Effects of Prebiotic Phytocompound Administration in Gestational Diabetic Dams and Its Influence on Offspring Cognitive Outcomes
Abstract
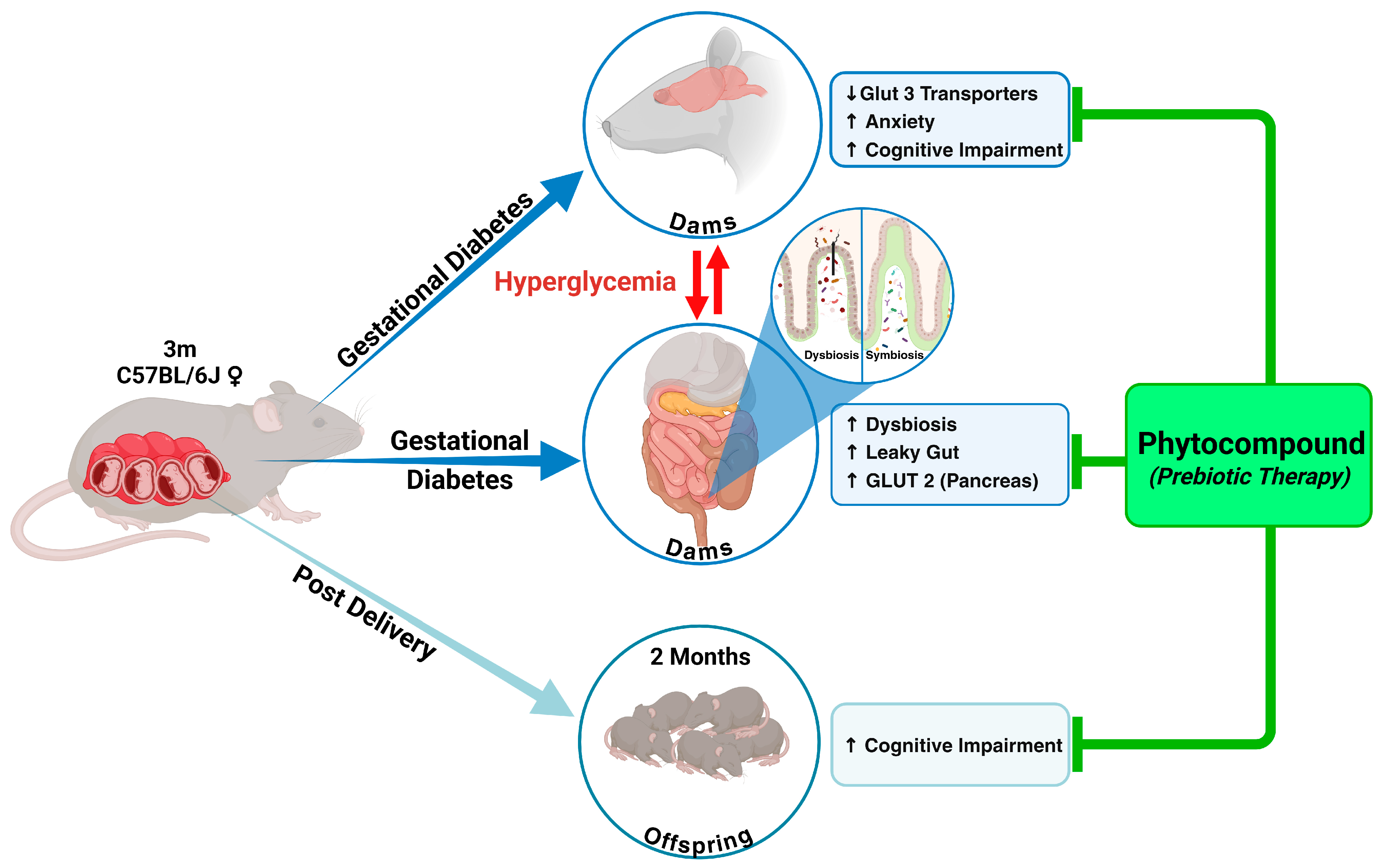
:1. Introduction
2. Results
2.1. GD in Dams Leads to Significantly Higher Blood Glucose Levels and Reduced Body Weight, and Both Were Reversed with Phytocompound Therapy
2.2. GD Dams Showed Significantly Elevated Levels of the Pro-Inflammatory Cytokine Interleukin 6 (IL-6) and Glucose Transporter 2 (GLUT2) Expression in the Pancreas During Pregnancy
2.3. GD in Dams During Pregnancy Led to the Loss of Beneficial Gut Microbiota and Impaired Gut Integrity, as Indicated by 16s RNA Sequencing and Reduced E-Cadherin Levels
2.4. GD Caused an Anxiety Phenotype with Cognitive Impairment in Both Dams and Offspring Born to GD Dams, Rescued by Phytocompound Treatment
2.5. Glucose Transporter 3 (GLUT3) in the Brain Was Significantly Altered Due to Gestational Diabetes in Pregnant Dams
3. Discussion
4. Materials and Methods
4.1. Plant Extract Preparation
4.2. Animals and Study Design
4.3. Metabolic Measurements
4.4. Oral Glucose Tolerance Test (OGTT)
4.5. Tissue Collection
4.6. mRNA Gene Expression in the Pancreas
4.7. Gut Content Collection and 16s RNA Gene Sequencing
4.8. Histopathology
4.9. Immunohistochemistry
4.10. Behavioral Analysis
4.11. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sweeting, A.; Hannah, W.; Backman, H.; Catalano, P.; Feghali, M.; Herman, W.H.; Hivert, M.F.; Immanuel, J.; Meek, C.; Oppermann, M.L.; et al. Epidemiology and Management of Gestational Diabetes. Lancet 2024, 404, 175–192. [Google Scholar] [CrossRef] [PubMed]
- Nakshine, V.S.; Jogdand, S.D. A Comprehensive Review of Gestational Diabetes Mellitus: Impacts on Maternal Health, Fetal Development, Childhood Outcomes, and Long-Term Treatment Strategies. Cureus 2023, 15, e47500. [Google Scholar] [CrossRef] [PubMed]
- Johns, E.C.; Denison, F.C.; Norman, J.E.; Reynolds, R.M. Gestational Diabetes Mellitus: Mechanisms, Treatment, and Complications. Trends Endocrinol. Metab. 2018, 29, 743–754. [Google Scholar] [CrossRef]
- Fitzgerald, E.; Hor, K.; Drake, A.J. Maternal Influences on Fetal Brain Development: The Role of Nutrition, Infection and Stress, and the Potential for Intergenerational Consequences. Early Hum. Dev. 2020, 150, 105190. [Google Scholar] [CrossRef] [PubMed]
- Semo, D.; Reinecke, H.; Godfrey, R. Gut Microbiome Regulates Inflammation and Insulin Resistance: A Novel Therapeutic Target to Improve Insulin Sensitivity. Signal Transduct. Target. Ther. 2024, 9, 35. [Google Scholar] [CrossRef]
