Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair
Abstract
1. Introduction
2. Mechanisms of Cardiac Regeneration: From Neonatal Regenerative Capacity to Post-Infarction Repair
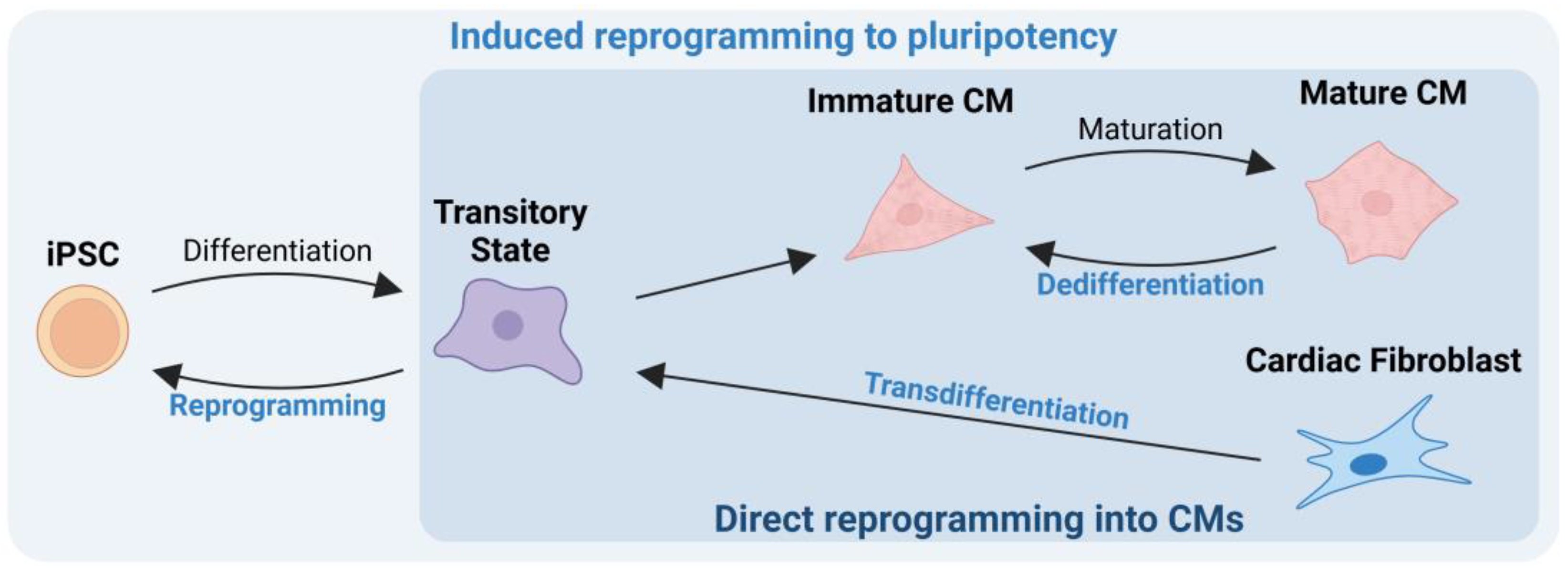
3. Induced Reprogramming to Pluripotency
| Cell Type | Description | Advantages | Disadvantages | Use in Clinical Trials |
|---|---|---|---|---|
| BMMNCs [6,34,35] | Mixed population containing HSCs and MSCs | Abundant, easily accessible, autologous transplantation | Limited differentiation potential | Improved myocardial performance after transplantation |
| HSCs [6,36,37] | Subpopulation of BMMNCs, can differentiate into all blood cells | Autologous transplantation, well-established isolation protocols | Limited abundance, weak differentiation potential | Limited therapeutic use |
| EPCs [6,38] | Can differentiate into endothelial cells, promote blood vessel growth | Homing capacity, paracrine signaling stimulates endothelial proliferation | Reduced differentiation capacity | Shown to improve heart function in some studies |
| MSCs [6,35,39] | Immunomodulatory, anti-inflammatory properties | Improve cardiac function, reduce inflammation Direct effect on cardiac tissue repair and regeneration The secretion of trophic factors improves cardiac function by tissue injury reduction, inhibition of fibrotic remodeling, angiogenesis, and activation of host tissue stem cell niches | Conflicting results in clinical trials | Most studied stem cells for cardiac injury |
| CSCs [6,35,40] | Isolated from heart tissue, can differentiate into CM | Preserve heart function, stimulate angiogenesis | Limited number of cells | Shown to improve heart function in animal models |
| ESCs [35,41] | Pluripotent, can differentiate into any cell type | High differentiation potential, self-renewal capacity | Ethical concerns, poor engraftment, risk of teratoma formation | Not used due to ethical and safety concerns |
| iPSCs [41] | Generated from adult cells, can differentiate into any cell type | Autologous transplantation, no ethical concerns, self-renewal capacity | Immature cells, risk of tumorigenicity, low numbers of pure and mature CMs | Shown to improve cardiac function in animal models |
Advances in Pluripotent Cell Differentiation into Functional Cardiac Cells
4. Direct Cell Reprogramming into Cardiomyocytes
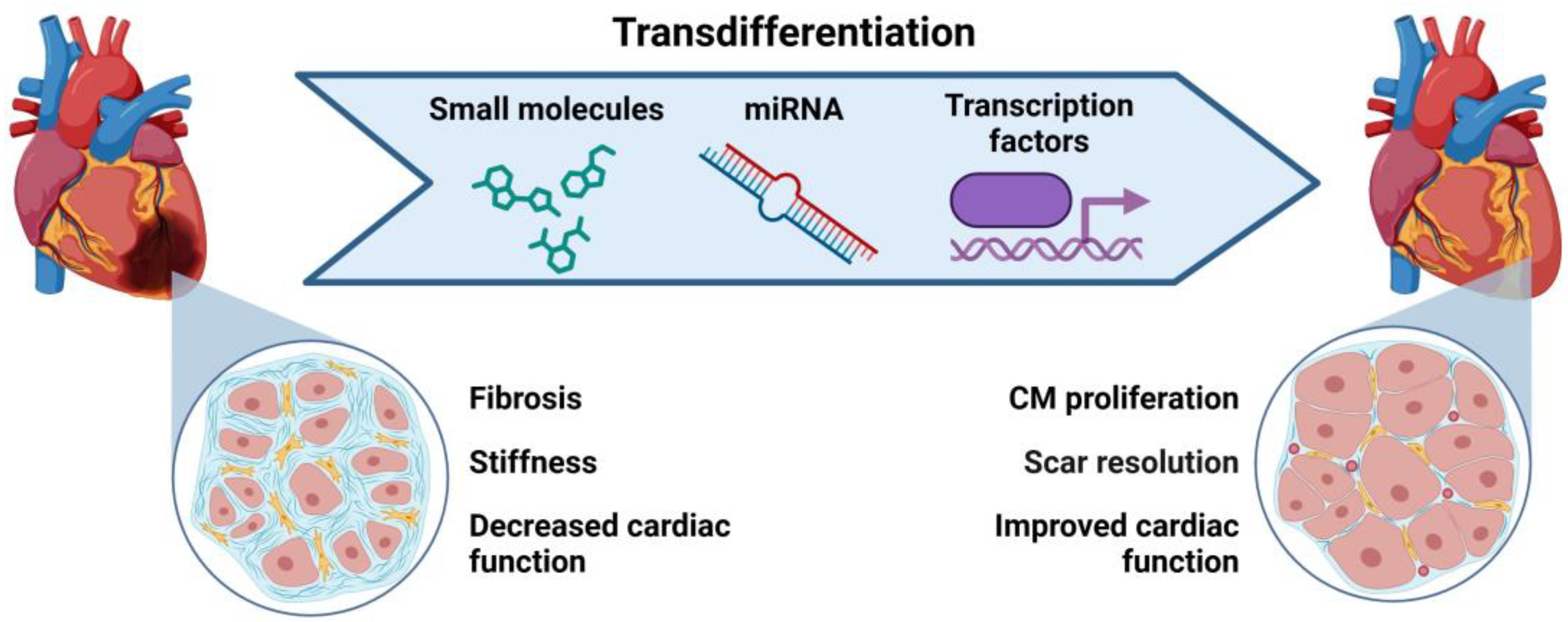
4.1. Transdifferentiation to Generate Cardiac Cells
Small Molecules in Cardiac Transdifferentiation
| Pathway/Function | Compound | References |
|---|---|---|
| TGF-β signaling inhibitor | SB431542 | [68,101] |
| RepSox | [91] | |
| A83-01 (TGF-type I receptor) | [105] | |
| OAC2 | [105] | |
| Wnt signaling activator | CHIR99021 | [68,91,105] |
| XAV939 | [68] | |
| MAPK/ERK signaling inhibitor | PD0325901 | [91] |
| Forskolin | [91,101] | |
| SC1 | [105] | |
| Rho-associated kinase (ROCK) pathway inhibitor | Y-27632 | [105] |
| IGF1/PI3K/Akt1 signaling pathway activator | FGF10 | [119] |
| VEGF | [119] | |
| SU16F (platelet-derived growth factor receptor (PDGFR) inhibitor) | [105] | |
| JNJ10198409 | [105] | |
| Leukemia inhibitory factor | LIF | [91] |
| RA receptor agonist | TTNPB | [91] |
| DNA methylation inhibitor | AS8351 | [105] |
| Histone deacetylation inhibitor | VPA | [91,100] |
| Parnate | [91] | |
| Histone methylation modulator | BIX-01294 | [68,105] |
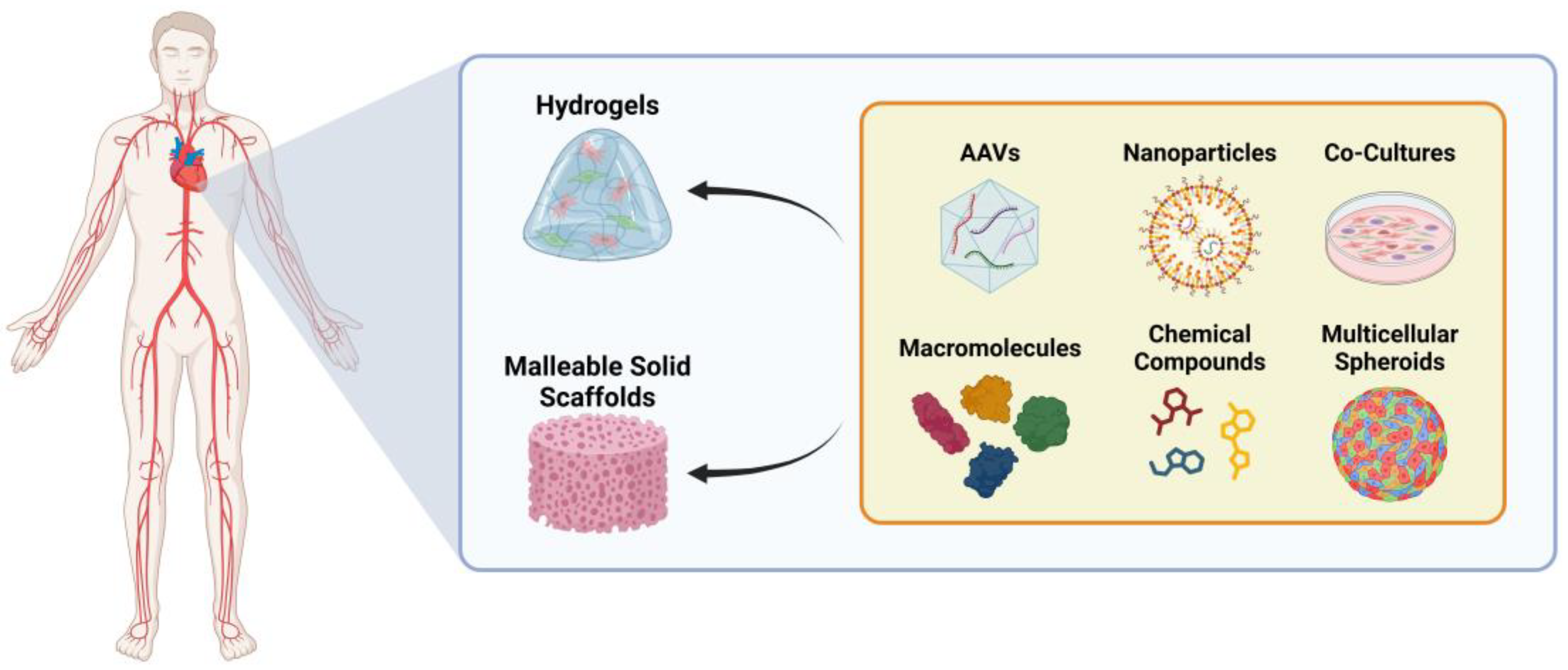
4.2. Delivery Methods for Reprogramming Factors
4.3. Challenges and Future Directions in Transdifferentiation
4.4. Dedifferentiation for Cardiac Repair
5. In Vivo Cardiac Repair: From Reprogramming Techniques to Heart Transplantation
6. Ethics and Equity in the Access to Cell Therapies for Heart Disease
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization (WHO). Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-%28cvds%29 (accessed on 3 April 2024).
