Expression, Distribution and Function of the Transient Receptor Potential Vanilloid Type 1 (TRPV1) in Endometrial Cancer
Abstract
:1. Introduction
2. Results
2.1. Patient Characteristics
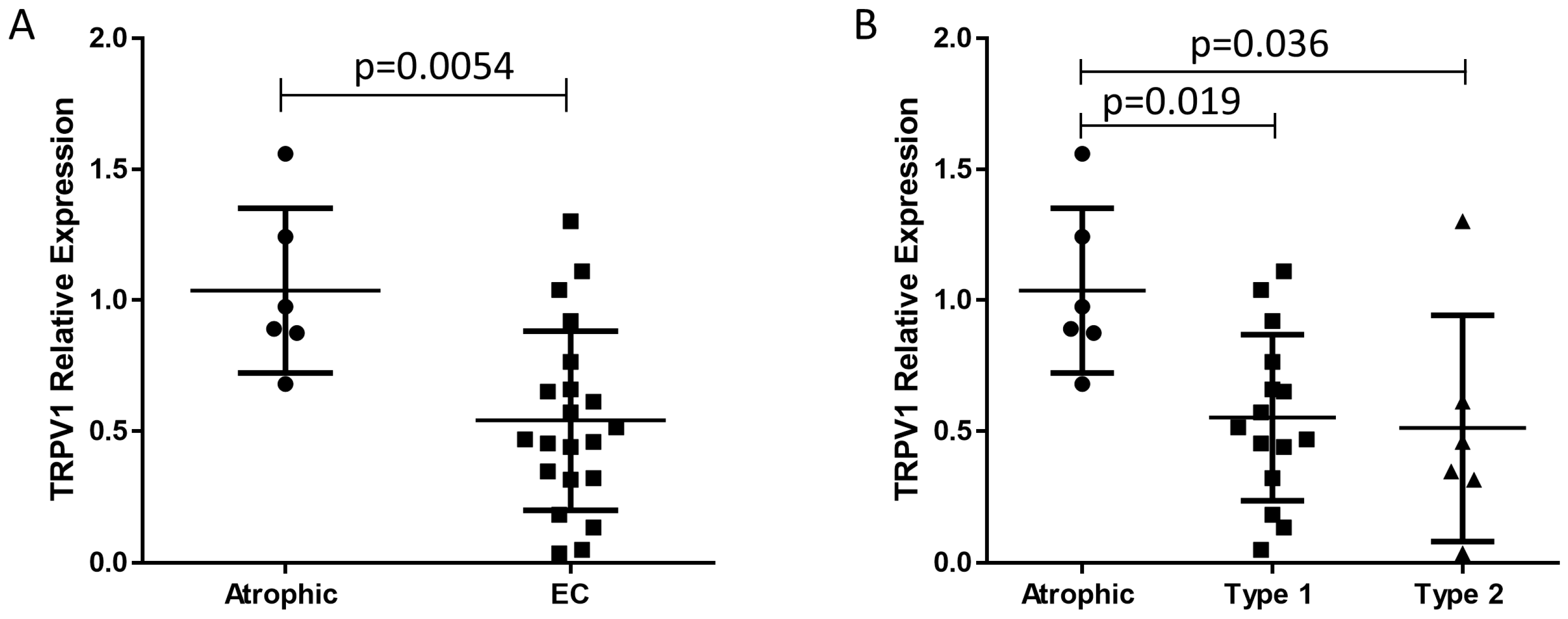
2.2. TRPV1 Transcript Levels
2.3. Identification and Location of TRPV1 Protein in EC
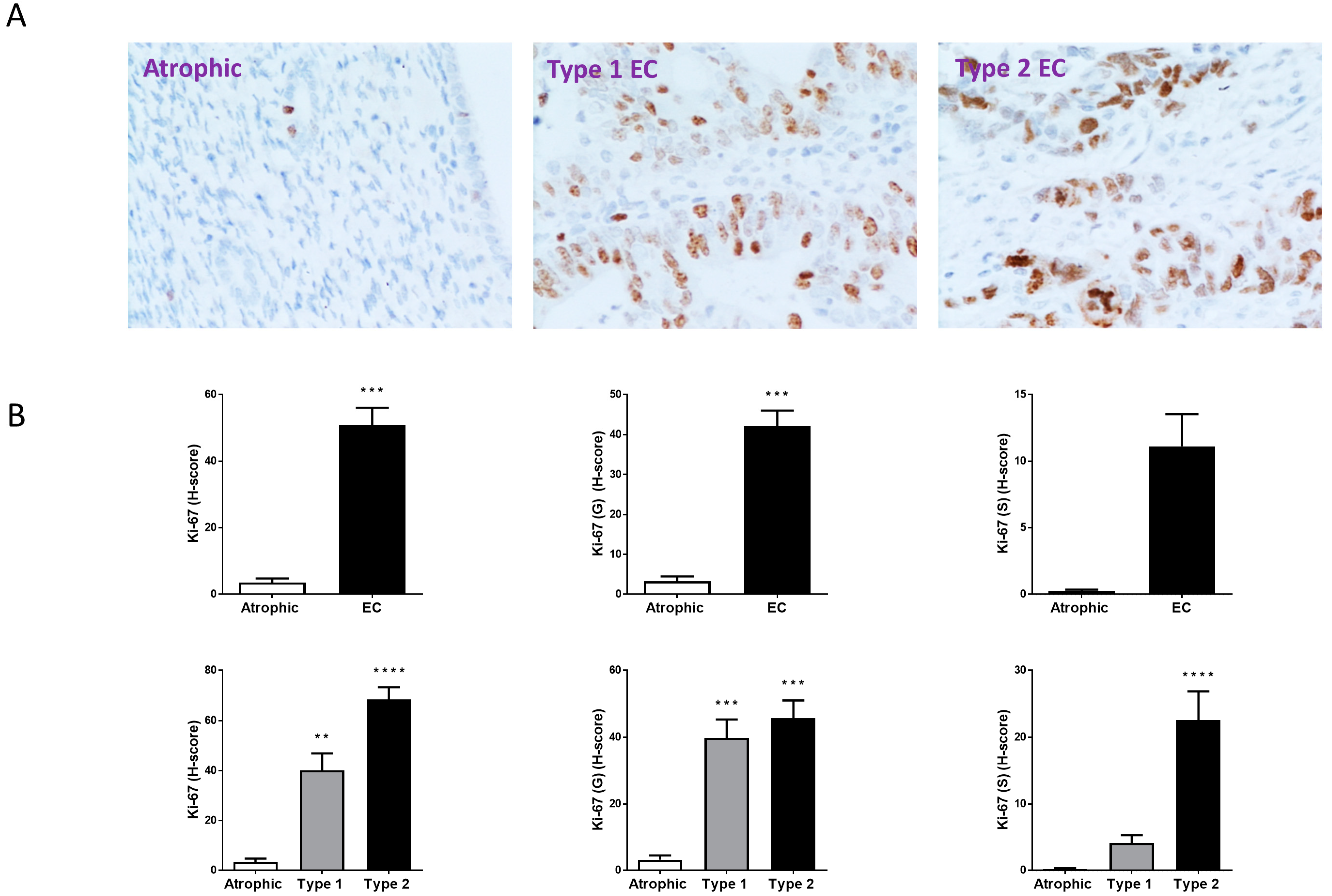
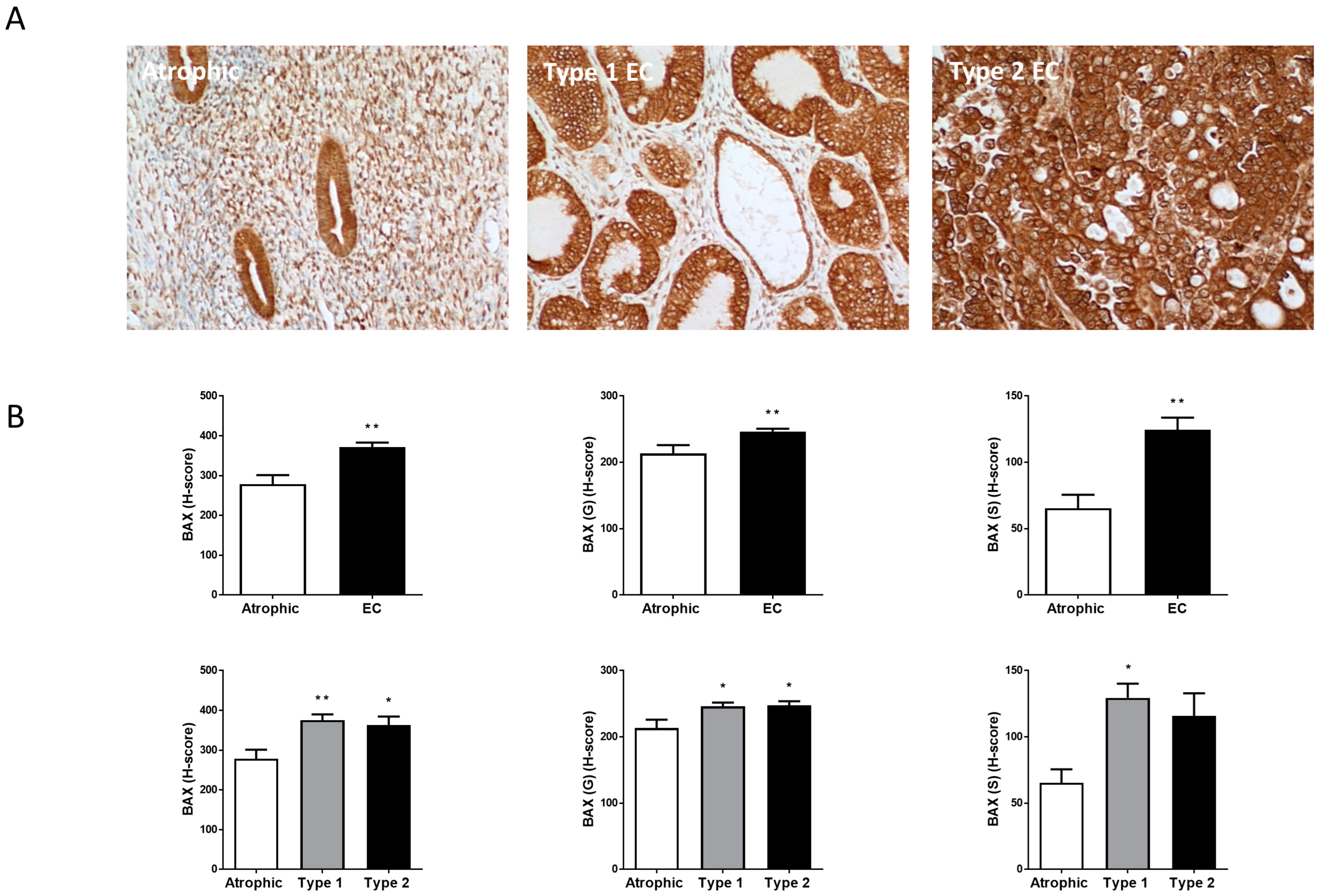
2.4. Ki-67, BAX and Bcl-2 Staining Patterns
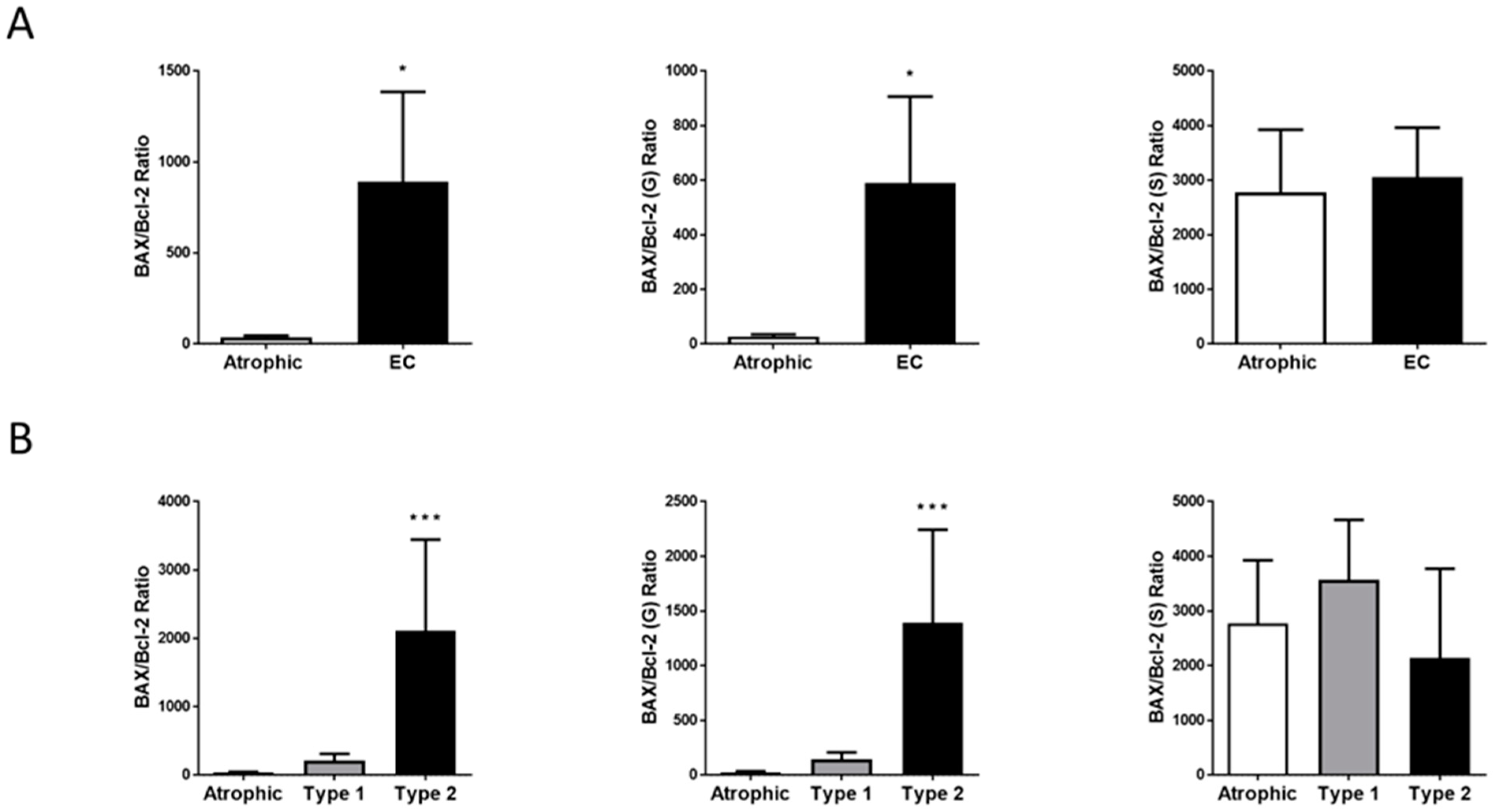
2.5. Hallmark of Apoptosis
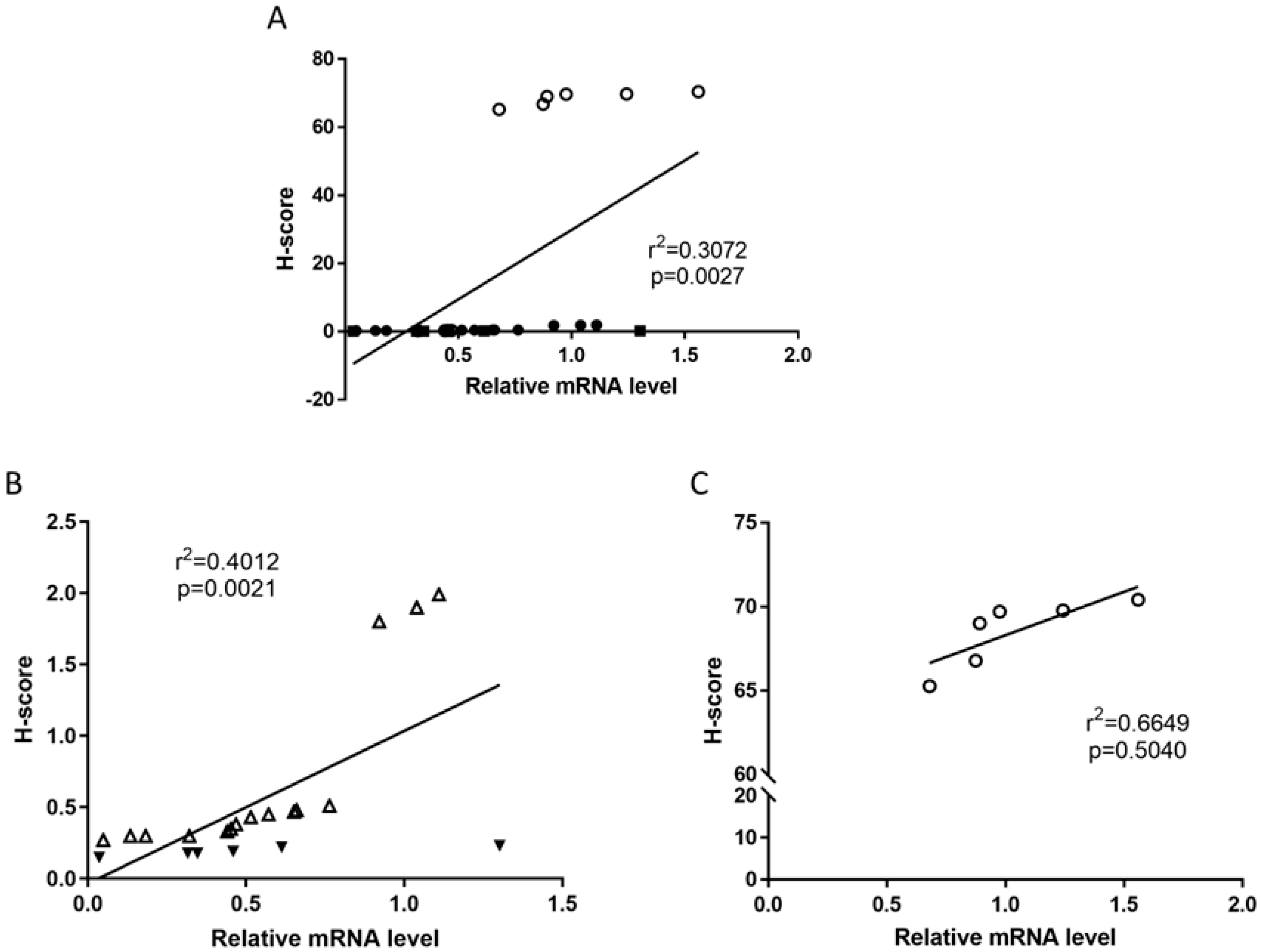
2.6. Relationships Between TRPV1 Protein Levels with mRNA Levels and That of Other ECS Proteins
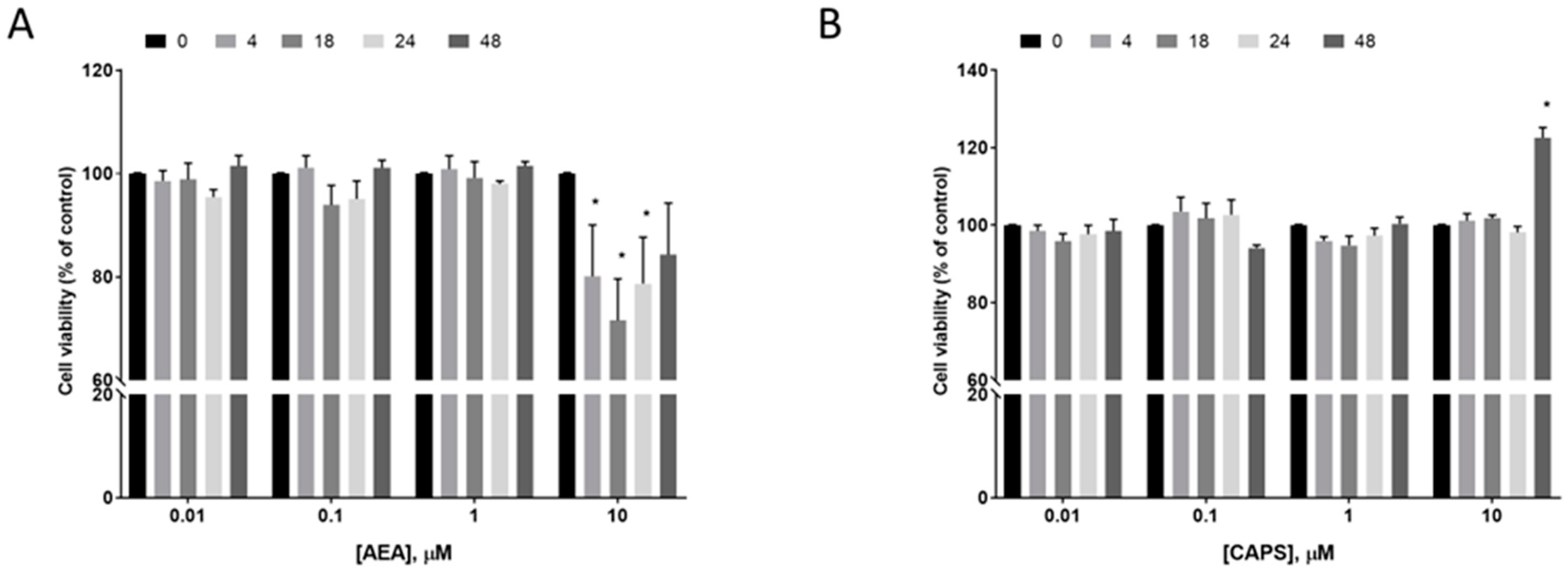
2.7. Effect of AEA and Capsaicin on EC Cell Survival
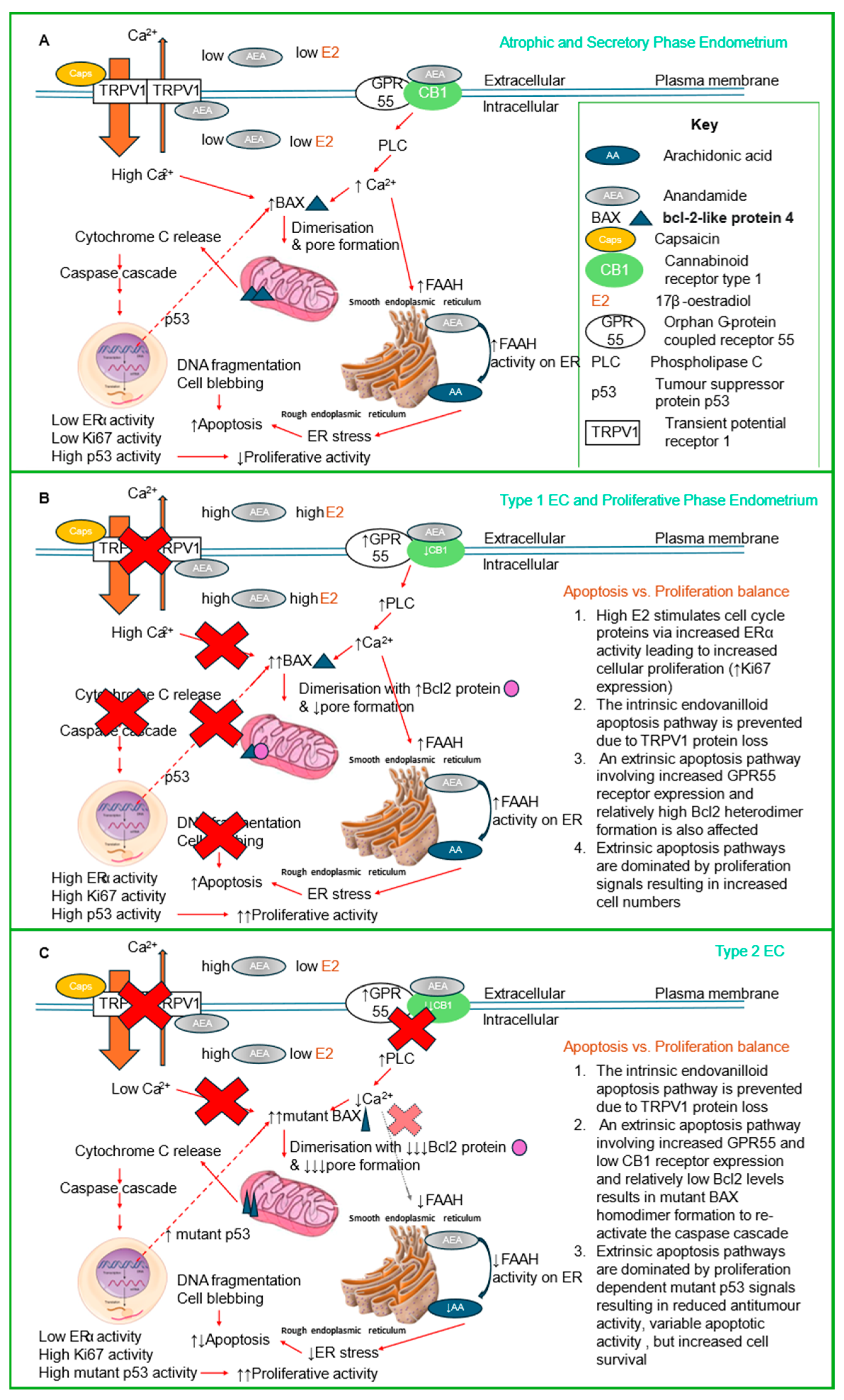
3. Discussion
4. Materials and Methods
4.1. Participants
4.2. Sample Collection
4.3. RNA Extraction, cDNA Synthesis and Quantitative Real-Time PCR
4.4. Identification and Tissue Localisation of TRPV1, CB1, CB2, NAPE-PLD, FAAH, Ki-67, BAX and Bcl-2 Protein Expression
4.5. Histomorphometric Analyses
4.6. Effect of AEA or Capsaicin on Ishikawa Cell Viability
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holman, L.L.; Lu, K.H. The epidemiology of endometrial cancer. Glob. Libr. Women’s Med. 2012, 41, 1–16. [Google Scholar] [CrossRef]
- American Cancer Society. Global Cancer Facts & Figures; American Cancer Society: Atlanta, GA, USA, 2018. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2023. CA A Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.A.; Althouse, A.D.; Freese, K.E.; Soisson, S.; Edwards, R.P.; Welburn, S.; Sukumvanich, P.; Comerci, J.; Kelley, J.; LaPorte, R.E.; et al. USA endometrial cancer projections to 2030: Should we be concerned? Future Oncol. 2014, 10, 2561–2568. [Google Scholar] [CrossRef]
- Evans, T.; Sany, O.; Pearmain, P.; Ganesan, R.; Blann, A.; Sundar, S. Differential trends in the rising incidence of endometrial cancer by type: Data from a UK population-based registry from 1994 to 2006. Br. J. Cancer 2011, 104, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Yasin, H.K.; Taylor, A.H.; Ayakannu, T. A Narrative Review of the Role of Diet and Lifestyle Factors in the Development and Prevention of Endometrial Cancer. Cancers 2021, 13, 2149. [Google Scholar] [CrossRef]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, T.; Taylor, A.H.; Bari, M.; Mastrangelo, N.; Maccarrone, M.; Konje, J.C. Expression and function of the endocannabinoid modulating enzymes fatty acid amide hydrolase and N-acylphosphatidylethanolamine-specific phospholipase D in endometrial carcinoma. Front. Oncol. 