- Biete, M.; Vasudevan, S. Gestational Diabetes Mellitus: Impacts on Fetal Neurodevelopment, Gut Dysbiosis, and the Promise of Precision Medicine. Front. Mol. Biosci. 2024, 11, 1420664. [Google Scholar] [CrossRef]
- Ansari, P.; Khan, J.T.; Chowdhury, S.; Reberio, A.D.; Kumar, S.; Seidel, V.; Abdel-Wahab, Y.H.A.; Flatt, P.R. Plant-Based Diets and Phytochemicals in the Management of Diabetes Mellitus and Prevention of Its Complications: A Review. Nutrients 2024, 16, 3709. [Google Scholar] [CrossRef]
- Jagadeesan, G.; Muniyandi, K.; Manoharan, A.L.; Nataraj, G.; Thangaraj, P. Understanding the Bioaccessibility, α-Amylase and α-Glucosidase Enzyme Inhibition Kinetics of Allmania nodiflora (L.) R.Br. Ex Wight Polyphenols during in Vitro Simulated Digestion. Food Chem. 2022, 372, 131294. [Google Scholar] [CrossRef]
- Holscher, H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes 2017, 8, 172–184. [Google Scholar] [CrossRef]
- Graham, M.L.; Janecek, J.L.; Kittredge, J.A.; Hering, B.J.; Schuurman, H.J. The Streptozotocin-Induced Diabetic Nude Mouse Model: Differences between Animals from Different Sources. Comp. Med 2011, 61, 356. [Google Scholar]
- Grupe, K.; Scherneck, S. Mouse Models of Gestational Diabetes Mellitus and Its Subtypes: Recent Insights and Pitfalls. Int. J. Mol. Sci. 2023, 24, 5982. [Google Scholar] [CrossRef]
- Hellerstrom, C.; And, I.S.; Eriksson, U.J. Is There an Animal Model for Gestational Diabetes? Diabetes 1985, 34 (Suppl. S2), 28–31. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wu, N.; Yu, W.; Li, L.; Ouyang, H.; Liu, X.; Qian, M.; Al-Mureish, A. Research Progress on the Experimental Animal Model of Gestational Diabetes Mellitus. Diabetes Metab. Syndr. Obes. 2020, 13, 4235–4247. [Google Scholar] [CrossRef] [PubMed]
- Paavilainen, E.; Tertti, K.; Nikkinen, H.; Veijola, R.; Vääräsmäki, M.; Loo, B.M.; Tossavainen, P.; Rönnemaa, T.; Niinikoski, H. Metformin versus Insulin Therapy for Gestational Diabetes: Effects on Offspring Anthropometrics and Metabolism at the Age of 9 Years: A Follow-up Study of Two Open-Label, Randomized Controlled Trials. Diabetes Obes. Metab. 2022, 24, 402–410. [Google Scholar] [CrossRef]
- Paul, P.; Priyambada, L.; Abraham, A.; Manimegalai, B.; Paul, T.V.; Princy, S.; Antonisamy, B.; Thomas, N.; Yenuberi, H.; Mathews, J.E. Follow-up of Offspring and Mothers with Gestational Diabetes Treated with Metformin or Glibenclamide: A Randomized Controlled Trial. Int. J. Gynaecol. Obstet. 2021, 152, 446–447. [Google Scholar] [CrossRef]
