Integrative Utilization of Transcriptomics and Metabolomics Sheds Light on Disparate Growth Performance of Whiteleg Shrimp, Litopenaeus vannamei
Abstract
1. Introduction
2. Results
2.1. Significant Growth Performance Differences Between NL and NS
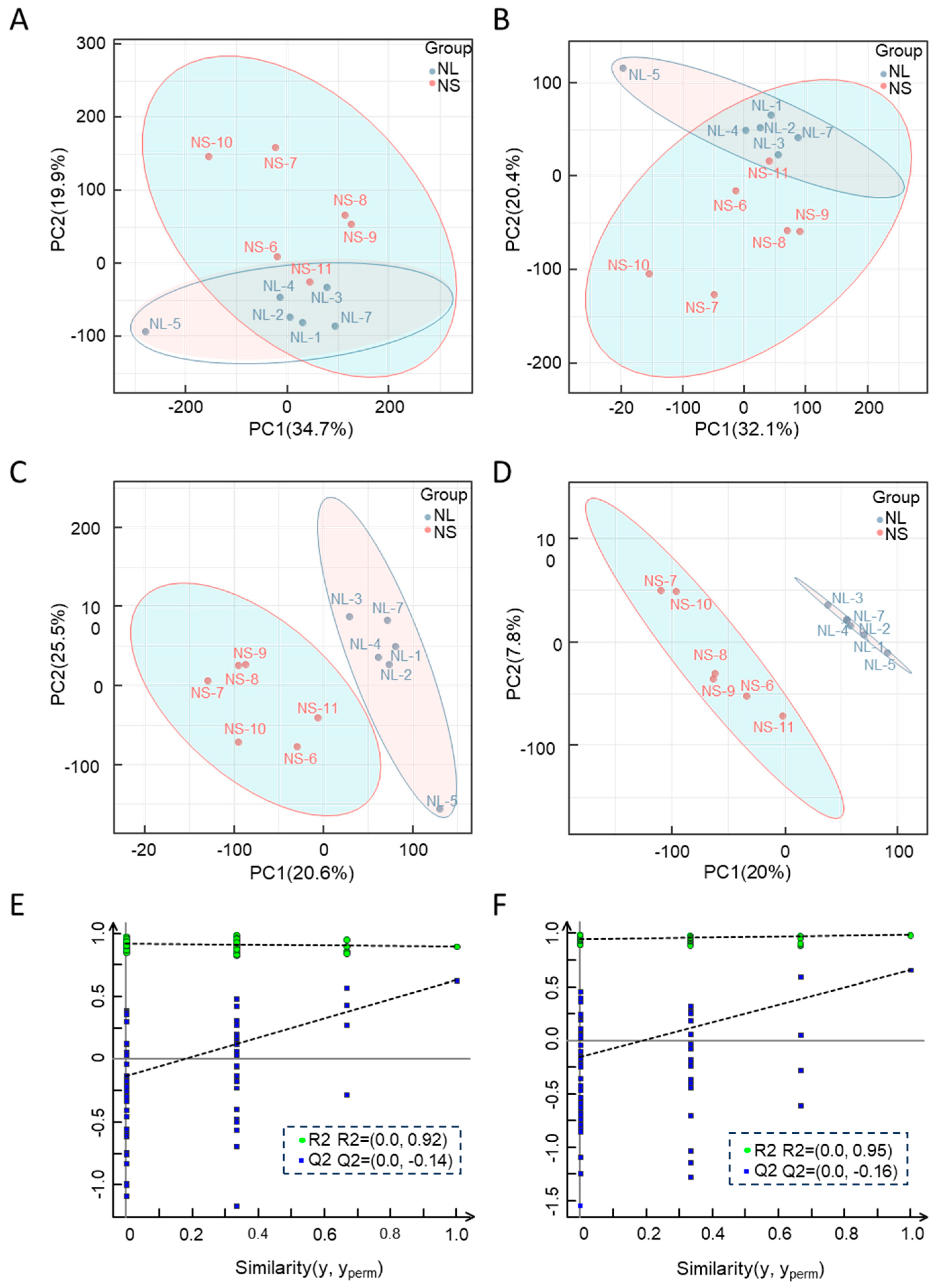
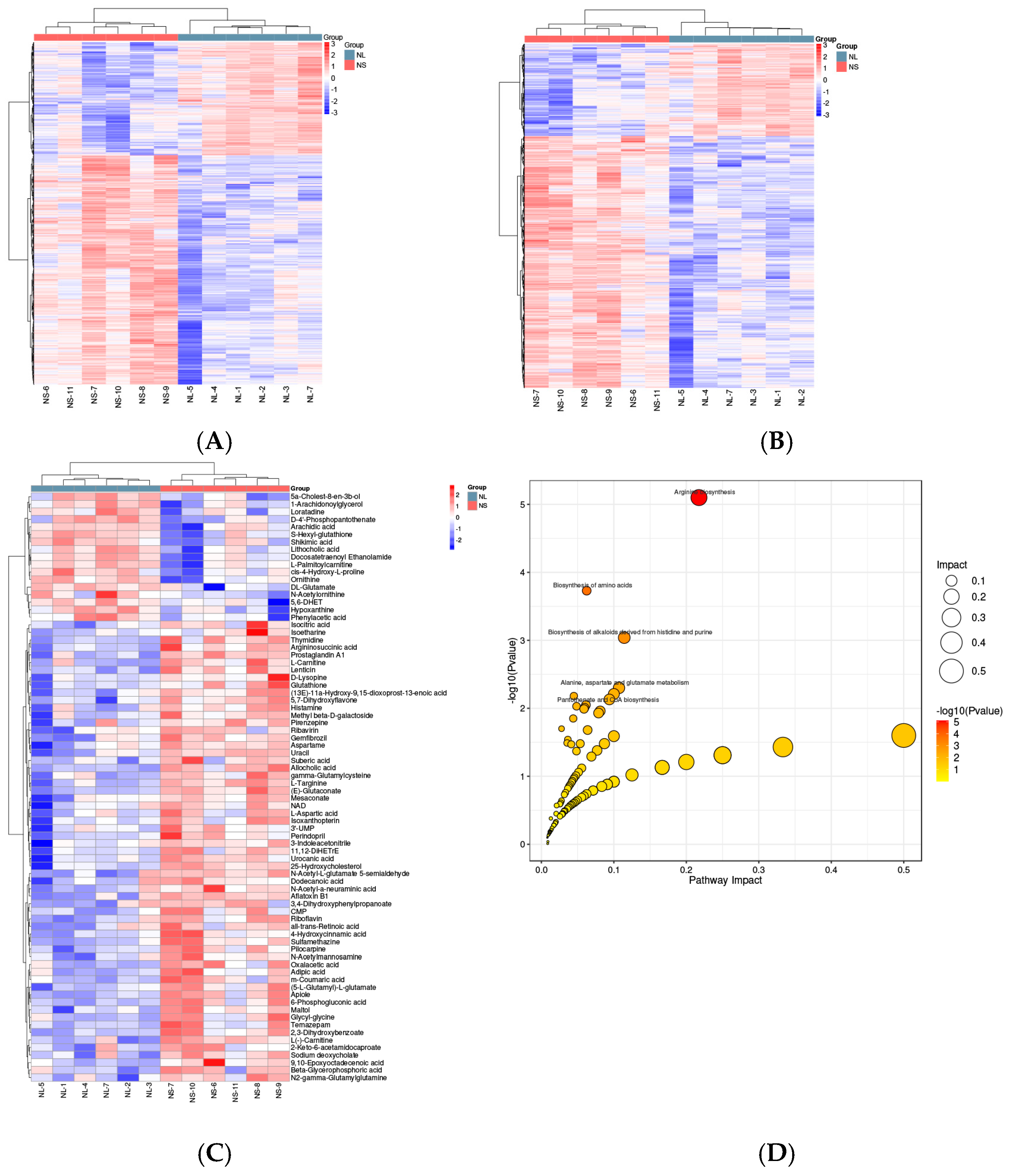
2.2. Metabolomic Analysis
2.3. Transcriptomic Analysis
2.4. Integrative Analysis of Metabolomics and Transcriptomics
3. Discussion
3.1. Fatty Acid Degradation
3.2. Retinol Metabolism
3.3. TCA Cycle
3.4. Glutathione Metabolism
3.5. Riboflavin Metabolism and Purine Metabolism
4. Materials and Methods
4.1. Experimental Animals
4.2. LC–MS/MS and Metabolomic Data Analysis
4.3. Transcriptomic Analysis for the Shriimp with Disparate Growth Performance
4.4. Integrative Metabolomics and Transcriptomics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PCA | principal component analysis |
| OPLS-DA | orthogonal projections to latent structures discriminant analysis |
| LC-MS/MS | Liquid Chromatography-Tandem Mass Spectrometry |
| TCA cycle | tricarboxylic acid cycle |
| PLS-DA | partial least squares-discriminant analysis |
| DMs | differential metabolites |
| DEGs | differentially expressed genes |
| TPM | transcripts per million |
| FDR | false discovery rate |
| qPCR | Quantitative Real-time PCR |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| GO | Gene Ontology |
| ACADVL | acyl-CoA dehydrogenase |
| HADHA | hydroxyl-CoA dehydrogenase |
| frmA | Formaldehyde Dehydrogenase |
| UGT | uridine diphosphate glycosyltransferase |
| ACLY | ATP citrate lyase |
| PK | pyruvate kinase |
| ACP2 | Acid Phosphatase 2 |
| GCLM | Glutamate-Cysteine Ligase Modifier Subunit |
| GST | Glutathione S-transferases |
| ANPEP | Alanyl Aminopeptidase |
| ROS | reactive oxygen species |
| FAD | flavin adenine dinucleotide |
| FMN | flavin mononucleotide |
| Ru5P | ribulose-5-phosphate |
| GTP | guanosine-5′-triphosphate |
References
- Katneni, V.K.; Shekhar, M.S.; Jangam, A.K.; Prabhudas, S.K.; Krishnan, K.; Kaikkolante, N.; Paran, B.C.; Baghel, D.S.; Koyadan, V.K.; Jena, J. Novel isoform sequencing based full-length transcriptome resource for Indian white shrimp, Penaeus indicus. Front. Mar. Sci. 2020, 7, 605098. [Google Scholar]
