Chicken Primordial Germ Cells Do Not Proliferate in Insulin-Lacking Media
Abstract
1. Introduction
2. Results
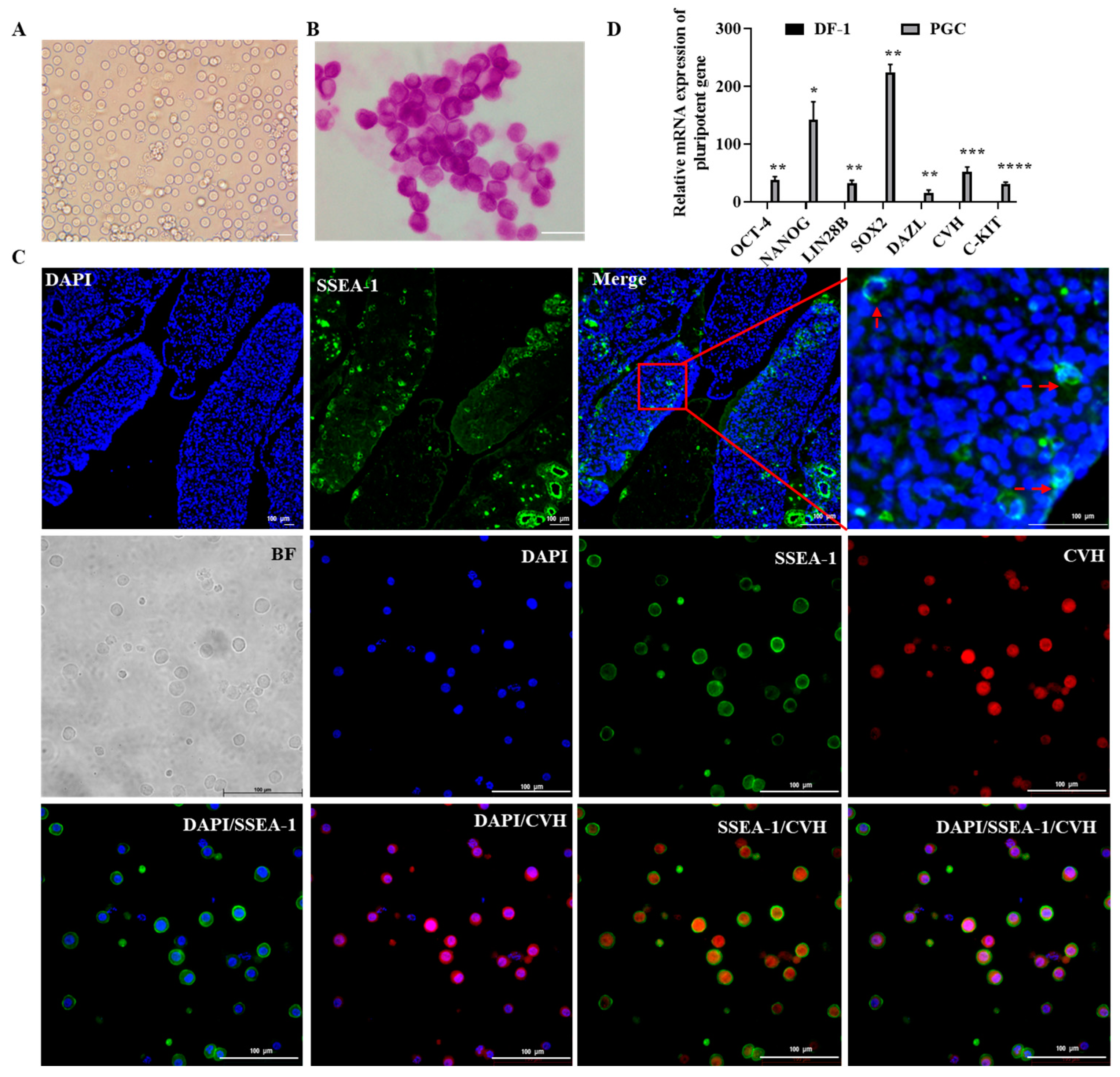
2.1. Culture and Characterization of Chicken Primordial Germ Cells
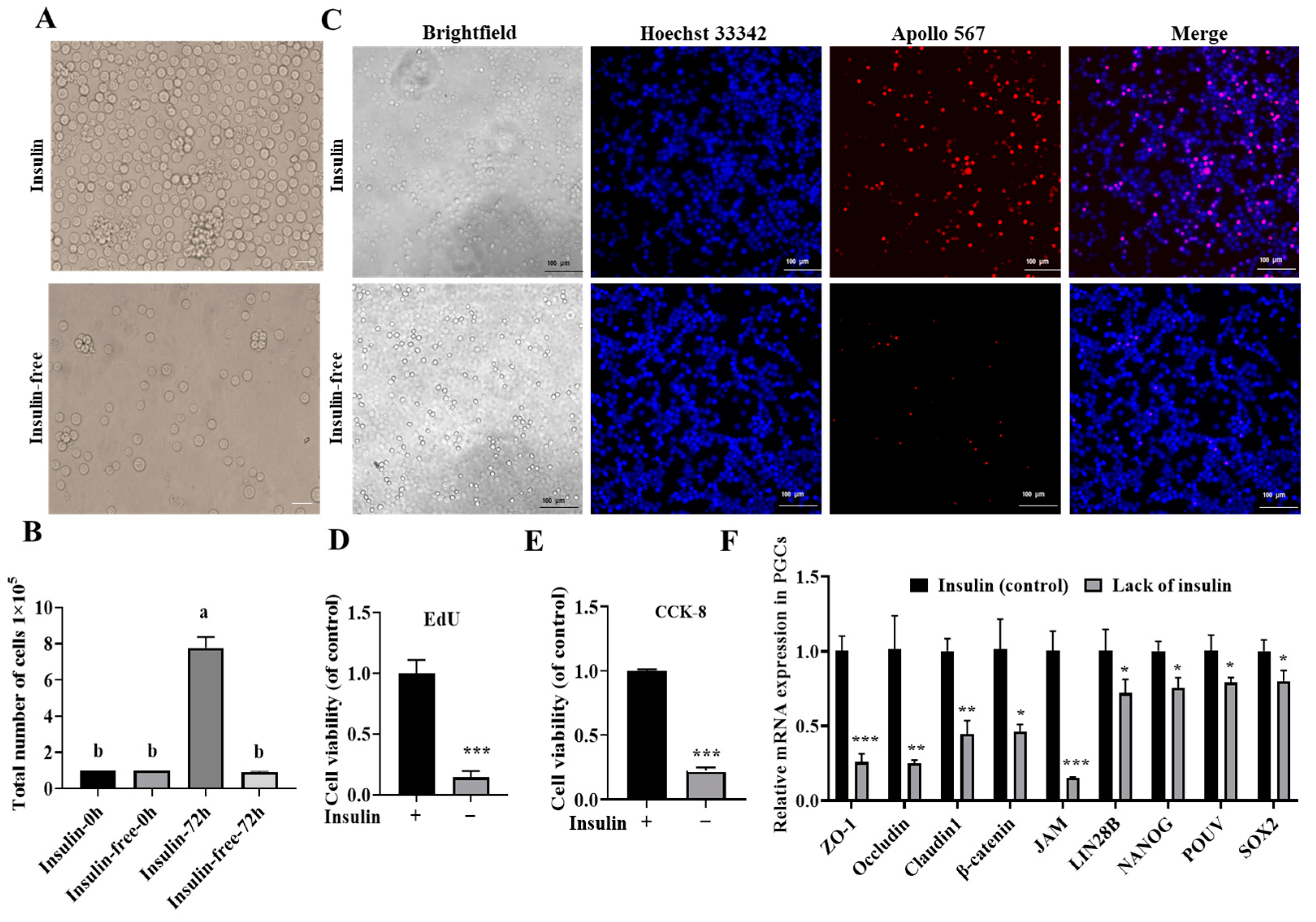
2.2. Insulin Is Indispensable for the Long-Term In Vitro Culture of PGCs
2.3. Non-Insulin Media Leads to Decreased Expression of Pluripotency Genes
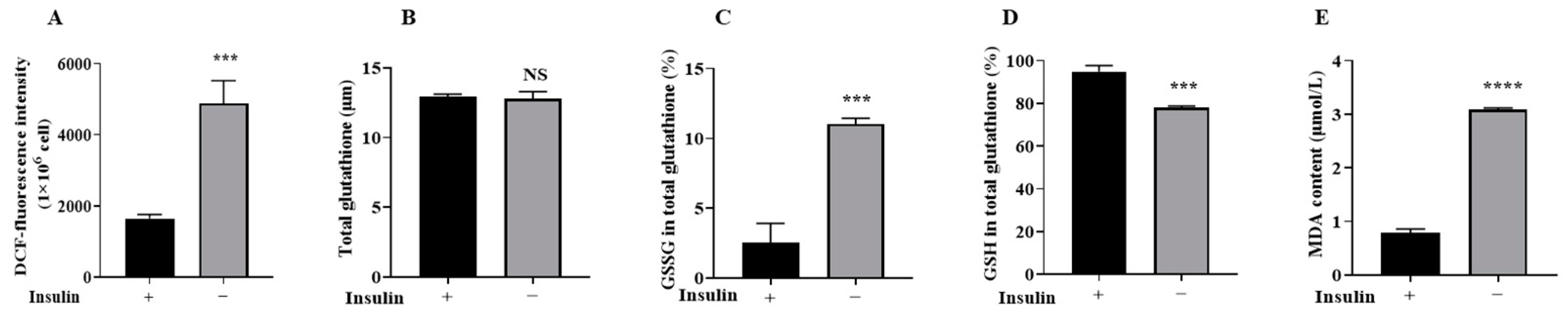
2.4. Non-Insulin Media Reduces Redox Capacity in PGCs
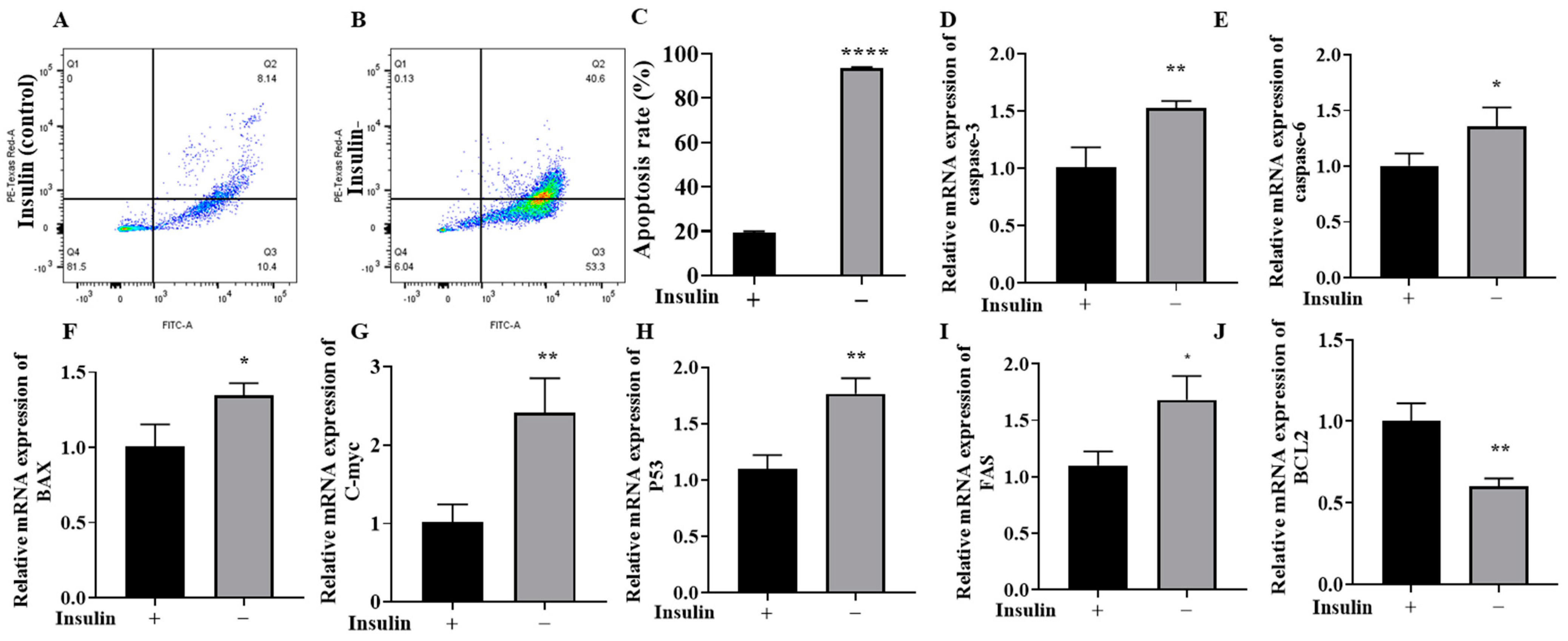
2.5. Non-Insulin Media Can Induce Apoptosis in PGCs
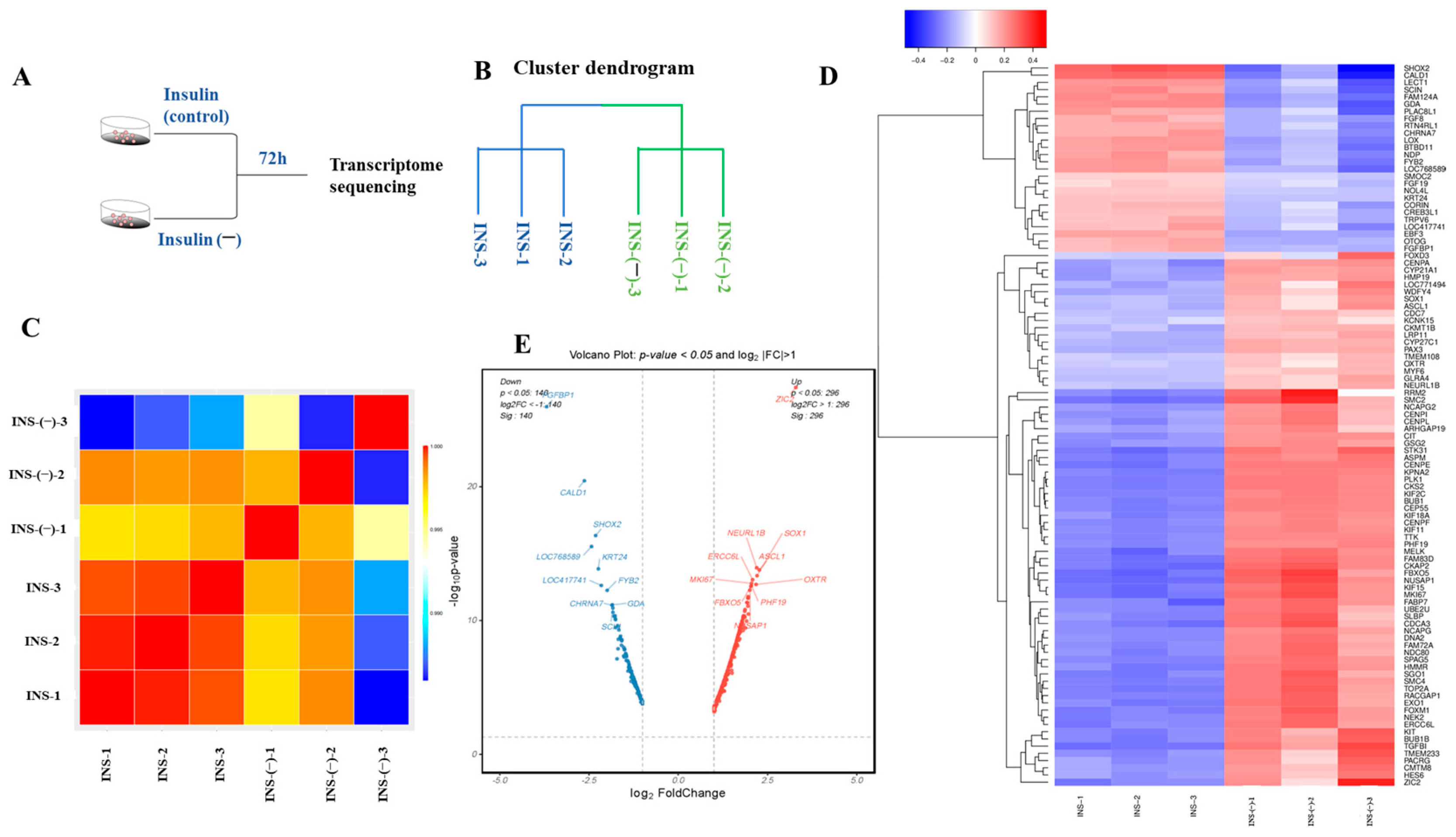
2.6. RNA-Seq Assessment of the Quality of the Data
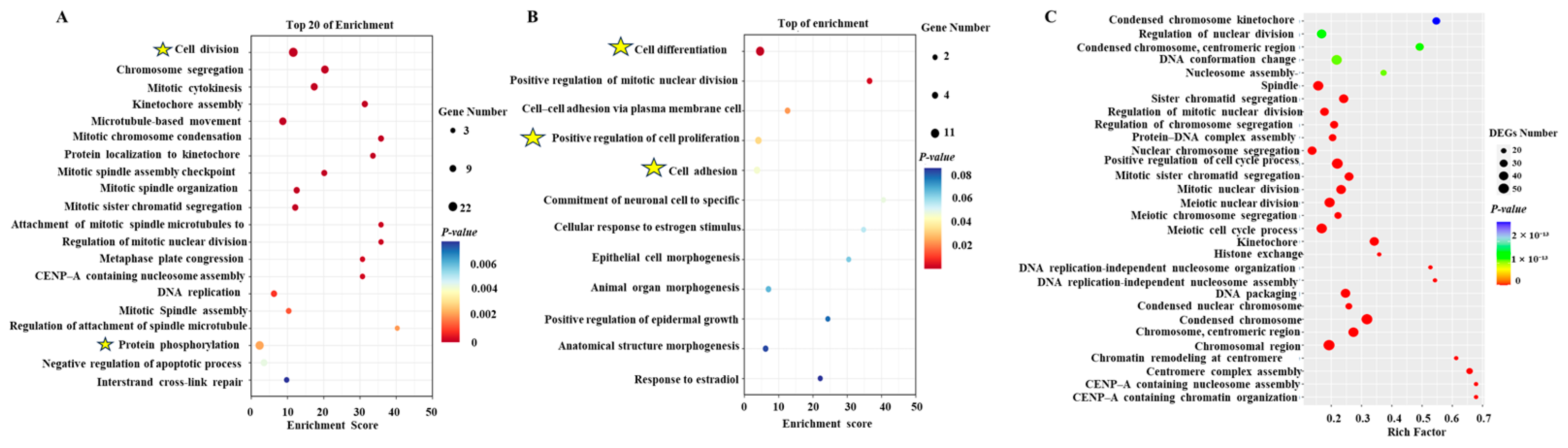
2.7. Enrichment Analysis of DEGs
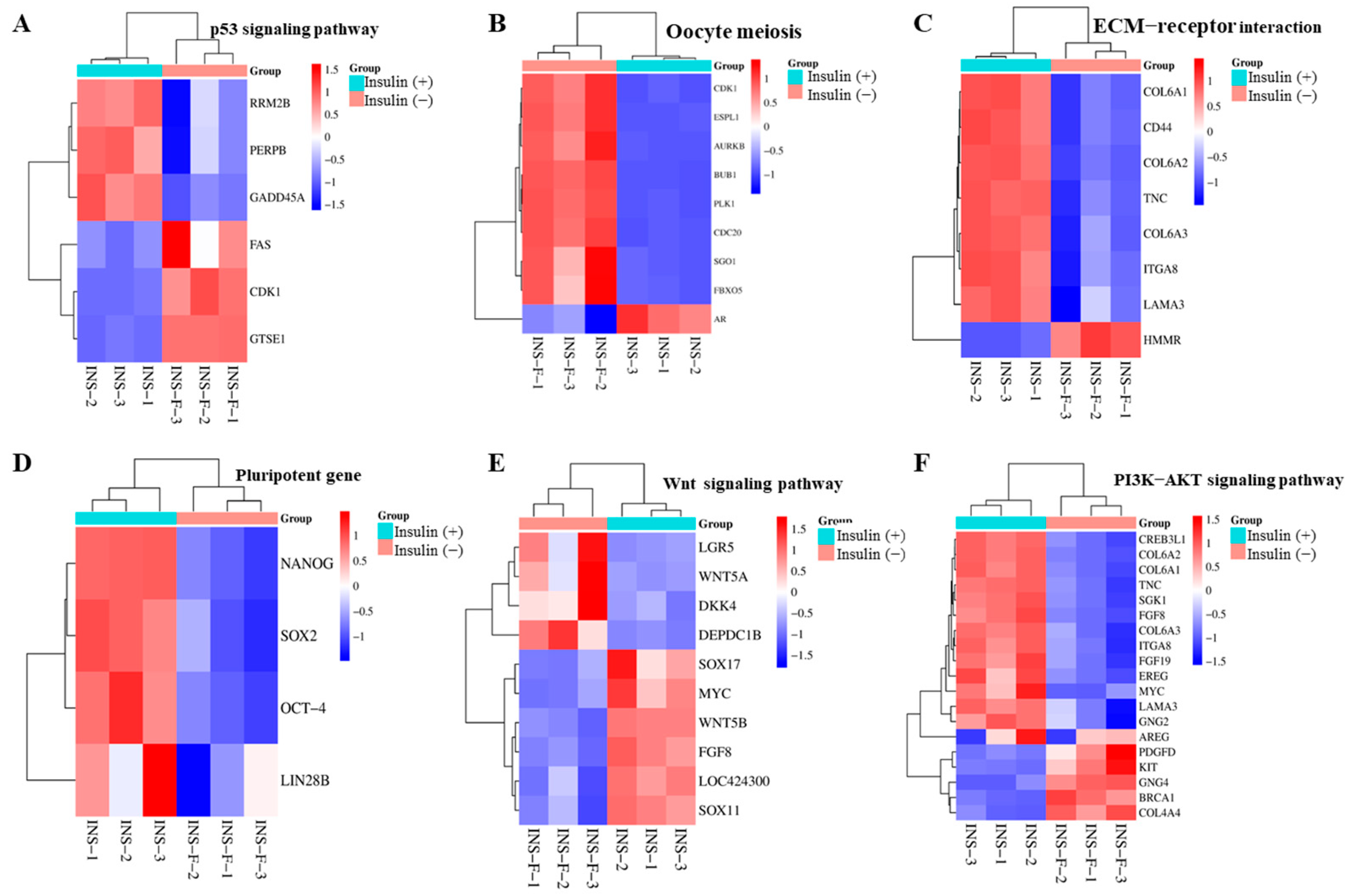
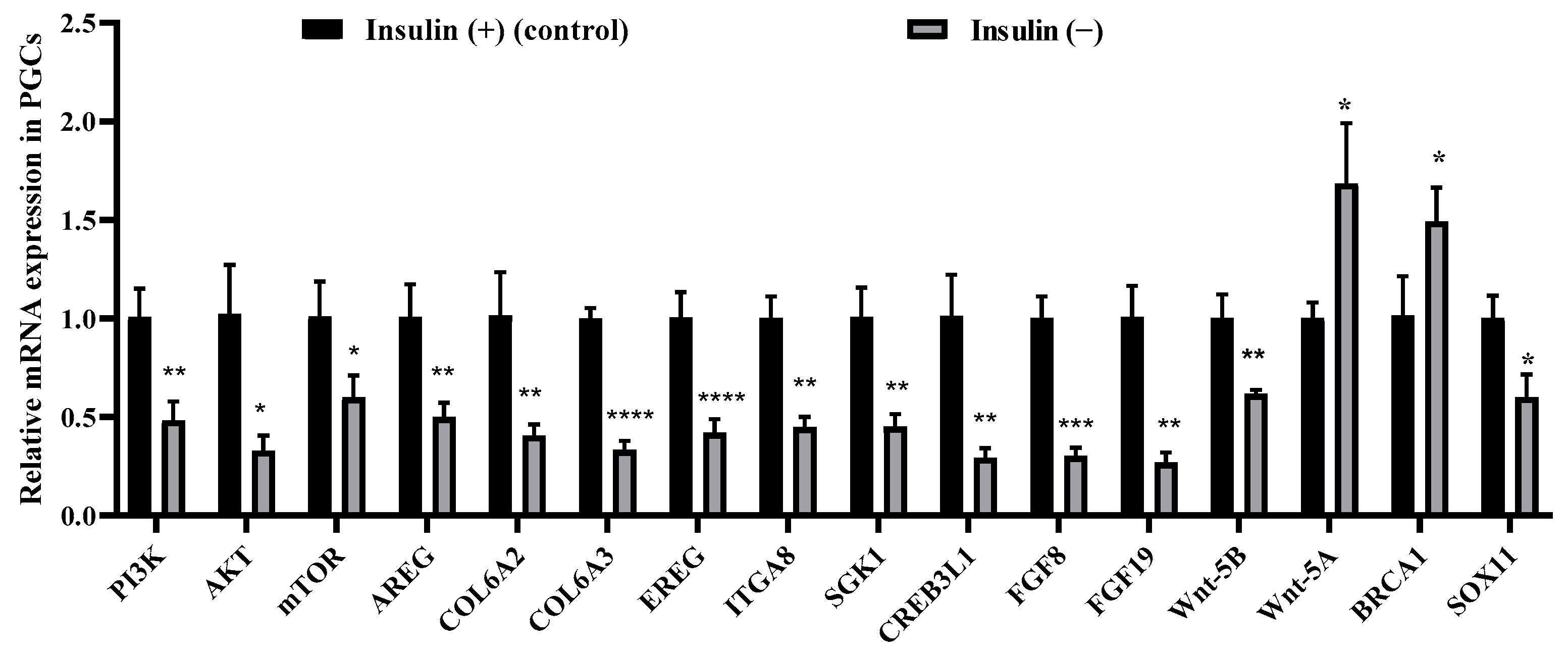
2.8. Non-Insulin Media Affect the PI3K/AKT and Wnt Signaling Pathways in PGCs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. PGC Culture Conditions
5.2. Periodic Acid–Schiff Stain
5.3. Immunohistochemistry
5.4. Experimental Design
5.5. Cell Counting Kit-8 (CCK-8) Assay
5.6. EdU Proliferation Assay
5.7. RNA Extraction and Real-Time Quantitative PCR (RT–qPCR)
5.8. Detection of Apoptosis by the FITC/PI Double-Staining Method
5.9. Reactive Oxygen Species Assay
5.10. Assay for GSH/GSSG/MDA
5.11. RNA Extraction, Library Preparation, and Illumina HiSeq Sequencing
5.12. Differential Expression Analysis and Functional Enrichment
5.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farzaneh, M.; Khoshnam, S.E.; Nokhbatolfoghahai, M. First scientific record of two cases of partial twinning in the chick embryo, Gallus gallus domesticus. Vet. Rec. Case Rep. 2016, 4, e000353. [Google Scholar]
- Trefil, P.; Aumann, D.; Koslová, A.; Mucksová, J.; Benešová, B.; Kalina, J.; Wurmser, C.; Fries, R.; Elleder, D.; Schusser, B.; et al. Male fertility restored by transplanting primordial germ cells into testes: A new way towards efficient transgenesis in chicken. Sci. Rep. 2017, 7, 14246. [Google Scholar]
- Han, J.Y.; Park, Y.H. Primordial germ cell-mediated transgenesis and genome editing in birds. J. Anim. Ence Biotechnol. 2018, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, M.E.; Gheyas, A.A.; Mason, A.S.; Nandi, S.; Taylor, L.; Sherman, A.; Smith, J.; Burt, D.W.; Hawken, R.; McGrew, M.J. Reviving rare chicken breeds using genetically engineered sterility in surrogate host birds. Proc. Natl. Acad. Sci. USA 2019, 116, 20930–20937. [Google Scholar] [PubMed]
- Whyte, J.; Glover, J.D.; Woodcock, M.; Brzeszczynska, J.; Taylor, L.; Sherman, A.; Kaiser, P.; McGrew, M.J. FGF, Insulin, and SMAD Signaling Cooperate for Avian Primordial Germ Cell Self-Renewal. Stem Cell Rep. 2015, 5, 1171–1182. [Google Scholar]
- Ramos, D.M.; Abdulmalik, S.; Arul, M.R.; Sardashti, N.; Banasavadi-Siddegowda, Y.K.; Nukavarapu, S.P.; Drissi, H.; Kumbar, S.G. Insulin-Functionalized Bioactive Fiber Matrices with Bone Marrow-Derived Stem Cells in Rat Achilles Tendon Regeneration. ACS Appl. Bio Mater. 2022, 5, 2851–2861. [Google Scholar]
- Li, P.; Wei, J.; Gao, X.; Wei, B.; Lin, H.; Huang, R.; Niu, Y.; Lim, K.; Jing, K.; Chu, J. Insulin Promotes the Proliferation of Human Umbilical Cord Matrix-Derived Mesenchymal Stem Cells by Activating the Akt-Cyclin D1 Axis. Stem Cells Int. 2017, 2017, 7371615. [Google Scholar]