- Rowan, J.A.; Rush, E.C.; Plank, L.D. Metformin in Gestational Diabetes The Offspring Follow Up (MiGTOFU): Associations between Maternal Characteristics and Size and Adiposity of Boys and Girls at Nine Years. Aust. N. Z. J. Obstet. Gynaecol. J. 2023, 63, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Rowan, J.A.; Rush, E.C.; Plank, L.D.; Lu, J.; Obolonkin, V.; Coat, S.; Hague, W.M. Metformin in Gestational Diabetes: The Offspring Follow-up (MiG TOFU): Body Composition and Metabolic Outcomes at 7–9 Years of Age. BMJ. Open Diabetes Res. Care 2018, 6, e000456. [Google Scholar] [CrossRef]
- Megur, A.; Daliri, E.B.M.; Baltriukienė, D.; Burokas, A. Prebiotics as a Tool for the Prevention and Treatment of Obesity and Diabetes: Classification and Ability to Modulate the Gut Microbiota. Int. J. Mol. Sci. 2022, 23, 6097. [Google Scholar] [CrossRef]
- Ayesha, I.E.; Monson, N.R.; Klair, N.; Patel, U.; Saxena, A.; Patel, D.; Venugopal, S. Probiotics and Their Role in the Management of Type 2 Diabetes Mellitus (Short-Term Versus Long-Term Effect): A Systematic Review and Meta-Analysis. Cureus 2023, 15, e46741. [Google Scholar] [CrossRef]
- Degruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Conesa, M.P.B.; Blixt, F.W.; Peesh, P.; Khan, R.; Korf, J.; Lee, J.; Jagadeesan, G.; Andersohn, A.; Das, T.K.; Tan, C.; et al. Stabilizing Histamine Release in Gut Mast Cells Mitigates Peripheral and Central Inflammation after Stroke. J. Neuroinflammation 2023, 20, 230. [Google Scholar] [CrossRef]
- Schneider, M.R.; Dahlhoff, M.; Horst, D.; Hirschi, B.; Trülzsch, K.; Müller-Höcker, J.; Vogelmann, R.; Allgäuer, M.; Gerhard, M.; Steininger, S.; et al. A Key Role for E-Cadherin in Intestinal Homeostasis and Paneth Cell Maturation. PLoS ONE 2010, 5, e14325. [Google Scholar] [CrossRef]
- Jirkof, P. Burrowing and Nest Building Behavior as Indicators of Well-Being in Mice. J. Neurosci. Methods 2014, 234, 139–146. [Google Scholar] [CrossRef]
- Kraeuter, A.K.; Guest, P.C.; Sarnyai, Z. The Y-Maze for Assessment of Spatial Working and Reference Memory in Mice. Methods Mol. Biol. 2019, 1916, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, K.K.W. Glial Fibrillary Acidic Protein: From Intermediate Filament Assembly and Gliosis to Neurobiomarker. Trends Neurosci. 2015, 38, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.M.; Mitchell, A.L.; Williamson, C. Metabolic Impact of Bile Acids in Gestation. Eur. J. Endocrinol. 2021, 184, R69–R83. [Google Scholar] [CrossRef]
