Abstract
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains a significant global health challenge, affecting millions annually and leading to substantial mortality, particularly in developing countries. The pathogen’s ability to persist latently and evade host immunity, combined with the emergence of drug-resistant strains, underscores the need for innovative therapeutic strategies. This review highlights the crucial role of interleukin-12 (IL-12) in coordinating immune responses against TB, focusing on its potential as an immunotherapy target. IL-12, a key Th1 cytokine, enhances cellular immunity by promoting Th1 cell differentiation and IFN-γ production, vital for Mtb clearance. By stimulating cytotoxic T lymphocytes and establishing immune memory, IL-12 supports robust host defense mechanisms. However, the complexity of IL-12 biology, including its roles in pro-inflammatory and regulatory pathways, necessitates a nuanced understanding for effective therapeutic use. Recent studies have shown how IL-12 impacts T cell synapse formation, exosome-mediated bystander activation, and interactions with other cytokines in shaping T cell memory. Genetic defects in the IL-12/IFN-γ axis link to susceptibility to mycobacterial diseases, highlighting its importance in TB immunity. The review also addresses challenges like cytokine imbalances seen in TNF-α/IFN-γ synergy, which exacerbate inflammation, and the implications for IL-12-based interventions. Research into modulating IL-12, including its use as an adjuvant and in recombinant vaccines, promises improved TB treatment outcomes and vaccine efficacy. The review concludes by stressing the need for continued investigation into IL-12’s molecular mechanisms towards precision immunotherapies to combat TB and its complications.
1. Introduction
Tuberculosis (TB), a severe chronic infectious disease caused by Mycobacterium tuberculosis (Mtb), remains a major global health threat [1]. According to the World Health Organization (WHO), TB affects approximately one-quarter of the global population, with tens of millions of new cases diagnosed annually, resulting in nearly one million deaths, predominantly in developing countries [2,3]. Despite advances in TB research and control, significant challenges persist, including the continued spread of the disease and the complexities of treatment. The dormancy of Mtb is a formidable challenge; even after standard treatment, dormant bacteria can persist in vivo and relapse unpredictably. This underscores the inadequacy of current treatment regimens in eradicating all bacteria, especially those in a dormant state, thus highlighting the need for new therapies targeting these latent bacteria [4]. Furthermore, the emergence of multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB compounds the difficulty of treatment [5,6]. Consequently, there is an urgent need for innovative treatment strategies, including immunotherapy, to enhance the host’s ability to eliminate bacteria, reduce recurrence risk, and improve overall treatment outcomes.
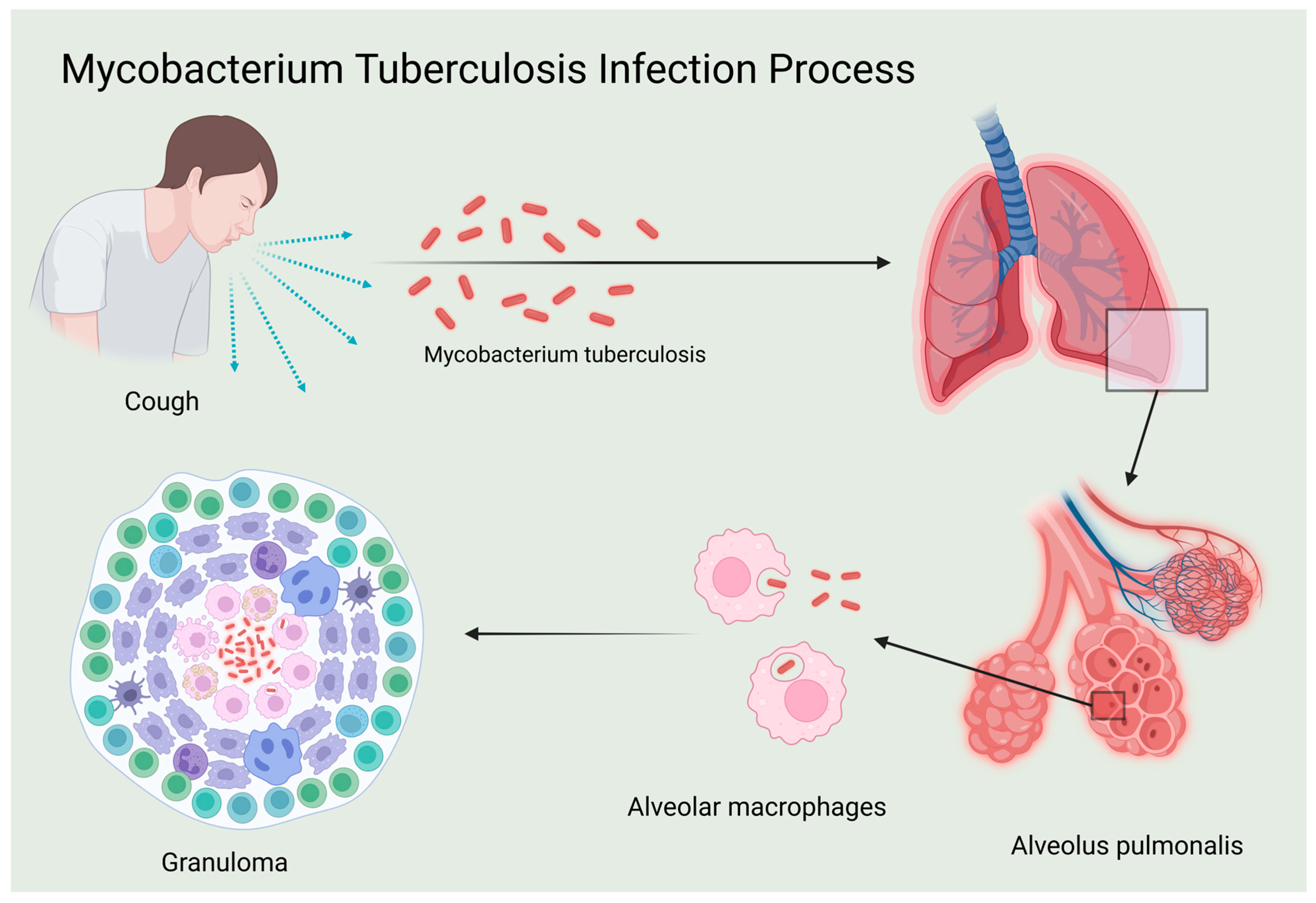
At the immunological level, the host’s immune system plays a crucial role in combating Mtb. TB initially infects macrophages and dendritic cells, impairing their antigen-presenting capabilities and leading to latency and potential recurrence. Most infected individuals develop latent tuberculosis infection (LTBI) [7]. During infection, the pathogen can evade the immune response and become dormant within lesions, maintaining a host–pathogen balance through granuloma formation. Resurgence of Mtb may occur when host immunity is compromised, with approximately 5% to 15% of LTBI cases progressing to active tuberculosis (ATB) [8]. Following infection, macrophages and dendritic cells release cytokines such as TNF-α, IL-1, IL-6, and IL-12, which are crucial in regulating immune response [9]. IL-12 is a critical cytokine that activates and enhances innate and cellular immune responses, notably by promoting the Th1 cellular immune response through stimulating Th1 cells to release interferon-gamma (IFN-γ), thereby enhancing the host’s ability to clear Mtb. In addition to promoting Th1 cell activity, IL-12 indirectly activates cytotoxic T lymphocytes (CTLs), which directly kill infected cells. Cellular immunity also involves the formation of immune memory, enabling a quicker and more effective response to subsequent infections by the same pathogen [10]. Therefore, IL-12 is pivotal in the immune response against Mtb and is crucial for controlling and eliminating TB bacteria (Figure 1).
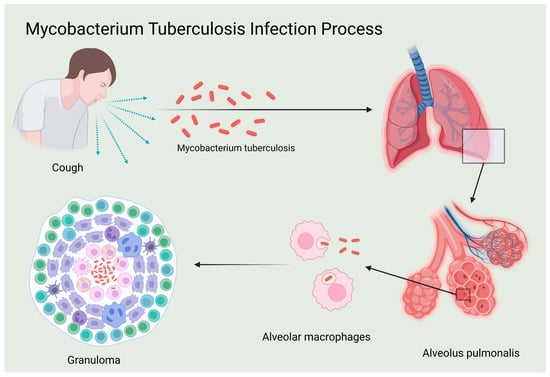
Figure 1.
Mycobacterium tuberculosis infection process.
In recent years, researchers have made significant efforts to deepen the understanding of the immunological mechanisms underlying tuberculosis, particularly the role of IL-12 in immune regulation. Further investigation into the function and regulation of IL-12 holds promise for developing more effective TB treatment and prevention strategies [11,12]. This paper aims to systematically explore the critical role and potential applications of IL-12 in the immune regulation of tuberculosis, providing new avenues for treatment and prevention.
2. Overview of IL-12
2.1. Structure and Molecular Biological Characteristics of IL-12 Family
The IL-12 family consists of several cytokines, including IL-12, IL-23, IL-27, and IL-35, which play crucial roles in immune regulation and inflammation. Among them, IL-12 is the most extensively studied for its role in Mtb infection.
IL-12 is a heterodimer composed of the p35 and p40 subunits [11], primarily synthesized by antigen-presenting cells (APCs) such as macrophages and dendritic cells. Upon binding to its receptor, IL-12 activates signaling pathways such as JAK-STAT, enhancing Th1 cell differentiation and promoting CTL activity, both of which are vital for the immune response to TB [12,13]. IL-23 shares the p40 subunit with IL-12 but includes a distinct p19 subunit. IL-23 is primarily involved in the differentiation and maintenance of Th17 cells, which are important in inflammatory responses. Although its role in TB is less studied, IL-23 may influence the inflammatory environment during active TB, particularly in cases of chronic inflammation or autoimmune involvement [14,15]. IL-27, composed of p28 and EBI3 subunits, regulates both Th1 and Th17 responses. It is involved in immune homeostasis and can modulate immune responses to prevent excessive inflammation. In the context of TB, IL-27 may play a dual role by promoting Th1 responses while controlling overactive immune reactions that could lead to tissue damage [10,16]. IL-35, a heterodimer formed by p35 and EBI3 subunits [17], is primarily produced by regulatory T cells (Tregs). It exhibits potent immunosuppressive activity and plays a key role in maintaining immune tolerance. IL-35 may contribute to the regulation of immune responses, balancing the need for strong defense against Mtb while preventing excessive inflammation that could harm the host [18,19,20,21].
Despite structural and functional disparities among IL-12 family members, their collective involvement significantly influences the body’s immune equilibrium and the onset and progression of various diseases.
2.2. IL-12 Production and Signal Transduction
2.2.1. Generation of IL-12
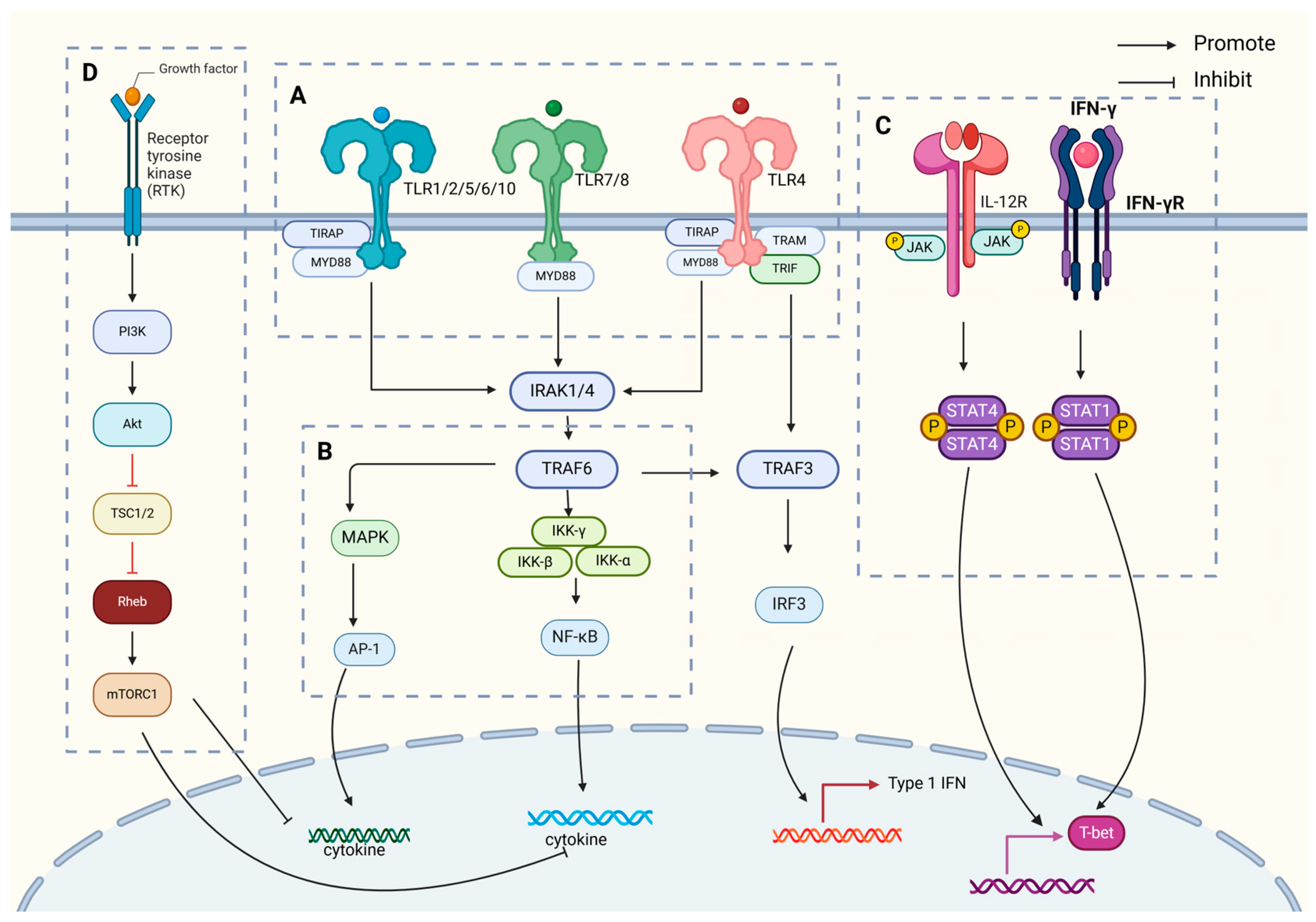
IL-12 is primarily synthesized by APCs, including dendritic cells, macrophages, neutrophils, and B lymphocytes, in response to antigenic stimuli. Its production is regulated by a complex interplay of immune signals, including cytokines such as TNF-α [22] and IL-1β [23], as well as by Th1 cells releasing IFN-γ. This results in a positive feedback loop, amplifying IL-12 production and promoting a robust immune response. IL-12 plays a crucial role in initiating adaptive immunity by supporting Th1 cell differentiation and enhancing CTL activity, both essential for effective immune defense (Figure 2).
