Adventitious and Hairy Root Cultures of Salvia apiana as a Source of Rosmarinic Acid
Abstract
1. Introduction
2. Results and Discussion
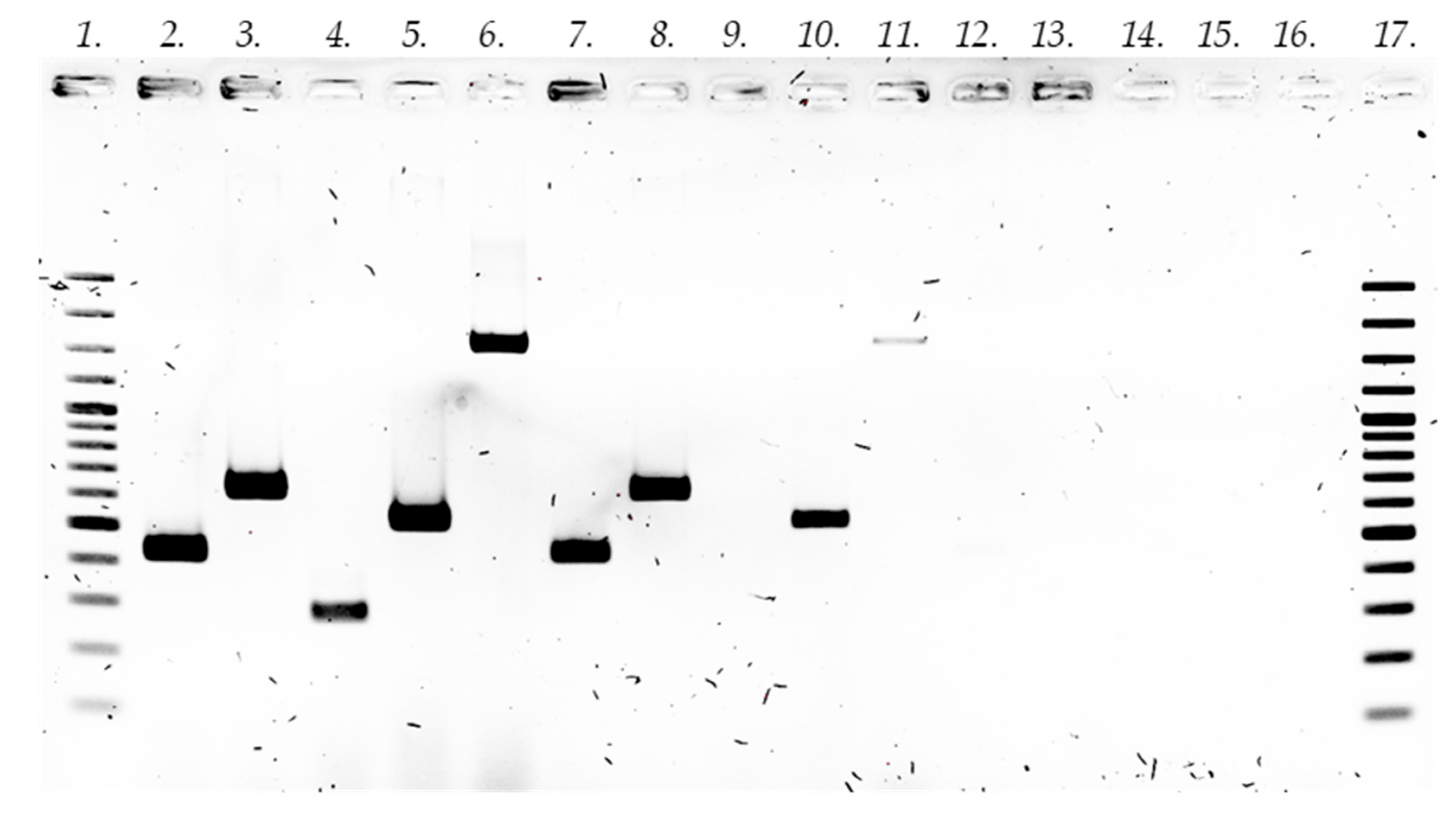
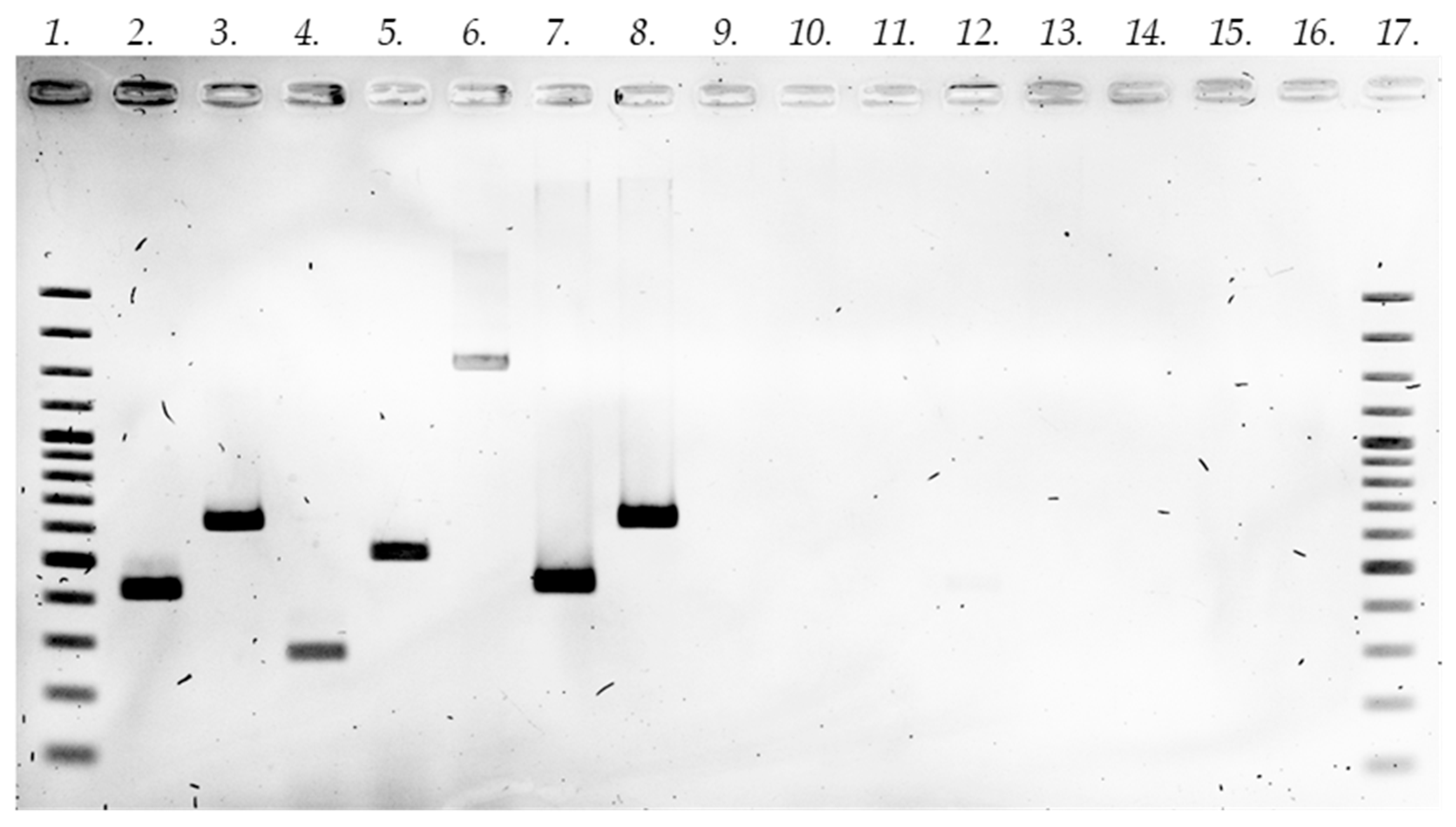
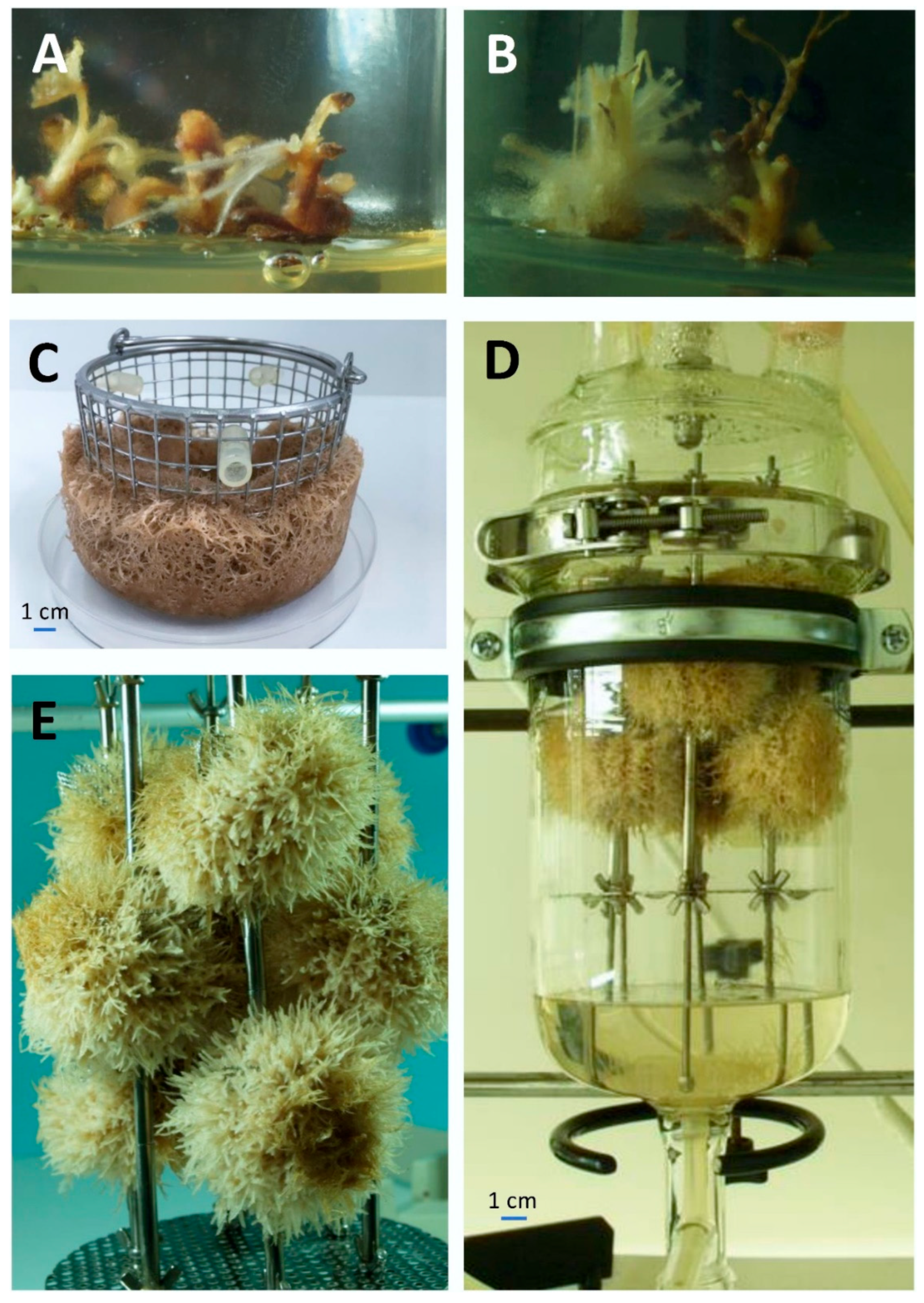
2.1. Establishment of In Vitro Root Cultures
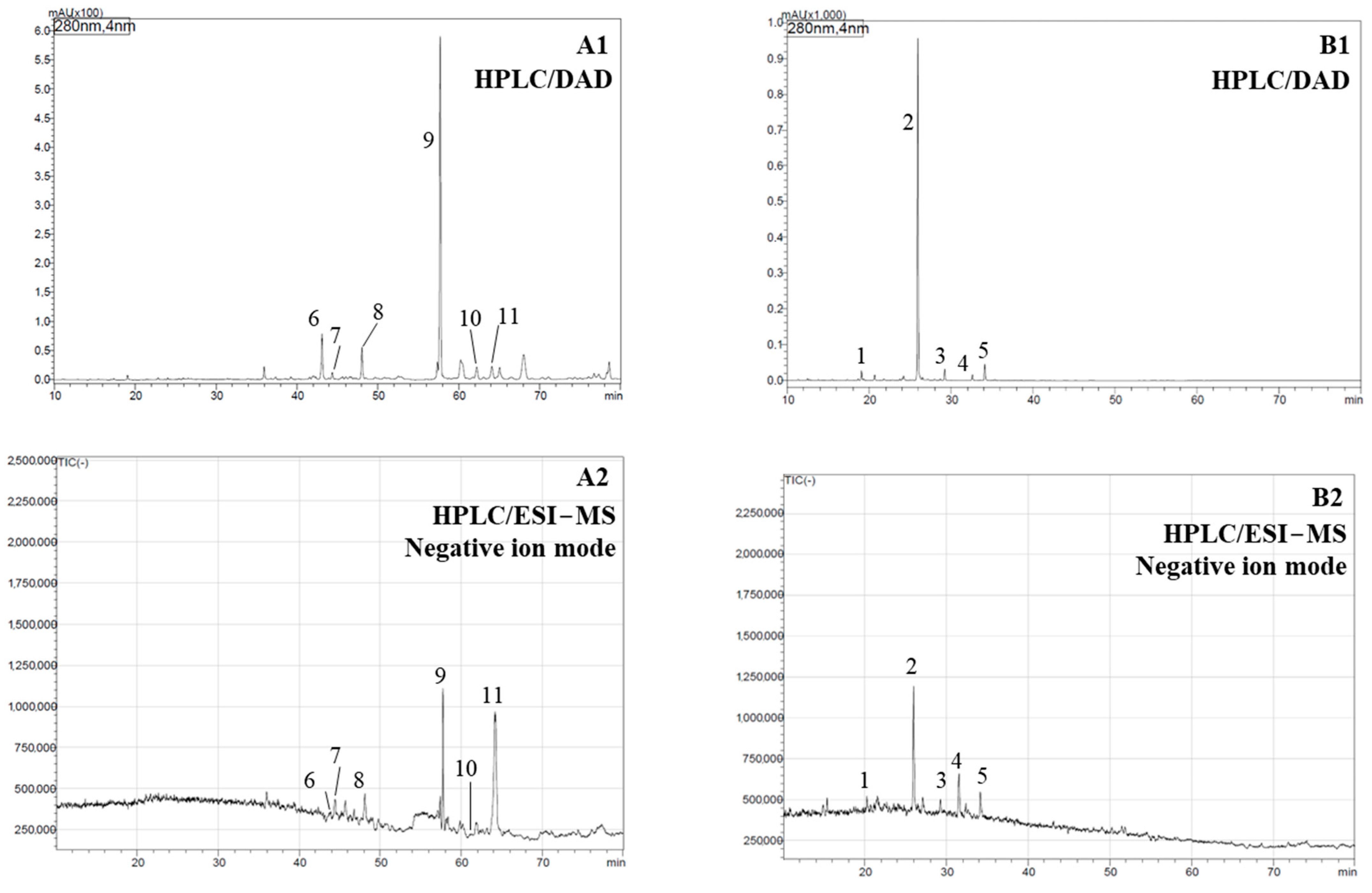
2.2. Phytochemical Analysis
| Peak No. a | Retention Time [min] | λmax [nm] | Pseudomolecular ion (m/z) | Tentative Identification | Plant Sample b | Ref. |
|---|---|---|---|---|---|---|
| 1 | 17.16 | 323 | [M − H]−: 385 | Sinapic acid hexose | NT | [41,42] |
| 2 | 25.93 | 229, 283, 331 | [M − H]−: 359 | Rosmarinic acid * | NT, TA1-3, TL1-2 | [33,43,44] |
| 3 | 29.21 | 328 | [M − H]−: 373 | Methyl rosmarinate | NT | [41] |
| 4 | 32.63 | 338 | [M − H]−: 313 | Salvianolic acid F | NT | [41] |
| 5 | 34.14 | 337 | [M − H]−: 313 | Salvianolic acid F isomer | NT | [41] |
| 6 | 43.02 | - | [M − H]−: 345 | Rosmanol | FG | [33,45] |
| 7 | 44.72 | 285 | [M − H]−: 347 | Hydroxycarnosic acid | FG | [44] |
| 8 | 50.79 | 260 | [M − H]−: 347 | Hydroxycarnosic acid isomer | FG | [33] |
| 9 | 58.66 | 222, 264 | [M + H]−: 297 | Cryptotanshinone * | FG | [33] |
| 10 | 62.67 | - | [M + H]−: 295 | Tanshinone IIA * | FG | [33] |
| 11 | 64.59 | - | [M + H]−: 315 | 1,6,6,9a-tetramethyl-1,2,4,5,5a,6,7,8,9,9a-decahydrophenanthro[1,2-b]furan-10,11-dione | FG | [33] |
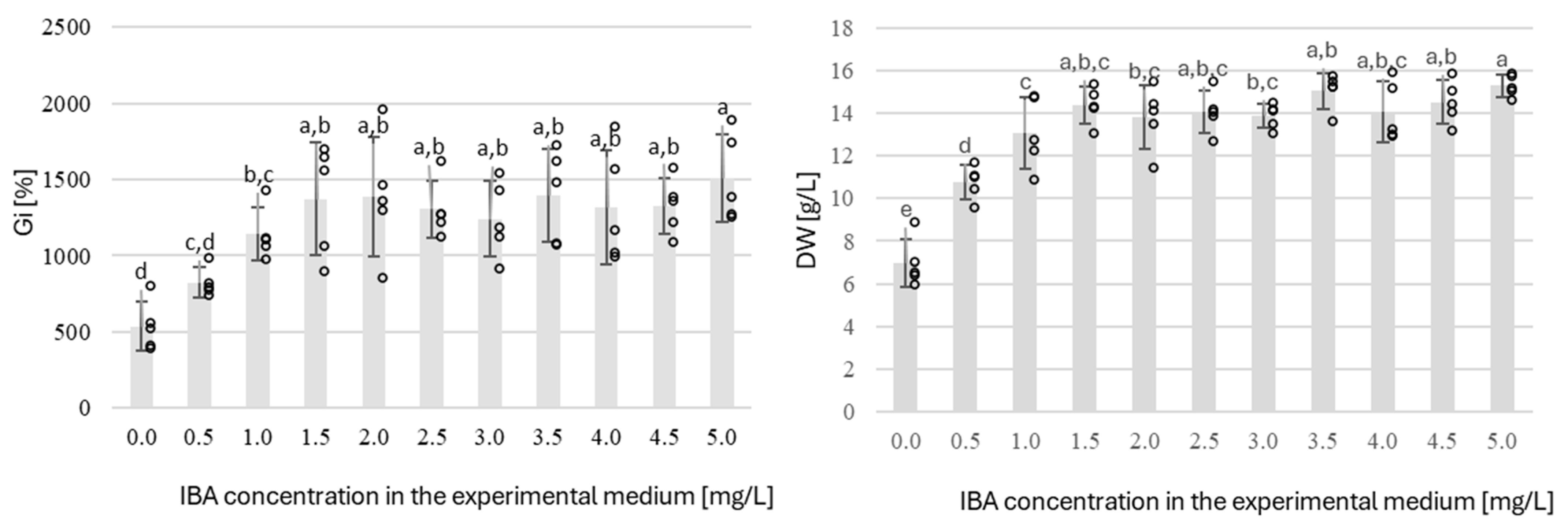
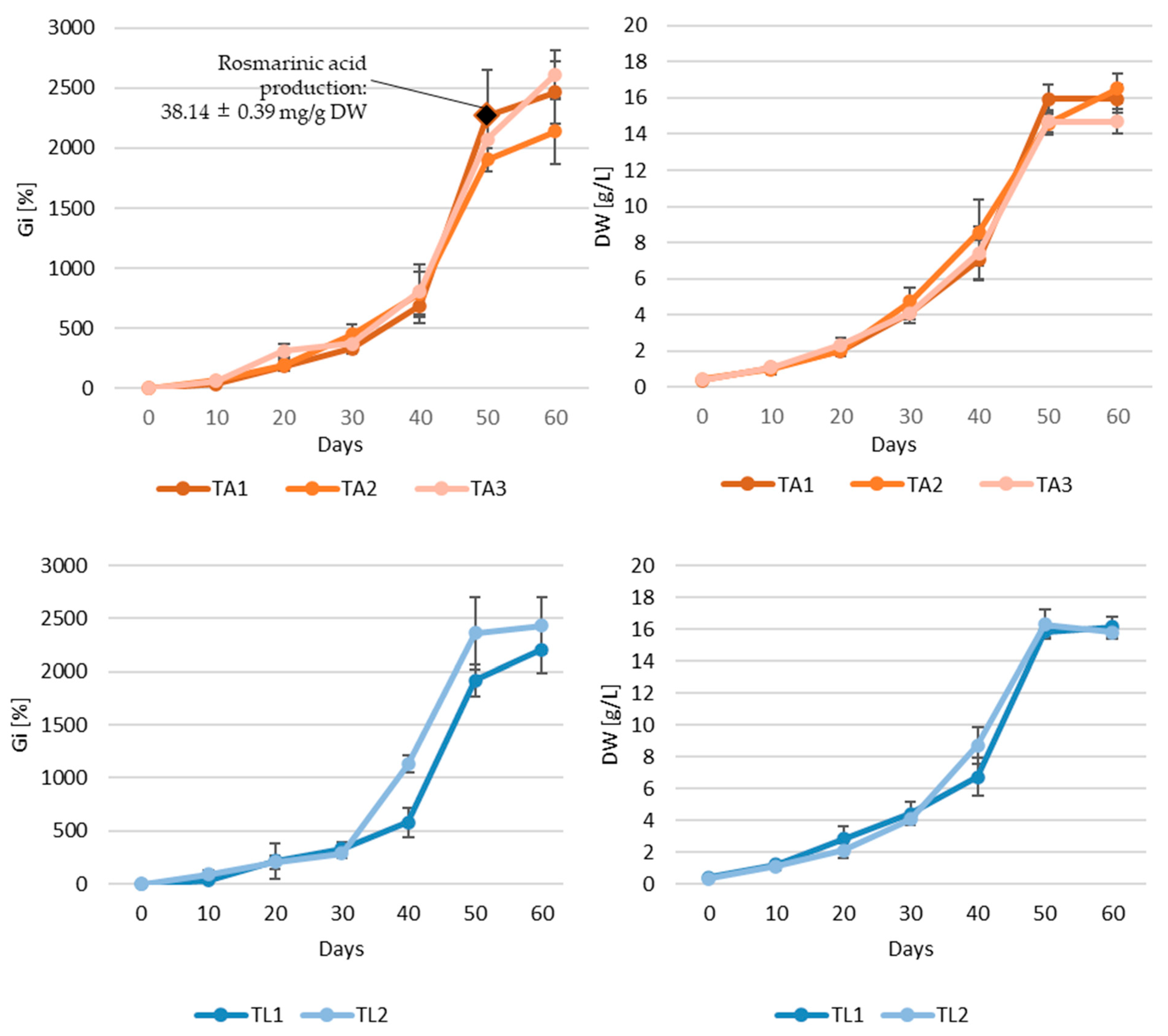
2.3. Shake Flask Cultures
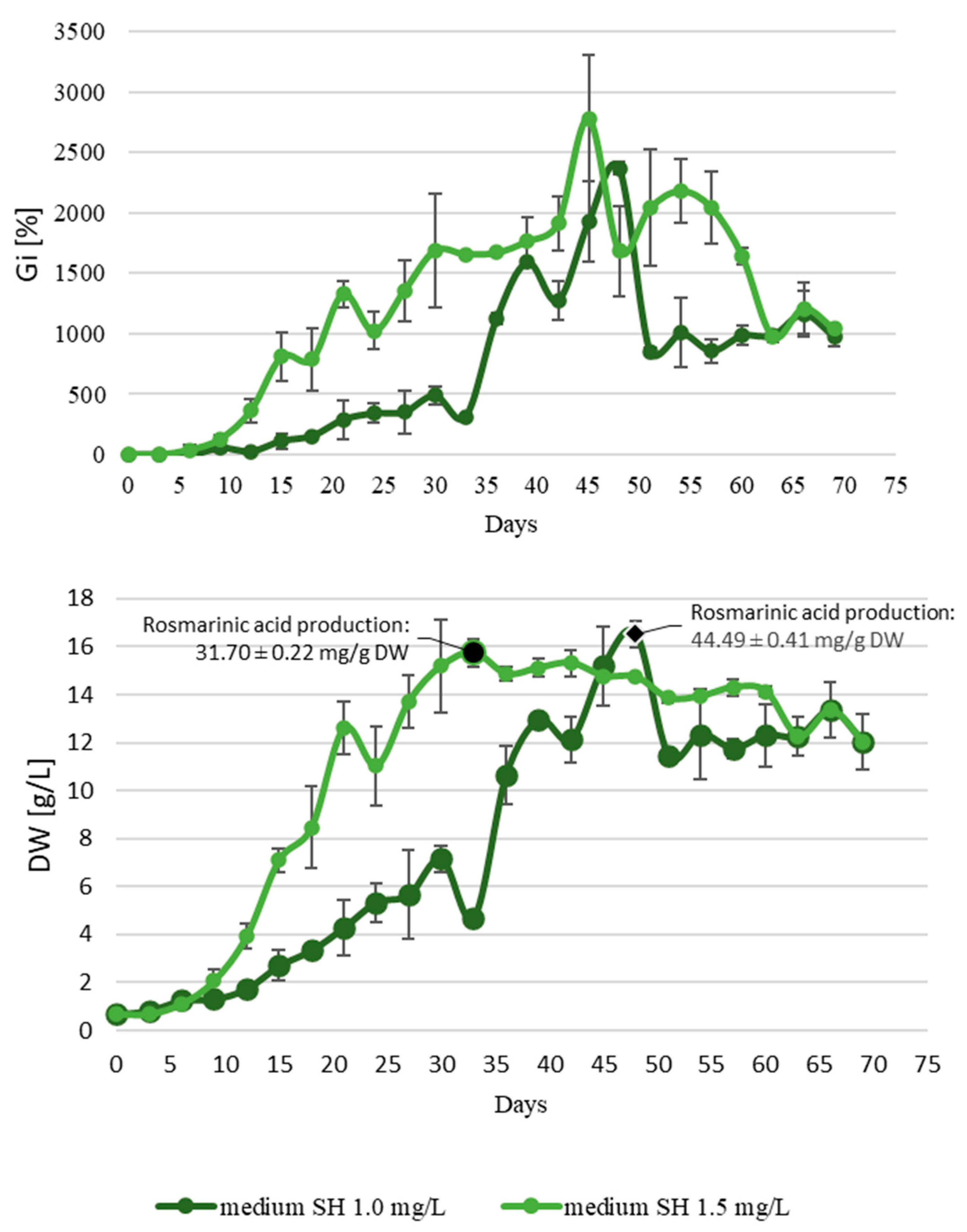
2.4. Bioreactor Experiments
3. Materials and Methods
3.1. Reagents and General Procedures
3.2. Plant Material
3.3. Establishment of Adventitious Root Culture
3.4. Establishment of Hairy Root Cultures and Confirmation of Transformation
3.5. Shake Flask Cultures
3.6. Bioreactor Experiments
3.7. Determination of Growth Parameters
3.8. Extraction Procedures
3.9. Qualitative and Quantitative HPLC-DAD-ESI-MS Analysis
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SH | Schenk–Hildebrandt |
| PGR | Plant growth regulator |
| IBA | Indole-3-butyric acid |
| NT | Adventitious root (non-transformed) |
| FG | Field grown |
| FW | Fresh weight |
| DW | Dried weight |
| Gi | Growth index |
| SB | The nutrient mist bioreactor |
| ICB | The modified air-lift bioreactor |
| RA | Rosmarinic acid |
| CT | Cryptotanshinone |
Appendix A

References
- Kabouche, A.; Kabouche, Z. Bioactive Diterpenoids of Salvia Species. Stud. Nat. Prod. Chem. 2008, 35, 753–833. [Google Scholar] [CrossRef]
- Bisio, A.; Pedrelli, F.; D’Ambola, M.; Labanca, F.; Schito, A.M.; Govaerts, R.; De Tommasi, N.; Milella, L. Quinone Diterpenes from Salvia Species: Chemistry, Botany, and Biological Activity. Phytochem. Rev. 2019, 18, 665–842. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Polyphenolics of Salvia—A Review. Phytochemistry 2002, 59, 117–140. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jiang, X.; Mashiguchi, K.; Yamaguchi, S.; Lu, S. Biosynthesis and Signal Transduction of Plant Growth Regulators and Their Effects on Bioactive Compound Production in Salvia miltiorrhiza (Danshen). Chin. Med. 2024, 19, 102. [Google Scholar] [CrossRef]
