Anther Transcriptome Analysis of Two Heat Tolerance-Differentiated Indica Rice Restorer Lines Reveals the Importance of Non-Structural Carbohydrates and ATP in the Regulation of Heat Tolerance
Abstract
1. Introduction
2. Results
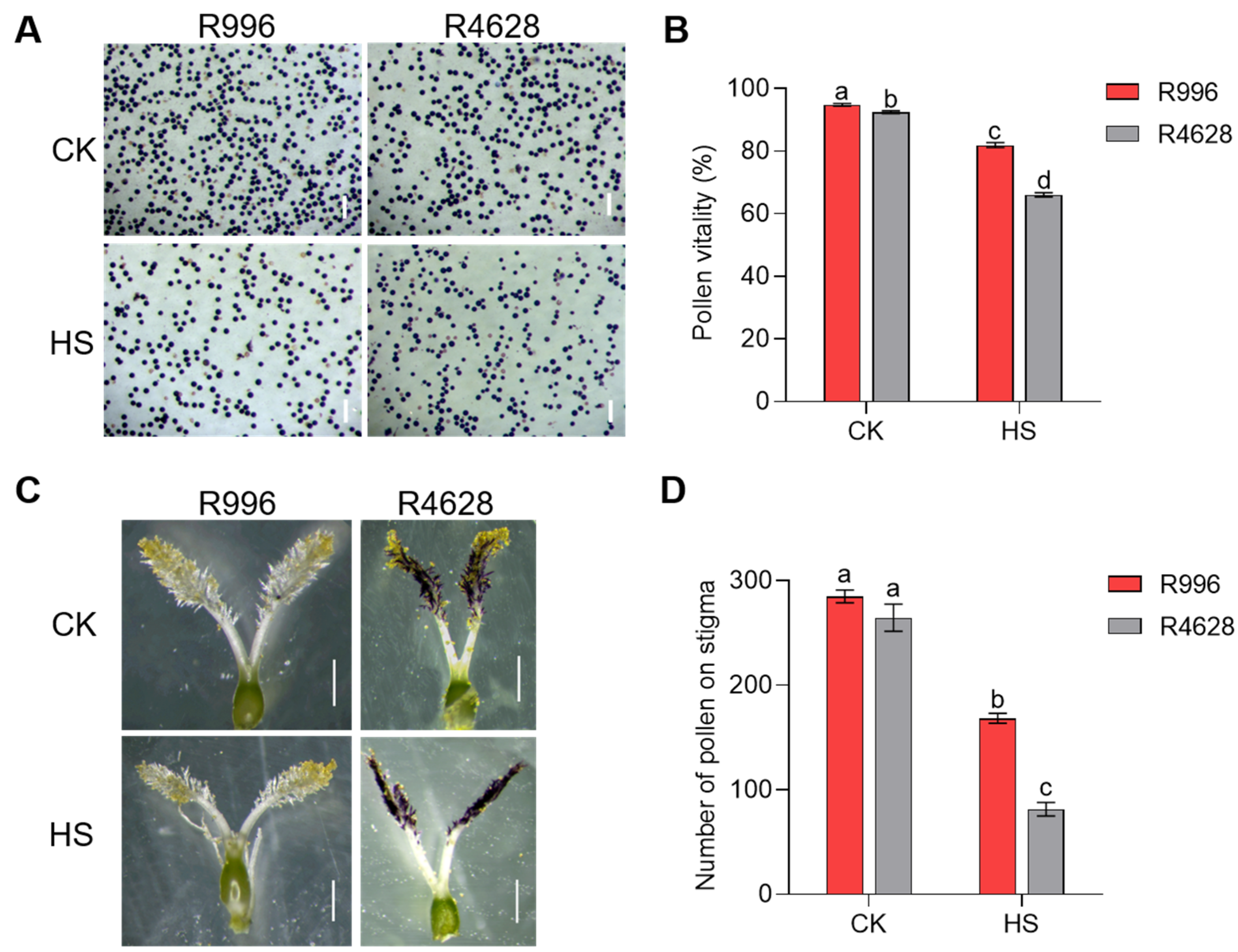