- Neven, A.C.H.; Mousa, A.; Boyle, J.A.; Teede, H.J. Endocrine and Metabolic Interactions in Healthy Pregnancies and Hyperinsulinemic Pregnancies Affected by Polycystic Ovary Syndrome, Diabetes and Obesity. Front. Endocrinol. 2023, 13, 993619. [Google Scholar] [CrossRef]
- Sonagra, A.D.; Biradar, S.M.; Murthy, D.S.J. Normal Pregnancy—A State of Insulin Resistance. J. Clin. Diagn. Res. 2014, 8, CC01. [Google Scholar] [CrossRef]
- Kampmann, U.; Knorr, S.; Fuglsang, J.; Ovesen, P. Review Article Determinants of Maternal Insulin Resistance during Pregnancy: An Updated Overview. J. Diabetes Res. 2019, 2019, 5320156. [Google Scholar] [CrossRef]
- Inoue, S.; Kozuma, Y.; Miyahara, M.; Yoshizato, T.; Tajiri, Y.; Hori, D.; Ushijima, K. Pathophysiology of Gestational Diabetes Mellitus in Lean Japanese Pregnant Women in Relation to Insulin Secretion or Insulin Resistance. Diabetol. Int. 2020, 11, 269–273. [Google Scholar] [CrossRef]
- Plows, J.F.; Stanley, J.L.; Baker, P.N.; Reynolds, C.M.; Vickers, M.H. The Pathophysiology of Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3342. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Goswami, S.; Giacomini, K.M.; Altman, R.B.; Klein, T.E. Metformin Pathways: Pharmacokinetics and Pharmacodynamics. Pharmacogenet. Genom. 2012, 22, 820. [Google Scholar] [CrossRef]
- Rena, G.; Hardie, D.G.; Pearson, E.R. The Mechanisms of Action of Metformin. Diabetologia 2017, 60, 1577. [Google Scholar] [CrossRef] [PubMed]
- Loan, A.; Syal, C.; Lui, M.; He, L.; Wang, J. Promising Use of Metformin in Treating Neurological Disorders: Biomarker-Guided Therapies. Neural Regen. Res. 2023, 19, 1045. [Google Scholar] [CrossRef]
- Tocci, V.; Mirabelli, M.; Salatino, A.; Sicilia, L.; Giuliano, S.; Brunetti, F.S.; Chiefari, E.; De Sarro, G.; Foti, D.P.; Brunetti, A. Metformin in Gestational Diabetes Mellitus: To Use or Not to Use, That Is the Question. Pharmaceuticals 2023, 16, 1318. [Google Scholar] [CrossRef]
- Cheng, M.; Ren, L.; Jia, X.; Wang, J.; Cong, B. Understanding the Action Mechanisms of Metformin in the Gastrointestinal Tract. Front. Pharmacol. 2024, 15, 1347047. [Google Scholar] [CrossRef]