- Yao, Y.; Zhai, Z.; Wang, Y. Evaluation of Insulin Medium or Chondrogenic Medium on Proliferation and Chondrogenesis of ATDC5 Cells. BioMed Res. Int. 2014, 2014, 569241. [Google Scholar]
- Chen, G.; Gulbranson, D.R.; Hou, Z.; Bolin, J.M.; Ruotti, V.; Probasco, M.D.; Smuga-Otto, K.; Howden, S.E.; Diol, N.R.; Propson, N.E.; et al. Chemically defined conditions for human iPSC derivation and culture HHS public access. Nat. Methods 2011, 8, 424–429. [Google Scholar] [CrossRef]
- Cañón, S.; Herranz, C.; Manzanares, M. Germ cell restricted expression of chick Nanog. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 2889–2894. [Google Scholar]
- Lavial, F.; Acloque, H.; Bertocchini, F.; Macleod, D.J.; Boast, S.; Bachelard, E.; Montillet, G.; Thenot, S.; Sang, H.M.; Stern, C.D.; et al. The Oct4 homologue PouV and Nanog regulate pluripotency in chicken embryonic stem cells. Development 2007, 134, 3549–3563. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Alev, C.; Wu, Y.; Nagai, H.; Sheng, G. Activin/TGF-beta signaling regulates Nanog expression in the epiblast during gastrulation. Mech. Dev. 2011, 128, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Okuzaki, Y.; Kaneoka, H.; Suzuki, T.; Hagihara, Y.; Nakayama, Y.; Murakami, S.; Murase, Y.; Kuroiwa, A.; Iijima, S.; Nishijima, K.I. PRDM14 and BLIMP1 control the development of chicken primordial germ cells. Dev. Biol. 2019, 455, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Omasa, T. What Kind of Signaling Maintains Pluripotency and Viability in Human-Induced Pluripotent Stem Cells Cultured on Laminin-511 with Serum-Free Medium? BioResearch Open Access 2016, 5, 84–93. [Google Scholar] [CrossRef]
- Ahmed, M.; Ffrench-Constant, C. Extracellular Matrix Regulation of Stem Cell Behavior. Curr. Stem Cell Rep. 2016, 2, 197–206. [Google Scholar]
- Li, L.; Bennett, S.A.; Wang, L. Role of E-cadherin and other cell adhesion molecules in survival and differentiation of human pluripotent stem cells. Cell Adhes. Migr. 2012, 6, 59–70. [Google Scholar] [CrossRef]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the stem cell niche for tissue regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef]
- Suzuki, K.; Kwon, S.J.; Saito, D.; Atsuta, Y. LIN28 is essential for the maintenance of chicken primordial germ cells. Cells Dev. 2023, 176, 203874. [Google Scholar] [CrossRef]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. MCP 2014, 13, 397–406. [Google Scholar] [CrossRef]
- Mishra, M.; Akatsu, H.; Heese, K. The novel protein MANI modulates neurogenesis and neurite-cone growth. J. Cell. Mol. Med. 2011, 15, 1713–1725. [Google Scholar] [CrossRef]
- González-Tarragó, V.; Elosegui-Artola, A.; Bazellières, E.; Oria, R.; Pérez-González, C.; Roca-Cusachs, P. Binding of ZO-1 to α5β1 integrins regulates the mechanical properties of α5β1-fibronectin links. Mol. Biol. Cell 2017, 28, 1847–1852. [Google Scholar] [CrossRef]
- Rouaud, F.; Sluysmans, S.; Flinois, A.; Shah, J.; Vasileva, E.; Citi, S. Scaffolding proteins of vertebrate apical junctions: Structure, functions and biophysics. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183399. [Google Scholar]
- Williams, A.S.; Kang, L.; Wasserman, D.H. The extracellular matrix and insulin resistance. Trends Endocrinol. Metab. TEM 2015, 26, 357–366. [Google Scholar] [PubMed]