2019, 9, 1363. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Willets, J.M.; Konje, J.C. The endocannabinoid system and sex steroid hormone-dependent cancers. Int. J. Endocrinol. 2013, 2013, 259676. [Google Scholar] [CrossRef] [PubMed]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Sawzdargo, M.; Nguyen, T.; Lee, D.K.; Lynch, K.R.; Cheng, R.; Heng, H.H.; George, S.R.; O’Dowd, B.F. Identification and cloning of three novel human G protein-coupled receptor genes GPR52, PsiGPR53 and GPR55: GPR55 is extensively expressed in human brain. Brain Res. Mol. Brain Res. 1999, 64, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Cannabinoid receptor expression in estrogen-dependent and estrogen-independent endometrial cancer. J. Recept. Signal Transduct. Res. 2018, 38, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Maccarrone, M.; Konje, J.C. Identification of novel predictive biomarkers for endometrial malignancies: N-acylethanolamines. Front. Oncol. 2019, 9, 430. [Google Scholar] [CrossRef]
- Risinger, J.I.; Maxwell, G.L.; Chandramouli, G.V.; Jazaeri, A.; Aprelikova, O.; Patterson, T.; Berchuck, A.; Barrett, J.C. Microarray analysis reveals distinct gene expression profiles among different histologic types of endometrial cancer. Cancer Res. 2003, 63, 6–11. [Google Scholar]
- Fonseca, B.M.; Correia-da-Silva, G.; Teixeira, N.A. Cannabinoid-induced cell death in endometrial cancer cells: Involvement of TRPV1 receptors in apoptosis. J. Physiol. Biochem. 2018, 74, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Guida, M.; Ligresti, A.; De Filippis, D.; D’Amico, A.; Petrosino, S.; Cipriano, M.; Bifulco, G.; Simonetti, S.; Orlando, P.; Insabato, L.; et al. The levels of the endocannabinoid receptor CB2 and its ligand 2-arachidonoylglycerol are elevated in endometrial carcinoma. Endocrinology 2010, 151, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J. Novel cannabinoid receptors. Br. J. Pharmacol. 2007, 152, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.D.; Williamson, E.M. Cannabinoids inhibit human keratinocyte proliferation through a non-CB1/CB2 mechanism and have a potential therapeutic value in the treatment of psoriasis. J. Dermatol. Sci. 2007, 45, 87–92. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Willets, J.M.; Brown, L.; Lambert, D.G.; McDonald, J.; Davies, Q.; Moss, E.L.; Konje, J.C. Validation of endogenous control reference genes for normalizing gene expression studies in endometrial carcinoma. Mol. Hum. Reprod. 2015, 21, 723–735. [Google Scholar] [CrossRef]
