The Identification of Novel Anti-Inflammatory Effects of Cannabigerol in the Kidney Tissue of Rats Subjected to a High-Fat High-Sucrose Diet
Abstract
1. Introduction
2. Results
2.1. The Influence of Cannabigerol on the Activity of the n-6 and n-3 Polyunsaturated Fatty Acid Metabolic Pathway and Ratio of n-6/n-3 Polyunsaturated Fatty Acid in the Kidney Tissue of Rats Subjected to a Standard Diet or High-Fat High-Sucrose Diet
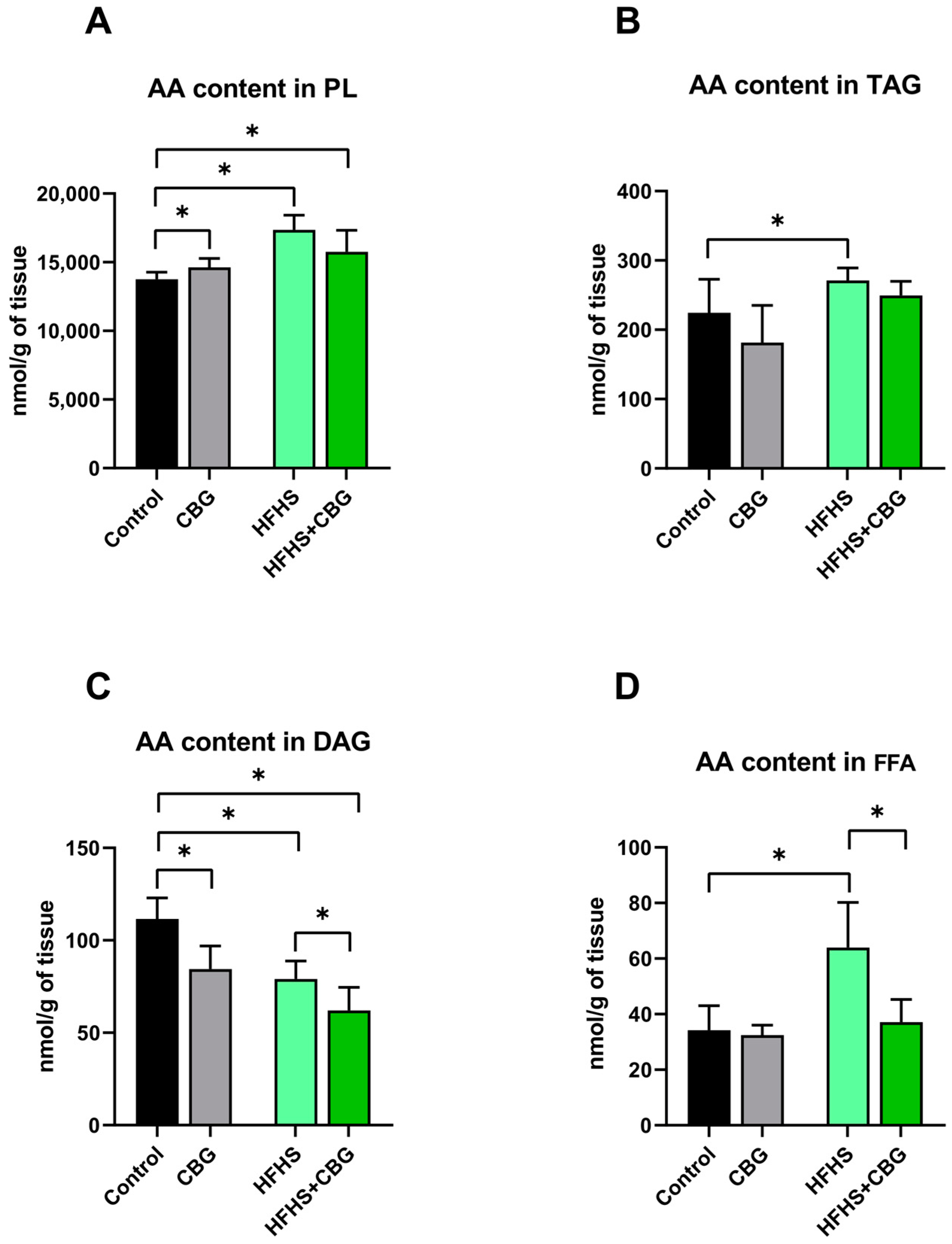
2.2. The Influence of Cannabigerol on the Concentration of Arachidonic Acid in the Kidney Tissue of Rats Subjected to a Standard Diet or High-Fat High-Sucrose Diet
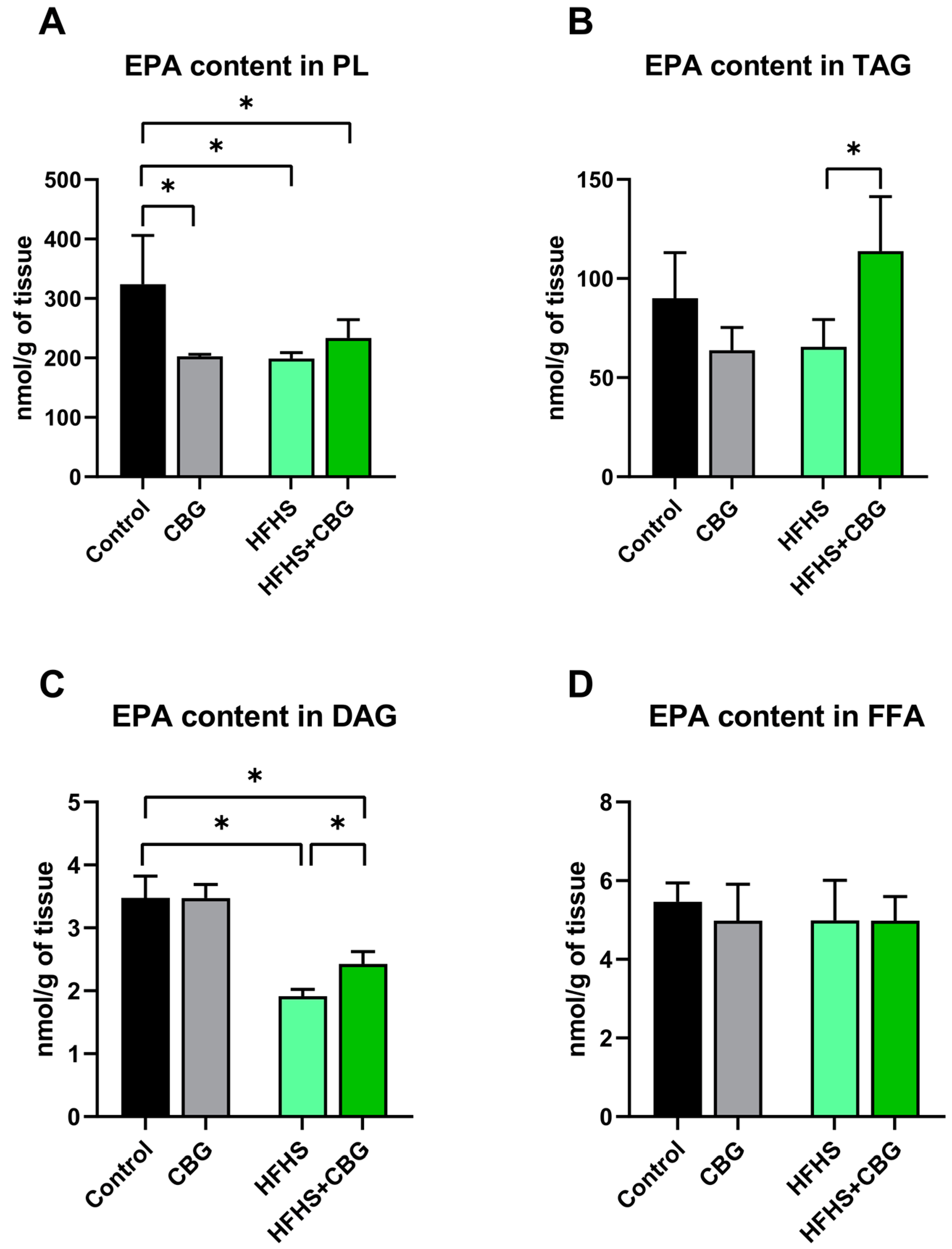
2.3. The Influence of Cannabigerol on the Concentration of Eicosapentaenoic Acid in the Kidney Tissue of Rats Subjected to a Standard Diet or High-Fat High-Sucrose Diet
2.4. The Influence of Cannabigerol on the Concentration of Docosahexaenoic Acid in the Kidney Tissue of Rats Subjected to a Standard Diet or High-Fat High Sucrose Diet
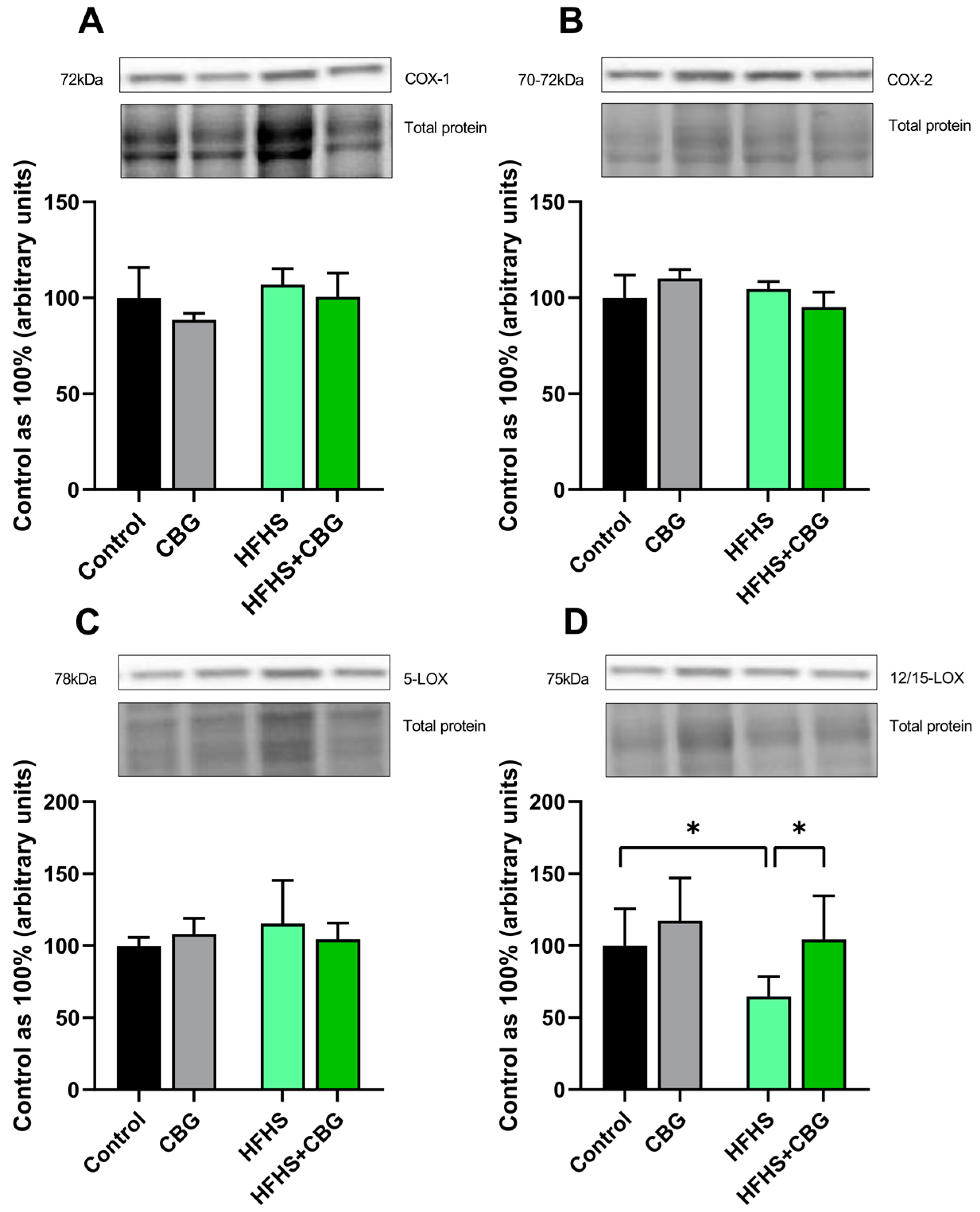
2.5. The Influence of Cannabigerol on the Expression of Proteins Regulating Arachidonic Acid Metabolism in the Kidney Tissue of Rats Subjected to a Standard Diet or High-Fat High-Sucrose Diet
2.6. The Influence of Cannabigerol on the Expression of Other Proteins Involved in the Development of the Inflammatory State in the Kidney Tissue of Rats Subjected to a Standard Diet or High-Fat High Sucrose Diet
2.7. The Influence of Cannabigerol on the Expression of Proteins Regulating the Fibrosis Process in the Kidney Tissue of Rats Subjected to a Standard Diet or High-Fat High-Sucrose Diet
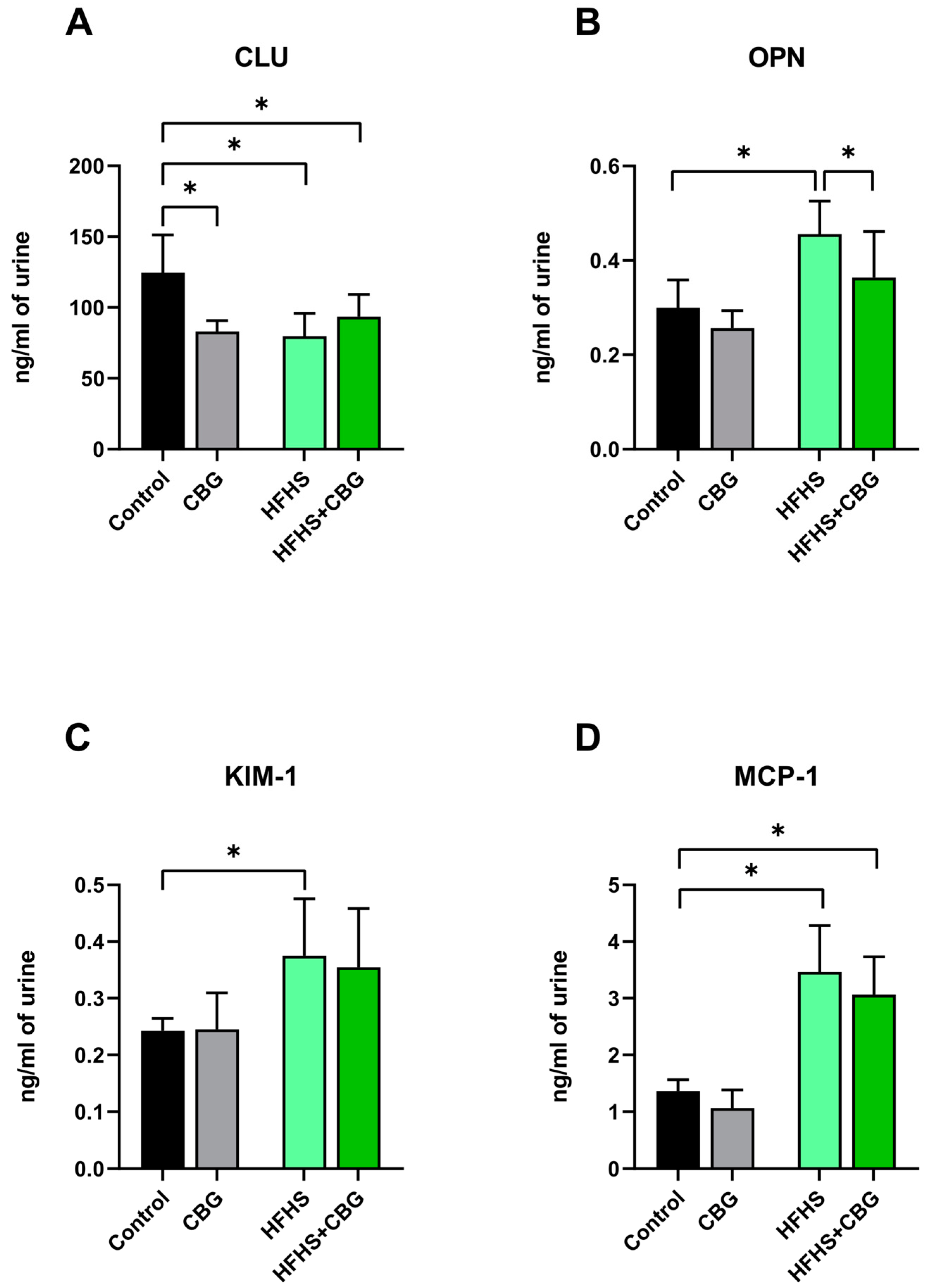
2.8. The Influence of Cannabigerol on the Concentration of Kidney Toxicity Markers in Urine Samples of Rats Subjected to a Standard Diet or High-Fat High-Sucrose Diet
3. Discussion
4. Materials and Methods
4.1. Experimental Procedures
4.2. Gas–Liquid Chromatography
4.3. Immunoblotting
4.4. Multiplex Immunoassay
4.5. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Becker, T.K.; Majmundar, M.; Harris, K.M.; National Academies of Sciences, Engineering, and Medicine; Division of Behavioral and Social Sciences and Education; Committee on National Statistics; Committee on Population; Committee on Rising Midlife Mortality Rates and Socioeconomic Disparities. High and Rising Mortality Rates Among Working-Age Adults; The National Academies Press: Washington, DC, USA, 2021; ISBN 9780309684736. [Google Scholar]
