Structural and Functional Characteristics of TLR19 in Barbel Chub Compared to TLR19 in Grass Carp
Abstract
1. Introduction
2. Results
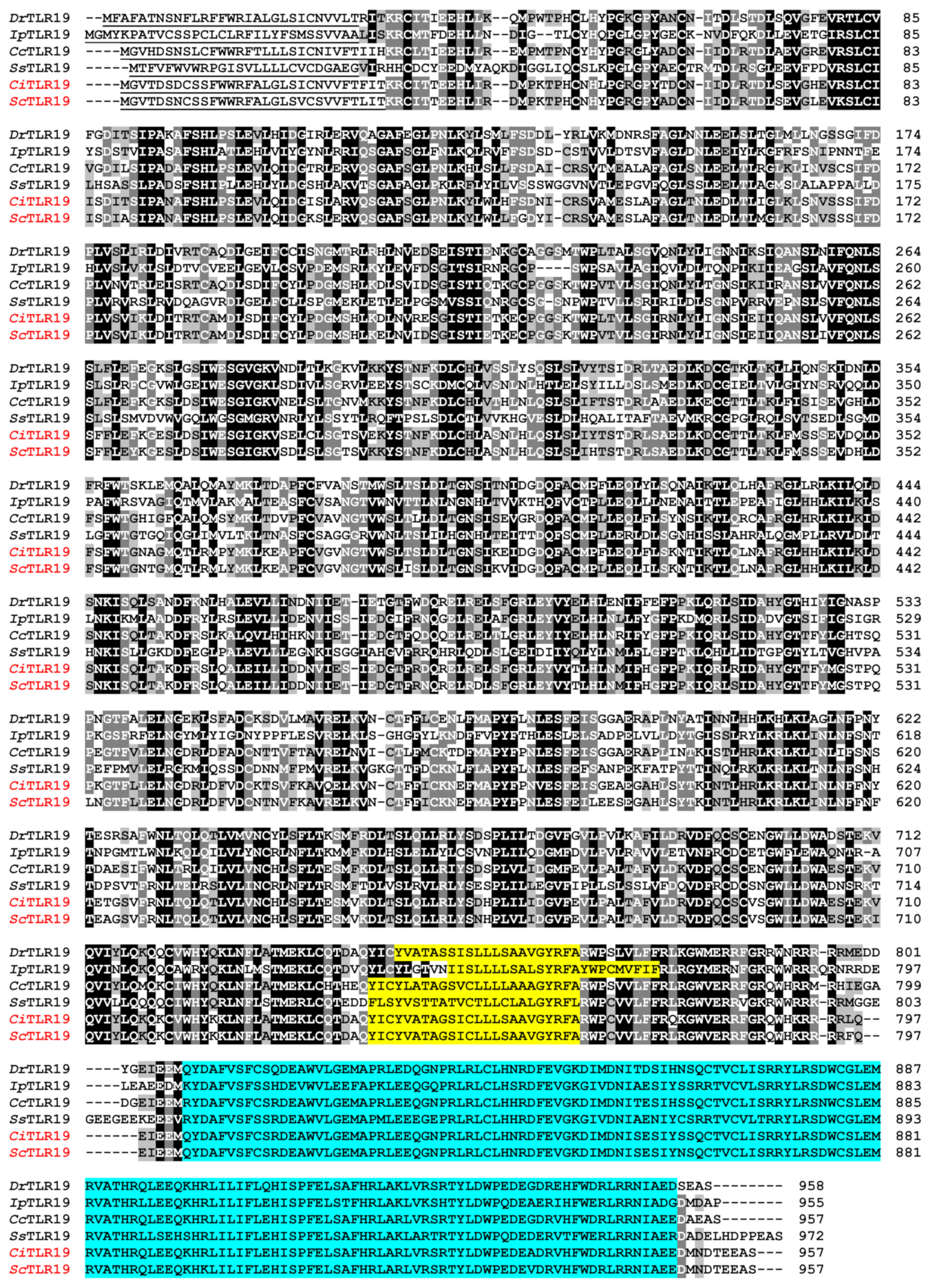
2.1. Sequence Similarity and Homology of TLR19s from Barbel Chub and Grass Carp
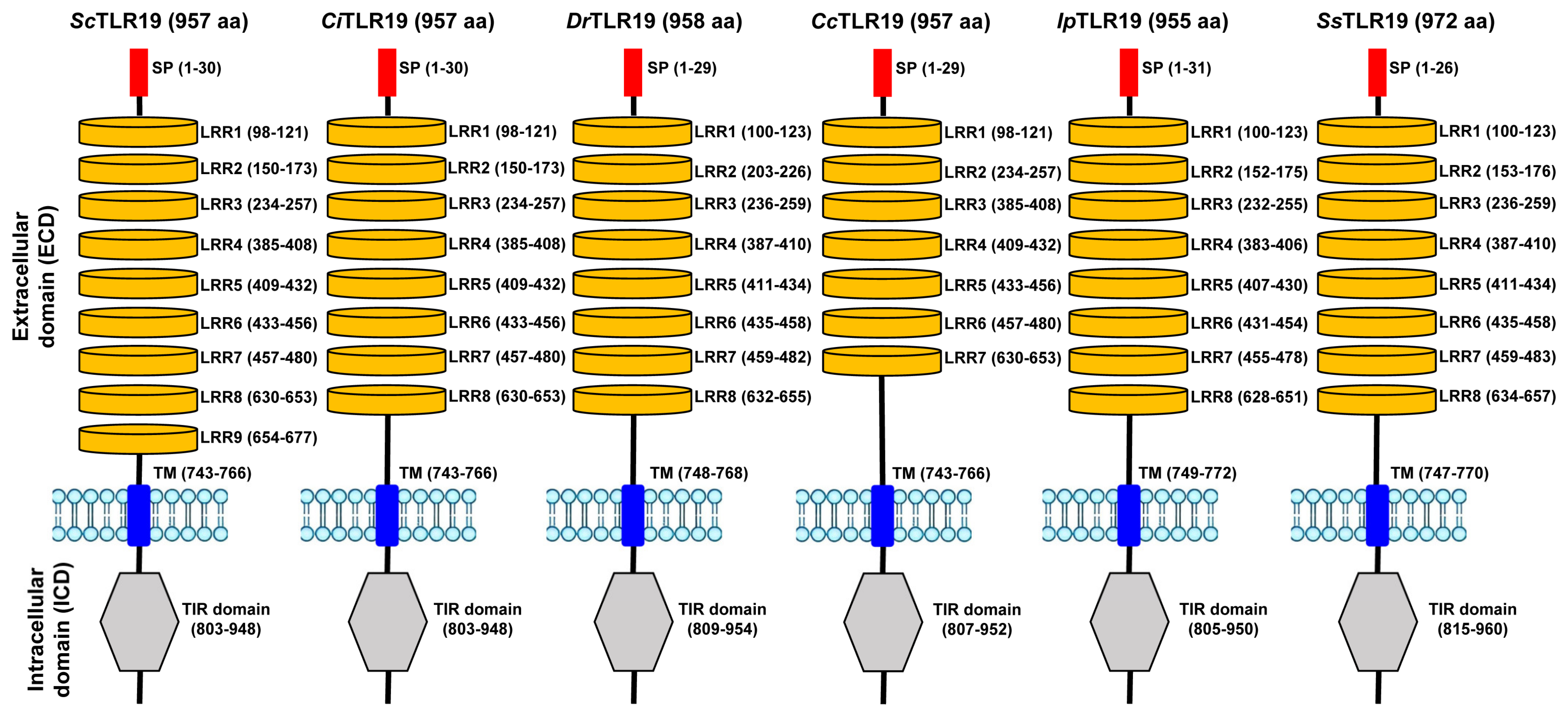
2.2. Protein Domain Composition of TLR19s from Barbel Chub and Grass Carp
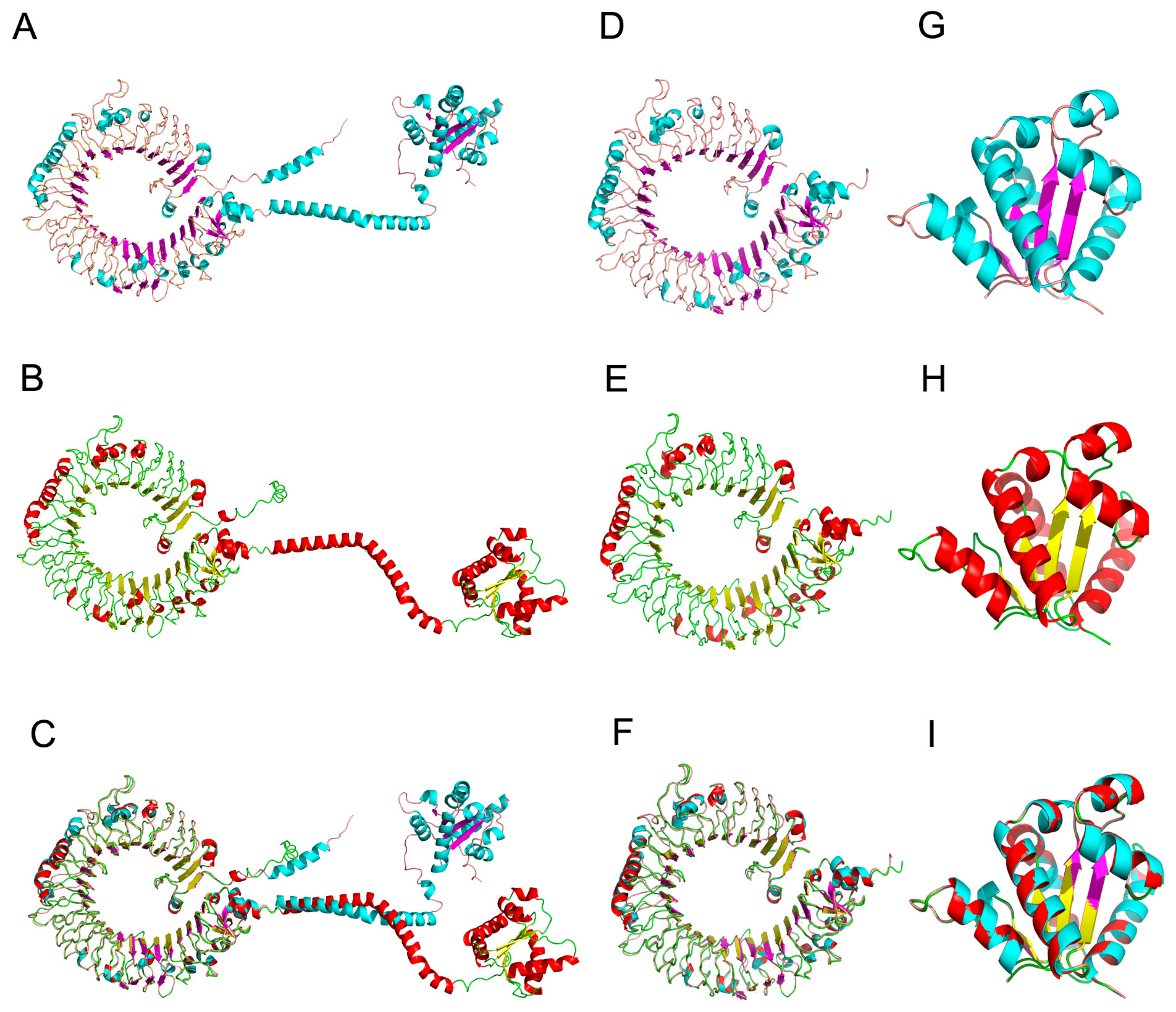
2.3. Three-Dimensional Structural Features of TLR19s from Barbel Chub and Grass Carp
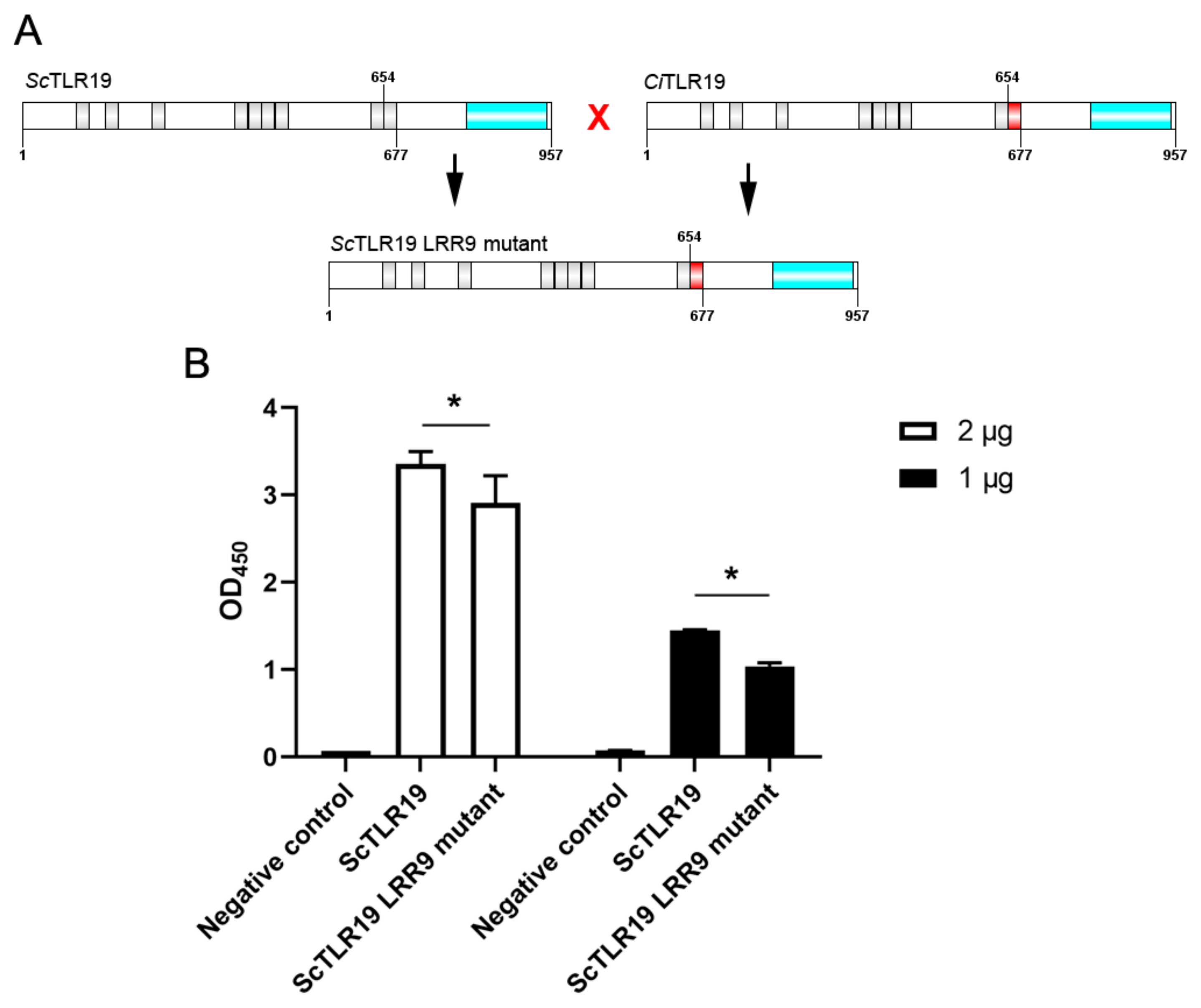
2.4. Differences in Binding Activity of TLR19 from Barbel Chub and Grass Carp to PolyI:C
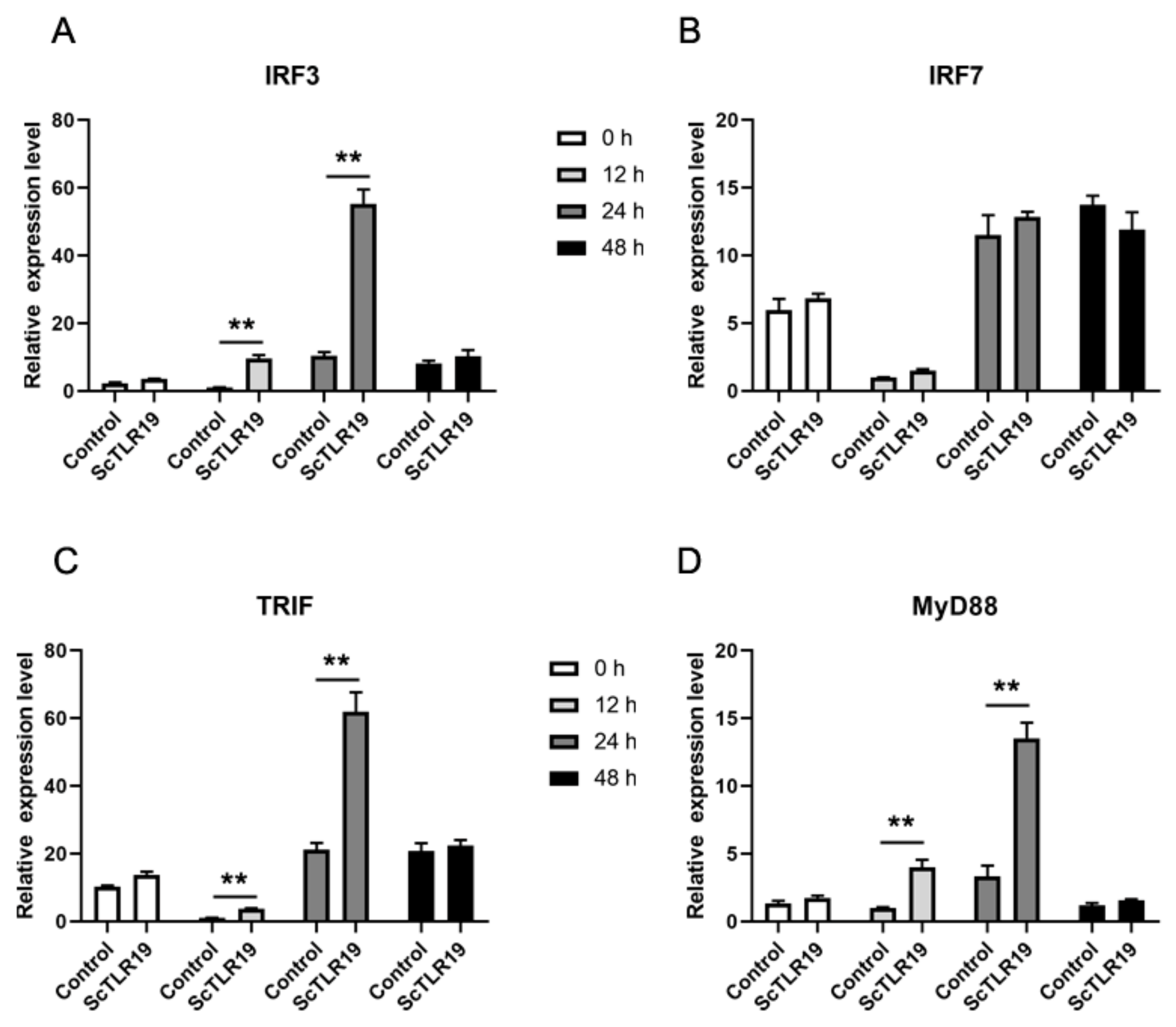
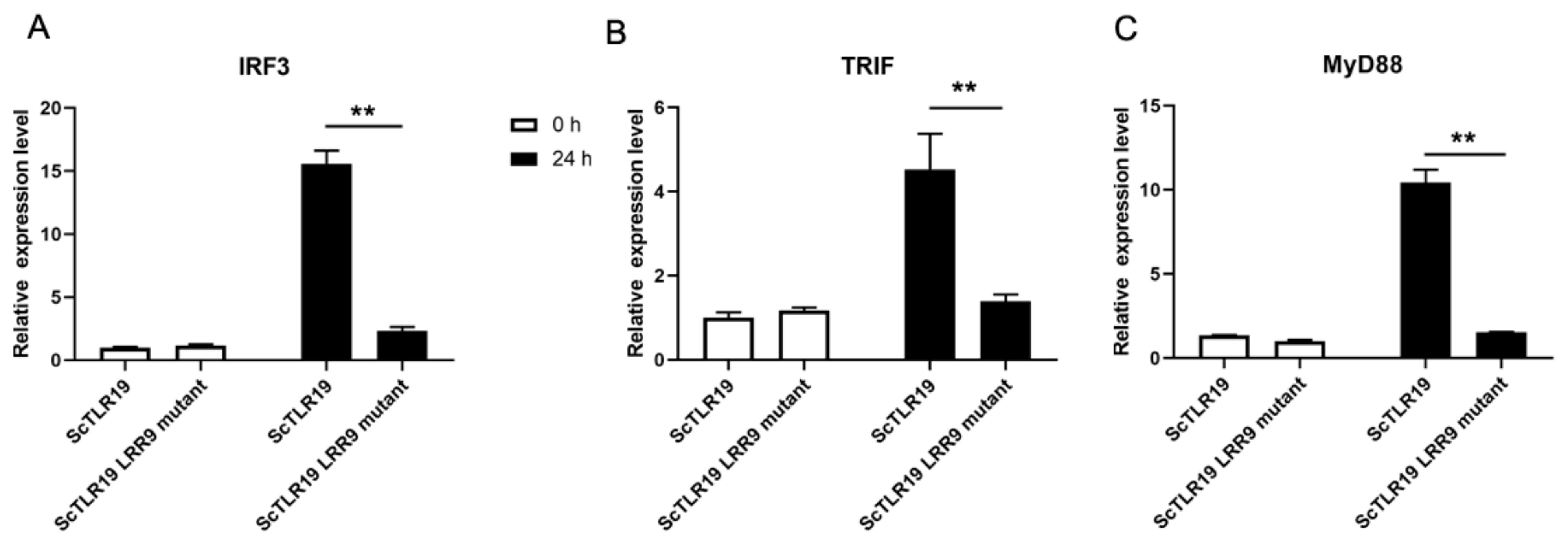
2.5. Differences in the Activation of Antiviral Immune Pathways by TLR19s from Barbel Chub and Grass Carp
3. Discussion
4. Materials and Methods
4.1. Cell Line and Viruses
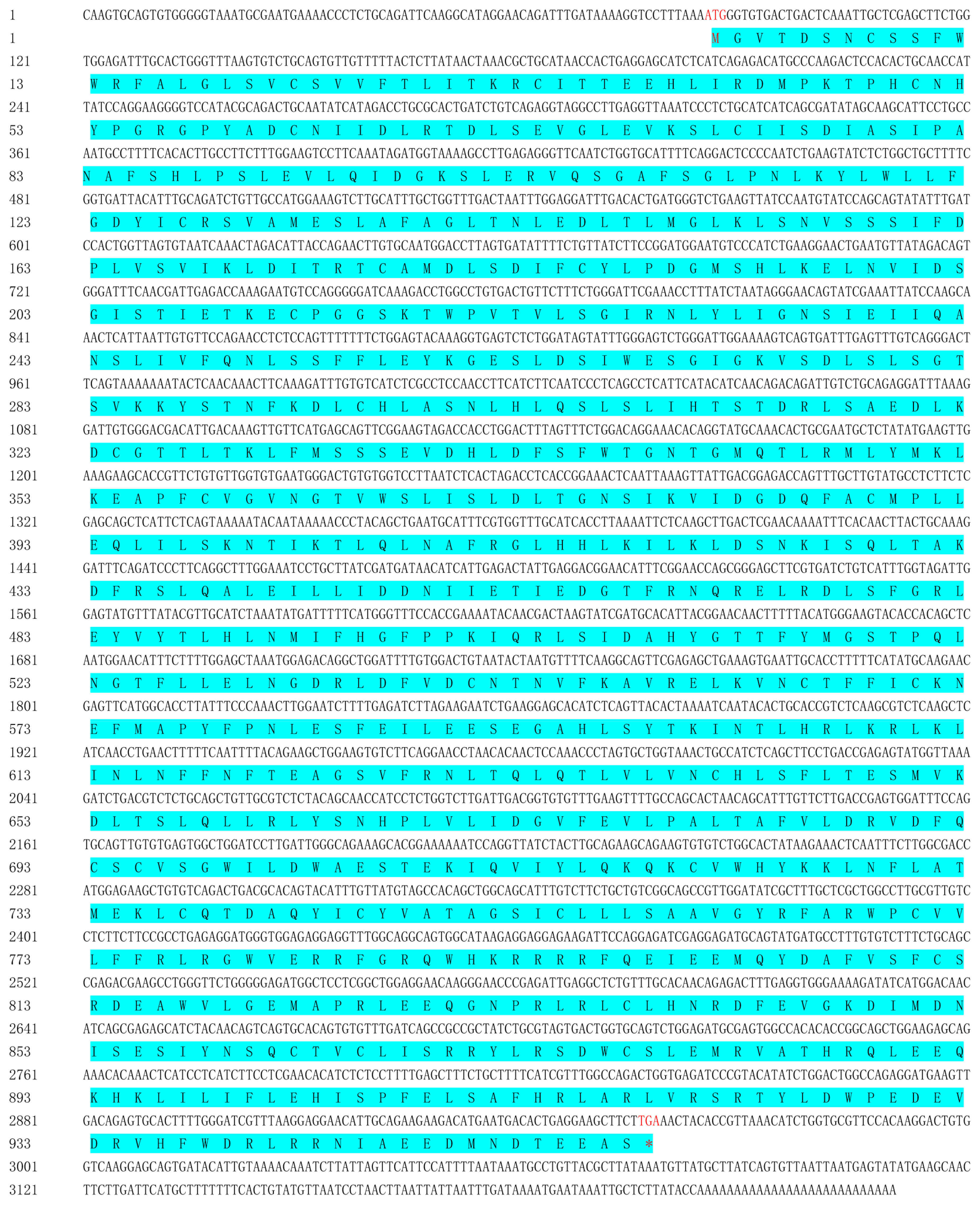
4.2. Gene Cloning of ScTLR19 by RACE
4.3. Sequence Analysis of TLR19s
4.4. Phylogenetic Analysis of TLR19s
4.5. Molecular Modeling and Docking
4.6. The Construction of ScTLR19 and ScTLR19 LRR9 Mutant Vectors
4.7. Transfection
4.8. ELISA
4.9. In Vitro GCRV Challenge Experiment
4.10. RNA Extraction, cDNA Synthesis, and Quantitative PCR (qPCR)
4.11. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, N.C.; Rise, M.L.; Christian, S.L. A Comparison of the Innate and Adaptive Immune Systems in Cartilaginous Fish, Ray-Finned Fish, and Lobe-Finned Fish. Front. Immunol. 2019, 10, 2292. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, L.V. Zebrafish as a Model for Fish Diseases in Aquaculture. Pathogens 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Manes, N.P.; Nita-Lazar, A. Molecular Mechanisms of the Toll-Like Receptor, STING, MAVS, Inflammasome, and Interferon Pathways. mSystems 2021, 6, e00336-21. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.; Zipfel, C.; Jones, J.D.G.; Ngou, B.P.M. Concerted actions of PRR- and NLR-mediated immunity. Essays Biochem. 2022, 66, 501–511. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Ghani, M.U.; Chen, J.F.; Khosravi, Z.; Wu, Q.S.; Liu, Y.J.; Zhou, J.J.; Zhong, L.P.; Cui, H.J. Unveiling the multifaceted role of toll-like receptors in immunity of aquatic animals: Pioneering strategies for disease management. Front. Immunol. 2024, 15, 1378111. [Google Scholar] [CrossRef]