- Fawzy, S.; Wang, W.; Wu, M.; Yi, G.; Huang, X. Effects of dietary different canthaxanthin levels on growth performance, antioxidant capacity, biochemical and immune-physiological parameters of white shrimp (Litopenaeus vannamei). Aquaculture 2022, 556, 738276. [Google Scholar]
- Kong, J.; Yan, Y.; Lu, X.; Luan, S.; Meng, X.; Dai, P.; Chen, B.; Cao, B.; Qiang, G.; Luo, K. Integrative phenotypic and gene expression data identify myostatin as a muscle growth inhibitor in Chinese shrimp Fenneropenaeus chinensis. Sci. Rep. 2020, 10, 5985. [Google Scholar]
- Zhu, L.; Li, C.; Su, X.; Guo, C.; Wang, Z.; Jin, C.; Li, Y.; Li, T. Identification and assessment of differentially expressed genes involved in growth regulation in Apostichopus japonicus. Genet. Mol. Res. 2013, 12, 3028–3037. [Google Scholar]
- Zhang, S.; Yang, H.; Liu, S.; Zhang, L.; Ru, X.; Xu, D. The influence of genetics factor on key growth traits and quantitative genetic analysis of sea cucumber Apostichopus japonicus (Selenka) heat-resistant and fast-growing strain. Aquac. Int. 2015, 23, 219–233. [Google Scholar] [CrossRef]
- Yin, J.; Li, Z.; Pan, C.; Ma, B.; Liu, Y.; Hu, C.; Luo, P. Understanding the internal differences behind unsynchronized growth in sea cucumber Holothuria leucospilota by integration of transcriptomic and metabolomic data. Aquac. Rep. 2023, 32, 101688. [Google Scholar]
- Tamayo, D.; Ibarrola, I.; Urrutia, M.B.; Navarro, E. The physiological basis for inter-individual growth variability in the spat of clams (Ruditapes philippinarum). Aquaculture 2011, 321, 113–120. [Google Scholar]
- Hao, R.; Wang, Z.; Yang, C.; Deng, Y.; Zheng, Z.; Wang, Q.; Du, X. Metabolomic responses of juvenile pearl oyster Pinctada maxima to different growth performances. Aquaculture 2018, 491, 258–265. [Google Scholar] [CrossRef]
- Pell, J.M.; Stewart, C.E.H.; Bates, P.C.; Aston, R. Chapter 17—Immunological Approaches to Modify Growth. In Low-Fat Meats; Hafs, H.D., Zimbelman, R.G., Eds.; Academic Press: Cambridge, MA, USA, 1994; pp. 303–319. [Google Scholar]
- Ma, B.; Liu, Y.; Pan, W.; Li, Z.; Ren, C.; Hu, C.; Luo, P. Integrative application of transcriptomics and metabolomics provides insights into unsynchronized growth in sea cucumber (Stichopus monotuberculatus). Int. J. Mol. Sci. 2022, 23, 15478. [Google Scholar] [CrossRef]
