The Role and Potential Application of IL-12 in the Immune Regulation of Tuberculosis
Abstract
1. Introduction
2. Overview of IL-12
2.1. Structure and Molecular Biological Characteristics of IL-12 Family
2.2. IL-12 Production and Signal Transduction
2.2.1. Generation of IL-12
2.2.2. Signal Transduction Regulation—Related Signaling Pathways of IL-12 Generation
- Major Signaling Pathway 1: Toll-like Receptor (TLR) Signaling Pathway
- Major Signaling Pathway 2: C-Type Lectin Receptor (CLR) Signaling Pathway
- Major Signaling Pathway 3: Intracellular Signal Transduction Pathway—JAK-STAT
- Others: PI3K/Akt Signaling Pathway
2.3. Modification/Regulation of IL-12 Transcription and Translation
2.4. Secretion of IL-12
2.5. Regulation of IL-12
- (1)
- Negative Regulation
- Exogenous Drug Interventions
- (2)
- Positive regulation
3. The Regulatory Mechanism of IL-12 on Immune Cells
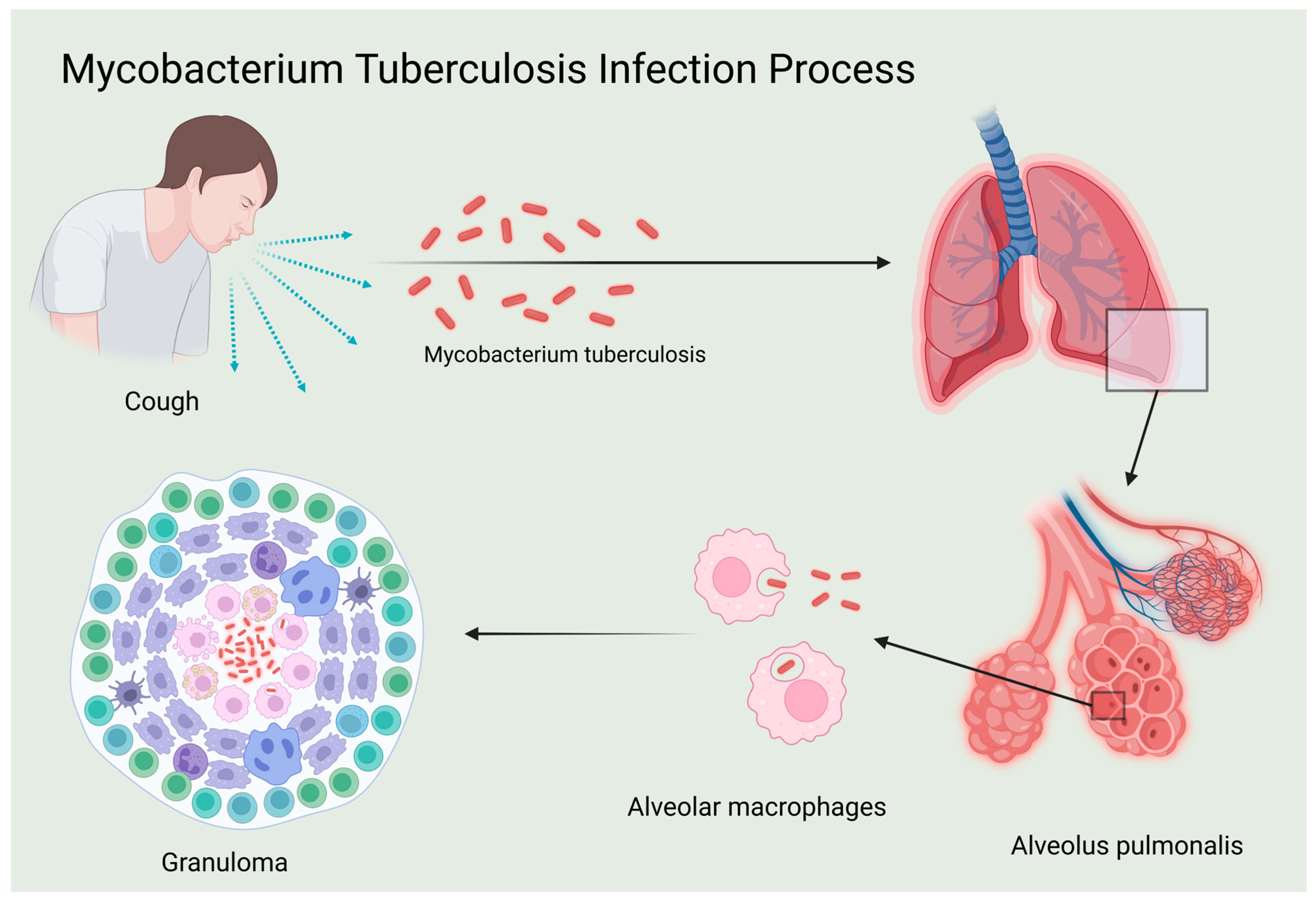
4. Role of IL-12 in the Immune Response to Tuberculosis
4.1. Influence of IL-12 in TB Pathogenesis
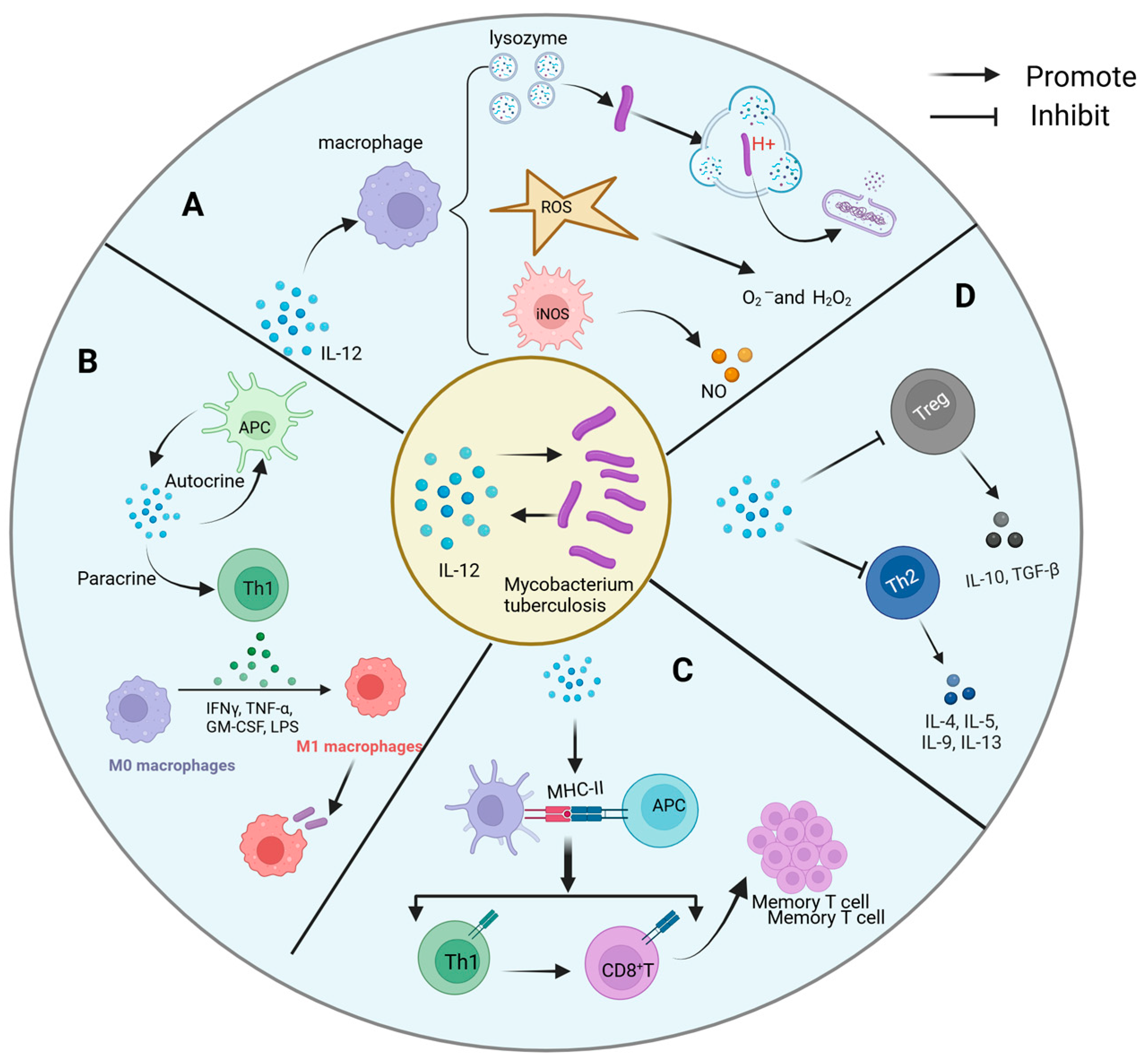
4.2. Interaction Mechanism Between IL-12 and Mtb
- A.
- Direct antibacterial activity
- B.