- Musale, V.; Murdoch, C.E.; Banah, A.K.; Hasib, A.; Hennayake, C.K.; Dong, B.; Lang, C.C.; Wasserman, D.H.; Kang, L. Limiting extracellular matrix expansion in diet-induced obese mice reduces cardiac insulin resistance and prevents myocardial remodelling. Mol. Metab. 2024, 86, 101970. [Google Scholar] [PubMed]
- Tharp, K.M.; Higuchi-Sanabria, R.; Timblin, G.A.; Ford, B.; Garzon-Coral, C.; Schneider, C.; Muncie, J.M.; Stashko, C.; Daniele, J.R.; Moore, A.S.; et al. Adhesion-mediated mechanosignaling forces mitohormesis. Cell Metab. 2021, 33, 1322–1341.e1313. [Google Scholar] [CrossRef]
- Jiménez-Uribe, A.P.; Gómez-Sierra, T.; Aparicio-Trejo, O.E.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Backstage players of fibrosis: NOX4, mTOR, HDAC, and S1P; companions of TGF-β. Cell. Signal. 2021, 87, 110123. [Google Scholar]
- Solari, C.; Petrone, M.V.; Vazquez Echegaray, C.; Cosentino, M.S.; Waisman, A.; Francia, M.; Barañao, L.; Miriuka, S.; Guberman, A. Superoxide dismutase 1 expression is modulated by the core pluripotency transcription factors Oct4, Sox2 and Nanog in embryonic stem cells. Mech. Dev. 2018, 154, 116–121. [Google Scholar]
- Bigarella, C.L.; Liang, R.; Ghaffari, S. Stem cells and the impact of ROS signaling. Development 2014, 141, 4206–4218. [Google Scholar]
- Sart, S.; Song, L.; Li, Y. Controlling Redox Status for Stem Cell Survival, Expansion, and Differentiation. Oxid. Med. Cell Longev. 2015, 105135. [Google Scholar]
- Godoy-Parejo, C.; Deng, C.; Liu, W.; Chen, G. Insulin Stimulates PI3K/AKT and Cell Adhesion to Promote the Survival of Individualized Human Embryonic Stem Cells. Stem Cells 2019, 37, 1030–1041. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, J.; Yang, J.; Xiao, L.; Hua, Q.; Zou, Y. PI3K/AKT/mTOR signaling participates in insulin-mediated regulation of pathological myopia-related factors in retinal pigment epithelial cells. BMC Ophthalmol. 2021, 21, 218. [Google Scholar] [CrossRef] [PubMed]
- Rulifson, I.C.; Karnik, S.K.; Heiser, P.W.; ten Berge, D.; Chen, H.; Gu, X.; Taketo, M.M.; Nusse, R.; Hebrok, M.; Kim, S.K. Wnt signaling regulates pancreatic beta cell proliferation. Proc. Natl. Acad. Sci. USA 2007, 104, 6247–6252. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Miyahara, D.; Nakamura, Y. Avian Primordial Germ Cells. Adv. Exp. Med. Biol. 2017, 1001, 1–18. [Google Scholar] [PubMed]
- Chen, M.J.; Zhou, J.Y.; Chen, Y.J.; Wang, X.Q.; Yan, H.C.; Gao, C.Q. The in ovo injection of methionine improves intestinal cell proliferation and differentiation in chick embryos by activating the JAK2/STAT3 signaling pathway. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2021, 7, 1031–1038. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed Cell-Death by Ferroptosis: Antioxidants as Mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef]
- Kaminskyy, V.O.; Zhivotovsky, B. Free radicals in cross talk between autophagy and apoptosis. Antioxid. Redox Signal. 2014, 21, 86–102. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Ge, X.; Zhang, F. Puerarin attenuates neurological deficits via Bcl-2/Bax/cleaved caspase-3 and Sirt3/SOD2 apoptotic pathways in subarachnoid hemorrhage mice. Biomed. Pharmacother. = Biomed. Pharmacother. 2019, 109, 726–733. [Google Scholar] [CrossRef]
- Sasaki, H.; Matsui, Y. Epigenetic events in mammalian germ-cell development: Reprogramming and beyond. Nat. Rev. Genet. 2008, 9, 129–140. [Google Scholar] [CrossRef]