- Rosas, C.; Cuzon, G.; Gaxiola, G.; Le Priol, Y.; Pascual, C.; Rossignyol, J.; Contreras, F.; Sanchez, A.; Van Wormhoudt, A. Metabolism and growth of juveniles of Litopenaeus vannamei: Effect of salinity and dietary carbohydrate levels. J. Exp. Mar. Biol. Ecol. 2001, 259, 1–22. [Google Scholar] [CrossRef]
- Muralisankar, T.; Kalaivani, P.; Thangal, S.; Santhanam, P. Growth, biochemical, antioxidants, metabolic enzymes and hemocytes population of the shrimp Litopenaeus vannamei exposed to acidified seawater. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2021, 239, 108843. [Google Scholar] [CrossRef] [PubMed]
- Quintino-Rivera, J.G.; Elizondo-González, R.; Gamboa-Delgado, J.; Guzmán-Villanueva, L.T.; Peña-Rodriguez, A. Metabolic turnover rate, digestive enzyme activities, and bacterial communities in the white shrimp Litopenaeus vannamei under compensatory growth. PeerJ 2023, 11, e14747. [Google Scholar] [CrossRef]
- Li, E.; Chen, L.; Zeng, C.; Chen, X.; Yu, N.; Lai, Q.; Qin, J.G. Growth, body composition, respiration and ambient ammonia nitrogen tolerance of the juvenile white shrimp, Litopenaeus vannamei, at different salinities. Aquaculture 2007, 265, 385–390. [Google Scholar] [CrossRef]
- Ren, X.; Wang, Q.; Shao, H.; Xu, Y.; Liu, P.; Li, J. Effects of low temperature on shrimp and crab physiology, behavior, and growth: A review. Front. Mar. Sci. 2021, 8, 746177. [Google Scholar] [CrossRef]
- Lan, X.; Peng, X.; Du, T.; Xia, Z.; Gao, Q.; Tang, Q.; Yi, S.; Yang, G. Alterations of the Gut Microbiota and Metabolomics Associated with the Different Growth Performances of Macrobrachium rosenbergii Families. Animals 2023, 13, 1539. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Zhang, X.; Zhang, Y.; Li, S.; Lu, L.; Xu, D.; Liu, X. Effects of dietary carbohydrate levels on the growth, glycometabolism, antioxidant capacity and metabolome of largemouth bass (Micropterus salmoides). Aquac. Res. 2022, 53, 3748–3758. [Google Scholar] [CrossRef]
- Tan, Y.; Li, Y.; Ren, L.; Fu, H.; Li, Q.; Liu, S. Integrative proteome and metabolome analyses reveal molecular basis underlying growth and nutrient composition in the Pacific oyster, Crassostrea gigas. J. Proteom. 2024, 290, 105021. [Google Scholar] [CrossRef]