- Taylor, A.H.; Tortolani, D.; Ayakannu, T.; Konje, J.C.; Maccarrone, M. (Endo)cannabinoids and Gynaecological Cancers. Cancers 2020, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Ren, G.; Shi, Y. The putative cannabinoid receptor GPR55 promotes cancer cell proliferation. Oncogene 2011, 30, 139–141. [Google Scholar] [CrossRef]
- Andradas, C.; Caffarel, M.M.; Perez-Gomez, E.; Salazar, M.; Lorente, M.; Velasco, G.; Guzman, M.; Sanchez, C. The orphan G protein-coupled receptor GPR55 promotes cancer cell proliferation via ERK. Oncogene 2011, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Pineiro, R.; Maffucci, T.; Falasca, M. The putative cannabinoid receptor GPR55 defines a novel autocrine loop in cancer cell proliferation. Oncogene 2011, 30, 142–152. [Google Scholar] [CrossRef]
- Kalogris, C.; Caprodossi, S.; Amantini, C.; Lambertucci, F.; Nabissi, M.; Morelli, M.B.; Farfariello, V.; Filosa, A.; Emiliozzi, M.C.; Mammana, G.; et al. Expression of transient receptor potential vanilloid-1 (TRPV1) in urothelial cancers of human bladder: Relation to clinicopathological and molecular parameters. Histopathology 2010, 57, 744–752. [Google Scholar] [CrossRef] [PubMed]
- Domotor, A.; Peidl, Z.; Vincze, A.; Hunyady, B.; Szolcsanyi, J.; Kereskay, L.; Szekeres, G.; Mozsik, G. Immunohistochemical distribution of vanilloid receptor, calcitonin-gene related peptide and substance P in gastrointestinal mucosa of patients with different gastrointestinal disorders. Inflammopharmacology 2005, 13, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Hartel, M.; di Mola, F.F.; Selvaggi, F.; Mascetta, G.; Wente, M.N.; Felix, K.; Giese, N.A.; Hinz, U.; Di Sebastiano, P.; Buchler, M.W.; et al. Vanilloids in pancreatic cancer: Potential for chemotherapy and pain management. Gut 2006, 55, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Ayakannu, T.; Taylor, A.H.; Konje, J.C. Expression of the putative cannabinoid receptor GPR55 is increased in endometrial carcinoma. Histochem. Cell Biol. 2021, 156, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Mutch, D.G. The new FIGO staging system for cancers of the vulva, cervix, endometrium and sarcomas. Gynecol. Oncol. 2009, 115, 325–328. [Google Scholar] [CrossRef]
