Enhancing the Quality of Indoor-Grown Basil Microgreens with Low-Dose UV-B or UV-C Light Supplementation
Abstract
1. Introduction
2. Results
2.1. Effects of UV Light on Plant Morphology and Biomass Accumulation
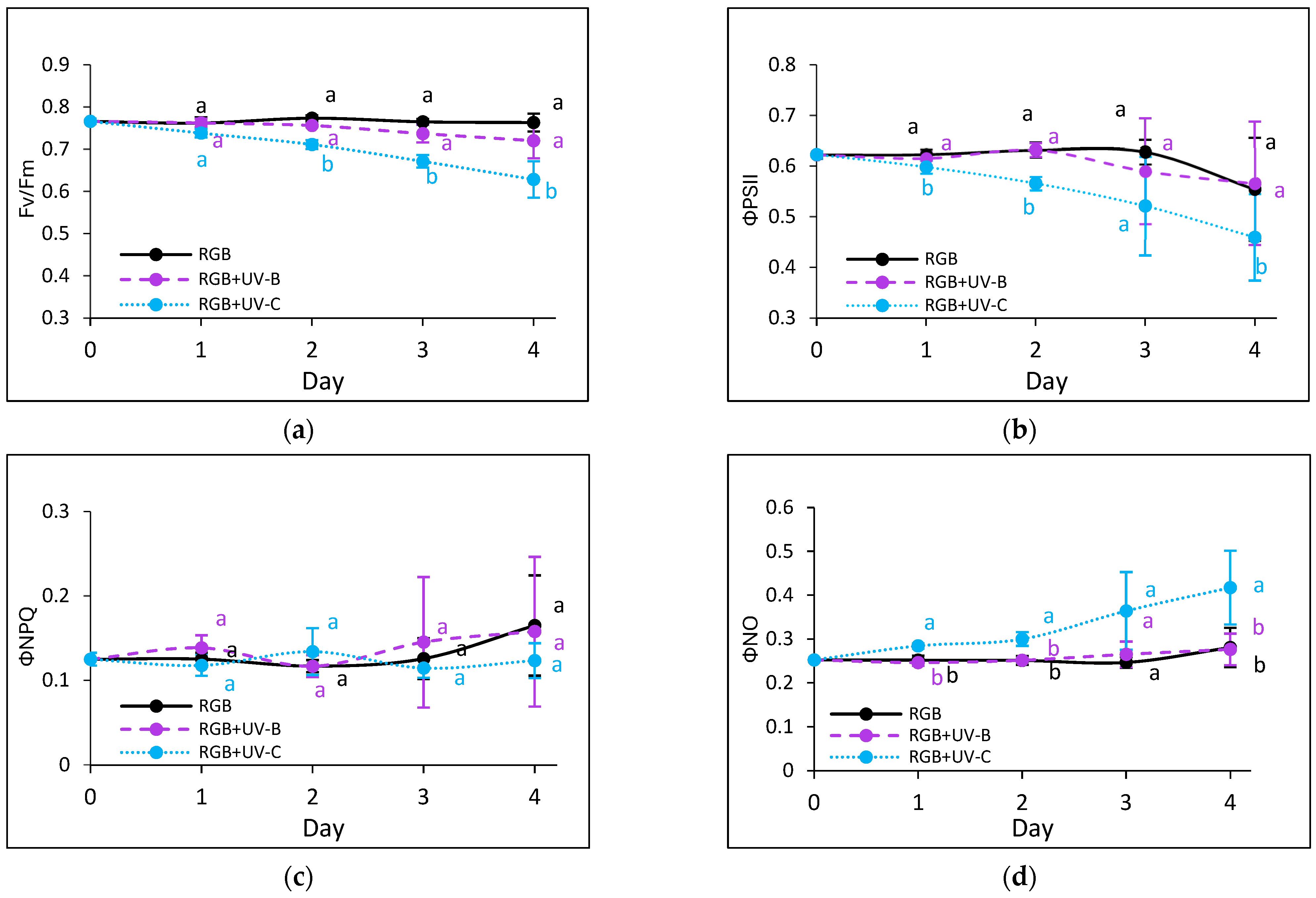
2.2. Photosynthetic Activity Under Short-Term Exposition to UV-B or UV-C Light
2.2.1. Effects of UV Light on Photosynthetic Pigments and Soluble Leaf Protein Content
2.2.2. Effects of UV Light on RuBisCO Abundance
2.2.3. Effects of UV Light on Photosynthetic Efficiency of PSII
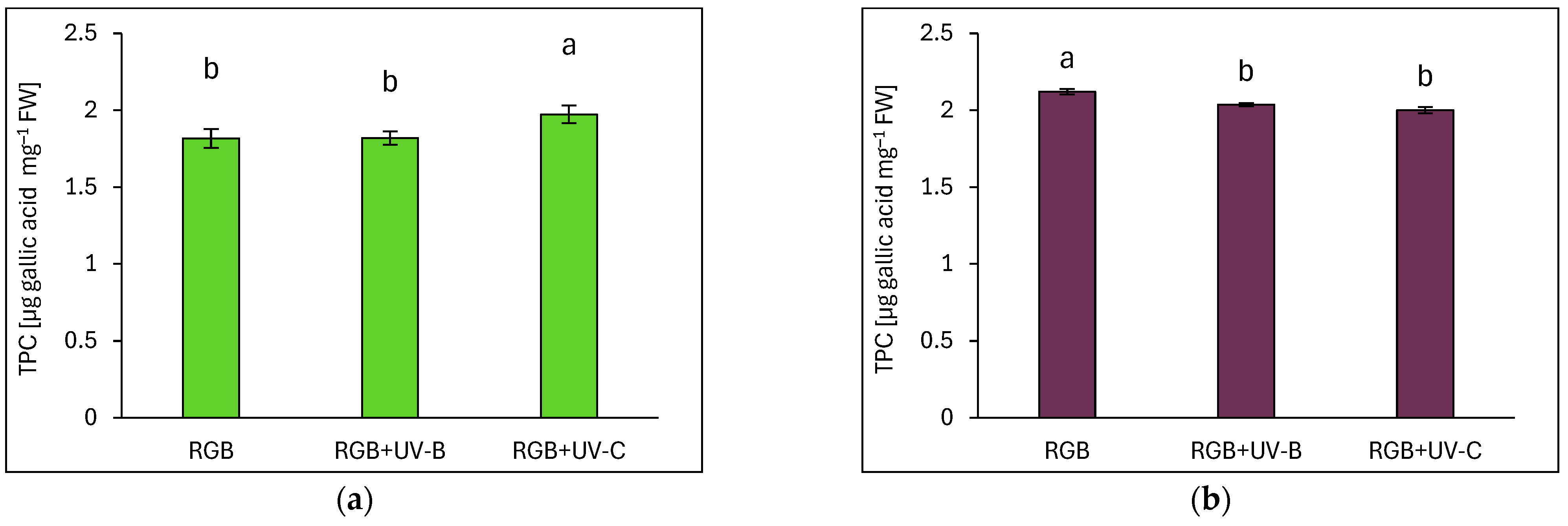
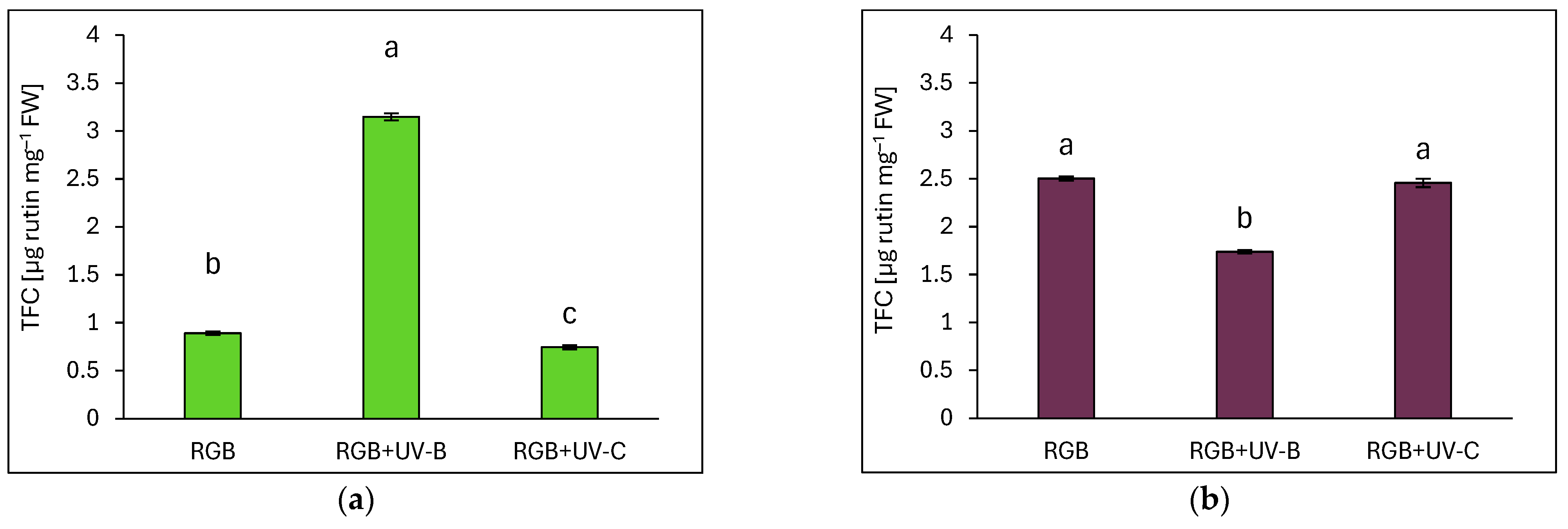
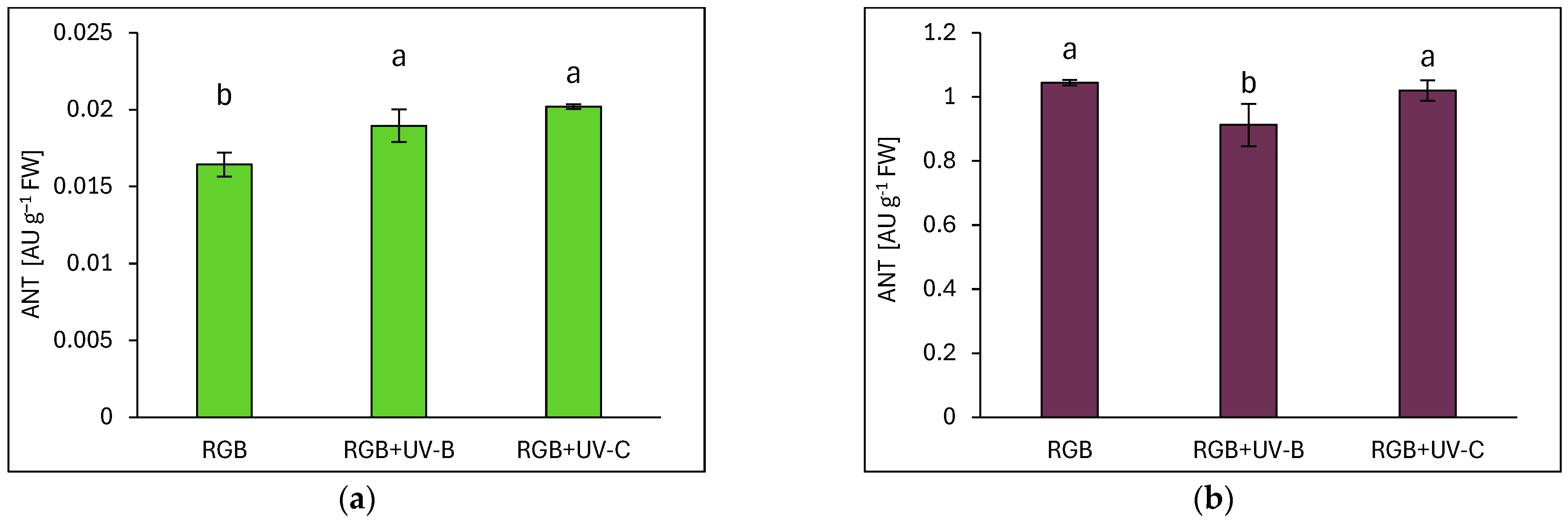
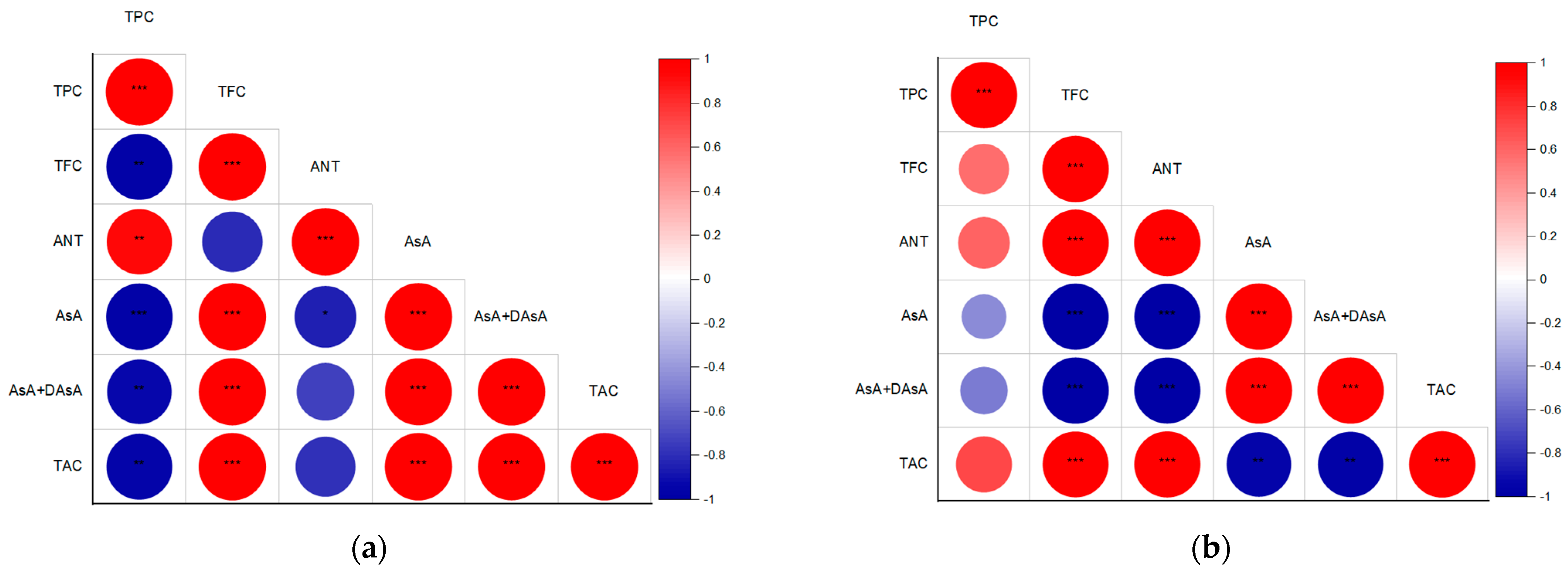
2.3. Antioxidant Capacity in Response to Supplemental UV-B or UV-C Light
2.3.1. Total Phenolic and Flavonoid Content
2.3.2. Anthocyanin Level
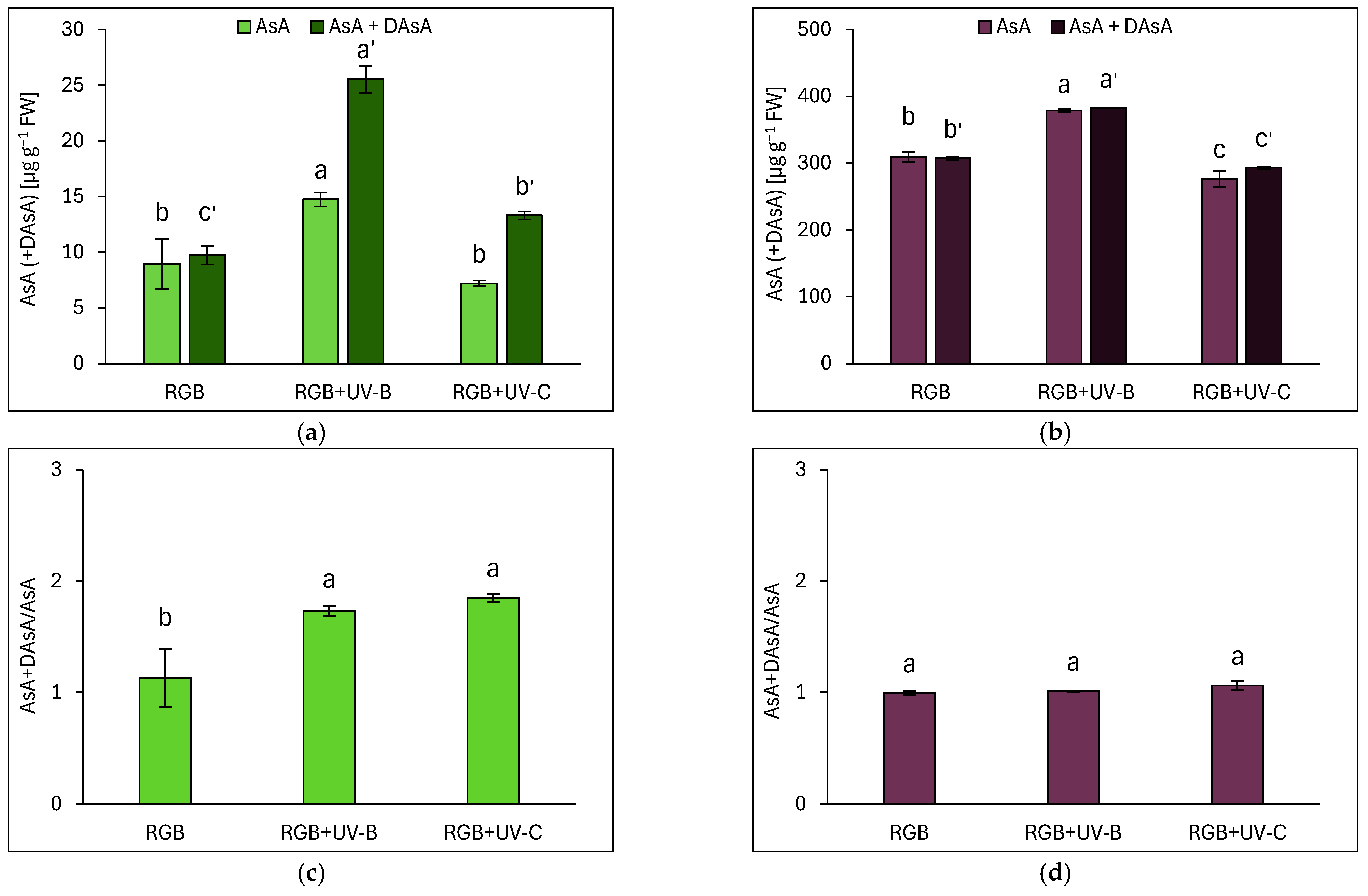
2.3.3. Ascorbic Acid Pool
2.3.4. Overall Antioxidant Capacity
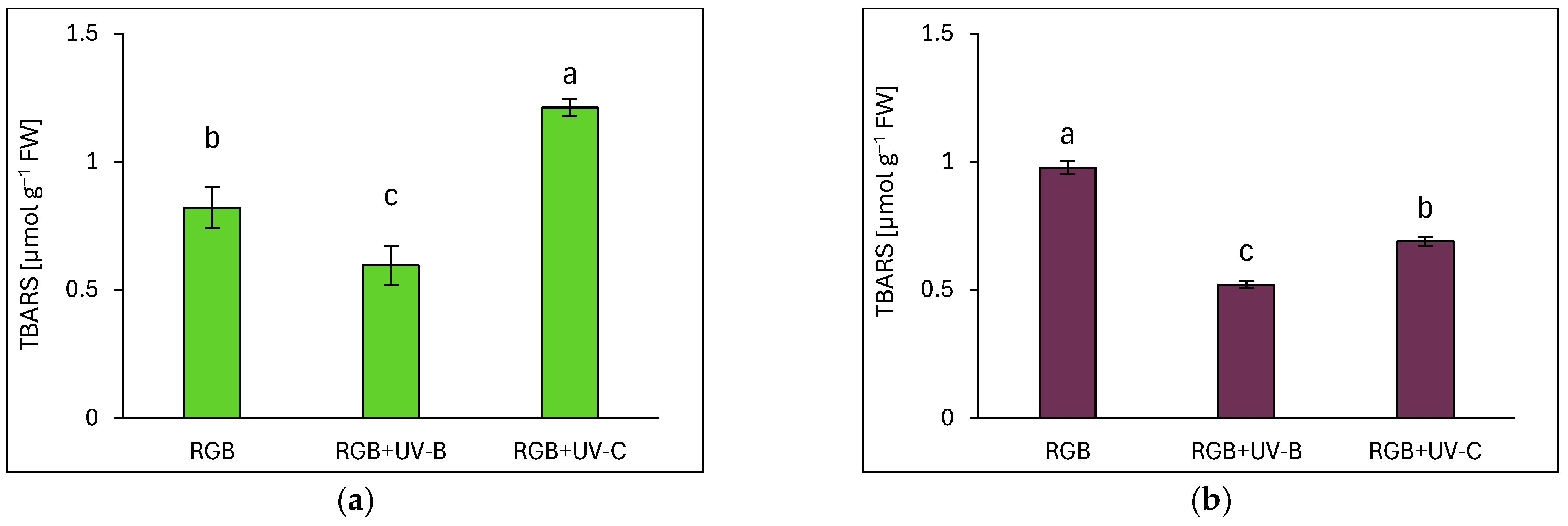
2.3.5. Effects of UV Light on Lipid Peroxidation
2.3.6. Immunoblot Analyses of UVR8 and CHS Protein
3. Discussion
3.1. Efficiency of RGB Spectrum Supplementation with UV-B or UV-C Light on Antioxidant Capacity, UVR8 and CHS Activation, and Morphology of Basil Cultivars
3.2. Condition of Photosynthetic Apparatus in Response to UV-B or UV-C Supplementation to the RGB Spectrum
4. Materials and Methods
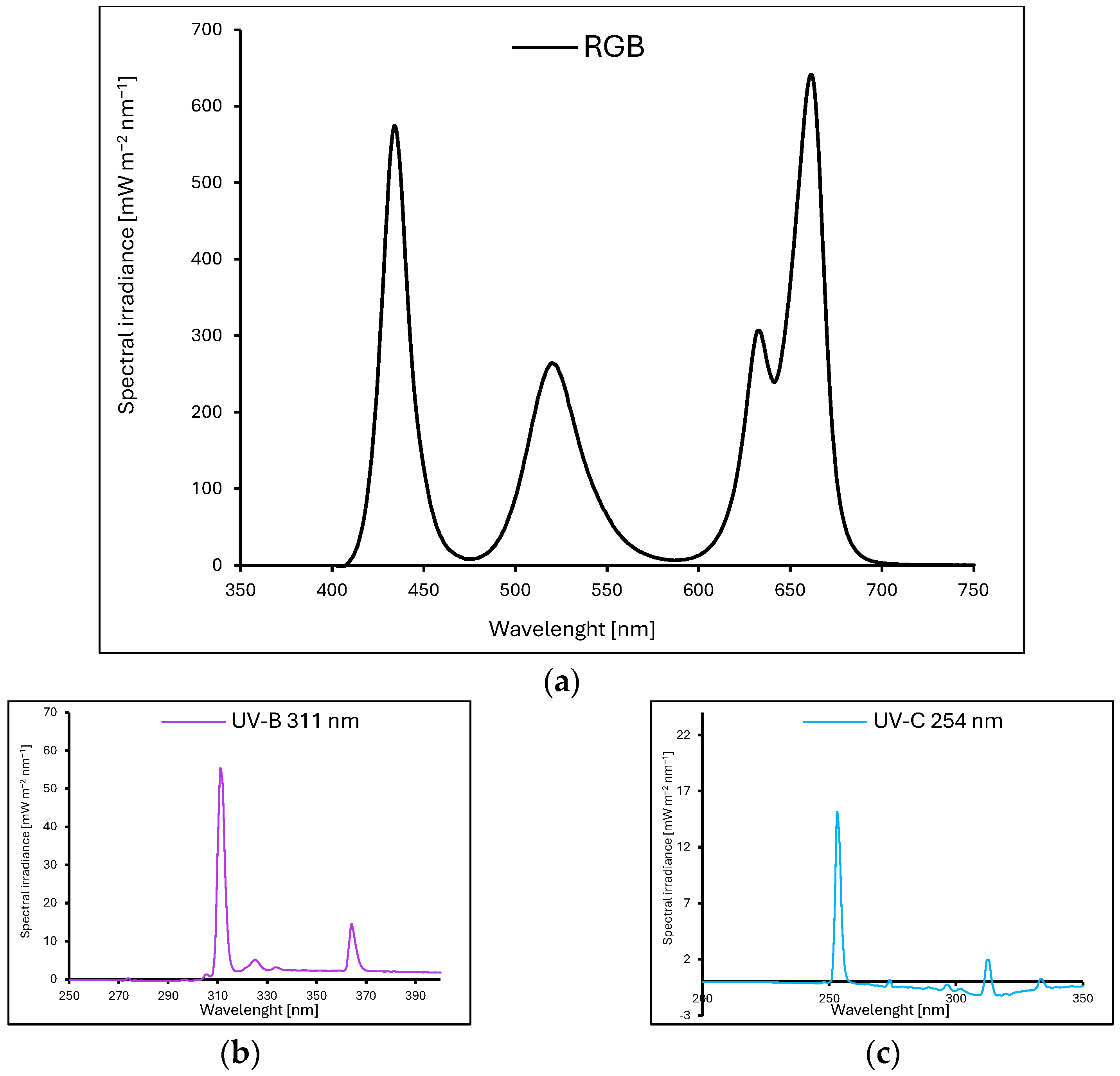
4.1. Plant Material, Growth Conditions, Light Treatment, and Leaf Biomass Analysis
4.2. Photosynthetic Pigment Determination
4.3. Soluble Protein Levels, RuBisCO Abundance, and Western Blot (WB) Analysis
4.4. Chlorophyll Fluorescence (ChF)
4.5. Estimation of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
4.6. The Ascorbate/Dehydroascorbate (AsA/DAsA) Ratio
4.7. Anthocyanin (ANT) Assay
4.8. Antioxidant Activity by DPPH Assay
4.9. Lipid Peroxidation
