160. [Google Scholar] [CrossRef]
- Salvador-Castell, M.; Demé, B.; Oger, P.; Peters, J. Lipid Phase Separation Induced by the Apolar Polyisoprenoid Squalane Demonstrates Its Role in Membrane Domain Formation in Archaeal Membranes. Langmuir 2020, 36, 7375–7382. [Google Scholar] [CrossRef]
- LoRicco, J.G.; Salvador-Castell, M.; Demé, B.; Peters, J.; Oger, P.M. Apolar Polyisoprenoids Located in the Midplane of the Bilayer Regulate the Response of an Archaeal-Like Membrane to High Temperature and Pressure. Front. Chem. 2020, 8, 594039. [Google Scholar] [CrossRef] [PubMed]
- Elling, F.J.; Könneke, M.; Nicol, G.W.; Stieglmeier, M.; Bayer, B.; Spieck, E.; de la Torre, J.R.; Becker, K.W.; Thomm, M.; Prosser, J.I.; et al. Chemotaxonomic Characterisation of the Thaumarchaeal Lipidome. Environ. Microbiol. 2017, 19, 2681–2700. [Google Scholar] [CrossRef] [PubMed]
- Bräuer, S.L.; Cadillo-Quiroz, H.; Ward, R.J.; Yavitt, J.B.; Zinder, S.H. Methanoregula boonei Gen. Nov., Sp. Nov., an Acidiphilic Methanogen Isolated from an Acidic Peat Bog. Int. J. Syst. Evol. Microbiol. 2011, 61, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Choquet, C.G.; Patel, G.B.; Beveridge, T.J.; Sprott, G.D. Formation of Unilamellar Liposomes from Total Polar Lipid Extracts of Methanogens. Appl. Environ. Microbiol. 1992, 58, 2894–2900. [Google Scholar] [CrossRef]
- Nemoto, N.; Shida, Y.; Shimada, H.; Oshima, T.; Yamagishi, A. Characterization of the Precursor of Tetraether Lipid Biosynthesis in the Thermoacidophilic Archaeon Thermoplasma acidophilum. Extremophiles 2003, 7, 235–243. [Google Scholar] [CrossRef]
- Itoh, Y.H.; Sugai, A.; Uda, I.; Itoh, T. The Evolution of Lipids. Adv. Space Res. 2001, 28, 719–724. [Google Scholar] [CrossRef]
- Zeng, Z.; Liu, X.-L.; Wei, J.H.; Summons, R.E.; Welander, P.V. Calditol-Linked Membrane Lipids Are Required for Acid Tolerance in Sulfolobus acidocaldarius. Proc. Natl. Acad. Sci. USA 2018, 115, 12932–12937. [Google Scholar] [CrossRef]
- Tourte, M.; Coffinet, S.; Wörmer, L.; Lipp, J.S.; Hinrichs, K.-U.; Oger, P.M. The Exploration of Thermococcus barophilus Lipidome Reveals the Widest Variety of Phosphoglycolipids in Thermococcales. Front. Microbiol. 2022, 13, 869479. [Google Scholar] [CrossRef]
- Kejžar, J.; Mrak, P.; Osojnik Črnivec, I.G.; Poklar Ulrih, N. Influence of Archaeal Lipids Isolated from Aeropyrum Pernix K1 on Physicochemical Properties of Sphingomyelin-Cholesterol Liposomes. Biochim. Biophys. Acta BBA-Biomembr. 2024, 1866, 184374. [Google Scholar] [CrossRef]
- Tourte, M.; Kuentz, V.; Schaeffer, P.; Grossi, V.; Cario, A.; Oger, P.M. Novel Intact Polar and Core Lipid Compositions in the Pyrococcus Model Species, P. furiosus and P. yayanosii, Reveal the Largest Lipid Diversity Amongst Thermococcales. Biomolecules 2020, 10, 830. [Google Scholar] [CrossRef]
- Shen, X.; Perreault, H. Characterization of Carbohydrates Using a Combination of Derivatization, High-Performance Liquid Chromatography and Mass Spectrometry. J. Chromatogr. A 1998, 811, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Cai, R.; An, Y.; Tang, H. Simultaneous Quantification of Five Stereoisomeric Hexoses in Nine Biological Matrices Using Ultrahigh Performance Liquid Chromatography with Tandem Mass Spectrometry. J. Anal. Test. 2020, 4, 249–256. [Google Scholar] [CrossRef]
- Christie, W.W. Thin-Layer Chromatography of Lipids. 2011. Available online: https://www.aocs.org/resource/thin-layer-chromatography-of-lipids/ (accessed on 21 May 2012).