- Cornier, M.-A.; Dabelea, D.; Hernandez, T.L.; Lindstrom, R.C.; Steig, A.J.; Stob, N.R.; Van Pelt, R.E.; Wang, H.; Eckel, R.H. The Metabolic Syndrome. Endocr. Rev. 2008, 29, 777–822. [Google Scholar] [CrossRef] [PubMed]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef]
- Jiang, S.; Liu, H.; Li, C. Dietary Regulation of Oxidative Stress in Chronic Metabolic Diseases. Foods 2021, 10, 1854. [Google Scholar] [CrossRef]
- Rapa, S.F.; Di Iorio, B.R.; Campiglia, P.; Heidland, A.; Marzocco, S. Inflammation and Oxidative Stress in Chronic Kidney Disease—Potential Therapeutic Role of Minerals, Vitamins and Plant-Derived Metabolites. Int. J. Mol. Sci. 2019, 21, 263. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.I.; Higgs, G.A. Eicosanoids and Inflammation. J. Pathol. 1988, 156, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Fu, X.; Chen, Q.; Patra, J.K.; Wang, D.; Wang, Z.; Gai, Z. Arachidonic Acid Metabolism and Kidney Inflammation. Int. J. Mol. Sci. 2019, 20, 3683. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.-C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed]
- Zatterale, F.; Longo, M.; Naderi, J.; Raciti, G.A.; Desiderio, A.; Miele, C.; Beguinot, F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2020, 10, 1607. [Google Scholar] [CrossRef]
- Webster, E.R.; Perkovic, A.; Neuen, B.L.; Tuttle, K.R.; Perkovic, V. Effects of Anti-Inflammatory Agents on Clinical Outcomes in People with Chronic Kidney Disease: A Systematic Review and Meta-Analysis of Randomised Control Trials. Clin. Kidney J. 2025, 18, sfaf001. [Google Scholar] [CrossRef]
- Leehey, D.; Zhang, X. Inflammation in Diabetic Kidney Disease: Focus on New Therapeutic Considerations. Med. Res. Arch. 2023, 11, 2. [Google Scholar] [CrossRef]
- Sen, T.; Curovic, V.R.; Jongs, N.; Laverman, G.D.; Kooy, A.; Persson, F.; Rossing, P.; Heerspink, H.J.L. Effects of Albuminuria-Lowering Treatments on Inflammation Markers: A Post Hoc Analysis from the ROTATE Trials. Diabetes Obes. Metab. 2023, 25, 2413–2418. [Google Scholar] [CrossRef]
- Huck, D.M.; Buckley, L.F.; Chandraker, A.; Blankstein, R.; Weber, B. Targeting Pharmacotherapies for Inflammatory and Cardiorenal Endpoints in Kidney Disease. J. Cardiovasc. Pharmacol. 2024, 83, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Bobulescu, I.A. Renal Lipid Metabolism and Lipotoxicity. Curr. Opin. Nephrol. Hypertens. 2010, 19, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Wolf, G.; Ziyadeh, F.N. Cellular and Molecular Mechanisms of Proteinuria in Diabetic Nephropathy. Nephron. Physiol. 2007, 106, 26–31. [Google Scholar] [CrossRef]
