Abstract
The utilization of photosynthetic microbes, such as cyanobacteria and microalgae, offers sustainable solutions to addressing global resource shortages and pollution. While these microorganisms have demonstrated significant potential in biomanufacturing, their industrial application is limited by suboptimal photosynthetic efficiency. Synthetic biology integrates molecular biology, systems biology, and engineering principles to provide a powerful tool for elucidating photosynthetic mechanisms and rationally optimizing photosynthetic platforms. This review summarizes recent advancements in regulating photosynthesis in cyanobacteria and microalgae via synthetic biology, focusing on strategies to enhance light energy absorption, optimize electron transport chains, and improve carbon assimilation. Furthermore, we discuss key challenges in translating these genetic modifications to large-scale bioproduction, highlighting specific bottlenecks in strain stability, metabolic burden, and process scalability. Finally, we propose potential solutions, such as AI-assisted metabolic engineering, synthetic microbial consortia, and next-generation photobioreactor designs, to overcome these limitations. Overall, while synthetic biology holds great promise for enhancing photosynthetic efficiency in cyanobacteria and microalgae, further research is needed to refine genetic strategies and develop scalable production systems.
1. Introduction
In the face of increasing global resource shortages and environmental pollution, the search for sustainable energy and production methods has become a critical priority [1,2]. Photosynthetic microorganism-based green biomanufacturing offers a promising pathway toward sustainable development [3]. Prokaryotic cyanobacteria and eukaryotic microalgae, two major platforms of photosynthetic microbial cell factory, each have distinct characteristics and potential [4,5]. Eukaryotic microalgae have attracted substantial attention on account of their capacity to flourish in outdoor settings and their high productivity in generating proteins and lipids [6]. In contrast, cyanobacteria, with their well-characterized genetic background and high genetic manipulability, have emerged as more designable and scalable chassis cells for photosynthesis processes [7,8]. By introducing heterologous metabolic pathways and modifying their metabolic networks, cyanobacteria have successfully produced various high-value products, including sucrose [9], inositol [10], 3-HB, 3-HV, 3-HP [11], and astaxanthin [12]. Microalgae also play a significant role in green biomanufacturing, particularly in the production of triglycerides (TAG) [13] and fatty acid esters (biodiesel) [14]. Additionally, they can generate valuable compounds, such as β-carotene [15], astaxanthin [16], lutein [17], and other carotenoids [18]. However, despite these advancements, the productivity of photosynthetic microorganisms remains lower than that of terrestrial plants, which are commonly utilized in biomass-based production systems [19,20]. While algae, cyanobacteria, and plants can all be processed through different types of biorefinery technologies, the inherent limitations of photosynthetic microorganisms, such as lower biomass accumulation and metabolic efficiency, present challenges for their large-scale industrial application. Notably, consortia between microalgae and N-fixing cyanobacteria have demonstrated promising biotechnological potential by enhancing nutrient availability, promoting mutualistic interactions, and potentially, improving overall biomass yield [21].
Photosynthetic microorganisms can capture nearly 50% of incident light energy; however, less than 10% of that energy is capable of being converted into chemical energy presented as biomass. The maximum theoretical solar-to-biomass energy conversion efficiency for oxygenic photosynthesis is estimated to be around 9–10% under ideal conditions [22]. However, in practice, the efficiency is significantly lower, with cyanobacteria achieving approximately 1–2% and microalgae reaching 4–8% [22,23]. This discrepancy is primarily due to various limiting factors, such as inefficient light absorption, suboptimal carbon fixation pathways, and energy losses in metabolic processes. Bridging this gap between theoretical and actual efficiency is crucial for improving the industrial viability of photosynthetic microorganisms in bio-based production systems. Photosynthesis takes place in the following two phases: the light reactions and the dark reactions [24]. During the light reactions, through their light-harvesting antenna system, photosynthetic microorganisms trap solar energy and then convey it to the reaction centers of the photosystem complexes, converting it into electrical energy. Electrons are transferred through various protein complexes and electron carriers within photosynthetic membrane, storing energy as ATP and NAD(P)H. In the dark reactions, ATP and NAD(P)H are used to fix CO2 and produce organic compounds. The systematic optimization of three key processes—light energy absorption and utilization, electron transport chains, and carbon assimilation—is widely recognized as an effective strategy for improving photosynthetic efficiency [25].
Synthetic biology represents an interdisciplinary domain that synergistically amalgamates biology, genomics, engineering, and informatics [26]. It approaches biological systems as engineered systems using a “bottom-up” methodology, following an iterative “design–build–test–learn” research strategy. This process moves from “units” to “devices” to “systems”, with the goal of redesigning natural organisms or creating new synthetic ones. The ultimate aim is to uncover the principles of life and transform biological systems for engineering applications [27,28,29]. Through synthetic biology, a deeper understanding of photosynthesis in photosynthetic microorganisms can be achieved, enabling the more effective regulation of photosynthetic efficiency. Despite this, several significant challenges remain for applying synthetic biology to regulate photosynthetic microbial cell factories; for example, (i) it is difficult to improve photosynthetic efficiency by modifying just one or a few genes; (ii) many unknown functional proteins impact the regulatory effect; (iii) synchronizing photosynthetic efficiency with cell growth optimization remains challenging; and (iv) the effects of genetic engineering are often difficult to translate into large-scale production processes. In this context, we provide an overview of the work and accomplishments from the past few years in regulating photosynthesis in microalgae and cyanobacterium through synthetic biology strategies (Figure 1), along with the major challenges and future perspectives. The efforts provide a foundation for further deciphering the photosynthesis mechanisms and improving photosynthetic efficiency in these cell systems (Table 1).
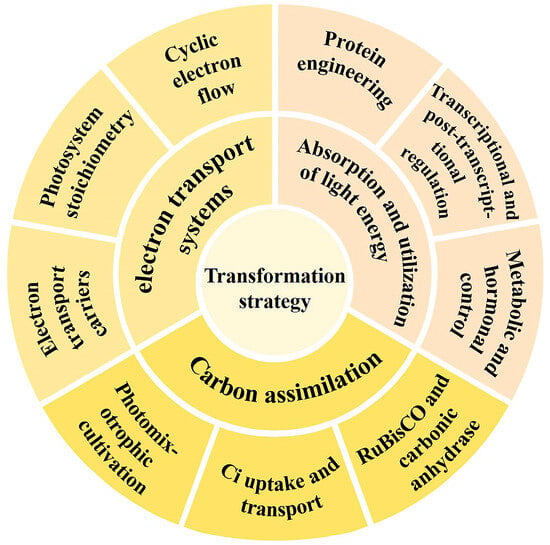
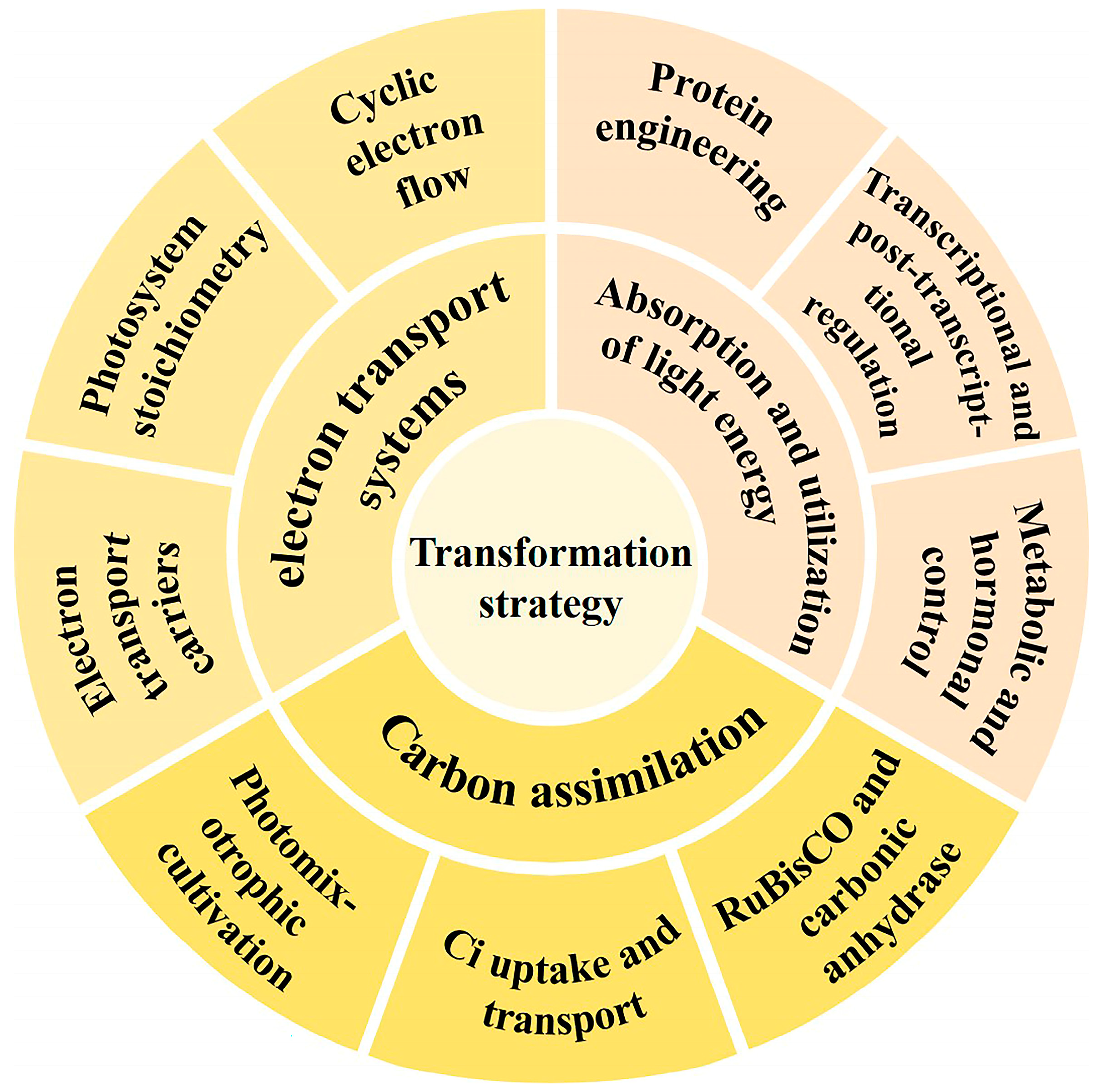
Figure 1.
Regulatory targets in photosynthesis. The goals of the transformation strategy are divided into three parts: absorption and utilization of light energy, electron transport systems, and carbon assimilation.

Table 1.
Regulation of photosynthesis in microalgae and cyanobacteria by synthetic biology.
2. Modulation of Absorption and Utilization of Light Energy
The initial phase of photosynthesis, designated as the primary reaction, encompasses the sequence of processes commencing from the excitation of chlorophyll molecules upon light absorption and culminating in the initiation of the first photochemical reaction [55]. This stage includes the absorption, transfer, and conversion of light energy by pigment molecules. Regulating the processes—light energy absorption, transfer, and conversion—has become a key strategy for improving the efficiency of light energy utilization (Figure 2). Photosynthetic microalgae and cyanobacteria share comparable internal antenna systems, which consist of protein complexes binding chlorophyll a (Chl-a) for light capture. However, their external light-harvesting pigment–protein complexes (LHCs) differ. In photosynthetic microalgae, the principal external light-harvesting complexes are the membrane-integral LHCII and several minor chlorophyll–protein complexes, which are responsible for capturing sunlight (Figure 2A). In cyanobacteria, the primary external light-harvesting complex is the phycobilisome (PBS). This complex is affixed to the cytoplasmic surface of the PSII reaction centers and assumes a pivotal role in light absorption (Figure 2B) [56]. Optimizing light absorption and utilization involves the following three major regulatory mechanisms: protein engineering, transcriptional control, and metabolic modulation. Below, we discuss recent advances in these regulatory strategies, categorized by their common mechanisms.
