Exploring Male-Specific Synaptic Plasticity in Major Depressive Disorder: A Single-Nucleus Transcriptomic Analysis Using Bioinformatics Methods
Abstract
:1. Introduction
2. Results
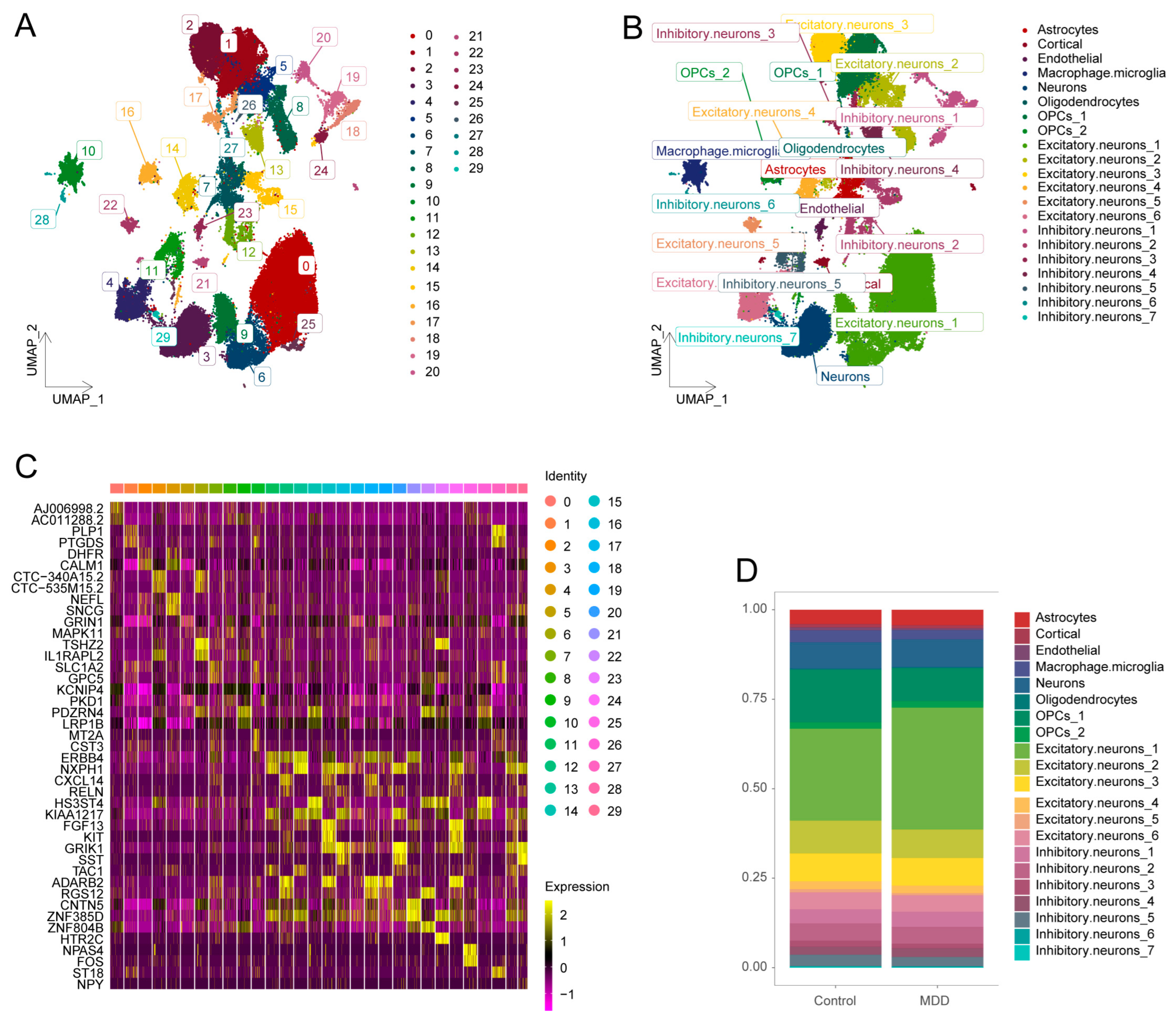
2.1. Identification of 21 Cell Clusters from Single-Nucleus RNA-Seq Data
2.2. Identification of Synaptic Plasticity Activity in Different Cell Subsets
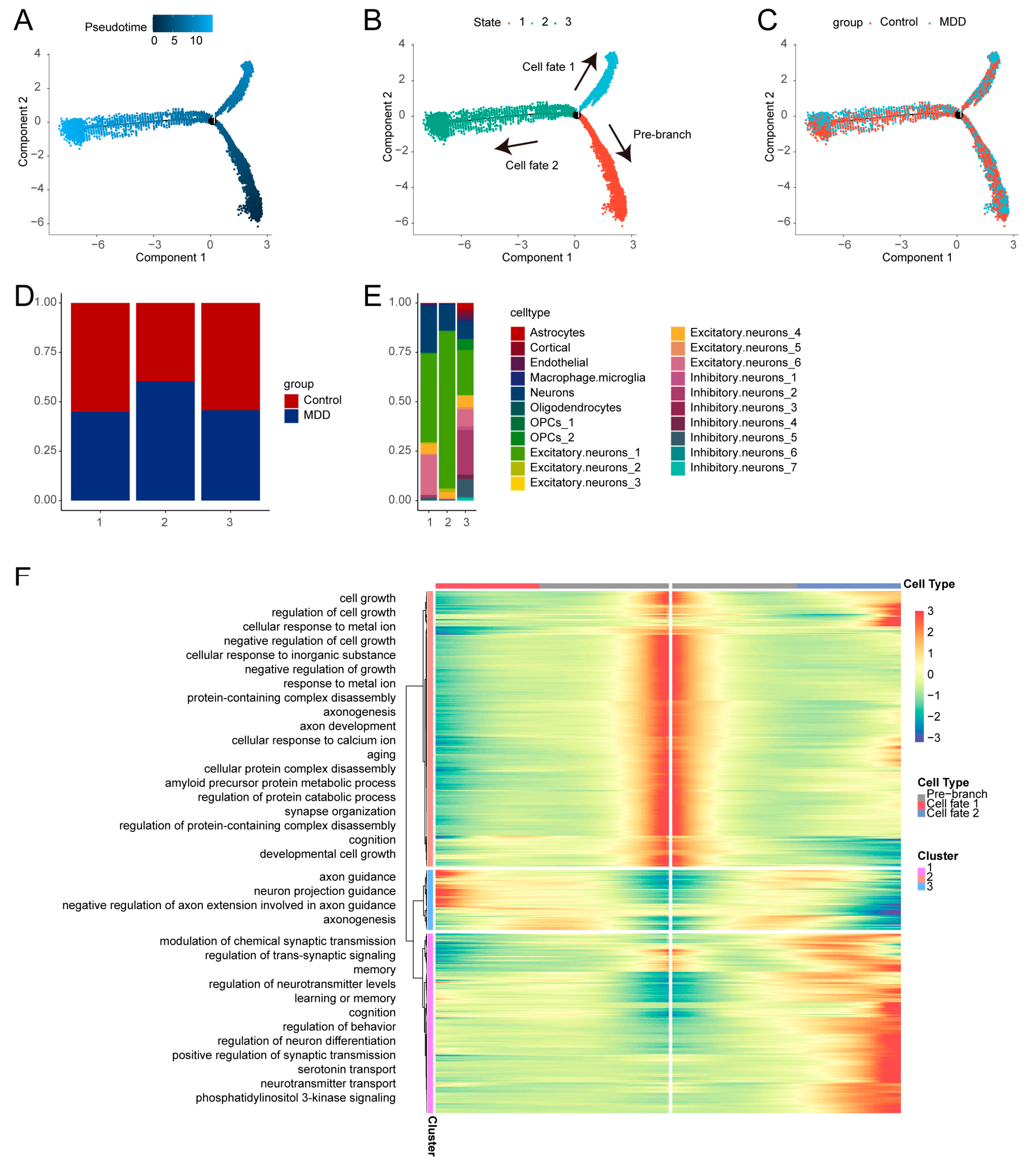
2.3. Pseudotime Trajectory Analysis of Cells with High Synaptic Plasticity Activity
2.4. Cell–Cell Communication and Ligand-Receptor Interaction Analysis