- Grdiša, M.; Jug-dujaković, M.; Lončarić, M.; Carović-Stanko, K.; Ninčević, T.; Liber, Z.; Radosavljević, I.; Šatović, Z. Dalmatian Sage (Salvia Officinalis L.): A Review of Biochemical Contents, Medical Properties and Genetic Diversity. Agric. Conspec. Sci. 2015, 80, 69–78. [Google Scholar]
- Birtić, S.; Dussort, P.; Pierre, F.-X.X.; Bily, A.C.; Roller, M. Carnosic Acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour. Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Luczkiewicz, M. White Sage (Salvia apiana)–a Ritual and Medicinal Plant of the Chaparral: Plant Characteristics in Comparison with Other Salvia Species. Planta Med. 2022, 88, 604–627. [Google Scholar] [CrossRef]
- Cvetkovikj, I.; Stefkov, G.; Karapandzova, M.; Kulevanova, S. Essential Oil Composition of Salvia fruticosa Mill. Populations from Balkan Peninsula. Maced. Pharm. Bull. 2015, 61, 19–26. [Google Scholar] [CrossRef]
- Li, M.-H.; Peng, Y.; Xiao, P.-G. Distribution of Tanshinones in the Genus Salvia (Family Lamiaceae) from China and Its Systematic Significance. J. Syst. Evol. 2010, 48, 118–122. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, W.; Huang, L. Tanshinones, Critical Pharmacological Components in Salvia miltiorrhiza. Front. Pharmacol. 2019, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Dougué Kentsop, R.A.; Devi, P.; Copetta, A.; Ruffoni, B.; Parisi, V.; Bisio, A.; Iobbi, V. Salvia Species: Biotechnological Strategies Applied to In Vitro Cultures for the Controlled Production of Bioactive Diterpenoids. Agronomy 2024, 14, 835. [Google Scholar] [CrossRef]
- Marchev, A.; Haas, C.; Schulz, S.; Georgiev, V.; Steingroewer, J.; Bley, T.; Pavlov, A. Sage in Vitro Cultures: A Promising Tool for the Production of Bioactive Terpenes and Phenolic Substances. Biotechnol. Lett. 2014, 36, 211–221. [Google Scholar] [CrossRef] [PubMed]
- D’Amelia, V.; Ruggiero, A.; Tranchida-Lombardo, V.; Leone, A.; Tucci, M.; Docimo, T. Biosynthesis of Salvia Specialized and Biotechnological to Increase Their Production. In Salvia Biotechnology; Georgiev, V., Pavlov, A., Eds.; Springer International Publishing: Cham, Switzerland, 2018; p. 241. ISBN 9783319739007. [Google Scholar]
- Kuźma, Ł. Biosynthesis of Biological Active Abietane Diterpenoids in Transformed Root Cultures of Salvia Species. In Plant Cell and Tissue Differentiation and Secondary Metabolites; Reference Series in Phytochemistry; Ramawat, K., Ekiert, H., Goyal, S., Eds.; Springer International Publishing: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Hussain, M.J.; Abbas, Y.; Nazli, N.; Fatima, S.; Drouet, S.; Hano, C.; Abbasi, B.H. Root Cultures, a Boon for the Production of Valuable Compounds: A Comparative Review. Plants 2022, 11, 439. [Google Scholar] [CrossRef]