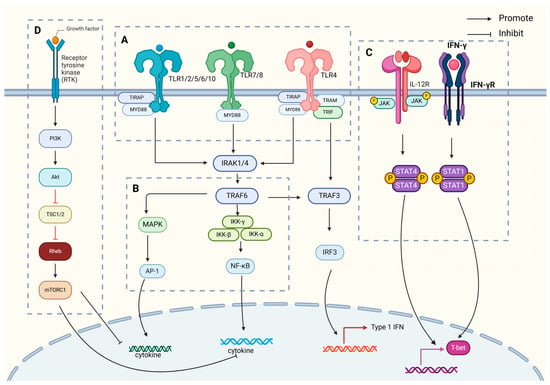
Figure 2.
IL-12 generation associated signal transduction pathway diagram. The key signal regulation mechanisms of IL-12 generation mainly include the following. (A) TLR-mediated dual-track signal initiation: After TLR recognizes PAMPs in the MyD88-dependent pathway, IRAK family members are summoned to form an activation complex, which activates downstream NF-κB and MAPK signals through TRAF6, directly promoting the transcription and secretion of IL-12 p40/p35 subunits. The TRIF pathway promotes IRF3 phosphorylation by activating TBK1/IKKε, which not only promotes the massive production of IFN-β, but also indirectly enhances the expression of IL-12 p35 subunit. (B) CLR-assisted signal enhancement: Recognition of sugar structures on the surface of specific pathogens by CLR activates the SYK/CARD9 axis, which further promotes IL-12 production through NF-κB, MAPK, and IRF pathways. (C) Establishment of JAK-STAT positive feedback loop: After IL-12 binds to its receptor, JAK kinase is activated, STAT1/4 is phosphorylated, and ISRE is bound to promote IL-12 expression. (D) Fine regulation of PI3K/Akt pathway: The dual regulatory role of PI3K/Akt signal IL-12 expression includes, on the one hand, the inhibition of IL-12 synthesis through mTOR, and on the other hand, promotion of IL-10 production to balance excessive inflammatory response. At the same time, PI3K regulates the expression of various inflammatory factors, including IL-12, by fine-tuning NF-κB activity.
2.2.2. Signal Transduction Regulation—Related Signaling Pathways of IL-12 Generation
- Major Signaling Pathway 1: Toll-like Receptor (TLR) Signaling Pathway
IL-12 production is regulated by APCs, specifically dendritic cells, through the TLR signaling pathway upon pathogen recognition. TLRs are receptors that bind pathogen-associated molecular patterns (PAMPs) such as bacterial lipopolysaccharides and viral nucleic acids. This engagement activates two key signaling cascades [24,25]: the MyD88-dependent and TRIF-dependent pathways.
In the MyD88-dependent pathway [26], MyD88 recruits IRAK proteins, leading to the activation of TRAF6 and subsequent NF-κB and MAPK signaling pathways. These pathways upregulate the transcription of IL-12 subunits (p40 and p35), promoting IL-12 production, which is crucial for initiating adaptive immunity by promoting Th1 cell differentiation and enhancing NK and CTL activity [27].
In the TRIF-dependent pathway, TRIF activates interferon regulatory factors (IRF3, IRF7) [28,29], enhancing IL-12 production by triggering the activation of TBK1 and IKK-ε, which further induce type I interferons (e.g., IFN-β) and inflammatory factors [30].
- Major Signaling Pathway 2: C-Type Lectin Receptor (CLR) Signaling Pathway
The CLR signaling pathway is critical for IL-12 production in response to pathogen recognition by antigen-presenting cells [31]. CLRs, expressed on dendritic cells and macrophages, bind glycosylated pathogen antigens such as bacterial polysaccharides and parasitic glycoproteins [32]. Ligand binding triggers downstream signaling through SYK and CARD9 [33,34], activating NF-κB and MAPK pathways, which enhance IL-12 gene transcription [34,35,36,37].
The activation of CLR also engages transcription factors from NF-κB, AP-1, and IRF families [35,36], which are essential for promoting IL-12 gene expression [37,38].
- Major Signaling Pathway 3: Intracellular Signal Transduction Pathway—JAK-STAT
The JAK-STAT pathway is essential for IL-12 signaling. Upon binding to its receptor IL-12R, IL-12 activates JAK kinases [39,40], which are phosphorylate STAT proteins (notably STAT1 and STAT4) [41,42,43]. These phosphorylated STATs form dimers and translocate to the nucleus, binding to IL-12 response elements (ISRE), promoting the transcription of IL-12 genes [42,43,44,45,46,47,48,49]. This pathway not only induces Th1 cell differentiation but also amplifies IFN-γ production, which further enhances IL-12 synthesis, creating a positive feedback loop that strengthens the immune response to pathogens.
- Others: PI3K/Akt Signaling Pathway
The PI3K/Akt pathway plays a dual role in regulating IL-12 production. The activation of PI3K by TLRs (especially TLR4) leads to Akt phosphorylation, which modulates inflammatory cytokine production [47,48,49]. On one hand, PI3K/Akt suppresses IL-12 production by targeting mTOR and other effectors [50,51,52], while on the other hand, it promotes the anti-inflammatory cytokine IL-10 [53], which helps regulate excessive inflammation [52]. PI3K also influences NF-κB activity, further regulating IL-12 production [54]. The complex regulation of IL-12 by PI3K highlights its role in balancing pro-inflammatory and anti-inflammatory responses [55].
2.3. Modification/Regulation of IL-12 Transcription and Translation
IL-12 production is regulated at multiple levels, including transcriptional and translational processes. Transcription of the IL-12 gene is initiated by signaling through TLRs and CLRs, which activate intracellular pathways like NF-κB and JAK-STAT. These signaling events ensure a prompt response to pathogen-associated signals, with transcription factors such as NF-κB, AP-1, and STATs directly binding to the IL-12 promoter to enhance transcription [56,57].
Co-transcription factors like p300/CBP (transcription coactivating protein 300/CREB binding protein) [54,58] and CBP (CREB binding protein) [59] further modulate IL-12 gene expression through epigenetic modifications, such as acetylation, which enhances gene promoter accessibility and transcriptional efficiency.
At the translational level, IL-12 production is controlled by factors influencing mRNA stability, translation initiation, and post-transcriptional modifications. mRNA stability is regulated by RNA-binding proteins such as AU-rich element-binding proteins [56], which affect IL-12 mRNA degradation [60]. Translation initiation is mediated by factors like eIF-2α, eIF-2B, and eIF-4E [57,61], which ensure efficient mRNA translation [57,60,62]. These processes are regulated by signaling pathways like mTOR and MAPK [63,64], linking extracellular stimuli to translation efficiency.
The mTOR pathway, in particular, is central to controlling ribosome function and adjusting IL-12 production in response to cellular needs, further ensuring efficient immune responses to diverse stimuli.
2.4. Secretion of IL-12
The secretion of IL-12 is a carefully orchestrated, multi-step process, beginning with the synthesis of the p35 and p40 subunits, which are transcribed and translated to form IL-12 dimers in the endoplasmic reticulum (ER) [65,66,67]. These dimers are then transported to the Golgi apparatus and early endosomes, where they undergo further modifications [68]. Following this, IL-12 dimers are carried in vesicles to the plasma membrane, where they fuse and release IL-12 into the extracellular space. This process is tightly regulated by various cytokines, including TNF-α and IFN-γ, as well as intercellular interactions, particularly between T cells and dendritic cells. Such regulatory mechanisms ensure the precise modulation of IL-12 secretion, which plays a crucial role in the coordination of immune responses.
2.5. Regulation of IL-12
To maintain immune system equilibrium and prevent hyperinflammatory responses, interleukin-12 (IL-12) synthesis and activity are modulated by a spectrum of positive and negative regulators.
- (1)
- Negative Regulation
Direct inhibitors of IL-12 production include IL-10, the transforming growth factor-β (TGF-β), interleukin-4 (IL-4), interleukin-13 (IL-13), and prostaglandin E2 (PGE2) [69]. IL-10 obstructs IL-12 production in antigen-presenting cells by inhibiting NF-κB and STAT4 signaling pathways [70,71]. TGF-β employs the Smad signaling mechanism to hinder IL-12 synthesis, fostering immune tolerance [72]. IL-4 and IL-13 promote Th2 immune responses, diminishing IL-12 production via Stat6 pathway activation [73]. PGE2 limits NF-κB activation through EP2/EP4 receptors, curbing IL-12 production.
Indirect inhibitory mechanisms on IL-12 function are multifaceted and include signaling molecules like suppressor of cytokine signaling 1 (SOCS1) and suppressor of cytokine signaling 3 (SOCS3). These harmful feedback proteins bind to JAK/STAT signaling pathway nodes, diminishing IL-12 signaling efficiency. Immune checkpoint molecules like cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) [74] and programmed cell death protein 1 (PD-1) [75] reduce IL-12 necessity by competitively binding costimulatory molecules, thus depleting T cell function. Although interferon-β (IFN-β) and interferon-α (IFN-α) occasionally boost IL-12 production, they also elevate SOCS protein expression [76], which dampens IL-12 function. TNF-α’s effect varies depending on the physiological and pathological context.
Moreover, upon activation, specific G-protein-coupled receptors, like adenosine A2A receptors, modulate immune cell function, including diminishing dendritic cells’ ability to produce IL-12 [77,78]. These harmful regulatory mechanisms collectively ensure appropriate IL-12 expression, averting excessive inflammation and autoimmune diseases (Table 1).

Table 1.
IL-12 Negative Regulators and Their Mechanisms of Action.
- Exogenous Drug Interventions
Exogenous drug interventions targeting IL-12 and IL-23, such as Ustekinumab [79,80] and Briakinumab [81], effectively block the shared p40 subunit of both cytokines. Consequently, the activation of Th1 and Th17 cells and the production of related pro-inflammatory cytokines are diminished, significantly impacting inflammatory diseases like psoriasis and psoriatic arthritis. Additionally, IL-23-specific inhibitors like Tildrakizumab and Risankizumab [79] indirectly affect IL-12 activity by binding the p19 subunit of IL-23, effectively controlling Th17 cell activation and inflammatory mediator secretion, thereby ameliorating related diseases. Furthermore, Pitrakinra, a competitive antagonist against the IL-4/IL-13 receptor, mainly treats asthma and allergic diseases by competitively binding the IL-12 receptor, mitigating the effects of IL-4 and IL-13, and easing airway inflammation.
- (2)
- Positive regulation
The positive regulation of IL-12 stands as a pivotal process in orchestrating pro-inflammatory responses, particularly those of the Th1 immune pathway, crucial for effective immune surveillance and host defense mechanisms. The regulatory cascade encompasses multifaceted interactions among an array of molecular constituents, cellular entities, and signaling pathways. Initially, the recognition of PAMPs by TLRs, notably TLR-2 [82], TLR-4 [83,84], TLR-5 [85,86], and TLR-9 [87,88], following the detection of bacterial lipopolysaccharides (LPS), flagellin, and diverse microbial components, precipitates downstream signaling events that robustly induce IL-12 production across monocytes, macrophages, and DCs. This T-cell-independent mechanism underpins the rapid innate immune response against early infections. Furthermore, a positive feedback loop involving IFN-γ potentiates IL-12 production, as its secretion by activated NK cells and T cells stimulates mononuclear/macrophages, perpetuating IL-12 synthesis and reinforcing the Th1 immune axis. Additionally, IL-18-mediated activation of NF-κB and MAPK signaling cascades augments IL-12 transcription and expression, intensifying IL-12’s biosynthesis and functional efficacy, and further bolstering Th1 immune response development. Despite the canonical categorization of IL-4 and IL-13 as Th2 cytokines, their nuanced interplay with dendritic cells can facilitate IL-12 subunit transcription, notably p40 and p35, thereby enhancing IL-12 production, notably in the later phases of treatment [89]. This interleukin-induced modulation, complemented by intercellular interactions such as CD40-CD40 ligand (CD40L) engagement between T cells and dendritic cells or macrophages [90,91], fosters IL-12 synthesis, mainly through the preferential induction of p35 gene transcription in dendritic cells, thereby promoting IL-12 heterodimer assembly. In sum, the positive regulation of IL-12 epitomizes a finely tuned interplay of signaling molecules and cellular interactions, safeguarding adequate pathogen clearance and immune homeostasis (Table 2).