- Mahapatra, S.; Ganguly, B.; Pani, S.; Saha, A.; Samanta, M. A comprehensive review on the dynamic role of toll-like receptors (TLRs) in frontier aquaculture research and as a promising avenue for fish disease management. Int. J. Biol. Macromol. 2023, 253, 126541. [Google Scholar] [CrossRef]
- Pietretti, D.; Wiegertjes, G.F. Ligand specificities of Toll-like receptors in fish: Indications from infection studies. Dev. Comp. Immunol. 2014, 43, 205–222. [Google Scholar] [CrossRef]
- Gao, D.; Li, W. Structures and recognition modes of toll-like receptors. Proteins 2017, 85, 3–9. [Google Scholar] [CrossRef]
- Bzówka, M.; Bagrowska, W.; Góra, A. Recent Advances in Studying Toll-like Receptors with the Use of Computational Methods. J. Chem. Inf. Model. 2023, 63, 3669–3687. [Google Scholar] [CrossRef]
- Chen, Y.H.; Wu, K.H.; Wu, H.P. Unraveling the Complexities of Toll-like Receptors: From Molecular Mechanisms to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 5037. [Google Scholar] [CrossRef] [PubMed]
- Kornilov, F.D.; Shabalkina, A.V. The architecture of transmembrane and cytoplasmic juxtamembrane regions of Toll-like receptors. Nat. Commun. 2023, 14, 1503. [Google Scholar] [CrossRef] [PubMed]
- Bryant, C.E. Rethinking Toll-like receptor signalling. Curr. Opin. Immunol. 2024, 91, 102460. [Google Scholar] [CrossRef]
- Kawai, T.; Ikegawa, M.; Ori, D.; Akira, S. Review Decoding Toll-like receptors: Recent insights and perspectives in innate immunity. Immunity 2024, 57, 649–673. [Google Scholar] [CrossRef]
- Sahoo, B.R. Structure of fish Toll-like receptors (TLR) and NOD-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef]
- Ji, J.; Rao, Y.; Wan, Q. Teleost-Specific TLR19 Localizes to Endosome, Recognizes dsRNA, Recruits TRIF, Triggers both IFN and NF-κB Pathways, and Protects Cells from Grass Carp Reovirus Infection. J. Immunol. 2018, 200, 573–585. [Google Scholar] [CrossRef]
- Su, J. Toll-like receptor signaling in teleosts. Sci. China Life Sci. 2025. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Z.; Fu, H.; Zhang, S.; Liu, J.; Chang, F.; Li, F.; Zhao, J.; Yin, D. Structural and evolutionary characteristics of fish-specific TLR19. Fish Shellfish Immunol. 2015, 47, 271–279. [Google Scholar] [CrossRef]
- Paria, A.; Muduli, C.; Rathore, G. Understanding the molecular response of non-mammalian toll-like receptor 22 (TLR22) in amphibious air-breathing catfish, Clarias magur (Hamilton, 1822) to bacterial infection or ligand stimulation through molecular cloning and expression profiling. Gene 2023, 866, 147351. [Google Scholar] [CrossRef]