- Rosengren, B.-I.; Sagstad, S.J.; Karlsen, T.V.; Wiig, H. Isolation of Interstitial Fluid and Demonstration of Local Proinflammatory Cytokine Production and Increased Absorptive Gradient in Chronic Peritoneal Dialysis. Am. J. Physiol.-Ren. Physiol. 2013, 304, F198–F206. [Google Scholar] [CrossRef]
- Brierley, D.I.; Samuels, J.; Duncan, M.; Whalley, B.J.; Williams, C.M. Cannabigerol Is a Novel, Well-Tolerated Appetite Stimulant in Pre-Satiated Rats. Psychopharmacology 2016, 233, 3603–3613. [Google Scholar] [CrossRef] [PubMed]
- Jastrząb, A.; Jarocka-Karpowicz, I.; Skrzydlewska, E. The Origin and Biomedical Relevance of Cannabigerol. Int. J. Mol. Sci. 2022, 23, 7929. [Google Scholar] [CrossRef]
- Erdemli, Z.; Gul, M.; Kayhan, E.; Gokturk, N.; Bag, H.G.; Erdemli, M.E. High-Fat and Carbohydrate Diet Caused Chronic Kidney Damage by Disrupting Kidney Function, Caspase-3, Oxidative Stress and Inflammation. Prostaglandins Other Lipid Mediat. 2024, 172, 106822. [Google Scholar] [CrossRef]
- Haliem Saleh, N.E.; Ibrahim, M.Y.; Saad, A.H.; Habeeb, W.N.; Saleh, R.K.; Abdel-Hakeem, E.A. High Caloric Carbohydrate Diets Promote Disturbed Metabolic Milieu and Induce Renal Injury in Albino Rats. Minia J. Med. Res. 2024. [Google Scholar] [CrossRef]
- Meléndez-Salcido, C.G.; Ramírez-Emiliano, J.; Pérez-Vázquez, V. Hypercaloric Diet Promotes Metabolic Disorders and Impaired Kidney Function. Curr. Pharm. Des. 2022, 28, 3127–3139. [Google Scholar] [CrossRef]
- Gonzalez-Becerra, K.; Barron-Cabrera, E.; Muñoz-Valle, J.F.; Torres-Castillo, N.; Rivera-Valdes, J.J.; Rodriguez-Echevarria, R.; Martinez-Lopez, E. A Balanced Dietary Ratio of N-6:N-3 Polyunsaturated Fatty Acids Exerts an Effect on Total Fatty Acid Profile in RBCs and Inflammatory Markers in Subjects with Obesity. Healthcare 2023, 11, 2333. [Google Scholar] [CrossRef] [PubMed]
- Voggel, J.; Fink, G.; Zelck, M.; Wohlfarth, M.; Post, J.M.; Bindila, L.; Rauh, M.; Amann, K.; Alejandre Alcázar, M.A.; Dötsch, J.; et al. Elevated N-3/n-6 PUFA Ratio in Early Life Diet Reverses Adverse Intrauterine Kidney Programming in Female Rats. J. Lipid Res. 2022, 63, 100283. [Google Scholar] [CrossRef] [PubMed]
- Vernail, V.L.; Bingaman, S.S.; Silberman, Y.; Raup-Konsavage, W.M.; Vrana, K.E.; Arnold, A.C. Acute Cannabigerol Administration Lowers Blood Pressure in Mice. Front. Physiol. 2022, 13, 871962. [Google Scholar] [CrossRef]