- Krol, A.; Kokotkiewicz, A.; Gorniak, M.; Naczk, A.M.; Zabiegala, B.; Gebalski, J.; Graczyk, F.; Zaluski, D.; Bucinski, A.; Luczkiewicz, M. Evaluation of the Yield, Chemical Composition and Biological Properties of Essential Oil from Bioreactor-Grown Cultures of Salvia apiana Microshoots. Sci. Rep. 2023, 13, 7141. [Google Scholar] [CrossRef] [PubMed]
- Schenk, R.U.; Hildebrandt, A.C. Medium and Techniques for Induction and Growth of Monocotyledonous and Dicotyledonous Plant Cell Cultures. Can. J. Bot. 1972, 50, 199–204. [Google Scholar] [CrossRef]
- Luczkiewicz, M.; Jesionek, A.; Kokotkiewicz, A.; Migas, P.; Mardarowicz, M.; Szreniawa-Sztajnert, A.; Zabiegala, B.; Bucinski, A. Production of Essential Oils from in Vitro Cultures of Caryopteris Species and Comparison of Their Concentrations with in Vivo Plants. Acta Physiol. Plant 2015, 37, 58. [Google Scholar] [CrossRef]
- Wilczańska-Barska, A.; Krauze-Baranowska, M.; Majdan, M.; Głód, D. Wild Type Root Cultures of Scutellaria Barbata. BioTechnologia 2011, 4, 369–377. [Google Scholar] [CrossRef]
- Shimomura, K.; Kitazawa, T.; Okamura, N.; Yagi, A. Tanshinone Production in Adventitious Roots and Regenerates of Salvia miltiorrhiza. J. Nat. Prod. 1991, 54, 1583–1587. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Skrzypek, Z.; Wysokińska, H. Diterpenoids and Triterpenoids in Hairy Roots of Salvia sclarea. Plant Cell Tissue Organ. Cult. 2006, 84, 171–179. [Google Scholar] [CrossRef]
- Kentsop, R.A.D.; Iobbi, V.; Donadio, G.; Ruffoni, B.; De Tommasi, N.; Bisio, A. Abietane Diterpenoids from the Hairy Roots of Salvia corrugata. Molecules 2021, 26, 5144. [Google Scholar] [CrossRef]
- Hu, Z.B.; Alfermann, A.W. Diterpenoid Production in Hairy Root Cultures of Salvia miltiorrhiza. Phytochemistry 1993, 32, 699–703. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Królicka, A.; Wysokińska, H. Establishment of Salvia officinalis L. Hairy Root Cultures for the Production of Rosmarinic Acid. Z. Fur Naturforschung Sect. C J. Biosci. 2006, 61, 351–356. [Google Scholar] [CrossRef]
- Khoshsokhan, F.; Babalar, M.; Salami, S.A.; Sheikhakbari-Mehr, R.; Mirjalili, M.H. Rosmarinic Acid Production in Hairy Root Cultures of Salvia nemorosa L. (Lamiaceae). Biocatal. Agric. Biotechnol. 2022, 45, 102494. [Google Scholar] [CrossRef]
- Attaran Dowom, S.; Abrishamchi, P.; Radjabian, T.; Salami, S.A. Elicitor-Induced Phenolic Acids Accumulation in Salvia virgata Jacq. Hairy Root Cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2022, 148, 107–117. [Google Scholar] [CrossRef]
- Wang, Q.J.; Zheng, L.P.; Yuan, H.Y.; Wang, J.W. Propagation of Salvia miltiorrhiza from Hairy Root Explants via Somatic Embryogenesis and Tanshinone Content in Obtained Plants. Ind. Crops Prod. 2013, 50, 648–653. [Google Scholar] [CrossRef]
- Kuźma, Ł.; Kisiel, W.; Królicka, A.; Wysokińska, H. Genetic Transformation of Salvia austriaca by Agrobacterium Rhizogenes and Diterpenoid Isolation. Pharmazie 2011, 66, 904–907. [Google Scholar] [CrossRef] [PubMed]