- Wei, X.Y.; Wang, J.; Guo, S.T.; Lv, Y.Y.; Li, Y.P.; Qin, C.J.; Zou, Y.C.; Shi, Q.C.; Hu, P.; Xiong, X.Q.; et al. Molecular characterization of a teleost-specific toll-like receptor 22 (tlr22) gene from yellow catfish (Pelteobagrus fulvidraco) and its transcriptional change in response to poly I:C and Aeromonas hydrophila stimuli. Fish Shellfish Immunol. 2023, 134, 108579. [Google Scholar] [CrossRef]
- Gan, Z.; Chen, S.N.; Huang, B.; Zou, J.; Nie, P. Fish type I and type II interferons: Composition, receptor usage, production and function. Rev. Aquac. 2020, 12, 773–804. [Google Scholar] [CrossRef]
- Zhao, F.; Li, Y.W.; Pan, H.J.; Shi, C.B.; Luo, X.C.; Li, A.X.; Wu, S.Q. Expression profiles of toll-like receptors in channel catfish (Ictalurus punctatus) after infection with Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2013, 35, 993–997. [Google Scholar] [CrossRef] [PubMed]
- Meijer, A.H.; Gabby Krens, S.F.; Medina Rodriguez, I.A.; He, S.; Bitter, W.; Ewa Snaar-Jagalska, B.; Spaink, H.P. Expression analysis of the Toll-like receptor and TIR domain adaptor families of zebrafish. Mol. Immunol. 2004, 40, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.T.; Zou, J.; Holland, J.W.; Martin, S.A.; Collet, B.; Kanellos, T.; Secombes, C.J. Identification and characterisation of TLR18-21 genes in Atlantic salmon (Salmo salar). Fish Shellfish Immunol. 2014, 41, 549–559. [Google Scholar] [CrossRef]
- Wang, K.L.; Ji, W.; Zhang, G.R.; Wei, K.J.; Shi, Z.C.; Zhang, X.T.; Zheng, H.; Fan, Q.X. Molecular characterization and expression analysis of three TLR genes in yellow catfish (Pelteobagrus fulvidraco): Responses to stimulation of Aeromonas hydrophila and TLR ligands. Fish Shellfish Immunol. 2017, 66, 466–479. [Google Scholar] [CrossRef]
- Lai, R.F.; Jakovlić, I.; Liu, H.; Zhan, F.B.; Wei, J.; Wang, W.M. Molecular characterization and immunological response analysis of toll-like receptors from the blunt snout bream (Megalobrama amblycephala). Dev. Comp. Immunol. 2017, 67, 471–475. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Lv, Z. Comparative Transcriptomics Analysis Reveals Unique Immune Response to Grass Carp Reovirus Infection in Barbel Chub (Squaliobarbus curriculus). Biology 2024, 13, 214. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, T.; Jin, S.; Wang, J.; Wang, J.; Luo, H.; Li, R.; Sun, T.; Zou, J.; Li, Y. Characterization and immune function of the interferon-β promoter stimulator-1 in the barbel chub, Squaliobarbus curriculus. Dev. Comp. Immunol. 2020, 104, 103571. [Google Scholar] [CrossRef]