4.10. Data Fitting and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Engler, N.; Krarti, M. Review of energy efficiency in controlled environment agriculture. Renew. Sustain. Energy Rev. 2021, 141, 110786. [Google Scholar] [CrossRef]
- Van Delden, S.H.; SharathKumar, M.; Butturini, M.; Graamans, L.J.A.; Heuvelink, E.; Kacira, M.; Kaiser, E.; Klamer, R.S.; Klerkx, L.; Kootstra, G.; et al. Current status and future challenges in implementing and upscaling vertical farming systems. Nat. Food 2021, 2, 944–956. [Google Scholar] [CrossRef] [PubMed]
- Gnauer, C.; Pichler, H.; Schmittner, C.; Tauber, M.; Christl, K.; Knapitsch, J.; Parapatits, M. A recommendation for suitable technologies for an indoor farming framework. Elektrotech. Inform. 2020, 137, 370–374. [Google Scholar] [CrossRef]
- Surjadinata, B.B.; Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. UVA, UVB and UVC light enhances the biosynthesis of phenolic antioxidants in fresh-cut carrot through a synergistic effect with wounding. Molecules 2017, 22, 668. [Google Scholar] [CrossRef] [PubMed]
- Brazaityte, A.; Virsile, A.; Jankauskiene, J.; Sakalauskiene, S.; Samuoliene, G.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Miliauskienė, J.; Vaštakaitė, V.; et al. Effect of supplemental UV-A irradiation in solid-state lighting on the growth and phytochemical content of microgreens. Int. Agrophys. 2015, 29, 13–22. [Google Scholar] [CrossRef]
- Budavári, N.; Pék, Z.; Helyes, L.; Takács, S.; Nemeskéri, E. An overview on the use of artificial lighting for sustainable lettuce and microgreens production in an indoor vertical farming system. Horticulturae 2024, 10, 938. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345, 128727. [Google Scholar] [CrossRef]
- Lee, J.-H.; Tanaka, S.; Goto, E. Growth and Biosynthesis of Phenolic Compounds of Canola (Brassica napus L.) to Different Ultraviolet (UV)-B Wavelengths in a Plant Factory with Artificial Light. Plants 2022, 11, 1732. [Google Scholar] [CrossRef]