- Christey, M.C. Use of Ri-Mediated Transformation for Production of Transgenic Plants. Vitr. Cell. Dev. Biol. Plant 2001, 37, 687–700. [Google Scholar] [CrossRef]
- Wojciechowska, M.; Owczarek, A.; Kiss, A.K.; Grąbkowska, R.; Olszewska, M.A.; Grzegorczyk-Karolak, I. Establishment of Hairy Root Cultures of Salvia bulleyana Diels for Production of Polyphenolic Compounds. J. Biotechnol. 2020, 318, 10–19. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Zhang, Y.L.; Song, J.Y. Production of Lithospermic Acid B and Rosmarinic Acid in Hairy Root Cultures of Salvia miltiorrhiza. J. Ind. Microbiol. Biotechnol. 1999, 22, 133–138. [Google Scholar] [CrossRef]
- Hu, J.; Wang, F.; Liang, F.; Wu, Z.; Jiang, R.; Li, J.; Chen, J.; Qiu, S.; Wang, J.; Zhang, Y.; et al. Identification of Abietane-Type Diterpenoids and Phenolic Acids Biosynthesis Genes in Salvia apiana Jepson Through Full-Length Transcriptomic and Metabolomic Profiling. Front. Plant Sci. 2022, 13, 919025. [Google Scholar] [CrossRef]
- Grzegorczyk, I.; Bilichowski, I.; Mikiciuk-Olasik, E.; Wysokińska, H. In Vitro Cultures of Salvia officinalis L. As a Source of Antioxidant Compounds. Acta Soc. Bot. Pol. 2005, 74, 17–21. [Google Scholar] [CrossRef]
- Caruso, J.L.; Callahan, J.; DeChant, C.; Winget, G.D.; Jayasimhulu, K. Carnosic Acid in Green Callus and Regenerated Shoots of Rosmarinus Officinalis. Plant Cell Rep. 2000, 19, 500–503. [Google Scholar] [CrossRef]
- Appiah, K.S.; Mardani, H.K.; Omari, R.A.; Eziah, V.Y.; Ofosu-Anim, J.; Onwona-Agyeman, S.; Amoatey, C.A.; Kawada, K.; Katsura, K.; Oikawa, Y.; et al. Involvement of Carnosic Acid in the Phytotoxicity of Rosmarinus Officinalis Leaves. Toxins 2018, 10, 498. [Google Scholar] [CrossRef]
- Del Baño, M.J.; Lorente, J.; Castillo, J.; Benavente-García, O.; Del Río, J.A.; Ortuño, A.; Quirin, K.W.; Gerard, D. Phenolic Diterpenes, Flavones, and Rosmarinic Acid Distribution during the Development of Leaves, Flowers, Stems, and Roots of Rosmarinus Officinalis. Antioxidant Activity. J. Agric. Food Chem. 2003, 51, 4247–4253. [Google Scholar] [CrossRef] [PubMed]
- Modarres, M.; Asili, J.; Lahouti, M.; Gangali, A.; Iranshahy, M.; Sahebkar, A. Simultaneous Determination of Rosmarinic Acid, Salvianolic Acid B and Caffeic Acid in Salvia leriifolia Benth. Root, Leaf and Callus Extracts Using a High-Performance Liquid Chromatography with Diode-Array Detection Technique. J. Liq. Chromatogr. Relat. Technol. 2014, 37, 1721–1730. [Google Scholar] [CrossRef]
- He, C.; Wei, J.; Jin, Y.; Chen, S. Bioactive Components of the Roots of Salvia miltiorrhizae: Changes Related to Harvest Time and Germplasm Line. Ind. Crops Prod. 2010, 32, 313–317. [Google Scholar] [CrossRef]
- Song, Z.; Li, X. Expression Profiles of Rosmarinic Acid Biosynthesis Genes in Two Salvia miltiorrhiza Lines with Differing Water-Soluble Phenolic Contents. Ind. Crops Prod. 2015, 71, 24–30. [Google Scholar] [CrossRef]