- Jin, S.; Zhao, X.; Wang, H.; Su, J.; Wang, J.; Ding, C.; Li, Y.; Xiao, T. Molecular characterization and expression of TLR7 and TLR8 in barbel chub (Squaliobarbus curriculus): Responses to stimulation of grass carp reovirus and lipopolysaccharide. Fish Shellfish. Immunol. 2018, 83, 292–307. [Google Scholar] [CrossRef]
- Wang, R.H.; Li, W.; Fan, Y.D.; Liu, Q.L.; Zeng, L.B.; Xiao, T.Y. Tlr22 structure and expression characteristic of barbel chub, Squaliobarbus curriculus provides insights into antiviral immunity against infection with grass carp reovirus. Fish Shellfish Immunol. 2017, 66, 120–128. [Google Scholar] [CrossRef]
- Li, Y.; Jin, S.; Zhao, X.; Luo, H.; Li, R.; Li, D.; Xiao, T. Sequence and expression analysis of the cytoplasmic pattern recognition receptor melanoma differentiation-associated gene 5 from the barbel chub Squaliobarbus curriculus. Fish Shellfish Immunol. 2019, 94, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Tang, H.; Sun, M.X.; Liu, Q.L.; Liao, Y.J.; Luo, H.; Li, R.; Wang, R.H.; Yang, H.; Wang, H.Q.; et al. The SIDT2/MDA5/IFN axis contributes to virus resistance in teleost fish. Aquaculture 2024, 583, 740568. [Google Scholar] [CrossRef]
- Rao, Y.; Su, J. Insights into the antiviral immunity against grass carp (Ctenopharyngodon idella) reovirus (GCRV) in grass carp. J. Immunol. Res. 2015, 2015, 670437. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q. Studies on Genetic Characteristics and Grass Carp Reovirus Resistance of F1 Hybrids between Grass Carp (Ctenopharyngodon idellus) and Barbel Chub (Squaliobarbus curriculus). Ph.D. Thesis, Hunan Agriculture University, Changsha, China, 2014. [Google Scholar]
- Sakaniwa, K.; Shimizu, T. Targeting the innate immune receptor TLR8 using small-molecule agents. Acta Crystallogr. Sect. D-Struct. Biol. 2020, 76, 621–629. [Google Scholar] [CrossRef]
- Prakasam, P.; Salam, A.A.A.; Ahamed, S.I.B. The pathogenic effect of SNPs on structure and function of human TLR4 using a computational approach. J. Biomol. Struct. Dyn. 2023, 41, 12387–12400. [Google Scholar] [CrossRef]
- Hsu, S.H.; Wu, C.T.; Sun, Y.J.; Chang, M.Y.; Li, C.; Ko, Y.C.; Chou, L.F.; Yang, C.W. Crystal structure of Leptospira LSS_01692 reveals a dimeric structure and induces inflammatory responses through Toll-like receptor 2-dependent NF-κB and MAPK signal transduction pathways. FEBS J. 2023, 290, 4513–4532. [Google Scholar] [CrossRef]