Table 2.
IL-12 Positive Regulators and Their Mechanisms of Action.
3. The Regulatory Mechanism of IL-12 on Immune Cells
IL-12 stands as a pivotal regulator in both adaptive and innate immune responses, exerting a profound influence on the activation and differentiation of Th1 cells. Firstly, IL-12 assumes a guiding role in driving Th1 cell differentiation [92], first demonstrated in IL-12p40-deficient murine models [93] and further characterized through STAT4-dependent signaling pathways [94].
Secondly, IL-12 contributes to the sustained proliferation and heightened activity of Th1 cells. Following differentiation, Th1 cells predominantly secrete IFN-γ, and IL-12 presence enhances IFN-γ production, establishing a reinforcing feedback loop that amplifies Th1 cell expansion and maintains their heightened activity. Additionally, IL-12 is crucial in preserving the Th1/Th2 cell balance by impeding Th2 cell differentiation [95]. Given the proclivity of Th1 cell-mediated responses towards cell-mediated immunity, their role is particularly pertinent in combating intracellular infections such as Mtb and hepatitis B virus (HBV) [96]. Noteworthy associations have been elucidated between IL-12 levels and serum HBV DNA content in patients with chronic hepatitis B, underscoring its clinical significance [97]. Moreover, IL-12 exerts regulatory influence over Th1/Th2 class cytokine production by peripheral blood mononuclear cells (PBMC), further emphasizing its integral role in immune homeostasis.
IL-12 plays a multifaceted role in regulating the function of NK cells, underscoring its significance in immune modulation [98]. Firstly, IL-12 directly or indirectly enhances NK cell activity, fostering proliferation and augmenting cytotoxicity, mainly when NK cells interact with APCs expressing IL-12. This interaction potentiates NK cell-mediated destruction of virus-infected and tumor cells, bolstering immune surveillance mechanisms. Secondly, IL-12 stimulation induces NK cells and selected lymphocytes, notably CD4+ Th1 cells, to secrete IFN-γ. Moreover, IL-12 signals, in concert with additional costimulatory cues such as IL-15 or TLR agonists [99], synergistically enhance NK cell-killing activity, amplifying their effector functions against pathogens and malignancies. Finally, the IL-12-mediated induction of IFN-γ production is a crucial determinant in steering the Th1 type immune response [100], vital for combating intracellular parasites and specific tumor types.
Under the regulatory influence of IL-12, macrophages demonstrate heightened activation properties, substantially enhancing their phagocytic and bactericidal capabilities [101,102]. Macrophages, adept at pathogen engulfment and destruction, further potentiate their cytotoxicity against infected cells by upregulating the production and release of pro-inflammatory mediators such as TNF-α and nitric oxide (NO) [103]. Concurrently, IL-12 optimizes macrophage antigen presentation proficiency, enabling these activated cells to intricately process and relay phagocytosed pathogen antigenic information to immune effectors like T cells, thereby augmenting overall immune responses. While facilitating the prompt initiation of inflammatory cascades and bolstering macrophage-mediated pathogen clearance, IL-12 also exerts precise control over the magnitude and duration of inflammatory reactions. This timely modulation ensures the inflammatory response remains regulated, preventing excessive tissue damage and maintaining immune homeostasis [104].
IL-12 plays a crucial role in the activation, differentiation, proliferation, and functional enhancement of cytotoxic T lymphocytes (CTLs, also known as CD8+ T cells), as well as in memory formation and tissue localization, underscoring its central importance in antiviral and antitumor immunity [105,106]. IL-12 synergizes with T cell receptor (TCR) signaling and costimulatory molecules to facilitate the differentiation of naïve CD8+ T cells into effector CTLs. This cytokine enhances the cytotoxic capacity of CTLs by upregulating the expression of lytic molecules such as perforin and granzyme B, thereby improving their efficacy in targeting virus-infected and tumor cells. Furthermore, IL-12 significantly induces CTLs to produce large amounts of IFN-γ [107], a potent antiviral and antitumor cytokine that not only directly inhibits pathogen replication and tumor growth but also exerts a wide range of immunoregulatory effects. These effects include enhancing macrophage antigen presentation, upregulating major histocompatibility complex (MHC) molecules, and promoting NK cell activation, thereby amplifying the overall immune response against pathogens and tumors. IL-12 is also instrumental in shaping the memory phenotype of CTLs, facilitating the formation of long-lasting immune memory [108]. CTLs stimulated by IL-12 can differentiate into memory T cells, which persist in the host and rapidly mount robust immune responses upon re-exposure to the same or similar antigens, providing durable protection. Additionally, IL-12 influences the migration and infiltration of CTLs into inflammatory sites or tumor microenvironments by modulating the expression of adhesion molecules and chemokine receptors, ensuring targeted cytotoxic effects where needed. Despite potential systemic toxicity issues in therapeutic applications, the pivotal role of IL-12 in immune regulation underscores its importance. Ongoing research aims to harness IL-12 or its analogs to enhance CTL activity safely and effectively, highlighting its potential in immunotherapeutic strategies.
4. Role of IL-12 in the Immune Response to Tuberculosis
IL-12, as a key pro-inflammatory cytokine, occupies a central position in the immune response to tuberculosis, and its normal function directly affects host resistance to Mtb infection and disease progression.
4.1. Influence of IL-12 in TB Pathogenesis
A. Susceptibility and disease progression: Genetic studies have revealed significant associations between polymorphisms in IL-12 and their receptor genes and individual TB susceptibility [109]. Specifically, defective or dysfunctional IL-12 genes may lead to reduced IL-12 biosynthesis, which in turn weakens the host’s innate immune defense against Mtb and increases the risk of infection [110]. After the onset of infection, changes in IL-12 levels reflect the strength and direction of the immune response. Low levels of IL-12 are often associated with elevated disease activity, deterioration, and poor response to therapy. Conversely, high levels of IL-12 tend to portend a better clinical prognosis, possibly stemming from a more effective anti-Mtb immune response and inflammatory control [111].
B. Immunomodulation and pathological processes: The central role of IL-12 in TB immunomodulation is reflected in its dual activation of the natural and adaptive immune systems. First, IL-12 stimulates the activation of macrophages and dendritic cells, enhancing their ability to phagocytose and kill Mtb. This is mainly through the upregulation of the NADPH oxidase-mediated oxidative burst [112], which induces the expression of acidified lysozyme and nitric oxide synthase (iNOS) [113], thereby promoting NO production, all of which are important mechanisms for the direct inhibition of Mtb growth. Second, IL-12 critically promotes Th1 cell differentiation and enhances macrophage function by stimulating IFN-γ production while initiating the synergistic secretion of Th1-type cytokines. In addition, IL-12 maintains an inflammatory microenvironment that favors Th1-type immune responses and prevents lung tissue damage caused by excessive inflammation, such as cavity formation and fibrosis, by suppressing Th2-type responses and the activity of regulatory T cells (Tregs) [114]. IL-12 deficiency may lead to an imbalance of immune responses, manifested by insufficient anti-Mtb immune effects or inflammatory responses that are excessive, exacerbating pathological changes in the lungs [115].
C. Therapeutic intervention and immune enhancement: IL-12 is widely regarded as a promising immunotherapeutic target given its key role in the immune response to TB. Currently, therapeutic strategies targeting IL-12 are divided into two main directions: First, direct supplementation of IL-12 to enhance immune response, including the use of recombinant IL-12 protein therapy or gene therapy to increase the level of IL-12 in the body, enhance the immune response of Th1 cells, and promote Mtb clearance. Preclinical studies, including seminal work by JoAnn Flynn’s group in murine TB models [116], have demonstrated that recombinant IL-12 administration significantly reduces pulmonary bacterial burden (1.5 log10 CFU reduction) and enhances granuloma resolution through IFN-γ-dependent macrophage activation, and IL-12 is even expected to be used for the prevention of TB relapse [117,118,119]. On the other hand, the modulation of IL-12-related signaling pathways by small molecule drugs or biologics is also a hot research topic. For example, the development of agonists targeting the IL-12 receptor or its downstream signaling molecules such as STAT4 can specifically enhance IL-12 signaling and promote immune cell function without increasing the risk of systemic inflammatory responses [120]. In addition, studies targeting negative regulators of IL-12 production, such as inhibitors of IL-10 or SOCS proteins [76,121], may help to deregulate the inhibition of IL-12 production and thus indirectly enhance its immune effects.
4.2. Interaction Mechanism Between IL-12 and Mtb
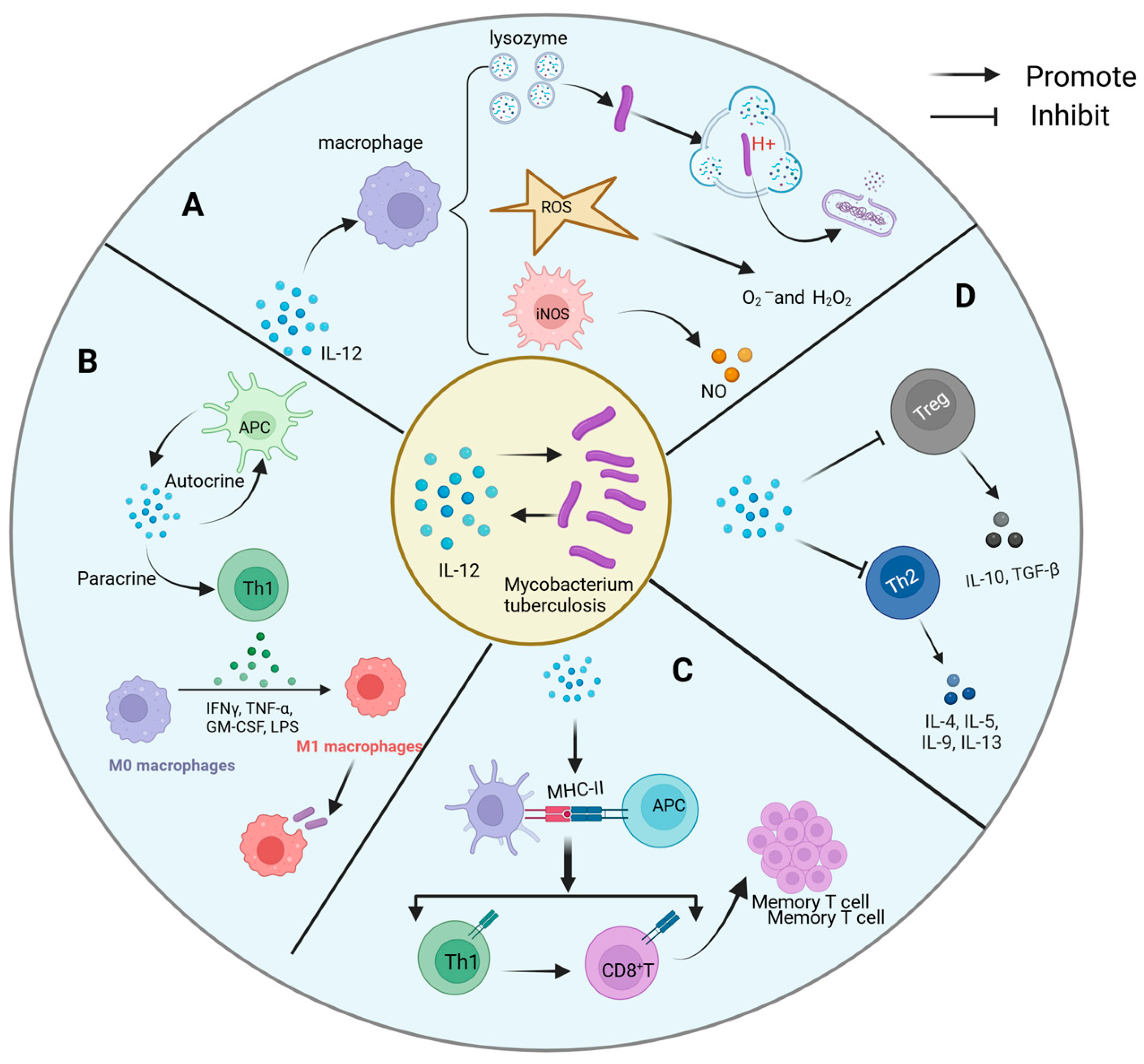
The interaction mechanism between IL-12 and Mtb is intricate and multifaceted, encompassing both direct and indirect pathways of influence (Figure 3).
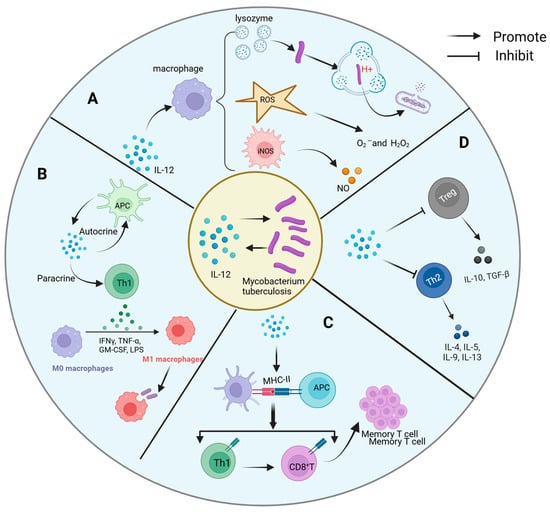
Figure 3.