- Lo, S.-L.; Chang, E.L. Purification and Characterization of a Liposomal-Forming Tetraether Lipid Fraction. Biochem. Biophys. Res. Commun. 1990, 167, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Salvador-Castell, M.; Demé, B.; Oger, P.; Peters, J. Structural Characterization of an Archaeal Lipid Bilayer as a Function of Hydration and Temperature. Int. J. Mol. Sci. 2020, 21, 1816. [Google Scholar] [CrossRef]
- Salvador-Castell, M.; Golub, M.; Erwin, N.; Demé, B.; Brooks, N.J.; Winter, R.; Peters, J.; Oger, P.M. Characterisation of a Synthetic Archeal Membrane Reveals a Possible New Adaptation Route to Extreme Conditions. Commun. Biol. 2021, 4, 1–13. [Google Scholar] [CrossRef]
- Sohlenkamp, C.; Geiger, O. Bacterial Membrane Lipids: Diversity in Structures and Pathways. FEMS Microbiol. Rev. 2016, 40, 133–159. [Google Scholar] [CrossRef]
- Meador, T.B.; Gagen, E.J.; Loscar, M.E.; Goldhammer, T.; Yoshinaga, M.Y.; Wendt, J.; Thomm, M.; Hinrichs, K.-U. Thermococcus kodakarensis Modulates Its Polar Membrane Lipids and Elemental Composition According to Growth Stage and Phosphate Availability. Front. Microbiol. 2014, 5, 10. [Google Scholar] [CrossRef]
- Nishihara, M.; Koga, Y. Two New Phospholipids, Hydroxyarchaetidylglycerol and Hydroxyarchaetidylethanolamine, from the Archaea Methanosarcina Barkeri. Biochim. Biophys. Acta BBA-Lipids Lipid Metab. 1995, 1254, 155–160. [Google Scholar] [CrossRef]
- Tarui, M.; Tanaka, N.; ToMuRA, K.; Ohga, M.; KoG, H.M.Y. Lipid Component Parts Analysis of the Hyperthermophilic Sulfate-ReducingArchaeon Archaeoglobus fuigidus. J. UOEH 2007, 29, 131–139. [Google Scholar]
- Nishihara, M.; Morii, H.; Matsuno, K.; Ohga, M.; Stetter, K.O.; Koga, Y. Structural Analysis by Reductive Cleavage with LiAlH4 of an Allyl Ether Choline-Phospholipid, Archaetidylcholine, from the Hyperthermophilic Methanoarchaeon Methanopyrus Kandleri. Archaea 2002, 1, 123–131. [Google Scholar] [CrossRef]
- Kodama, M.; Miyata, T. Effect of the Head Group of Phospholipids on the Acyl-Chain Packing and Structure of Their Assemblies as Revealed by Microcalorimetry and Electron Microscopy. Colloids Surf. Physicochem. Eng. Asp. 1996, 109, 283–289. [Google Scholar] [CrossRef]
- Winter, R.; Jeworrek, C. Effect of Pressure on Membranes. Soft Matter 2009, 5, 3157–3173. [Google Scholar] [CrossRef]
- Ruiz, J.; LoRicco, J.G.; Soulère, L.; Castell, M.S.; Grélard, A.; Kauffmann, B.; Dufourc, E.J.; Demé, B.; Popowycz, F.; Peters, J. Membrane Plasticity Induced by Myo -Inositol Derived Archaeal Lipids: Chemical Synthesis and Biophysical Characterization. Phys. Chem. Chem. Phys. 2023, 25, 16273–16287. [Google Scholar] [CrossRef]
- van de Vossenberg, J.L.C.M.; Driessen, A.J.M.; Zillig, W.; Konings, W.N. Bioenergetics and Cytoplasmic Membrane Stability of the Extremely Acidophilic, Thermophilic Archaeon Picrophilus Oshimae. Extremophiles 1998, 2, 67–74. [Google Scholar] [CrossRef]
- Tenchov, B.; Vescio, E.M.; Sprott, G.D.; Zeidel, M.L.; Mathai, J.C. Salt Tolerance of Archaeal Extremely Halophilic Lipid Membranes. J. Biol. Chem. 2006, 281, 10016–10023. [Google Scholar] [CrossRef]
- Chong, P.L.-G.; Zein, M.; Khan, T.K.; Winter, R. Structure and Conformation of Bipolar Tetraether Lipid Membranes Derived from Thermoacidophilic Archaeon Sulfolobus acidocaldarius as Revealed by Small-Angle X-Ray Scattering and High-Pressure FT-IR Spectroscopy. J. Phys. Chem. B 2003, 107, 8694–8700. [Google Scholar] [CrossRef]