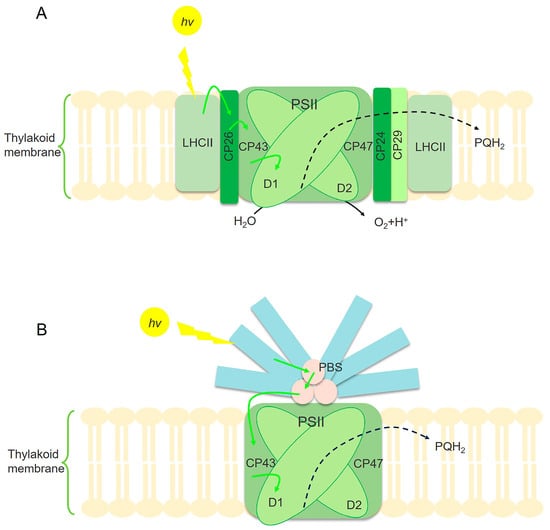
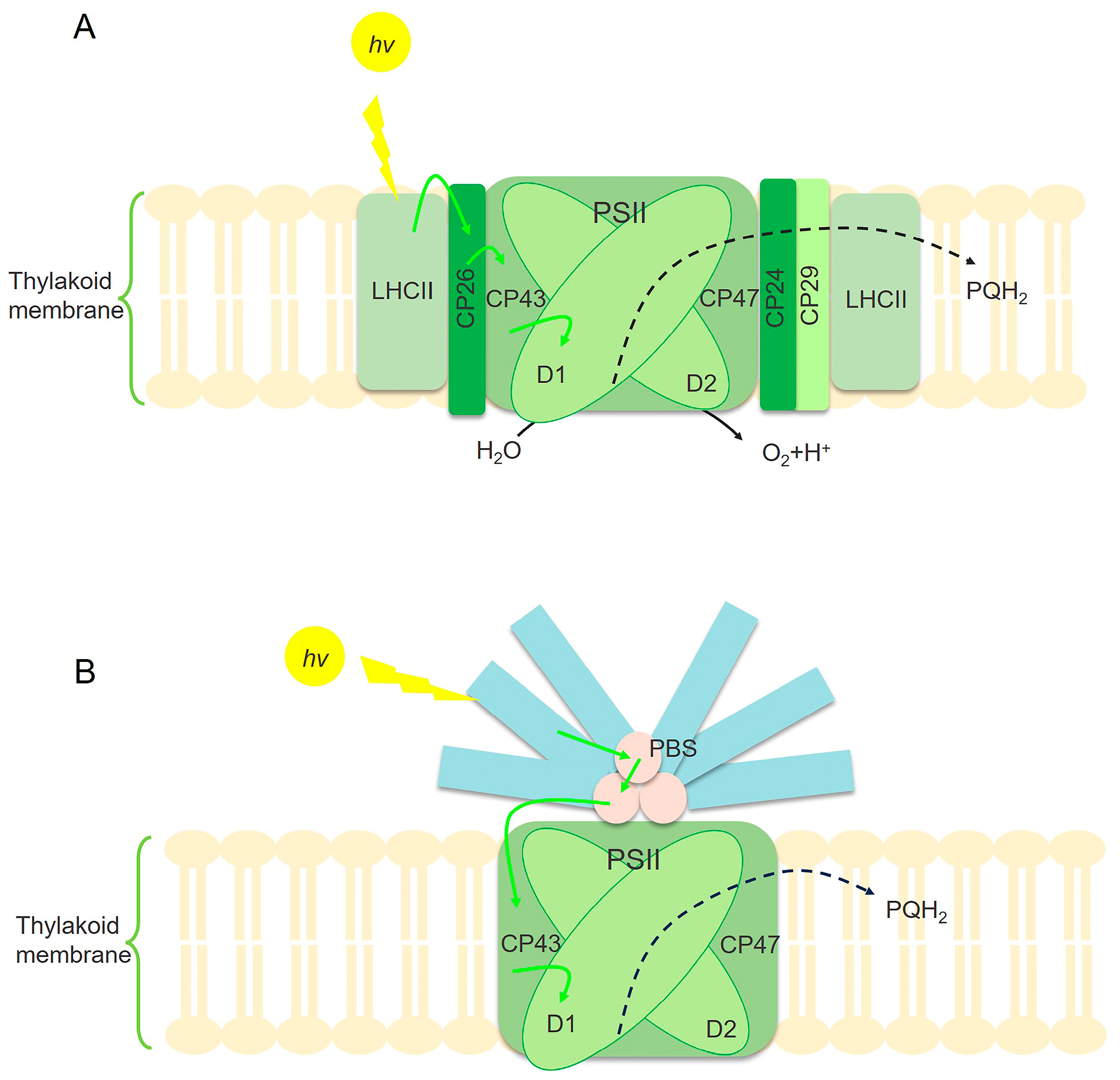
Figure 2.
Illustration of the mechanism of light energy absorption and utilization in microalgae (A) and cyanobacteria (B). PSII is the main site for cyanobacteria and photosynthetic microalgae to absorb light energy. The center of the PSII core is formed by D1 and D2 proteins. CP43 and CP47 are protein complexes that bind to several Chl-a molecules, serving as the inner antenna of PSII. Photosynthetic microalgae utilize the main membrane-integral LHC and some secondary chlorophyll protein complexes to capture sunlight, such as CP24, CP26, and CP29 (A). However, the main light-harvesting complexes of PSII in cyanobacteria are PBSs (B) [56]. The yellow arrow represents light energy absorption. The green arrow represents the transfer of excitation energy. The black dashed arrow represents electronic transmission.
2.1. Protein Engineering for Light Absorption Optimization
Protein engineering offers a direct approach to modifying light-harvesting complexes for improved efficiency [57]. This strategy involves engineering key proteins responsible for light capture, photoprotection, and energy transfer to enhance robustness under fluctuating light conditions. Lu et al. engineered a hlr1 mutant in Nannochloropsis oceanica, leading to alterations in photosystem I (PSI) and a reduction in reactive oxygen species (ROS) production [30]. This improved high-light tolerance, demonstrating that target genetic modifications can enhance photosynthetic robustness. In addition, researchers found that certain enzymes in photosynthetic microorganisms, though not directly involved in photosynthesis, can impact pigment production. For example, the PhoD-type alkaline phosphatase (AP) gene influences both pigment synthesis and photosynthesis efficiency. Using CRISPR technology, Li et al. engineered the PhoD_45757 mutant (mPhoD44) in the diatom Phaeodactylum tricornutum. In comparison to the WT, this led to a 37% elevation in the content of Chl-a and a 49% increase in the content of chlorophyll c [31]. Another member of the AP family, PhoA, has shown similar effects. Zhang et al. created a PhoA mutant using CRISPR/Cas9, which reduced the enzyme’s organophosphate (DOP) hydrolase activity. However, this modification resulted in substantial augmentations in the levels of cellular pigments, carbon, and lipids. Moreover, it brought about improvements in the photosynthetic rate, growth rate, and the transcriptional levels of related metabolic pathways [32]. These studies illustrate how synthetic biology can reprogram photosynthesis at the protein level, allowing for enhanced light capture and photoprotection.
2.2. Transcriptional and Post-Transcriptional Regulation of Light-Harvesting Efficiency
Beyond direct protein modifications, transcriptional and post-transcriptional regulation plays a critical role in controlling LHC abundance and pigment composition in response to light conditions. Agarwal et al. identified the LHC-regulating Myb (LRM) transcription factor, which governs LHC gene expression in Phaeodactylum tricornutum [34]. Knocking down LRM impaired dynamic light absorption adjustments, highlighting the potential of transcriptional engineering for fine-tuning photosynthetic responses. NAB1, a nucleic acid-binding protein in Chlamydomonas reinhardtii (C. reinhardtii), regulates light-harvesting antenna complex expression [58]. Engineered strains with NAB1-controlled chlorophyllide a oxygenase (CAO) expression exhibited twice the biomass productivity, while maintaining optimal state transitions and photoprotection [33]. Regulating the size of the light-harvesting antenna enhances non-photochemical quenching (NPQ) processes and state transitions, increasing photosynthetic rates and significantly improving light energy utilization efficiency under low-light conditions, ultimately leading to greater biomass accumulation.
2.3. Metabolic and Hormonal Control of Photosynthetic Pigments
Synthetic biology approaches can enhance precursor availability for pigment synthesis, thereby improving light absorption and energy conversion. For instance, engineering carotenoid biosynthesis pathways has shown promise for improving photoprotection and minimizing oxidative damage, paving the way for metabolically optimized strains [59]. In addition to metabolic modifications, hormonal regulation also plays a critical role in modulating photosynthetic pigment levels. Zhang et al. demonstrated that the exogenous application of abscisic acid (ABA) downregulated the LHCA1 gene in Nannochloropsis haitanensis, reducing light capture and inhibiting photosynthesis [35]. This reduced light capture and photosynthetic performance, suggesting that phytohormone-responsive circuits could be engineered as synthetic regulators to optimize photosynthetic output. Together, these studies highlight that metabolic engineering and hormonal regulation provide additional mechanisms for optimizing photosynthetic efficiency by modulating pigment production and antenna size.
3. Adjustment of Electron Transport Systems
The electron transfer process during photosynthesis represents a crucial stage. It encompasses two photosystems, namely Photosystem I (PSI) and Photosystem II (PSII). These two photosystems collaborate to transform light energy into chemical energy [60]. Adopting a systematic optimization approach for the electron transfer process may serve as an effective strategy to improve photosynthetic efficiency (Figure 3).
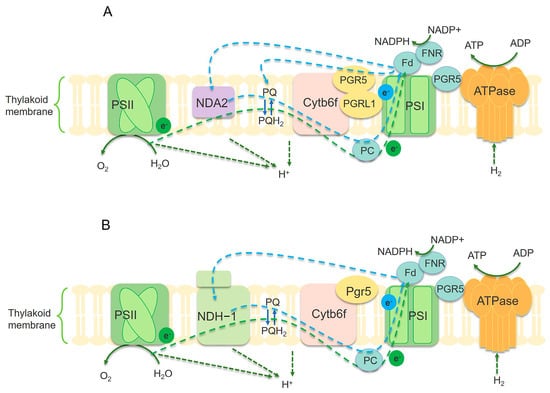
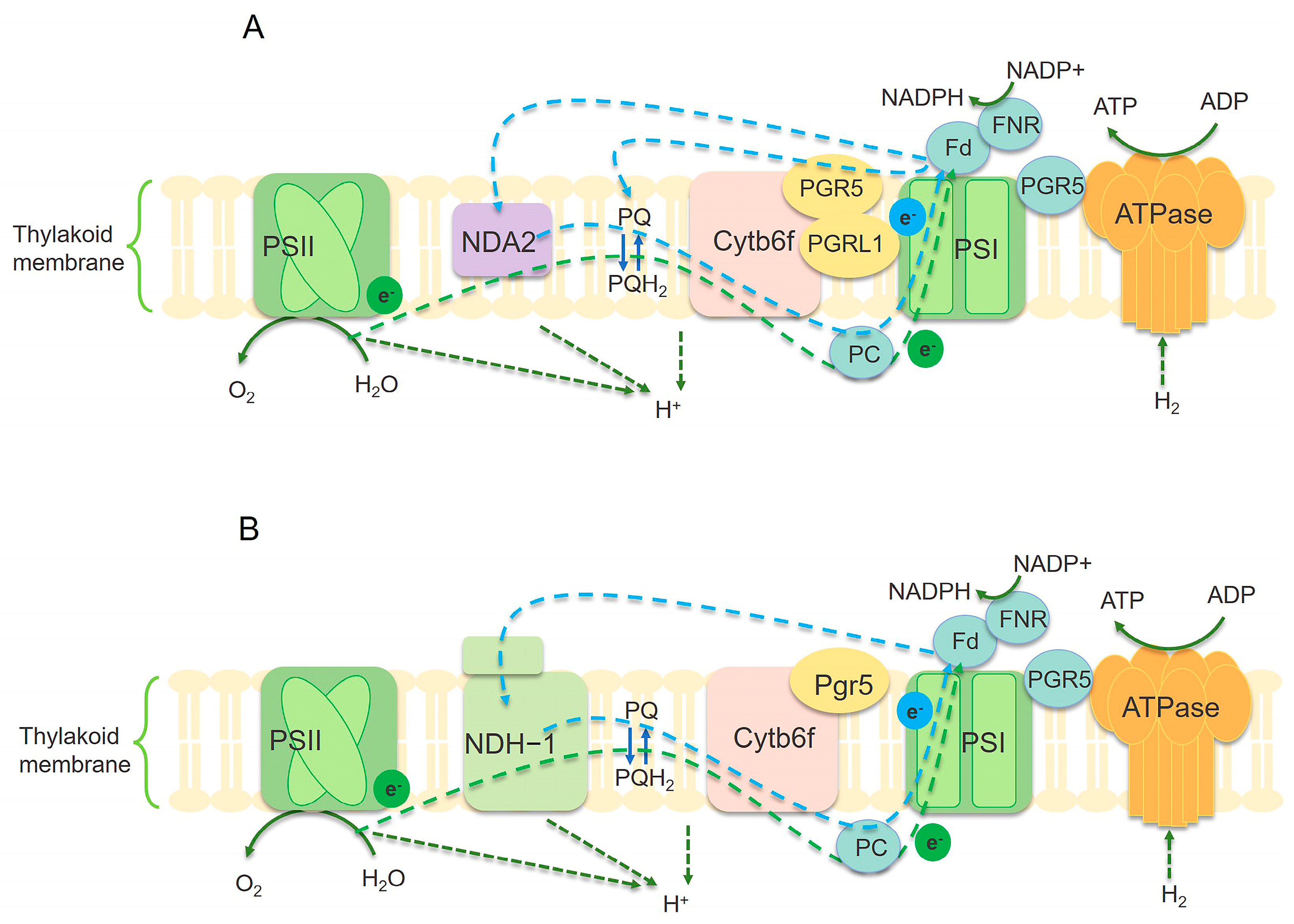
Figure 3.