- Grzegorczyk-Karolak, I.; Krzemińska, M.; Kiss, A.K.; Olszewska, M.A.; Owczarek, A. Phytochemical Profile and Antioxidant Activity of Aerial and Underground Parts of Salvia bulleyana Diels. Plants. Metab. 2020, 10, 497. [Google Scholar] [CrossRef]
- Zengin, G.; Atasagun, B.; Zakariyyah Aumeeruddy, M.; Saleem, H.; Mollica, A.; Babak Bahadori, M.; Mahomoodally, M.F. Phenolic Profiling and in Vitro Biological Properties of Two Lamiaceae Species (Salvia modesta and Thymus argaeus): A Comprehensive Evaluation. Ind. Crops Prod. 2019, 128, 308–314. [Google Scholar] [CrossRef]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. Phytochemical Composition and Bioactive Effects of Salvia africana, Salvia officinalis ‘Icterina’ and Salvia mexicana Aqueous Extracts. Molecules 2019, 24, 4327. [Google Scholar] [CrossRef] [PubMed]
- Afonso, A.F.; Pereira, O.R.; Fernandes, Â.S.F.; Calhelha, R.C.; Silva, A.M.S.; Ferreira, I.C.F.R.; Cardoso, S.M. The Health-Benefits and Phytochemical Profile of Salvia Apiana and Salvia Farinacea Var. Victoria Blue Decoctions. Antioxidants 2019, 8, 241. [Google Scholar] [CrossRef] [PubMed]
- Velamuri, R.; Sharma, Y.; Fagan, J.; Schaefer, J. Application of UHPLC-ESI-QTOF-MS in Phytochemical Profiling of Sage (Salvia officinalis) and Rosemary (Rosmarinus officinalis). Planta Medica Int. Open 2020, 7, e133–e144. [Google Scholar] [CrossRef]
- González, A.G.; Aguiar, Z.E.; Grillo, T.A.; Luis, J.G. Diterpenes and Diterpene Quinones from the Roots of Salvia apiana. Phytochemistry 1992, 31, 1691–1695. [Google Scholar] [CrossRef]
- Wang, F.; Bao, Y.; Yang, F.; Yuan, L.; Han, X.; Huang, Y.; Wei, Y.; Zhang, L.; Yang, Z.; Yang, D. Content Determination and Chemical Clustering Analysis of Tanshinone and Salvianolic Acid in Salvia spp. Metabolites 2024, 14, 441. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, D.; Liang, Z.; Liu, J.; Yan, K.; Zhu, Y.; Yang, S. Climatic Factors Control the Geospatial Distribution of Active Ingredients in Salvia miltiorrhiza Bunge in China. Sci. Rep. 2019, 9, 904. [Google Scholar] [CrossRef]
- Tada, H.; Ikeda, Y.; Omoto, T.; Shimomurat, K.; Ishimaru, K. Rosmarinic Acid and Related Phenolics in Adventitious Root Cultures of Ocimum basilicum L. Plant Tissue Cult. Lett. 1996, 13, 69–71. [Google Scholar] [CrossRef]
- Ruffoni, B.; Bertoli, A.; Pistelli, L.; Pistelli, L. Micropropagation of Salvia Wagneriana Polak and Hairy Root Cultures with Rosmarinic Acid Production. Nat. Prod. Res. 2016, 30, 2538–2544. [Google Scholar] [CrossRef]
- Shi, M.; Luo, X.; Ju, G.; Li, L.; Huang, S.; Zhang, T.; Wang, H.; Kai, G. Enhanced Diterpene Tanshinone Accumulation and Bioactivity of Transgenic Salvia miltiorrhiza Hairy Roots by Pathway Engineering. J. Agric. Food Chem. 2016, 64, 2523–2530. [Google Scholar] [CrossRef]