- Calapai, F.; Cardia, L.; Esposito, E.; Ammendolia, I.; Mondello, C.; Lo Giudice, R.; Gangemi, S.; Calapai, G.; Mannucci, C. Pharmacological Aspects and Biological Effects of Cannabigerol and Its Synthetic Derivatives. Evid. Based Complement. Altern. Med. 2022, 2022, 3336516. [Google Scholar] [CrossRef]
- Poli, A.; Agostoni, C.; Visioli, F. Dietary Fatty Acids and Inflammation: Focus on the n-6 Series. Int. J. Mol. Sci. 2023, 24, 4567. [Google Scholar] [CrossRef]
- Su, H.; Lei, C.-T.; Zhang, C. Interleukin-6 Signaling Pathway and Its Role in Kidney Disease: An Update. Front. Immunol. 2017, 8, 405. [Google Scholar] [CrossRef]
- Bielawiec, P.; Harasim-Symbor, E.; Sztolsztener, K.; Konstantynowicz-Nowicka, K.; Chabowski, A. Attenuation of Oxidative Stress and Inflammatory Response by Chronic Cannabidiol Administration Is Associated with Improved N-6/n-3 PUFA Ratio in the White and Red Skeletal Muscle in a Rat Model of High-Fat Diet-Induced Obesity. Nutrients 2021, 13, 1603. [Google Scholar] [CrossRef]
- Lefkowith, J.B.; Klahr, S. Polyunsaturated Fatty Acids and Renal Disease. Exp. Biol. Med. 1996, 213, 13–23. [Google Scholar] [CrossRef]
- Luetić, M.; Vitlov Uljević, M.; Mašek, T.; Benzon, B.; Vukojević, K.; Filipović, N. PUFAs Supplementation Affects the Renal Expression of Pannexin 1 and Connexins in Diabetic Kidney of Rats. Histochem. Cell Biol. 2020, 153, 165–175. [Google Scholar] [CrossRef]
- Alashmali, S.M.; Lin, L.; Trépanier, M.-O.; Cisbani, G.; Bazinet, R.P. The Effects of N-6 Polyunsaturated Fatty Acid Deprivation on the Inflammatory Gene Response to Lipopolysaccharide in the Mouse Hippocampus. J. Neuroinflammation 2019, 16, 237. [Google Scholar] [CrossRef]
- Jannas-Vela, S.; Espinosa, A.; Candia, A.A.; Flores-Opazo, M.; Peñailillo, L.; Valenzuela, R. The Role of Omega-3 Polyunsaturated Fatty Acids and Their Lipid Mediators on Skeletal Muscle Regeneration: A Narrative Review. Nutrients 2023, 15, 871. [Google Scholar] [CrossRef] [PubMed]
- Pizzini, A.; Lunger, L.; Sonnweber, T.; Weiss, G.; Tancevski, I. The Role of Omega-3 Fatty Acids in the Setting of Coronary Artery Disease and COPD: A Review. Nutrients 2018, 10, 1864. [Google Scholar] [CrossRef]
- Han, E.; Yun, Y.; Kim, G.; Lee, Y.-H.; Wang, H.J.; Lee, B.-W.; Cha, B.S.; Kim, B.S.; Kang, E.S. Effects of Omega-3 Fatty Acid Supplementation on Diabetic Nephropathy Progression in Patients with Diabetes and Hypertriglyceridemia. PLoS ONE 2016, 11, e0154683. [Google Scholar] [CrossRef]