- Ge, C.; Yu, M.; Petitte, J.N.; Zhang, C. Epidermal growth factor-induced proliferation of chicken primordial germ cells: Involvement of calcium/protein kinase C and NFKB1. Biol. Reprod. 2009, 80, 528–536. [Google Scholar] [CrossRef]
- Choi, J.W.; Kim, S.; Kim, T.M.; Kim, Y.M.; Seo, H.W.; Park, T.S.; Jeong, J.W.; Song, G.; Han, J.Y. Basic fibroblast growth factor activates MEK/ERK cell signaling pathway and stimulates the proliferation of chicken primordial germ cells. PLoS ONE 2010, 5, e12968. [Google Scholar] [CrossRef]
- Garcia, M.A.; Nelson, W.J.; Chavez, N. Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb. Perspect. Biol. 2018, 10, a029181. [Google Scholar]
- Shim, S.R.; Kook, S.; Kim, J.I.; Song, W.K. Degradation of focal adhesion proteins paxillin and p130cas by caspases or calpains in apoptotic rat-1 and L929 cells. Biochem. Biophys. Res. Commun. 2001, 286, 601–608. [Google Scholar] [PubMed]
- Okamura, D.; Kimura, T.; Nakano, T.; Matsui, Y. Cadherin-mediated cell interaction regulates germ cell determination in mice. Development 2003, 130, 6423–6430. [Google Scholar] [PubMed]
- Xu, J.; Kausalya, P.J.; Phua, D.C.; Ali, S.M.; Hossain, Z.; Hunziker, W. Early embryonic lethality of mice lacking ZO-2, but Not ZO-3, reveals critical and nonredundant roles for individual zonula occludens proteins in mammalian development. Mol. Cell. Biol. 2008, 28, 1669–1678. [Google Scholar] [PubMed]
- González-Mariscal, L.; Domínguez-Calderón, A.; Raya-Sandino, A.; Ortega-Olvera, J.M.; Vargas-Sierra, O.; Martínez-Revollar, G. Tight junctions and the regulation of gene expression. Semin. Cell Dev. Biol. 2014, 36, 213–223. [Google Scholar]
- Severson, E.A.; Jiang, L.; Ivanov, A.I.; Mandell, K.J.; Nusrat, A.; Parkos, C.A. Cis-dimerization mediates function of junctional adhesion molecule A. Mol. Biol. Cell 2008, 19, 1862–1872. [Google Scholar]
- Yoneyama, Y.; Lanzerstorfer, P.; Niwa, H.; Umehara, T.; Shibano, T.; Yokoyama, S.; Chida, K.; Weghuber, J.; Hakuno, F.; Takahashi, S.I. IRS-1 acts as an endocytic regulator of IGF-I receptor to facilitate sustained IGF signaling. eLife 2018, 7, e32893. [Google Scholar] [CrossRef]
- Yazıcı, D.; Sezer, H. Insulin Resistance, Obesity and Lipotoxicity. Adv. Exp. Med. Biol. 2017, 960, 277–304. [Google Scholar]
- Blázquez, E.; Velázquez, E.; Hurtado-Carneiro, V.; Ruiz-Albusac, J.M. Insulin in the brain: Its pathophysiological implications for States related with central insulin resistance, type 2 diabetes and Alzheimer’s disease. Front. Endocrinol. 2014, 5, 161. [Google Scholar] [CrossRef]
- Talbot, K. Brain insulin resistance in Alzheimer’s disease and its potential treatment with GLP-1 analogs. Neurodegener. Dis. Manag. 2014, 4, 31–40. [Google Scholar] [CrossRef]
- Zhou, L.; Li, H.; Sun, T.; Wen, X.; Niu, C.; Li, M.; Li, W.; Hoffman, A.R.; Hu, J.-F.; Cui, J. HULC targets the IGF1R–PI3K-AKT axis in trans to promote breast cancer metastasis and cisplatin resistance. Cancer Lett. 2022, 548, 215861. [Google Scholar] [CrossRef] [PubMed]
- Naeemipour, M.; Dehghani, H.; Bassami, M.; Bahrami, A. Expression dynamics of pluripotency genes in chicken primordial germ cells before and after colonization of the genital ridges. Mol. Reprod. Dev. 2013, 80, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Won, S.; Jung, K.M.; Woo, S.J.; Lee, H.; Kim, Y.M.; Kim, H.; Han, J.Y. Chicken blastoderms and primordial germ cells possess a higher expression of DNA repair genes and lower expression of apoptosis genes to preserve their genome stability. Sci. Rep. 2022, 12, 49. [Google Scholar] [CrossRef] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Ufer, C.; Wang, C.C. The Roles of Glutathione Peroxidases during Embryo Development. Front. Mol. Neurosci. 2011, 4, 12. [Google Scholar] [CrossRef]