- Cheng, Q.; He, Y.; Li, G.; Liu, Y.; Gao, W.; Huang, L. Effects of Combined Elicitors on Tanshinone Metabolic Profiling and SmCPS Expression in Salvia miltiorrhiza Hairy Root Cultures. Molecules 2013, 18, 7473–7485. [Google Scholar] [CrossRef]
- Contreras, A.; Leroy, B.; Mariage, P.A.; Wattiez, R. Proteomic Analysis Reveals Novel Insights into Tanshinones Biosynthesis in Salvia miltiorrhiza Hairy Roots. Sci. Rep. 2019, 9, 5768. [Google Scholar] [CrossRef]
- Sarhadi, E.; Ebrahimi, S.N.; Hadjiakhoondi, A.; Manayi, A. Chemical Composition and Antioxidant Activity of Root Essential Oil of Different Salvia leriifolia Populations. J. Essent. Oil Bear. Plants 2021, 24, 209–217. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Bulgakov, V.P.; Inyushkina, Y.V.; Fedoreyev, S.A. Rosmarinic Acid and Its Derivatives: Biotechnology and Applications. Crit. Rev. Biotechnol. 2012, 32, 203–217. [Google Scholar] [CrossRef]
- Marchev, A.S.; Yordanova, Z.P.; Georgiev, M.I. Critical Reviews in Biotechnology Green (Cell) Factories for Advanced Production of Plant Secondary Metabolites. Biotechnology 2020, 40, 443–458. [Google Scholar] [CrossRef]
- Marchev, A.S.; Vasileva, L.V.; Amirova, K.M.; Savova, M.S.; Koycheva, I.K.; Balcheva-Sivenova, Z.P.; Vasileva, S.M.; Georgiev, M.I. Rosmarinic Acid—From Bench to Valuable Applications in Food Industry. Trends Food Sci. Technol. 2021, 117, 182–193. [Google Scholar] [CrossRef]
- Rahmat, E.; Kang, Y. Adventitious Root Culture for Secondary Metabolite Production in Medicinal Plants: A Review. J. Plant Biotechnol. 2019, 46, 143–157. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Alegre, L. Subcellular Compartmentation of the Diterpene Carnosic Acid and Its Derivatives in the Leaves of Rosemary. Plant Physiol. 2001, 125, 1094–1102. [Google Scholar] [CrossRef]
- Karam, N.S.; Jawad, F.M.; Arikat, N.A.; Shibli, R.A. Growth and Rosmarinic Acid Accumulation in Callus, Cell Suspension, and Root Cultures of Wild Salvia fruticosa. Plant Cell Tissue Organ. Cult. 2003, 73, 117–121. [Google Scholar] [CrossRef]
- Sarropoulou, V.; Paloukopoulou, C.; Karioti, A.; Maloupa, E.; Grigoriadou, K. Rosmarinic Acid Production from Origanum dictamnus L. Root Liquid Cultures In Vitro. Plants 2023, 12, 299. [Google Scholar] [CrossRef]
- Batra, J.; Dutta, A.; Singh, D.; Kumar, S.; Sen, J. Growth and Terpenoid Indole Alkaloid Production in Catharanthus Roseus Hairy Root Clones in Relation to Left- and Right-Termini-Linked Ri T-DNA Gene Integration. Plant Cell Rep. 2004, 23, 148–154. [Google Scholar] [CrossRef]
- Li, B.; Wang, B.; Li, H.; Peng, L.; Ru, M.; Liang, Z.; Yan, X.; Zhu, Y. Establishment of Salvia castanea Diels f. Tomentosa Stib. Hairy Root Cultures and the Promotion of Tanshinone Accumulation and Gene Expression with Ag+, Methyl Jasmonate, and Yeast Extract Elicitation. Protoplasma 2016, 253, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Hu, Z.; Tan, R.X.; Wu, J. Efficient Production and Recovery of Diterpenoid Tanshinones in Salvia miltiorrhiza Hairy Root Cultures with in Situ Adsorption, Elicitation and Semi-Continuous Operation. J. Biotechnol. 2005, 119, 416–424. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, S.; Mishra, S.; Srivastava, V. Bioreactor Technology for Hairy Roots Cultivation. In Bioprocessing of Plant In Vitro Systems; Pavlov, A., Bley, T., Eds.; Springer International Publishing AG: Cham, Switzerland, 2018; pp. 483–506. [Google Scholar]