Mechanism of interaction between IL-12 and Mycobacterium tuberculosis. The complex interaction mechanism between IL-12 and Mtb includes the following: (A) direct antibacterial action: IL-12 indirectly enhances the defense against Mtb by promoting macrophage activation. It promotes the production of ROS and NO, as well as the activation of acidifying lysozyme, mechanisms that work together to disrupt the structure of Mtb, inhibit its growth, and optimize the host’s first line of defense against Mtb. (B) Activation and differentiation of immune cells: IL-12 activates Th1 through autocrine and paracrine, resulting in the production of a large amount of IFN-γ, which strengthens the phagocytosis and bactericidal ability of macrophages. (C) Antigen presentation and memory formation: IL-12 can promote the maturation of APCs and enhance the expression of MHC molecules, activate specific Th1 and CD8+ T cells, and lay the foundation for the establishment of long-term immune memory. (D) Fine regulation of inflammatory microenvironment: IL-12 not only simply promotes Th1 response, but also inhibits Th2 response and Treg activity, preventing the imbalance of immune response, so as to achieve effective control of Mtb infection.
- A.
- Direct antibacterial activity
Regarding direct antibacterial activity, although IL-12 lacks inherent bactericidal capabilities, it significantly potentiates the effectiveness of immune cells against Mtb by orchestrating macrophage activation. Mechanistically, IL-12 operates on multiple fronts: Firstly, it fosters the generation of reactive oxygen species (ROS) within macrophages, including superoxide anions and hydrogen peroxide. These ROS molecules exert oxidative stress on Mtb, leading to structural damage and inhibition of bacterial proliferation. Secondly, IL-12 facilitates the upregulation of acidifying lysozyme expression and activity, thereby facilitating the degradation of Mtb’s cell wall components, including its characteristic mycobacteria acid, within acidic phagolysosomes. This disruption compromises bacterial integrity. Additionally, IL-12 stimulates the synthesis of NO, which disrupts essential life processes of Mtb, such as DNA synthesis, energy metabolism, and protein synthesis. Collaborating with ROS and other antibacterial mechanisms, NO amplifies the inhibitory effect on Mtb proliferation [122]. Through these concerted cellular activities, IL-12 fortifies macrophage defense mechanisms against Mtb, fundamentally restraining bacterial replication and survival within host cells.
- B.
- Immune cell activation and differentiation
IL-12 assumes a pivotal role in orchestrating the adaptive immune response, particularly in the activation and differentiation of T helper 1 (Th1) cells, which are crucial players in combating Mtb. Through diverse pathways, IL-12 potentiates the functionality of Th1 cells and mobilizes other immune effectors against Mtb. Primarily, IL-12 elicits robust IFN-γ production by Th1 cells, a pivotal cytokine in anti-Mtb immunity. IFN-γ exerts multifaceted effects, augmenting the antimicrobial prowess of macrophages by enhancing Mtb clearance mechanisms. This includes upregulating major histocompatibility complex class II (MHC-II) molecules, bolstering phagocytic activity, and promoting ROS and NO production within macrophages [123].
Furthermore, IL-12 sustains the proliferation and viability of Th1 cells via autocrine and paracrine pathways, ensuring continuous IFN-γ secretion. Additionally, IL-12 fosters immune memory formation by enhancing the expression of costimulatory molecules on Th1 cell surfaces, facilitating their interaction with APCs. Notably, Th1 cytokines such as IFN-γ and TNF-α, induced by IL-12, not only directly impact macrophage function but also activate cytotoxic CD8+ T cells and NK cells, bolstering the collective immune response against Mtb.
- C.
- Antigen presentation and immune memory
IL-12 plays a crucial role in enhancing Th1 cell differentiation and IFN-γ production, both of which are essential for controlling intracellular pathogens [124]. Additionally, recent studies have demonstrated that IL-12 can modulate the activity of regulatory T cells, further influencing the immune response. For instance, a study [125] has shown that IL-12 not only influences the differentiation of Th1 cells but also plays a significant role in regulating immune tolerance and modulating inflammatory responses. IL-12 exerts a profound influence on antigen presentation and immune memory, which are pivotal components of the host defense against Mtb. By promoting DC maturation and enhancing the expression of major histocompatibility complex MHC class I and II molecules, IL-12 significantly augments the efficiency of Mtb antigen presentation. This orchestrated process ensures the effective activation of specific T cells, particularly Th1 and CD8+ T cells, upon the initial Mtb encounter. Subsequently, following the resolution of infection, the Th1-polarized immune milieu fostered by IL-12 facilitates the generation and perpetuation of memory T cells [108]. These enduring memory T cells persist within the host for extended durations, poised to mount rapid and robust immune responses upon re-exposure to Mtb. This mechanism effectively curtails or prevents tuberculosis recurrence, underscoring the crucial role of IL-12 in long-term immune memory maintenance and host defense against Mtb.
- D.
- Shaping of the inflammatory microenvironment
IL-12 plays a pivotal role in shaping the inflammatory microenvironment by finely regulating cytokine networks, thereby ensuring dominance of the Th1-type immune response while concurrently suppressing Th2-type responses [95] and regulatory T cell (Tregs) activity [114]. This intricate regulatory mechanism, documented in prior studies, mitigates immunosuppression and tissue destruction, thus fostering effective anti-Mtb immune response. By orchestrating this targeted modulation of the inflammatory milieu, IL-12 prevents lung damage resulting from excessive inflammation, while concurrently promoting the efficient clearance of Mtb within the lesion site. This nuanced regulatory function underscores the critical importance of IL-12 in maintaining immune homeostasis and optimizing host defense mechanisms against Mtb infection.
4.3. IL-12 and Drug-Resistance Mycobacterium Tuberculosis
The molecular interplay between IL-12 and Mtb drug resistance is rooted in the cytokine’s dual role as a regulator of host immunity and a target of pathogen-driven immune evasion. IL-12, produced by DCs and macrophages upon Mtb recognition, drives Th1 differentiation via STAT4 signaling, leading to IFN-γ production—a critical mediator of macrophage bactericidal activity through NO synthesis and autophagy activation. However, Mtb employs sophisticated mechanisms to suppress IL-12 signaling, indirectly fostering drug resistance. For instance, Mtb-derived LAM binds TLR2 to inhibit DCs maturation, reducing IL-12 secretion and skewing the immune response toward an IL-10-dominated anti-inflammatory state. This immunosuppressive shift not only impairs bacterial clearance but also creates a permissive environment for subpopulations of Mtb to persist under suboptimal drug concentrations, enabling the accumulation of resistance-conferring mutations [126,127]. Notably, IL-12 deficiency exacerbates metabolic dysregulation in macrophages, such as heightened glycolysis via HIF-1αand impaired mitochondrial oxidative phosphorylation, which Mtb exploits to evade antimicrobial stress. Furthermore, Mtb strains with drug-resistance mutations exhibit altered cell wall lipids, which directly suppress IL-12 transcription by recruiting HDACs to the IL12B promoter, thereby dampening Th1 responses. Conversely, IL-12 enhances antigen presentation by upregulating MHC-II expression and counteracts IL-10-mediated inhibition of NF-κB, restoring pro-inflammatory signaling necessary for drug efficacy [128]. Emerging evidence also highlights IL-12’s role in modulating non-coding RNAs, which regulate pathways like autophagy and apoptosis, processes critical for eliminating drug-tolerant Mtb persisters [129,130]. These molecular interactions underscore IL-12 as a pivotal node in the host–pathogen conflict, where its suppression by Mtb not only compromises immune control but also indirectly perpetuates drug resistance by fostering bacterial survival and genetic adaptation [131]. Therapeutic strategies targeting IL-12 restoration—such as nanoparticle-delivered IL-12 mRNA or IL-10 receptor antagonists—may thus synergize with existing antibiotics to disrupt this vicious cycle [132].
5. Clinical Application of IL-12 in Diagnosis, Treatment, and Prognosis Evaluation of Tuberculosis
5.1. Diagnosis
- A.
- Importance of IL-12 in Tuberculosis Diagnosis
Previous investigations have established a correlation between IL-12 levels and TB severity and treatment outcomes [133]. Consequently, assessing IL-12 concentrations in TB patients’ serum or cerebrospinal fluid holds promise as a valuable diagnostic tool. Such measurements offer insightful data for evaluating disease activity, prognostication, and tailoring individualized treatment strategies.
- B.
- Potential Role of IL-12 and Other Cytokines in Tuberculosis Diagnosis
Ren et al. observed elevated serum levels of various cytokines, including IL-1β, IL-6, IL-8, IL-12p70, TNF-α, and IFN-γ, in patients with tuberculosis and tuberculosis pneumonia compared to healthy individuals. Particularly noteworthy was the significant elevation observed in patients with tuberculosis pneumonia. This suggests that a comprehensive assessment of multiple factors may enhance the accuracy of TB and chronic pulmonary aspergillosis (CPA) diagnosis [134]. Such multifactorial testing approaches not only aid in precise disease diagnosis but also deepen insights into the intricate immune responses elicited during infections with Mtb and Aspergillus fumigatus [135].
- C.
- Potential Use of Host Biomarkers in Tuberculosis Diagnosis
In tuberculosis (TB) diagnosis, a recent study investigating the utility of host biomarkers in pulmonary tuberculosis (PTB) diagnosis revealed notable findings [136]. Comparative analysis of serum samples from 55 PTB patients and 106 individuals with other respiratory diseases (ORDs) unveiled significant differences in several biomarkers, including IP10, IL6, IL2, IL1β, TNF-α, IFN-γ, and IL12p70 between the two groups. Notably, a biomarker combination comprising IP10, IL6, TNF-α, IL1β, IL1ra, and IL12p70 exhibited exceptional diagnostic performance in PTB diagnosis. This had an area under the receiver operating characteristic (ROC) curve reaching up to 90%, indicating its remarkable diagnostic utility. This discovery offers a valuable repertoire of biomarkers for the swift screening of active TB, poised to play a pivotal adjunctive diagnostic role in clinical settings.
Moreover, Yu et al. have substantiated through animal experimental studies that Mtb infection in mice leads to elevated levels of IL-35 and inducible T regulatory type 35 (iTr35) subsets, concomitant with increased bacterial burden and lung lesions. IL-35 and iTr35 cells may exert immunosuppressive effects in chronic Mtb infection [137]. In patients with ATB, leukocytes and peripheral blood mononuclear cells exhibited significantly increased mRNA expression of IL-35 and its subunits p35 and EBI3. Following anti-TB drug treatment, serum IL-35 levels and p35 or EBI3 expression showed a reduction, suggesting the potential of IL-35 as a biomarker for TB immune status and prognosis assessment [19,20].
- D.
- Superiority of IL-27 in the Diagnosis of Tuberculous Pleurisy
As a member of the IL-12 cytokine family, IL-27 exhibits unique diagnostic utility in tuberculous pleurisy. Unlike IL-12’s role in driving Th1 immunity, IL-27 regulates immune balance while serving as a biomarker for effusion differentiation, which exhibits notable sensitivity and specificity in diagnosing tuberculous pleurisy, as evidenced by meta-analytical investigations [138]. Specifically, IL-27 demonstrates remarkable efficacy in distinguishing tuberculous pleural effusion from malignant pleural effusion. Furthermore, when combined with IFN-γ and/or adenosine deaminase (ADA), IL-27 markedly enhances diagnostic sensitivity and specificity to 100%, further substantiating its central role in tuberculous pleurisy diagnosis. Domestic research endeavors corroborate the diagnostic efficacy of IL-27, either as a standalone marker or in conjunction with IFN-γ and ADA, not only in accurately identifying tuberculous pleurisy but also in notably augmenting the specificity of diagnostic assays. These collective findings underscore the heightened diagnostic value of IL-27 compared to alternative markers in the context of tuberculous pleurisy diagnosis [139].
- E.
- Diagnostic Value of IL-12 in Diseases Associated with Immune Dysfunction
The diagnostic significance of IL-12 in immune dysfunction is profound, particularly in patients afflicted with primary immunodeficiency syndrome (PIDs) characterized by mutations in IL-12 and IFN-γ-related immune pathway genes. Such mutations markedly elevate susceptibility to nontuberculous mycobacteriosis (NTM disease). These insights underscore the pivotal role of IL-12 in identifying and understanding immune dysregulation, offering valuable implications for diagnostic strategies and therapeutic interventions in afflicted individuals [140,141,142].
5.2. Treatment
The clinical application of IL-12 in tuberculosis treatment has yet to be directly incorporated into conventional therapeutic regimens. However, its potential role in immune modulation and antituberculosis therapy has garnered extensive investigation and exploration.
A. Immuno-enhancer and Adjuvant Combination Therapy: IL-12 exhibits the capacity to bolster Th1 type immune responses, augment the activity of NK cells and T cells, and induce IFN-γ production, thereby enhancing the host’s defense against Mtb. Theoretically, supplementing IL-12 or enhancing its activity could bolster the immune-mediated clearance of TB, particularly in immunocompromised or drug-resistant TB patients. A 2021 study [143] explored the potential of IL-12 in improving CD4+ T cell function, which, in turn, may enhance the efficacy of antibiotic therapy in TB. In a study by Nolt and Flynn [144], IL-12 was administered to C57BL/6 mice via respiratory aerosolization over eight weeks. These mice were infected with Mtb. Results demonstrated that IL-12 significantly improved the survival rate of CD4+ T-cell deficient mice and reduced bacterial load, suggesting the potential of IL-12 to directly or indirectly impede Mtb growth and ameliorate disease status. Moreover, IL-12 was found to upregulate the expression of Mtb-specific IL-21 at TB lesion sites, notably enhancing the proportion of IL-21+IFN-γ+CD4+ T cells, indicative of its role in bolstering local immune responses for effective Mtb control [145]. While IL-12 currently does not serve as a frontline drug for TB treatment, investigations have explored its potential utility in combination with traditional anti-TB drugs to expedite recovery and diminish bacterial load by fortifying the host’s immune response. Such approaches hold promise for addressing drug-resistant or complex TB cases.