- Tang, T.; Zhong, W.; Yang, L.; He, M.; Jiang, S.; Yin, D.; Guo, J.; Gao, Z. In vitro and in vivo anti-oomycetes activities and mechanisms of linalool against Saprolegnia ferax. Aquaculture 2024, 578, 740031. [Google Scholar] [CrossRef]
- Xian, J.; Wang, N.; Zhao, P.; Zhang, Y.; Meng, J.; Ma, X.; Guo, X.; Wang, Z.; Bo, X. Molecular characterization and immune protection of the 3-hydroxyacyl-CoA dehydrogenase gene in Echinococcus granulosus. Parasites Vectors 2021, 14, 489. [Google Scholar] [CrossRef]
- Bonds, A.C.; Yuan, T.; Werman, J.M.; Jang, J.; Lu, R.; Nesbitt, N.M.; Garcia-Diaz, M.; Sampson, N.S. Post-translational succinylation of Mycobacterium tuberculosis enoyl-CoA hydratase EchA19 slows catalytic hydration of cholesterol catabolite 3-oxo-chol-4,22-diene-24-oyl-CoA. ACS Infect. Dis. 2020, 6, 2214–2224. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Kakimoto, P.A.; Kowaltowski, A.J. Diet-sensitive sources of reactive oxygen species in liver mitochondria: Role of very long chain acyl-CoA dehydrogenases. PLoS ONE 2013, 8, e77088. [Google Scholar]
- Hulme, H.E.; Meikle, L.M.; Wessel, H.; Strittmatter, N.; Swales, J.; Thomson, C.; Nilsson, A.; Nibbs, R.J.; Milling, S.; Andren, P.E. Mass spectrometry imaging identifies palmitoylcarnitine as an immunological mediator during Salmonella Typhimurium infection. Sci. Rep. 2017, 7, 2786. [Google Scholar]
- Bi, B.; Yuan, Y.; Zhao, Y.; He, M.; Song, H.; Kong, L.; Gao, Y. Effect of crowding stress on growth performance, the antioxidant system and humoral immunity in hybrid sturgeon. Aquac. Rep. 2023, 28, 101468. [Google Scholar]
- Al-Bakheit, A.; Traka, M.; Saha, S.; Mithen, R.; Melchini, A. Accumulation of palmitoylcarnitine and its effect on pro-inflammatory pathways and calcium influx in prostate cancer. Prostate 2016, 76, 1326–1337. [Google Scholar] [CrossRef]
- Ji, D.-W.; Li, F.; Hu, L.-H.; Luo, K.; Zhang, J.-M.; Yan, M.-C.; Zhang, M. Metabolomics analysis revealed biochemical changes in hepatopancreas and ovary of Litopenaeus vannamei during ovarian maturation. Aquac. Rep. 2022, 25, 101250. [Google Scholar] [CrossRef]