- Mahapatra, S.; Ganguly, B.; Pani, S.; Jena, N.; Bej, A.; Saha, A.; Samanta, M. Toll-like receptor 21 in Labeo rohita recognizes double-stranded RNA and lipopolysaccharides by engaging the critical motifs in the LRR domain and gets activated against bacterial assaults. Biochem. Biophys. Res. Commun. 2024, 739, 150581. [Google Scholar] [CrossRef]
- Huang, C.; Chen, S.; Zhang, T.; Li, D.; Huang, Z.; Huang, J.; Qin, Y.; Chen, B.; Cheng, G.; Ma, F.; et al. TLR3 Ligand PolyI:C Prevents Acute Pancreatitis Through the Interferon-β/Interferon-α/β Receptor Signaling Pathway in a Caerulein-Induced Pancreatitis Mouse Model. Front. Immunol. 2019, 10, 980. [Google Scholar] [CrossRef]
- Takada, E.; Okahira, S.; Sasai, M.; Funami, K.; Seya, T.; Matsumoto, M. C-terminal LRRs of human Toll-like receptor 3 control receptor dimerization and signal transmission. Mol. Immunol. 2007, 44, 3633–3640. [Google Scholar] [CrossRef]
- Sahoo, B.R.; Basu, M.; Swain, B.; Maharana, J.; Dikhit, M.R.; Jayasankar, P.; Samanta, M. Structural insights of rohu TLR3, its binding site analysis with fish reovirus dsRNA, poly I:C and zebrafish TRIF. Int. J. Biol. Macromol. 2012, 51, 531–543. [Google Scholar] [CrossRef]
- Chakrapani, V.; Rasal, K.D.; Kumar, S.; Mohapatra, S.D.; Sundaray, J.K.; Jayasankar, P.; Barman, H.K. In Silico Analysis of nsSNPs of Carp TLR22 Gene Affecting its Binding Ability with Poly I:C. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 641–652. [Google Scholar] [CrossRef]
- Gutierrez, E.G.; Ortega, J. Uncovering selection pressures on the IRF gene family in bats’ immune system. Immunogenetics 2025, 77, 10. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.T.; Tzvetkov, E.; Pereira, A. TLR7 and TLR8 Differentially Activate the IRF and NF-κB Pathways in Specific Cell Types to Promote Inflammation. Immunohorizons 2020, 4, 93–107. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, X.; Cai, J.; OuYang, Z.; Wei, S.; Wei, J.; Qin, Q. Identification of orange-spotted grouper (Epinephelus coioides) interferon regulatory factor 3 involved in antiviral immune response against fish RNA virus. Fish Shellfish Immunol. 2015, 42, 345–352. [Google Scholar] [CrossRef]
- Krishnan, R.; Kurcheti, P.P.; Mushtaq, Z.; K, J.; Naik, T.V. Interferon-regulatory factors, IRF3 and IRF7 in Asian seabass, Lates calcarifer: Characterization, ontogeny and transcriptional modulation upon challenge with nervous necrosis virus. Fish Shellfish Immunol. 2019, 89, 468–476. [Google Scholar] [CrossRef]