- Marincsák, R.; Tóth, B.; Czifra, G.; Márton, I.; Rédl, P.; Tar, I.; Tóth, L.; Kovács, L.; Bíró, T. Increased expression of TRPV1 in squamous cell carcinoma of the human tongue. Oral. Diseases 2009, 15, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Czifra, G.; Varga, A.; Nyeste, K.; Marincsák, R.; Tóth, B.I.; Kovács, I.; Kovács, L.; Bíró, T. Increased expressions of cannabinoid receptor-1 and transient receptor potential vanilloid-1 in human prostate carcinoma. J. Cancer Res. Clin. Oncol. 2009, 135, 507–514. [Google Scholar] [CrossRef]
- Ayakannu, T.; Taylor, A.H.; Marczylo, T.H.; Konje, J.C. New insights of uterine leiomyoma pathogenesis: Endocannabinoid system. Med. Sci. Monit. Basic. Res. 2019, 25, 76–87. [Google Scholar] [CrossRef]
- Mistretta, F.; Buffi, N.M.; Lughezzani, G.; Lista, G.; Larcher, A.; Fossati, N.; Abrate, A.; Dell’Oglio, P.; Montorsi, F.; Guazzoni, G.; et al. Bladder cancer and urothelial impairment: The role of TRPV1 as potential drug target. Biomed. Res. Int. 2014, 2014, 987149. [Google Scholar] [CrossRef] [PubMed]
- Lozano, C.; Cordova, C.; Marchant, I.; Zuniga, R.; Ochova, P.; Ramirez-Barrantes, R.; Gonzalez-Arriagada, W.A.; Rodriguez, B.; Olivero, P. Intracellular aggregated TRPV1 is associated with lower survival in breast cancer patients. Breast Cancer Targets Ther. 2018, 10, 161–168. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Chiang, C.; Xiao, T.; Chen, Y.; Zhao, Y.; Zheng, D. The impact of TRPV1 on cancer pathogenesis and therapy: A systematic review. Int. J. Biol. Sci. 2021, 17, 2034–2049. [Google Scholar] [CrossRef] [PubMed]
- Han, G.H.; Chay, D.B.; Nam, S.; Cho, H.; Chung, J.Y.; Kim, J.H. Prognostic significance of transient receptor potential vanilloid type 1 (TRPV1) and phosphatase and tension homolog (PTEN) in epithelial ovarian cancer. Cancer Genom. Proteom. 2020, 17, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Culjkovic-Kraljacic, B.; Borden, K.L.B. The impact of post-transcriptional control: Better living through RNA regulons. Front. Genet. 2018, 9, 512. [Google Scholar] [CrossRef] [PubMed]
- McKallip, R.J.; Nagarkatti, M.; Nagarkatti, P.S. Delta-9-tetrahydrocannabinol enhances breast cancer growth and metastasis by suppression of the antitumor immune response. J. Immunol. 2005, 174, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, H.; Ning, W.; Backlund, M.G.; Dey, S.K.; DuBois, R.N. Loss of cannabinoid receptor 1 accelerates intestinal tumor growth. Cancer Res. 2008, 68, 6468–6476. [Google Scholar] [CrossRef]
- Cudaback, E.; Marrs, W.; Moeller, T.; Stella, N. The expression level of CB1 and CB2 receptors determines their efficacy at inducing apoptosis in astrocytomas. PLoS ONE 2010, 5, e8702. [Google Scholar] [CrossRef]