Illustration of electron transfer mechanism in microalgae (A) and cyanobacteria (B). The blue dashed arrow represents the circulating electron flow (CEF) around PSI. The green arc-shaped dashed line represents the linear electron flow (LEF) between PSII and PSI. NDH-1 complex mediates CEF in cyanobacteria (B). There is no NDH-1 in photosynthetic microalgae, replaced by NDA2 (A), and PGR5/PGRL1 also mediates CEF. PQ/PQH2: plastoquinone. PC: plastocyanin. Fd: ferredoxin. FNR: ferredoxin-NADP(H) reductase. Cytb6f: cytochrome b6-f complex [60,61].
3.1. Regulation of Electron Transport Carriers
Plastoquinone (PQ) functions as a crucial electron carrier within both the photosynthetic and respiratory electron transport chains. In cyanobacteria and microalgae, it facilitates the conveyance of reducing power and energy [60]. In a study by Fan et al., when the enzyme geranylgeranyl diphosphate:4-hydroxybenzoate geranylgeranyl transferase (LepGT) was overexpressed in Synechocystis sp. PCC 6803 (Synechocystis 6803), the content of plastoquinone (PQ) decreased by 22.18%. Unexpectedly, such a reduction gave rise to an improvement in both the photosynthetic rate and the respiration rate [36]. Compared to the WT, the strain overexpressing LepGT showed an 11.55% increase in electron transport efficiency. NADP+ and NAD+ serve as indispensable electron acceptors within the realm of energy metabolism [36]. Specifically, NADP+ functions as an electron acceptor in the light-dependent reactions of photosynthesis. Moreover, its biosynthesis is of great significance for photoautotrophic growth [36]. By modulating PQ biosynthesis, the engineered Synechocystis strains exhibited enhanced electron transport efficiency, improved PSII activity, and optimized ATP/NADPH production, leading to an overall increase in photosynthetic rate and biomass accumulation.
3.2. Regulation of Photosystem Stoichiometry
PSI and PSII serve as indispensable constituents of the photosynthetic electron transport chain. The PSI core complex contains the reaction center pigment P700, which functions as an electron acceptor, and the PSI LHC, which transfers electrons from phycobilins to ferredoxin [60,62]. Alterations in the stoichiometry of Photosystem I (PSI) and Photosystem II (PSII), frequently occurring as a reaction to diverse light qualities, can act as a compensatory mechanism in the thylakoid membrane to equalize light absorption between the two photosystems [63]. Photosynthetic microorganisms can adjust the relative abundance of their light-harvesting complexes, leading to different PSI-to-PSII ratios, which can significantly impact the photosynthetic efficiency. Within the cyanobacterial strain Synechocystis 6803, PSI is typically 2 to 4 times more abundant than PSII [64]. In an experiment by Moore et al., researchers modified the promoter region of the psaAB operon to adjust the PSI-to-PSII ratios, creating strains with reduced PSI level. These modified strains demonstrated higher productivity under high-light intensities, as they reached saturation at these levels, optimizing their photosynthetic capacity [37]. The reduction in PSI content in Synechocystis 6803 led to enhanced light utilization, improved linear electron transport, and better growth under high-light conditions, ultimately increasing productivity. The BtpA protein (biogenesis of thylakoid proteins A) is known to regulate PSI levels post-transcriptionally by stabilizing the core PSI protein PsaA in Synechocystis 6803. In an associated study, Knoot et al. engineered a CRISPR interference (CRISPRi) system using dCas12a (dCpf1) to precisely control PSI levels in Synechococcus sp. UTEX 2973 (Synechococcus 2973). This system achieved over 90% inhibition of high-abundance gene targets in the absence of inducers. By targeting the homologous btpA gene in Synechococcus 2973, researchers reduced PSI levels to 13% of the control group1 mM. Additionally, the Chl-a content per cell in the modified strain dropped to less than 25% of that in the uninduced control. When IPTG was removed by refreshing the culture medium, PSI levels returned to normal within 48 h, indicating that PSI expression in these strains could be reversibly controlled [38]. Physical induction can also alter the PSII/PSI ratio. For instance, Balan et al. exposed Chlorella vulgaris to UVB radiation, which specifically stimulated PSII, increasing antenna size 10-fold. Under low-light laboratory conditions, a larger antenna size enables PSII to capture photons more efficiently, thereby promoting the enhancement of photosynthetic efficiency. This treatment produced a PSII-to-PSI ratio that was 2 to 7 times higher than that in control group strains grown solely under photosynthetically active radiation (PAR) at 5000 lux without UV exposure. Strains with an increased PSII/PSI ratio exhibited significantly higher photosynthetic efficiency and growth, achieving a maximum specific growth rate 4.3 times greater than that of the control group [39].
3.3. Regulation of Cyclic Electron Flow (CEF)
CEF, powered by PSI, synthesizes ATP without generating extra NADPH [65,66,67,68]. CEF operates primarily through two pathways, including the “antimycin A-insensitive” pathway [69], which involves the NADH dehydrogenase-like complex (NDH) (Figure 3B) [70,71], and the “antimycin A-sensitive” pathway, also known as the “PGR5-dependent CEF” pathway, involving the thylakoid proteins proton gradient regulation 5 (PGR5) and PGR5-like photosynthetic phenotype 1 (PGRL1) (Figure 3A) [72,73]. CEF plays a crucial role in preserving the redox equilibrium of the photosynthetic machinery and in precisely adjusting the NADPH/ATP ratio, particularly when light conditions are fluctuating [74]. According to research conducted by Jokel et al., the green alga C. reinhardtii was used to construct knockout mutants for the PGR5 and PGRL1 genes. The growth of the pgr5 mutant was severely impaired under mild fluctuating light (FL) conditions (20 μmol photons m−2 s−1 for 5 min, followed by 200 μmol photons m−2 s−1 for 30 s) and completely inhibited under more intense FL conditions (20 μmol photons m−2 s−1 for 5 min, followed by 600 μmol photons m−2 s−1 for 30 s). Under these conditions, both pgr5 and pgrl1 mutants showed varying degrees of growth inhibition. Immunoblot analysis indicated damage to the photosynthetic machinery, in the pgr5 mutant, the PsaA subunit of the PSI was reduced fivefold, while in the pgrl1 mutant, the Cyt f subunit of the Cyt b6f complex was reduced by 50%. Many cyanobacterial species have genes encoding PGR5-like proteins, but their role in photosynthesis remains incompletely elucidated [40]. As reported in a scientific investigation carried out by Margulis et al., overexpression of the pgr5 gene in Synechocystis 6803 led to a fivefold increase in chlorophyll accumulation and a corresponding increase in active PSI units, with the maximum P700 oxidation level (∆Amax) reaching approximately 250% that of the WT [41]. Conversely, Selão et al. knocked out pgr5 in Synechococcus sp. PCC 7002 (Synechococcus 7002), leading to an increased cellular [NADPH]/[NADP+] ratio and enhanced the NADP+-dependent lactate dehydrogenase (LDH) activity. This modification boosted the reduction in pyruvate to d-lactate, leading to nearly a sevenfold increase in d-lactate accumulation (≈1000 mg/L vs. ≈150 mg/L in the WT) under conditions of 25 °C and 0.04% (v/v) CO2 [42]. In C. reinhardtii, an additional NADPH-dependent and Fd-independent pathway involving the type II NAD(P)H dehydrogenase (NDA2) has been identified [75,76]. Baltz et al. successfully overexpressed the NDA2 gene in C. reinhardtii, resulting in a significant increase in hydrogen production via an indirect pathway, achieving almost twice the hydrogen production rate of the non-overexpressing strains. Although the PsaD subunit levels were reduced in the engineered strain, PSI activity remained unaffected. Additionally, in the dark, the reduction rate of P700+ was more rapid in the strain overexpressing NDA2 compared to that in the control, indicating that NDA2 overexpression effectively enhanced CEF activity [43].
4. Regulation of Carbon Assimilation
Carbon assimilation is a key step in photosynthesis, through which photosynthetic microalgae convert inorganic carbon (CO2) into organic carbon (such as glucose and starch) (Figure 4). This process provides the energy and raw materials for their growth and development [77]. Enhancing carbon assimilation is crucial for improving biomass productivity, optimizing biofuel synthesis, and increasing photosynthetic efficiency. This section categorizes advancements in carbon assimilation into three major strategies, each focusing on a key regulatory mechanism.
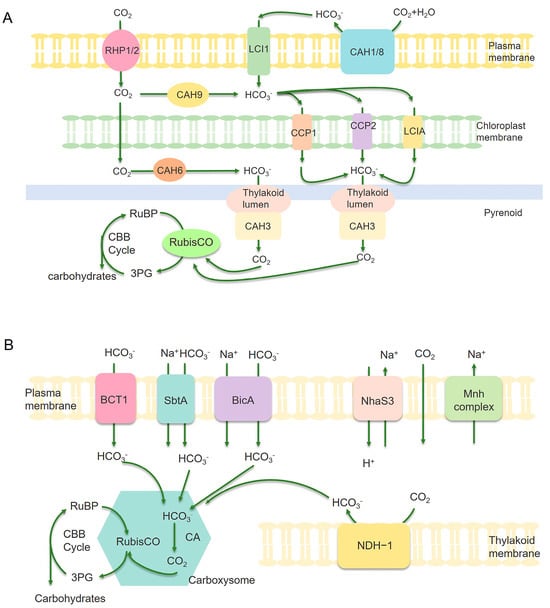
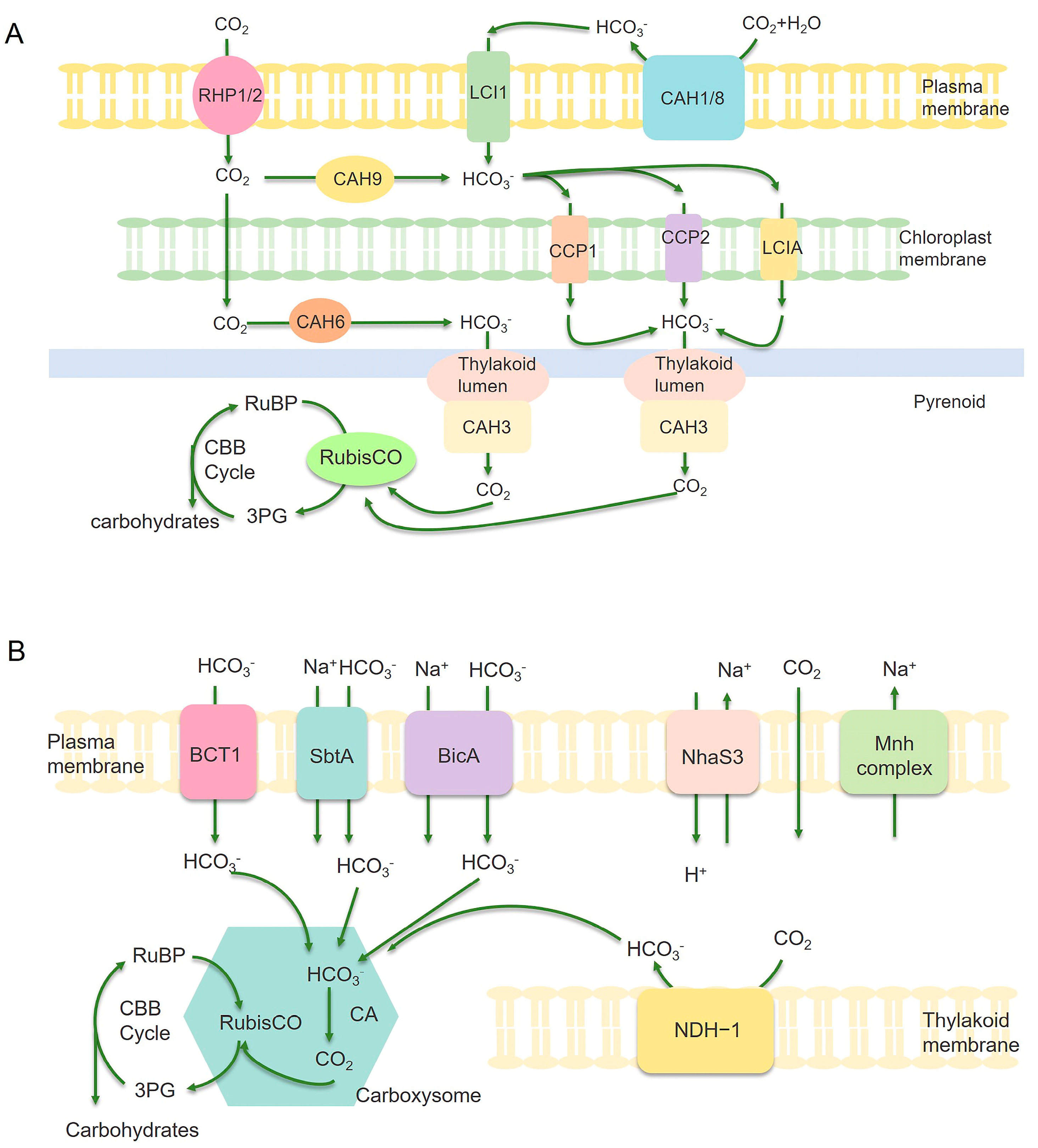
Figure 4.