B. Prevention of Recurrence and Transmission: Modulating cytokine levels such as IL-12 contribute to diminishing the risk of tuberculosis recurrence post-cure or inhibiting the potential for latent TB bacteria to reactivate within the body. The utilization of IL-12 holds promise in preventing TB recurrence, thereby mitigating TB dissemination and prevalence within the community.
C. The Potential Role of IL-12 in Tuberculosis Treatment: While the role of IL-12 in conventional CD4+ T cell immunity is well-established, its impact on the non-CD4+-expressing subset of γδT cells T cells, particularly Vγ2Vδ2 T cells, remains incompletely elucidated [146,147]. Vγ2Vδ2 T cells play a pivotal role in combating Mtb infection, as they recognize Mtb metabolites isopentenyl pyrophosphate (IPP) and hydroxy-methylbutenyl pyrophosphate (HMBPP) and swiftly secrete Th1 cytokines like IFN-γ and TNF-α [147]. Recent investigations have unveiled that IL-12 may foster the expansion and differentiation of HMBPP-activated Vγ2Vδ2 T cells via PI3K/AKT and STAT4 signaling pathways, endowing them with heightened antibacterial activity and memory phenotype and facilitating the release of diverse antibacterial cytokines and cytotoxic particles. Thus, IL-12 exerts a direct and indirect beneficial impact on antituberculosis immunity [148,149].
5.3. Prognosis Assessment
In a clinical study conducted in India to assess the changes in serum biomarkers among patients with active tuberculosis following treatment with antituberculosis drugs, it was observed that individuals with pulmonary tuberculosis (APTB) exhibited elevated serum levels of pro-inflammatory cytokines, including IL-12p40, IFN-γ, TNF-α, IL-1β, and IL-6 at baseline compared to healthy controls (p < 0.01). After six months of antituberculosis drug therapy, the levels of IFN-γ, TNF-α, IL-1β, IL-12p40, and IL-6 in APTB patients approached values observed in the healthy population. This trend suggests a potential role for IL-12 in the prognostic evaluation of ATB, however, further investigations are warranted to substantiate these findings [150]. Additionally, the post-treatment expression of serum IL-35 was observed to decrease compared to pre-treatment levels, implicating a potential role for IL-35 in the assessment of tuberculosis prognosis [20,151].
5.4. Prevention
In striving for an effective TB vaccine strategy, the aim is to prevent both primary infection and post-exposure disease, halt the reactivation of latent infection, and act as a complement to standard TB treatment. Currently, novel TB vaccines undergoing clinical trials encompass a variety of types, including viral vector vaccines, recombinant protein/adjuvant vaccines, whole cell/extract vaccines, attenuated/recombinant live vaccines, and DNA vaccines. Notably, IL-12 has shown promising results when employed as an adjuvant expressed via a plasmid carrier. Its incorporation into a specific DNA promoter as an adjuvant added to the MVA85A vaccine notably augments the immune response to Mtb infection [152]. This adjuvant strategy bolsters vaccine protection by enhancing the vaccine-induced Th1-type immune response, which is particularly beneficial for individuals with diminished responsiveness to the BCG vaccine. Furthermore, the synergistic combination of IL-12 with other adjuvants, such as TLR agonists and alum, holds the potential to optimize vaccine formulations, enhance vaccine immunogenicity, and reduce vaccine dose or frequency requirements [153,154].
5.5. Disease and Susceptibility to Tuberculosis
In addition to well-established risk factors such as diabetes [155,156] and HIV [157], other immune-related diseases may heighten susceptibility to Mtb infection. Autoimmune conditions like rheumatoid arthritis and systemic lupus erythematosus, often managed with immunosuppressive therapies, compromise immune function, thereby increasing tuberculosis susceptibility. Furthermore, chronic lung ailments, notably chronic obstructive pulmonary disease (COPD) [158], represent a significant risk factor for TB susceptibility due to lung tissue damage and impaired airway clearance.
Alterations in IL-12 levels play a pivotal role in host immune function in these diseases. Metabolic dysregulation in diabetic patients notably impairs the function of innate immune cells, resulting in reduced secretion of key cytokines like IL-1β, IL-12, and IL-18 and diminished IFN-γ response upon stimulation. Such immunological changes contribute to heightened tuberculosis vulnerability, likely stemming from chronic inflammation and metabolic irregularities inherent in the disease state. In individuals with AIDS, HIV-induced immune compromise may inhibit IL-12 production, further exacerbating susceptibility to TB.
The immune system often operates in a hyperactive or compromised state for patients with other immune disorders and COPD, potentially impacting IL-12 production. Consequently, variations in IL-12 levels may significantly influence host resistance to Mtb, escalating infection risk and disease progression. Notably, for patients with Mendelian susceptibility to mycobacterial disease (MSMD) [159,160], while the genetic etiology remains unclear for some, IL-12 and IL-23-dependent IFN-γ immunity emerge as critical for Mtb resistance, offering invaluable avenues for further investigation.
5.6. Potential Strategies for Leveraging IL-12 Against Mtb Drug Resistance
Combination of Immunotherapy with Traditional Antibiotic Treatment: Given that IL-12 significantly enhances the host’s immune response against Mtb, it is proposed to use IL-12 as an adjunctive therapy in combination with existing anti-TB drugs. This approach aims to improve treatment efficacy and reduce the emergence of drug-resistant strains. For example, IL-12 expressed via a plasmid carrier has been shown to enhance vaccine-induced Th1-type immune responses [161]. Personalized approaches may involve monitoring IL-12 levels and adjusting treatments accordingly [162]. Development of Novel IL-12 Agonists: Exploration of new small molecule drugs or vaccine components that specifically enhance IL-12 signaling pathways could provide more effective immune protection without causing unnecessary systemic inflammation. A novel vaccine (HVJ-liposome/HSP65 DNA+IL-12 DNA) has been evaluated against TB using the cynomolgus monkey model, showing promise in enhancing immune responses [163].
6. Summary
In summary, IL-12 emerges as a pivotal player in the immune modulation of tuberculosis, as illustrated in Figure 1, which shows the process of Mycobacterium tuberculosis infection. This infection process is critical to understanding how IL-12 mediates immune responses, including the differentiation of Th1 cells, enhancement of cytotoxic T lymphocyte function, and promotion of immune memory formation. The intricate production, secretion, and regulatory mechanisms of IL-12, depicted in Figure 2, profoundly influence the magnitude of immune responses, infection outcomes, and disease progression. The key signal regulation mechanisms of IL-12 generation, shown in Figure 2, highlight the involvement of various signaling pathways. These include TLR-mediated dual-track signal initiation (A), CLR-assisted signal enhancement (B), the establishment of a JAK-STAT positive feedback loop (C), and the fine regulation of the PI3K/Akt pathway (D). Each of these pathways plays a crucial role in IL-12 production and underscores the complexity of immune regulation in tuberculosis.
IL-12’s direct and indirect actions against tuberculosis pathogens, through the facilitation of Th1 cell differentiation, augmentation of cytotoxic T lymphocyte function, and promotion of immune memory formation, underscore its indispensable role in infection containment and pathogen eradication. Furthermore, IL-12’s homeostatic regulation, including its modulation of positive and negative regulators like IL-10 and TGF-β, alongside the advent of drug intervention strategies like IL-12/IL-23 inhibitors, underscore the multifaceted nature of its involvement in immune homeostasis maintenance and therapeutic interventions.
Clinically, IL-12 level fluctuations serve as a crucial indicator for tuberculosis diagnosis, treatment response monitoring, and prognosis assessment. Combined detection with other cytokines enhances diagnostic accuracy, while IL-27 and other IL-12 family members exhibit notable advantages in diagnosing specific TB presentations such as tuberculous pleurisy. These insights enrich our comprehension of TB immune pathophysiology and offer novel avenues for personalized treatment and precision medicine.
In conclusion, as a pivotal immune regulatory molecule, IL-12’s role in tuberculosis elucidates the disease’s immune regulatory network. It furnishes a scientific foundation for novel therapeutic and preventive strategies such as vaccine formulation and immunotherapy optimization [164,165,166,167,168,169]. Future investigations should delve deeper into IL-12’s mechanisms and regulatory network, analyzing its dynamics across various disease stages and populations to craft more efficacious disease control and eradication strategies and bolster global TB prevention and management efforts.
Author Contributions
H.W. and G.R. wrote the paper. Y.L. edited the figures. X.L. obtained the funding and managed the project. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National High Level Hospital Clinical Research Funding (2022-PUMCH-C-013).
Data Availability Statement
The authors declare that all data were generated in-house and that no paper mill was used.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peña, D.; Rovetta, A.I.; Hernández Del Pino, R.E.; Amiano, N.O.; Pasquinelli, V.; Pellegrini, J.M.; Tateosian, N.L.; Rolandelli, A.; Gutierrez, M.; Musella, R.M.; et al. A Mycobacterium tuberculosis Dormancy Antigen Differentiates Latently Infected Bacillus Calmette-Guérin-vaccinated Individuals. EBioMedicine 2015, 2, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Kanjoori, A.H.; Hosseinian-Far, A.; Hasheminezhad, R.; Mansouri, K.; Mohammadi, M. Global prevalence of drug-resistant tuberculosis: A systematic review and meta-analysis. Infect. Dis. Poverty 2023, 12, 57. [Google Scholar] [CrossRef]
- Swain, S.S.; Sharma, D.; Hussain, T.; Pati, S. Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerg. Microbes Infect. 2020, 9, 1651–1663. [Google Scholar] [CrossRef]
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’Ambrosio, L.; Centis, R.; Goletti, D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe 2021, 17, 210079. [Google Scholar] [CrossRef]
- Zellweger, J.P.; Sotgiu, G.; Corradi, M.; Durando, P. The diagnosis of latent tuberculosis infection (LTBI): Currently available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med. Lav. 2020, 111, 170–183. [Google Scholar] [CrossRef]
- Lovey, A.; Verma, S.; Kaipilyawar, V.; Ribeiro-Rodrigues, R.; Husain, S.; Palaci, M.; Dietze, R.; Ma, S.; Morrison, R.D.; Sherman, D.R.; et al. Early alveolar macrophage response and IL-1R-dependent T cell priming determine transmissibility of Mycobacterium tuberculosis strains. Nat. Commun. 2022, 13, 884. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef]
- Presky, D.H.; Yang, H.; Minetti, L.J.; Chua, A.O.; Nabavi, N.; Wu, C.Y.; Gately, M.K.; Gubler, U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 14002–14007. [Google Scholar] [CrossRef]
- Ma, X.; Trinchieri, G. Regulation of interleukin-12 production in antigen-presenting cells. Adv. Immunol. 2001, 79, 55–92. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Paul, W.E. Regulation of T(H)1 differentiation—Controlling the controllers. Nat. Immunol. 2002, 3, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef]