- Immune cell activation and differentiation
- C.
- Antigen presentation and immune memory
- D.
- Shaping of the inflammatory microenvironment
4.3. IL-12 and Drug-Resistance Mycobacterium Tuberculosis
5. Clinical Application of IL-12 in Diagnosis, Treatment, and Prognosis Evaluation of Tuberculosis
5.1. Diagnosis
- A.
- Importance of IL-12 in Tuberculosis Diagnosis
- B.
- Potential Role of IL-12 and Other Cytokines in Tuberculosis Diagnosis
- C.
- Potential Use of Host Biomarkers in Tuberculosis Diagnosis
- D.
- Superiority of IL-27 in the Diagnosis of Tuberculous Pleurisy
- E.
- Diagnostic Value of IL-12 in Diseases Associated with Immune Dysfunction
5.2. Treatment
5.3. Prognosis Assessment
5.4. Prevention
5.5. Disease and Susceptibility to Tuberculosis
5.6. Potential Strategies for Leveraging IL-12 Against Mtb Drug Resistance
6. Summary
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peña, D.; Rovetta, A.I.; Hernández Del Pino, R.E.; Amiano, N.O.; Pasquinelli, V.; Pellegrini, J.M.; Tateosian, N.L.; Rolandelli, A.; Gutierrez, M.; Musella, R.M.; et al. A Mycobacterium tuberculosis Dormancy Antigen Differentiates Latently Infected Bacillus Calmette-Guérin-vaccinated Individuals. EBioMedicine 2015, 2, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Bagcchi, S. WHO’s Global Tuberculosis Report 2022. Lancet Microbe 2023, 4, e20. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2022; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Lewinsohn, D.M.; Leonard, M.K.; LoBue, P.A.; Cohn, D.L.; Daley, C.L.; Desmond, E.; Keane, J.; Lewinsohn, D.A.; Loeffler, A.M.; Mazurek, G.H.; et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017, 64, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Salari, N.; Kanjoori, A.H.; Hosseinian-Far, A.; Hasheminezhad, R.; Mansouri, K.; Mohammadi, M. Global prevalence of drug-resistant tuberculosis: A systematic review and meta-analysis. Infect. Dis. Poverty 2023, 12, 57. [Google Scholar] [CrossRef]
- Swain, S.S.; Sharma, D.; Hussain, T.; Pati, S. Molecular mechanisms of underlying genetic factors and associated mutations for drug resistance in Mycobacterium tuberculosis. Emerg. Microbes Infect. 2020, 9, 1651–1663. [Google Scholar] [CrossRef]
- Migliori, G.B.; Ong, C.W.M.; Petrone, L.; D’Ambrosio, L.; Centis, R.; Goletti, D. The definition of tuberculosis infection based on the spectrum of tuberculosis disease. Breathe 2021, 17, 210079. [Google Scholar] [CrossRef]
- Zellweger, J.P.; Sotgiu, G.; Corradi, M.; Durando, P. The diagnosis of latent tuberculosis infection (LTBI): Currently available tests, future developments, and perspectives to eliminate tuberculosis (TB). Med. Lav. 2020, 111, 170–183. [Google Scholar] [CrossRef]
- Lovey, A.; Verma, S.; Kaipilyawar, V.; Ribeiro-Rodrigues, R.; Husain, S.; Palaci, M.; Dietze, R.; Ma, S.; Morrison, R.D.; Sherman, D.R.; et al. Early alveolar macrophage response and IL-1R-dependent T cell priming determine transmissibility of Mycobacterium tuberculosis strains. Nat. Commun. 2022, 13, 884. [Google Scholar] [CrossRef]
- Pflanz, S.; Timans, J.C.; Cheung, J.; Rosales, R.; Kanzler, H.; Gilbert, J.; Hibbert, L.; Churakova, T.; Travis, M.; Vaisberg, E.; et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 2002, 16, 779–790. [Google Scholar] [CrossRef]
- Presky, D.H.; Yang, H.; Minetti, L.J.; Chua, A.O.; Nabavi, N.; Wu, C.Y.; Gately, M.K.; Gubler, U. A functional interleukin 12 receptor complex is composed of two beta-type cytokine receptor subunits. Proc. Natl. Acad. Sci. USA 1996, 93, 14002–14007. [Google Scholar] [CrossRef]
- Ma, X.; Trinchieri, G. Regulation of interleukin-12 production in antigen-presenting cells. Adv. Immunol. 2001, 79, 55–92. [Google Scholar] [CrossRef] [PubMed]
- O’Shea, J.J.; Paul, W.E. Regulation of T(H)1 differentiation—Controlling the controllers. Nat. Immunol. 2002, 3, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Oppmann, B.; Lesley, R.; Blom, B.; Timans, J.C.; Xu, Y.; Hunte, B.; Vega, F.; Yu, N.; Wang, J.; Singh, K.; et al. Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 2000, 13, 715–725. [Google Scholar] [CrossRef]
- Parham, C.; Chirica, M.; Timans, J.; Vaisberg, E.; Travis, M.; Cheung, J.; Pflanz, S.; Zhang, R.; Singh, K.P.; Vega, F.; et al. A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rbeta1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 2002, 168, 5699–5708. [Google Scholar] [CrossRef]
- Li, J.; Gran, B.; Zhang, G.X.; Rostami, A.; Kamoun, M. IL-27 subunits and its receptor (WSX-1) mRNAs are markedly up-regulated in inflammatory cells in the CNS during experimental autoimmune encephalomyelitis. J. Neurol. Sci. 2005, 232, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.H.; Chen, H.Y.; Huang, C.; Yan, J.M.; Yin, Z.; Zhang, X.L.; Pan, Q. Accumulation of EBI3 induced by virulent Mycobacterium tuberculosis inhibits apoptosis in murine macrophages. Pathog. Dis. 2019, 77, ftz007. [Google Scholar] [CrossRef]
- Correale, J.; Marrodan, M.; Carnero Contentti, E. Interleukin-35 is a critical regulator of immunity during helminth infections associated with multiple sclerosis. Immunology 2021, 164, 569–586. [Google Scholar] [CrossRef]
- Dai, Y.C.; Wang, W.D.; Zhang, J.A.; Chen, C.; Luo, H.L.; Xu, H.; Peng, Y.; Luo, H.; Yang, X.R.; Chen, X.; et al. MTB driven B cells producing IL-35 and secreting high level of IL-10 in the patients with active pulmonary tuberculosis. Mol. Immunol. 2019, 112, 175–181. [Google Scholar] [CrossRef]
- Kong, B.; Liu, G.B.; Zhang, J.A.; Fu, X.X.; Xiang, W.Y.; Gao, Y.C.; Lu, Y.B.; Wu, X.J.; Qiu, F.; Wang, W.D.; et al. Elevated serum IL-35 and increased expression of IL-35-p35 or -EBI3 in CD4(+)CD25(+) T cells in patients with active tuberculosis. Am. J. Transl. Res. 2016, 8, 623–633. [Google Scholar]
- Collison, L.W.; Chaturvedi, V.; Henderson, A.L.; Giacomin, P.R.; Guy, C.; Bankoti, J.; Finkelstein, D.; Forbes, K.; Workman, C.J.; Brown, S.A.; et al. IL-35-mediated induction of a potent regulatory T cell population. Nat. Immunol. 2010, 11, 1093–1101. [Google Scholar] [CrossRef]
- Ma, X. TNF-alpha and IL-12: A balancing act in macrophage functioning. Microbes Infect. 2001, 3, 121–129. [Google Scholar] [CrossRef]
- Queiroz-Junior, C.M.; Silva, M.J.; Corrêa, J.D.; Madeira, M.F.; Garlet, T.P.; Garlet, G.P.; Cunha, F.Q.; Teixeira, M.M.; da Silva, T.A. A controversial role for IL-12 in immune response and bone resorption at apical periodontal sites. Clin. Dev. Immunol. 2010, 2010, 327417. [Google Scholar] [CrossRef]
- Yang, H.; Wei, J.; Zhang, H.; Song, W.; Wei, W.; Zhang, L.; Qian, K.; He, S. Upregulation of Toll-like Receptor (TLR) expression and release of cytokines from mast cells by IL-12. Cell Physiol. Biochem. 2010, 26, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Akira, S. TLR signaling pathways. Semin. Immunol. 2004, 16, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.; Barton, G.M. MyD88: A central player in innate immune signaling. F1000Prime Rep. 2014, 6, 97. [Google Scholar] [CrossRef]
- Martin, S.F.; Dudda, J.C.; Bachtanian, E.; Lembo, A.; Liller, S.; Dürr, C.; Heimesaat, M.M.; Bereswill, S.; Fejer, G.; Vassileva, R.; et al. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J. Exp. Med. 2008, 205, 2151–2162. [Google Scholar] [CrossRef]