- Urban, L.; Charles, F.; de Miranda, M.R.A.; Aarrouf, J. Understanding the physiological effects of UV-C light and exploiting its agronomic potential before and after harvest. Plant Physiol. Biochem. 2016, 105, 1–11. [Google Scholar] [CrossRef]
- Xie, Y.; Miao, T.; Lyu, S.; Huang, Y.; Shu, M.; Li, S.; Xiong, T. Arabidopsis ERD15 regulated by BBX24 plays a positive role in UV-B signaling. Plant Sci. 2024, 343, 112077. [Google Scholar] [CrossRef]
- Tissot, N.; Ulm, R. Cryptochrome-mediated blue-light signalling modulates UVR8 photoreceptor activity and contributes to UV-B tolerance in Arabidopsis. Nat. Commun. 2020, 11, 1323. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; He, K.; Stolc, V.; Lee, H.; Figueroa, P.; Gao, Y.; Tongprasit, W.; Zhao, H.; Lee, I.; Deng, X.W. Analysis of transcription factor HY5 genomic binding sites revealed its hierarchical role in light regulation of development. Plant Cell 2007, 19, 731–749. [Google Scholar] [CrossRef]
- Yadav, A.; Ravindran, N.; Singh, D.; Rahul, P.V.; Datta, S. Role of Arabidopsis BBX proteins in light signaling. J. Plant Biochem. Biotechnol. 2020, 29, 623–635. [Google Scholar] [CrossRef]
- Ferreyra, M.L.F.; Serra, P.; Casati, P. Recent advances on the roles of flavonoids as plant protective molecules after UV and high light exposure. Physiol. Plant. 2021, 173, 736–749. [Google Scholar] [CrossRef] [PubMed]
- Pacak, I.; Trojak, M.; Skowron, E. The use of UV-A radiation for biofortification of lettuce and basil plants with antioxidant phenolic and flavonoid compounds. Folia Biol. Oecol. 2024, 18, 110–121. [Google Scholar] [CrossRef]
- Skowron, E.; Trojak, M.; Pacak, I. Effects of UV-B and UV-C Spectrum Supplementation on the Antioxidant Properties and Photosynthetic Activity of Lettuce Cultivars. Int. J. Mol. Sci. 2024, 25, 9298. [Google Scholar] [CrossRef] [PubMed]