2.5. Analysis of DEGs Associated with Synaptic Plasticity in MDD
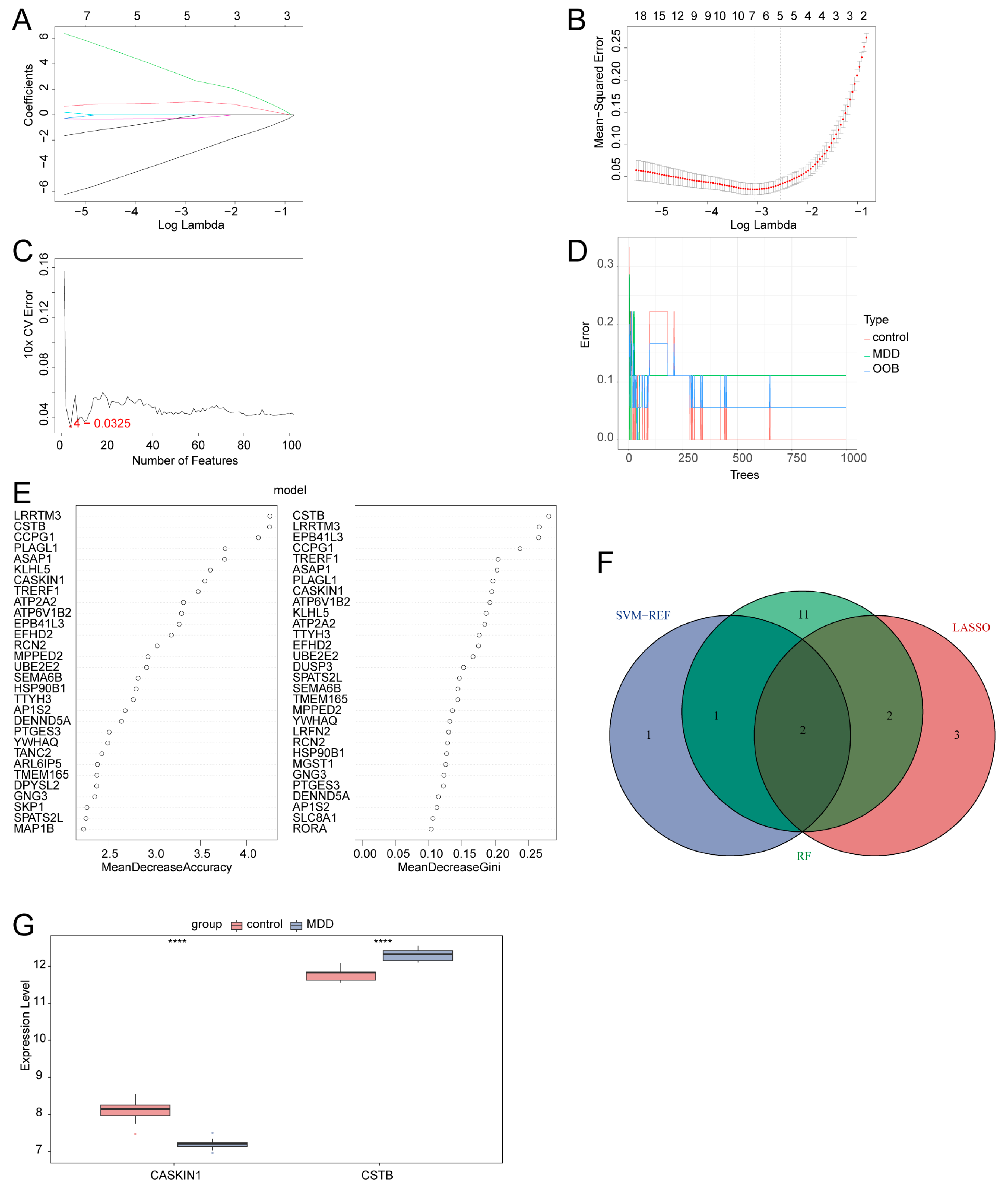
2.6. Identification of Two Hub Genes Using Multiple Machine Learning Methods
2.7. Signaling Pathways Analysis
2.8. GSEA of the DEGs
2.9. Interaction Network Analysis of the Hub Genes
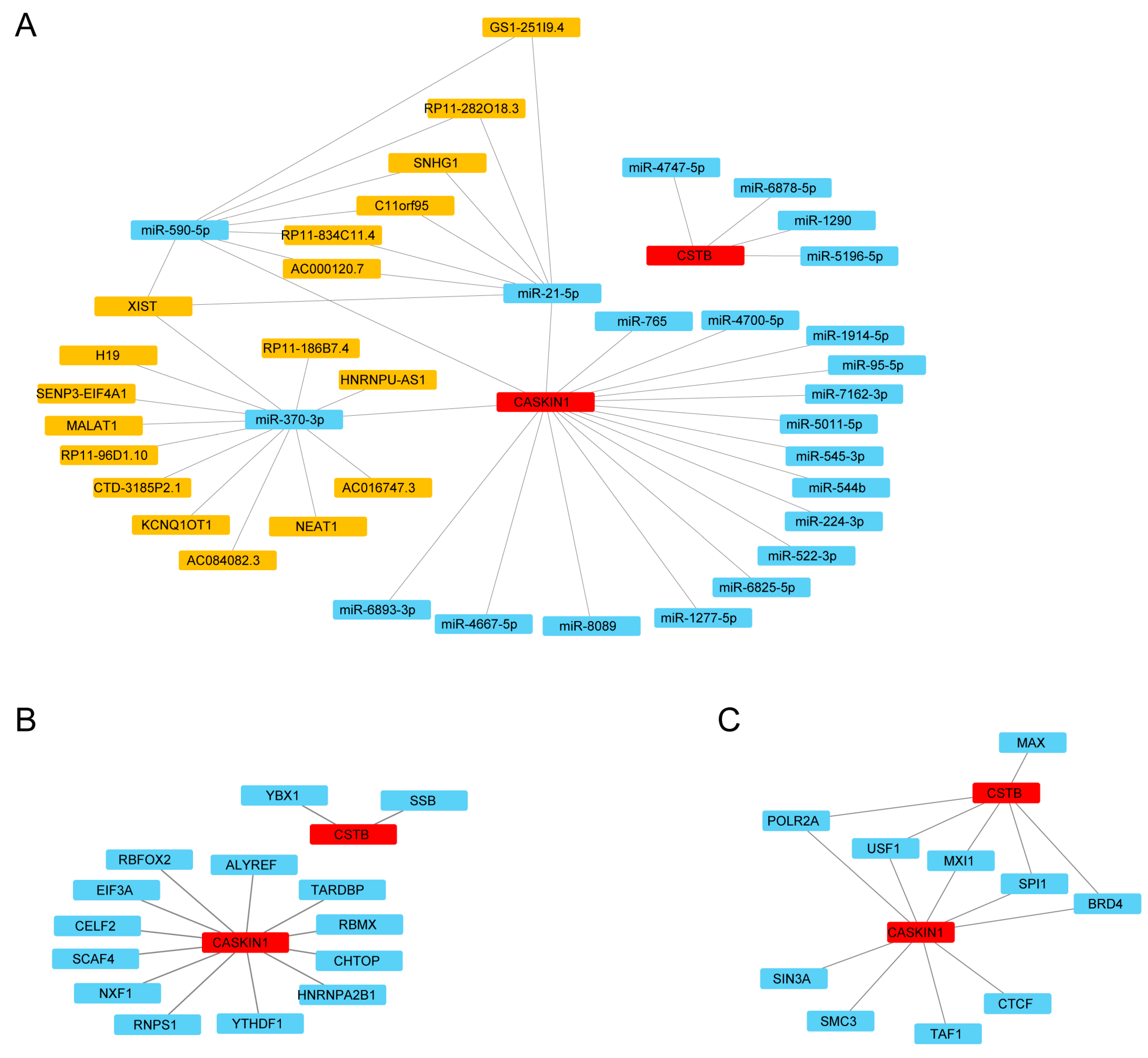
2.10. Construction of ceRNA, RBP, and TF Regulatory Networks
3. Discussion
4. Materials and Methods
4.1. Single-Nucleus RNA-Seq Data Processing
4.2. Identification of Synaptic Plasticity Activity in Different Cell Subsets
4.3. Pseudotime Trajectory Analysis