- Lima, C.F.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Phenolic compounds protect HepG2 cells from oxidative damage: Relevance of glutathione levels. Life Sci. 2006, 79, 2056–2068. [Google Scholar] [CrossRef]
- An, J.; Hu, J.; Shang, Y.; Zhong, Y.; Zhang, X.; Yu, Z. The cytotoxicity of organophosphate flame retardants on HepG2, A549 and Caco-2 cells. J. Environ. Sci. Health. Part A Toxic/Hazard. Subst. Environ. Eng. 2016, 51, 980–988. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Jiang, P.; Song, Y.; Zhao, Y.; Zheng, Y. Focal Adhesion Kinase Inhibitor Inhibits the Oxidative Damage Induced by Central Venous Catheter via Abolishing Focal Adhesion Kinase-Protein Kinase B Pathway Activation. BioMed Res. Int. 2021, 2021, 6685493. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Y.; Zhang, J.; Hu, C.; Jiang, J.; Li, Y.; Peng, Z. ROS-induced lipid peroxidation modulates cell death outcome: Mechanisms behind apoptosis, autophagy, and ferroptosis. Arch. Toxicol. 2023, 97, 1439–1451. [Google Scholar] [CrossRef]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef]
- Matsathit, U.; Sermwittayawong, D.; Komolgriengkrai, M.; Khimmaktong, W. Glucan-rich polysaccharides obtained from split gill mushroom [Schizophyllum commune (Fr.)] ameliorate hyperglycemia by enhancing insulin and GLUT2 pancreas in type 2 diabetic rats. Histol. Histopathol. 2024, 39, 191–203. [Google Scholar]
- Dandona, P.; Ghanim, H.; Bandyopadhyay, A.; Korzeniewski, K.; Ling Sia, C.; Dhindsa, S.; Chaudhuri, A. Insulin suppresses endotoxin-induced oxidative, nitrosative, and inflammatory stress in humans. Diabetes Care 2010, 33, 2416–2423. [Google Scholar] [PubMed]
- Francés, D.E.; Ronco, M.T.; Monti, J.A.; Ingaramo, P.I.; Pisani, G.B.; Parody, J.P.; Pellegrino, J.M.; Sanz, P.M.; Carrillo, M.C.; Carnovale, C.E. Hyperglycemia induces apoptosis in rat liver through the increase of hydroxyl radical: New insights into the insulin effect. J. Endocrinol. 2010, 205, 187–200. [Google Scholar] [PubMed]
- Lee, C.K.; Weaks, R.L.; Johnson, G.A.; Bazer, F.W.; Piedrahita, J.A. Effects of protease inhibitors and antioxidants on In vitro survival of porcine primordial germ cells. Biol. Reprod. 2000, 63, 887–897. [Google Scholar] [PubMed]
- Teng, H.; Sui, X.; Zhou, C.; Shen, C.; Yang, Y.; Zhang, P.; Guo, X.; Huo, R. Fatty acid degradation plays an essential role in proliferation of mouse female primordial germ cells via the p53-dependent cell cycle regulation. Cell Cycle 2016, 15, 425–431. [Google Scholar]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar]
- Deng, J.; Shimamura, T.; Perera, S.; Carlson, N.E.; Cai, D.; Shapiro, G.I.; Wong, K.K.; Letai, A. Proapoptotic BH3-Only BCL-2 Family Protein BIM Connects Death Signaling from Epidermal Growth Factor Receptor Inhibition to the Mitochondrion. Cancer Res. 2007, 67, 11867–11875. [Google Scholar]
- Sarosiek, K.A.; Letai, A. Directly targeting the mitochondrial pathway of apoptosis for cancer therapy using BH3 mimetics—Recent successes, current challenges and future promise. FEBS J. 2016, 283, 3523–3533. [Google Scholar]