- Eskandarzade, P.; Mehrjerdi, M.Z.; Gruda, N.S.; Aliniaeifard, S. Phytochemical compositions and antioxidant activity of green and purple basils altered by light intensity and harvesting time. Heliyon 2024, 10, e30931. [Google Scholar] [CrossRef]
- Thongtip, A.; Mosaleeyanon, K.; Janta, S.; Wanichananan, P.; Chutimanukul, P.; Thepsilvisut, O.; Chutimanukul, P. Assessing light spectrum impact on growth and antioxidant properties of basil family microgreens. Sci. Rep. 2024, 14, 27875. [Google Scholar] [CrossRef]
- Jacobo-Velázquez, D.A.; Moreira-Rodríguez, M.; Benavides, J. UVA and UVB radiation as innovative tools to biofortify horticultural crops with nutraceuticals. Horticulturae 2022, 8, 387. [Google Scholar] [CrossRef]
- Weiland, M.; Weßler, C.F.; Filler, T.; Glaab, J.; Lobo Ploch, N.; Winterwerber, U.; Wiesner-Reinhold, M.; Schreiner, M.; Neugart, S. A comparison of consistent UV treatment versus inconsistent UV treatment in horticultural production of lettuce. Photochem. Photobiol. Sci. 2023, 22, 1611–1624. [Google Scholar] [CrossRef]
- Qian, M.; Kalbina, I.; Rosenqvist, E.; Jansen, M.A.; Strid, Å. Supplementary UV-A and UV-B radiation differentially regulate morphology in Ocimum basilicum. Photochem. Photobiol. Sci. 2023, 22, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Semenova, N.A.; Smirnov, A.A.; Ivanitskikh, A.S.; Izmailov, A.Y.; Dorokhov, A.S.; Proshkin, Y.A.; Yanykin, D.V.; Sarimov, R.R.; Gudkov, S.V.; Chilingaryan, N.O. Impact of ultraviolet radiation on the pigment content and essential oil accumulation in sweet basil (Ocimum basilicum L.). Appl. Sci. 2022, 12, 7190. [Google Scholar] [CrossRef]
- Santin, M.; Becagli, M.; Sciampagna, M.C.; Mannucci, A.; Ranieri, A.; Castagna, A. Integrative effect of UV-B and some organic amendments on growth, phenolic and flavonoid compounds, and antioxidant activity of basil (Ocimum basilicum L.) plants. Horticulturae 2023, 9, 894. [Google Scholar] [CrossRef]