Illustration of carbon assimilation mechanism in microalgae (A) and cyanobacteria (B). (A) CAH1, CAH3, CAH6, CAH8, and CAH9 are various subtypes of carbonic anhydrase. Transport parking space RPH1/RPH2/LC1 on the plasma membrane and LCIA/CCP1/CCP2 located on the chloroplast envelope are Ci transporters. Arrows represent carbon assimilation pathways. (B) BCT1, SbtA, and BicA are bicarbonate transporters that can actively transport HCO3− into the cytoplasm. The carboxysome is an important component of cyanobacteria CCM, which contains enzymes RuBisCO and CA inside. CA converts bicarbonate into CO2 in the carboxysome, thereby increasing the concentration of CO2 near the key enzyme RuBisCO and promoting CO2 conversion. NDH-1 complex can convert CO2 into HCO3−, which can prevent CO2 leakage from the carboxysome. RuBisCO catalyzes the reaction of Riboulose-1,5-bisphosphate (RuBP) with CO2 to produce 3-phosphoglycerate (3-PG), thereby achieving CO2 fixation [78,79].
4.1. Improving Ci (HCO3−/CO2) Uptake and Transport
Efficient carbon assimilation begins with the uptake and concentration of Ci (HCO3−/CO2). Cyanobacteria and microalgae utilize CO2-concentrating mechanisms (CCM) to actively capture CO2, thereby boosting photosynthetic rates. Engineering Ci uptake and transport systems has emerged as a promising strategy for improving CO2 fixation and biomass yield. For example, Gupta et al. examined the impact of two distinct ribosome binding site (RBS) sequences along with three different promoters (PcpcB, Pcpc560, and PrbcL2) on the expression of sodium-dependent bicarbonate transporter SbtA in Synechococcus 7002. The optimized construct using Pcpc560 and RBS2, resulted in a 90% increase in biomass under air or 1% CO2 conditions, demonstrating the effectiveness of transporter engineering for enhanced carbon uptake [44]. Wang et al. analyzed BicA, a bicarbonate transporter belonging to the SLC26 family, which plays a critical role in CCM in Synechocystis 6803. The deletion of ndhD3, ndhD4, cmpA, sbtA, and bicA resulted in growth arrest under both ambient CO2 conditions, highlighting their essential role in HCO3− uptake and transport. However, the overexpression of BicA restored growth, demonstrating its potential for bioengineering CO2 uptake in heterologous hosts [45]. In Synechococcus elongatus PCC 7942 (Synechococcus 7942), a mutant strain engineered for α-farnesene production showed growth inhibition under ambient CO2 conditions due to the deletion of β-carboxysome and the CCM genes. The overexpression of carbonic anhydrase and bicarbonate transporters restored growth and increased α-farnesene production to 5.0 ± 0.6 mg/L, demonstrating that Ci uptake optimization contributed to the enhanced carbon flux for bioproduct synthesis [46]. These studies underscore the importance of Ci transporters in improving carbon capture, providing opportunities for synthetic biology applications in biofuel and biochemical production.
4.2. Optimizing Carbon Fixation Through RuBisCO and Carbonic Anhydrase Engineering
Ribulose-1,5-bisphosphate carboxylase/oxygenase (RuBisCO) assumes the responsibility of catalyzing the fixation of CO2 during the photosynthesis process. Notwithstanding its crucial function, RuBisCO is regarded as an enzyme with low efficiency, making it a primary target for endeavors in directed evolution [80,81,82]. Huang et al. demonstrated that the deletion of RuBisCO accumulation factor 1 (Raf1) in Synechococcus 7942 reduced RuBisCO RbcL8S8 holoenzyme by 30% and maximum CO2 fixation capacity by 39% [47]. Bolay et al. identified sll0998 (rbcR), a trans-acting transcriptional regulator, as a key modulator of RuBisCO gene expression in the Synechocystis 6803 [48]. The knockout of rbcR led to reduced RuBisCO expression, impaired growth, and decreased CO2 assimilation, indicating its regulatory role. Lu et al. knocked out SeXPK, a phosphoenolpyruvate carboxykinase in the Synechococcus 7942 strain, leading to a 63% increase in RuBisCO carbon fixation efficiency without affecting growth [49]. Carbonic anhydrase (CA) is an ancient zinc-containing metalloenzyme that catalyzes the interconversion between CO2 and HCO3−, playing a critical role in carbon assimilation and transport (Figure 4) [83]. Engineering CA activity provides an effective means to enhance CO2 conversion rates and optimize metabolic flux toward biomass accumulation. Fan et al. studied the effects of acetazolamide (a CA-specific inhibitor) on Phaeodactylum tricornutum and Nannochloropsis oceanica, finding significant reductions in growth rate and photosynthetic activity [50]. Lin et al. overexpressed MICA, a highly active CA isoform, in Chlorella sorokiniana and Chlorella vulgaris, leading to higher CO2 fixation rates, increased biomass yield, and enhanced lipid production [51]. Together, these studies demonstrated that engineering the activity of CA and RuBisCO provides an effective means to increase photosynthetic performance and enhance carbon flux toward biomass accumulation.
4.3. Exploring Photomixotrophic Cultivation for Greater Carbon Utilizaiton
Some microalgae and cyanobacteria can grow either photoautotrophically or photomixotrophically, simultaneously utilizing CO2 and organic carbon sources, like xylose, glucose, glycerol, or acetate [84]. The use of dual carbon sources enhances the growth and metabolic activity of photosynthetic microorganisms, offering attractive opportunities for biotechnology applications [85]. Although cyanobacteria can naturally utilize glucose, the central carbon metabolism leads to the release of carbon atoms in the form of CO2, resulting in a net loss of carbon. To address this, Song et al. engineered a synthetic nonoxidative cyclic glycolysis (NOG) pathway in Synechocystis 6803 to increase the carbon flux from glucose to the key intracellular precursor acetyl-CoA, successfully doubling the intracellular pool of the acetyl-CoA pool [52]. Moreover, to reduce competition for glucose, the native Entner–Doudoroff (ED) pathway was knocked out in the engineered strain carrying the NOG pathway, further increasing acetyl-CoA level by up to 280%. Xylose, which constitutes a significant part of lignocellulose and stands as the second-most prevalent sugar in nature following glucose, represents a promising renewable resource for the production of biofuels and chemicals [52]. Some model cyanobacteria have been engineered to utilize xylose by introducing a major facilitator superfamily transporter, XylE, alongside the native substrate, glucose, to enhance the production of heterologous ethylene and 2,3-butanediol [86,87]. More recently, Pressley et al. investigated the metabolic state of xylose-consuming mixotrophic Synechococcus 7942 during the production of 2,3-butanediol. They found that the overexpression of phosphoribulokinase (Prk) and ribulose-5-phosphate-3-epimerase (Rpe) led to a 29% increase in 2,3-butanediol titer, while the knockout of cp12 boosted production by 53% [53]. C. reinhardtii, recognized as a model photosynthetic organism, is also capable of mixotrophic growth, making use of supplementary organic carbon sources, which demonstrates its metabolic flexible incorporating diverse carbon intermediates [88]. Additionally, its potential in bioremediation and bioproduct synthesis has garnered increasing attention, as it can efficiently remove contaminants from wastewater, while also serving as a platform for biofuel and high-value compound production [89]. Yao et al. achieved formate tolerance exceeding 40 mM in C. reinhardtii through a combination of co-substrate addition, evolutionary approaches, and growth condition optimization. The study demonstrated that formate not only enhanced growth and photosynthetic activity but also increased biomass by 27% and photosynthetic oxygen evolution by 60% [54]. These findings suggest that photomixotrophic cultivation offers higher metabolic flexibility and greater carbon conversion efficiency, making it a promising strategy for industrial bioproduction.
5. Challenges of Regulating Photosynthesis Efficiency Through Synthetic Biology
Photosynthetic microorganisms, with their remarkable ability to absorb carbon dioxide and perform oxygen-producing photosynthesis, have emerged as a top choice for green biomanufacturing. By leveraging synthetic biology to regulate the photosynthesis process in these microorganisms, we can control their photosynthetic rate and growth and even modify them to produce high-value chemicals. For example, introducing exogenous synthetic pathways into cyanobacteria can enable the production of compounds, such as sucrose [9], inositol [10],3-HB,3-HV,3-HP [11], and astaxanthin [12]. Optimizing the photosynthetic processes of microalgae and cyanobacteria using synthetic biology holds significant potential for expanding their industrial applications in biomanufacturing. However, the complexity of photosynthesis, the unknown proteins involved in photosynthesis, and the limitations of engineering tools and ecological adaptation present significant challenges in leveraging synthetic biology to regulate photosynthesis.
5.1. Isolated Gene Engineering Insufficient to Achieve Measurable Improvements
In cyanobacteria or microalgae, modifying one or a few individual genes often has a limited impact on enhancing photosynthetic efficiency. This is due to the complex, multi-gene collaborative nature of photosynthesis, which involves interconnected processes, such as light capture, electron transfer, and carbon fixation. Altering a single component is unlikely to overcome constraints in other processes, thereby failing to achieve a significant improvement in overall photosynthetic efficiency. Melis demonstrated that, while reducing antenna size can enhance the photosynthetic efficiency of individual cells, this modification offers limited benefits for overall productivity under high-density culture conditions [22]. Kirst et al. deleted the TLA3-CpSRP43 gene in C. reinhardtii, leading to a reduction in antenna size. However, the anticipated increase in photosynthetic efficiency was not observed, suggesting that the impact of a single genetic modification may be limited [90]. In their article, Angermayr et al. emphasized that, while various genetic modifications have been applied to the metabolic pathways of cyanobacteria, the inherent complexity of photosynthesis means that altering a single or a few genes is typically insufficient to achieve significant improvements in photosynthetic efficiency. Instead, systematic and multi-level engineering strategies are required [7]. All these findings demonstrate that, in cyanobacteria or microalgae, modifying one or a few genes individually may result in only limited improvements in photosynthetic efficiency. A comprehensive approach that considers all aspects of photosynthesis and employs systematic optimization is essential.
5.2. Unknown Proteins Involved in Photosynthesis Emerged as Potential Factors Influencing the Regulation
Unknown proteins may play crucial roles in regulating photosynthesis in cyanobacteria and microalgae and serve as potential variables in synthetic biology design. For instance, an unannotated protein identified in C. reinhardtii appears to be implicated in the assembly and maintenance of the stability of the PSII supramolecular complex, with its mutation causing significant impairment of photosynthesis [91]. Recent studies have identified that small proteins in cyanobacteria play key roles in photosynthesis, contributing to cellular redox regulation, carbon/nitrogen balance, and various metabolic activities. For example, as Krauspe et al. discovered, in Synechocystis 6803, the diminutive protein NblD assumes a critical role in the degradation process of phycobilisomes during nitrogen-restricted circumstances. Simultaneously, it is indispensable for preserving the cellular quantities of amino acids, nitrogen-bearing metabolites, and organic acids [92]. The microalgal nitrogen-fixing bacterial consortia have been explored for bioremediation, biofuel production, and biofertilization applications. However, uncharacterized molecular components within these consortia play a role in regulating photosynthetic efficiency and nitrogen assimilation, which suggests that further research into unknown proteins may uncover new regulatory mechanisms [21]. Given the abundance of unknown proteins in microalgae and cyanobacteria, manipulating specific genes or pathways may reveal regulatory interactions between these unknown proteins and the target genes. Such interactions could significantly impact photosystem function, metabolic network balance, and biological adaptability.