- Liu, T.-W.; Heden, T.D.; Matthew Morris, E.; Fritsche, K.L.; Vieira-Potter, V.J.; Thyfault, J.P. High-Fat Diet Alters Serum Fatty Acid Profiles in Obesity Prone Rats: Implications for In Vitro Studies. Lipids 2015, 50, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Sonnweber, T.; Pizzini, A.; Nairz, M.; Weiss, G.; Tancevski, I. Arachidonic Acid Metabolites in Cardiovascular and Metabolic Diseases. Int. J. Mol. Sci. 2018, 19, 3285. [Google Scholar] [CrossRef]
- Ogata, Y.; Ishidoya, S.; Fukuzaki, A.; Kaneto, H.; Takeda, A.; Ohyama, C.; Orikasa, S.; Arai, Y. Upregulated Expression of Transforming Growth Factor-Beta, Type IV Collagen, and Plasminogen Activator Inhibitor-1 MRNA Are Decreased after Release of Unilateral Ureteral Obstruction. Tohoku J. Exp. Med. 2002, 197, 159–168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Turolo, S.; Edefonti, A.; Mazzocchi, A.; Syren, M.L.; Morello, W.; Agostoni, C.; Montini, G. Role of Arachidonic Acid and Its Metabolites in the Biological and Clinical Manifestations of Idiopathic Nephrotic Syndrome. Int. J. Mol. Sci. 2021, 22, 5452. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, J.; Zhang, W.; Guo, Z.; Ma, Q. Arachidonic Acid Metabolism in Health and Disease. MedComm 2023, 4, e363. [Google Scholar] [CrossRef]
- Mutsaers, H.A.M.; Nørregaard, R. Prostaglandin E2 Receptors as Therapeutic Targets in Renal Fibrosis. Kidney Res. Clin. 2022, 41, 4–13. [Google Scholar] [CrossRef]
- Simon, L.S. Role and Regulation of Cyclooxygenase-2 during Inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- Wang, B.; Wu, L.; Chen, J.; Dong, L.; Chen, C.; Wen, Z.; Hu, J.; Fleming, I.; Wang, D.W. Metabolism Pathways of Arachidonic Acids: Mechanisms and Potential Therapeutic Targets. Signal Transduct. Target Ther. 2021, 6, 94. [Google Scholar] [CrossRef]
- López-Parra, M.; Clària, J.; Planagumà, A.; Titos, E.; Masferrer, J.L.; Woerner, B.M.; Koki, A.T.; Jiménez, W.; Altuna, R.; Arroyo, V.; et al. Cyclooxygenase-1 Derived Prostaglandins Are Involved in the Maintenance of Renal Function in Rats with Cirrhosis and Ascites. Br. J. Pharmacol. 2002, 135, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Gao, B.; Li, M.; Yao, L.; Wang, S.; Chen, M.; Li, H.; Ma, C.; Ji, A.; Li, Y. Hydrogen Sulfide Mitigates Kidney Injury in High Fat Diet-Induced Obese Mice. Oxid. Med. Cell Longev. 2016, 2016, 2715718. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Chiang, N. Endogenous Pro-Resolving and Anti-Inflammatory Lipid Mediators: A New Pharmacologic Genus. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S200–S215. [Google Scholar] [CrossRef] [PubMed]