- Kandpal, M.; Indari, O.; Baral, B.; Jakhmola, S.; Tiwari, D.; Bhandari, V.; Pandey, R.K.; Bala, K.; Sonawane, A.; Jha, H.C. Dysbiosis of Gut Microbiota from the Perspective of the Gut–Brain Axis: Role in the Provocation of Neurological Disorders. Metabolites 2022, 12, 1064. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.J.; Correia, R.G.; Cruz, A.F.; Martins, J.M.; Rodrigues, M.S.; Gomes, C.A.; Ambrósio, A.F.; Baptista, F.I. Sex Differences in Offspring Neurodevelopment, Cognitive Performance and Microglia Morphology Associated with Maternal Diabetes: Putative Targets for Insulin Therapy. Brain Behav. Immun. Health 2020, 5, 100075. [Google Scholar] [CrossRef]
- Aggarwal, N.; Kitano, S.; Puah, G.R.Y.; Kittelmann, S.; Hwang, I.Y.; Chang, M.W. Microbiome and Human Health: Current Understanding, Engineering, and Enabling Technologies. Chem. Rev. 2023, 123, 31–72. [Google Scholar] [CrossRef]
- Dunlop, A.L.; Mulle, J.G.; Ferranti, E.P.; Edwards, S.; Dunn, A.B.; Corwin, E.J. Maternal Microbiome and Pregnancy Outcomes That Impact Infant Health: A Review. Adv. Neonatal Care 2015, 15, 377–385. [Google Scholar] [CrossRef]
- Singh, P.; Elhaj, D.A.I.; Ibrahim, I.; Abdullahi, H.; Al Khodor, S. Maternal Microbiota and Gestational Diabetes: Impact on Infant Health. J. Transl. Med. 2023, 21, 364. [Google Scholar] [CrossRef] [PubMed]
- Mustad, V.A.; Huynh, D.T.T.; López-Pedrosa, J.M.; Campoy, C.; Rueda, R. The Role of Dietary Carbohydrates in Gestational Diabetes. Nutrients 2020, 12, 385. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S. Roles of Diet-Associated Gut Microbial Metabolites on Brain Health: Cell-to-Cell Interactions between Gut Bacteria and the Central Nervous System. Adv. Nutr. 2024, 15, 100136. [Google Scholar] [CrossRef]
- Yang, L.; Sakandar, H.A.; Sun, Z.; Zhang, H. Recent Advances of Intestinal Microbiota Transmission from Mother to Infant. J. Funct. Foods 2021, 87, 104719. [Google Scholar] [CrossRef]
- Sanz, Y. Gut Microbiota and Probiotics in Maternal and Infant Health. Am. J. Clin. Nutr. 2011, 94, S2000–S2005. [Google Scholar] [CrossRef] [PubMed]
- Soto, A.; Martín, V.; Jiménez, E.; Mader, I.; Rodríguez, J.M.; Fernández, L. Lactobacilli and Bifidobacteria in Human Breast Milk: Influence of Antibiotherapy and Other Host and Clinical Factors. J. Pediatr. Gastroenterol. Nutr. 2014, 59, 78–88. [Google Scholar] [CrossRef]
- Mueller, N.T.; Bakacs, E.; Combellick, J.; Grigoryan, Z.; Dominguez-Bello, M.G. The Infant Microbiome Development: Mom Matters. Trends Mol. Med. 2015, 21, 109–117. [Google Scholar] [CrossRef]
- Hickman, B.; Salonen, A.; Ponsero, A.J.; Jokela, R.; Kolho, K.L.; de Vos, W.M.; Korpela, K. Gut Microbiota Wellbeing Index Predicts Overall Health in a Cohort of 1000 Infants. Nat. Commun. 2024, 15, 8323. [Google Scholar] [CrossRef]