- Parham, C.; Chirica, M.; Timans, J.; Vaisberg, E.; Travis, M.; Cheung, J.; Pflanz, S.; Zhang, R.; Singh, K.P.; Vega, F.; et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002, 168, 5699–5708. [Google Scholar] [CrossRef]
- Li, J.; Gran, B.; Zhang, G.X.; Rostami, A.; Kamoun, M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2005, 232, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.H.; Chen, H.Y.; Huang, C.; Yan, J.M.; Yin, Z.; Zhang, X.L.; Pan, Q. Accumulation of EBI3 induced by virulent Mycobacterium tuberculosis inhibits apoptosis in murine macrophages. Pathog. Dis. 2019, 77, ftz007. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M.; Carnero Contentti, E. Interleukin-35 is a critical regulator of immunity during helminth infections associated with multiple sclerosis. Immunology 2021, 164, 569–586. [Google Scholar] [CrossRef]
- Dai, Y.C.; Wang, W.D.; Zhang, J.A.; Chen, C.; Luo, H.L.; Xu, H.; Peng, Y.; Luo, H.; Yang, X.R.; Chen, X.; et al. MTB driven B cells producing IL-35 and secreting high level of IL-10 in the patients with active pulmonary tuberculosis. Mol. Immunol. 2019, 112, 175–181. [Google Scholar] [CrossRef]
- Kong, B.; Liu, G.B.; Zhang, J.A.; Fu, X.X.; Xiang, W.Y.; Gao, Y.C.; Lu, Y.B.; Wu, X.J.; Qiu, F.; Wang, W.D.; et al. Elevated serum IL-35 and increased expression of IL-35-p35 or -EBI3 in CD4(+)CD25(+) T cells in patients with active tuberculosis. Am. J. Transl. Res. 2016, 8, 623–633. [Google Scholar]
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A.; et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010, 11, 1093–1101. [Google Scholar] [CrossRef]
- Ma, X. TNF-alpha and IL-12: A balancing act in macrophage functioning. Microbes Infect. 2001, 3, 121–129. [Google Scholar] [CrossRef]
- Queiroz-Junior, C.M.; Silva, M.J.; Corrêa, J.D.; Madeira, M.F.; Garlet, T.P.; Garlet, G.P.; Cunha, F.Q.; Teixeira, M.M.; da Silva, T.A. A controversial role for IL-12 in immune response and bone resorption at apical periodontal sites. Clin. Dev. Immunol. 2010, 2010, 327417. [Google Scholar] [CrossRef]
- Yang, H.; Wei, J.; Zhang, H.; Song, W.; Wei, W.; Zhang, L.; Qian, K.; He, S. Upregulation of Toll-like Receptor (TLR) expression and release of cytokines from mast cells by IL-12. Cell Physiol. Biochem. 2010, 26, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Martin, S.F.; Dudda, J.C.; Bachtanian, E.; Lembo, A.; Liller, S.; Dürr, C.; Heimesaat, M.M.; Bereswill, S.; Fejer, G.; Vassileva, R.; et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J. Exp. Med. 2008, 205, 2151–2162. [Google Scholar] [CrossRef]
- Durand, J.K.; Zhang, Q.; Baldwin, A.S. Roles for the IKK-Related Kinases TBK1 and IKKε in Cancer. Cells 2018, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.L.; Gioia, R.; Gatot, J.S.; Patrascu, F.; Carpentier, I.; Chapelle, J.P.; O’Neill, L.; Beyaert, R.; Piette, J.; Chariot, A. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem. Sci. 2008, 33, 171–180. [Google Scholar] [CrossRef]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef]
- Fischer, S.; Stegmann, F.; Gnanapragassam, V.S.; Lepenies, B. From structure to function—Ligand recognition by myeloid C-type lectin receptors. Comput. Struct. Biotechnol. J. 2022, 20, 5790–5812. [Google Scholar] [CrossRef]
- Wevers, B.A.; Kaptein, T.M.; Zijlstra-Willems, E.M.; Theelen, B.; Boekhout, T.; Geijtenbeek, T.B.; Gringhuis, S.I. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host. Microbe 2014, 15, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.D.; Smith, P.R.; Despres, H.W.; Snyder, J.P.; Hogan, T.C.; Rodriguez, P.D.; Amiel, E. Glycogen Metabolism Supports Early Glycolytic Reprogramming and Activation in Dendritic Cells in Response to Both TLR and Syk-Dependent CLR Agonists. Cells 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, R.; Li, J.; Li, J. The Role of C-Type Lectin Receptor Signaling in the Intestinal Microbiota-Inflammation-Cancer Axis. Front. Immunol. 2022, 13, 894445. [Google Scholar] [CrossRef]
- Kingeter, L.M.; Lin, X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell Mol. Immunol. 2012, 9, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cui, D.; Chao, X.; Chen, P.; Liu, J.; Wang, Y.; Su, T.; Li, M.; Xu, R.; Zhu, Y.; et al. Transcriptome Analysis Identifies Strategies Targeting Immune Response-Related Pathways to Control Enterotoxigenic Escherichia coli Infection in Porcine Intestinal Epithelial Cells. Front. Vet. Sci. 2021, 8, 677897. [Google Scholar] [CrossRef]
- Kaden, S.A.; Kurig, S.; Vasters, K.; Hofmann, K.; Zaenker, K.S.; Schmitz, J.; Winkels, G. Enhanced dendritic cell-induced immune responses mediated by the novel C-type lectin receptor mDCAR1. J. Immunol. 2009, 183, 5069–5078. [Google Scholar] [CrossRef]
- Chen, C.H.; Floyd, H.; Olson, N.E.; Magaletti, D.; Li, C.; Draves, K.; Clark, E.A. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood 2006, 107, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.; Trifinopoulos, J.; Sexl, V.; Putz, E.M. JAK/STAT Cytokine Signaling at the Crossroad of NK Cell Development and Maturation. Front. Immunol. 2019, 10, 2590. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Tanaka, Y. JAK inhibitors for rheumatoid arthritis. Expert. Opin. Investig. Drugs 2023, 32, 333–344. [Google Scholar] [CrossRef]
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ synergy amplifies senescence-associated inflammation and SARS-CoV-2 receptor expression via hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646. [Google Scholar] [CrossRef]
- Au-Yeung, N.; Mandhana, R.; Horvath, C.M. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. Jakstat 2013, 2, e23931. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, Y.; Tilborghs, S.; Jacobs, J.; De Waele, J.; Quatannens, D.; Deben, C.; Prenen, H.; Pauwels, P.; Trinh, X.B.; Wouters, A.; et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020, 60, 41–56. [Google Scholar] [CrossRef]
- Wesoly, J.; Szweykowska-Kulinska, Z.; Bluyssen, H.A. STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim. Pol. 2007, 54, 27–38. [Google Scholar]
- Nowicka, H.; Sekrecka, A.; Blaszczyk, K.; Kluzek, K.; Chang, C.Y.; Wesoly, J.; Lee, C.K.; Bluyssen, H.A.R. ISGF3 and STAT2/IRF9 Control Basal and IFN-Induced Transcription through Genome-Wide Binding of Phosphorylated and Unphosphorylated Complexes to Common ISRE-Containing ISGs. Int. J. Mol. Sci. 2023, 24, 17635. [Google Scholar] [CrossRef] [PubMed]
- Bechara, R.; Antonios, D.; Azouri, H.; Pallardy, M. Nickel Sulfate Promotes IL-17A Producing CD4+ T Cells by an IL-23-Dependent Mechanism Regulated by TLR4 and Jak-STAT Pathways. J. Investig. Dermatol. 2017, 137, 2140–2148. [Google Scholar] [CrossRef]
- Lin, Y.; Kuang, W.; Wu, B.; Xie, C.; Liu, C.; Tu, Z. IL-12 induces autophagy in human breast cancer cells through AMPK and the PI3K/Akt pathway. Mol. Med. Rep. 2017, 16, 4113–4118. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, T.; Tian, C.; Ma, K.; Gou, J.; Huang, R.; Li, S.; Li, Q.; Xu, C.; Li, L.; et al. Kinsenoside attenuates liver fibro-inflammation by suppressing dendritic cells via the PI3K-AKT-FoxO1 pathway. Pharmacol. Res. 2022, 177, 106092. [Google Scholar] [CrossRef]
- Quan, J.H.; Chu, J.Q.; Kwon, J.; Choi, I.W.; Ismail, H.A.; Zhou, W.; Cha, G.H.; Zhou, Y.; Yuk, J.M.; Jo, E.K.; et al. Intracellular Networks of the PI3K/AKT and MAPK Pathways for Regulating Toxoplasma gondii-Induced IL-23 and IL-12 Production in Human THP-1 Cells. PLoS ONE 2015, 10, e0141550. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Chen, L.; Xu, W.; Wu, B. Photobiomodulation activates undifferentiated macrophages and promotes M1/M2 macrophage polarization via PI3K/AKT/mTOR signaling pathway. Lasers Med. Sci. 2023, 38, 86. [Google Scholar] [CrossRef]
- Nandagopal, N.; Ali, A.K.; Komal, A.K.; Lee, S.H. The Critical Role of IL-15-PI3K-mTOR Pathway in Natural Killer Cell Effector Functions. Front. Immunol. 2014, 5, 187. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Q.; Liu, X.; Shan, Y. IL-12 and IL-10 production are differentially regulated by phosphatidylinositol 3-kinase in mast cells. Scand. J. Immunol. 2012, 75, 266–272. [Google Scholar] [CrossRef]
- Carter, A.N.; Born, H.A.; Levine, A.T.; Dao, A.T.; Zhao, A.J.; Lee, W.L.; Anderson, A.E. Wortmannin Attenuates Seizure-Induced Hyperactive PI3K/Akt/mTOR Signaling, Impaired Memory, and Spine Dysmorphology in Rats. eNeuro 2017, 4, ENEURO.0354–16.2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lu, J.; Wei, L.; Wang, X.; Xu, X.; Dong, M.; Huang, B. Histone acetyltransferase activity of p300 enhances the activation of IL-12 p40 promoter. Mol. Immunol. 2004, 41, 1241–1246. [Google Scholar] [CrossRef]
- Li, Y.; Xia, J.; Jiang, N.; Xian, Y.; Ju, H.; Wei, Y.; Zhang, X. Corin protects H(2)O(2)-induced apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes. Biomed. Pharmacother. 2018, 97, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cardoso, S.C.; Bekker, C.P.J.; Boes, M.; Radstake, T.; Angiolilli, C. CXCL4 is a driver of cytokine mRNA stability in monocyte-derived dendritic cells. Mol. Immunol. 2019, 114, 524–534. [Google Scholar] [CrossRef]
- Dawson, J.E.; Bah, A.; Zhang, Z.; Vernon, R.M.; Lin, H.; Chong, P.A.; Vanama, M.; Sonenberg, N.; Gradinaru, C.C.; Forman-Kay, J.D. Non-cooperative 4E-BP2 folding with exchange between eIF4E-binding and binding-incompatible states tunes cap-dependent translation inhibition. Nat. Commun. 2020, 11, 3146. [Google Scholar] [CrossRef]
- Lu, J.; Sun, H.; Wang, X.; Liu, C.; Xu, X.; Li, F.; Huang, B. Interleukin-12 p40 promoter activity is regulated by the reversible acetylation mediated by HDAC1 and p300. Cytokine 2005, 31, 46–51. [Google Scholar] [CrossRef]
- Guindi, C.; Cloutier, A.; Gaudreau, S.; Zerif, E.; McDonald, P.P.; Tatsiy, O.; Asselin, C.; Dupuis, G.; Gris, D.; Amrani, A.A. Role of the p38 MAPK/C/EBPβ Pathway in the Regulation of Phenotype and IL-10 and IL-12 Production by Tolerogenic Bone Marrow-Derived Dendritic Cells. Cells 2018, 7, 256. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.P.; Chénard, V.; Sikström, K.; Zheng, L.; Alain, T.; Gandin, V.; Avizonis, D.; Arguello, M.; Zakaria, C.; et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013, 18, 698–711. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef]
- Saraf, A.; Luo, J.; Morris, D.R.; Storm, D.R. Phosphorylation of eukaryotic translation initiation factor 4E and eukaryotic translation initiation factor 4E-binding protein (4EBP) and their upstream signaling components undergo diurnal oscillation in the mouse hippocampus: Implications for memory persistence. J. Biol. Chem. 2014, 289, 20129–20138. [Google Scholar] [CrossRef]
- Shemesh, A.; Pickering, H.; Roybal, K.T.; Lanier, L.L. Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion. J. Exp. Med. 2022, 219, e20212434. [Google Scholar] [CrossRef]
- Yang, R.; Yang, E.; Shen, L.; Modlin, R.L.; Shen, H.; Chen, Z.W. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth. J. Immunol. 2018, 200, 2405–2417. [Google Scholar] [CrossRef]
- Hildenbrand, K.; Aschenbrenner, I.; Franke, F.C.; Devergne, O.; Feige, M.J. Biogenesis and engineering of interleukin 12 family cytokines. Trends Biochem. Sci. 2022, 47, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Goodall, J.C.; Wu, C.; Zhang, Y.; McNeill, L.; Ellis, L.; Saudek, V.; Gaston, J.S. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. USA 2010, 107, 17698–17703. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.; Belmadani, S.; Matrougui, K. Disrupting Interleukin 12 Improves Microvascular Endothelial Function in Type 2 Diabetes Through ER Stress CHOP and Oxidative Stress Mechanisms. Diabetes Metab. Syndr. Obes. 2022, 15, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Uronen-Hansson, H.; Allen, J.; Osman, M.; Squires, G.; Klein, N.; Callard, R.E. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: Integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology 2004, 111, 173–178. [Google Scholar] [CrossRef]