- Thygesen, K.; Alpert, J.S.; White, H.D. Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2007, 50, 2173–2195. [Google Scholar] [PubMed]
- Jopling, C.; Boue, S.; Belmonte, J.C.I. Dedifferentiation, Transdifferentiation and Reprogramming: Three Routes to Regeneration. Nat. Rev. Mol. Cell Biol. 2011, 12, 79–89. [Google Scholar] [CrossRef]
- Bergmann, O.; Bhardwaj, R.D.; Bernard, S.; Zdunek, S.; Barnabé-Heide, F.; Walsh, S.; Zupicich, J.; Alkass, K.; Buchholz, B.A.; Druid, H.; et al. Evidence for Cardiomyocyte Renewal in Humans. Science 2009, 324, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, K.; Zhang, Y.; Seinfeld, J.; Galang, G.; Tseliou, E.; Cheng, K.; Sun, B.; Aminzadeh, M.; Marbán, E. Cardiomyocyte Proliferation and Progenitor Cell Recruitment Underlie Therapeutic Regeneration after Myocardial Infarction in the Adult Mouse Heart. EMBO Mol. Med. 2013, 5, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.D.; Ferreira, A.; Fernandes, M.T.; Silva, B.M.; Esteves, F.; Leitão, H.S.; Bragança, J.; Calado, S.M. Human Stem Cells for Cardiac Disease Modeling and Preclinical and Clinical Applications—Are We on the Road to Success? Cells 2023, 12, 1727. [Google Scholar] [CrossRef]
- Mehta, A.S.; Singh, A. Insights into Regeneration Tool Box: An Animal Model Approach. Dev. Biol. 2019, 453, 111–129. [Google Scholar]
- Wang, J.; An, M.; Haubner, B.J.; Penninger, J.M. Cardiac Regeneration: Options for Repairing the Injured Heart. Front. Cardiovasc. Med. 2023, 9, 981982. [Google Scholar]
- Johnson, J.; Mohsin, S.; Houser, S.R. Cardiomyocyte Proliferation as a Source of New Myocyte Development in the Adult Heart. Int. J. Mol. Sci. 2021, 22, 7764. [Google Scholar] [CrossRef]
- Huh, S.; Song, H.R.; Jeong, G.R.; Jang, H.; Seo, N.H.; Lee, J.H.; Yi, J.Y.; Lee, B.; Choi, H.W.; Do, J.T.; et al. Suppression of the Erk–Srf Axis Facilitates Somatic Cell Reprogramming. Exp. Mol. Med. 2018, 50, e448. [Google Scholar] [CrossRef]
- Kong, Y.P.; Carrion, B.; Singh, R.K.; Putnam, A.J. Matrix Identity and Tractional Forces Influence Indirect Cardiac Reprogramming. Sci. Rep. 2013, 3, 3474. [Google Scholar] [CrossRef]
- Tani, H.; Sadahiro, T.; Yamada, Y.; Isomi, M.; Yamakawa, H.; Fujita, R.; Abe, Y.; Akiyama, T.; Nakano, K.; Kuze, Y.; et al. Direct Reprogramming Improves Cardiac Function and Reverses Fibrosis in Chronic Myocardial Infarction. Circulation 2023, 147, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.-N.; Burke, Z.D.; Tosh, D. Transdifferentiation, Metaplasia and Tissue Regeneration. Organogenesis 2004, 1, 36–44. [Google Scholar] [PubMed]
- Zhao, M.T.; Ye, S.; Su, J.; Garg, V. Cardiomyocyte Proliferation and Maturation: Two Sides of the Same Coin for Heart Regeneration. Front. Cell Dev. Biol. 2020, 8, 594226. [Google Scholar]
- Lam, N.T.; Sadek, H.A. Neonatal Heart Regeneration Comprehensive Literature Review. Circulation 2018, 138, 421–423. [Google Scholar]
- Payumo, A.Y.; Chen, X.; Hirose, K.; Chen, X.; Hoang, A.; Khyeam, S.; Yu, H.; Wang, J.; Chen, Q.; Powers, N.; et al. Adrenergic-Thyroid Hormone Interactions Drive Postnatal Thermogenesis and Loss of Mammalian Heart Regenerative Capacity. Circulation 2021, 144, 1000–1003. [Google Scholar] [CrossRef] [PubMed]
- Chingale, M.; Zhu, D.; Cheng, K.; Huang, K. Bioengineering Technologies for Cardiac Regenerative Medicine. Front. Bioeng. Biotechnol. 2021, 9, 681705. [Google Scholar]
- Dispersyn, G.D.; Mesotten, L.; Meuris, B.; Maes, A.; Mortelmans, L.; Flameng, W.; Ramaekers, F.; Borgers, M. Dissociation of Cardiomyocyte Apoptosis and Dedifferentiation in Infarct Border Zones. Eur. Heart J. 2002, 23, 849–857. [Google Scholar] [CrossRef]
- Nguyen, P.D.; de Bakker, D.E.M.; Bakkers, J. Cardiac Regenerative Capacity: An Evolutionary Afterthought? Cell. Mol. Life Sci. 2021, 78, 5107–5122. [Google Scholar] [CrossRef] [PubMed]
- Sharov, V.G.; Sabbah, H.N.; Ali, A.S.; Shimoyama, H.; Lesch, M.; Goldstein, S. Abnormalities of Cardiocytes in Regions Bordering Fibrous Scars of Dogs with Heart Failure. Int. J. Cardiol. 1997, 60, 273–279. [Google Scholar]
- Marín-Juez, R.; Marass, M.; Gauvrit, S.; Rossi, A.; Lai, S.L.; Materna, S.C.; Black, B.L.; Stainier, D.Y.R. Fast Revascularization of the Injured Area Is Essential to Support Zebrafish Heart Regeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 11237–11242. [Google Scholar] [CrossRef]
- Lai, S.-L.; Marín-Juez, R.; Luís Moura, P.; Kuenne, C.; Kuan Han Lai, J.; Taddese Tsedeke, A.; Guenther, S.; Looso, M.; Stainier, D.Y. Reciprocal Analyses in Zebrafish and Medaka Reveal That Harnessing the Immune Response Promotes Cardiac Regeneration. eLife 2017, 6, e25605. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Immune System and the Remodeling Infarcted Heart: Cell Biological Insights and Therapeutic Opportunities. J. Cardiovasc. Pharmacol. 2014, 63, 185–195. [Google Scholar] [PubMed]
- Venugopal, H.; Hanna, A.; Humeres, C.; Frangogiannis, N.G. Properties and Functions of Fibroblasts and Myofibroblasts in Myocardial Infarction. Cells 2022, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Shults, N.V.; Suzuki, Y.J.; Shults, N.V.; Suzuki, Y.J. Evidence for the Role of Cell Reprogramming in Naturally Occurring Cardiac Repair. In Muscle Cell and Tissue—Novel Molecular Targets and Current Advances; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Saparov, A.; Ogay, V.; Nurgozhin, T.; Chen, W.C.W.; Mansurov, N.; Issabekova, A.; Zhakupova, J. Role of the Immune System in Cardiac Tissue Damage and Repair Following Myocardial Infarction. Inflamm. Res. 2017, 66, 739–751. [Google Scholar] [CrossRef]
- Cao, J.; Poss, K.D. The Epicardium as a Hub for Heart Regeneration. Nat. Rev. Cardiol. 2018, 15, 631–647. [Google Scholar]
- Liu, G.; David, B.T.; Trawczynski, M.; Fessler, R.G. Advances in Pluripotent Stem Cells: History, Mechanisms, Technologies, and Applications. Stem Cell Rev. Rep. 2020, 16, 3–32. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Feyen, D.A.M.; McKeithan, W.L.; Bruyneel, A.A.N.; Spiering, S.; Hörmann, L.; Ulmer, B.; Zhang, H.; Briganti, F.; Schweizer, M.; Hegyi, B.; et al. Metabolic Maturation Media Improve Physiological Function of Human IPSC-Derived Cardiomyocytes. Cell Rep. 2020, 32, 107925. [Google Scholar] [CrossRef]
- Correia, C.; Koshkin, A.; Duarte, P.; Hu, D.; Teixeira, A.; Domian, I.; Serra, M.; Alves, P.M. Distinct Carbon Sources Affect Structural and Functional Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Sci. Rep. 2017, 7, 8590. [Google Scholar] [CrossRef]
- Kim, C. Disease Modeling and Cell Based Therapy with IPSC: Future Therapeutic Option with Fast and Safe Application. Blood Res. 2014, 49, 7–14. [Google Scholar]
- Attar, A.; Hosseinpour, A.; Hosseinpour, H.; Kazemi, A. Major Cardiovascular Events after Bone Marrow Mononuclear Cell Transplantation Following Acute Myocardial Infarction: An Updated Post-BAMI Meta-Analysis of Randomized Controlled Trials. BMC Cardiovasc. Disord. 2022, 22, 259. [Google Scholar] [CrossRef] [PubMed]
- Martin-Puig, S.; Wang, Z.; Chien, K.R. Lives of a Heart Cell: Tracing the Origins of Cardiac Progenitors. Cell Stem Cell 2008, 2, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Morrison, S.J.; Scadden, D.T. The Bone Marrow Niche for Haematopoietic Stem Cells. Nature 2014, 505, 327–334. [Google Scholar] [PubMed]