- Liñán-Cabello, M.A.; Paniagua-Michel, J. Induction factors derived from carotenoids and vitamin A during the ovarian maturation of Litopenaeus vannamei. Aquac. Int. 2004, 12, 583–592. [Google Scholar]
- Kin Ting Kam, R.; Deng, Y.; Chen, Y.; Zhao, H. Retinoic acid synthesis and functions in early embryonic development. Cell 2012, 2, 11. [Google Scholar]
- Laubenstein, T.D.; Jarrold, M.D.; Rummer, J.L.; Munday, P.L. Beneficial effects of diel CO2 cycles on reef fish metabolic performance are diminished under elevated temperature. Sci. Total Environ. 2020, 735, 139084. [Google Scholar]
- Liu, M.-J.; Gao, J.; Guo, H.-Y.; Zhu, K.-C.; Liu, B.-S.; Zhang, N.; Sun, J.-H.; Zhang, D.-C. Transcriptomics Reveal the Effects of Breeding Temperature on Growth and Metabolism in the Early Developmental Stage of Platax teira. Biology 2023, 12, 1161. [Google Scholar] [CrossRef]
- Wen, B.; Jin, S.-R.; Chen, Z.-Z.; Gao, J.-Z.; Wang, L.; Liu, Y.; Liu, H.-P. Plasticity of energy reserves and metabolic performance of discus fish (Symphysodon aequifasciatus) exposed to low-temperature stress. Aquaculture 2017, 481, 169–176. [Google Scholar] [CrossRef]
- Eroglu, A.; Dogan, Z.; Kanak, E.; Atli, G.; Canli, M. Effects of heavy metals (Cd, Cu, Cr, Pb, Zn) on fish glutathione metabolism. Environ. Sci. Pollut. Res. 2015, 22, 3229–3237. [Google Scholar]
- Zhou, J.; Wang, W.-N.; Wang, A.-L.; He, W.-Y.; Zhou, Q.-T.; Liu, Y.; Xu, J. Glutathione S-transferase in the white shrimp Litopenaeus vannamei: Characterization and regulation under pH stress. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2009, 150, 224–230. [Google Scholar] [PubMed]
- Solopova, A.; Bottacini, F.; Venturi degli Esposti, E.; Amaretti, A.; Raimondi, S.; Rossi, M.; Van Sinderen, D. Riboflavin biosynthesis and overproduction by a derivative of the human gut commensal Bifidobacterium longum subsp. infantis ATCC 15697. Front. Microbiol. 2020, 11, 573335. [Google Scholar]
- Saedisomeolia, A.; Ashoori, M. Riboflavin in human health: A review of current evidences. Adv. Food Nutr. Res. 2018, 83, 57–81. [Google Scholar] [PubMed]
- Yang, B.; Sun, Y.; Fu, S.; Xia, M.; Su, Y.; Liu, C.; Zhang, C.; Zhang, D. Improving the production of riboflavin by introducing a mutant ribulose 5-phosphate 3-epimerase gene in Bacillus subtilis. Front. Bioeng. Biotechnol. 2021, 9, 704650. [Google Scholar]