- Gustafsson, K.; Wang, X.; Severa, D.; Eriksson, M.; Kimby, E.; Merup, M.; Christensson, B.; Flygare, J.; Sander, B. Expression of cannabinoid receptors type 1 and type 2 in non-Hodgkin lymphoma: Growth inhibition by receptor activation. Int. J. Cancer 2008, 123, 1025–1033. [Google Scholar] [CrossRef]
- Chung, S.C.; Hammarsten, P.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Mancini, G.; Lutz, B.; Bergh, A.; Fowler, C.J. A high cannabinoid CB1 receptor immunoreactivity is associated with disease severity and outcome in prostate cancer. Eur. J. Cancer 2009, 45, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Michalski, C.W.; Oti, F.E.; Erkan, M.; Sauliunaite, D.; Bergmann, F.; Pacher, P.; Batkai, S.; Müller, M.W.; Giese, N.A.; Friess, H.; et al. Cannabinoids in pancreatic cancer: Correlation with survival and pain. Int. J. Cancer 2008, 122, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, S.B.; Lindgren, T.; Jonsson, M.; Jacobsson, S.O.P. Cannabinoid receptor-independent cytotoxic effects of cannabinoids in human colorectal carcinoma cells: Synergism with 5-fluorouracil. Cancer Chemother. Pharmacol. 2009, 63, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Ligresti, A.; De Petrocellis, L.; Hernán Pérez de la Ossa, D.; Aberturas, R.; Cristino, L.; Moriello, A.S.; Finizio, A.; Gil, M.E.; Torres, A.I.; Molpeceres, J.; et al. Exploiting nanotechnologies and TRPV1 channels to investigate the putative anandamide membrane transporter. PLoS ONE 2010, 5, e10239. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.Y.; Gavva, N.R. Therapeutic potential of vanilloid receptor TRPV1 agonists and antagonists as analgesics: Recent advances and setbacks. Brain Res. Rev. 2009, 60, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.M.; Newstead, S.; Swartz, K.J.; Sansom, M.S. Capsaicin interaction with TRPV1 channels in a lipid bilayer: Molecular dynamics simulation. Biophys. J. 2015, 108, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Devor, E.J.; Gonzalez-Bosquet, J.; Thiel, K.W.; Leslie, K.K. Genomic characterization of five commonly used endometrial cancer cell lines. Int. J. Oncol. 2020, 57, 1348–1357. [Google Scholar] [CrossRef] [PubMed]
- Chipuk, J.E.; Kuwana, T.; Bouchier-Hayes, L.; Droin, N.M.; Newmeyer, D.D.; Schuler, M.; Green, D.R. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 2004, 303, 1010–1014. [Google Scholar] [CrossRef]