5.3. Challenge of Balancing Cell Growth and Product Synthesis in Photosynthesis Regulation
Employing synthetic biology to regulate photosynthesis in microalgae and cyanobacteria holds potential to enhance photosynthetic efficiency and redirect energy and carbon flow toward target products. However, it may also impact cell growth and adaptation due to resource reallocation or metabolic burden [93]. Li et al. constructed ADP-glucose pyrophosphorylase-deficient mutants by inserting mutations, resulting in a lipid content 3.5 times higher than that of WT. However, at the same time, the growth of the mutants was damaged, and the PSII quantum yield was significantly lower than that of WT [94]. They also revealed that enhancing the lipid synthesis pathway in microalgae limits protein and pigment synthesis due to the preferential allocation of carbon and energy to lipid production. Anne M. et al. engineered the cyanobacterium Synechococcus 7942 through genetic knockout of the free fatty acid (FFA)-recycling acyl-ACP synthetase and heterologous expression of thioesterase to release FFAs. However, the engineered strain exhibited reduced photosynthetic yield, Chl-a degradation, and altered cellular localization of light-harvesting pigments, including phycocyanin and allophycocyanin [95]. Song et al. modified the central carbon metabolic route. They achieved this by incorporating a newly designed NOG-like module and eliminating a native ED pathway to boost the acetyl-CoA and lipid content, yet the cell growth in the engineered strain was remarkably decreased [52]. All these findings demonstrated the importance of collaboratively considering both cell growth and product synthesis when engineering photosynthetic cell factories.
5.4. Optimization Achieved in the Laboratory Proves Difficult to Reproduce in Large-Scale High-Density Cultivation
Translating genetic engineering advancements from laboratory-scale research to industrial-scale production presents significant challenges. These challenges primarily stem from strain instability, metabolic burden, process scalability, and bioreactor design constraints. Addressing these bottlenecks requires a multifaceted approach, integrating strain engineering, process optimization, and advanced bioreactor technologies. The key barriers to the commercial application of photosynthetic microalgae include regulatory constraints, strain instability in industrial settings, and economic feasibility. The genetic engineering strategy provides more insights for the large-scale production of photosynthetic microalgae, such as adaptive laboratory evolution, metabolic burden minimization, and process intensification strategies in bioreactor cultivation. Various types of photobioreactors have been developed for the cultivation of cyanobacteria and microalgae, each optimized for specific growth conditions and scalability. Bubble column photobioreactors feature a cylindrical structure divided into aerated and non-aerated zones to mimic airlift operation (7–24 cm in diameter). These reactors are typically illuminated by fluorescent tubes or LEDs distributed peripherally, requiring transparent materials (e.g., glass) for external lighting, while internal light sources allow the use of opaque materials. Stirred tank reactors rely on external or internal light sources (e.g., LEDs or optical fibers), with diameter optimization ensuring sufficient light intensity at the center. Optical fibers can couple natural and artificial light to adapt to diurnal variations, while red LEDs, due to their low energy consumption, are employed in wastewater treatment. Tubular reactors consist of a solar receiver (long tubes or helical structures optimized for light capture) and an airlift system (degassing the bubble column for oxygen removal), often illuminated by fluorescent lamps in laboratory settings. Airlift reactors utilize gas-driven circulation (via riser and downcomer zones) combined with external natural light, embedded LEDs, or hybrid lighting to balance efficient mass transfer and uniform light distribution. Each reactor type is tailored through differentiated designs (e.g., light source arrangement, structural optimization) to meet microalgal cultivation needs, spanning from laboratory pre-culture to industrial-scale production [96,97]. Nonetheless, when a single-species cultivation method is adopted to yield a high-density cell mass or the peak titers of particular products in photobioreactors, the over-absorption of photons by the cells in the top layer may prevent the light from reaching the cells deeper down [22]. For instance, Li et al. engineered a β-caryophyllene synthesis pathway in Synechococcus 2973, optimizing the expression of key synthases and precursor supply. In shake flask experiments, β-caryophyllene production reached approximately 121.22 μg/L. However, during high-density cultivation in a photobioreactor, although the yield increased to about 212.37 μg/L, the improvement was marginal compared to low-density cultures, suggesting that the benefits of enhanced light capture are constrained under high-density conditions [98].
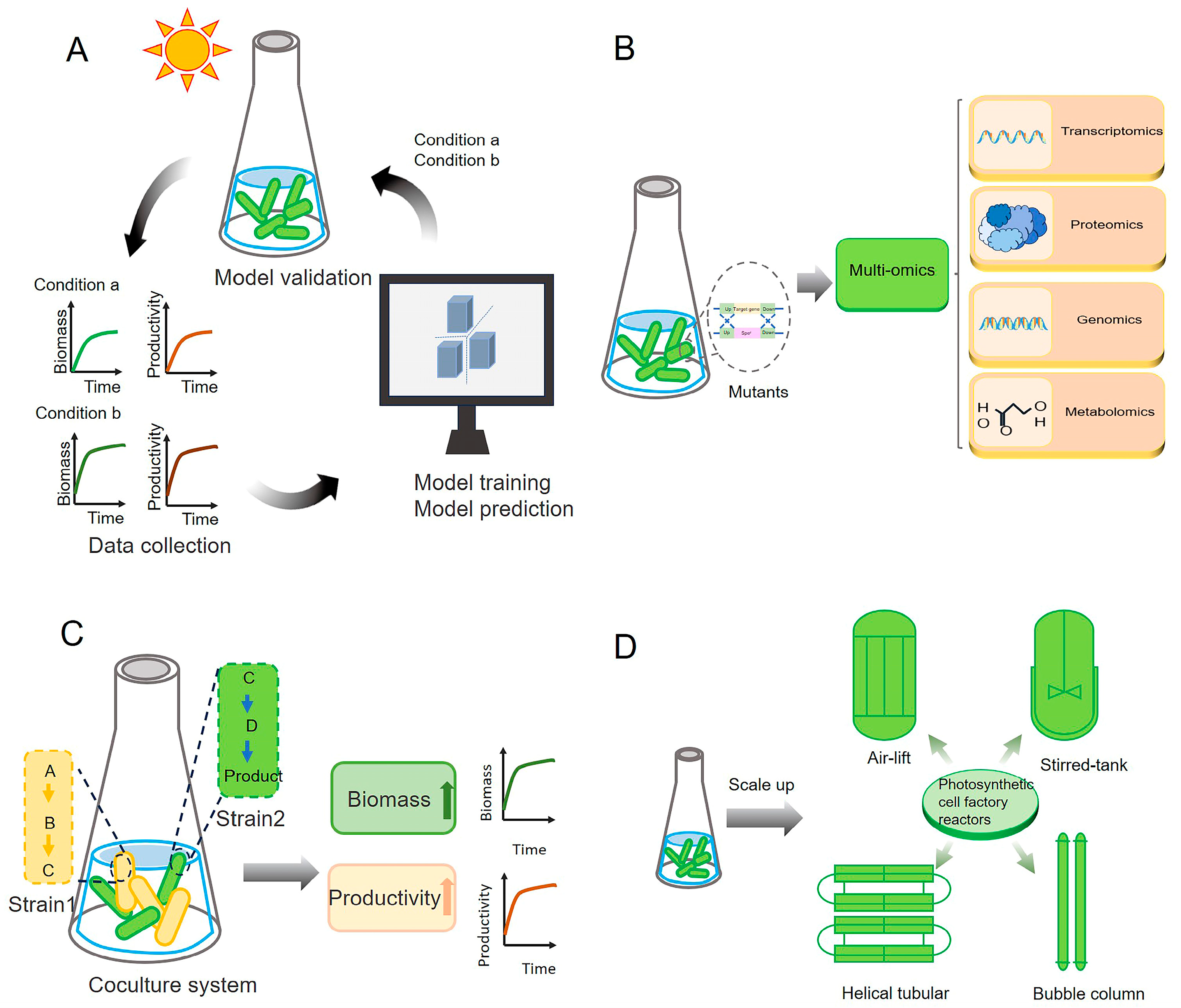
6. Perspectives and Future Direction
6.1. AI-Guided Metabolic Engineering for Photosynthetic Optimization
With the aid of artificial intelligence, particularly machine learning, synthetic biology can identify regulatory targets within complex biological systems, enabling the precise optimization of microalgal biotransformation processes and cyanobacterial metabolic regulation (Figure 5A). Through the analysis of available data, machine learning algorithms are capable of calculating the most suitable environmental and light conditions for the cultivation of microalgae. They can also forecast biofuel yields, enhance energy conversion efficiency, and guarantee a higher-quality biofuel [99]. Several studies have demonstrated the feasibility of AI-based tools for improving photosynthetic efficiency. Kugler et al. combined constraint-based metabolic modeling and machine learning to identify key bottlenecks limiting CO2 fixation in Synechocystis 6803. Their model accurately predicted rate-limiting steps in the CBB cycle, enabling the rational engineering of more efficient CO2 assimilation pathways [100]. Long et al. applied AI algorithms to optimize light intensity and nutrient availability in microalgal cultures. The system successfully increased biomass productivity by 32% compared to conventional manual optimization [101]. By applying decision tree algorithms, Ahmet Coşguna et al. determined 11 sets of conditions that foster high microalgal productivity and 13 factors that could boost lipid content [102]. Machine learning tools can additionally function as a precious conduit for comprehending cellular physiology and forecasting rate-limiting steps within genome-scale metabolic networks. Consequently, they offer guidance for the bioengineering of cyanobacteria. All the discoveries derived from machine learning offer a pathway to gain insights into cellular physiology and anticipate the rate-limiting steps in metabolic networks. This, in turn, provides guidance for those engaged in the biomanufacturing of cyanobacteria.
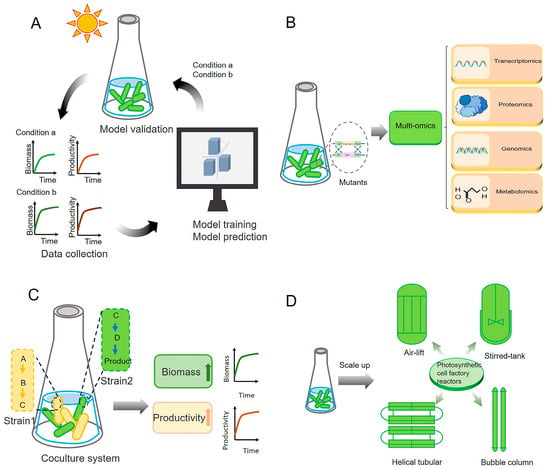
Figure 5.
Prospects and further directions for regulating photosynthesis through synthetic biology. (A) Optimizing photosynthesis with artificial intelligence. Critical data, such as growth conditions, photosynthetic efficiency, and production yields, are collected using advanced sensors and monitoring systems. AI algorithms analyze the collected data to construct comprehensive system models, which predict optimal conditions and intervention strategies for enhancing photosynthesis, guiding experimental designs and engineering efforts. (B) Characterization of unknown proteins. Mutants deficient in specific proteins can be constructed and analyzed using integrative omics approaches to identify and characterize previously unknown proteins that may be involved in photosynthesis. (C) Construction of artificial coculture systems. Artificial coculture systems can be designed to distribute the metabolic burden of microalgal cell factories across different strains. This strategy simultaneously enhances both photosynthetic efficiency and product yield. (D) Development of photosynthetic cell factory reactors. By integrating data from cell factory cultivations at various scales, tailored designs for large-scale photosynthetic cell factory bioreactors can be developed, further enhancing the advantages of high-density cultivation.
6.2. Unveiling the Functions of Unknown Proteins via Integrated Approach
Understanding the functions of unknown proteins provides deeper insights into the photosynthetic mechanisms of cyanobacteria and microalgae. This knowledge facilitates the optimization of critical processes, such as light reactions, carbon fixation, and photoprotection. In addition to enhancing photosynthetic efficiency, it paves the way for innovative approaches to bioenergy and bioproduct synthesis. System modeling enables the prediction of unknown protein functions by integrating data from genomics, proteomics, transcriptomics, and metabolomics (Figure 5B). Recent omics-based studies have started to uncover novel proteins that influence photosynthetic performance. Nishiguchi et al. used multi-omics analysis to identify previously unknown proteins involved in carbon assimilation in Synechocystis 6803. Their findings provided a functional basis for metabolic rewiring strategies to enhance CO2 fixation [103]. Based on their model predictions, they successfully engineered a high ethanol-yielding strain of Synechocystis 6803, achieving an ethanol production of approximately 120 mg/L [103]. Using omics technologies, functional prediction, and experimental validation to uncover the roles of unknown proteins can greatly enhance the predictability and success rates of synthetic biology projects. However, the lack of comprehensive large datasets and accurate ORF prediction tools for small prokaryotic proteins poses significant challenges to identifying unknown small proteins and regulatory factors. To overcome these obstacles, integrating proteogenomic analysis with ribosome profiling offers a powerful approach to discovering more small proteins [104]. Additionally, applying machine learning to identify unknown proteins and regulatory factors holds great potential for providing valuable insights for synthetic biology [105].