- Durand, J.K.; Zhang, Q.; Baldwin, A.S. Roles for the IKK-Related Kinases TBK1 and IKKε in Cancer. Cells 2018, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.L.; Gioia, R.; Gatot, J.S.; Patrascu, F.; Carpentier, I.; Chapelle, J.P.; O’Neill, L.; Beyaert, R.; Piette, J.; Chariot, A. Are the IKKs and IKK-related kinases TBK1 and IKK-epsilon similarly activated? Trends Biochem. Sci. 2008, 33, 171–180. [Google Scholar] [CrossRef]
- Yum, S.; Li, M.; Fang, Y.; Chen, Z.J. TBK1 recruitment to STING activates both IRF3 and NF-κB that mediate immune defense against tumors and viral infections. Proc. Natl. Acad. Sci. USA 2021, 118, e2100225118. [Google Scholar] [CrossRef]
- Fischer, S.; Stegmann, F.; Gnanapragassam, V.S.; Lepenies, B. From structure to function—Ligand recognition by myeloid C-type lectin receptors. Comput. Struct. Biotechnol. J. 2022, 20, 5790–5812. [Google Scholar] [CrossRef]
- Wevers, B.A.; Kaptein, T.M.; Zijlstra-Willems, E.M.; Theelen, B.; Boekhout, T.; Geijtenbeek, T.B.; Gringhuis, S.I. Fungal engagement of the C-type lectin mincle suppresses dectin-1-induced antifungal immunity. Cell Host. Microbe 2014, 15, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Curtis, K.D.; Smith, P.R.; Despres, H.W.; Snyder, J.P.; Hogan, T.C.; Rodriguez, P.D.; Amiel, E. Glycogen Metabolism Supports Early Glycolytic Reprogramming and Activation in Dendritic Cells in Response to Both TLR and Syk-Dependent CLR Agonists. Cells 2020, 9, 715. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, R.; Li, J.; Li, J. The Role of C-Type Lectin Receptor Signaling in the Intestinal Microbiota-Inflammation-Cancer Axis. Front. Immunol. 2022, 13, 894445. [Google Scholar] [CrossRef]
- Kingeter, L.M.; Lin, X. C-type lectin receptor-induced NF-κB activation in innate immune and inflammatory responses. Cell Mol. Immunol. 2012, 9, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Cui, D.; Chao, X.; Chen, P.; Liu, J.; Wang, Y.; Su, T.; Li, M.; Xu, R.; Zhu, Y.; et al. Transcriptome Analysis Identifies Strategies Targeting Immune Response-Related Pathways to Control Enterotoxigenic Escherichia coli Infection in Porcine Intestinal Epithelial Cells. Front. Vet. Sci. 2021, 8, 677897. [Google Scholar] [CrossRef]
- Kaden, S.A.; Kurig, S.; Vasters, K.; Hofmann, K.; Zaenker, K.S.; Schmitz, J.; Winkels, G. Enhanced dendritic cell-induced immune responses mediated by the novel C-type lectin receptor mDCAR1. J. Immunol. 2009, 183, 5069–5078. [Google Scholar] [CrossRef]
- Chen, C.H.; Floyd, H.; Olson, N.E.; Magaletti, D.; Li, C.; Draves, K.; Clark, E.A. Dendritic-cell-associated C-type lectin 2 (DCAL-2) alters dendritic-cell maturation and cytokine production. Blood 2006, 107, 1459–1467. [Google Scholar] [CrossRef] [PubMed]
- Gotthardt, D.; Trifinopoulos, J.; Sexl, V.; Putz, E.M. JAK/STAT Cytokine Signaling at the Crossroad of NK Cell Development and Maturation. Front. Immunol. 2019, 10, 2590. [Google Scholar] [CrossRef]
- Kubo, S.; Nakayamada, S.; Tanaka, Y. JAK inhibitors for rheumatoid arthritis. Expert. Opin. Investig. Drugs 2023, 32, 333–344. [Google Scholar] [CrossRef]
- Kandhaya-Pillai, R.; Yang, X.; Tchkonia, T.; Martin, G.M.; Kirkland, J.L.; Oshima, J. TNF-α/IFN-γ synergy amplifies senescence-associated inflammation and SARS-CoV-2 receptor expression via hyper-activated JAK/STAT1. Aging Cell 2022, 21, e13646. [Google Scholar] [CrossRef]
- Au-Yeung, N.; Mandhana, R.; Horvath, C.M. Transcriptional regulation by STAT1 and STAT2 in the interferon JAK-STAT pathway. Jakstat 2013, 2, e23931. [Google Scholar] [CrossRef] [PubMed]
- Verhoeven, Y.; Tilborghs, S.; Jacobs, J.; De Waele, J.; Quatannens, D.; Deben, C.; Prenen, H.; Pauwels, P.; Trinh, X.B.; Wouters, A.; et al. The potential and controversy of targeting STAT family members in cancer. Semin. Cancer Biol. 2020, 60, 41–56. [Google Scholar] [CrossRef]
- Wesoly, J.; Szweykowska-Kulinska, Z.; Bluyssen, H.A. STAT activation and differential complex formation dictate selectivity of interferon responses. Acta Biochim. Pol. 2007, 54, 27–38. [Google Scholar]
- Nowicka, H.; Sekrecka, A.; Blaszczyk, K.; Kluzek, K.; Chang, C.Y.; Wesoly, J.; Lee, C.K.; Bluyssen, H.A.R. ISGF3 and STAT2/IRF9 Control Basal and IFN-Induced Transcription through Genome-Wide Binding of Phosphorylated and Unphosphorylated Complexes to Common ISRE-Containing ISGs. Int. J. Mol. Sci. 2023, 24, 17635. [Google Scholar] [CrossRef] [PubMed]
- Bechara, R.; Antonios, D.; Azouri, H.; Pallardy, M. Nickel Sulfate Promotes IL-17A Producing CD4+ T Cells by an IL-23-Dependent Mechanism Regulated by TLR4 and Jak-STAT Pathways. J. Investig. Dermatol. 2017, 137, 2140–2148. [Google Scholar] [CrossRef]
- Lin, Y.; Kuang, W.; Wu, B.; Xie, C.; Liu, C.; Tu, Z. IL-12 induces autophagy in human breast cancer cells through AMPK and the PI3K/Akt pathway. Mol. Med. Rep. 2017, 16, 4113–4118. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, T.; Tian, C.; Ma, K.; Gou, J.; Huang, R.; Li, S.; Li, Q.; Xu, C.; Li, L.; et al. Kinsenoside attenuates liver fibro-inflammation by suppressing dendritic cells via the PI3K-AKT-FoxO1 pathway. Pharmacol. Res. 2022, 177, 106092. [Google Scholar] [CrossRef]
- Quan, J.H.; Chu, J.Q.; Kwon, J.; Choi, I.W.; Ismail, H.A.; Zhou, W.; Cha, G.H.; Zhou, Y.; Yuk, J.M.; Jo, E.K.; et al. Intracellular Networks of the PI3K/AKT and MAPK Pathways for Regulating Toxoplasma gondii-Induced IL-23 and IL-12 Production in Human THP-1 Cells. PLoS ONE 2015, 10, e0141550. [Google Scholar] [CrossRef]
- Tian, T.; Wang, Z.; Chen, L.; Xu, W.; Wu, B. Photobiomodulation activates undifferentiated macrophages and promotes M1/M2 macrophage polarization via PI3K/AKT/mTOR signaling pathway. Lasers Med. Sci. 2023, 38, 86. [Google Scholar] [CrossRef]
- Nandagopal, N.; Ali, A.K.; Komal, A.K.; Lee, S.H. The Critical Role of IL-15-PI3K-mTOR Pathway in Natural Killer Cell Effector Functions. Front. Immunol. 2014, 5, 187. [Google Scholar] [CrossRef]
- Song, C.; Zhang, Q.; Liu, X.; Shan, Y. IL-12 and IL-10 production are differentially regulated by phosphatidylinositol 3-kinase in mast cells. Scand. J. Immunol. 2012, 75, 266–272. [Google Scholar] [CrossRef]
- Carter, A.N.; Born, H.A.; Levine, A.T.; Dao, A.T.; Zhao, A.J.; Lee, W.L.; Anderson, A.E. Wortmannin Attenuates Seizure-Induced Hyperactive PI3K/Akt/mTOR Signaling, Impaired Memory, and Spine Dysmorphology in Rats. eNeuro 2017, 4, ENEURO.0354–16.2017. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lu, J.; Wei, L.; Wang, X.; Xu, X.; Dong, M.; Huang, B. Histone acetyltransferase activity of p300 enhances the activation of IL-12 p40 promoter. Mol. Immunol. 2004, 41, 1241–1246. [Google Scholar] [CrossRef]
- Li, Y.; Xia, J.; Jiang, N.; Xian, Y.; Ju, H.; Wei, Y.; Zhang, X. Corin protects H(2)O(2)-induced apoptosis through PI3K/AKT and NF-κB pathway in cardiomyocytes. Biomed. Pharmacother. 2018, 97, 594–599. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cardoso, S.C.; Bekker, C.P.J.; Boes, M.; Radstake, T.; Angiolilli, C. CXCL4 is a driver of cytokine mRNA stability in monocyte-derived dendritic cells. Mol. Immunol. 2019, 114, 524–534. [Google Scholar] [CrossRef]