- Castro, L.F.P.D.; Dopson, M.; Friedman, R. Biological Membranes in Extreme Conditions: Simulations of Anionic Archaeal Tetraether Lipid Membranes. PLoS ONE 2016, 11, e0155287. [Google Scholar] [CrossRef]
- Kucerka, N.; Nieh, M.; Pencer, J.; Sachs, J.; Katsaras, J. What Determines the Thickness of a Biological Membrane. Gen. Physiol. Biophys. 2009, 28, 117–125. [Google Scholar] [CrossRef]
- Misuraca, L.; LoRicco, J.; Oger, P.; Peters, J.; Demé, B. Incorporation and Localisation of Alkanes in a Protomembrane Model by Neutron Diffraction. Biochim. Biophys. Acta BBA-Biomembr. 2023, 1865, 184119. [Google Scholar] [CrossRef]
- Zeng, Z.; Chen, H.; Yang, H.; Chen, Y.; Yang, W.; Feng, X.; Pei, H.; Welander, P.V. Identification of a Protein Responsible for the Synthesis of Archaeal Membrane-Spanning GDGT Lipids. Nat. Commun. 2022, 13, 1545. [Google Scholar] [CrossRef]
- Kučerka, N.; Nagle, J.F.; Sachs, J.N.; Feller, S.E.; Pencer, J.; Jackson, A.; Katsaras, J. Lipid Bilayer Structure Determined by the Simultaneous Analysis of Neutron and X-Ray Scattering Data. Biophys. J. 2008, 95, 2356–2367. [Google Scholar] [CrossRef] [PubMed]
- Shinoda, W.; Shinoda, K.; Baba, T.; Mikami, M. Molecular Dynamics Study of Bipolar Tetraether Lipid Membranes. Biophys. J. 2005, 89, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Untersteller, É.; Fritz, B.; Bliériot, Y.; Sinaÿ, P. The Structure of Calditol Isolated from the Thermoacidophilic Archaebacterium Sulfolobus acidocaldarius. CR Acad. Sci.-Ser. IIC-Chem. 1999, 2, 429–433. [Google Scholar] [CrossRef]
- Uda, I.; Sugai, A.; Itoh, Y.H.; Itoh, T. Variation in Molecular Species of Polar Lipids from Thermoplasma acidophilum Depends on Growth Temperature. Lipids 2001, 36, 103–105. [Google Scholar] [CrossRef]
- Zhou, A. Energy Flux Controls Tetraether Lipid Cyclization in Sulfolobus acidocaldarius. Environ. Microbiol. 2020, 22, 343–353. [Google Scholar] [CrossRef]
- Tourte, M.; Schaeffer, P.; Grossi, V.; Oger, P.M. Membrane Adaptation in the Hyperthermophilic Archaeon Pyrococcus Furiosus Relies upon a Novel Strategy Involving Glycerol Monoalkyl Glycerol Tetraether Lipids. Environ. Microbiol. 2022, 24, 2029–2046. [Google Scholar] [CrossRef]
- Tourte, M.; Schaeffer, P.; Grossi, V.; Oger, P.M. Functionalized Membrane Domains: An Ancestral Feature of Archaea? Front. Microbiol. 2020, 11, 526. [Google Scholar] [CrossRef]
- Wagner, M.; van Wolferen, M.; Wagner, A.; Lassak, K.; Meyer, B.H.; Reimann, J.; Albers, S.-V. Versatile Genetic Tool Box for the Crenarchaeote Sulfolobus acidocaldarius. Front. Microbiol. 2012, 3, 214. [Google Scholar] [CrossRef]
- Gonthier, J.; Barrett, M.A.; Aguettaz, O.; Baudoin, S.; Bourgeat-Lami, E.; Demé, B.; Grimm, N.; Hauß, T.; Kiefer, K.; Lelièvre-Berna, E.; et al. BerILL: The Ultimate Humidity Chamber for Neutron Scattering. J. Neutron Res. 2019, 21, 65–76. [Google Scholar] [CrossRef]
- Cristiglio, V.; Giroud, B.; Didier, L.; Demé, B. D16 Is Back to Business: More Neutrons, More Space, More Fun. Neutron News 2015, 26, 22–24. [Google Scholar] [CrossRef]
- Buffet, J.-C.; Cristiglio, V.; Cuccaro, S.; Demé, B.; Guérard, B.; Marchal, J.; Pentenero, J.; Sartor, N.; Turi, J. Development of a Large-Area Curved Trench-MWPC 3He Detector for the D16 Neutron Diffractometer at the ILL. EPJ Web Conf. 2023, 286, 03010. [Google Scholar] [CrossRef]
- Arnold, O.; Bilheux, J.C.; Borreguero, J.M.; Buts, A.; Campbell, S.I.; Chapon, L.; Doucet, M.; Draper, N.; Ferraz Leal, R.; Gigg, M.A.; et al. Mantid—Data Analysis and Visualization Package for Neutron Scattering and µSR Experiments. Nucl. Instrum. Methods Phys. Res. Sect. Accel. Spectrom. Detect. Assoc. Equip. 2014, 764, 156–166. [Google Scholar] [CrossRef]