6.3. Enhancing Microbial Synergy via Building Artificial Coculture Systems
Microbial consortia—engineered communities of photosynthetic microbes and heterotrophic bacteria—offer enhanced metabolic exchange, nutrient recycling, and CO2 fixation compared to monocultures (Figure 5C). Engineering microbial consortia can overcome metabolic bottlenecks in single-strain systems by distributing the metabolic load across multiple strains, reducing the burden on any one strain for both growth and product synthesis [106]. Artificial coculture systems can be classified into several types based on functional interactions, including photoautotroph–heterotroph consortia, diazotrophic cyanobacteria–microalgae consortia, and microalgae–yeast/bacteria consortia. Several successful synthetic coculture models have been experimentally validated for biotechnological applications. Zhang et al. constructed an artificial cyanobacteria–E. coli coculture system, where the engineered Synechococcus 2973 provided fixed carbon for E. coli, enabling the production of 3-hydroxypropionic acid (3-HP) [9]. Xu et al. constructed a microalgae–yeast coculture system and found that both biomass and lipid yield were increased [107]. In addition, the artificial coculture system demonstrates excellent robustness and greater adaptability to changing environments. Take an example, Heys et al. found that various coculture systems, formed by combining sucrose-secreting cyanobacteria with three different heterotrophic bacteria (Saccharomyces cerevisiae, E. coli, or Bacillus subtilis), can be successfully established, demonstrating the robustness of autotrophic–heterotrophic mixed bacterial systems [108]. Moreover, Kruyer et al. demonstrated an artificial photosynthetic coculture system that produces rocket fuel and is capable of adapting to the harsh conditions of the Martian environment [109], a finding that was later confirmed by Ramalho et al. [110]. Diazotrophic cyanobacteria, such as Anabaena spp. and Nostoc spp., can fix atmospheric nitrogen, providing a sustainable nitrogen source for microalgae growth without the cost of chemical fertilizers [21]. The artificial coculture systems are likely to be further optimized through the careful consideration of metabolic compatibility, nutritional requirements, and environmental conditions, paving the way for broader applications.
6.4. Designing Photosynthetic Cell Factory Reactors for Large-Scale Production
The design of photosynthetic cell factory reactors is a critical step in optimizing photosynthesis, enhancing target product yields, and enabling sustainable production (Figure 5D). In practical applications, maintaining the stability and efficiency of photosynthetic cell factories in large-scale cultivation remains a critical challenge that needs to be addressed. For the study of photosynthesis in microalgae and cyanobacteria, the photobioreactor (PBR) represents a key technological advancement [111]. With the advancement of sensors, Internet of Things (IoT) technologies, and artificial intelligence (AI), future photobioreactors will become increasingly intelligent. They will possess the ability to conduct the real-time surveillance and modification of environmental variables, including temperature, light intensity, pH value, CO2 concentration, oxygen content, and more, ensuring that microalgae or cyanobacteria can perform photosynthesis under optimal growth conditions [112]. An automated control system can be established based on the growth cycle, dynamically optimizing parameters, such as gas input, light intensity, and temperature, based on real-time data to maximize biomass production and photosynthetic efficiency. In addition, future photobioreactors should focus more on the recovery and utilization of CO2, particularly in the treatment of industrial waste gases. The photosynthesis of microalgae and cyanobacteria can be harnessed to absorb and convert CO2, helping to reduce greenhouse gas emissions. Gaseous industrial wastes, for instance, those emitted by power plants, steel mills, and fertilizer plants, can be introduced into the photobioreactor, where microalgae’s photosynthesis transforms CO2 into biomass [113]. By optimizing the concentration and dissolution of CO2, the photosynthetic efficiency of microalgae can be further enhanced, particularly through the precise control of gas concentrations in closed reactors.
7. Conclusions
Photosynthetic microorganisms, such as microalgae and cyanobacteria, are attracting growing interest due to their prospects in eco-friendly biomanufacturing. Recent advancements in synthetic biology have successfully enhanced photosynthetic efficiency by engineering light-harvesting complexes, optimizing electron transport, and improving carbon fixation pathways. Experimental studies have demonstrated that targeted modifications, such as the rational design of antenna complexes, transcriptional control of photosynthetic genes, and metabolic engineering of carbon flux, can lead to measurable improvements in biomass productivity and biofuel yield. For instance, engineered strains of C. reinhardtii with optimized light-harvesting antenna sizes have exhibited double the biomass productivity of wild-type strains [33]. In cyanobacteria, the application of constraint-based modeling combined with machine learning has identified rate-limiting steps in the CBB cycle, leading to improved carbon fixation efficiencies [100]. Moreover, synthetic microbial coculture systems have demonstrated enhanced growth rates and product titers, as seen in engineered cyanobacteria-E. coli consortia that increased 3-HP production under photoautotrophic conditions [9]. While these successes illustrate the potential of synthetic biology, challenges remain, including the functional characterization of unknown proteins involved in photosynthesis and the optimization of growth-product trade-offs. The integration of AI-driven metabolic modeling and real-time bioprocess control is beginning to refine experimental design and strain engineering, though further validation is needed to confirm long-term scalability and industrial feasibility. As synthetic biology continues to merge with AI, multi-omics approaches, and advanced bioreactor designs, future developments will likely focus on the precise regulation of photosynthetic pathways, enhanced stress tolerance, and more efficient bioproduction systems. These innovations will not only boost the economic viability of photosynthetic biomanufacturing but also contribute to sustainable carbon capture and bioresource utilization.
Author Contributions
S.F., K.M. and X.S. wrote the draft of the manuscript; T.S., X.S., L.C. and W.Z. drafted and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by grants from the Ministry of Ecology and Environment Key Laboratory on Biodiversity and Biosafety and the National Key R&D Program of China (grant number 2024YFA0919700).
Data Availability Statement
All the data have been submitted, and there is no other available data and materials.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Abbreviations
The following abbreviations are used in this manuscript:
| 3-HB | 3-hydroxybutyric acid |
| 3-HV | 3-hydroxyvaleric acid |
| 3-HP | 3-hydroxypropionic acid |
| TAG | Triglycerides |
| ATP | Adenosine triphosphate |
| LHCs | Light-harvesting pigment–protein complexes |
| LHCRs | Chlorophyll a-binding proteins |
| PSI | Photosystem I |
| ABA | Abscisic acid |
| NAB1 | Nucleic acid binding 1 |
| CAO | Chlorophyllide a oxygenase |
| AP | Alkaline phosphatase |
| PSII | Photosystem II |
| PQ/PQH2 | Plastoquinone |
| DOP | Organophosphate |
| CCCP | Carbonyl cyanide m-chlorophenyl hydrazone |
| TCA cycle | Tricarboxylic acid cycle |
| CRISPRi | CRISPR interference |
| dCpf1 | dCas12a |
| PAR | Photosynthetically active radiation |
| CEF | Cyclic electron flow |
| NDH | NADH dehydrogenase-like complex |
| PGR5 | Proton gradient regulation 5 |
| PGRL1 | PGR5-like photosynthetic phenotype 1 |
| FL | Fluctuating light |
| NDA2 | Type II NAD(P)H dehydrogenase |
| Ci | Inorganic carbon |
| RBS | Ribosome binding site |
| CCM | CO2-concentrating mechanisms |
| CA | Carbonic anhydrase |
| CCMs | Carbon-concentrating mechanisms |
| Chl-a | Chlorophyll-a |
| RuBisCO | Ribulose-1,5-bisphosphate carboxylase/oxygenase |
| Raf1 | RuBisCO accumulation factor 1 |
| BN-PAGE | Blue native polyacrylamide gel electrophoresis |
| NOG | Nonoxidative cyclic glycolysis |
| ED | Entner-doudoroff |
| Rpe | Ribulose-5-phosphate-3-epimerase |
| Prk | Phosphoribulokinase |
| PBR | Photobioreactor |
| IoT | Internet of Things |
| LEF | Linear electron flow |
| PC | Plastocyanin |
| Fd | Ferredoxin |
| FNR | Ferredoxin-NADP(H) reductase |
| Cytb6f | Cytochrome b6-f complex. |
References
- Gustafson, A.; Marlon, J.R.; Goldberg, M.H.; Wang, X.; Ballew, M.T.; Rosenthal, S.A.; Leiserowitz, A. Blame where blame is due: Many Americans support suing fossil fuel companies for global warming damages. Environment 2020, 62, 30–35. [Google Scholar]
- Herrington, G. Update to limits to growth: Comparing the World3 model with empirical data. J. Ind. Ecol. 2021, 25, 614–626. [Google Scholar]
- Cui, J.; Sun, H.; Chen, R.; Sun, J.; Mo, G.; Luan, G.; Lu, X. Multiple routes toward engineering efficient cyanobacterial photosynthetic biomanufacturing technologies. Green Carbon 2023, 1, 210–226. [Google Scholar] [CrossRef]
- Zahra, Z.; Choo, D.H.; Lee, H.; Parveen, A. Cyanobacteria: Review of current potentials and applications. Environments 2020, 7, 13. [Google Scholar] [CrossRef]
- Rizwan, M.; Mujtaba, G.; Memon, S.A.; Lee, K.; Rashid, N. Exploring the potential of microalgae for new biotechnology applications and beyond: A review. Renew. Sustain. Energy Rev. 2018, 92, 394–404. [Google Scholar]
- Wijffels, R.H.; Kruse, O.; Hellingwerf, K.J. Potential of industrial biotechnology with cyanobacteria and eukaryotic microalgae. Curr. Opin. Biotechnol. 2013, 24, 405–413. [Google Scholar]
- Angermayr, S.A.; Hellingwerf, K.J.; Lindblad, P.; Teixeira de Mattos, M.J. Energy biotechnology with cyanobacteria. Curr. Opin. Biotechnol. 2009, 20, 257–263. [Google Scholar] [PubMed]
- Lu, X. A perspective: Photosynthetic production of fatty acid-based biofuels in genetically engineered cyanobacteria. Biotechnol. Adv. 2010, 28, 742–746. [Google Scholar]
- Zhang, L.; Chen, L.; Diao, J.; Song, X.; Shi, M.; Zhang, W. Construction and analysis of an artificial consortium based on the fast-growing cyanobacterium Synechococcus elongatus UTEX 2973 to produce the platform chemical 3-hydroxypropionic acid from CO2. Biotechnol. Biofuels 2020, 13, 82. [Google Scholar]
- Wang, X.; Chen, L.; Liu, J.; Sun, T.; Zhang, W. Light-driven biosynthesis of myo-Inositol directly from CO2 in Synechocystis sp. PCC 6803. Front. Microbiol. 2020, 11, 566117. [Google Scholar] [CrossRef]
- Song, X.; Wang, Y.; Diao, J.; Li, S.; Chen, L.; Zhang, W. Direct photosynthetic production of plastic building block chemicals from CO2. Adv. Exp. Med. Biol. 2018, 1080, 215–238. [Google Scholar]