- Dawson, J.E.; Bah, A.; Zhang, Z.; Vernon, R.M.; Lin, H.; Chong, P.A.; Vanama, M.; Sonenberg, N.; Gradinaru, C.C.; Forman-Kay, J.D. Non-cooperative 4E-BP2 folding with exchange between eIF4E-binding and binding-incompatible states tunes cap-dependent translation inhibition. Nat. Commun. 2020, 11, 3146. [Google Scholar] [CrossRef]
- Lu, J.; Sun, H.; Wang, X.; Liu, C.; Xu, X.; Li, F.; Huang, B. Interleukin-12 p40 promoter activity is regulated by the reversible acetylation mediated by HDAC1 and p300. Cytokine 2005, 31, 46–51. [Google Scholar] [CrossRef]
- Guindi, C.; Cloutier, A.; Gaudreau, S.; Zerif, E.; McDonald, P.P.; Tatsiy, O.; Asselin, C.; Dupuis, G.; Gris, D.; Amrani, A.A. Role of the p38 MAPK/C/EBPβ Pathway in the Regulation of Phenotype and IL-10 and IL-12 Production by Tolerogenic Bone Marrow-Derived Dendritic Cells. Cells 2018, 7, 256. [Google Scholar] [CrossRef]
- Morita, M.; Gravel, S.P.; Chénard, V.; Sikström, K.; Zheng, L.; Alain, T.; Gandin, V.; Avizonis, D.; Arguello, M.; Zakaria, C.; et al. mTORC1 controls mitochondrial activity and biogenesis through 4E-BP-dependent translational regulation. Cell Metab. 2013, 18, 698–711. [Google Scholar] [CrossRef]
- Thoreen, C.C.; Chantranupong, L.; Keys, H.R.; Wang, T.; Gray, N.S.; Sabatini, D.M. A unifying model for mTORC1-mediated regulation of mRNA translation. Nature 2012, 485, 109–113. [Google Scholar] [CrossRef]
- Saraf, A.; Luo, J.; Morris, D.R.; Storm, D.R. Phosphorylation of eukaryotic translation initiation factor 4E and eukaryotic translation initiation factor 4E-binding protein (4EBP) and their upstream signaling components undergo diurnal oscillation in the mouse hippocampus: Implications for memory persistence. J. Biol. Chem. 2014, 289, 20129–20138. [Google Scholar] [CrossRef]
- Shemesh, A.; Pickering, H.; Roybal, K.T.; Lanier, L.L. Differential IL-12 signaling induces human natural killer cell activating receptor-mediated ligand-specific expansion. J. Exp. Med. 2022, 219, e20212434. [Google Scholar] [CrossRef]
- Yang, R.; Yang, E.; Shen, L.; Modlin, R.L.; Shen, H.; Chen, Z.W. IL-12+IL-18 Cosignaling in Human Macrophages and Lung Epithelial Cells Activates Cathelicidin and Autophagy, Inhibiting Intracellular Mycobacterial Growth. J. Immunol. 2018, 200, 2405–2417. [Google Scholar] [CrossRef]
- Hildenbrand, K.; Aschenbrenner, I.; Franke, F.C.; Devergne, O.; Feige, M.J. Biogenesis and engineering of interleukin 12 family cytokines. Trends Biochem. Sci. 2022, 47, 936–949. [Google Scholar] [CrossRef] [PubMed]
- Goodall, J.C.; Wu, C.; Zhang, Y.; McNeill, L.; Ellis, L.; Saudek, V.; Gaston, J.S. Endoplasmic reticulum stress-induced transcription factor, CHOP, is crucial for dendritic cell IL-23 expression. Proc. Natl. Acad. Sci. USA 2010, 107, 17698–17703. [Google Scholar] [CrossRef] [PubMed]
- Radwan, E.; Belmadani, S.; Matrougui, K. Disrupting Interleukin 12 Improves Microvascular Endothelial Function in Type 2 Diabetes Through ER Stress CHOP and Oxidative Stress Mechanisms. Diabetes Metab. Syndr. Obes. 2022, 15, 2633–2642. [Google Scholar] [CrossRef] [PubMed]
- Uronen-Hansson, H.; Allen, J.; Osman, M.; Squires, G.; Klein, N.; Callard, R.E. Toll-like receptor 2 (TLR2) and TLR4 are present inside human dendritic cells, associated with microtubules and the Golgi apparatus but are not detectable on the cell surface: Integrity of microtubules is required for interleukin-12 production in response to internalized bacteria. Immunology 2004, 111, 173–178. [Google Scholar] [CrossRef]
- Kalim, K.W.; Groettrup, M. Prostaglandin E2 inhibits IL-23 and IL-12 production by human monocytes through down-regulation of their common p40 subunit. Mol. Immunol. 2013, 53, 274–282. [Google Scholar] [CrossRef]
- Aste-Amezaga, M.; Ma, X.; Sartori, A.; Trinchieri, G. Molecular mechanisms of the induction of IL-12 and its inhibition by IL-10. J. Immunol. 1998, 160, 5936–5944. [Google Scholar] [CrossRef]
- D’Andrea, A.; Aste-Amezaga, M.; Valiante, N.M.; Ma, X.; Kubin, M.; Trinchieri, G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J. Exp. Med. 1993, 178, 1041–1048. [Google Scholar] [CrossRef]
- Van Weyenbergh, J.; MP, P.S.; Báfica, A.; Cardoso, S.; Wietzerbin, J.; Barral-Netto, M. IFN-beta and TGF-beta differentially regulate IL-12 activity in human peripheral blood mononuclear cells. Immunol. Lett. 2001, 75, 117–122. [Google Scholar] [CrossRef]
- Finnegan, A.; Grusby, M.J.; Kaplan, C.D.; O’Neill, S.K.; Eibel, H.; Koreny, T.; Czipri, M.; Mikecz, K.; Zhang, J. IL-4 and IL-12 regulate proteoglycan-induced arthritis through Stat-dependent mechanisms. J. Immunol. 2002, 169, 3345–3352. [Google Scholar] [CrossRef]
- Vom Berg, J.; Vrohlings, M.; Haller, S.; Haimovici, A.; Kulig, P.; Sledzinska, A.; Weller, M.; Becher, B. Intratumoral IL-12 combined with CTLA-4 blockade elicits T cell-mediated glioma rejection. J. Exp. Med. 2013, 210, 2803–2811. [Google Scholar] [CrossRef] [PubMed]
- Garris, C.S.; Arlauckas, S.P.; Kohler, R.H.; Trefny, M.P.; Garren, S.; Piot, C.; Engblom, C.; Pfirschke, C.; Siwicki, M.; Gungabeesoon, J.; et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity 2018, 49, 1148–1161.e1147. [Google Scholar] [CrossRef]
- Qin, H.; Holdbrooks, A.T.; Liu, Y.; Reynolds, S.L.; Yanagisawa, L.L.; Benveniste, E.N. SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 2012, 189, 3439–3448. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Kuhel, D.G.; Chen, J.F.; Schwarzschild, M.A.; Deitch, E.A.; Mabley, J.G.; Marton, A.; Szabó, C. Adenosine inhibits IL-12 and TNF-α production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000, 14, 2065–2074. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.M.; Ross, W.G.; Agbai, O.N.; Frazier, R.; Figler, R.A.; Rieger, J.; Linden, J.; Ernst, P.B. The A2B adenosine receptor impairs the maturation and immunogenicity of dendritic cells. J. Immunol. 2009, 182, 4616–4623. [Google Scholar] [CrossRef]
- Bai, F.; Li, G.G.; Liu, Q.; Niu, X.; Li, R.; Ma, H. Short-Term Efficacy and Safety of IL-17, IL-12/23, and IL-23 Inhibitors Brodalumab, Secukinumab, Ixekizumab, Ustekinumab, Guselkumab, Tildrakizumab, and Risankizumab for the Treatment of Moderate to Severe Plaque Psoriasis: A Systematic Review and Network Meta-Analysis of Randomized Controlled Trials. J. Immunol. Res. 2019, 2019, 2546161. [Google Scholar] [CrossRef]
- Sands, B.E.; Sandborn, W.J.; Panaccione, R.; O’Brien, C.D.; Zhang, H.; Johanns, J.; Adedokun, O.J.; Li, K.; Peyrin-Biroulet, L.; Van Assche, G.; et al. Ustekinumab as Induction and Maintenance Therapy for Ulcerative Colitis. N. Engl. J. Med. 2019, 381, 1201–1214. [Google Scholar] [CrossRef]
- MacDonald, J.K.; Nguyen, T.M.; Khanna, R.; Timmer, A. Anti-IL-12/23p40 antibodies for induction of remission in Crohn’s disease. Cochrane Database Syst. Rev. 2016, 11, Cd007572. [Google Scholar] [CrossRef]
- Tamiya, S.; Yoshikawa, E.; Ogura, M.; Kuroda, E.; Suzuki, K.; Yoshioka, Y. Neutrophil-Mediated Lung Injury Both via TLR2-Dependent Production of IL-1α and IL-12 p40, and TLR2-Independent CARDS Toxin after Mycoplasma pneumoniae Infection in Mice. Microbiol. Spectr. 2021, 9, e0158821. [Google Scholar] [CrossRef]