- Mahmud, S.; Alam, S.; Emon, N.U.; Boby, U.H.; Kamruzzaman; Ahmed, F.; Monjur-Al-Hossain, A.S.M.; Tahamina, A.; Rudra, S.; Ajrin, M. Opportunities and Challenges in Stem Cell Therapy in Cardiovascular Diseases: Position Standing in 2022. Saudi Pharm. J. 2022, 30, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ip, J.E.; Huang, J.; Zhang, L.; Matsushita, K.; Liew, C.C.; Pratt, R.E.; Dzau, V.J. Essential Role of ICAM-1/CD18 in Mediating EPC Recruitment, Angiogenesis, and Repair to the Infarcted Myocardium. Circ. Res. 2006, 99, 315–322. [Google Scholar] [CrossRef]
- Razeghian-Jahromi, I.; Matta, A.G.; Canitrot, R.; Zibaeenezhad, M.J.; Razmkhah, M.; Safari, A.; Nader, V.; Roncalli, J. Surfing the Clinical Trials of Mesenchymal Stem Cell Therapy in Ischemic Cardiomyopathy. Stem Cell Res. Ther. 2021, 12, 361. [Google Scholar]
- Wang, X.; Hu, Q.; Nakamura, Y.; Lee, J.; Zhang, G.E.; From, A.H.L.; Zhang, J. The Role of the Sca-1/CD31 Cardiac Progenitor Cell Population in Postinfarction Left Ventricular Remodeling. Stem Cells 2006, 24, 1779–1788. [Google Scholar] [CrossRef]
- Rawat, N.; Singh, M.K. Induced Pluripotent Stem Cell: A Headway in Reprogramming with Promising Approach in Regenerative Biology. Vet. World 2017, 10, 640–649. [Google Scholar]
- Xie, Y.; Liu, J.; Qian, L. Direct Cardiac Reprogramming Comes of Age: Recent Advance and Remaining Challenges. Semin. Cell Dev. Biol. 2022, 122, 37–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, N.; Huang, Y.; Spencer, C.I.; Fu, J.D.; Yu, C.; Liu, K.; Nie, B.; Xu, T.; Li, K.; et al. Expandable Cardiovascular Progenitor Cells Reprogrammed from Fibroblasts. Cell Stem Cell 2016, 18, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Lassar, A.B.; Paterson, B.M.; Weintraub, H. Transfection of a DNA Locus That Mediates the Conversion of LOTV2 Fibroblasts to Myoblasts. Cell 1986, 47, 649–656. [Google Scholar] [CrossRef]
- Davis, R.L.; Weintraub, H.; Lassar, A.B. Expression of a Single Transfected CDNA Converts Fibmblasts to Myoblasts. Cell 1987, 51, 987–1000. [Google Scholar] [CrossRef]
- Ebrahimi, B. Cardiac Progenitor Reprogramming for Heart Regeneration. Cell Regen. 2018, 7, 1–6. [Google Scholar] [CrossRef]
- Boyle, A.J.; Schulman, S.P.; Hare, J.M. Stem Cell Therapy for Cardiac Repair: Ready for the next Step. Circulation 2006, 114, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.; Deyett, A.; Ilmer, T.; Haendeler, S.; Torres Caballero, A.; Novatchkova, M.; Netzer, M.A.; Ceci Ginistrelli, L.; Mancheno Juncosa, E.; Bhattacharya, T.; et al. Multi-Chamber Cardioids Unravel Human Heart Development and Cardiac Defects. Cell 2023, 186, 5587–5605.e27. [Google Scholar] [CrossRef] [PubMed]
- Christoffels, V.; Jensen, B. Cardiac Morphogenesis: Specification of the Four-Chambered Heart. Cold Spring Harb. Perspect. Biol. 2020, 12, a037143. [Google Scholar] [CrossRef]
- Bruneau, B.G. Signaling and Transcriptional Networks in Heart Development and Regeneration. Cold Spring Harb. Perspect. Biol. 2013, 5, a008292. [Google Scholar] [CrossRef]
- Wiesinger, A.; Boink, G.J.J.; Christoffels, V.M.; Devalla, H.D. Retinoic Acid Signaling in Heart Development: Application in the Differentiation of Cardiovascular Lineages from Human Pluripotent Stem Cells. Stem Cell Rep. 2021, 16, 2589–2606. [Google Scholar] [CrossRef]
- Inácio, J.M.; Nunes, M.M.; Almeida, M.; Cristo, F.; Anjos, R.; Belo, J.A. Gene-Edited Human-Induced Pluripotent Stem Cell Lines to Elucidate DAND5 Function throughout Cardiac Differentiation. Cells 2023, 12, 520. [Google Scholar] [CrossRef]
- He, X.; Liang, J.; Paul, C.; Huang, W.; Dutta, S.; Wang, Y. Advances in Cellular Reprogramming-Based Approaches for Heart Regenerative Repair. Cells 2022, 11, 3914. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Zhang, Y.; Buikema, J.W.; Serpooshan, V.; Chirikian, O.; Kosaric, N.; Churko, J.M.; Dzilic, E.; Shieh, A.; Burridge, P.W.; et al. Stage-Specific Effects of Bioactive Lipids on Human IPSC Cardiac Differentiation and Cardiomyocyte Proliferation. Sci. Rep. 2018, 8, 6618. [Google Scholar] [CrossRef]
- Cristo, F.; Inácio, J.M.; de Almeida, S.; Mendes, P.; Martins, D.S.; Maio, J.; Anjos, R.; Belo, J.A. Functional Study of DAND5 Variant in Patients with Congenital Heart Disease and Laterality Defects. BMC Med. Genet. 2017, 18, 77. [Google Scholar] [CrossRef]
- Feric, N.T.; Radisic, M. Maturing Human Pluripotent Stem Cell-Derived Cardiomyocytes in Human Engineered Cardiac Tissues. Adv. Drug Deliv. Rev. 2016, 96, 110–134. [Google Scholar] [PubMed]
- Scesa, G.; Adami, R.; Bottai, D. IPSC Preparation and Epigenetic Memory: Does the Tissue Origin Matter? Cells 2021, 10, 1470. [Google Scholar] [CrossRef]
- Poetsch, M.S.; Strano, A.; Guan, K. Human Induced Pluripotent Stem Cells: From Cell Origin, Genomic Stability, and Epigenetic Memory to Translational Medicine. Stem Cells 2022, 40, 546–555. [Google Scholar]
- Gładych, M.; Andrzejewska, A.; Oleksiewicz, U.; Estécio, M.R.H. Epigenetic Mechanisms of Induced Pluripotency. Wspolczesna Onkol. 2015, 1A, A30–A38. [Google Scholar]
- Van Den Hurk, M.; Kenis, G.; Bardy, C.; Van Den Hove, D.L.; Gage, F.H.; Steinbusch, H.W.; Rutten, B.P. Transcriptional and Epigenetic Mechanisms of Cellular Reprogramming to Induced Pluripotency. Epigenomics 2016, 8, 1131–1149. [Google Scholar] [CrossRef]
- Altomare, C.; Pianezzi, E.; Cervio, E.; Bolis, S.; Biemmi, V.; Benzoni, P.; Camici, G.G.; Moccetti, T.; Barile, L.; Vassalli, G. Human-Induced Pluripotent Stem Cell-Derived Cardiomyocytes from Cardiac Progenitor Cells: Effects of Selective Ion Channel Blockade. Europace 2016, 18, iv67–iv76. [Google Scholar] [CrossRef]
- Pianezzi, E.; Altomare, C.; Bolis, S.; Balbi, C.; Torre, T.; Rinaldi, A.; Camici, G.G.; Barile, L.; Vassalli, G. Role of Somatic Cell Sources in the Maturation Degree of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Biochim. Biophys. Acta Mol. Cell Res. 2020, 1867, 118538. [Google Scholar] [CrossRef]
- Gähwiler, E.K.N.; Motta, S.E.; Martin, M.; Nugraha, B.; Hoerstrup, S.P.; Emmert, M.Y. Human IPSCs and Genome Editing Technologies for Precision Cardiovascular Tissue Engineering. Front. Cell Dev. Biol. 2021, 9, 639699. [Google Scholar] [CrossRef]
- Liu, Z.; Zhou, J.; Wang, H.; Zhao, M.; Wang, C. Current Status of Induced Pluripotent Stem Cells in Cardiac Tissue Regeneration and Engineering. Regen. Med. Res. 2013, 1, 6. [Google Scholar] [CrossRef] [PubMed]
- Grath, A.; Dai, G. Direct Cell Reprogramming for Tissue Engineering and Regenerative Medicine. J. Biol. Eng. 2019, 13, 14. [Google Scholar] [CrossRef]
- Jugdutt, B.I. Ventricular Remodeling after Infarction and the Extracellular Collagen Matrix: When Is Enough Enough? Circulation 2003, 108, 1395–1403. [Google Scholar] [CrossRef] [PubMed]
- Sadahiro, T.; Ieda, M. Direct Cardiac Reprogramming for Cardiovascular Regeneration and Differentiation. Keio J. Med. 2020, 69, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Ifkovits, J.L.; Addis, R.C.; Epstein, J.A.; Gearhart, J.D. Inhibition of TGFβ Signaling Increases Direct Conversion of Fibroblasts to Induced Cardiomyocytes. PLoS ONE 2014, 9, e89678. [Google Scholar] [CrossRef]
- Zhao, Y.; Londono, P.; Cao, Y.; Sharpe, E.J.; Proenza, C.; O’Rourke, R.; Jones, K.L.; Jeong, M.Y.; Walker, L.A.; Buttrick, P.M.; et al. High-Efficiency Reprogramming of Fibroblasts into Cardiomyocytes Requires Suppression of Pro-Fibrotic Signalling. Nat. Commun. 2015, 6, 8243. [Google Scholar] [CrossRef]
- Mosimann, C.; Hausmann, G.; Basler, K. β-Catenin Hits Chromatin: Regulation of Wnt Target Gene Activation. Nat. Rev. Mol. Cell Biol. 2009, 10, 276–286. [Google Scholar] [CrossRef]