- Pessa-Morikawa, T.; Husso, A.; Kärkkäinen, O.; Koistinen, V.; Hanhineva, K.; Iivanainen, A.; Niku, M. Maternal Microbiota-Derived Metabolic Profile in Fetal Murine Intestine, Brain and Placenta. BMC Microbiol. 2022, 22, 46. [Google Scholar] [CrossRef]
- Caricilli, A.M.; Saad, M.J.A. The Role of Gut Microbiota on Insulin Resistance. Nutrients 2013, 5, 829–851. [Google Scholar] [CrossRef]
- He, F.F.; Li, Y.M. Role of Gut Microbiota in the Development of Insulin Resistance and the Mechanism Underlying Polycystic Ovary Syndrome: A Review. J. Ovarian Res. 2020, 13, 73. [Google Scholar] [CrossRef] [PubMed]
- Luck, B.; Engevik, M.A.; Ganesh, B.P.; Lackey, E.P.; Lin, T.; Balderas, M.; Major, A.; Runge, J.; Luna, R.A.; Sillitoe, R.V.; et al. Bifidobacteria Shape Host Neural Circuits during Postnatal Development by Promoting Synapse Formation and Microglial Function. Sci. Rep. 2020, 10, 7737. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Peng, J.; Li, F.; Wong, F.S.; Wen, L. Evaluation of Different Mucosal Microbiota Leads to Gut Microbiota-Based Prediction of Type 1 Diabetes in NOD Mice. Sci. Rep. 2018, 8, 15451. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, J.; Chen, H.; Jin, X.; Lin, Z.; Fan, C.; Shan, Z.; Teng, W.; Li, J. Dynamic Changes in the Gut Microbiota during Three Consecutive Trimesters of Pregnancy and Their Correlation with Abnormal Glucose and Lipid Metabolism. Eur. J. Med. Res. 2024, 29, 117. [Google Scholar] [CrossRef] [PubMed]
- Soderborg, T.K.; Carpenter, C.M.; Janssen, R.C.; Weir, T.L.; Robertson, C.E.; Ir, D.; Young, B.E.; Krebs, N.F.; Hernandez, T.L.; Barbour, L.A.; et al. Gestational Diabetes Is Uniquely Associated with Altered Early Seeding of the Infant Gut Microbiota. Front. Endocrinol. 2020, 11, 603021. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, T.; Zhang, Y.; Hu, Q.; Wang, X.; Chang, H.; Mao, J.H.; Snijders, A.M.; Xia, Y. Contribution of Trace Element Exposure to Gestational Diabetes Mellitus through Disturbing the Gut Microbiome. Environ. Int. 2021, 153, 106520. [Google Scholar] [CrossRef]
- Byers, S.L.; Wiles, M.V.; Dunn, S.L.; Taft, R.A. Mouse Estrous Cycle Identification Tool and Images. PLoS ONE 2012, 7, e35538. [Google Scholar] [CrossRef]
- Wang, J.; Wang, C.; Li, S.; Li, W.; Yuan, G.; Pan, Y.; Chen, H. Anti-Diabetic Effects of Inonotus Obliquus Polysaccharides in Streptozotocin-Induced Type 2 Diabetic Mice and Potential Mechanism via PI3K-Akt Signal Pathway. Biomed. Pharmacother. 2017, 95, 1669–1677. [Google Scholar] [CrossRef]