- Kalim, K.W.; Groettrup, M. Prostaglandin E2 inhibits IL-23 and IL-12 production by human monocytes through down-regulation of their common p40 subunit. Mol. Immunol. 2013, 53, 274–282. [Google Scholar] [CrossRef]
- Aste-Amezaga, M.; Ma, X.; Sartori, A.; Trinchieri, G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1998, 160, 5936–5944. [Google Scholar] [CrossRef]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef]
- Van Weyenbergh, J.; MP, P.S.; Báfica, A.; Cardoso, S.; Wietzerbin, J.; Barral-Netto, M. IFN-beta and TGF-beta differentially regulate IL-12 activity in human peripheral blood mononuclear cells. Immunol. Lett. 2001, 75, 117–122. [Google Scholar] [CrossRef]
- Finnegan, A.; Grusby, M.J.; Kaplan, C.D.; O’Neill, S.K.; Eibel, H.; Koreny, T.; Czipri, M.; Mikecz, K.; Zhang, J. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J. Immunol. 2002, 169, 3345–3352. [Google Scholar] [CrossRef]
- Vom Berg, J.; Vrohlings, M.; Haller, S.; Haimovici, A.; Kulig, P.; Sledzinska, A.; Weller, M.; Becher, B. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J. Exp. Med. 2013, 210, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e1147. [Google Scholar] [CrossRef]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Kuhel, D.G.; Chen, J.F.; Schwarzschild, M.A.; Deitch, E.A.; Mabley, J.G.; Marton, A.; Szabó, C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000, 14, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Ross, W.G.; Agbai, O.N.; Frazier, R.; Figler, R.A.; Rieger, J.; Linden, J.; Ernst, P.B. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J. Immunol. 2009, 182, 4616–4623. [Google Scholar] [CrossRef]
- Bai, F.; Li, G.G.; Liu, Q.; Niu, X.; Li, R.; Ma, H. Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Immunol. Res. 2019, 2019, 2546161. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- MacDonald, J.K.; Nguyen, T.M.; Khanna, R.; Timmer, A. Anti-IL-12/23p40 antibodies for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 11, Cd007572. [Google Scholar] [CrossRef]
- Tamiya, S.; Yoshikawa, E.; Ogura, M.; Kuroda, E.; Suzuki, K.; Yoshioka, Y. Neutrophil-Mediated Lung Injury Both via TLR2-Dependent Production of IL-1α and IL-12 p40, and TLR2-Independent CARDS Toxin after Mycoplasma pneumoniae Infection in Mice. Microbiol. Spectr. 2021, 9, e0158821. [Google Scholar] [CrossRef]
- Kim, H.S.; Chung, D.H. TLR4-mediated IL-12 production enhances IFN-γ and IL-1β production, which inhibits TGF-β production and promotes antibody-induced joint inflammation. Arthritis Res. Ther. 2012, 14, R210. [Google Scholar] [CrossRef]
- Posseme, C.; Llibre, A.; Charbit, B.; Bondet, V.; Rouilly, V.; Saint-André, V.; Boussier, J.; Bergstedt, J.; Smith, N.; Townsend, L.; et al. Early IFNβ secretion determines variable downstream IL-12p70 responses upon TLR4 activation. Cell Rep. 2022, 39, 110989. [Google Scholar] [CrossRef]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Genetically determined high activity of IL-12 and IL-18 in ulcerative colitis and TLR5 in Crohns disease were associated with non-response to anti-TNF therapy. Pharmacogenomics J. 2018, 18, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Frazão, J.B.; Errante, P.R.; Condino-Neto, A. Toll-like receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Arch. Immunol. Ther. Exp. 2013, 61, 427–443. [Google Scholar] [CrossRef]
- Theiner, G.; Rössner, S.; Dalpke, A.; Bode, K.; Berger, T.; Gessner, A.; Lutz, M.B. TLR9 cooperates with TLR4 to increase IL-12 release by murine dendritic cells. Mol. Immunol. 2008, 45, 244–252. [Google Scholar] [CrossRef]
- Mandour, M.F.; Soe, P.P.; Castonguay, A.S.; Van Snick, J.; Coutelier, J.P. Inhibition of IL-12 heterodimers impairs TLR9-mediated prevention of early mouse plasmacytoma cell growth. Front. Med. 2022, 9, 1057252. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. Mast cells to dendritic cells: Let IL-13 shut your IL-12 down. J. Allergy Clin. Immunol. 2021, 147, 2073–2074. [Google Scholar] [CrossRef]
- Abdi, K.; Laky, K.; Padhan, K.; Petrovas, C.; Skinner, J.; Kabat, J.; Dorward, D.W.; Brzostowski, J.; Long, E.O.; Trinchieri, G.; et al. Cutting Edge: Quantitative Determination of CD40L Threshold for IL-12 and IL-23 Production from Dendritic Cells. J. Immunol. 2018, 201, 2879–2884. [Google Scholar] [CrossRef]
- Bullens, D.M.; Kasran, A.; Thielemans, K.; Bakkus, M.; Ceuppens, J.L. CD40L-induced IL-12 production is further enhanced by the Th2 cytokines IL-4 and IL-13. Scand. J. Immunol. 2001, 53, 455–463. [Google Scholar] [CrossRef]
- Ashour, D.; Arampatzi, P.; Pavlovic, V.; Förstner, K.U.; Kaisho, T.; Beilhack, A.; Erhard, F.; Lutz, M.B. IL-12 from endogenous cDC1, and not vaccine DC, is required for Th1 induction. JCI Insight 2020, 5, e135143. [Google Scholar] [CrossRef]
- Cooper, A.M.; Magram, J.; Ferrante, J.; Orme, I.M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 1997, 186, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wakeham, J.; Wang, J.; Magram, J.; Croitoru, K.; Harkness, R.; Dunn, P.; Zganiacz, A.; Xing, Z. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette-Guérin in IL-12-deficient mice. J. Immunol. 1998, 160, 6101–6111. [Google Scholar] [CrossRef] [PubMed]
- Qadir, K.; Metwali, A.; Blum, A.M.; Li, J.; Elliott, D.E.; Weinstock, J.V. TGF-beta and IL-10 regulation of IFN-gamma produced in Th2-type schistosome granulomas requires IL-12. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G940–G946. [Google Scholar] [CrossRef] [PubMed]
- Arababadi, M.K.; Pourfathollah, A.A.; Jafarzadeh, A.; Hassanshahi, G.; Daneshmandi, S.; Shamsizadeh, A.; Kennedy, D. Non-association of IL-12 +1188 and IFN-γ +874 polymorphisms with cytokines serum level in occult HBV infected patients. Saudi J. Gastroenterol. 2011, 17, 30–35. [Google Scholar] [CrossRef]
- Zhou, F.; Xiong, H.; Zhen, S.; Chen, A.; Huang, M.; Luo, Y. Serum levels of IL-12, IL-18, and IL-21 are indicators of viral load in patients chronically infected with HBV. Braz. J. Med. Biol. Res. 2022, 55, e12320. [Google Scholar] [CrossRef]
- Abdi, K.; Laky, K.; Abshari, M.; Hill, E.M.; Lantz, L.; Singh, N.J.; Long, E.O. Dendritic cells Trigger IFN-γ secretion by NK cells independent of IL-12 and IL-18. Eur. J. Immunol. 2022, 52, 1431–1440. [Google Scholar] [CrossRef]
- Terrén, I.; Sandá, V.; Amarilla-Irusta, A.; Lopez-Pardo, A.; Sevilla, A.; Astarloa-Pando, G.; Amo, L.; Zenarruzabeitia, O.; Scorrano, L.; Borrego, F. IL-12/15/18-induced cell death and mitochondrial dynamics of human NK cells. Front. Immunol. 2023, 14, 1211839. [Google Scholar] [CrossRef]
- Mah, A.Y.; Cooper, M.A. Metabolic Regulation of Natural Killer Cell IFN-γ Production. Crit. Rev. Immunol. 2016, 36, 131–147. [Google Scholar] [CrossRef]
- Robinson, C.M.; Nau, G.J. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J. Infect. Dis. 2008, 198, 359–366. [Google Scholar] [CrossRef]
- McClean, C.M.; Tobin, D.M. Macrophage form, function, and phenotype in mycobacterial infection: Lessons from tuberculosis and other diseases. Pathog. Dis. 2016, 74, ftw068. [Google Scholar] [CrossRef]
- Hmama, Z.; Peña-Díaz, S.; Joseph, S.; Av-Gay, Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol. Rev. 2015, 264, 220–232. [Google Scholar] [CrossRef]
- Dao, D.N.; Kremer, L.; Guérardel, Y.; Molano, A.; Jacobs, W.R., Jr.; Porcelli, S.A.; Briken, V. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect. Immun. 2004, 72, 2067–2074. [Google Scholar] [CrossRef]
- Markiewicz, M.A.; Wise, E.L.; Buchwald, Z.S.; Cheney, E.E.; Hansen, T.H.; Suri, A.; Cemerski, S.; Allen, P.M.; Shaw, A.S. IL-12 enhances CTL synapse formation and induces self-reactivity. J. Immunol. 2009, 182, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Mehta, H.; Lovell, A.; Papaioannou, E.; Fairbanks, L. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8(+) T cells. Eur. J. Immunol. 2016, 46, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jay, S.M.; Wang, Y.; Wu, S.W.; Xiao, Z. IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8(+) T cells. Sci. Rep. 2017, 7, 13365. [Google Scholar] [CrossRef] [PubMed]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, J.; Khalifa, E.H.; Khattak, F.A.; Khan, A.S.; Farooq, S.U.; Osman, S.M.A.; Salih, M.M.; Ullah, N.; Khan, T.A. Novel mutations in genes of the IL-12/IFN-γ axis cause susceptibility to tuberculosis. J. Infect. Public Health 2023, 16, 1368–1378. [Google Scholar] [CrossRef]
- Kelsey, A.; Chirch, L.M.; Payette, M.J. Tuberculosis and interleukin blocking monoclonal antibodies: Is there risk? Dermatol. Online J. 2018, 24, 9. [Google Scholar] [CrossRef]
- Nekooie-Marnany, N.; Deswarte, C.; Ostadi, V.; Bagherpour, B.; Taleby, E.; Ganjalikhani-Hakemi, M.; Le Voyer, T.; Rahimi, H.; Rosain, J.; Pourmoghadas, Z.; et al. Impaired IL-12- and IL-23-Mediated Immunity Due to IL-12Rβ1 Deficiency in Iranian Patients with Mendelian Susceptibility to Mycobacterial Disease. J. Clin. Immunol. 2018, 38, 787–793. [Google Scholar] [CrossRef]
- Pérez, D.; Muñoz, M.C.; Molina, J.M.; Muñoz-Caro, T.; Silva, L.M.; Taubert, A.; Hermosilla, C.; Ruiz, A. Eimeria ninakohlyakimovae induces NADPH oxidase-dependent monocyte extracellular trap formation and upregulates IL-12 and TNF-α, IL-6 and CCL2 gene transcription. Vet. Parasitol. 2016, 227, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Kees, T.; Almeida, A.S.; Liu, B.; He, X.Y.; Ng, D.; Han, X.; Spector, D.L.; McNeish, I.A.; Gimotty, P.; et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell 2021, 39, 1361–1374.e9. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020, 30, 1039–1051.e5. [Google Scholar] [CrossRef] [PubMed]
- Latsi, P.; Pantelidis, P.; Vassilakis, D.; Sato, H.; Welsh, K.I.; du Bois, R.M. Analysis of IL-12 p40 subunit gene and IFN-gamma G5644A polymorphisms in Idiopathic Pulmonary Fibrosis. Respir. Res. 2003, 4, 6. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Triebold, K.J.; Sypek, J.; Wolf, S.; Bloom, B.R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 1995, 155, 2515–2524. [Google Scholar] [PubMed]
- Chen, Y.Y.; Lin, C.W.; Huang, W.F.; Chang, J.R.; Su, I.J.; Hsu, C.H.; Cheng, H.Y.; Hsu, S.C.; Dou, H.Y. Recombinant bacille Calmette-Guerin coexpressing Ag85b, CFP10, and interleukin-12 elicits effective protection against Mycobacterium tuberculosis. J. Microbiol. Immunol. Infect. 2017, 50, 90–96. [Google Scholar] [CrossRef]
- Mata-Espinosa, D.A.; Francisco-Cruz, A.; Marquina-Castillo, B.; Barrios-Payan, J.; Ramos-Espinosa, O.; Bini, E.I.; Xing, Z.; Hernández-Pando, R. Immunotherapeutic effects of recombinant adenovirus encoding interleukin 12 in experimental pulmonary tuberculosis. Scand J. Immunol. 2019, 89, e12743. [Google Scholar] [CrossRef]
- Gerace, E.; Pasquali, P.; Oesch, B.; Falduto, M.; Mandanici, F.; Fiasconaro, M.; Vitale, M.; Di Marco Lo Presti, V.; Amato, B. Stimulation of Bovine Whole-Blood Samples Cultured in Media Supplemented with Recombinant Interleukin-7 (IL-7) and IL-12 Extends the Life Span of the Gamma Interferon Assay To Detect Mycobacterium bovis-Infected Cattle. J. Clin. Microbiol. 2016, 54, 2315–2320. [Google Scholar] [CrossRef]
- Iida, K.; Suzuki, K.; Yokota, M.; Nakagomi, D.; Wakashin, H.; Iwata, A.; Kawashima, H.; Takatori, H.; Nakajima, H. STAT4 is required for IFN-β-induced MCP-1 mRNA expression in murine mast cells. Int. Arch. Allergy Immunol. 2011, 155 (Suppl. S1), 71–76. [Google Scholar] [CrossRef]
- Hussain, S.; Rasool, R.; Shafi, T.; Gull, A.; Qureshi, T.A.; Jan, R.; Shah, Z.A. Evaluation of SOCS5 mRNA and its association with serum IL-12 levels and rs41379147 SNP in various subsets of allergic disorders: A case control study. Mol. Immunol. 2023, 162, 102–110. [Google Scholar] [CrossRef]
- Pahan, K.; Sheikh, F.G.; Liu, X.; Hilger, S.; McKinney, M.; Petro, T.M. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J. Biol. Chem. 2001, 276, 7899–7905. [Google Scholar] [CrossRef] [PubMed]