- Li, X.-H.; Li, Q.; Jiang, L.; Deng, C.; Liu, Z.; Fu, Y.; Zhang, M.; Tan, H.; Feng, Y.; Shan, Z.; et al. Generation of Functional Human Cardiac Progenitor Cells by High-Efficiency Protein Transduction. Stem Cells Transl. Med. 2015, 4, 1415–1424. [Google Scholar] [CrossRef]
- Wang, L.; Liu, Z.; Yin, C.; Asfour, H.; Chen, O.; Li, Y.; Bursac, N.; Liu, J.; Qian, L. Stoichiometry of Gata4, Mef2c, and Tbx5 Influences the Efficiency and Quality of Induced Cardiac Myocyte Reprogramming. Circ. Res. 2015, 116, 237–244. [Google Scholar] [CrossRef]
- Jayawardena, T.M.; Finch, E.A.; Zhang, L.; Zhang, H.; Hodgkinson, C.P.; Pratt, R.E.; Rosenberg, P.B.; Mirotsou, M.; Dzau, V.J. MicroRNA Induced Cardiac Reprogramming in Vivo Evidence for Mature Cardiac Myocytes and Improved Cardiac Function. Circ. Res. 2014, 116, 418–424. [Google Scholar] [CrossRef]
- Jayawardena, T.M.; Egemnazarov, B.; Finch, E.A.; Zhang, L.; Payne, J.A.; Pandya, K.; Zhang, Z.; Rosenberg, P.; Mirotsou, M.; Dzau, V.J. Cellular Biology MicroRNA-Mediated In Vitro and In Vivo Direct Reprogramming of Cardiac Fibroblasts to Cardiomyocytes. Circ. Res. 2012, 110, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, Y.; Liu, J.; Qian, L. Direct Cell Reprogramming: Approaches, Mechanisms and Progress. Nat. Rev. Mol. Cell Biol. 2021, 22, 410–424. [Google Scholar] [PubMed]
- López-Muneta, L.; Miranda-Arrubla, J.; Carvajal-Vergara, X. The Future of Direct Cardiac Reprogramming: Any Gmt Cocktail Variety? Int. J. Mol. Sci. 2020, 21, 7950. [Google Scholar] [CrossRef]
- Ieda, M.; Fu, J.D.; Delgado-Olguin, P.; Vedantham, V.; Hayashi, Y.; Bruneau, B.G.; Srivastava, D. Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes by Defined Factors. Cell 2010, 142, 375–386. [Google Scholar] [CrossRef]
- Srivastava, D. Reprogramming Approaches to Cardiovascular Disease: From Developmental Biology to Regenerative Medicine. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology; Springer: Tokyo, Japan, 2016; pp. 3–10. ISBN 9784431546283. [Google Scholar]
- Liu, Z.; Wang, L.; Welch, J.D.; Ma, H.; Zhou, Y.; Vaseghi, H.R.; Yu, S.; Wall, J.B.; Alimohamadi, S.; Zheng, M.; et al. Single-Cell Transcriptomics Reconstructs Fate Conversion from Fibroblast to Cardiomyocyte. Nature 2017, 551, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Lalit, P.A.; Salick, M.R.; Nelson, D.O.; Squirrell, J.M.; Shafer, C.M.; Patel, N.G.; Saeed, I.; Schmuck, E.G.; Markandeya, Y.S.; Wong, R.; et al. Lineage Reprogramming of Fibroblasts into Proliferative Induced Cardiac Progenitor Cells by Defined Factors. Cell Stem Cell 2016, 18, 354–367. [Google Scholar] [CrossRef]
- Song, K.; Nam, Y.J.; Luo, X.; Qi, X.; Tan, W.; Huang, G.N.; Acharya, A.; Smith, C.L.; Tallquist, M.D.; Neilson, E.G.; et al. Heart Repair by Reprogramming Non-Myocytes with Cardiac Transcription Factors. Nature 2012, 485, 599–604. [Google Scholar] [CrossRef]
- Christoforou, N.; Chellappan, M.; Adler, A.F.; Kirkton, R.D.; Wu, T.; Addis, R.C.; Bursac, N.; Leong, K.W. Transcription Factors MYOCD, SRF, Mesp1 and SMARCD3 Enhance the Cardio-Inducing Effect of GATA4, TBX5, and MEF2C during Direct Cellular Reprogramming. PLoS ONE 2013, 8, e63577. [Google Scholar] [CrossRef]
- Sharma, A.; Zhang, Y.; Wu, S.M. Harnessing the Induction of Cardiomyocyte Proliferation for Cardiac Regenerative Medicine. Curr. Treat. Options Cardiovasc. Med. 2015, 17, 404. [Google Scholar]
- Zhao, H.; Zhang, Y.; Xu, X.; Sun, Q.; Yang, C.; Wang, H.; Yang, J.; Yang, Y.; Yang, X.; Liu, Y.; et al. Sall4 and Myocd Empower Direct Cardiac Reprogramming from Adult Cardiac Fibroblasts After Injury. Front. Cell Dev. Biol. 2021, 9, 608367. [Google Scholar] [CrossRef]
- Mollinari, C.; Zhao, J.; Lupacchini, L.; Garaci, E.; Merlo, D.; Pei, G. Transdifferentiation: A New Promise for Neurodegenerative Diseases. Cell Death Dis. 2018, 9, 830. [Google Scholar]
- Ebert, A.D.; Diecke, S.; Chen, I.Y.; Wu, J.C. Reprogramming and Transdifferentiation for Cardiovascular Development and Regenerative Medicine: Where Do We Stand? EMBO Mol. Med. 2015, 7, 1090–1103. [Google Scholar] [CrossRef]
- Ginis, I.; Luo, Y.; Miura, T.; Thies, S.; Brandenberger, R.; Gerecht-Nir, S.; Amit, M.; Hoke, A.; Carpenter, M.K.; Itskovitz-Eldor, J.; et al. Differences between Human and Mouse Embryonic Stem Cells. Dev. Biol. 2004, 269, 360–380. [Google Scholar] [CrossRef] [PubMed]
- Christoforou, N.; Chakraborty, S.; Kirkton, R.D.; Adler, A.F.; Addis, R.C.; Leong, K.W. Core Transcription Factors, MicroRNAs, and Small Molecules Drive Transdifferentiation of Human Fibroblasts Towards the Cardiac Cell Lineage. Sci. Rep. 2017, 7, 40285. [Google Scholar] [CrossRef]
- Fu, J.D.; Stone, N.R.; Liu, L.; Spencer, C.I.; Qian, L.; Hayashi, Y.; Delgado-Olguin, P.; Ding, S.; Bruneau, B.G.; Srivastava, D. Direct Reprogramming of Human Fibroblasts Toward a Cardiomyocyte-Like State. Stem Cell Rep. 2013, 1, 235–247. [Google Scholar] [CrossRef]
- Wada, R.; Muraoka, N.; Inagawa, K.; Yamakawa, H.; Miyamoto, K.; Sadahiro, T.; Umei, T.; Kaneda, R.; Suzuki, T.; Kamiya, K.; et al. Induction of Human Cardiomyocyte-Like Cells from Fibroblasts by Defined Factors. Proc. Natl. Acad. Sci. USA 2013, 110, 12667–12672. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, C.; Xu, X.; Gu, H.; Ye, Y.; Jiang, C.; Qiu, Z.; Xie, X. Direct Reprogramming of Mouse Fibroblasts into Cardiomyocytes with Chemical Cocktails. Cell Res. 2015, 25, 1013–1024. [Google Scholar] [CrossRef]
- Aalikhani, M.; Alikhani, M.; Khajeniazi, S.; Khosravi, A.; Bazi, Z.; Kianmehr, A. Positive Effect of MiR-2392 on Fibroblast to Cardiomyocyte-like Cell Fate Transition: An In Silico and In Vitro Study. Gene 2023, 879, 147598. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, Z.; Welch, J.D.; Gao, X.; Wang, L.; Garbutt, T.; Keepers, B.; Ma, H.; Prins, J.F.; Shen, W.; et al. Single-Cell Transcriptomic Analyses of Cell Fate Transitions During Human Cardiac Reprogramming. Cell Stem Cell 2019, 25, 149–164.e9. [Google Scholar] [CrossRef]
- Garbutt, T.A.; Zhou, Y.; Keepers, B.; Liu, J.; Qian, L. An Optimized Protocol for Human Direct Cardiac Reprogramming. Star Protoc. 2020, 1, 100010. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.J.; Song, K.; Luo, X.; Daniel, E.; Lambeth, K.; West, K.; Hill, J.A.; Di Maio, J.M.; Baker, L.A.; Bassel-Duby, R.; et al. Reprogramming of Human Fibroblasts toward a Cardiac Fate. Proc. Natl. Acad. Sci. USA 2013, 110, 5588–5593. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Liang, J.; Huang, W.; Ma, J.; Park, K.H.; Wu, Z.; Chen, P.; Zhu, H.; Ma, J.J.; Cai, W.; et al. CRISPR Activation of Endogenous Genes Reprograms Fibroblasts into Cardiovascular Progenitor Cells for Myocardial Infarction Therapy. Mol. Ther. 2022, 30, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, X.; Zhao, L.; Zuo, S.; Chen, X.; Zhang, L.; Lin, Z.; Zhao, X.; Qin, Y.; Zhou, X.; et al. Lineage Reprogramming of Fibroblasts into Induced Cardiac Progenitor Cells by CRISPR/Cas9-Based Transcriptional Activators. Acta Pharm. Sin. B 2020, 10, 313–326. [Google Scholar] [CrossRef]
- Soufi, A.; Donahue, G.; Zaret, K.S. Facilitators and Impediments of the Pluripotency Reprogramming Factors’ Initial Engagement with the Genome. Cell 2012, 151, 994–1004. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, L.; Vaseghi, H.R.; Liu, Z.; Lu, R.; Alimohamadi, S.; Yin, C.; Fu, J.D.; Wang, G.G.; Liu, J.; et al. Bmi1 Is a Key Epigenetic Barrier to Direct Cardiac Reprogramming. Cell Stem Cell 2016, 18, 382–395. [Google Scholar] [CrossRef] [PubMed]
- Morez, C.; Noseda, M.; Paiva, M.A.; Belian, E.; Schneider, M.D.; Stevens, M.M. Enhanced Efficiency of Genetic Programming toward Cardiomyocyte Creation through Topographical Cues. Biomaterials 2015, 70, 94–104. [Google Scholar] [CrossRef]