4.4. Cell–CellCommunication and Ligand-Receptor Interaction Analysis
4.5. Bulk RNA-Seq Data Processing
4.6. Differential Expression Analysis
4.7. Machine Learning Analysis
4.8. Functional Enrichment Analysis
4.9. Co-Expressed Genes Interaction Network Construction
4.10. CeRNA Network Construction
4.11. RBP-mRNA Network Construction
4.12. mRNA-TF Network Construction
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDD | Major depressive disorder |
| HPA | Hpothalamic–pituitary–adrenal |
| BDNF | Bain-derived neurotrophic factor |
| mTOR | Mammalian target of rapamycin |
| SSRIs | Selective serotonin-reuptake inhibitors |
| TCAs | Tricyclic antidepressants |
| scRNA-seq | Single-cell RNA sequencing |
| snRNA-seq | Single-nucleus RNA sequencing |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| GSEA | Gene set enrichment analysis |
| GEO | Gene set enrichment analysis |
| PCA | Principal component analysis |
| PCs | Principal components |
| UMAP | Uniform manifold approximation and projection |
| DEGs | Differentially expressed genes |
| AUC | Area under the curve |
| BEAM | Branch expression analysis modeling |
| UMIs | Unique molecular identifiers |
| LASSO | Least absolute shrinkage and selection operator |
| RF | Random forest |
| SVM-RFE | Support vector machine–recursive feature elimination |
| GSVA | Gene set variation analysis |
| RBP | RNA binding protein |
| TFs | Transcription factors |
| MDG | Mean decrease Gini |
| MDA | Mean decrease accuracy |
| NES | Normalized enrichment score |
| LTP | Long-term potentiation |
| LTD | Long-term potentiation |
References
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- World Health Organization. Suicide Prevention. Available online: https://www.who.int/health-topics/suicide#tab=tab_1 (accessed on 12 August 2024).