- Gaviglio, E.A.; Peralta Ramos, J.M.; Arroyo, D.S.; Bussi, C.; Iribarren, P.; Rodriguez-Galan, M.C. Systemic sterile induced-co-expression of IL-12 and IL-18 drive IFN-γ-dependent activation of microglia and recruitment of MHC-II-expressing inflammatory monocytes into the brain. Int. Immunopharmacol. 2022, 105, 108546. [Google Scholar] [CrossRef] [PubMed]
- Pflanz, S.; Hibbert, L.; Mattson, J.; Rosales, R.; Vaisberg, E.; Bazan, J.F.; Phillips, J.H.; McClanahan, T.K.; de Waal Malefyt, R.; Kastelein, R.A. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004, 172, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.D.; Counoupas, C.; Daniel, L.; Zhang, G.; Cook, S.J.; Cootes, T.A.; Stifter, S.A.; Bowen, D.G.; Triccas, J.A.; Bertolino, P.; et al. TCR Affinity Controls the Dynamics but Not the Functional Specification of the Antimycobacterial CD4(+) T Cell Response. J. Immunol. 2021, 206, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.; Alaouna, M.; Cholo, M.C.; Hull, R. Is targeting dysregulation in apoptosis splice variants in Mycobacterium tuberculosis (MTB) host interactions and splicing factors resulting in immune evasion by MTB strategies a possibility? Tuberculosis 2020, 124, 101964. [Google Scholar] [CrossRef]
- Liu, D.; Huang, F.; Zhang, G.; He, W.; Ou, X.; He, P.; Zhao, B.; Zhu, B.; Liu, F.; Li, Z.; et al. Whole-genome sequencing for surveillance of tuberculosis drug resistance and determination of resistance level in China. Clin. Microbiol. Infect. 2022, 28, 731-e9. [Google Scholar] [CrossRef]
- Abdalla, A.E.; Lambert, N.; Duan, X.; Xie, J. Interleukin-10 Family and Tuberculosis: An Old Story Renewed. Int. J. Biol. Sci. 2016, 12, 710–717. [Google Scholar] [CrossRef]
- Dokrungkoon, T.; Tulyaprawat, O.; Suwannakarn, K.; Ngamskulrungroj, P. In vitro modeling of isoniazid resistance mechanisms in Mycobacterium tuberculosis H37Rv. Front. Microbiol. 2023, 14, 1171861. [Google Scholar] [CrossRef]
- Thibodeau, J.; Bourgeois-Daigneault, M.C.; Huppé, G.; Tremblay, J.; Aumont, A.; Houde, M.; Bartee, E.; Brunet, A.; Gauvreau, M.E.; de Gassart, A.; et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur. J. Immunol. 2008, 38, 1225–1230. [Google Scholar] [CrossRef]
- Guirado, E.; Schlesinger, L.S.; Kaplan, G. Macrophages in tuberculosis: Friend or foe. Semin. Immunopathol. 2013, 35, 563–583. [Google Scholar] [CrossRef]
- Li, W.; Deng, G.; Li, M.; Liu, X.; Wang, Y. Roles of Mucosal Immunity against Mycobacterium tuberculosis Infection. Tuberc. Res. Treat. 2012, 2012, 791728. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, T.M.; Demkow, U.; Michalowska-Mitczuk, D.; Filewska, M.; Bialas, B.; Zycinska, K.; Obrowski, M.H.; Kus, J.; Skopinska-Rozewska, E. Angiogenic activity of sera from pulmonary tuberculosis patients in relation to IL-12p40 and TNFα serum levels. Lung 2011, 189, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Li, H.; Guo, C.; Shang, Y.; Wang, W.; Zhang, X.; Li, S.; Pang, Y. Serum Cytokine Biomarkers for Use in Diagnosing Pulmonary Tuberculosis versus Chronic Pulmonary Aspergillosis. Infect. Drug Resist. 2023, 16, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.M.W.; Soeroto, A.Y.; Putriyani, G.; Hanifah, W.; Permata, A.; Annisa, J.; Fithriyana, I.; Verweij, P.E.; van Laarhoven, A.; de Veerdonk, F.L.V.; et al. Aspergillus fumigatus-specific antibodies in patients with chronic tuberculosis. Int. J. Tuberc. Lung. Dis. 2020, 24, 853–856. [Google Scholar] [CrossRef]
- Namuganga, A.R.; Nsereko, M.; Bagaya, B.S.; Mayanja-Kizza, H.; Chegou, N.N. Differential expression of host protein biomarkers among symptomatic clinic attendees finally diagnosed with tuberculosis and other respiratory diseases with or without latent Mycobacterium tuberculosis infection. Immunol. Lett. 2023, 253, 8–18. [Google Scholar] [CrossRef]
- Yu, F.; Zhu, X.; Li, Q.; Xu, W.; Gao, Y.; Wen, Y.; Zhang, Q.; Dou, J. Elevated IL-35 level and iTr35 subset increase the bacterial burden and lung lesions in Mycobacterium tuberculosis-infected mice. Open Life Sci. 2022, 17, 312–320. [Google Scholar] [CrossRef]
- Antonangelo, L.; Faria, C.S.; Sales, R.K. Tuberculous pleural effusion: Diagnosis & management. Expert Rev. Respir. Med. 2019, 13, 747–759. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, Y.; Liu, H.; Dou, Q. Age: Pleural fluid ADA ratio and other indicators for differentiating between tubercular and malignant pleural effusions. Medicine 2022, 101, e29788. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Mansouri, D.; Khosravi, A.; Garssen, J.; Velayati, A.; Adcock, I.M. Cancers Related to Immunodeficiencies: Update and Perspectives. Front. Immunol. 2016, 7, 365. [Google Scholar] [CrossRef]
- Wani, S.R.; Wattal, C.; Raveendran, R. Epidemiology and risk factors associated with NTM pulmonary and extrapulmonary infections in a high tuberculosis endemic Region. Indian J. Med. Microbiol. 2020, 38, 169–175. [Google Scholar] [CrossRef]
- Namkoong, H.; Hasegawa, N.; Betsuyaku, T. Susceptibility genes for nontuberculous mycobacterial disease. Nihon Rinsho Meneki Gakkai Kaishi 2017, 40, 60–67. [Google Scholar] [CrossRef]
- Costa, D.L.; Amaral, E.P.; Namasivayam, S.; Mittereder, L.R.; Andrade, B.B.; Sher, A. Enhancement of CD4(+) T Cell Function as a Strategy for Improving Antibiotic Therapy Efficacy in Tuberculosis: Does It Work? Front. Cell Infect. Microbiol. 2021, 11, 672527. [Google Scholar] [CrossRef]
- Nolt, D.; Flynn, J.L. Interleukin-12 therapy reduces the number of immune cells and pathology in lungs of mice infected with Mycobacterium tuberculosis. Infect. Immun. 2004, 72, 2976–2988. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Lao, S.; Yang, B.; Yu, S.; Zhang, Y.; Wu, C. Mycobacterium tuberculosis-Specific IL-21+IFN-γ+CD4+ T Cells Are Regulated by IL-12. PLoS ONE 2016, 11, e0147356. [Google Scholar] [CrossRef]
- Shen, L.; Huang, D.; Qaqish, A.; Frencher, J.; Yang, R.; Shen, H.; Chen, Z.W. Fast-acting γδ T-cell subpopulation and protective immunity against infections. Immunol. Rev. 2020, 298, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W. Multifunctional immune responses of HMBPP-specific Vγ2Vδ2 T cells in M. tuberculosis and other infections. Cell Mol. Immunol. 2013, 10, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yang, E.; Guo, M.; Yang, R.; Huang, G.; Peng, Y.; Sha, W.; Wang, F.; Shen, L. Adjunctive Zoledronate + IL-2 administrations enhance anti-tuberculosis Vγ2Vδ2 T-effector populations, and improve treatment outcome of multidrug-resistant tuberculosis1. Emerg. Microbes Infect. 2022, 11, 1790–1805. [Google Scholar] [CrossRef]
- Shen, L.; Frencher, J.; Huang, D.; Wang, W.; Yang, E.; Chen, C.Y.; Zhang, Z.; Wang, R.; Qaqish, A.; Larsen, M.H.; et al. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl. Acad. Sci. USA 2019, 116, 6371–6378. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Ahmed, A.M.; Choudhuri, S.; Sen, A.; Hazra, A.; Pal, N.K.; Bhattacharya, B.; Bahar, B. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Mol. Immunol. 2014, 62, 159–168. [Google Scholar] [CrossRef]
- Yao, R.; Wu, Y.; Ma, B. Important role of interleukin-35 in infectious diseases and its significance. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 848–852. [Google Scholar] [CrossRef]
- Morelli, M.P.; Del Medico Zajac, M.P.; Pellegrini, J.M.; Amiano, N.O.; Tateosian, N.L.; Calamante, G.; Gherardi, M.M.; García, V.E. IL-12 DNA Displays Efficient Adjuvant Effects Improving Immunogenicity of Ag85A in DNA Prime/MVA Boost Immunizations. Front. Cell Infect. Microbiol. 2020, 10, 581812. [Google Scholar] [CrossRef]
- Fang, D.; Wang, R.; Fan, X.; Li, M.; Qian, C.; Cao, B.; Yu, J.; Liu, H.; Lou, Y.; Wan, K. Recombinant BCG vaccine expressing multistage antigens of Mycobacterium tuberculosis provides long-term immunity against tuberculosis in BALB/c mice. Hum. Vaccin. Immunother. 2024, 20, 2299607. [Google Scholar] [CrossRef] [PubMed]
- Burkert, S.; Schumann, R.R. RNA Sensing of Mycobacterium tuberculosis and Its Impact on TB Vaccination Strategies. Vaccines 2020, 8, 67. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wei, M.; Yin, D.; Tang, Y.; Jia, W.; Wang, C.; Guo, J.; Li, A.; Gong, Y. Associations between type 1 diabetes and pulmonary tuberculosis: A bidirectional mendelian randomization study. Diabetol. Metab. Syndr. 2024, 16, 60. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Zheng, Z.; Shao, X.; Yang, P.; Yang, X.; Nan, K. Unraveling genetic causality between type 2 diabetes and pulmonary tuberculosis on the basis of Mendelian randomization. Diabetol. Metab. Syndr. 2023, 15, 228. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Xiang, Y.; Guo, W.; Zhu, Q.; Wu, S.; Tan, Y.; Yan, Y.; Shen, L.; Feng, Y.; Liang, K. Phenotype and function of peripheral blood γδ T cells in HIV infection with tuberculosis. Front. Cell Infect. Microbiol. 2022, 12, 1071880. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Dhooria, S.; Muthu, V.; Salzer, H.J.F.; Agarwal, R. Burden, clinical features, and outcomes of post-tuberculosis chronic obstructive lung diseases. Curr. Opin. Pulm. Med. 2024, 30, 156–166. [Google Scholar] [CrossRef]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef]
- Philippot, Q.; Ogishi, M.; Bohlen, J.; Puchan, J.; Arias, A.A.; Nguyen, T.; Martin-Fernandez, M.; Conil, C.; Rinchai, D.; Momenilandi, M.; et al. Human IL-23 is essential for IFN-γ-dependent immunity to mycobacteria. Sci. Immunol. 2023, 8, eabq5204. [Google Scholar] [CrossRef]
- Reeme, A.E.; Miller, H.E.; Robinson, R.T. IL12B expression is sustained by a heterogenous population of myeloid lineages during tuberculosis. Tuberculosis 2013, 93, 343–356. [Google Scholar] [CrossRef][Green Version]
- Abdulahi, I.; Hafiz, T.R. Multidrug Resistant Tuberculosis in HIV Positive Patients and its Effect on IL-10 and IL-12. Egypt. J. Med. Microbiol. 2023, 32, 61–68. [Google Scholar]
- Okada, M.; Kita, Y.; Nakajima, T.; Kanamaru, N.; Hashimoto, S.; Nagasawa, T.; Kaneda, Y.; Yoshida, S.; Nishida, Y.; Fukamizu, R.; et al. Evaluation of a novel vaccine (HVJ-liposome/HSP65 DNA+IL-12 DNA) against tuberculosis using the cynomolgus monkey model of TB. Vaccine 2007, 25, 2990–2993. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.E.; Erasmus, J.H.; Reese, V.A.; Pecor, T.; Archer, J.; Kandahar, A.; Hsu, F.C.; Nicholes, K.; Reed, S.G.; Baldwin, S.L.; et al. An RNA-Based Vaccine Platform for Use against Mycobacterium tuberculosis. Vaccines 2023, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Farris, E.; Swanson, R.V.; Das, S.; Yang, Y.; Martin, T.; Khader, S.A. Saponin TQL1055 adjuvant-containing vaccine confers protection upon Mycobacterium tuberculosis challenge in mice. Hum. Vaccin. Immunother. 2024, 20, 2302070. [Google Scholar] [CrossRef]
- Baldwin, S.L.; Reese, V.A.; Larsen, S.E.; Pecor, T.; Brown, B.P.; Granger, B.; Podell, B.K.; Fox, C.B.; Reed, S.G.; Coler, R.N. Therapeutic efficacy against Mycobacterium tuberculosis using ID93 and liposomal adjuvant formulations. Front. Microbiol. 2022, 13, 935444. [Google Scholar] [CrossRef]
- Kwon, K.W.; Kang, T.G.; Lee, A.; Jin, S.M.; Lim, Y.T.; Shin, S.J.; Ha, S.J. Protective Efficacy and Immunogenicity of Rv0351/Rv3628 Subunit Vaccine Formulated in Different Adjuvants Against Mycobacterium tuberculosis Infection. Immune Netw. 2023, 23, e16. [Google Scholar] [CrossRef] [PubMed]
- Horváti, K.; Pályi, B.; Henczkó, J.; Balka, G.; Szabó, E.; Farkas, V.; Biri-Kovács, B.; Szeder, B.; Fodor, K. A Convenient Synthetic Method to Improve Immunogenicity of Mycobacterium tuberculosis Related T-Cell Epitope Peptides. Vaccines 2019, 7, 101. [Google Scholar] [CrossRef]
- Petrone, L.; Peruzzu, D.; Altera, A.M.G.; Salmi, A.; Vanini, V.; Cuzzi, G.; Coppola, A.; Mellini, V.; Gualano, G.; Palmieri, F.; et al. Therapy modulates the response to T cell epitopes over the spectrum of tuberculosis infection. J. Infect. 2024, 89, 106295. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).