- Nelson, M.C.; Morrison, H.G.; Benjamino, J.; Grim, S.L.; Graf, J. Analysis, Optimization and Verification of Illumina-Generated 16s RRNA Gene Amplicon Surveys. PLoS ONE 2014, 9, e94249. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pẽa, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME Allows Analysis of High-Throughput Community Sequencing Data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Honarpisheh, P.; Reynolds, C.R.; Conesa, M.P.B.; Manchon, J.F.M.; Putluri, N.; Bhattacharjee, M.B.; Urayama, A.; McCullough, L.D.; Ganesh, B.P. Dysregulated Gut Homeostasis Observed Prior to the Accumulation of the Brain Amyloid-β in Tg2576 Mice. Int. J. Mol. Sci. 2020, 21, 1711. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.P.; Klopfleisch, R.; Loh, G.; Blaut, M. Commensal Akkermansia Muciniphila Exacerbates Gut Inflammation in Salmonella Typhimurium-Infected Gnotobiotic Mice. PLoS ONE 2013, 8, e74963. [Google Scholar] [CrossRef]
- Das, T.K.; Blasco-Conesa, M.P.; Korf, J.; Honarpisheh, P.; Chapman, M.R.; Ganesh, B.P. Bacterial Amyloid Curli Associated Gut Epithelial Neuroendocrine Activation Predominantly Observed in Alzheimer’s Disease Mice with Central Amyloid-β Pathology. J. Alzheimer’s Dis. 2022, 88, 191–205. [Google Scholar] [CrossRef]
- Noh, B.; Blasco-Conesa, M.P.; Rahman, S.M.; Monga, S.; Ritzel, R.; Guzman, G.; Lai, Y.J.; Ganesh, B.P.; Urayama, A.; McCullough, L.D.; et al. Iron Overload Induces Cerebral Endothelial Senescence in Aged Mice and in Primary Culture in a Sex-Dependent Manner. Aging Cell 2023, 22, e13977. [Google Scholar] [CrossRef] [PubMed]
- Deacon, R.M.J. Assessing Nest Building in Mice. Nat. Protoc. 2006, 1, 1117–1119. [Google Scholar] [CrossRef]
- Hollister, E.B.; Riehle, K.; Luna, R.A.; Weidler, E.M.; Rubio-Gonzales, M.; Mistretta, T.A.; Raza, S.; Doddapaneni, H.V.; Metcalf, G.A.; Muzny, D.M.; et al. Structure and Function of the Healthy Pre-Adolescent Pediatric Gut Microbiome. Microbiome 2015, 3, 36. [Google Scholar] [CrossRef]
- Spychala, M.S.; Venna, V.R.; Jandzinski, M.; Doran, S.J.; Durgan, D.J.; Ganesh, B.P.; Ajami, N.J.; Putluri, N.; Graf, J.; Bryan, R.M.; et al. Age-Related Changes in the Gut Microbiota Influence Systemic Inflammation and Stroke Outcome. Ann. Neurol. 2018, 84, 23–36. [Google Scholar] [CrossRef]
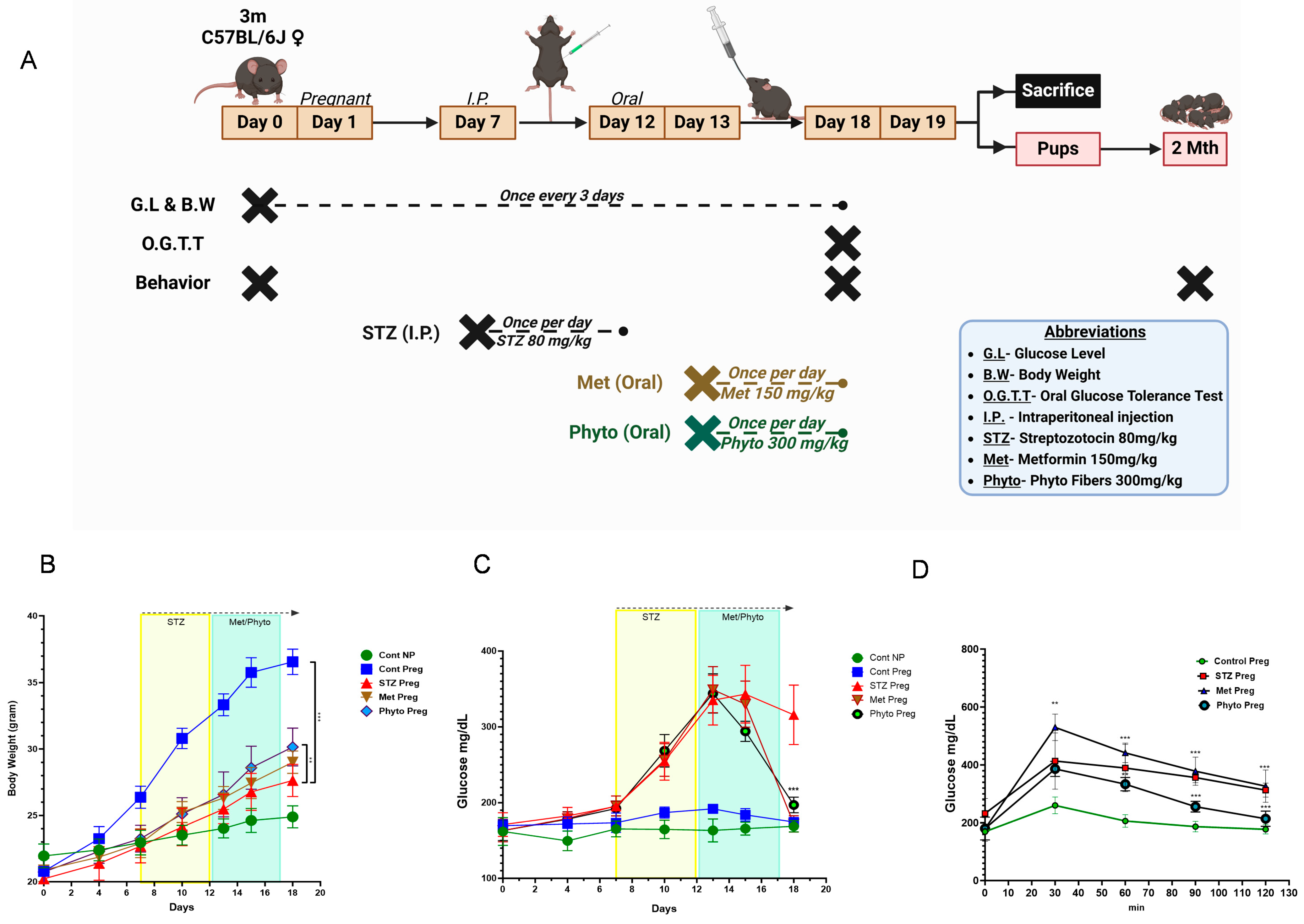
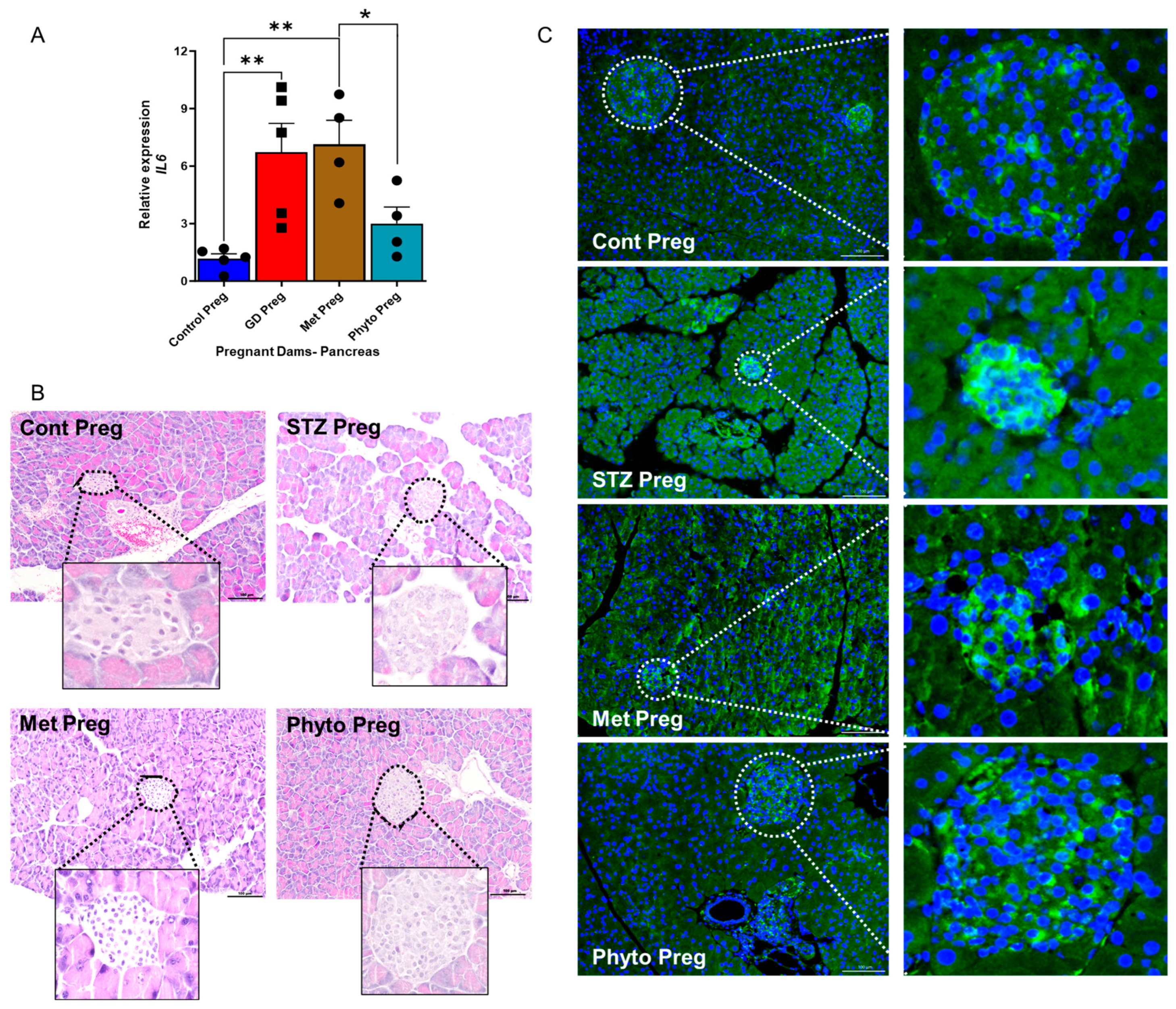
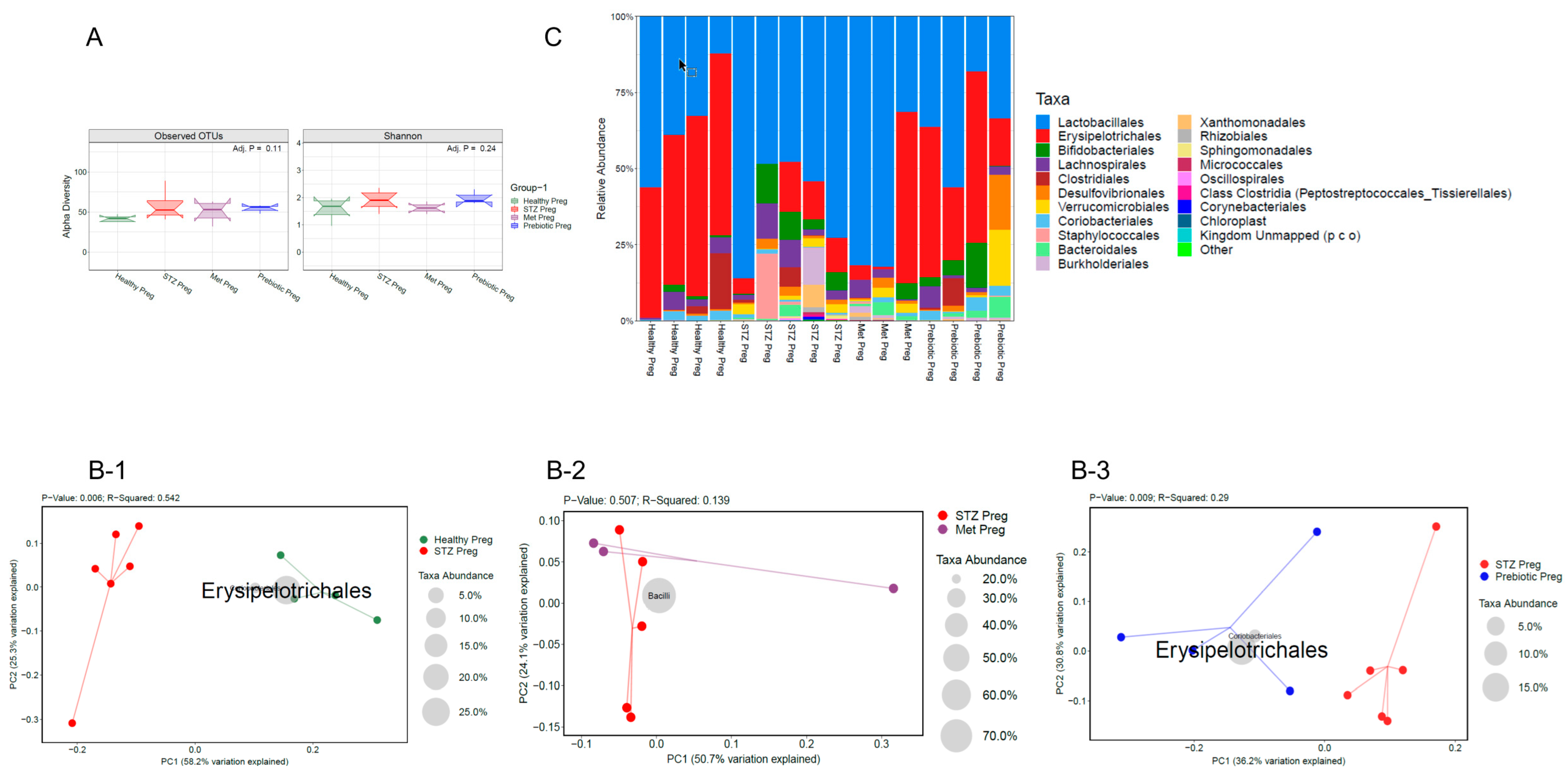
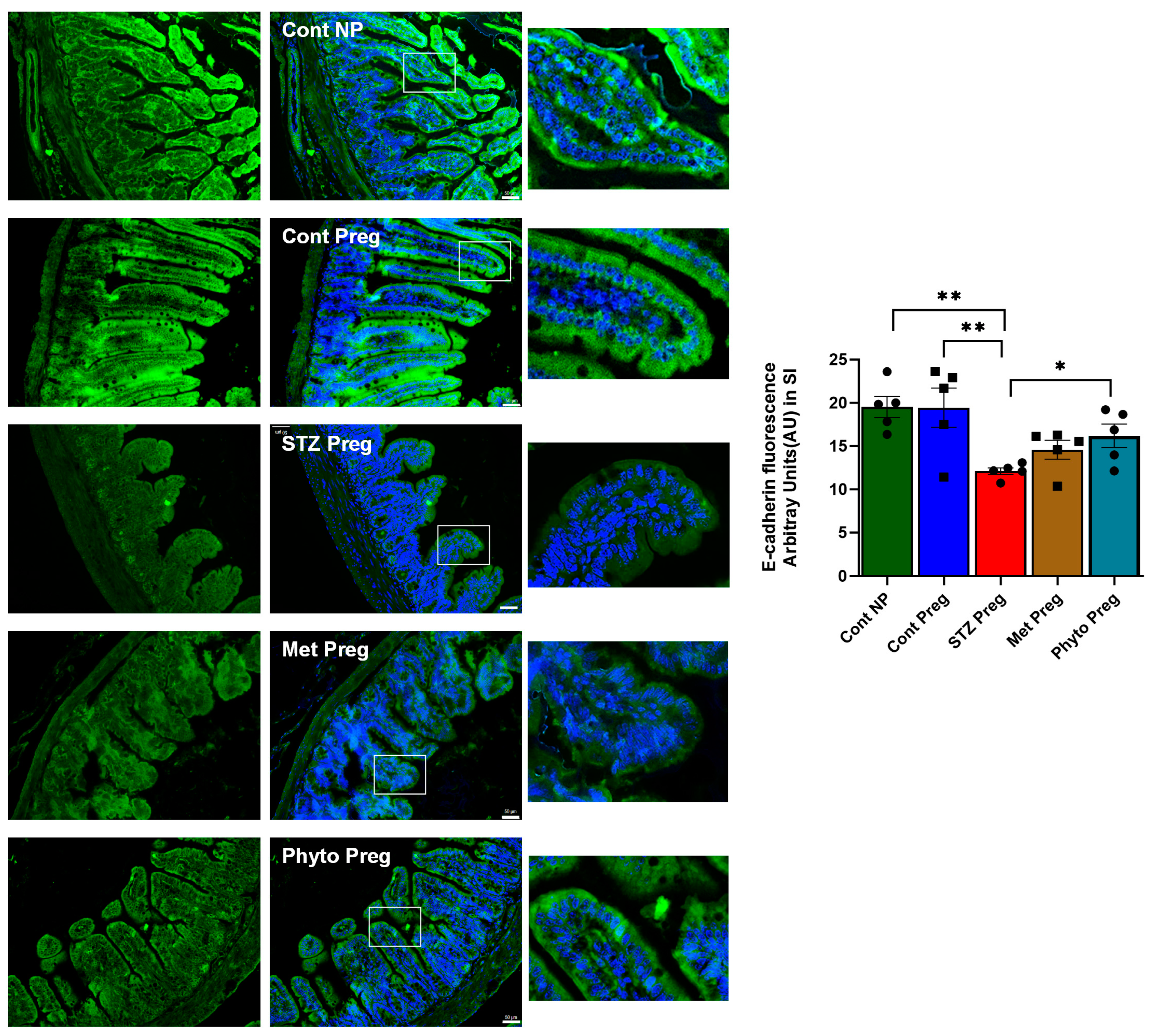

| Animal Groups | IP | Oral | |