- Kim, H.S.; Chung, D.H. TLR4-mediated IL-12 production enhances IFN-γ and IL-1β production, which inhibits TGF-β production and promotes antibody-induced joint inflammation. Arthritis Res. Ther. 2012, 14, R210. [Google Scholar] [CrossRef]
- Posseme, C.; Llibre, A.; Charbit, B.; Bondet, V.; Rouilly, V.; Saint-André, V.; Boussier, J.; Bergstedt, J.; Smith, N.; Townsend, L.; et al. Early IFNβ secretion determines variable downstream IL-12p70 responses upon TLR4 activation. Cell Rep. 2022, 39, 110989. [Google Scholar] [CrossRef]
- Bank, S.; Andersen, P.S.; Burisch, J.; Pedersen, N.; Roug, S.; Galsgaard, J.; Turino, S.Y.; Brodersen, J.B.; Rashid, S.; Rasmussen, B.K.; et al. Genetically determined high activity of IL-12 and IL-18 in ulcerative colitis and TLR5 in Crohns disease were associated with non-response to anti-TNF therapy. Pharmacogenomics J. 2018, 18, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Frazão, J.B.; Errante, P.R.; Condino-Neto, A. Toll-like receptors’ pathway disturbances are associated with increased susceptibility to infections in humans. Arch. Immunol. Ther. Exp. 2013, 61, 427–443. [Google Scholar] [CrossRef]
- Theiner, G.; Rössner, S.; Dalpke, A.; Bode, K.; Berger, T.; Gessner, A.; Lutz, M.B. TLR9 cooperates with TLR4 to increase IL-12 release by murine dendritic cells. Mol. Immunol. 2008, 45, 244–252. [Google Scholar] [CrossRef]
- Mandour, M.F.; Soe, P.P.; Castonguay, A.S.; Van Snick, J.; Coutelier, J.P. Inhibition of IL-12 heterodimers impairs TLR9-mediated prevention of early mouse plasmacytoma cell growth. Front. Med. 2022, 9, 1057252. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Conti, P. Mast cells to dendritic cells: Let IL-13 shut your IL-12 down. J. Allergy Clin. Immunol. 2021, 147, 2073–2074. [Google Scholar] [CrossRef]
- Abdi, K.; Laky, K.; Padhan, K.; Petrovas, C.; Skinner, J.; Kabat, J.; Dorward, D.W.; Brzostowski, J.; Long, E.O.; Trinchieri, G.; et al. Cutting Edge: Quantitative Determination of CD40L Threshold for IL-12 and IL-23 Production from Dendritic Cells. J. Immunol. 2018, 201, 2879–2884. [Google Scholar] [CrossRef]
- Bullens, D.M.; Kasran, A.; Thielemans, K.; Bakkus, M.; Ceuppens, J.L. CD40L-induced IL-12 production is further enhanced by the Th2 cytokines IL-4 and IL-13. Scand. J. Immunol. 2001, 53, 455–463. [Google Scholar] [CrossRef]
- Ashour, D.; Arampatzi, P.; Pavlovic, V.; Förstner, K.U.; Kaisho, T.; Beilhack, A.; Erhard, F.; Lutz, M.B. IL-12 from endogenous cDC1, and not vaccine DC, is required for Th1 induction. JCI Insight 2020, 5, e135143. [Google Scholar] [CrossRef]
- Cooper, A.M.; Magram, J.; Ferrante, J.; Orme, I.M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with mycobacterium tuberculosis. J. Exp. Med. 1997, 186, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Wakeham, J.; Wang, J.; Magram, J.; Croitoru, K.; Harkness, R.; Dunn, P.; Zganiacz, A.; Xing, Z. Lack of both types 1 and 2 cytokines, tissue inflammatory responses, and immune protection during pulmonary infection by Mycobacterium bovis bacille Calmette-Guérin in IL-12-deficient mice. J. Immunol. 1998, 160, 6101–6111. [Google Scholar] [CrossRef] [PubMed]
- Qadir, K.; Metwali, A.; Blum, A.M.; Li, J.; Elliott, D.E.; Weinstock, J.V. TGF-beta and IL-10 regulation of IFN-gamma produced in Th2-type schistosome granulomas requires IL-12. Am. J. Physiol. Gastrointest. Liver Physiol. 2001, 281, G940–G946. [Google Scholar] [CrossRef] [PubMed]
- Arababadi, M.K.; Pourfathollah, A.A.; Jafarzadeh, A.; Hassanshahi, G.; Daneshmandi, S.; Shamsizadeh, A.; Kennedy, D. Non-association of IL-12 +1188 and IFN-γ +874 polymorphisms with cytokines serum level in occult HBV infected patients. Saudi J. Gastroenterol. 2011, 17, 30–35. [Google Scholar] [CrossRef]
- Zhou, F.; Xiong, H.; Zhen, S.; Chen, A.; Huang, M.; Luo, Y. Serum levels of IL-12, IL-18, and IL-21 are indicators of viral load in patients chronically infected with HBV. Braz. J. Med. Biol. Res. 2022, 55, e12320. [Google Scholar] [CrossRef]
- Abdi, K.; Laky, K.; Abshari, M.; Hill, E.M.; Lantz, L.; Singh, N.J.; Long, E.O. Dendritic cells Trigger IFN-γ secretion by NK cells independent of IL-12 and IL-18. Eur. J. Immunol. 2022, 52, 1431–1440. [Google Scholar] [CrossRef]
- Terrén, I.; Sandá, V.; Amarilla-Irusta, A.; Lopez-Pardo, A.; Sevilla, A.; Astarloa-Pando, G.; Amo, L.; Zenarruzabeitia, O.; Scorrano, L.; Borrego, F. IL-12/15/18-induced cell death and mitochondrial dynamics of human NK cells. Front. Immunol. 2023, 14, 1211839. [Google Scholar] [CrossRef]
- Mah, A.Y.; Cooper, M.A. Metabolic Regulation of Natural Killer Cell IFN-γ Production. Crit. Rev. Immunol. 2016, 36, 131–147. [Google Scholar] [CrossRef]
- Robinson, C.M.; Nau, G.J. Interleukin-12 and interleukin-27 regulate macrophage control of Mycobacterium tuberculosis. J. Infect. Dis. 2008, 198, 359–366. [Google Scholar] [CrossRef]
- McClean, C.M.; Tobin, D.M. Macrophage form, function, and phenotype in mycobacterial infection: Lessons from tuberculosis and other diseases. Pathog. Dis. 2016, 74, ftw068. [Google Scholar] [CrossRef]
- Hmama, Z.; Peña-Díaz, S.; Joseph, S.; Av-Gay, Y. Immunoevasion and immunosuppression of the macrophage by Mycobacterium tuberculosis. Immunol. Rev. 2015, 264, 220–232. [Google Scholar] [CrossRef]
- Dao, D.N.; Kremer, L.; Guérardel, Y.; Molano, A.; Jacobs, W.R., Jr.; Porcelli, S.A.; Briken, V. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect. Immun. 2004, 72, 2067–2074. [Google Scholar] [CrossRef]
- Markiewicz, M.A.; Wise, E.L.; Buchwald, Z.S.; Cheney, E.E.; Hansen, T.H.; Suri, A.; Cemerski, S.; Allen, P.M.; Shaw, A.S. IL-12 enhances CTL synapse formation and induces self-reactivity. J. Immunol. 2009, 182, 1351–1361. [Google Scholar] [CrossRef] [PubMed]
- Noble, A.; Mehta, H.; Lovell, A.; Papaioannou, E.; Fairbanks, L. IL-12 and IL-4 activate a CD39-dependent intrinsic peripheral tolerance mechanism in CD8(+) T cells. Eur. J. Immunol. 2016, 46, 1438–1448. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jay, S.M.; Wang, Y.; Wu, S.W.; Xiao, Z. IL-12 stimulates CTLs to secrete exosomes capable of activating bystander CD8(+) T cells. Sci. Rep. 2017, 7, 13365. [Google Scholar] [CrossRef] [PubMed]
- Raeber, M.E.; Zurbuchen, Y.; Impellizzieri, D.; Boyman, O. The role of cytokines in T-cell memory in health and disease. Immunol. Rev. 2018, 283, 176–193. [Google Scholar] [CrossRef]
- Ahmad, S.; Ahmed, J.; Khalifa, E.H.; Khattak, F.A.; Khan, A.S.; Farooq, S.U.; Osman, S.M.A.; Salih, M.M.; Ullah, N.; Khan, T.A. Novel mutations in genes of the IL-12/IFN-γ axis cause susceptibility to tuberculosis. J. Infect. Public Health 2023, 16, 1368–1378. [Google Scholar] [CrossRef]
- Kelsey, A.; Chirch, L.M.; Payette, M.J. Tuberculosis and interleukin blocking monoclonal antibodies: Is there risk? Dermatol. Online J. 2018, 24, 9. [Google Scholar] [CrossRef]
- Nekooie-Marnany, N.; Deswarte, C.; Ostadi, V.; Bagherpour, B.; Taleby, E.; Ganjalikhani-Hakemi, M.; Le Voyer, T.; Rahimi, H.; Rosain, J.; Pourmoghadas, Z.; et al. Impaired IL-12- and IL-23-Mediated Immunity Due to IL-12Rβ1 Deficiency in Iranian Patients with Mendelian Susceptibility to Mycobacterial Disease. J. Clin. Immunol. 2018, 38, 787–793. [Google Scholar] [CrossRef]