- Bektik, E.; Sun, Y.; Dennis, A.T.; Sakon, P.; Yang, D.; Deschênes, I.; Fu, J.D. Inhibition of Creb-Cbp Signaling Improves Fibroblast Plasticity for Direct Cardiac Reprogramming. Cells 2021, 10, 1572. [Google Scholar] [CrossRef]
- Bektik, E.; Fu, J.D. Ameliorating the Fibrotic Remodeling of the Heart through Direct Cardiac Reprogramming. Cells 2019, 8, 679. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Xing, J.; Zhou, J.; Li, H. Chemical Transdifferentiation of Somatic Cells: Unleashing the Power of Small Molecules. Biomedicines 2023, 11, 2913. [Google Scholar] [CrossRef]
- Cathomen, T.; Schambach, A. Zinc Positive: Tailored Genome Engineering Meets Reprogramming. Gene Ther. 2010, 17, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Huang, Y.; Zheng, J.; Spencer, I.; Zhang, Y.; Fu, J.-D.; Nie, B.; Xie, M.; Zhang, M.; Wang, H.; et al. Conversion of human Fibroblasts into Functional Cardiomyocytes by Small Molecules. Science 2016, 352, 1213–1216. [Google Scholar] [CrossRef]
- Qin, H.; Zhao, A.; Fu, X. Small Molecules for Reprogramming and Transdifferentiation. Cell. Mol. Life Sci. 2017, 74, 3553–3575. [Google Scholar] [PubMed]
- Shafi, O.; Zahra, K.; Shah, H.H. Dysregulations in Cardiogenic Mechanisms by TGF-Beta and Angiotensin II in Cardiac Remodeling Post-Ischemic Injury: A Systematic Review. medRxiv 2024. [Google Scholar]
- Tan, F.; Qian, C.; Tang, K.; Abd-Allah, S.M.; Jing, N. Inhibition of Transforming Growth Factor β (TGF-β) Signaling Can Substitute for Oct4 Protein in Reprogramming and Maintain Pluripotency. J. Biol. Chem. 2015, 290, 4500–4511. [Google Scholar] [CrossRef]
- Okuyama, T.; Yamagishi, R.; Shimada, J.; Ikeda, M.; Maruoka, Y.; Kaneko, H. Structural and Mechanistic Insights into Nuclear Transport and Delivery of the Critical Pluripotency Factor Oct4 to DNA. J. Biomol. Struct. Dyn. 2018, 36, 767–778. [Google Scholar] [CrossRef]
- Du, J.; Wu, Y.; Ai, Z.; Shi, X.; Chen, L.; Guo, Z. Mechanism of SB431542 in Inhibiting Mouse Embryonic Stem Cell Differentiation. Cell Signal 2014, 26, 2107–2116. [Google Scholar] [CrossRef]
- Zhu, S.; Li, W.; Zhou, H.; Wei, W.; Ambasudhan, R.; Lin, T.; Kim, J.; Zhang, K.; Ding, S. Reprogramming of Human Primary Somatic Cells by OCT4 and Chemical Compounds. Cell Stem Cell 2010, 7, 651–655. [Google Scholar]
- Ichida, J.K.; Blanchard, J.; Lam, K.; Son, E.Y.; Chung, J.E.; Egli, D.; Loh, K.M.; Carter, A.C.; Di Giorgio, F.P.; Koszka, K.; et al. A Small-Molecule Inhibitor of Tgf-β Signaling Replaces Sox2 in Reprogramming by Inducing Nanog. Cell Stem Cell 2009, 5, 491–503. [Google Scholar] [CrossRef]
- Wang, H.; Hao, J.; Hong, C.C. Cardiac Induction of Embryonic Stem Cells by a Small Molecule Inhibitor of Wnt/β-Catenin Signaling. Acs Chem. Biol. 2011, 6, 192–197. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Y.; Li, C.; Ai, K.; Li, K.; Li, H.; Yang, J. The Evolutionarily Conserved MAPK/Erk Signaling Promotes Ancestral T-Cell Immunity in Fish via c-Myc-Mediated Glycolysis. J. Biol. Chem. 2020, 295, 3000–3016. [Google Scholar] [CrossRef] [PubMed]
- Bain, J.; Plater, L.; Elliott, M.; Shpiro, N.; Hastie, C.J.; Mclauchlan, H.; Klevernic, I.; Arthur, J.S.C.; Alessi, D.R.; Cohen, P. The Selectivity of Protein Kinase Inhibitors: A Further Update. Biochem. J. 2007, 408, 297–315. [Google Scholar] [CrossRef]
- Barrett, S.D.; Bridges, A.J.; Dudley, D.T.; Saltiel, A.R.; Fergus, J.H.; Flamme, C.M.; Delaney, A.M.; Kaufman, M.; LePage, S.; Leopold, W.R.; et al. The Discovery of the Benzhydroxamate MEK Inhibitors CI-1040 and PD 0325901. Bioorg Med. Chem. Lett. 2008, 18, 6501–6504. [Google Scholar] [CrossRef]
- Chen, S.; Tae Do, J.; Zhang, Q.; Yao, S.; Yan, F.; Peters, E.C.; Schö ler, H.R.; Schultz, P.G.; Ding, S. Self-Renewal of Embryonic Stem Cells by a Small Molecule. Proc. Natl. Acad. Sci. USA 2006, 103, 17266–17271. [Google Scholar] [CrossRef]
- Zhou, H.; Dickson, M.E.; Kim, M.S.; Bassel-Duby, R.; Olson, E.N. Akt1/Protein Kinase B Enhances Transcriptional Reprogramming of Fibroblasts to Functional Cardiomyocytes. Proc. Natl. Acad. Sci. USA 2015, 112, 11864–11869. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Muraoka, N.; Miyamoto, K.; Sadahiro, T.; Isomi, M.; Haginiwa, S.; Kojima, H.; Umei, T.; Akiyama, M.; Kuishi, Y.; et al. Fibroblast Growth Factors and Vascular Endothelial Growth Factor Promote Cardiac Reprogramming Under Defined Conditions. Stem Cell Rep. 2015, 5, 1128–1142. [Google Scholar] [CrossRef] [PubMed]
- Abad, M.; Hashimoto, H.; Zhou, H.; Morales, M.G.; Chen, B.; Bassel-Duby, R.; Olson, E.N. Notch Inhibition Enhances Cardiac Reprogramming by Increasing MEF2C Transcriptional Activity. Stem Cell Rep. 2017, 8, 548–560. [Google Scholar] [CrossRef]
- Kanda, M.; Nagai, T.; Takahashi, T.; Liu, M.L.; Kondou, N.; Naito, A.T.; Akazawa, H.; Sashida, G.; Iwama, A.; Komuro, I.; et al. Leukemia Inhibitory Factor Enhances Endogenous Cardiomyocyte Regeneration after Myocardial Infarction. PLoS ONE 2016, 11, e0156562. [Google Scholar] [CrossRef]
- Drowley, L.; McPheat, J.; Nordqvist, A.; Peel, S.; Karlsson, U.; Martinsson, S.; Müllers, E.; Dellsén, A.; Knight, S.; Barrett, I.; et al. Discovery of Retinoic Acid Receptor Agonists as Proliferators of Cardiac Progenitor Cells through a Phenotypic Screening Approach. Stem Cells Transl. Med. 2020, 9, 47–60. [Google Scholar] [CrossRef]
- Wobus, A.M.; Rohwedel, J.; Maltsev, V.; Hescheler, J.; Hescheler, J. In Vitro Differentiation of Embryonic Stem Cells into Cardiomyocytes or Skeletal Muscle Cells Is Specifically Modulated by Retinoic Acid. Roux’s Arch. Dev. Biol. 1994, 204, 36–45. [Google Scholar] [CrossRef]
- Bilbija, D.; Elmabsout, A.A.; Sagave, J.; Haugen, F.; Bastani, N.; Dahl, C.P.; Gullestad, L.; Sirsjö, A.; Blomhoff, R.; Valen, G. Expression of Retinoic Acid Target Genes in Coronary Artery Disease. Int. J. Mol. Med. 2014, 33, 677–686. [Google Scholar] [CrossRef]
- Zawada, D.; Kornherr, J.; Meier, A.B.; Santamaria, G.; Dorn, T.; Nowak-Imialek, M.; Ortmann, D.; Zhang, F.; Lachmann, M.; Dreßen, M.; et al. Retinoic Acid Signaling Modulation Guides In Vitro Specification of Human Heart Field-Specific Progenitor Pools. Nat. Commun. 2023, 14, 1722. [Google Scholar] [CrossRef]
- Huang, C.; Tu, W.; Fu, Y.; Wang, J.; Xie, X. Chemical-Induced Cardiac Reprogramming In Vivo. Cell Res. 2018, 28, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Kubicek, S.; O’Sullivan, R.J.; August, E.M.; Hickey, E.R.; Zhang, Q.; Teodoro, M.L.L.; Rea, S.; Mechtler, K.; Kowalski, J.A.; Homon, C.A.; et al. Reversal of H3K9me2 by a Small-Molecule Inhibitor for the G9a Histone Methyltransferase. Mol. Cell 2007, 25, 473–481. [Google Scholar] [CrossRef]
- Lawlor, L.; Yang, X.B. Harnessing the HDAC–Histone Deacetylase Enzymes, Inhibitors and How These Can Be Utilised in Tissue Engineering. Int. J. Oral Sci. 2019, 11, 20. [Google Scholar]
- Li, W.; Zhou, H.Y.; Abujarour, R.; Zhu, S.; Joo, J.Y.; Lin, T.; Hao, E.; Schöler, H.R.; Hayek, A.; Ding, S. Generation of Human-Induced Pluripotent Stem Cells in the Absence of Exogenous Sox2. Stem Cells 2009, 27, 2992–3000. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, K.; Akiyama, M.; Tamura, F.; Isomi, M.; Yamakawa, H.; Sadahiro, T.; Muraoka, N.; Kojima, H.; Haginiwa, S.; Kurotsu, S.; et al. Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction. Cell Stem Cell 2018, 22, 91–103.e5. [Google Scholar] [CrossRef]
- Wolfram, J.A.; Donahue, J.K. Gene Therapy to Treat Cardiovascular Disease. J. Am. Heart Assoc. 2013, 2, e000119. [Google Scholar] [PubMed]