- Wong, A.C.-N.; Dobson, A.J.; Douglas, A.E. Gut microbiota dictates the metabolic response of Drosophila to diet. J. Exp. Biol. 2014, 217, 1894–1901. [Google Scholar]
- LeBlanc, J.G.; Milani, C.; De Giori, G.S.; Sesma, F.; Van Sinderen, D.; Ventura, M. Bacteria as vitamin suppliers to their host: A gut microbiota perspective. Curr. Opin. Biotechnol. 2013, 24, 160–168. [Google Scholar]
- Sanjeewani, K.; Lee, K.-J. Dietary riboflavin requirement of pacific white shrimp (Litopenaeus vannamei). Aquac. Nutr. 2023, 2023, 6685592. [Google Scholar]
- Chen, B.; Zhong, P.; Wu, X.; Peng, K.; Sun, Y.; Chen, X.; Zhao, H.; Xu, Z.; Liu, J.; Li, H. Construction of a genetic linkage map, QTLs mapping for low salinity and growth-related traits and identification of the candidate genes in Pacific white shrimp (Litopenaeus vannamei). Aquac. Rep. 2022, 22, 100978. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Ma, B.; Li, P.; Chen, T.; Ren, C.; Hu, C.; Luo, P. Integrative Utilization of Transcriptomics and Metabolomics Sheds Light on Disparate Growth Performance of Whiteleg Shrimp, Litopenaeus vannamei. Int. J. Mol. Sci. 2025, 26, 3133. https://doi.org/10.3390/ijms26073133
Zhang X, Ma B, Li P, Chen T, Ren C, Hu C, Luo P. Integrative Utilization of Transcriptomics and Metabolomics Sheds Light on Disparate Growth Performance of Whiteleg Shrimp, Litopenaeus vannamei. International Journal of Molecular Sciences. 2025; 26(7):3133. https://doi.org/10.3390/ijms26073133
Chicago/Turabian StyleZhang, Xin, Bo Ma, Pengying Li, Ting Chen, Chunhua Ren, Chaoqun Hu, and Peng Luo. 2025. "Integrative Utilization of Transcriptomics and Metabolomics Sheds Light on Disparate Growth Performance of Whiteleg Shrimp, Litopenaeus vannamei" International Journal of Molecular Sciences 26, no. 7: 3133. https://doi.org/10.3390/ijms26073133
APA StyleZhang, X., Ma, B., Li, P., Chen, T., Ren, C., Hu, C., & Luo, P. (2025). Integrative Utilization of Transcriptomics and Metabolomics Sheds Light on Disparate Growth Performance of Whiteleg Shrimp, Litopenaeus vannamei. International Journal of Molecular Sciences, 26(7), 3133. https://doi.org/10.3390/ijms26073133