- Ricci, C.; Pastukh, V.; Mozaffari, M.; Schaffer, S.W. Insulin withdrawal induces apoptosis via a free radical-mediated mechanism. Can. J. Physiol. Pharmacol. 2007, 85, 455–464. [Google Scholar]
- Okamoto, T.; Coultas, L.; Metcalf, D.; van Delft, M.F.; Glaser, S.P.; Takiguchi, M.; Strasser, A.; Bouillet, P.; Adams, J.M.; Huang, D.C. Enhanced stability of Mcl1, a prosurvival Bcl2 relative, blunts stress-induced apoptosis, causes male sterility, and promotes tumorigenesis. Proc. Natl. Acad. Sci. USA 2014, 111, 261–266. [Google Scholar]
- Pagliassotti, M.J.; Wei, Y.; Wang, D. Insulin protects liver cells from saturated fatty acid-induced apoptosis via inhibition of c-Jun NH2 terminal kinase activity. Endocrinology 2007, 148, 3338–3345. [Google Scholar] [PubMed]
- Chawengsaksophak, K.; Svingen, T.; Ng, E.T.; Epp, T.; Spiller, C.M.; Clark, C.; Cooper, H.; Koopman, P. Loss of Wnt5a disrupts primordial germ cell migration and male sexual development in mice. Biol. Reprod. 2012, 86, 1–12. [Google Scholar]
- Kimura, T.; Nakamura, T.; Murayama, K.; Umehara, H.; Yamano, N.; Watanabe, S.; Taketo, M.M.; Nakano, T. The stabilization of beta-catenin leads to impaired primordial germ cell development via aberrant cell cycle progression. Dev. Biol. 2006, 300, 545–553. [Google Scholar] [PubMed]
- Laird, D.J.; Altshuler-Keylin, S.; Kissner, M.D.; Zhou, X.; Anderson, K.V. Ror2 enhances polarity and directional migration of primordial germ cells. PLoS Genet. 2011, 7, e1002428. [Google Scholar]
- Zhang, L.; Ma, L.; Yan, T.; Han, X.; Xu, J.; Xu, J.; Xu, X. Activated mitochondrial apoptosis in hESCs after dissociation involving the PKA/p-p53/Bax signaling pathway. Exp. Cell Res. 2018, 369, 226–233. [Google Scholar]
- Lessman, C.A. Effect of insulin on meiosis reinitiation induced in vitro by three progestogens in oocytes of the goldfish (Carassius auratus). Dev. Biol. 1985, 107, 259–263. [Google Scholar]
- Maitra, S.; Das, D.; Ghosh, P.; Hajra, S.; Roy, S.S.; Bhattacharya, S. High cAMP attenuation of insulin-stimulated meiotic G2-M1 transition in zebrafish oocytes: Interaction between the cAMP-dependent protein kinase (PKA) and the MAPK3/1 pathways. Mol. Cell. Endocrinol. 2014, 393, 109–119. [Google Scholar] [PubMed]
- Lessman, C.A.; Schuetz, A.W. Role of follicle wall in meiosis reinitiation induced by insulin in Rana pipiens oocytes. Am. J. Physiol. 1981, 241, E51–E56. [Google Scholar]
- Wang, J.J.; Xu, F.B.; Hu, S.; Xu, Y.M.; Wang, X.Z. Identification and validation of cortisol-related hub biomarkers and the related pathogenesis of biomarkers in Ischemic Stroke. Brain Behav. 2024, 14, e3358. [Google Scholar]
- Sowers, M.R.; Wilson, A.L.; Kardia, S.R.; Chu, J.; McConnell, D.S. CYP1A1 and CYP1B1 polymorphisms and their association with estradiol and estrogen metabolites in women who are premenopausal and perimenopausal. Am. J. Med. 2006, 119, S44–S51. [Google Scholar]
- You, Z.; Yuan, J.; Wang, Y.; Sun, Y.; Ni, A.; Li, Y.; Ma, H.; Ma, T.; Chen, J. Integrated transcriptomic analysis on chicken ovary reveals CYP21A1 affects follicle granulosa cell development and steroid hormone synthesis. Poult. Sci. 2024, 103, 103589. [Google Scholar] [CrossRef] [PubMed]
- Bruce, J.I.E.; Sánchez-Alvarez, R.; Sans, M.D.; Sugden, S.A.; Qi, N.; James, A.D.; Williams, J.A. Insulin protects acinar cells during pancreatitis by preserving glycolytic ATP supply to calcium pumps. Nat. Commun. 2021, 12, 4386. [Google Scholar] [CrossRef] [PubMed]