- Diao, J.; Song, X.; Zhang, L.; Cui, J.; Chen, L.; Zhang, W. Tailoring cyanobacteria as a new platform for highly efficient synthesis of astaxanthin. Metab. Eng. 2020, 61, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Fayyaz, M.; Chew, K.W.; Show, P.L.; Ling, T.C.; Ng, I.-S.; Chang, J.-S. Genetic engineering of microalgae for enhanced biorefinery capabilities. Biotechnol. Adv. 2020, 43, 107554. [Google Scholar] [CrossRef]
- Chung, Y.-S.; Lee, J.-W.; Chung, C.-H. Molecular challenges in microalgae towards cost-effective production of quality biodiesel. Renew. Sustain. Energy Rev. 2017, 74, 139–144. [Google Scholar]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as sources of carotenoids. Mar. Drugs 2011, 9, 625–644. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams III, W.W. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 2149–2153. [Google Scholar] [CrossRef]
- Cordero, B.F.; Couso, I.; León, R.; Rodríguez, H.; Vargas, M.Á. Enhancement of carotenoids biosynthesis in Chlamydomonas reinhardtii by nuclear transformation using a phytoene synthase gene isolated from Chlorella zofingiensis. Appl. Microbiol. Biotechnol. 2011, 91, 341–351. [Google Scholar] [CrossRef]
- Spolaore, P.; Joannis-Cassan, C.; Duran, E.; Isambert, A. Commercial applications of microalgae. J. Biosci. Bioeng. 2006, 101, 87–96. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Vecchi, V.; Barera, S.; Bassi, R.; Dall’Osto, L. Potential and challenges of improving photosynthesis in algae. Plants 2020, 9, 67. [Google Scholar] [CrossRef]
- Llamas, A.; Leon-Miranda, E.; Tejada-Jimenez, M. Microalgal and nitrogen-fixing bacterial consortia: From interaction to biotechnological potential. Plants 2023, 12, 2476. [Google Scholar] [CrossRef] [PubMed]
- Melis, A. Solar energy conversion efficiencies in photosynthesis: Minimizing the chlorophyll antennae to maximize efficiency. Plant Sci. 2009, 177, 272–280. [Google Scholar]
- Xie, Y.; Khoo, K.S.; Chew, K.W.; Devadas, V.V.; Phang, S.J.; Lim, H.R.; Rajendran, S.; Show, P.L. Advancement of renewable energy technologies via artificial and microalgae photosynthesis. Bioresour. Technol. 2022, 363, 127830. [Google Scholar]
- Dong, H.; Li, F.; Xuan, X.; Ahiakpa, J.K.; Tao, J.; Zhang, X.; Ge, P.; Wang, Y.; Gai, W.; Zhang, Y. The genetic basis and improvement of photosynthesis in tomato. Hortic. Plant J. 2024, 11, 69–84. [Google Scholar]
- Arnon, D.I. The light reactions of photosynthesis. Proc. Natl. Acad. Sci. USA 1971, 68, 2883–2892. [Google Scholar] [CrossRef]
- Zhang, X.-E.; Liu, C.; Dai, J.; Yuan, Y.; Gao, C.; Feng, Y.; Wu, B.; Wei, P.; You, C.; Wang, X.; et al. Enabling technology and core theory of synthetic biology. Sci. China Life Sci. 2023, 66, 1742–1785. [Google Scholar]
- Pouvreau, B.; Vanhercke, T.; Singh, S. From plant metabolic engineering to plant synthetic biology: The evolution of the design/build/test/learn cycle. Plant Sci. 2018, 273, 3–12. [Google Scholar]
- Ke, J.; Wang, B.; Yoshikuni, Y. Microbiome engineering: Synthetic biology of plant-associated microbiomes in sustainable agriculture. Trends Biotechnol. 2021, 39, 244–261. [Google Scholar]
- Rourke, M. Access and benefit-sharing DNA Componentry for plant synthetic biology: Bioparts expressed in plant chassis. Plants People Planet. 2022, 4, 76–83. [Google Scholar]
- Lu, Y.; Gan, Q.; Iwai, M.; Alboresi, A.; Burlacot, A.; Dautermann, O.; Takahashi, H.; Crisanto, T.; Peltier, G.; Morosinotto, T.; et al. Role of an ancient light-harvesting protein of PSI in light absorption and photoprotection. Nat. Commun. 2021, 12, 679. [Google Scholar]
- Li, J.; Zhang, K.; Li, L.; Wang, Y.; Lin, S. Unsuspected functions of alkaline phosphatase PhoD in the diatom Phaeodactylum tricornutum. Algal Res. 2022, 68, 102873. [Google Scholar]
- Zhang, K.; Li, J.; Zhou, Z.; Huang, R.; Lin, S. Roles of alkaline phosphatase PhoA in algal metabolic regulation under phosphorus-replete conditions. J. Phycol. 2021, 57, 703–707. [Google Scholar] [CrossRef]
- Negi, S.; Perrine, Z.; Friedland, N.; Kumar, A.; Tokutsu, R.; Minagawa, J.; Berg, H.; Barry, A.N.; Govindjee, G.; Sayre, R. Light regulation of light-harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae. Plant J. 2020, 103, 584–603. [Google Scholar]
- Agarwal, A.; Di, R.; Falkowski, P.G. Light-harvesting complex gene regulation by a MYB-family transcription factor in the marine diatom, Phaeodactylum tricornutum. Photosynth. Res. 2022, 153, 59–70. [Google Scholar]
- Zhang, C.; Chen, J.; Yang, R.; Luo, Q.; Wang, T.; Zhang, P.; Chen, H. Abscisic acid activates desiccation tolerance responses in intertidal seaweed Neoporphyra haitanensis. Front. Mar. Sci. 2022, 9, 1007193. [Google Scholar]
- Fan, J.; Zhou, D.; Chen, C.; Wu, J.; Wu, H. Reprogramming the metabolism of Synechocystis PCC 6803 by regulating the plastoquinone biosynthesis. Synth. Syst. Biotechnol. 2021, 6, 351–359. [Google Scholar] [PubMed]
- Moore, V.; Vermaas, W. Functional consequences of modification of the photosystem I/photosystem II ratio in the cyanobacterium Synechocystis sp. PCC 6803. J. Bacteriol. 2024, 206, e00454-23. [Google Scholar]
- Knoot, C.J.; Biswas, S.; Pakrasi, H.B. Tunable repression of key photosynthetic processes using Cas12a CRISPR interference in the fast-growing cyanobacterium Synechococcus sp. UTEX 2973. ACS Synth. Biol. 2019, 9, 132–143. [Google Scholar]
- Balan, R.; Suraishkumar, G. Simultaneous increases in specific growth rate and specific lipid content of Chlorella vulgaris through UV-induced reactive species. Biotechnol. Prog. 2014, 30, 291–299. [Google Scholar]
- Jokel, M.; Johnson, X.; Peltier, G.; Aro, E.M.; Allahverdiyeva, Y. Hunting the main player enabling Chlamydomonas reinhardtii growth under fluctuating light. Plant J. 2018, 94, 822–835. [Google Scholar]
- Margulis, K.; Zer, H.; Lis, H.; Schoffman, H.; Murik, O.; Shimakawa, G.; Krieger-Liszkay, A.; Keren, N. Over expression of the cyanobacterial Pgr5-homologue leads to pseudoreversion in a gene coding for a putative esterase in Synechocystis 6803. Life 2020, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Selão, T.T.; Jebarani, J.; Ismail, N.A.; Norling, B.; Nixon, P.J. Enhanced production of D-lactate in cyanobacteria by re-routing photosynthetic cyclic and pseudo-cyclic electron flow. Front. Plant Sci. 2020, 10, 1700. [Google Scholar]
- Baltz, A.; Dang, K.-V.; Beyly, A.; Auroy, P.; Richaud, P.; Cournac, L.; Peltier, G. Plastidial expression of type II NAD (P) H dehydrogenase increases the reducing state of plastoquinones and hydrogen photoproduction rate by the indirect pathway in Chlamydomonas reinhardtii. Plant Physiol. 2014, 165, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Srivastava, S. The effect of promoter and rbs combination on the growth and glycogen productivity of sodium-dependent bicarbonate transporter (SbtA) overexpressing Synechococcus sp. PCC 7002 cells. Front. Microbiol. 2021, 12, 607411. [Google Scholar]
- Wang, C.; Sun, B.; Zhang, X.; Huang, X.; Zhang, M.; Guo, H.; Chen, X.; Huang, F.; Chen, T.; Mi, H.; et al. Structural mechanism of the active bicarbonate transporter from cyanobacteria. Nat. Plants 2019, 5, 1184–1193. [Google Scholar]
- Lee, H.J.; Choi, J.-i.; Woo, H.M. Biocontainment of engineered Synechococcus elongatus PCC 7942 for photosynthetic production of α-farnesene from CO2. J Agric Food Chem 2021, 69, 698–703. [Google Scholar] [PubMed]
- Huang, F.; Kong, W.W.; Sun, Y.; Chen, T.; Dykes, G.F.; Jiang, Y.L.; Liu, L.N. Rubisco accumulation factor 1 (Raf1) plays essential roles in mediating Rubisco assembly and carboxysome biogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 17418–17428. [Google Scholar]
- Bolay, P.; Schlüter, S.; Grimm, S.; Riediger, M.; Hess, W.R.; Klähn, S. The transcriptional regulator RbcR controls ribulose-1, 5-bisphosphate carboxylase/oxygenase (RuBisCO) genes in the cyanobacterium Synechocystis sp. PCC 6803. New Phytol. 2022, 235, 432–445. [Google Scholar] [CrossRef]
- Lu, K.-J.; Chang, C.-W.; Wang, C.-H.; Chen, F.Y.-H.; Huang, I.Y.; Huang, P.-H.; Yang, C.-H.; Wu, H.-Y.; Wu, W.-J.; Hsu, K.-C.; et al. An ATP-sensitive phosphoketolase regulates carbon fixation in cyanobacteria. Nat. Metab. 2023, 5, 1111–1126. [Google Scholar]
- Fan, W.; Liu, Y.; Xu, X.; Dong, X.; Wang, H. Effects of HCO3− and CO2 conversion rates on carbon assimilation strategies in marine microalgae: Implication by stable carbon isotope analysis of fatty acids. Plant Physiol. Biochem. 2024, 209, 108530. [Google Scholar] [CrossRef]
- Lin, W.R.; Lai, Y.C.; Sung, P.K.; Tan, S.I.; Chang, C.H.; Chen, C.Y.; Chang, J.S.; Ng, I.S. Enhancing carbon capture and lipid accumulation by genetic carbonic anhydrase in microalgae. J. Taiwan Inst. Chem. Eng. 2018, 93, 131–141. [Google Scholar]
- Song, X.; Diao, J.; Yao, J.; Cui, J.; Sun, T.; Chen, L.; Zhang, W. Engineering a central carbon metabolism pathway to increase the intracellular acetyl-CoA pool in Synechocystis sp. PCC 6803 grown under photomixotrophic conditions. ACS Synth. Biol. 2021, 10, 836–846. [Google Scholar]
- Pressley, S.R.; Gonzales, J.N.; Atsumi, S. Efficient utilization of xylose requires CO2 fixation in Synechococcus elongatus PCC 7942. Metab. Eng. 2024, 86, 115–123. [Google Scholar] [PubMed]
- Yao, S.; Cao, X.; Wang, Y.; Li, D.; Wang, W.; Chen, R.; Li, C. Enhancing overall carbon fixation and energy conversion with formate in green microalga Chlamydomonas reinhardtii. Algal Res. 2023, 72, 103108. [Google Scholar] [CrossRef]
- Yoshihara, K.; Kumazaki, S. Primary processes in plant photosynthesis: Photosystem I reaction center. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 22–32. [Google Scholar]
- Shevela, D.; Kern, J.F.; Govindjee, G.; Messinger, J. Solar energy conversion by photosystem II: Principles and structures. Photosynth. Res. 2023, 156, 279–307. [Google Scholar]
- Hitchcock, A.; Hunter, C.N.; Sobotka, R.; Komenda, J.; Dann, M.; Leister, D. Redesigning the photosynthetic light reactions to enhance photosynthesis–the PhotoRedesign consortium. Plant J. 2022, 109, 23–34. [Google Scholar] [PubMed]
- Mussgnug, J.H.; Wobbe, L.; Elles, I.; Claus, C.; Hamilton, M.; Fink, A.; Kahmann, U.; Kapazoglou, A.; Mullineaux, C.W.; Hippler, M.; et al. NAB1 is an RNA binding protein involved in the light-regulated differential expression of the light-harvesting antenna of Chlamydomonas reinhardtii. Plant Cell 2005, 17, 3409–3421. [Google Scholar]
- Stra, A.; Almarwaey, L.O.; Alagoz, Y.; Moreno, J.C.; Al-Babili, S. Carotenoid metabolism: New insights and synthetic approaches. Front. Plant Sci. 2023, 13, 1072061. [Google Scholar] [CrossRef]
- Nikkanen, L.; Solymosi, D.; Jokel, M.; Allahverdiyeva, Y. Regulatory electron transport pathways of photosynthesis in cyanobacteria and microalgae: Recent advances and biotechnological prospects. Physiol. Plant. 2021, 173, 514–525. [Google Scholar] [CrossRef]
- Allahverdiyeva, Y.; Suorsa, M.; Tikkanen, M.; Aro, E.-M. Photoprotection of photosystems in fluctuating light intensities. J. Exp. Bot. 2015, 66, 2427–2436. [Google Scholar]