- Harroun, T.A.; Katsaras, J.; Wassall, S.R. Cholesterol Is Found To Reside in the Center of a Polyunsaturated Lipid Membrane. Biochemistry 2008, 47, 7090–7096. [Google Scholar] [CrossRef] [PubMed]
- Hrubovčák, P.; Kondela, T.; Ermakova, E.; Kučerka, N. Application of Small-Angle Neutron Diffraction to the Localization of General Anesthetics in Model Membranes. Eur. Biophys. J. 2019, 48, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, V.; Husson, F. The Structure of the Liquid-Crystalline Phases of Lipid-Water Systems. J. Cell Biol. 1962, 12, 207–219. [Google Scholar] [CrossRef]
- Ayesa, U.; Chong, P.L.-G. Polar Lipid Fraction E from Sulfolobus acidocaldarius and Dipalmitoylphosphatidylcholine Can Form Stable yet Thermo-Sensitive Tetraether/Diester Hybrid Archaeosomes with Controlled Release Capability. Int. J. Mol. Sci. 2020, 21, 8388. [Google Scholar] [CrossRef]
- Abdelrasoul, A.; Doan, H.; Lohi, A.; Abdelrasoul, A.; Doan, H.; Lohi, A. Applications of Biomimetic and Bioinspired Membranes. In Biomimetic and Bioinspired Membranes for New Frontiers in Sustainable Water Treatment Technology; IntechOpen: London, UK, 2017; ISBN 978-953-51-3662-0. [Google Scholar]
- Hanford, M.J.; Peeples, T.L. Archaeal Tetraether Lipids. Appl. Biochem. Biotechnol. 2002, 97, 45–62. [Google Scholar] [CrossRef]
- Lipscomb, G.L.; Stirrett, K.; Schut, G.J.; Yang, F.; Jenney, F.E.; Scott, R.A.; Adams, M.W.W.; Westpheling, J. Natural Competence in the Hyperthermophilic Archaeon Pyrococcus Furiosus Facilitates Genetic Manipulation: Construction of Markerless Deletions of Genes Encoding the Two Cytoplasmic Hydrogenases. Appl. Environ. Microbiol. 2011, 77, 2232–2238. [Google Scholar] [CrossRef]
- Peterse, F.; Hopmans, E.C.; Schouten, S.; Mets, A.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S. Identification and Distribution of Intact Polar Branched Tetraether Lipids in Peat and Soil. Org. Geochem. 2011, 42, 1007–1015. [Google Scholar] [CrossRef]
- Brock, T.D.; Brock, K.M.; Belly, R.T.; Weiss, R.L. Sulfolobus: A New Genus of Sulfur-Oxidizing Bacteria Living at Low pH and High Temperature. Arch. Mikrobiol. 1972, 84, 54–68. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saracco, M.; Schaeffer, P.; Tourte, M.; Albers, S.-V.; Louis, Y.; Peters, J.; Demé, B.; Fontanay, S.; Oger, P.M. Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability. Int. J. Mol. Sci. 2025, 26, 3045. https://doi.org/10.3390/ijms26073045
Saracco M, Schaeffer P, Tourte M, Albers S-V, Louis Y, Peters J, Demé B, Fontanay S, Oger PM. Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability. International Journal of Molecular Sciences. 2025; 26(7):3045. https://doi.org/10.3390/ijms26073045
Chicago/Turabian StyleSaracco, Margot, Philippe Schaeffer, Maxime Tourte, Sonja-Verena Albers, Yoann Louis, Judith Peters, Bruno Demé, Stephane Fontanay, and Philippe M. Oger. 2025. "Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability" International Journal of Molecular Sciences 26, no. 7: 3045. https://doi.org/10.3390/ijms26073045
APA StyleSaracco, M., Schaeffer, P., Tourte, M., Albers, S.-V., Louis, Y., Peters, J., Demé, B., Fontanay, S., & Oger, P. M. (2025). Bilayer-Forming Lipids Enhance Archaeal Monolayer Membrane Stability. International Journal of Molecular Sciences, 26(7), 3045. https://doi.org/10.3390/ijms26073045