- Gao, G.; Bish, L.T.; Sleeper, M.M.; Mu, X.; Sun, L.; Lou, Y.; Duan, J.; Hu, C.; Wang, L.; Sweeney, H.L. Transendocardial Delivery of AAV6 Results in Highly Efficient and Global Cardiac Gene Transfer in Rhesus Macaques. Hum. Gene Ther. 2011, 22, 979–984. [Google Scholar] [CrossRef]
- Bish, L.T.; Sleeper, M.M.; Brainard, B.; Cole, S.; Russell, N.; Withnall, E.; Arndt, J.; Reynolds, C.; Davison, E.; Sanmiguel, J.; et al. Percutaneous Transendocardial Delivery of Self-Complementary Adeno-Associated Virus 6 Achieves Global Cardiac Gene Transfer in Canines. Mol. Ther. 2008, 16, 1953–1959. [Google Scholar] [CrossRef]
- Inagaki, K.; Fuess, S.; Storm, T.A.; Gibson, G.A.; Mctiernan, C.F.; Kay, M.A.; Nakai, H. Robust Systemic Transduction with AAV9 Vectors in Mice: Efficient Global Cardiac Gene Transfer Superior to That of AAV8. Mol. Ther. 2006, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.Y.; Jeong, S.N.; Kang, J.I.; Lee, S.W. Chimeric Adeno-Associated Virus-Mediated Cardiovascular Reprogramming for Ischemic Heart Disease. Acs Omega 2018, 3, 5918–5925. [Google Scholar] [CrossRef]
- Chamberlain, K.; Riyad, J.M.; Weber, T. Cardiac Gene Therapy with Adeno-Associated Virus-Based Vectors. Curr. Opin. Cardiol. 2017, 32, 275–282. [Google Scholar]
- Yamada, K.P.; Tharakan, S.; Ishikawa, K. Consideration of Clinical Translation of Cardiac AAV Gene Therapy. Cell Gene Ther. Insights 2020, 6, 609–615. [Google Scholar] [CrossRef]
- Yang, L.; Xue, S.; Du, M.; Lian, F. Highly Efficient MicroRNA Delivery Using Functionalized Carbon Dots for Enhanced Conversion of Fibroblasts to Cardiomyocytes. Int. J. Nanomed. 2021, 16, 3741–3754. [Google Scholar] [CrossRef]
- Kim, H.; Song, B.-W.; Park, S.-J.; Choi, S.W.; Moon, H.; Hwang, K.-C.; Kang, S.-W.; Moon, S.-H.; Yang, Y.; Chan Kwon, I.; et al. Ultraefficient Extracellular Vesicle-Guided Direct Reprogramming of Fibroblasts into Functional Cardiomyocytes. Sci. Adv. 2022, 8, eabj6621. [Google Scholar] [PubMed]
- Chang, Y.; Lee, E.; Kim, J.; Kwon, Y.W.; Kwon, Y.; Kim, J. Efficient In Vivo Direct Conversion of Fibroblasts into Cardiomyocytes Using a Nanoparticle-Based Gene Carrier. Biomaterials 2019, 192, 500–509. [Google Scholar] [CrossRef]
- Feldman, K.S.; Zahid, M. In Vivo Imaging of Transduction Efficiencies of Cardiac Targeting Peptide. J. Vis. Exp. 2020, 2020, 1–13. [Google Scholar] [CrossRef]
- Wang, Q.; Song, Y.; Chen, J.; Li, Q.; Gao, J.; Tan, H.; Zhu, Y.; Wang, Z.; Li, M.; Yang, H.; et al. Direct In Vivo Reprogramming with Non-Viral Sequential Targeting Nanoparticles Promotes Cardiac Regeneration. Biomaterials 2021, 276, 121028. [Google Scholar] [CrossRef]
- Guan, Y.; Niu, H.; Wen, J.; Dang, Y.; Zayed, M.; Guan, J. Rescuing Cardiac Cells and Improving Cardiac Function by Targeted Delivery of Oxygen-Releasing Nanoparticles After or Even Before Acute Myocardial Infarction. Acs Nano 2022, 16, 19551–19566. [Google Scholar] [CrossRef]
- Dvir, T.; Bauer, M.; Schroeder, A.; Tsui, J.H.; Anderson, D.G.; Langer, R.; Liao, R.; Kohane, D.S. Nanoparticles Targeting the Infarcted Heart. Nano Lett. 2011, 11, 4411–4414. [Google Scholar] [CrossRef]
- Omidian, H.; Babanejad, N.; Cubeddu, L.X. Nanosystems in Cardiovascular Medicine: Advancements, Applications, and Future Perspectives. Pharmaceutics 2023, 15, 1935. [Google Scholar] [CrossRef] [PubMed]
- Saludas, L.; Oliveira, C.C.; Roncal, C.; Ruiz-Villalba, A.; Prósper, F.; Garbayo, E.; Blanco-Prieto, M.J. Extracellular Vesicle-Based Therapeutics for Heart Repair. Nanomaterials 2021, 11, 570. [Google Scholar] [CrossRef]
- Sun, H.; Wang, X.; Pratt, R.E.; Dzau, V.J.; Hodgkinson, C.P. C166 EVs Potentiate MiR Cardiac Reprogramming via MiR-148a-3p. J. Mol. Cell. Cardiol. 2024, 190, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Lazaropoulos, M.P.; Elrod, J.W. Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ. Res. 2020, 127, 427–447. [Google Scholar] [PubMed]
- Pikir, B.S.; Andrianto, A.; Suryawan, I.G.R.; Hermawan, H.O.; Kartikasari, D.P.; Harsoyo, P.M. MicroRNA-1 Induces Transdifferentiation of Peripheral Blood CD34+ Cells into Cardiomyocytes-Like Cells. Indones. Biomed. J. 2022, 14, 269–275. [Google Scholar] [CrossRef]
- Paul, A.; Hasan, A.; Al Kindi, H.; Gaharwar, A.K.; Rao, V.T.S.; Nikkhah, M.; Shin, S.R.; Krafft, D.; Dokmeci, M.R.; Shum-Tim, D.; et al. Injectable Graphene Oxide/Hydrogel-Based Angiogenic Gene Delivery System for Vasculogenesis and Cardiac Repair. Acs Nano 2014, 8, 8050–8062. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C. Dedifferentiation: Inspiration for Devising Engineering Strategies for Regenerative Medicine. NPJ Regen. Med. 2020, 5, 14. [Google Scholar]
- Porrello, E.R.; Mahmoud, A.I.; Simpson, E.; Johnson, B.A.; Grinsfelder, D.; Canseco, D.; Mammen, P.P.; Rothermel, B.A.; Olson, E.N.; Sadek, H.A. Regulation of Neonatal and Adult Mammalian Heart Regeneration by the MiR-15 Family. Proc. Natl. Acad. Sci. USA 2013, 110, 187–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Gago-Lopez, N.; Li, N.; Zhang, Z.; Alver, N.; Liu, Y.; Martinson, A.M.; Mehri, A.; MacLellan, W.R. Single-Cell Imaging and Transcriptomic Analyses of Endogenous Cardiomyocyte Dedifferentiation and Cycling. Cell Discov. 2019, 5, 30. [Google Scholar] [CrossRef]
- Wang, C.; Liu, W.; Shen, Y.; Chen, J.; Zhu, H.; Yang, X.; Jiang, X.; Wang, Y.; Zhou, J. Cardiomyocyte Dedifferentiation and Remodeling in 3D Scaffolds to Generate the Cellular Diversity of Engineering Cardiac Tissues. Biomater. Sci. 2019, 7, 4636–4650. [Google Scholar] [CrossRef] [PubMed]
- Taegtmeyer, H.; Sen, S.; Vela, D. Return to the Fetal Gene Program: A Suggested Metabolic Link to Gene Expression in the Heart. In Proceedings of the Annals of the New York Academy of Sciences; Blackwell Publishing Inc.: Hoboken, NJ, USA, 2010; Volume 1188, pp. 191–198. [Google Scholar]
- Guo, Q.Y.; Yang, J.Q.; Feng, X.X.; Zhou, Y.J. Regeneration of the Heart: From Molecular Mechanisms to Clinical Therapeutics. Mil. Med. Res. 2023, 10, 18. [Google Scholar] [CrossRef]
- Zhang, D.; Ning, J.; Ramprasath, T.; Yu, C.; Zheng, X.; Song, P.; Xie, Z.; Zou, M.H. Kynurenine Promotes Neonatal Heart Regeneration by Stimulating Cardiomyocyte Proliferation and Cardiac Angiogenesis. Nat. Commun. 2022, 13, 6371. [Google Scholar] [CrossRef]
- Ikeda, S.; Mizushima, W.; Sciarretta, S.; Abdellatif, M.; Zhai, P.; Mukai, R.; Fefelova, N.; Oka, S.I.; Nakamura, M.; Del Re, D.P.; et al. Hippo Deficiency Leads to Cardiac Dysfunction Accompanied by Cardiomyocyte Dedifferentiation During Pressure Overload. Circ. Res. 2019, 124, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Pöling, J.; Gajawada, P.; Lörchner, H.; Polyakova, V.; Szibor, M.; Böttger, T.; Warnecke, H.; Kubin, T.; Braun, T. The Janus Face of OSM-Mediated Cardiomyocyte Dedifferentiation during Cardiac Repair and Disease. Cell Cycle 2012, 11, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Kubin, T.; Pöling, J.; Kostin, S.; Gajawada, P.; Hein, S.; Rees, W.; Wietelmann, A.; Tanaka, M.; Lörchner, H.; Schimanski, S.; et al. Oncostatin M Is a Major Mediator of Cardiomyocyte Dedifferentiation and Remodeling. Cell Stem Cell 2011, 9, 420–432. [Google Scholar] [CrossRef]
- Tanaka, E.M.; Gann, A.A.F.; Gates, P.B.; Brockes, J.P. Newt Myotubes Reenter the Cell Cycle by Phosphorylation of the Retinoblastoma Protein. J. Cell Biol. 1997, 136, 155–165. [Google Scholar] [CrossRef]