- Börgeson, E.; Docherty, N.G.; Murphy, M.; Rodgers, K.; Ryan, A.; O’Sullivan, T.P.; Guiry, P.J.; Goldschmeding, R.; Higgins, D.F.; Godson, C. Lipoxin A4 and Benzo-Lipoxin A4 Attenuate Experimental Renal Fibrosis. FASEB J. 2011, 25, 2967–2979. [Google Scholar] [CrossRef]
- Börgeson, E.; Johnson, A.M.F.; Lee, Y.S.; Till, A.; Syed, G.H.; Ali-Shah, S.T.; Guiry, P.J.; Dalli, J.; Colas, R.A.; Serhan, C.N.; et al. Lipoxin A4 Attenuates Obesity-Induced Adipose Inflammation and Associated Liver and Kidney Disease. Cell Metab. 2015, 22, 125–137. [Google Scholar] [CrossRef]
- Wardyn, J.D.; Ponsford, A.H.; Sanderson, C.M. Dissecting Molecular Cross-Talk between Nrf2 and NF-ΚB Response Pathways. Biochem. Soc. Trans. 2015, 43, 621–626. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, K.C.; Lee, J.H. Anti-Inflammatory Effects of Cannabigerol In Vitro and In Vivo Are Mediated Through the JAK/STAT/NFκB Signaling Pathway. Cells 2025, 14, 83. [Google Scholar] [CrossRef]
- Wang, X.; Chen, J.; Xu, J.; Xie, J.; Harris, D.C.H.; Zheng, G. The Role of Macrophages in Kidney Fibrosis. Front. Physiol. 2021, 12, 705838. [Google Scholar] [CrossRef]
- Zhu, M.M.; Ma, Y.; Tang, M.; Pan, L.; Liu, W.L. Hypoxia-Induced Upregulation of Matrix Metalloproteinase 9 Increases Basement Membrane Degradation by Downregulating Collagen Type IV Alpha 1 Chain. Physiol. Res. 2022, 71, 825–834. [Google Scholar] [CrossRef]
- Ibeh, C.L.; Yiu, A.J.; Kanaras, Y.L.; Paal, E.; Birnbaumer, L.; Jose, P.A.; Bandyopadhyay, B.C. Evidence for a Regulated Ca2+ Entry in Proximal Tubular Cells and Its Implication in Calcium Stone Formation. J. Cell Sci. 2019, 132, jcs225268. [Google Scholar] [CrossRef] [PubMed]
- Vassiliadis, J.; Bracken, C.; Matthews, D.; O’Brien, S.; Schiavi, S.; Wawersik, S. Calcium Mediates Glomerular Filtration through Calcineurin and MTORC2/Akt Signaling. J. Am. Soc. Nephrol. 2011, 22, 1453. [Google Scholar] [CrossRef]
- Djenoune, L.; Tomar, R.; Dorison, A.; Ghobrial, I.; Schenk, H.; Hegermann, J.; Beverly-Staggs, L.; Hidalgo-Gonzalez, A.; Little, M.H.; Drummond, I.A. Autonomous Calcium Signaling in Human and Zebrafish Podocytes Controls Kidney Filtration Barrier Morphogenesis. J. Am. Soc. Nephrol. 2021, 32, 1697–1712. [Google Scholar] [CrossRef]
- Milinkeviciute, G.; Green, K.N. Clusterin/Apolipoprotein J, Its Isoforms and Alzheimer’s Disease. Front. Aging Neurosci. 2023, 15, 1167886. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Guan, Q.; Liu, X.; Wang, H.; Gleave, M.E.; Nguan, C.Y.C.; Du, C. Relationship of Clusterin with Renal Inflammation and Fibrosis after the Recovery Phase of Ischemia-Reperfusion Injury. BMC Nephrol. 2016, 17, 133. [Google Scholar] [CrossRef]