- Pérez, D.; Muñoz, M.C.; Molina, J.M.; Muñoz-Caro, T.; Silva, L.M.; Taubert, A.; Hermosilla, C.; Ruiz, A. Eimeria ninakohlyakimovae induces NADPH oxidase-dependent monocyte extracellular trap formation and upregulates IL-12 and TNF-α, IL-6 and CCL2 gene transcription. Vet. Parasitol. 2016, 227, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Kees, T.; Almeida, A.S.; Liu, B.; He, X.Y.; Ng, D.; Han, X.; Spector, D.L.; McNeish, I.A.; Gimotty, P.; et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell 2021, 39, 1361–1374.e9. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Tomita, Y.; Jankowska-Gan, E.; Lema, D.A.; Arvedson, M.P.; Nair, A.; Bracamonte-Baran, W.; Zhou, Y.; Meyer, K.K.; Zhong, W.; et al. Treg-Cell-Derived IL-35-Coated Extracellular Vesicles Promote Infectious Tolerance. Cell Rep. 2020, 30, 1039–1051.e5. [Google Scholar] [CrossRef] [PubMed]
- Latsi, P.; Pantelidis, P.; Vassilakis, D.; Sato, H.; Welsh, K.I.; du Bois, R.M. Analysis of IL-12 p40 subunit gene and IFN-gamma G5644A polymorphisms in Idiopathic Pulmonary Fibrosis. Respir. Res. 2003, 4, 6. [Google Scholar] [CrossRef]
- Flynn, J.L.; Goldstein, M.M.; Triebold, K.J.; Sypek, J.; Wolf, S.; Bloom, B.R. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 1995, 155, 2515–2524. [Google Scholar] [PubMed]
- Chen, Y.Y.; Lin, C.W.; Huang, W.F.; Chang, J.R.; Su, I.J.; Hsu, C.H.; Cheng, H.Y.; Hsu, S.C.; Dou, H.Y. Recombinant bacille Calmette-Guerin coexpressing Ag85b, CFP10, and interleukin-12 elicits effective protection against Mycobacterium tuberculosis. J. Microbiol. Immunol. Infect. 2017, 50, 90–96. [Google Scholar] [CrossRef]
- Mata-Espinosa, D.A.; Francisco-Cruz, A.; Marquina-Castillo, B.; Barrios-Payan, J.; Ramos-Espinosa, O.; Bini, E.I.; Xing, Z.; Hernández-Pando, R. Immunotherapeutic effects of recombinant adenovirus encoding interleukin 12 in experimental pulmonary tuberculosis. Scand J. Immunol. 2019, 89, e12743. [Google Scholar] [CrossRef]
- Gerace, E.; Pasquali, P.; Oesch, B.; Falduto, M.; Mandanici, F.; Fiasconaro, M.; Vitale, M.; Di Marco Lo Presti, V.; Amato, B. Stimulation of Bovine Whole-Blood Samples Cultured in Media Supplemented with Recombinant Interleukin-7 (IL-7) and IL-12 Extends the Life Span of the Gamma Interferon Assay To Detect Mycobacterium bovis-Infected Cattle. J. Clin. Microbiol. 2016, 54, 2315–2320. [Google Scholar] [CrossRef]
- Iida, K.; Suzuki, K.; Yokota, M.; Nakagomi, D.; Wakashin, H.; Iwata, A.; Kawashima, H.; Takatori, H.; Nakajima, H. STAT4 is required for IFN-β-induced MCP-1 mRNA expression in murine mast cells. Int. Arch. Allergy Immunol. 2011, 155 (Suppl. S1), 71–76. [Google Scholar] [CrossRef]
- Hussain, S.; Rasool, R.; Shafi, T.; Gull, A.; Qureshi, T.A.; Jan, R.; Shah, Z.A. Evaluation of SOCS5 mRNA and its association with serum IL-12 levels and rs41379147 SNP in various subsets of allergic disorders: A case control study. Mol. Immunol. 2023, 162, 102–110. [Google Scholar] [CrossRef]
- Pahan, K.; Sheikh, F.G.; Liu, X.; Hilger, S.; McKinney, M.; Petro, T.M. Induction of nitric-oxide synthase and activation of NF-kappaB by interleukin-12 p40 in microglial cells. J. Biol. Chem. 2001, 276, 7899–7905. [Google Scholar] [CrossRef] [PubMed]
- Gaviglio, E.A.; Peralta Ramos, J.M.; Arroyo, D.S.; Bussi, C.; Iribarren, P.; Rodriguez-Galan, M.C. Systemic sterile induced-co-expression of IL-12 and IL-18 drive IFN-γ-dependent activation of microglia and recruitment of MHC-II-expressing inflammatory monocytes into the brain. Int. Immunopharmacol. 2022, 105, 108546. [Google Scholar] [CrossRef] [PubMed]
- Pflanz, S.; Hibbert, L.; Mattson, J.; Rosales, R.; Vaisberg, E.; Bazan, J.F.; Phillips, J.H.; McClanahan, T.K.; de Waal Malefyt, R.; Kastelein, R.A. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J. Immunol. 2004, 172, 2225–2231. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, N.D.; Counoupas, C.; Daniel, L.; Zhang, G.; Cook, S.J.; Cootes, T.A.; Stifter, S.A.; Bowen, D.G.; Triccas, J.A.; Bertolino, P.; et al. TCR Affinity Controls the Dynamics but Not the Functional Specification of the Antimycobacterial CD4(+) T Cell Response. J. Immunol. 2021, 206, 2875–2887. [Google Scholar] [CrossRef] [PubMed]
- Dlamini, Z.; Alaouna, M.; Cholo, M.C.; Hull, R. Is targeting dysregulation in apoptosis splice variants in Mycobacterium tuberculosis (MTB) host interactions and splicing factors resulting in immune evasion by MTB strategies a possibility? Tuberculosis 2020, 124, 101964. [Google Scholar] [CrossRef]
- Liu, D.; Huang, F.; Zhang, G.; He, W.; Ou, X.; He, P.; Zhao, B.; Zhu, B.; Liu, F.; Li, Z.; et al. Whole-genome sequencing for surveillance of tuberculosis drug resistance and determination of resistance level in China. Clin. Microbiol. Infect. 2022, 28, 731-e9. [Google Scholar] [CrossRef]
- Abdalla, A.E.; Lambert, N.; Duan, X.; Xie, J. Interleukin-10 Family and Tuberculosis: An Old Story Renewed. Int. J. Biol. Sci. 2016, 12, 710–717. [Google Scholar] [CrossRef]
- Dokrungkoon, T.; Tulyaprawat, O.; Suwannakarn, K.; Ngamskulrungroj, P. In vitro modeling of isoniazid resistance mechanisms in Mycobacterium tuberculosis H37Rv. Front. Microbiol. 2023, 14, 1171861. [Google Scholar] [CrossRef]
- Thibodeau, J.; Bourgeois-Daigneault, M.C.; Huppé, G.; Tremblay, J.; Aumont, A.; Houde, M.; Bartee, E.; Brunet, A.; Gauvreau, M.E.; de Gassart, A.; et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur. J. Immunol. 2008, 38, 1225–1230. [Google Scholar] [CrossRef]
- Guirado, E.; Schlesinger, L.S.; Kaplan, G. Macrophages in tuberculosis: Friend or foe. Semin. Immunopathol. 2013, 35, 563–583. [Google Scholar] [CrossRef]
- Li, W.; Deng, G.; Li, M.; Liu, X.; Wang, Y. Roles of Mucosal Immunity against Mycobacterium tuberculosis Infection. Tuberc. Res. Treat. 2012, 2012, 791728. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, T.M.; Demkow, U.; Michalowska-Mitczuk, D.; Filewska, M.; Bialas, B.; Zycinska, K.; Obrowski, M.H.; Kus, J.; Skopinska-Rozewska, E. Angiogenic activity of sera from pulmonary tuberculosis patients in relation to IL-12p40 and TNFα serum levels. Lung 2011, 189, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Li, H.; Guo, C.; Shang, Y.; Wang, W.; Zhang, X.; Li, S.; Pang, Y. Serum Cytokine Biomarkers for Use in Diagnosing Pulmonary Tuberculosis versus Chronic Pulmonary Aspergillosis. Infect. Drug Resist. 2023, 16, 2217–2226. [Google Scholar] [CrossRef] [PubMed]
- Dewi, I.M.W.; Soeroto, A.Y.; Putriyani, G.; Hanifah, W.; Permata, A.; Annisa, J.; Fithriyana, I.; Verweij, P.E.; van Laarhoven, A.; de Veerdonk, F.L.V.; et al. Aspergillus fumigatus-specific antibodies in patients with chronic tuberculosis. Int. J. Tuberc. Lung. Dis. 2020, 24, 853–856. [Google Scholar] [CrossRef]
- Namuganga, A.R.; Nsereko, M.; Bagaya, B.S.; Mayanja-Kizza, H.; Chegou, N.N. Differential expression of host protein biomarkers among symptomatic clinic attendees finally diagnosed with tuberculosis and other respiratory diseases with or without latent Mycobacterium tuberculosis infection. Immunol. Lett. 2023, 253, 8–18. [Google Scholar] [CrossRef]