- Valussi, M.; Besser, J.; Wystub-Lis, K.; Zukunft, S.; Richter, M.; Kubin, T.; Boettger, T.; Braun, T. Repression of Osmr and Fgfr1 by MiR-1/133a Prevents Cardiomyocyte Dedifferentiation and Cell Cycle Entry in the Adult Heart. Sci. Adv. 2021, 7, eabi6648. [Google Scholar] [CrossRef]
- Beauchemin, M.; Smith, A.; Yin, V.P. Dynamic Microrna-101a and Fosab Expression Controls Zebrafish Heart Regeneration. Development 2015, 142, 4029–4037. [Google Scholar] [CrossRef]
- Yin, V.P.; Lepilina, A.; Smith, A.; Poss, K.D. Regulation of Zebrafish Heart Regeneration by MiR-133. Dev. Biol. 2012, 365, 319–327. [Google Scholar] [CrossRef]
- Heallen, T.; Morikawa, Y.; Leach, J.; Tao, G.; Willerson, J.T.; Johnson, R.L.; Martin, J.F. Hippo Signaling Impedes Adult Heart Regeneration. Development 2013, 140, 4683–4690. [Google Scholar] [CrossRef] [PubMed]
- Flinn, M.A.; Jeffery, B.E.; O’Meara, C.C.; Link, B.A. Yap Is Required for Scar Formation but Not Myocyte Proliferation During Heart Regeneration in Zebrafish. Cardiovasc. Res. 2019, 115, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Heallen, T.; Zhang, M.; Wang, J.; Bonilla-Claudio, M.; Klysik, E.; Johnson, R.L.; Martin, J.F. Hippo Pathway Inhibits Wnt Signaling to Restrain Cardiomyocyte Proliferation and Heart Size. Science 2011, 332, 458–461. [Google Scholar] [CrossRef] [PubMed]
- Neininger, A.C.; Long, J.H.; Baillargeon, S.M.; Burnette, D.T. A Simple and Flexible High-Throughput Method for the Study of Cardiomyocyte Proliferation. Sci. Rep. 2019, 9, 15917. [Google Scholar] [CrossRef]
- Von Gise, A.; Lin, Z.; Schlegelmilch, K.; Honor, L.B.; Pan, G.M.; Buck, J.N.; Ma, Q.; Ishiwata, T.; Zhou, B.; Camargo, F.D.; et al. YAP1, the Nuclear Target of Hippo Signaling, Stimulates Heart Growth Through Cardiomyocyte Proliferation but Not Hypertrophy. Proc. Natl. Acad. Sci. USA 2012, 109, 2394–2399. [Google Scholar] [CrossRef]
- Del Re, D.P. Hippo-Yap Signaling in Cardiac and Fibrotic Remodeling. Curr. Opin. Physiol. 2022, 26, 100492. [Google Scholar] [PubMed]
- Liu, R.; Jagannathan, R.; Li, F.; Lee, J.; Balasubramanyam, N.; Kim, B.S.; Yang, P.; Yechoor, V.K.; Moulik, M. Tead1 Is Required for Perinatal Cardiomyocyte Proliferation. PLoS ONE 2019, 14, e0212017. [Google Scholar] [CrossRef]
- Wang, X.; Ha, T.; Liu, L.; Hu, Y.; Kao, R.; Kalbfleisch, J.; Williams, D.; Li, C. TLR3 Mediates Repair and Regeneration of Damaged Neonatal Heart through Glycolysis Dependent YAP1 Regulated MiR-152 Expression. Cell Death Differ. 2018, 25, 966–982. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Inácio, J.M.; Sousa, I.; Guimarães, A.R.; Franco, D.; Moura, G.; Belo, J.A. MiRNAs in Heart Development and Disease. Int. J. Mol. Sci. 2024, 25, 1673. [Google Scholar] [CrossRef]
- Eulalio, A.; Mano, M.; Ferro, M.D.; Zentilin, L.; Sinagra, G.; Zacchigna, S.; Giacca, M. Functional Screening Identifies MiRNAs Inducing Cardiac Regeneration. Nature 2012, 492, 376–381. [Google Scholar] [CrossRef]
- Chen, J.; Huang, Z.P.; Seok, H.Y.; Ding, J.; Kataoka, M.; Zhang, Z.; Hu, X.; Wang, G.; Lin, Z.; Wang, S.; et al. Mir-17-92 Cluster Is Required for and Sufficient to Induce Cardiomyocyte Proliferation in Postnatal and Adult Hearts. Circ. Res. 2013, 112, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Lesizza, P.; Prosdocimo, G.; Martinelli, V.; Sinagra, G.; Zacchigna, S.; Giacca, M. Single-Dose Intracardiac Injection of Pro-Regenerative MicroRNAs Improves Cardiac Function After Myocardial Infarction. Circ. Res. 2017, 120, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, Y.; Wang, T.; Zhou, N.; Kong, J.; Chen, L.; Snitow, M.; Morley, M.; Li, D.; Petrenko, N.; et al. A MicroRNA-Hippo Pathway That Promotes Cardiomyocyte Proliferation and Cardiac Regeneration in Mice. Sci. Transl. Med. 2015, 7, 279ra38. [Google Scholar] [CrossRef]
- Torrini, C.; Cubero, R.J.; Dirkx, E.; Braga, L.; Ali, H.; Prosdocimo, G.; Gutierrez, M.I.; Collesi, C.; Licastro, D.; Zentilin, L.; et al. Common Regulatory Pathways Mediate Activity of MicroRNAs Inducing Cardiomyocyte Proliferation. Cell Rep. 2019, 27, 2759–2771.e5. [Google Scholar] [CrossRef] [PubMed]
- Gabisonia, K.; Prosdocimo, G.; Aquaro, G.D.; Carlucci, L.; Zentilin, L.; Secco, I.; Ali, H.; Braga, L.; Gorgodze, N.; Bernini, F.; et al. MicroRNA Therapy Stimulates Uncontrolled Cardiac Repair After Myocardial Infarction in Pigs. Nature 2019, 569, 418–422. [Google Scholar] [CrossRef]
- Fan, Y.; Ho, B.X.; Pang, J.K.S.; Pek, N.M.Q.; Hor, J.H.; Ng, S.Y.; Soh, B.S. Wnt/β-Catenin-Mediated Signaling Re-Activates Proliferation of Matured Cardiomyocytes. Stem Cell Res. Ther. 2018, 9, 338. [Google Scholar] [CrossRef]
- Zhao, L.; Borikova, A.L.; Ben-Yair, R.; Guner-Ataman, B.; MacRae, C.A.; Lee, R.T.; Geoffrey Burns, C.; Burns, C.E. Notch Signaling Regulates Cardiomyocyte Proliferation during Zebrafish Heart Regeneration. Proc. Natl. Acad. Sci. USA 2014, 111, 1403–1408. [Google Scholar] [CrossRef]
- Beigi, F.; Schmeckpeper, J.; Pow-Anpongkul, P.; Payne, J.A.; Zhang, L.; Zhang, Z.; Huang, J.; Mirotsou, M.; Dzau, V.J. C3orf58, a Novel Paracrine Protein, Stimulates Cardiomyocyte Cell-Cycle Progression through the PI3K-AKT-CDK7 Pathway. Circ. Res. 2013, 113, 372–380. [Google Scholar] [CrossRef]
- Yue, Z.; Chen, J.; Lian, H.; Pei, J.; Li, Y.; Chen, X.; Song, S.; Xia, J.; Zhou, B.; Feng, J.; et al. PDGFR-β Signaling Regulates Cardiomyocyte Proliferation and Myocardial Regeneration. Cell Rep. 2019, 28, 966–978.e4. [Google Scholar] [CrossRef]
- Gemberling, M.; Karra, R.; Dickson, A.L.; Poss, K.D. Nrg1 Is an Injury-Induced Cardiomyocyte Mitogen for the Endogenous Heart Regeneration Program in Zebrafish. eLife 2015, 4, e05871. [Google Scholar] [CrossRef]
- Bersell, K.; Arab, S.; Haring, B.; Kühn, B. Neuregulin1/ErbB4 Signaling Induces Cardiomyocyte Proliferation and Repair of Heart Injury. Cell 2009, 138, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Raya, A.; Koth, C.M.; Bü scher, D.; Kawakami, Y.; Itoh, T.; Marina Raya, R.; Sternik, G.; Tsai, H.-J.; Rodríguez-Esteban, C.; Carlos Izpisú a-Belmonte, J. Activation of Notch Signaling Pathway Precedes Heart Regeneration in Zebrafish´Angel. Proc. Natl. Acad. Sci. USA 2003, 100, 11889–11895. [Google Scholar] [PubMed]
- Cui, M.; Atmanli, A.; Morales, M.G.; Tan, W.; Chen, K.; Xiao, X.; Xu, L.; Liu, N.; Bassel-Duby, R.; Olson, E.N. Nrf1 Promotes Heart Regeneration and Repair by Regulating Proteostasis and Redox Balance. Nat. Commun. 2021, 12, 5270. [Google Scholar] [CrossRef]
- Doppler, S.A.; Deutsch, M.A.; Serpooshan, V.; Li, G.; Dzilic, E.; Lange, R.; Krane, M.; Wu, S.M. Mammalian Heart Regeneration. Circ. Res. 2017, 120, 630–632. [Google Scholar] [PubMed]
- D’Uva, G.; Aharonov, A.; Lauriola, M.; Kain, D.; Yahalom-Ronen, Y.; Carvalho, S.; Weisinger, K.; Bassat, E.; Rajchman, D.; Yifa, O.; et al. ERBB2 Triggers Mammalian Heart Regeneration by Promoting Cardiomyocyte Dedifferentiation and Proliferation. Nat. Cell Biol. 2015, 17, 627–638. [Google Scholar] [CrossRef]
- Zhao, L.; Ben-Yair, R.; Burns, C.E.; Burns, C.G. Endocardial Notch Signaling Promotes Cardiomyocyte Proliferation in the Regenerating Zebrafish Heart through Wnt Pathway Antagonism. Cell Rep. 2019, 26, 546–554.e5. [Google Scholar] [CrossRef]
- Karra, R.; Knecht, A.K.; Kikuchi, K.; Poss, K.D. Myocardial NF-ΚB Activation Is Essential for Zebrafish Heart Regeneration. Proc. Natl. Acad. Sci. USA 2015, 112, 13255–13260. [Google Scholar] [CrossRef]
- Wu, C.C.; Kruse, F.; Vasudevarao, M.D.; Junker, J.P.; Zebrowski, D.C.; Fischer, K.; Noël, E.S.; Grün, D.; Berezikov, E.; Engel, F.B.; et al. Spatially Resolved Genome-Wide Transcriptional Profiling Identifies BMP Signaling as Essential Regulator of Zebrafish Cardiomyocyte Regeneration. Dev. Cell 2016, 36, 36–49. [Google Scholar] [CrossRef]