- Milić Komić, S.; Živanović, B.; Dumanović, J.; Kolarž, P.; Sedlarević Zorić, A.; Morina, F.; Vidocić, M.; Veljović Jovanović, S. Differential antioxidant response to supplemental UV-B irradiation and sunlight in three basil varieties. Int. J. Mol. Sci. 2023, 24, 15350. [Google Scholar] [CrossRef]
- Yang, G.Y.; Sun, M.Y.; Brewer, L.; Tang, Z.K.; Nieuwenhuizen, N.; Cooney, J.; Xu, S.Z.; Sheng, J.W.; Andre, C.; Xue, C.; et al. Allelic variation of BBX24 is a dominant determinant controlling red coloration and dwarfism in pear. Plant Biotechnol. J. 2024, 6, 1468–1490. [Google Scholar] [CrossRef]
- Podolec, R.; Wagnon, T.B.; Leonardelli, M.; Johansson, H.; Ulm, R. Arabidopsis B-box transcription factors BBX20-22 promote UVR8 photoreceptor-mediated UV-B responses. Plant J. 2022, 111, 422–439. [Google Scholar] [CrossRef]
- Tattini, M.; Sebastiani, F.; Brunetti, C.; Fini, A.; Torre, S.; Gori, A.; Centritto, M.; Ferrini, F.; Landi, M.; Guidi, L. Dissecting molecular and physiological response mechanisms to high solar radiation in cyanic and acyanic leaves: A case study on red and green basil. J. Exp. Bot. 2017, 68, 2425–2437. [Google Scholar] [CrossRef]
- Nazir, M.; Asad Ullah, M.; Mumtaz, S.; Siddiquah, A.; Shah, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Molecules 2020, 25, 1072. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, B.H.; Khan, T.; Khurshid, R.; Nadeem, M.; Drouet, S.; Hano, C. UV-C mediated accumulation of pharmacologically significant phytochemicals under light regimes in in vitro culture of Fagonia indica (L.). Sci. Rep. 2021, 11, 679. [Google Scholar] [CrossRef]
- Rabelo, M.C.; Bang, W.Y.; Nair, V.; Alves, R.E.; Jacobo-Velázquez, D.A.; Sreedharan, S.; Alcântara de Miranda, M.R.; Cisneros-Zevallos, L. UVC light modulates vitamin C and phenolic biosynthesis in acerola fruit: Role of increased mitochondria activity and ROS production. Sci. Rep. 2020, 10, 21972. [Google Scholar] [CrossRef]