- Rush, A.J.; Trivedi, M.H.; Wisniewski, S.R.; Nierenberg, A.A.; Stewart, J.W.; Warden, D.; Niederehe, G.; Thase, M.E.; Lavori, P.W.; Lebowitz, B.D.; et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: A STAR*D report. Am. J. Psychiatry 2006, 163, 1905–1917. [Google Scholar] [PubMed]
- Gerhard, D.M.; Wohleb, E.S.; Duman, R.S. Emerging treatment mechanisms for depression: Focus on glutamate and synaptic plasticity. Drug Discov. Today 2016, 21, 454–464. [Google Scholar]
- Nedic Erjavec, G.; Sagud, M.; Nikolac Perkovic, M.; Svob Strac, D.; Konjevod, M.; Tudor, L.; Uzun, S.; Pivac, N. Depression: Biological markers and treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110139. [Google Scholar]
- Stetler, C.; Miller, G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011, 73, 114–126. [Google Scholar]
- Ren, F.; Guo, R. Synaptic Microenvironment in Depressive Disorder: Insights from Synaptic Plasticity. Neuropsychiatr. Dis. Treat. 2021, 17, 157–165. [Google Scholar] [PubMed]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar]
- Marsden, W.N. Synaptic plasticity in depression: Molecular, cellular and functional correlates. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 43, 168–184. [Google Scholar]
- Citri, A.; Malenka, R.C. Synaptic Plasticity: Multiple Forms, Functions, and Mechanisms. Neuropsychopharmacology 2008, 33, 18–41. [Google Scholar]
- Magee, J.C.; Grienberger, C. Synaptic Plasticity Forms and Functions. Annu. Rev. Neurosci. 2020, 43, 95–117. [Google Scholar] [CrossRef]
- Kandel, E.R. The molecular biology of memory storage: A dialogue between genes and synapses. Science 2001, 294, 1030–1038. [Google Scholar] [CrossRef]
- Vose, L.R.; Stanton, P.K. Synaptic Plasticity, Metaplasticity and Depression. Curr. Neuropharmacol. 2017, 15, 71–86. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef]
- MacQueen, G.M.; Yucel, K.; Taylor, V.H.; Macdonald, K.; Joffe, R. Posterior hippocampal volumes are associated with remission rates in patients with major depressive disorder. Biol. Psychiatry 2008, 64, 880–883. [Google Scholar] [CrossRef]
- Kang, H.J.; Voleti, B.; Hajszan, T.; Rajkowska, G.; Stockmeier, C.A.; Licznerski, P.; Lepack, A.; Majik, M.S.; Jeong, L.S.; Banasr, M.; et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat. Med. 2012, 18, 1413–1417. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef]
- Castrén, E.; Hen, R. Neuronal plasticity and antidepressant actions. Trends Neurosci. 2013, 36, 259–267. [Google Scholar] [CrossRef]
- Ru, Q.; Lu, Y.; Saifullah, A.B.; Blanco, F.A.; Yao, C.; Cata, J.P.; Li, D.P.; Tolias, K.F.; Li, L. TIAM1-mediated synaptic plasticity underlies comorbid depression-like and ketamine antidepressant-like actions in chronic pain. J. Clin. Investig. 2022, 132, e158545. [Google Scholar] [CrossRef] [PubMed]
- Lake, B.B.; Chen, S.; Sos, B.C.; Fan, J.; Kaeser, G.E.; Yung, Y.C.; Duong, T.E.; Gao, D.; Chun, J.; Kharchenko, P.V.; et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 2018, 36, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.X.; Terry, J.M.; Belgrader, P.; Ryvkin, P.; Bent, Z.W.; Wilson, R.; Ziraldo, S.B.; Wheeler, T.D.; McDermott, G.P.; Zhu, J.; et al. Massively parallel digital transcriptional profiling of single cells. Nat. Commun. 2017, 8, 14049. [Google Scholar]