- Ye, C.; Wan, C.; Chen, J.; Li, G.; Li, Y.X.; Wang, Y.; Tao, Q.; Peng, L.C.; Fang, R.D. Cathelicidin CATH-B1 Inhibits Pseudorabies Virus Infection via Direct Interaction and TLR4/JNK/IRF3-Mediated Interferon Activation. J. Virol. 2023, 97, e0070623. [Google Scholar] [CrossRef]
- Liu, B.M.; Li, N.L.; Wang, R.X.; Li, X.F.; Li, Z.A.; Marion, T.N.; Li, K. Key roles for phosphorylation and the Coiled-coil domain in TRIM56-mediated positive regulation of TLR3-TRIF- dependent innate immunity. J. Biol. Chem. 2024, 300, 107249. [Google Scholar] [CrossRef]
- Saeng-Chuto, K.; Madapong, A.; Kaeoket, K.; Piñeyro, P.E.; Tantituvanont, A.; Nilubol, D. Co-infection of porcine deltacoronavirus and porcine epidemic diarrhea virus induces early TRAF6-mediated NF-κB and IRF7 signaling pathways through TLRs. Sci. Rep. 2022, 12, 19443. [Google Scholar] [CrossRef]
- Owen, A.M.; Luan, L.; Burelbach, K.R.; McBride, M.A.; Stothers, C.L.; Boykin, O.A.; Sivanesam, K.; Schaedel, J.F.; Patil, T.K.; Wang, J.; et al. MyD88-dependent signaling drives toll-like receptor-induced trained immunity in macrophages. Front. Immunol. 2022, 13, 1044662. [Google Scholar] [CrossRef]
- Díaz-Dinamarca, D.A.; Salazar, M.L.; Escobar, D.F.; Castillo, B.N.; Valdebenito, B.; Díaz, P.; Manubens, A.; Salazar, F.; Troncoso, M.F.; Lavandero, S.; et al. Surface immunogenic protein from Streptococcus agalactiae and Fissurella latimarginata hemocyanin are TLR4 ligands and activate MyD88- and TRIF dependent signaling pathways. Front. Immunol. 2023, 14, 1186188. [Google Scholar] [CrossRef]
- Saikh, K.U.; Anam, K.; Sultana, H.; Ahmed, R.; Kumar, S.; Srinivasan, S.; Ahmed, H. Targeting Myeloid Differentiation Primary Response Protein 88 (MyD88) and Galectin-3 to Develop Broad-Spectrum Host-Mediated Therapeutics against SARS-CoV-2. Int. J. Mol. Sci. 2024, 25, 8421. [Google Scholar] [CrossRef] [PubMed]
- Ahmed-Hassan, H.; Abdul-Cader, M.S.; Sabry, M.A.; Hamza, E.; Abdul-Careem, M.F. Toll-like receptor (TLR)4 signalling induces myeloid differentiation primary response gene (MYD) 88 independent pathway in avian species leading to type I interferon production and antiviral response. Virus Res. 2018, 256, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xu, X.; Shen, Y.; Fang, Y.; Zhang, J.; Bai, Y.; Gu, S.; Wang, R.; Chen, T.; Li, J. Myeloid differentiation factor 88 (Myd88) is involved in the innate immunity of black carp (Mylopharyngodon piceus) defense against pathogen infection. Fish Shellfish Immunol. 2019, 94, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ding, S.; He, Y.; Ren, J.; Li, W.; Zhang, Q. Northeast Chinese lamprey (Lethenteron morii) MyD88: Identification, expression, and functional characterization. Fish Shellfish Immunol. 2019, 94, 539–547. [Google Scholar] [CrossRef]