- Vass, I.; Szilárd, A.; Sicora, C. Adverse effects of UV-B light on the structure and function of the photosynthetic apparatus. In Handbook of Photosynthesis, 2nd ed.; Pessarakli, M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 827–843. [Google Scholar]
- Kataria, S.; Jajoo, A.; Guruprasad, K.N. Impact of increasing Ultraviolet-B (UV-B) radiation on photosynthetic processes. J. Photochem. Photobiol. B 2014, 137, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Ri, I.; Pak, S.; Pak, U.; Yun, C.; Tang, Z. How does UV-B radiation influence the photosynthesis and secondary metabolism of Schisandra chinensis leaves? Ind. Crop. Prod. 2024, 208, 117832. [Google Scholar] [CrossRef]
- Zhang, H.; Song, W.; Zheng, L. Pre-harvest low-dose UV-B irradiation improves the accumulation of bioactive component and post-harvest shelf-life of Mesembryanthemum crystallinum. J. Agric. Food Res. 2025, 19, 101737. [Google Scholar] [CrossRef]
- Kosobryukhov, A.; Khudyakova, A.; Kreslavski, V. Impact of UV radiation on photosynthetic apparatus: Adaptive and damaging mechanisms. In Plant Ecophysiology and Adaptation Under Climate Change: Mechanisms and Perspectives I; Spinger: Berlin/Heidelberg, Germany, 2020; pp. 555–576. [Google Scholar] [CrossRef]
- Bijlsma, J.; de Bruijn, W.J.; Velikov, K.P.; Vincken, J.P. Unravelling discolouration caused by iron-flavonoid interactions: Complexation, oxidation, and formation of networks. Food Chem. 2022, 370, 131292. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Tsugama, D.; Liu, S.; Takano, T. A rapid chemical method for lysing Arabidopsis cells for protein analysis. Plant Methods 2011, 7, 22. [Google Scholar] [CrossRef]