- Geng, T.; Xia, L.; Li, F.; Xia, J.; Zhang, Y.; Wang, Q.; Yang, B.; Montgomery, S.; Cui, H.; Gong, D. The role of endoplasmic reticulum stress and insulin resistance in the occurrence of goose fatty liver. Biochem. Biophys. Res. Commun. 2015, 465, 83–87. [Google Scholar] [CrossRef]
- Thapa, N.; Chen, M.; Horn, H.T.; Choi, S.; Wen, T.; Anderson, R.A. Phosphatidylinositol-3-OH kinase signalling is spatially organized at endosomal compartments by microtubule-associated protein 4. Nat. Cell Biol. 2020, 22, 1357–1370. [Google Scholar] [CrossRef] [PubMed]
- Alva, J.A.; Lee, G.E.; Escobar, E.E.; Pyle, A.D. Phosphatase and tensin homolog regulates the pluripotent state and lineage fate choice in human embryonic stem cells. Stem Cells 2011, 29, 1952–1962. [Google Scholar] [CrossRef]
- Armstrong, L.; Hughes, O.; Yung, S.; Hyslop, L.; Stewart, R.; Wappler, I.; Peters, H.; Walter, T.; Stojkovic, P.; Evans, J.; et al. The role of PI3K/AKT, MAPK/ERK and NFkappabeta signalling in the maintenance of human embryonic stem cell pluripotency and viability highlighted by transcriptional profiling and functional analysis. Hum. Mol. Genet. 2006, 15, 1894–1913. [Google Scholar] [CrossRef]
- Jirmanova, L.; Afanassieff, M.; Gobert-Gosse, S.; Markossian, S.; Savatier, P. Differential contributions of ERK and PI3-kinase to the regulation of cyclin D1 expression and to the control of the G1/S transition in mouse embryonic stem cells. Oncogene 2002, 21, 5515–5528. [Google Scholar] [CrossRef]
- McLean, A.B.; D’Amour, K.A.; Jones, K.L.; Krishnamoorthy, M.; Kulik, M.J.; Reynolds, D.M.; Sheppard, A.M.; Liu, H.; Xu, Y.; Baetge, E.E.; et al. Activin a efficiently specifies definitive endoderm from human embryonic stem cells only when phosphatidylinositol 3-kinase signaling is suppressed. Stem Cells 2007, 25, 29–38. [Google Scholar] [CrossRef]
- Paling, N.R.; Wheadon, H.; Bone, H.K.; Welham, M.J. Regulation of embryonic stem cell self-renewal by phosphoinositide 3-kinase-dependent signaling. J. Biol. Chem. 2004, 279, 48063–48070. [Google Scholar] [CrossRef]
- Shepherd, P.R.; Navé, B.T.; Siddle, K. Insulin stimulation of glycogen synthesis and glycogen synthase activity is blocked by wortmannin and rapamycin in 3T3-L1 adipocytes: Evidence for the involvement of phosphoinositide 3-kinase and p70 ribosomal protein-S6 kinase. Biochem. J. 1995, 305 Pt 1, 25–28. [Google Scholar] [CrossRef]
- Kurtzhals, P.; Schaffer, L.; Sorensen, A.; Kristensen, C.; Jonassen, I.; Schmid, C.; Trub, T. Correlations of receptor binding and metabolic and mitogenic potencies of insulin analogs designed for clinical use. Diabetes 2000, 49, 999–1005. [Google Scholar] [PubMed]
- Tennagels, N.; Werner, U. The metabolic and mitogenic properties of basal insulin analogues. Arch. Physiol. Biochem. 2013, 119, 1–14. [Google Scholar] [CrossRef]
- Wu, L.; Lin, Q.; Chatla, S.; Amarachintha, S.; Wilson, A.F.; Atale, N.; Gao, Z.J.; Joseph, J.; Wolff, E.V.; Du, W. LepR+ niche cell-derived AREG compromises hematopoietic stem cell maintenance under conditions of DNA repair deficiency and aging. Blood 2023, 142, 1529–1542. [Google Scholar]
- Wong, S.K.; Mohamad, N.V.; Jayusman, P.A.; Ibrahim, N. A Review on the Crosstalk between Insulin and Wnt/β-Catenin Signalling for Bone Health. Int. J. Mol. Sci. 2023, 24, 12441. [Google Scholar] [CrossRef] [PubMed]