- Lake, B.B.; Codeluppi, S.; Yung, Y.C.; Gao, D.; Chun, J.; Kharchenko, P.V.; Linnarsson, S.; Zhang, K. A comparative strategy for single-nucleus and single-cell transcriptomes confirms accuracy in predicted cell-type expression from nuclear RNA. Sci. Rep. 2017, 7, 6031. [Google Scholar]
- Nagy, C.; Maitra, M.; Tanti, A.; Suderman, M.; Théroux, J.F.; Davoli, M.A.; Perlman, K.; Yerko, V.; Wang, Y.C.; Tripathy, S.J.; et al. Single-nucleus transcriptomics of the prefrontal cortex in major depressive disorder implicates oligodendrocyte precursor cells and excitatory neurons. Nat. Neurosci. 2020, 23, 771–781. [Google Scholar]
- Maitra, M.; Mitsuhashi, H.; Rahimian, R.; Chawla, A.; Yang, J.; Fiori, L.M.; Davoli, M.A.; Perlman, K.; Aouabed, Z.; Mash, D.C.; et al. Cell type specific transcriptomic differences in depression show similar patterns between males and females but implicate distinct cell types and genes. Nat. Commun. 2023, 14, 2912. [Google Scholar]
- Xie, Y.; Chen, L.; Wang, L.; Liu, T.; Zheng, Y.; Si, L.; Ge, H.; Xu, H.; Xiao, L.; Wang, G. Single-nucleus transcriptomic analysis reveals the relationship between gene expression in oligodendrocyte lineage and major depressive disorder. J. Transl. Med. 2024, 22, 109. [Google Scholar]
- Howard, D.M.; Adams, M.J.; Shirali, M.; Clarke, T.K.; Marioni, R.E.; Davies, G.; Coleman, J.R.I.; Alloza, C.; Shen, X.; Barbu, M.C.; et al. Genome-wide association study of depression phenotypes in UK Biobank identifies variants in excitatory synaptic pathways. Nat. Commun. 2018, 9, 1470. [Google Scholar]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar]
- Lynch, M.A. Long-term potentiation and memory. Physiol. Rev. 2004, 84, 87–136. [Google Scholar]
- Colakoglu, G.; Bergstrom-Tyrberg, U.; Berglund, E.O.; Ranscht, B. Contactin-1 regulates myelination and nodal/paranodal domain organization in the central nervous system. Proc. Natl. Acad. Sci. USA 2014, 111, E394–E403. [Google Scholar] [PubMed]
- Mei, L.; Xiong, W.C. Neuregulin 1 in neural development, synaptic plasticity and schizophrenia. Nat. Rev. Neurosci. 2008, 9, 437–452. [Google Scholar] [PubMed]
- Stafford, R.L.; Hinde, E.; Knight, M.J.; Pennella, M.A.; Ear, J.; Digman, M.A.; Gratton, E.; Bowie, J.U. Tandem SAM domain structure of human Caskin1: A presynaptic, self-assembling scaffold for CASK. Structure 2011, 19, 1826–1836. [Google Scholar]
- Katano, T.; Takao, K.; Abe, M.; Yamazaki, M.; Watanabe, M.; Miyakawa, T.; Sakimura, K.; Ito, S. Distribution of Caskin1 protein and phenotypic characterization of its knockout mice using a comprehensive behavioral test battery. Mol. Brain 2018, 11, 63. [Google Scholar]
- Bencsik, N.; Pusztai, S.; Borbély, S.; Fekete, A.; Dülk, M.; Kis, V.; Pesti, S.; Vas, V.; Szűcs, A.; Buday, L.; et al. Dendritic spine morphology and memory formation depend on postsynaptic Caskin proteins. Sci. Rep. 2019, 9, 16843. [Google Scholar]
- Joensuu, T.; Tegelberg, S.; Reinmaa, E.; Segerstråle, M.; Hakala, P.; Pehkonen, H.; Korpi, E.R.; Tyynelä, J.; Taira, T.; Hovatta, I.; et al. Gene expression alterations in the cerebellum and granule neurons of Cstb−/− mouse are associated with early synaptic changes and inflammation. PLoS ONE 2014, 9, e89321. [Google Scholar]
- D’Amato, E.; Kokaia, Z.; Nanobashvili, A.; Reeben, M.; Lehesjoki, A.E.; Saarma, M.; Lindvall, O. Seizures induce widespread upregulation of cystatin B, the gene mutated in progressive myoclonus epilepsy, in rat forebrain neurons. Eur. J. Neurosci. 2000, 12, 1687–1695. [Google Scholar]