- Skowron, E.; Trojak, M. Effect of exogenously-applied abscisic acid, putrescine and hydrogen peroxide on drought tolerance of barley. Biologia 2021, 76, 453–468. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Laby, R.J.; Kincaid, M.S.; Kim, D.; Gibson, S.I. The Arabidopsis sugar-insensitive mutants sis4 and sis5 are defective in abscisic acid synthesis and response. Plant J. 2000, 23, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Mehmood, A.; Javid, S.; Khan, M.F.; Ahmad, K.S.; Mustafa, A. In vitro total phenolics, total flavonoids, antioxidant and antibacterial activities of selected medicinal plants using different solvent systems. BMC Chem. 2022, 16, 64. [Google Scholar] [CrossRef] [PubMed]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]









| Parameter | Treatment | ||
|---|---|---|---|
| RGB | RGB+UV-B | RGB+UV-C | |
| Chlorophyll a + b [mg g−1 FW] | 1.405 ± 0.013 a | 0.878 ± 0.006 b | 1.423 ± 0.005 a |
| Chlorophyll a [mg g−1 FW] | 1.000 ± 0.010 a | 0.617 ± 0.004 c | 0.991 ± 0.004 b |
| Chlorophyll b [mg g−1 FW] | 0.405 ± 0.003 b | 0.261 ± 0.002 c | 0.432 ± 0.002 a |
| Chlorophyll a/b | 2.470 ± 0.006 a | 2.364 ± 0.011 b | 2.295 ± 0.012 c |
| Carotenoids [mg g−1 FW] | 0.161 ± 0.001 a | 0.113 ± 0.000 c | 0.144 ± 0.001 b |
| Chlorophyll a + b/carotenoids | 8.732 ± 0.006 b | 7.799 ± 0.034 c | 9.905 ± 0.054 a |
| Soluble leaf proteins [mg g−1 FW] | 60.32 ± 0.83 c | 74.52 ± 0.33 b | 78.09 ± 0.90 a |
| Parameter | Treatment | ||
|---|---|---|---|
| RGB | RGB+UV-B | RGB+UV-C | |
| Chlorophyll a + b [mg g−1 FW] | 1.384 ± 0.008 b | 0.786 ± 0.003 c | 1.419 ± 0.007 a |
| Chlorophyll a [mg g−1 FW] | 0.939 ± 0.006 b | 0.526 ± 0.002 c | 0.966 ± 0.005 a |
| Chlorophyll b [mg g−1 FW] | 0.444 ± 0.003 b | 0.261 ± 0.001 c | 0.453 ± 0.003 a |
| Chlorophyll a/b | 2.115 ± 0.003 b | 2.015 ± 0.005 c | 2.132 ± 0.009 a |
| Carotenoids [mg g−1 FW] | 0.181 ± 0.001 b | 0.089 ± 0.001 c | 0.188 ± 0.001 a |