- Muller, C.; Lynch, D.L.; Hurst, D.P.; Reggio, P.H. A closer look at anandamide interaction with TRPV1. Front. Mol. Biosci. 2020, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Leary, A.; Creutzberg, C.; Abu-Rustum, N.; Darai, E. Endometrial cancer. Lancet 2016, 387, 1094–1108. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Zhu, Y.; Eno, C.O.; Liu, Y.; Deleeuw, L.; Burlison, J.A.; Chaires, J.B.; Trent, J.O.; Li, C. Activation of the proapoptotic Bcl-2 protein Bax by a small molecule induces tumor cell apoptosis. Mol. Cell Biol. 2014, 34, 1198–1207. [Google Scholar] [CrossRef] [PubMed]
- Vandenabeele, P.; Bultynck, G.; Savvides, S.N. Pore-forming proteins as drivers of membrane permeabilization in cell death pathways. Nat. Rev. Mol. Cell Biol. 2023, 24, 312–333. [Google Scholar] [CrossRef]
- Wang, K.; Gross, A.; Waksman, G.; Korsmeyer, S.J. Mutagenesis of the BH3 domain of BAX identifies residues critical for dimerization and killing. Mol. Cell Biol. 1998, 18, 6083–6089. [Google Scholar] [CrossRef] [PubMed]
- Hao, Q.; Chen, J.; Lu, H.; Zhou, X. The ARTS of p53-dependent mitochondrial apoptosis. J. Mol. Cell Biol. 2023, 14, mjac074. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J. p53, the cellular gatekeeper for growth and division. Cell 1997, 88, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Menendez-Perez, C.; Rivas-Santisteban, R.; Del Valle, E.; Tolivia, J.; Navarro, A.; Franco, R.; Martinez-Pinilla, E. Heteromers Formed by GPR55 and Either Cannabinoid CB(1) or CB(2) Receptors Are Upregulated in the Prefrontal Cortex of Multiple Sclerosis Patients. Int. J. Mol. Sci. 2024, 25, 4176. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and extrinsic pathways of apoptosis: Role in cancer development and prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar] [CrossRef]
- Amantini, C.; Ballarini, P.; Caprodossi, S.; Nabissi, M.; Morelli, M.B.; Lucciarini, R.; Cardarelli, M.A.; Mammana, G.; Santoni, G. Triggering of transient receptor potential vanilloid type 1 (TRPV1) by capsaicin induces Fas/CD95-mediated apoptosis of urothelial cancer cells in an ATM-dependent manner. Carcinogenesis 2009, 30, 1320–1329. [Google Scholar] [CrossRef]
- Contassot, E.; Tenan, M.; Schnüriger, V.; Pelte, M.-F.; Dietrich, P.-Y. Arachidonyl ethanolamide induces apoptosis of uterine cervix cancer cells via aberrantly expressed vanilloid receptor-1. Gynecol. Oncol. 2004, 93, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, O.; Morelli, M.B.; Annibali, D.; Aguzzi, C.; Zeppa, L.; Tuyaerts, S.; Amantini, C.; Amant, F.; Ferretti, B.; Maggi, F.; et al. The Effects of Cannabidiol and Prognostic Role of TRPV2 in Human Endometrial Cancer. Int. J. Mol. Sci. 2020, 21, 5409. [Google Scholar] [CrossRef] [PubMed]