- Kuźma, Ł.; Kaiser, M.; Wysokińska, H. The Production and Antiprotozoal Activity of Abietane Diterpenes in Salvia Austriaca Hairy Roots Grown in Shake Flasks and Bioreactor. Prep. Biochem. Biotechnol. 2017, 47, 58–66. [Google Scholar] [CrossRef]
- Jaremicz, Z.; Luczkiewicz, M.; Kokotkiewicz, A.; Krolicka, A.; Sowinski, P. Production of Tropane Alkaloids in Hyoscyamus Niger (Black Henbane) Hairy Roots Grown in Bubble-Column and Spray Bioreactors. Biotechnol. Lett. 2014, 36, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Grzegorczyk, I.; Wysokińska, H. Antioxidant Compounds in Salvia officinalis L. Shoot and Hairy Root Cultures in the Nutrient Sprinkle Bioreactor. Acta Soc. Bot. Pol. 2011, 79, 7–10. [Google Scholar] [CrossRef]
- Mishra, B.N.; Ranjan, R. Growth of Hairy-Root Cultures in Various Bioreactors for the Production of Secondary Metabolites. Biotechnol. Appl. Biochem. 2008, 49, 1–10. [Google Scholar] [CrossRef]
- Mirmazloum, I.; Slavov, A.K.; Marchev, A.S. The Untapped Potential of Hairy Root Cultures and Their Multiple Applications. Int. J. Mol. Sci. 2024, 25, 12682. [Google Scholar] [CrossRef]
- Baque, M.A.; Moh, S.-H.; Lee, E.-J.; Zhong, J.-J.; Paek, K.-Y. Production of Biomass and Useful Compounds from Adventitious Roots of High-Value Added Medicinal Plants Using Bioreactor. Biotechnol. Adv. 2012, 30, 1255–1267. [Google Scholar] [CrossRef]
- Bekesiova, I.; Nap, J.-P.; Mlynarova, L. Isolation of High Quality DNA and RNA from Leaves of the Carnivorous Plant Drosera rotundifolia. Plant Mol. Biol. Rep. 1999, 17, 269–277. [Google Scholar]
- Szopa, A.; Kokotkiewicz, A.; Luczkiewicz, M.; Ekiert, H. Schisandra Lignans Production Regulated by Different Bioreactor Type. J. Biotechnol. 2017, 247, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, M.; Imran, M.; Aslam Gondal, T.; Imran, A.; Shahbaz, M.; Muhammad Amir, R.; Wasim Sajid, M.; Batool Qaisrani, T.; Atif, M.; Hussain, G.; et al. Therapeutic Potential of Rosmarinic Acid: A Comprehensive Review. Appl. Sci. 2019, 9, 3139. [Google Scholar] [CrossRef]

| Compound | Roots of the Intact Plant | Adventitious Root Culture | Transformed Root Culture | ||||
|---|---|---|---|---|---|---|---|
| TA1 | TA2 | TA3 | TL1 | TL2 | |||
| Rosmarinic acid | ND | 17.22 ± 0.33 | 12.76 ± 0.09 | 11.01 ± 0.28 | 9.83 ± 0.19 | 15.32 ± 0.39 | 12.97 ± 0.06 |
| Cryptotanshinone | 3.71 ± 0.20 | ND | ND | ND | ND | ND | ND |
| Experiment Designation | Root Line a | Culture Type | Lighting Conditions | PGRs (mg/L) | Growth Period (days) | Harvesting Interval (days) | No of Replicates Per Treatment | Phytochemical Analysis (day) b |