- Brezitski, K.D.; Goff, A.W.; DeBenedittis, P.; Karra, R. A Roadmap to Heart Regeneration Through Conserved Mechanisms in Zebrafish and Mammals. Curr. Cardiol. Rep. 2021, 23, 29. [Google Scholar] [CrossRef]
- Gupta, V.; Gemberling, M.; Karra, R.; Rosenfeld, G.E.; Evans, T.; Poss, K.D. An Injury-Responsive Gata4 Program Shapes the Zebrafish Cardiac Ventricle. Curr. Biol. 2013, 23, 1221–1227. [Google Scholar] [CrossRef]
- Mohamed, T.M.A.; Ang, Y.S.; Radzinsky, E.; Zhou, P.; Huang, Y.; Elfenbein, A.; Foley, A.; Magnitsky, S.; Srivastava, D. Regulation of Cell Cycle to Stimulate Adult Cardiomyocyte Proliferation and Cardiac Regeneration. Cell 2018, 173, 104–116.e12. [Google Scholar] [CrossRef] [PubMed]
- Tane, S.; Kubota, M.; Okayama, H.; Ikenishi, A.; Yoshitome, S.; Iwamoto, N.; Satoh, Y.; Kusakabe, A.; Ogawa, S.; Kanai, A.; et al. Repression of Cyclin D1 Expression Is Necessary for the Maintenance of Cell Cycle Exit in Adult Mammalian Cardiomyocytes. J. Biol. Chem. 2014, 289, 18033–18044. [Google Scholar] [CrossRef] [PubMed]
- Pasumarthi, K.B.S.; Nakajima, H.; Nakajima, H.O.; Soonpaa, M.H.; Field, L.J. Targeted Expression of Cyclin D2 Results in Cardiomyocyte DNA Synthesis and Infarct Regression in Transgenic Mice. Circ. Res. 2005, 96, 110–118. [Google Scholar] [CrossRef]
- Cheng, R.K.; Asai, T.; Tang, H.; Dashoush, N.H.; Kara, R.J.; Costa, K.D.; Naka, Y.; Wu, E.X.; Wolgemuth, D.J.; Chaudhry, H.W. Cyclin A2 Induces Cardiac Regeneration after Myocardial Infarction and Prevents Heart Failure. Circ. Res. 2007, 100, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Li, X.; Zheng, H.; Si, X.; Li, B.; Wei, G.; Li, C.; Chen, Y.; Chen, Y.; Liao, W.; et al. Loss of Super-Enhancer-Regulated CircRNA Nfix Induces Cardiac Regeneration after Myocardial Infarction in Adult Mice. Circulation 2019, 139, 2857–2876. [Google Scholar] [CrossRef]
- Huang, W.; Feng, Y.; Liang, J.; Yu, H.; Wang, C.; Wang, B.; Wang, M.; Jiang, L.; Meng, W.; Cai, W.; et al. Loss of MicroRNA-128 Promotes Cardiomyocyte Proliferation and Heart Regeneration. Nat. Commun. 2018, 9, 700. [Google Scholar] [CrossRef]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The Oxygen-Rich Postnatal Environment Induces Cardiomyocyte Cell-Cycle Arrest through DNA Damage Response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef]
- Guimarães-Camboa, N.; Stowe, J.; Aneas, I.; Sakabe, N.; Cattaneo, P.; Henderson, L.; Kilberg, M.S.; Johnson, R.S.; Chen, J.; McCulloch, A.D.; et al. HIF1α Represses Cell Stress Pathways to Allow Proliferation of Hypoxic Fetal Cardiomyocytes. Dev. Cell 2015, 33, 507–521. [Google Scholar] [CrossRef]
- Kimura, W.; Xiao, F.; Canseco, D.C.; Muralidhar, S.; Thet, S.; Zhang, H.M.; Abderrahman, Y.; Chen, R.; Garcia, J.A.; Shelton, J.M.; et al. Hypoxia Fate Mapping Identifies Cycling Cardiomyocytes in the Adult Heart. Nature 2015, 523, 226–230. [Google Scholar] [CrossRef]
- White, H.D.; Chew, D.P. Acute Myocardial Infarction. Lancet 2008, 372, 570–584. [Google Scholar] [CrossRef]
- Piperata, A.; Caraffa, R.; Bifulco, O.; Avesani, M.; Apostolo, A.; Gerosa, G.; Bottio, T. Marginal Donors and Organ Shortness: Concomitant Surgical Procedures During Heart Transplantation: A Literature Review. J. Cardiovasc. Med. 2022, 23, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, D.; Liang, P.; Li, Y.; Cao, F. The Current Dilemma and Breakthrough of Stem Cell Therapy in Ischemic Heart Disease. Front. Cell Dev. Biol. 2021, 9, 636136. [Google Scholar] [CrossRef] [PubMed]
- Masumoto, H.; Nakane, T.; Tinney, J.P.; Yuan, F.; Ye, F.; Kowalski, W.J.; Minakata, K.; Sakata, R.; Yamashita, J.K.; Keller, B.B. The Myocardial Regenerative Potential of Three-Dimensional Engineered Cardiac Tissues Composed of Multiple Human IPS Cell-Derived Cardiovascular Cell Lineages. Sci. Rep. 2016, 6, 29933. [Google Scholar] [CrossRef]
- Tang, J.; Wang, J.; Huang, K.; Ye, Y.; Su, T.; Qiao, L.; Hensley, M.T.; Caranasos, T.G.; Zhang, J.; Gu, Z.; et al. Cardiac Cell-Integrated Microneedle Patch for Treating Myocardial Infarction. Sci. Adv. 2018, 4, eaat9365. [Google Scholar]
- Haake, K.; Ackermann, M.; Lachmann, N. Concise Review: Towards the Clinical Translation of Induced Pluripotent Stem Cell-Derived Blood Cells—Ready for Take-Off. Stem Cells Transl. Med. 2019, 8, 332–339. [Google Scholar]
- Daley, G.Q.; Hyun, I.; Apperley, J.F.; Barker, R.A.; Benvenisty, N.; Bredenoord, A.L.; Breuer, C.K.; Caulfield, T.; Cedars, M.I.; Frey-Vasconcells, J.; et al. Setting Global Standards for Stem Cell Research and Clinical Translation: The 2016 ISSCR Guidelines. Stem Cell Rep. 2016, 6, 787–797. [Google Scholar]
- González, F.; Boué, S.; Belmonte, J.C.I. Methods for Making Induced Pluripotent Stem Cells: Reprogramming à La Carte. Nat. Rev. Genet. 2011, 12, 231–242. [Google Scholar] [CrossRef]
- Ban, H.; Nishishita, N.; Fusaki, N.; Tabata, T.; Saeki, K.; Shikamura, M.; Takada, N.; Inoue, M.; Hasegawa, M.; Kawamata, S.; et al. Efficient Generation of Transgene-Free Human Induced Pluripotent Stem Cells (IPSCs) by Temperature-Sensitive Sendai Virus Vectors. Proc. Natl. Acad. Sci. USA 2011, 108, 14234–14239. [Google Scholar] [CrossRef]
- Fink, K.D.; Crane, A.T.; Lévêque, X.; Dues, D.J.; Huffman, L.D.; Moore, A.C.; Story, D.T.; DeJonge, R.E.; Antcliff, A.; Starski, P.A.; et al. Intrastriatal Transplantation of Adenovirus-Generated Induced Pluripotent Stem Cells for Treating Neuropathological and Functional Deficits in a Rodent Model of Huntington’s Disease. Stem Cells Transl. Med. 2014, 3, 620–631. [Google Scholar] [CrossRef]
- Center for iPS Cell Research and Application (CiRA), K.U. IPS Cell Stock for Regenerative Medicine. Available online: https://www.cira.kyoto-u.ac.jp/e/research/stock.html (accessed on 4 September 2024).
- Cyranoski, D. Japanese Man Is First to Receive “reprogrammed” Stem Cells from Another Person. Nature 2017. [Google Scholar] [CrossRef]
- Vannucci, L.; Lai, M.; Chiuppesi, F.; Ceccherini-Nelli, L.; Pistello, M. Viral Vectors: A Look Back and Ahead on Gene Transfer Technology. New Microbiol. 2013, 36, 1–22. [Google Scholar] [PubMed]
- Hacein-Bey-Abina, S.; Von Kalle, C.; Schmidt, M.; McCormack, M.P.; Wulffraat, N.; Leboulch, P.; Lim, A.; Osborne, C.S.; Pawliuk, R.; Morillon, E.; et al. LMO2-Associated Clonal T Cell Proliferation in Two Patients after Gene Therapy for SCID-X1. Science 2003, 302, 415–419. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almeida, M.; Inácio, J.M.; Vital, C.M.; Rodrigues, M.R.; Araújo, B.C.; Belo, J.A. Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair. Int. J. Mol. Sci. 2025, 26, 3063. https://doi.org/10.3390/ijms26073063
Almeida M, Inácio JM, Vital CM, Rodrigues MR, Araújo BC, Belo JA. Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair. International Journal of Molecular Sciences. 2025; 26(7):3063. https://doi.org/10.3390/ijms26073063
Chicago/Turabian StyleAlmeida, Micael, José M. Inácio, Carlos M. Vital, Madalena R. Rodrigues, Beatriz C. Araújo, and José A. Belo. 2025. "Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair" International Journal of Molecular Sciences 26, no. 7: 3063. https://doi.org/10.3390/ijms26073063
APA StyleAlmeida, M., Inácio, J. M., Vital, C. M., Rodrigues, M. R., Araújo, B. C., & Belo, J. A. (2025). Cell Reprogramming, Transdifferentiation, and Dedifferentiation Approaches for Heart Repair. International Journal of Molecular Sciences, 26(7), 3063. https://doi.org/10.3390/ijms26073063