- Allen, J.F. Photosynthesis of ATP—Electrons, proton pumps, rotors, and poise. Cell 2002, 110, 273–276. [Google Scholar] [CrossRef]
- Chow, W.S.; Melis, A.; Anderson, J. Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc. Natl. Acad. Sci. USA 1990, 87, 7502–7506. [Google Scholar] [CrossRef]
- Fujimori, T.; Higuchi, M.; Sato, H.; Aiba, H.; Muramatsu, M.; Hihara, Y.; Sonoike, K. The mutant of sll1961, which encodes a putative transcriptional regulator, has a defect in regulation of photosystem stoichiometry in the cyanobacterium Synechocystis sp. PCC 6803. Plant Physiol. 2005, 139, 408–416. [Google Scholar] [CrossRef]
- Nawrocki, W.J.; Bailleul, B.; Picot, D.; Cardol, P.; Rappaport, F.; Wollman, F.A.; Joliot, P. The mechanism of cyclic electron flow. Biochim. Biophys. Acta Bioenerg. 2019, 1860, 433–438. [Google Scholar] [CrossRef]
- Alric, J.; Johnson, X. Alternative electron transport pathways in photosynthesis: A confluence of regulation. Curr. Opin. Plant Biol. 2017, 37, 78–86. [Google Scholar]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Annu. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Labs, M.; Rühle, T.; Leister, D. The antimycin A-sensitive pathway of cyclic electron flow: From 1963 to 2015. Photosynth. Res. 2016, 129, 231–238. [Google Scholar] [CrossRef]
- Tagawa, K.; Tsujimoto, H.Y.; Arnon, D.I. Role of chloroplast ferredoxin in the energy conversion process of photosynthesis. Proc. Natl. Acad. Sci. USA 1963, 49, 567–572. [Google Scholar] [CrossRef]
- Yamamoto, H.; Peng, L.; Fukao, Y.; Shikanai, T. An Src homology 3 domain-like fold protein forms a ferredoxin binding site for the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant Cell 2011, 23, 1480–1493. [Google Scholar] [CrossRef]
- Schuller, J.M.; Birrell, J.A.; Tanaka, H.; Konuma, T.; Wulfhorst, H.; Cox, N.; Schuller, S.K.; Thiemann, J.; Lubitz, W.; Sétif, P.; et al. Structural adaptations of photosynthetic complex I enable ferredoxin-dependent electron transfer. Science 2019, 363, 257–260. [Google Scholar]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- DalCorso, G.; Pesaresi, P.; Masiero, S.; Aseeva, E.; Schünemann, D.; Finazzi, G.; Joliot, P.; Barbato, R.; Leister, D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in arabidopsis. Cell 2008, 132, 273–285. [Google Scholar] [CrossRef]
- Dann, M.; Leister, D. Evidence that cyanobacterial Sll1217 functions analogously to PGRL1 in enhancing PGR5-dependent cyclic electron flow. Nat. Commun. 2019, 10, 5299. [Google Scholar]
- Jans, F.; Mignolet, E.; Houyoux, P.-A.; Cardol, P.; Ghysels, B.; Cuiné, S.; Cournac, L.; Peltier, G.; Remacle, C.; Franck, F. A type II NAD(P)H dehydrogenase mediates light-independent plastoquinone reduction in the chloroplast of Chlamydomonas. Proc. Natl. Acad. Sci. USA 2008, 105, 20546–20551. [Google Scholar]
- Alric, J. Cyclic electron flow around photosystem I in unicellular green algae. Photosynth. Res. 2010, 106, 47–56. [Google Scholar]
- Champigny, M.L. Regulation of photosynthetic carbon assimilation at the cellular level: A review. Photosynth. Res. 1985, 6, 273–286. [Google Scholar]
- Singh, S.K.; Sundaram, S.; Sinha, S.; Rahman, M.A.; Kapur, S. Recent advances in CO2 uptake and fixation mechanism of cyanobacteria and microalgae. Crit. Rev. Environ. Sci. Technol. 2016, 46, 1297–1323. [Google Scholar]
- Tomar, V.; Sidhu, G.K.; Nogia, P.; Mehrotra, R.; Mehrotra, S. Regulatory components of carbon concentrating mechanisms in aquatic unicellular photosynthetic organisms. Plant Cell Rep. 2017, 36, 1671–1688. [Google Scholar]
- Carmo-Silva, E.; Scales, J.C.; Madgwick, P.J.; Parry, M.A. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ. 2015, 38, 1817–1832. [Google Scholar]
- Hanson, M.R.; Lin, M.T.; Carmo-Silva, A.E.; Parry, M.A. Towards engineering carboxysomes into C3 plants. Plant J. 2016, 87, 38–50. [Google Scholar] [CrossRef]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and metabolic maintenance of Rubisco. Annu. Rev. Plant Biol. 2017, 68, 29–60. [Google Scholar] [CrossRef]
- Mondal, M.; Khanra, S.; Tiwari, O.; Gayen, K.; Halder, G. Role of carbonic anhydrase on the way to biological carbon capture through microalgae—A mini review. Environ. Prog. Sustain. Energy 2016, 35, 1605–1615. [Google Scholar] [CrossRef]
- Matson, M.M.; Atsumi, S. Photomixotrophic chemical production in cyanobacteria. Curr. Opin. Biotechnol. 2018, 50, 65–71. [Google Scholar] [CrossRef]
- Luan, G.; Zhang, S.; Wang, M.; Lu, X. Progress and perspective on cyanobacterial glycogen metabolism engineering. Biotechnol. Adv. 2019, 37, 771–786. [Google Scholar] [CrossRef]
- Lee, T.C.; Xiong, W.; Paddock, T.; Carrieri, D.; Chang, F.; Chiu, H.F.; Ungerer, J.; Juo, S.H.H.; Maness, P.C.; Yu, J. Engineered xylose utilization enhances bio-products productivity in the cyanobacterium Synechocystis sp. PCC 6803. Metab. Eng. 2015, 30, 179–189. [Google Scholar] [CrossRef]
- McEwen, J.T.; Kanno, M.; Atsumi, S. 2, 3 Butanediol production in an obligate photoautotrophic cyanobacterium in dark conditions via diverse sugar consumption. Metab. Eng. 2016, 36, 28–36. [Google Scholar] [CrossRef]
- Moon, M.; Kim, C.W.; Park, W.K.; Yoo, G.; Choi, Y.E.; Yang, J.W. Mixotrophic growth with acetate or volatile fatty acids maximizes growth and lipid production in Chlamydomonas reinhardtii. Algal Res. 2013, 2, 352–357. [Google Scholar] [CrossRef]
- Bellido-Pedraza, C.M.; Torres, M.J.; Llamas, A. The Microalgae Chlamydomonas for Bioremediation and Bioproduct Production. Cells 2024, 13, 1137. [Google Scholar] [CrossRef]
- Kirst, H.; Garcia-Cerdan, J.G.; Zurbriggen, A.; Ruehle, T.; Melis, A. Truncated photosystem chlorophyll antenna size in the green microalga Chlamydomonas reinhardtii upon deletion of the TLA3-CpSRP43 gene. Plant Physiol. 2012, 160, 2251–2260. [Google Scholar] [CrossRef]
- Nickelsen, J.; Rengstl, B. Photosystem II assembly: From cyanobacteria to plants. Annu. Rev. Plant Biol. 2013, 64, 609–635. [Google Scholar] [PubMed]
- Krauspe, V.; Fahrner, M.; Spät, P.; Steglich, C.; Frankenberg-Dinkel, N.; Maček, B.; Schilling, O.; Hess, W.R. Discovery of a small protein factor involved in the coordinated degradation of phycobilisomes in cyanobacteria. Proc. Natl. Acad. Sci. USA 2021, 118, e2012277118. [Google Scholar]
- Wu, G.; Yan, Q.; Jones, J.A.; Tang, Y.J.; Fong, S.S.; Koffas, M.A.G. Metabolic burden: Cornerstones in synthetic biology and metabolic engineering applications. Trends Biotechnol. 2016, 34, 652–664. [Google Scholar]
- Li, Y.; Han, D.; Hu, G.; Sommerfeld, M.; Hu, Q. Inhibition of starch synthesis results in overproduction of lipids in Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2010, 107, 258–268. [Google Scholar] [PubMed]
- Ruffing, A.M.; Jones, H.D.T. Physiological effects of free fatty acid production in genetically engineered Synechococcus elongatus PCC 7942. Biotechnol. Bioeng. 2012, 109, 2190–2199. [Google Scholar] [CrossRef] [PubMed]
- Benner, P.; Meier, L.; Pfeffer, A.; Krüger, K.; Oropeza Vargas, J.E.; Weuster-Botz, D. Lab-scale photobioreactor systems: Principles, applications, and scalability. Bioprocess Biosyst. Eng. 2022, 45, 791–813. [Google Scholar]
- Hincapie, E.; Stuart, B.J. Design, construction, and validation of an internally lit air-lift photobioreactor for growing algae. Front. Energy Res. 2015, 2, 65. [Google Scholar]
- Li, S.; Sun, T.; Chen, L.; Zhang, W. Light and carbon dioxide-driven synthesis of high-density fuel in Synechococcus elongates UTEX 2973. Sheng Wu Gong Cheng Xue Bao = Chin. J. Biotechnol. 2020, 36, 2126–2138. [Google Scholar]
- Wang, Z.; Peng, X.; Xia, A.; Shah, A.A.; Huang, Y.; Zhu, X.; Zhu, X.; Liao, Q. The role of machine learning to boost the bioenergy and biofuels conversion. Bioresour. Technol. 2022, 343, 126099. [Google Scholar]
- Kugler, A.; Stensjö, K. Machine learning predicts system-wide metabolic flux control in cyanobacteria. Metab. Eng. 2024, 82, 171–182. [Google Scholar]
- Long, B.; Fischer, B.; Zeng, Y.; Amerigian, Z.; Li, Q.; Bryant, H.; Li, M.; Dai, S.Y.; Yuan, J.S. Machine learning-informed and synthetic biology-enabled semi-continuous algal cultivation to unleash renewable fuel productivity. Nat. Commun. 2022, 13, 541. [Google Scholar] [CrossRef] [PubMed]
- Coşgun, A.; Günay, M.E.; Yıldırım, R. Exploring the critical factors of algal biomass and lipid production for renewable fuel production by machine learning. Renew. Energy 2021, 163, 1299–1317. [Google Scholar] [CrossRef]
- Nishiguchi, H.; Hiasa, N.; Uebayashi, K.; Liao, J.; Shimizu, H.; Matsuda, F. Transomics data-driven, ensemble kinetic modeling for system-level understanding and engineering of the cyanobacteria central metabolism. Metab. Eng. 2019, 52, 273–283. [Google Scholar] [CrossRef]
- Cao, X.; Khitun, A.; Harold, C.M.; Bryant, C.J.; Zheng, S.-J.; Baserga, S.J.; Slavoff, S.A. Nascent alt-protein chemoproteomics reveals a pre-60S assembly checkpoint inhibitor. Nat. Chem. Biol. 2022, 18, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.J.; Shin, M.S.; Oh, Y.J.; Oh, H.S.; Ryu, K.H. Identification of protein functions using a machine-learning approach based on sequence-derived properties. Proteome Sci. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Matuszyńska, A.; Ebenhöh, O.; Zurbriggen, M.D.; Ducat, D.C.; Axmann, I.M. A new era of synthetic biology—Microbial community design. Synth. Biol. 2024, 9, ysae011. [Google Scholar]
- Xu, Z.; Theodoropoulos, C.; Pittman, J.K. Optimization of a Chlorella–Saccharomyces co–culture system for enhanced metabolite productivity. Algal Res. 2024, 79, 103455. [Google Scholar] [CrossRef]
- Hays, S.G.; Yan, L.L.; Silver, P.A.; Ducat, D.C. Synthetic photosynthetic consortia define interactions leading to robustness and photoproduction. J. Biol. Eng. 2017, 11, 1–14. [Google Scholar] [CrossRef]
- Kruyer, N.S.; Realff, M.J.; Sun, W.; Genzale, C.L.; Peralta-Yahya, P. Designing the bioproduction of Martian rocket propellant via a biotechnology-enabled in situ resource utilization strategy. Nat. Commun. 2021, 12, 6166. [Google Scholar] [CrossRef]
- Ramalho, T.P.; Chopin, G.; Salman, L.; Baumgartner, V.; Heinicke, C.; Verseux, C. On the growth dynamics of the cyanobacterium Anabaena sp. PCC 7938 in Martian regolith. Npj Microgravity 2022, 8, 43. [Google Scholar]
- Penloglou, G.; Pavlou, A.; Kiparissides, C. Recent advancements in photo-bioreactors for microalgae cultivation: A brief overview. Processes 2024, 12, 1104. [Google Scholar] [CrossRef]
- Tummawai, T.; Rohitatisha Srinophakun, T.; Padungthon, S.; Sukpancharoen, S. Application of artificial intelligence and image processing for the cultivation of Chlorella sp. using tubular photobioreactors. ACS Omega 2024, 9, 46017–46029. [Google Scholar] [PubMed]
- Molazadeh, M.; Ahmadzadeh, H.; Pourianfar, H.R.; Lyon, S.; Rampelotto, P.H. The use of microalgae for coupling wastewater treatment with CO2 biofixation. Front. Bioeng. Biotechnol. 2019, 7, 42. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).