|---|---|---|---|---|---|---|---|---|
| EX0 | NT | agitated | darkness | IBA (1.0) | 21 | - | - | 21 |
| TA1 | agitated c | darkness | - | 21 | - | - | 21 | |
| TA2 | agitated c | darkness | - | 21 | - | - | 21 | |
| TA3 | agitated c | darkness | - | 21 | - | - | 21 | |
| TL1 | agitated c | darkness | - | 21 | - | - | 21 | |
| TL2 | agitated c | darkness | - | 21 | - | - | 21 | |
| EX1 | NT | agitated c | darkness | IBA (0.5–5.0 with 0.5 increments) | 48 | - | 6 | - |
| EX2 | NT | agitated c | darkness | IBA (1.0) | 69 | 3 | 3 | 48 |
| NT | agitated c | darkness | IBA (1.5) | 69 | 3 | 3 | 33 | |
| NT | agitated c | photoperiod | IBA (1.0) | 45 | 3 | 3 | - | |
| EX3 | NT | ICB d | darkness | IBA (1.0) | 40 | - | 3 | 40 |
| NT | ICB d | darkness | IBA (1.0) | 48 | - | 3 | 48 | |
| NT | ICB d | darkness | IBA (1.5) | 40 | - | 3 | 40 | |
| NT | ICB d | darkness | IBA (1.5) | 48 | - | 3 | 48 | |
| NT | SB e | darkness | IBA (1.0) | 40 | - | 3 | 40 | |
| NT | SB e | darkness | IBA (1.0) | 48 | - | 3 | 48 | |
| NT | SB e | darkness | IBA (1.5) | 40 | - | 3 | 40 | |
| NT | SB e | darkness | IBA (1.5) | 48 | - | 3 | 48 | |
| EX4 | TA1 | agitated c | darkness | - | 60 | 10 | 6 | 50 |
| TA2 | agitated c | darkness | - | 60 | 10 | 6 | - | |
| TA3 | agitated c | darkness | - | 60 | 10 | 6 | - | |
| TL1 | agitated c | darkness | - | 60 | 10 | 6 | - | |
| TL2 | agitated c | darkness | - | 60 | 10 | 6 | - | |
| EX5 | TA1 | ICB d | darkness | - | 60 | - | 3 | 60 |
| TA1 | SB e | darkness | - | 60 | - | 3 | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krol, A.; Kokotkiewicz, A.; Krolicka, A.; Hinc, K.; Badura, A.; Lorenc, A.; Marzec-Wroblewska, U.; Bucinski, A.; Kuzma, L.; Luczkiewicz, M. Adventitious and Hairy Root Cultures of Salvia apiana as a Source of Rosmarinic Acid. Int. J. Mol. Sci. 2025, 26, 3138. https://doi.org/10.3390/ijms26073138
Krol A, Kokotkiewicz A, Krolicka A, Hinc K, Badura A, Lorenc A, Marzec-Wroblewska U, Bucinski A, Kuzma L, Luczkiewicz M. Adventitious and Hairy Root Cultures of Salvia apiana as a Source of Rosmarinic Acid. International Journal of Molecular Sciences. 2025; 26(7):3138. https://doi.org/10.3390/ijms26073138
Chicago/Turabian StyleKrol, Agata, Adam Kokotkiewicz, Aleksandra Krolicka, Krzysztof Hinc, Anna Badura, Andzelika Lorenc, Urszula Marzec-Wroblewska, Adam Bucinski, Lukasz Kuzma, and Maria Luczkiewicz. 2025. "Adventitious and Hairy Root Cultures of Salvia apiana as a Source of Rosmarinic Acid" International Journal of Molecular Sciences 26, no. 7: 3138. https://doi.org/10.3390/ijms26073138
APA StyleKrol, A., Kokotkiewicz, A., Krolicka, A., Hinc, K., Badura, A., Lorenc, A., Marzec-Wroblewska, U., Bucinski, A., Kuzma, L., & Luczkiewicz, M. (2025). Adventitious and Hairy Root Cultures of Salvia apiana as a Source of Rosmarinic Acid. International Journal of Molecular Sciences, 26(7), 3138. https://doi.org/10.3390/ijms26073138