- Pizzella, A.; Penna, E.; Abate, N.; Frenna, E.; Canafoglia, L.; Ragona, F.; Russo, R.; Chambery, A.; Perrone-Capano, C.; Cappello, S.; et al. Pathological Deficit of Cystatin B Impairs Synaptic Plasticity in EPM1 Human Cerebral Organoids. Mol. Neurobiol. 2024, 61, 4318–4334. [Google Scholar]
- Goldsmith, D.R.; Rapaport, M.H.; Miller, B.J. A meta-analysis of blood cytokine network alterations in psychiatric patients: Comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 2016, 21, 1696–1709. [Google Scholar]
- Bhatt, S.; Nagappa, A.N.; Patil, C.R. Role of oxidative stress in depression. Drug Discov. Today 2020, 25, 1270–1276. [Google Scholar]
- Dong, X.; Pi, Q.; Yuemaierabola, A.; Guo, W.; Tian, H. Silencing LINC00294 Restores Mitochondrial Function and Inhibits Apoptosis of Glioma Cells under Hypoxia via the miR-21-5p/CASKIN1/cAMP Axis. Oxid. Med. Cell. Longev. 2021, 2021, 8240015. [Google Scholar]
- Lv, X.; Zhao, K.; Lan, Y.; Li, Z.; Ding, N.; Su, J.; Lu, H.; Song, D.; Gao, F.; He, W. miR-21a-5p Contributes to Porcine Hemagglutinating Encephalomyelitis Virus Proliferation via Targeting CASK-Interactive Protein1 In vivo and vitro. Front. Microbiol. 2017, 8, 304. [Google Scholar]
- Zhou, Y.; Xiong, L.; Chen, J.; Wang, Q. Integrative Analyses of scRNA-seq, Bulk mRNA-seq, and DNA Methylation Profiling in Depressed Suicide Brain Tissues. Int. J. Neuropsychopharmacol. 2023, 26, 840–855. [Google Scholar]
- Ma, F.; Bian, H.; Jiao, W.; Zhang, N. Single-cell RNA-seq reveals the role of YAP1 in prefrontal cortex microglia in depression. BMC Neurol. 2024, 24, 191. [Google Scholar]
- Van de Sande, B.; Flerin, C.; Davie, K.; De Waegeneer, M.; Hulselmans, G.; Aibar, S.; Seurinck, R.; Saelens, W.; Cannoodt, R.; Rouchon, Q.; et al. A scalable SCENIC workflow for single-cell gene regulatory network analysis. Nat. Protoc. 2020, 15, 2247–2276. [Google Scholar]
- Qiu, X.; Mao, Q.; Tang, Y.; Wang, L.; Chawla, R.; Pliner, H.A.; Trapnell, C. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 2017, 14, 979–982. [Google Scholar]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Zhu, X.; Yang, F.; Liu, Y.; Ba, H.; Huang, P.; Wang, H.; Bian, Y.; Li, C.; Zhang, S. Exploring Male-Specific Synaptic Plasticity in Major Depressive Disorder: A Single-Nucleus Transcriptomic Analysis Using Bioinformatics Methods. Int. J. Mol. Sci. 2025, 26, 3135. https://doi.org/10.3390/ijms26073135
Chen J, Zhu X, Yang F, Liu Y, Ba H, Huang P, Wang H, Bian Y, Li C, Zhang S. Exploring Male-Specific Synaptic Plasticity in Major Depressive Disorder: A Single-Nucleus Transcriptomic Analysis Using Bioinformatics Methods. International Journal of Molecular Sciences. 2025; 26(7):3135. https://doi.org/10.3390/ijms26073135
Chicago/Turabian StyleChen, Ji, Xiumei Zhu, Fan Yang, Yanan Liu, Huajie Ba, Ping Huang, Hongyan Wang, Yingnan Bian, Chengtao Li, and Suhua Zhang. 2025. "Exploring Male-Specific Synaptic Plasticity in Major Depressive Disorder: A Single-Nucleus Transcriptomic Analysis Using Bioinformatics Methods" International Journal of Molecular Sciences 26, no. 7: 3135. https://doi.org/10.3390/ijms26073135
APA StyleChen, J., Zhu, X., Yang, F., Liu, Y., Ba, H., Huang, P., Wang, H., Bian, Y., Li, C., & Zhang, S. (2025). Exploring Male-Specific Synaptic Plasticity in Major Depressive Disorder: A Single-Nucleus Transcriptomic Analysis Using Bioinformatics Methods. International Journal of Molecular Sciences, 26(7), 3135. https://doi.org/10.3390/ijms26073135