- Yu, F.; Zhu, X.; Li, Q.; Xu, W.; Gao, Y.; Wen, Y.; Zhang, Q.; Dou, J. Elevated IL-35 level and iTr35 subset increase the bacterial burden and lung lesions in Mycobacterium tuberculosis-infected mice. Open Life Sci. 2022, 17, 312–320. [Google Scholar] [CrossRef]
- Antonangelo, L.; Faria, C.S.; Sales, R.K. Tuberculous pleural effusion: Diagnosis & management. Expert Rev. Respir. Med. 2019, 13, 747–759. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, Y.; Zhang, Y.; Liu, H.; Dou, Q. Age: Pleural fluid ADA ratio and other indicators for differentiating between tubercular and malignant pleural effusions. Medicine 2022, 101, e29788. [Google Scholar] [CrossRef]
- Mortaz, E.; Tabarsi, P.; Mansouri, D.; Khosravi, A.; Garssen, J.; Velayati, A.; Adcock, I.M. Cancers Related to Immunodeficiencies: Update and Perspectives. Front. Immunol. 2016, 7, 365. [Google Scholar] [CrossRef]
- Wani, S.R.; Wattal, C.; Raveendran, R. Epidemiology and risk factors associated with NTM pulmonary and extrapulmonary infections in a high tuberculosis endemic Region. Indian J. Med. Microbiol. 2020, 38, 169–175. [Google Scholar] [CrossRef]
- Namkoong, H.; Hasegawa, N.; Betsuyaku, T. Susceptibility genes for nontuberculous mycobacterial disease. Nihon Rinsho Meneki Gakkai Kaishi 2017, 40, 60–67. [Google Scholar] [CrossRef]
- Costa, D.L.; Amaral, E.P.; Namasivayam, S.; Mittereder, L.R.; Andrade, B.B.; Sher, A. Enhancement of CD4(+) T Cell Function as a Strategy for Improving Antibiotic Therapy Efficacy in Tuberculosis: Does It Work? Front. Cell Infect. Microbiol. 2021, 11, 672527. [Google Scholar] [CrossRef]
- Nolt, D.; Flynn, J.L. Interleukin-12 therapy reduces the number of immune cells and pathology in lungs of mice infected with Mycobacterium tuberculosis. Infect. Immun. 2004, 72, 2976–2988. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jiang, Y.; Lao, S.; Yang, B.; Yu, S.; Zhang, Y.; Wu, C. Mycobacterium tuberculosis-Specific IL-21+IFN-γ+CD4+ T Cells Are Regulated by IL-12. PLoS ONE 2016, 11, e0147356. [Google Scholar] [CrossRef]
- Shen, L.; Huang, D.; Qaqish, A.; Frencher, J.; Yang, R.; Shen, H.; Chen, Z.W. Fast-acting γδ T-cell subpopulation and protective immunity against infections. Immunol. Rev. 2020, 298, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.W. Multifunctional immune responses of HMBPP-specific Vγ2Vδ2 T cells in M. tuberculosis and other infections. Cell Mol. Immunol. 2013, 10, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Yang, E.; Guo, M.; Yang, R.; Huang, G.; Peng, Y.; Sha, W.; Wang, F.; Shen, L. Adjunctive Zoledronate + IL-2 administrations enhance anti-tuberculosis Vγ2Vδ2 T-effector populations, and improve treatment outcome of multidrug-resistant tuberculosis1. Emerg. Microbes Infect. 2022, 11, 1790–1805. [Google Scholar] [CrossRef]
- Shen, L.; Frencher, J.; Huang, D.; Wang, W.; Yang, E.; Chen, C.Y.; Zhang, Z.; Wang, R.; Qaqish, A.; Larsen, M.H.; et al. Immunization of Vγ2Vδ2 T cells programs sustained effector memory responses that control tuberculosis in nonhuman primates. Proc. Natl. Acad. Sci. USA 2019, 116, 6371–6378. [Google Scholar] [CrossRef]
- Chowdhury, I.H.; Ahmed, A.M.; Choudhuri, S.; Sen, A.; Hazra, A.; Pal, N.K.; Bhattacharya, B.; Bahar, B. Alteration of serum inflammatory cytokines in active pulmonary tuberculosis following anti-tuberculosis drug therapy. Mol. Immunol. 2014, 62, 159–168. [Google Scholar] [CrossRef]
- Yao, R.; Wu, Y.; Ma, B. Important role of interleukin-35 in infectious diseases and its significance. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 848–852. [Google Scholar] [CrossRef]
- Morelli, M.P.; Del Medico Zajac, M.P.; Pellegrini, J.M.; Amiano, N.O.; Tateosian, N.L.; Calamante, G.; Gherardi, M.M.; García, V.E. IL-12 DNA Displays Efficient Adjuvant Effects Improving Immunogenicity of Ag85A in DNA Prime/MVA Boost Immunizations. Front. Cell Infect. Microbiol. 2020, 10, 581812. [Google Scholar] [CrossRef]
- Fang, D.; Wang, R.; Fan, X.; Li, M.; Qian, C.; Cao, B.; Yu, J.; Liu, H.; Lou, Y.; Wan, K. Recombinant BCG vaccine expressing multistage antigens of Mycobacterium tuberculosis provides long-term immunity against tuberculosis in BALB/c mice. Hum. Vaccin. Immunother. 2024, 20, 2299607. [Google Scholar] [CrossRef] [PubMed]
- Burkert, S.; Schumann, R.R. RNA Sensing of Mycobacterium tuberculosis and Its Impact on TB Vaccination Strategies. Vaccines 2020, 8, 67. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, W.; Wei, M.; Yin, D.; Tang, Y.; Jia, W.; Wang, C.; Guo, J.; Li, A.; Gong, Y. Associations between type 1 diabetes and pulmonary tuberculosis: A bidirectional mendelian randomization study. Diabetol. Metab. Syndr. 2024, 16, 60. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, W.; Zheng, Z.; Shao, X.; Yang, P.; Yang, X.; Nan, K. Unraveling genetic causality between type 2 diabetes and pulmonary tuberculosis on the basis of Mendelian randomization. Diabetol. Metab. Syndr. 2023, 15, 228. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Xiang, Y.; Guo, W.; Zhu, Q.; Wu, S.; Tan, Y.; Yan, Y.; Shen, L.; Feng, Y.; Liang, K. Phenotype and function of peripheral blood γδ T cells in HIV infection with tuberculosis. Front. Cell Infect. Microbiol. 2022, 12, 1071880. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, I.S.; Dhooria, S.; Muthu, V.; Salzer, H.J.F.; Agarwal, R. Burden, clinical features, and outcomes of post-tuberculosis chronic obstructive lung diseases. Curr. Opin. Pulm. Med. 2024, 30, 156–166. [Google Scholar] [CrossRef]
- Bustamante, J.; Boisson-Dupuis, S.; Abel, L.; Casanova, J.L. Mendelian susceptibility to mycobacterial disease: Genetic, immunological, and clinical features of inborn errors of IFN-γ immunity. Semin. Immunol. 2014, 26, 454–470. [Google Scholar] [CrossRef]
- Philippot, Q.; Ogishi, M.; Bohlen, J.; Puchan, J.; Arias, A.A.; Nguyen, T.; Martin-Fernandez, M.; Conil, C.; Rinchai, D.; Momenilandi, M.; et al. Human IL-23 is essential for IFN-γ-dependent immunity to mycobacteria. Sci. Immunol. 2023, 8, eabq5204. [Google Scholar] [CrossRef]
- Reeme, A.E.; Miller, H.E.; Robinson, R.T. IL12B expression is sustained by a heterogenous population of myeloid lineages during tuberculosis. Tuberculosis 2013, 93, 343–356. [Google Scholar] [CrossRef][Green Version]
- Abdulahi, I.; Hafiz, T.R. Multidrug Resistant Tuberculosis in HIV Positive Patients and its Effect on IL-10 and IL-12. Egypt. J. Med. Microbiol. 2023, 32, 61–68. [Google Scholar]
- Okada, M.; Kita, Y.; Nakajima, T.; Kanamaru, N.; Hashimoto, S.; Nagasawa, T.; Kaneda, Y.; Yoshida, S.; Nishida, Y.; Fukamizu, R.; et al. Evaluation of a novel vaccine (HVJ-liposome/HSP65 DNA+IL-12 DNA) against tuberculosis using the cynomolgus monkey model of TB. Vaccine 2007, 25, 2990–2993. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.E.; Erasmus, J.H.; Reese, V.A.; Pecor, T.; Archer, J.; Kandahar, A.; Hsu, F.C.; Nicholes, K.; Reed, S.G.; Baldwin, S.L.; et al. An RNA-Based Vaccine Platform for Use against Mycobacterium tuberculosis. Vaccines 2023, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Farris, E.; Swanson, R.V.; Das, S.; Yang, Y.; Martin, T.; Khader, S.A. Saponin TQL1055 adjuvant-containing vaccine confers protection upon Mycobacterium tuberculosis challenge in mice. Hum. Vaccin. Immunother. 2024, 20, 2302070. [Google Scholar] [CrossRef]