- Kaleta, B. The Role of Osteopontin in Kidney Diseases. Inflamm. Res. 2019, 68, 93–102. [Google Scholar] [CrossRef]
- Torino, C.; Carbone, F.; Pizzini, P.; Mezzatesta, S.; D’Arrigo, G.; Gori, M.; Liberale, L.; Moriero, M.; Michelauz, C.; Frè, F.; et al. Osteopontin and Clinical Outcomes in Hemodialysis Patients. Biomedicines 2024, 12, 2605. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, S.S.; Siddiqui, K.; Mujammami, M.; Alotaibi, O.; Alanazi, S.S.; Rafiullah, M. Determinant of Osteopontin Levels in Microvascular Complications in Patients with Diabetes. Int. J. Gen. Med. 2022, 15, 4433–4440. [Google Scholar] [CrossRef]
- Viedt, C.; Orth, S.R. Monocyte Chemoattractant Protein-1 (MCP-1) in the Kidney: Does It More than Simply Attract Monocytes? Nephrol. Dial. Transpl. 2002, 17, 2043–2047. [Google Scholar] [CrossRef]
- Karmakova, T.A.; Sergeeva, N.S.; Kanukoev, K.Y.; Alekseev, B.Y.; Kaprin, A.D. Kidney Injury Molecule 1 (KIM-1): A Multifunctional Glycoprotein and Biological Marker (Review). Sovrem. Tekhnologii. Med. 2021, 13, 64–80. [Google Scholar] [CrossRef]
- Sinha, S.K.; Mellody, M.; Carpio, M.B.; Damoiseaux, R.; Nicholas, S.B. Osteopontin as a Biomarker in Chronic Kidney Disease. Biomedicines 2023, 11, 1356. [Google Scholar] [CrossRef] [PubMed]
- Layton, G.R.; Antoun, I.; Copperwheat, A.; Khan, Z.L.; Bhandari, S.S.; Somani, R.; Ng, A.; Zakkar, M. Osteopontin as a Biomarker for Coronary Artery Disease. Cells 2025, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Marco, E.M.; Romero-Zerbo, S.Y.; Viveros, M.P.; Bermudez-Silva, F.J. The Role of the Endocannabinoid System in Eating Disorders: Pharmacological Implications. Behav. Pharmacol. 2012, 23, 526–536. [Google Scholar] [CrossRef]
- Wargent, E.T.; Zaibi, M.S.; Silvestri, C.; Hislop, D.C.; Stocker, C.J.; Stott, C.G.; Guy, G.W.; Duncan, M.; Di Marzo, V.; Cawthorne, M.A. The Cannabinoid Δ(9)-Tetrahydrocannabivarin (THCV) Ameliorates Insulin Sensitivity in Two Mouse Models of Obesity. Nutr. Diabetes 2013, 3, e68. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Wang, S.T.; Peter, F. Gas-Liquid Chromatographic Determination of Fatty Acid Composition of Cholesteryl Esters in Human Serum Using Silica Sep-Pak Cartridges. J. Chromatogr. B Biomed. Sci. Appl. 1983, 276, 249–256. [Google Scholar] [CrossRef]



| Control | CBG | HFHS | HFHS+CBG | ||