- Yamamoto, M.; Sato, S.; Hemmi, H.; Hoshino, K.; Kaisho, T.; Sanjo, H.; Takeuchi, O.; Sugiyama, M.; Okabe, M.; Takeda, K.; et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science 2003, 301, 640–643. [Google Scholar] [CrossRef]
- Chen, Y.J.; Lin, J.H.; Zhao, Y.; Ma, X.P.; Yi, H.S. Toll-like receptor 3 (TLR3) regulation mechanisms and roles in antiviral innate immune responses. J. Zhejiang Univ. Sci. B 2021, 22, 609–632. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.E.; Cho, H.; Jung, H.G.; Lee, W.; Seo, H.Y.; Lee, S.H.; Ahn, D.G.; Kim, S.J.; Yu, J.W.; et al. Antiviral efficacy of orally delivered neoagarohexaose, a nonconventional TLR4 agonist, against norovirus infection in mice. Biomaterials 2020, 263, 120391. [Google Scholar] [CrossRef]
- Luo, H.; Li, Y.; Li, D.; Zou, J.; Xiao, T. Sequence structure and immune function of signal transduction and transcriptional activator STAT1 of barbel chub (Squaliobarbus curriculus). J. Fish. China 2021, 45, 381–395. [Google Scholar] [CrossRef]
- Campanella, J.J.; Bitincka, L.; Smalley, J. MatGAT: An application that generates similarity/identity matrices using protein or DNA sequences. BMC Bioinform. 2003, 4, 29. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

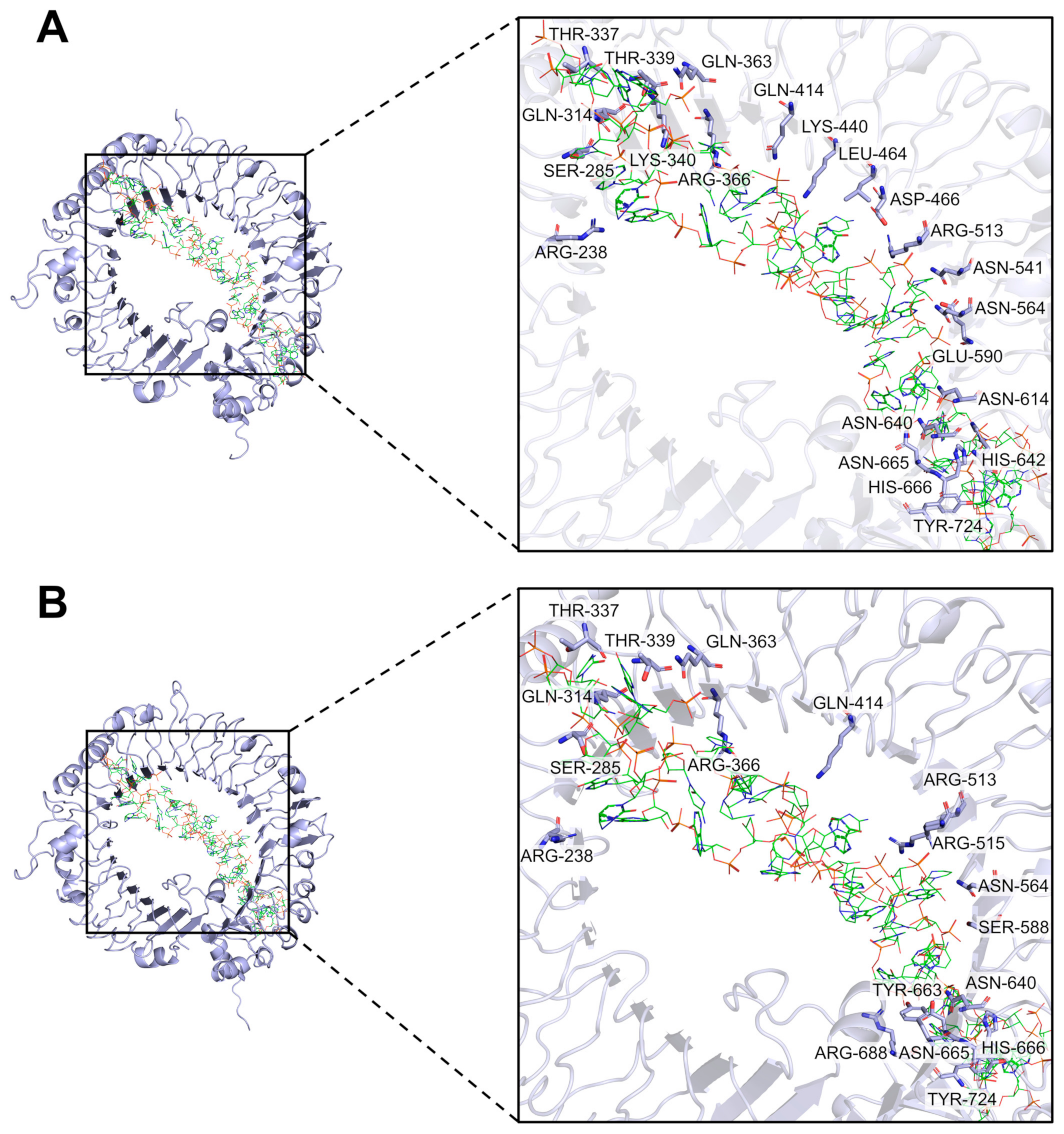

| CiTLR19-ECD and Poly (I:C) | ScTLR19-ECD and Poly (I:C) | |
|---|---|---|
| Docking score | −474.90 | −512.31 |
| Confidence score | 0.9985 | 0.9993 |
| Primers | Sequences (5′-3′) | Applications |
|---|---|---|
| ScTLR19 5′ | TCCAGGTGGTCTACTTCCGAACTGCTC | RACE |
| ScTLR19 3′W | ACTAATGTTTTCAAGGCAGTTCGAGAGC | RACE |
| ScTLR19 3′N | CACTGCACCGTCTCAAGCGTCT | RACE |
| UPM | CTAATACGACTCACTATAGGGC | RACE |
| ScTLR19-cF | CTGCCAATGCCTTTTCACAC | PCR |
| ScTLR19-cR | GCAAACAGAGCCTCAATCTCG | PCR |
| ScTLR19-oF | tcgagctcaagcttcgaattcATGGGTGTGACTGACTCAAATTGC | Construction of overexpression vector |
| ScTLR19-oR | ggatcccgggcccgcggtaccAGAAGCTTCCTCAGTGTCATTCATG | Construction of overexpression vector |
| ScTLR19-qF | TTCACACTTGCCTTCTTTGGA | qPCR |
| ScTLR19-qR | TAATCACCGAAAAGCAGCCAGA | qPCR |
| MyD88-qF | GAAGCCCGTCCAGGTTCCA | qPCR |
| MyD88-qR | TGAAGGCATCAAAGGTCTCCG | qPCR |
| TRIF-qF | TCGAGGAGGAAGGCTGGCACTT | qPCR |
| TRIF-qF | TGAGCGTGAAGCAGACAGCAGC | qPCR |
| IRF7-qF | CGCCTGTGTTCGTCACTCGT | qPCR |
| IRF7-qR | GGTGGTTGGAAAGCGTATTGG | qPCR |