| Chlorophyll a + b/carotenoids | 7.646 ± 0.026 b | 8.805 ± 0.025 a | 7.553 ± 0.037 c |
| Soluble leaf proteins [mg g−1 FW] | 127.74 ± 0.67 a | 109.36 ± 0.22 b | 106.26 ± 0.86 c |
| Treatment, Wavelength Peak (nm) | Daily Time Exposure (min), Diurnal Time | Total Time (h) | Total Irradiance (W m−2) | Irradiance (PAR) (W m−2) | Peak-to-Background Irradiance Ratio * | Cumulative Dose (kJ m−2) | |||
|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | ||||||
| UV-B, 311 | 15 12.00–12.15 p.m. | 30 12.00–12.30 p.m. | 60 12.00–1.00 p.m. | 120 12.00–2.00 p.m. | 3.75 | 1.1572 | 0.253 | 5.32 | 15.622 |
| UV-C, 254 | 7.5 12.00–12.08 p.m. | 15 12.00–12.15 p.m. | 30 12.00–12.30 p.m. | 60 12.00–1.00 p.m. | 1.875 | 0.8901 | 0.177 | 10.1 | 6.008 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowron, E.; Trojak, M.; Pacak, I.; Węzigowska, P.; Szymkiewicz, J. Enhancing the Quality of Indoor-Grown Basil Microgreens with Low-Dose UV-B or UV-C Light Supplementation. Int. J. Mol. Sci. 2025, 26, 2352. https://doi.org/10.3390/ijms26052352
Skowron E, Trojak M, Pacak I, Węzigowska P, Szymkiewicz J. Enhancing the Quality of Indoor-Grown Basil Microgreens with Low-Dose UV-B or UV-C Light Supplementation. International Journal of Molecular Sciences. 2025; 26(5):2352. https://doi.org/10.3390/ijms26052352
Chicago/Turabian StyleSkowron, Ernest, Magdalena Trojak, Ilona Pacak, Paulina Węzigowska, and Julia Szymkiewicz. 2025. "Enhancing the Quality of Indoor-Grown Basil Microgreens with Low-Dose UV-B or UV-C Light Supplementation" International Journal of Molecular Sciences 26, no. 5: 2352. https://doi.org/10.3390/ijms26052352
APA StyleSkowron, E., Trojak, M., Pacak, I., Węzigowska, P., & Szymkiewicz, J. (2025). Enhancing the Quality of Indoor-Grown Basil Microgreens with Low-Dose UV-B or UV-C Light Supplementation. International Journal of Molecular Sciences, 26(5), 2352. https://doi.org/10.3390/ijms26052352