|---|---|---|---|
| Gestational Day 7–11 | Gestational Day 13–17 | ||
| Group I | Non-pregnant Control Group (NPC) | - | - |
| Group II | Pregnant Control Group (PC) | Citrate Buffer saline | H2O |
| Group III | Gestational Diabetic Group (GD) | 80 mg/kg STZ in citrate buffer, pH 4.5 | H2O |
| Group IV | Metformin Treatment Group (MT) | 80 mg/kg STZ in citrate buffer, pH 4.5 | 150 mg/kg metformin in H2O |
| Group V | Phytocompound Treatment Group (PT) | 80 mg/kg STZ in citrate buffer, pH 4.5 | 300 mg/kg plant metabolite in H2O |
| Name of the Antibodies | Catalogue No | Company’s Name | Dilution Factor |
|---|---|---|---|
| Ecad | ab11512 | Abcam (Cambridge, UK) | 1:500 |
| GLUT2 | 66889-1-IG150UL | Thermo Fisher Scientific | 1:300 |
| GLUT3 | MA5-32697 | Thermo Fisher Scientific | 1:300 |
| GFAP | sc-33673 | Santa Cruz Biotechnology (Dallas, TX, USA) | 1:500 |
| Donkey anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 647 | A-31573 | Thermo Fisher Scientific | 1:1000 |
| Goat Anti-Mouse IgG H&L (Alexa Fluor® 488) | ab150113 | Abcam | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagadeesan, G.; Das, T.K.; Mendoza, J.M.; Alrousan, G.; Blasco-Conesa, M.P.; Thangaraj, P.; Ganesh, B.P. Effects of Prebiotic Phytocompound Administration in Gestational Diabetic Dams and Its Influence on Offspring Cognitive Outcomes. Int. J. Mol. Sci. 2025, 26, 3140. https://doi.org/10.3390/ijms26073140
Jagadeesan G, Das TK, Mendoza JM, Alrousan G, Blasco-Conesa MP, Thangaraj P, Ganesh BP. Effects of Prebiotic Phytocompound Administration in Gestational Diabetic Dams and Its Influence on Offspring Cognitive Outcomes. International Journal of Molecular Sciences. 2025; 26(7):3140. https://doi.org/10.3390/ijms26073140
Chicago/Turabian StyleJagadeesan, Gayathri, Tushar K. Das, Jennifer M. Mendoza, Ghalya Alrousan, Maria P. Blasco-Conesa, Parimelazhagan Thangaraj, and Bhanu Priya Ganesh. 2025. "Effects of Prebiotic Phytocompound Administration in Gestational Diabetic Dams and Its Influence on Offspring Cognitive Outcomes" International Journal of Molecular Sciences 26, no. 7: 3140. https://doi.org/10.3390/ijms26073140
APA StyleJagadeesan, G., Das, T. K., Mendoza, J. M., Alrousan, G., Blasco-Conesa, M. P., Thangaraj, P., & Ganesh, B. P. (2025). Effects of Prebiotic Phytocompound Administration in Gestational Diabetic Dams and Its Influence on Offspring Cognitive Outcomes. International Journal of Molecular Sciences, 26(7), 3140. https://doi.org/10.3390/ijms26073140