| IRF3-qF | AAACGTGTTCTGTACAAACCC | qPCR |
| IRF3-qR | CTCAAGCCCCAAATAGAGCTG | qPCR |
| β-actin-qF | GCTATGTGGCTCTTGACTTCG | qPCR |
| β-actin-qR | GGGCACCTGAACCTCTCATT | qPCR |
| Species | TLRs | Accession Numbers |
|---|---|---|
| Tachysurus fulvidraco | TLR19 | QLL99515.1 |
| Ctenopharyngodon idella | TLR19 | AUF71965.1 |
| Squaliobarbus curriculus | TLR19 | QJX15323.1 |
| Ictaluruspunctatus | TLR19 | AEI59675.1 |
| Danio rerio | TLR19 | NP001352353.1 |
| Tachysurus vachellii | TLR19 | WJQ78152.1 |
| Salmosalar | TLR19 | CDH93609.2 |
| Cyprinus carpio | TLR19 | BAU98390.1 |
| Megalobrama amblycephala | TLR19 | APT35508.1 |
| Homo sapiens | TLR1 | CAG38593.1 |
| Homo sapiens | TLR2 | AAH33756.1 |
| Homo sapiens | TLR3 | ABC86910.1 |
| Homo sapiens | TLR4 | AAF05316.1 |
| Homo sapiens | TLR5 | AAI09119.1 |
| Homo sapiens | TLR6 | BAA78631.1 |
| Homo sapiens | TLR7 | AAZ99026.1 |
| Homo sapiens | TLR8 | AAZ95441.1 |
| Homo sapiens | TLR9 | AAZ95520.1 |
| Homo sapiens | TLR10 | AAY78491.1 |
| Mus musculus | TLR1 | NP001263374.1 |
| Mus musculus | TLR2 | NP036035.3 |
| Mus musculus | TLR3 | AAH99937.1 |
| Mus musculus | TLR4 | NP067272.1 |
| Mus musculus | TLR5 | AAI25248.1 |
| Mus musculus | TLR6 | BAA78632.1 |
| Mus musculus | TLR7 | AAI32386.1 |
| Mus musculus | TLR8 | AAI32055.1 |
| Mus musculus | TLR9 | EDL21125.1 |
| Mus musculus | TLR11 | NP991388.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lv, Z.; Zhang, M.; Xu, Y.; Qin, B.; Yang, H.; Wei, R.; Xiao, T. Structural and Functional Characteristics of TLR19 in Barbel Chub Compared to TLR19 in Grass Carp. Int. J. Mol. Sci. 2025, 26, 3103. https://doi.org/10.3390/ijms26073103
Lv Z, Zhang M, Xu Y, Qin B, Yang H, Wei R, Xiao T. Structural and Functional Characteristics of TLR19 in Barbel Chub Compared to TLR19 in Grass Carp. International Journal of Molecular Sciences. 2025; 26(7):3103. https://doi.org/10.3390/ijms26073103
Chicago/Turabian StyleLv, Zhao, Mengyuan Zhang, Yang Xu, Beibei Qin, Hong Yang, Ruizhong Wei, and Tiaoyi Xiao. 2025. "Structural and Functional Characteristics of TLR19 in Barbel Chub Compared to TLR19 in Grass Carp" International Journal of Molecular Sciences 26, no. 7: 3103. https://doi.org/10.3390/ijms26073103
APA StyleLv, Z., Zhang, M., Xu, Y., Qin, B., Yang, H., Wei, R., & Xiao, T. (2025). Structural and Functional Characteristics of TLR19 in Barbel Chub Compared to TLR19 in Grass Carp. International Journal of Molecular Sciences, 26(7), 3103. https://doi.org/10.3390/ijms26073103