- Baldwin, S.L.; Reese, V.A.; Larsen, S.E.; Pecor, T.; Brown, B.P.; Granger, B.; Podell, B.K.; Fox, C.B.; Reed, S.G.; Coler, R.N. Therapeutic efficacy against Mycobacterium tuberculosis using ID93 and liposomal adjuvant formulations. Front. Microbiol. 2022, 13, 935444. [Google Scholar] [CrossRef]
- Kwon, K.W.; Kang, T.G.; Lee, A.; Jin, S.M.; Lim, Y.T.; Shin, S.J.; Ha, S.J. Protective Efficacy and Immunogenicity of Rv0351/Rv3628 Subunit Vaccine Formulated in Different Adjuvants Against Mycobacterium tuberculosis Infection. Immune Netw. 2023, 23, e16. [Google Scholar] [CrossRef] [PubMed]
- Horváti, K.; Pályi, B.; Henczkó, J.; Balka, G.; Szabó, E.; Farkas, V.; Biri-Kovács, B.; Szeder, B.; Fodor, K. A Convenient Synthetic Method to Improve Immunogenicity of Mycobacterium tuberculosis Related T-Cell Epitope Peptides. Vaccines 2019, 7, 101. [Google Scholar] [CrossRef]
- Petrone, L.; Peruzzu, D.; Altera, A.M.G.; Salmi, A.; Vanini, V.; Cuzzi, G.; Coppola, A.; Mellini, V.; Gualano, G.; Palmieri, F.; et al. Therapy modulates the response to T cell epitopes over the spectrum of tuberculosis infection. J. Infect. 2024, 89, 106295. [Google Scholar] [CrossRef]

| Classification | Name | Mechanism | Effect |
|---|---|---|---|
| Direct Negative Regulation of IL-12 | IL-10 | Inhibits NF-κB and STAT4 activity, prevents transcription of IL-12 p35 and p40 genes. | Inhibits IL-12 synthesis and secretion, promotes Th2 immune response, inhibits Th1 immune response. |
| TGF-β | Inhibits IL-12 transcription via Smad signaling pathway and other non-Smad pathways. | It suppresses IL-12 biosynthesis, maintains immune tolerance, and suppresses the excessive inflammatory response. | |
| IL-4 and IL-13 | Induce bias toward Th2 polarization via Stat6-mediated signaling pathway, inhibiting IL-12 production. | Inhibition of IL-12 production promotes Th2 immune response and diminishes Th1 immune response. | |
| PGE2 | Inhibits NF-κB activation and reduces IL-12 production via EP2 and EP4 receptors. | Regulates IL-12 synthesis to prevent excessive inflammation. | |
| Indirect Negative Regulation of IL-12 | SOCS1 and SOCS3 | Block IL-12 signaling by binding to JAK kinases or STAT proteins. | Indirectly reduces IL-12 function and regulates cell signaling pathways. |
| CTLA-4 | Inhibits T-cell activation and proliferation by competitively binding to the costimulatory molecule CD80/CD86. | Indirectly reduces T-cell activity and affects IL-12 demand and effects. | |
| PD-1 | Binds to PD-L1/PD-L2, leading to T-cell depletion and immunosuppression, affecting IL-12 production by antigen-presenting cells. | Indirectly affects IL-12 production and effects, regulating immune responses. | |
| IFN-β and IFN-α | Affect IL-12 production through complex positive and negative feedback mechanisms, including upregulation of SOCS protein expression. | Indirectly inhibit IL-12 biosynthesis under certain conditions. | |
| TNF | Promotes IL-12 production under normal conditions, but in chronic inflammation or hyperinflammatory responses, high levels of TNF-α inhibit IL-12 production. | Regulates IL-12 production and immune responses according to conditions. | |
| GPCRs | Indirectly inhibit IL-12 production by altering the functional state of immune cells. | Inhibit IL-12 production by altering signaling pathways. | |
| Exogenous Drugs | IL-12 and IL-23 inhibitors: Ustekinumab, Briakinumab | Bind to and block the shared p40 subunit of IL-12 and IL-23. | Down-regulate Th1-mediated inflammation by inhibiting IL-12 signaling, effectively controlling diseases dominated by Th1 immune response. |
| IL-23 specific inhibitors: Tildrakizumab and Risankizumab | Bind specifically to the p19 subunit of IL-23. | Indirectly affect IL-12 activity, controlling Th17 cell activation and secretion of inflammatory mediators such as IL-17. | |
| Competitive antagonist of IL-4/IL-13 receptor: Pitrakinra | Competitively binds to IL-12 receptor. | Blocks Th2 immune response, alleviating airway inflammation and reducing IL-4 and IL-13 effects, primarily used in asthma and allergic diseases. |
| Classification | Name | Mechanism | Effect |
|---|---|---|---|
| Cytokines | IFN-γ | Activates JAK-STAT signaling pathway, promoting IL-12 transcription and synthesis. | Enhances IL-12 expression in macrophages and dendritic cells, fostering Th1 cell response. |
| IL-4/IL-13 | Exhibits a bimodal effect: initially inhibits p40 production, later strongly enhances p40 production. | Stimulates IL-12 heterodimer production by upregulating transcription of p40 and p35 genes. | |
| IL-18 | Activates IL-18R signaling pathway, enhancing NF-κB and MAPK pathways, leading to increased IL-12 transcription and expression. | Amplifies IL-12 production and bioactivity, synergistically promoting Th1-type immune response. | |
| Pattern Recognition Receptor Agonists | TLRs (TLR-2, -4, -5, -9) agonists | Initiates transcription factors such as NF-κB, AP-1, augmenting IL-12 transcription and synthesis. | Promotes immune response by recognizing PAMPs (e.g., LPS, flagellin), robustly inducing IL-12 production in monocytes/macrophages and DCs without T cells. |
| Self-microbial products (bacteria, intracellular parasites, fungi, dsRNA, CpG DNA) | Binds to TLR, triggering transcription factor signaling, promoting IL-12 production. | Significantly boosts IL-12 expression, enhancing immune response. | |
| Immune Cell-to-Cell Interaction | Molecule CD40 Ligand (CD40L, CD154) | CD40L on T cells binds to CD40 on DCs or macrophages, promoting IL-12 expression. | Facilitates optimal IL-12 production through T cell and APC interaction, with CD40 stimulation preferentially inducing p35 gene transcription. |
| Transcription Factor Cascade Response | IRF Transcription Factors (IRF1, IRF2, IRF5, IRF7, IRF8) | Regulate IL-12 gene transcription, with deficiencies leading to impaired IL-12 expression, affecting p35 and p40 expression. | Key transcriptional regulators ensure timely IL-12 production. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Ruan, G.; Li, Y.; Liu, X. The Role and Potential Application of IL-12 in the Immune Regulation of Tuberculosis. Int. J. Mol. Sci. 2025, 26, 3106. https://doi.org/10.3390/ijms26073106
Wang H, Ruan G, Li Y, Liu X. The Role and Potential Application of IL-12 in the Immune Regulation of Tuberculosis. International Journal of Molecular Sciences. 2025; 26(7):3106. https://doi.org/10.3390/ijms26073106
Chicago/Turabian StyleWang, Hangxing, Guiren Ruan, Yuanchun Li, and Xiaoqing Liu. 2025. "The Role and Potential Application of IL-12 in the Immune Regulation of Tuberculosis" International Journal of Molecular Sciences 26, no. 7: 3106. https://doi.org/10.3390/ijms26073106
APA StyleWang, H., Ruan, G., Li, Y., & Liu, X. (2025). The Role and Potential Application of IL-12 in the Immune Regulation of Tuberculosis. International Journal of Molecular Sciences, 26(7), 3106. https://doi.org/10.3390/ijms26073106