- McLaren, J.; Prentice, A.; Charnock-Jones, D.S.; Sharkey, A.M.; Smith, S.K. Immunolocalization of the apoptosis regulating proteins Bcl-2 and Bax in human endometrium and isolated peritoneal fluid macrophages in endometriosis. Hum. Reprod. 1997, 12, 146–152. [Google Scholar] [CrossRef]
- Kargl, J.; Balenga, N.; Parzmair, G.P.; Brown, A.J.; Heinemann, A.; Waldhoer, M. The cannabinoid receptor CB1 modulates the signaling properties of the lysophosphatidylinositol receptor GPR55. J. Biol. Chem. 2012, 287, 44234–44248. [Google Scholar] [CrossRef]
- Sakuragi, N.; Salah-eldin, A.E.; Watari, H.; Itoh, T.; Inoue, S.; Moriuchi, T.; Fujimoto, S. Bax, Bcl-2, and p53 expression in endometrial cancer. Gynecol. Oncol. 2002, 86, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Konje, J.C.; Ayakannu, T. Identification of Potentially Novel Molecular Targets of Endometrial Cancer Using a Non-Biased Proteomic Approach. Cancers 2023, 15, 4665. [Google Scholar] [CrossRef]
- Vaskivuo, T.E.; Stenbäck, F.; Karhumaa, P.; Risteli, J.; Dunkel, L.; Tapanainen, J.S. Apoptosis and apoptosis-related proteins in human endometrium. Mol. Cell Endocrinol. 2000, 165, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, H.; Yoon, H. ER Stress-Mediated Signaling: Action Potential and Ca(2+) as Key Players. Int. J. Mol. Sci. 2016, 17, 1558. [Google Scholar] [CrossRef]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Bisogno, T.; Trevisani, M.; Al-Hayani, A.; De Petrocellis, L.; Fezza, F.; Tognetto, M.; Petros, T.J.; Krey, J.F.; Chu, C.J.; et al. An endogenous capsaicin-like substance with high potency at recombinant and native vanilloid VR1 receptors. Proc. Natl. Acad. Sci. USA 2002, 99, 8400–8405. [Google Scholar] [CrossRef] [PubMed]
- Creasman, W. Revised FIGO staging for carcinoma of the endometrium. Int. J. Gynaecol. Obstet. 2009, 105, 109. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Habayeb, O.M.H.; Taylor, A.H.; Bell, S.C.; Taylor, D.J.; Konje, J.C. Expression of the endocannabinoid system in human first trimester placenta and its role in trophoblast proliferation. Endocrinology 2008, 149, 5052–5060. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.H.; Guzail, M.; Wahab, M.; Thompson, J.R.; Al-Azzawi, F. Quantitative histomorphometric analysis of gonadal steroid receptor distribution in the normal human endometrium through the menstrual cycle. Histochem. Cell Biol. 2005, 123, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Thornton, M.J.; Taylor, A.H.; Mulligan, K.; Al-Azzawi, F.; Lyon, C.C.; O’Driscoll, J.; Messenger, A.G. Oestrogen receptor beta is the predominant oestrogen receptor in human scalp skin. Exp. Dermatol. 2003, 12, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.M.; Kim, Y.K.; Chung, J.H. Increased expression of TRPV1 channel in intrinsically aged and photoaged human skin in vivo. Exp. Dermatol. 2009, 18, 431–436. [Google Scholar] [CrossRef]


| RT-Qpcr * | |||
|---|---|---|---|
| Tissue Type | Designation | Age (Years) | BMI (kg/m2) |
| Control | Atrophic (6) | 60.67 ± 4.27 | 26.67 ± 6.50 |
| Type 1 EC | Grade 1 (6) | 66.17 ± 16.14 | 33.50 ± 8.92 |
| Grade 2 (6) | 66.50 ± 10.25 | 32.00 ± 5.97 | |
| Grade 3 (3) | 72.67 ± 12.06 | 35.33 ± 6.11 | |
| Type 2 EC | Serous (3) | 59.00 ± 3.46 | 37.67 ± 2.52 |
| Carcinosarcoma (3) | 50.00 ± 5.00 | 36.67 ± 6.43 | |
| Immunohistochemistry | |||
| Tissue type | Designation | Age (years) | BMI (kg/m2) |
| Control | Atrophic (6) | 60.67 ± 4.27 | 26.67 ± 6.50 |
| Type 1 EC | Grade 1 (6) | 62.50 ± 13.90 | 33.00 ± 8.76 |
| Grade 2 (6) | 65.17 ± 9.86 | 34.83 ± 5.56 | |
| Grade 3 (6) | 66.83 ± 7.88 | 31.50 ± 3.08 | |
| Type 2 EC | Serous (4) | 70.25 ± 10.97 | 33.00 ± 6.83 |
| Carcinosarcoma (6) | 58.33 ± 7.42 | 35.50 ± 5.99 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayakannu, T.; Taylor, A.H.; Konje, J.C. Expression, Distribution and Function of the Transient Receptor Potential Vanilloid Type 1 (TRPV1) in Endometrial Cancer. Int. J. Mol. Sci. 2025, 26, 3104. https://doi.org/10.3390/ijms26073104
Ayakannu T, Taylor AH, Konje JC. Expression, Distribution and Function of the Transient Receptor Potential Vanilloid Type 1 (TRPV1) in Endometrial Cancer. International Journal of Molecular Sciences. 2025; 26(7):3104. https://doi.org/10.3390/ijms26073104
Chicago/Turabian StyleAyakannu, Thangesweran, Anthony H. Taylor, and Justin C. Konje. 2025. "Expression, Distribution and Function of the Transient Receptor Potential Vanilloid Type 1 (TRPV1) in Endometrial Cancer" International Journal of Molecular Sciences 26, no. 7: 3104. https://doi.org/10.3390/ijms26073104
APA StyleAyakannu, T., Taylor, A. H., & Konje, J. C. (2025). Expression, Distribution and Function of the Transient Receptor Potential Vanilloid Type 1 (TRPV1) in Endometrial Cancer. International Journal of Molecular Sciences, 26(7), 3104. https://doi.org/10.3390/ijms26073104