|---|---|---|---|---|---|
| PL | n-6 PUFA | 1.449 ± 0.076 | 1.556 ± 0.113 | 1.624 ± 0.061 * | 1.684 ± 0.125 * |
| n-3 PUFA | 37.016 ± 5.335 | 40.438 ± 4.072 | 30.708 ± 2.953 * | 33.529 ± 3.996 | |
| n-6/n-3 PUFA | 0.039 ± 0.004 | 0.040 ± 0.005 | 0.054 ± 0.006 * | 0.048 ± 0.006 | |
| TAG | n-6 PUFA | 0.054 ± 0.008 | 0.053 ± 0.006 | 0.069 ± 0.008 * | 0.042 ± 0.005 * # |
| n-3 PUFA | 0.798 ± 0.146 | 0.953 ± 0.155 | 0.819 ± 0.117 | 0.577 ± 0.096 # | |
| n-6/n-3 PUFA | 0.074 ± 0.007 | 0.062 ± 0.016 | 0.076 ± 0.003 | 0.075 ± 0.021 | |
| DAG | n-6 PUFA | 0.574 ± 0.078 | 0.543 ± 0.009 | 0.655 ± 0.050 | 0.569 ± 0.044 # |
| n-3 PUFA | 2.802 ± 0.245 | 2.642 ± 0.053 | 2.591 ± 0.589 | 2.450 ± 0.200 | |
| n-6/n-3 PUFA | 0.236 ± 0.013 | 0.233 ± 0.027 | 0.232 ± 0.026 | 0.196 ± 0.033 | |
| FFA | n-6 PUFA | 0.419 ± 0.073 | 0.431 ± 0.040 | 0.491 ± 0.042 | 0.472 ± 0.078 |
| n-3 PUFA | 1.322 ± 0.118 | 1.623 ± 0.098 * | 1.547 ± 0.199 | 1.127 ± 0.252 | |
| n-6/n-3 PUFA | 0.326 ± 0.041 | 0.277 ± 0.029 | 0.296 ± 0.036 | 0.334 ± 0.078 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepaniuk, A.; Sztolsztener, K.; Konstantynowicz-Nowicka, K.; Harasim-Symbor, E.; Bielawiec, P.; Chabowski, A. The Identification of Novel Anti-Inflammatory Effects of Cannabigerol in the Kidney Tissue of Rats Subjected to a High-Fat High-Sucrose Diet. Int. J. Mol. Sci. 2025, 26, 3114. https://doi.org/10.3390/ijms26073114
Stepaniuk A, Sztolsztener K, Konstantynowicz-Nowicka K, Harasim-Symbor E, Bielawiec P, Chabowski A. The Identification of Novel Anti-Inflammatory Effects of Cannabigerol in the Kidney Tissue of Rats Subjected to a High-Fat High-Sucrose Diet. International Journal of Molecular Sciences. 2025; 26(7):3114. https://doi.org/10.3390/ijms26073114
Chicago/Turabian StyleStepaniuk, Anna, Klaudia Sztolsztener, Karolina Konstantynowicz-Nowicka, Ewa Harasim-Symbor, Patrycja Bielawiec, and Adrian Chabowski. 2025. "The Identification of Novel Anti-Inflammatory Effects of Cannabigerol in the Kidney Tissue of Rats Subjected to a High-Fat High-Sucrose Diet" International Journal of Molecular Sciences 26, no. 7: 3114. https://doi.org/10.3390/ijms26073114
APA StyleStepaniuk, A., Sztolsztener, K., Konstantynowicz-Nowicka, K., Harasim-Symbor, E., Bielawiec, P., & Chabowski, A. (2025). The Identification of Novel Anti-Inflammatory Effects of Cannabigerol in the Kidney Tissue of Rats Subjected to a High-Fat High-Sucrose Diet. International Journal of Molecular Sciences, 26(7), 3114. https://doi.org/10.3390/ijms26073114