- Heller, R.S.; Dichmann, D.S.; Jensen, J.; Miller, C.; Wong, G.; Madsen, O.D.; Serup, P. Expression patterns of Wnts, Frizzleds, sFRPs, and misexpression in transgenic mice suggesting a role for Wnts in pancreas and foregut pattern formation. Dev. Dyn. 2010, 225, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.P.; Collazo, A.; Trinh, L.A.; Pan, L.; Moens, C.B.; Crump, J.G. Wnt-dependent epithelial transitions drive pharyngeal pouch formation. Dev. Cell 2013, 24, 296–309. [Google Scholar]
- Wang, M.; Zhang, C.; Huang, C.; Cheng, S.; He, N.; Wang, Y.; Ahmed, M.F.; Zhao, R.; Jin, J.; Zuo, Q.; et al. Regulation of fibroblast growth factor 8 (FGF8) in chicken embryonic stem cells differentiation into spermatogonial stem cells. J. Cell. Biochem. 2018, 119, 2396–2407. [Google Scholar]
- Chen, Q.; Zhang, F.; Dong, L.; Wu, H.; Xu, J.; Li, H.; Wang, J.; Zhou, Z.; Liu, C.; Wang, Y.; et al. SIDT1-dependent absorption in the stomach mediates host uptake of dietary and orally administered microRNAs. Cell Res. 2021, 31, 247–258. [Google Scholar] [CrossRef]

| DMEM Basal Medium | |||
|---|---|---|---|
| Composition | Source | Concentration | Volume |
| DMEM | Meilunbio-PWL037, Dalian, China | 75% | 37.5 mL |
| Ultra-filtered water | Sigma-W3500, Shanghai, China | 24% | 12 mL |
| CaCl2·2H2O | Sigma-C7902, Shanghai, China | 0.15 mM | 0.5 mL |
| DMEM basal medium | Discard 3.368ml | 46.632 mL | |
| B-27TM supplement (control) | Gibco-17504044, Shanghai, China | 1× | 1 mL |
| B-27TM supplement, minus insulin (experimental group) | Gibco-A1895601, Shanghai, China | 1× | 1 mL |
| GlutaMax | Gibco-35050061, Shanghai, China | 2 mM | 0.5 mL |
| MEM NEAA | Gibco-11140050, Shanghai, China | 1× | 0.5 mL |
| 2-Mercaptoethanol | Gibco-21985023, Shanghai, China | 0.1 mM | 91 µL |
| Chicken serum | Gibco-16110082, Shanghai, China | 0.2% | 100 µL |
| EmbryoMax nucleosides | Sigma-ES-008-D, Shanghai, China | 1× | 0.5 mL |
| Sodium pyruvate | Gibco-11360070, Shanghai, China | 1.2 mM | 0.6 mL |
| Ovalbumln | Sigma-A5503, Shanghai, China | 0.2% | 0.1 g |
| Sodium heparin | MCE-HY-17567A, Shanghai, China | 0.01% | 50 µL |
| Basic fibroblast growth factor | MCE-HY-P70600, Shanghai, China | 1× | 20 µL |
| Human Activin A | MCE-HY-P70311, Shanghai, China | 1× | 25 µL |
| Pen strep | Gibco-15070063, Shanghai, China | 1× | 0.5 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Wu, J.; Peng, Y.; Qian, H.; Lv, X.; Li, F.; Jin, K.; Niu, Y.; Song, J.; Han, W.; et al. Chicken Primordial Germ Cells Do Not Proliferate in Insulin-Lacking Media. Int. J. Mol. Sci. 2025, 26, 3122. https://doi.org/10.3390/ijms26073122
Liu X, Wu J, Peng Y, Qian H, Lv X, Li F, Jin K, Niu Y, Song J, Han W, et al. Chicken Primordial Germ Cells Do Not Proliferate in Insulin-Lacking Media. International Journal of Molecular Sciences. 2025; 26(7):3122. https://doi.org/10.3390/ijms26073122
Chicago/Turabian StyleLiu, Xin, Jun Wu, Yixiu Peng, Hongwu Qian, Xiaoqian Lv, Fan Li, Kai Jin, Yingjie Niu, Jiuzhou Song, Wei Han, and et al. 2025. "Chicken Primordial Germ Cells Do Not Proliferate in Insulin-Lacking Media" International Journal of Molecular Sciences 26, no. 7: 3122. https://doi.org/10.3390/ijms26073122
APA StyleLiu, X., Wu, J., Peng, Y., Qian, H., Lv, X., Li, F., Jin, K., Niu, Y., Song, J., Han, W., Chen, G., Li, B., & Zuo, Q. (2025). Chicken Primordial Germ Cells Do Not Proliferate in Insulin-Lacking Media. International Journal of Molecular Sciences, 26(7), 3122. https://doi.org/10.3390/ijms26073122