2.1. R996 Is More Resistant to HS than R4628
2.2. Decrease in Stigma Pollen Number and Loss of Pollen Viability Are Responsible for Reduced Seed Setting Rate Under HS
2.3. RNA-Seq Results of Transcriptome Samples
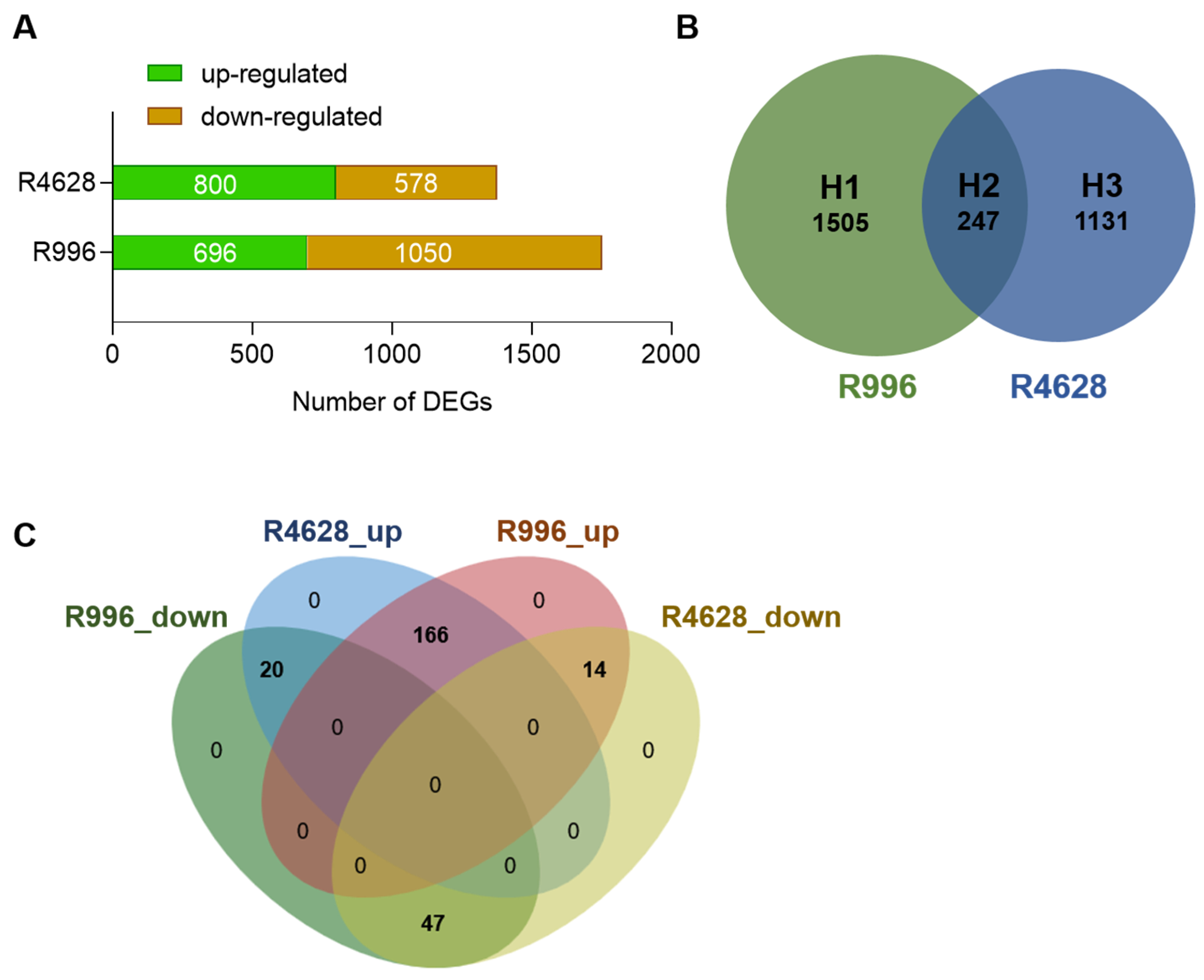
2.4. Differentially Expressed Gene Analysis
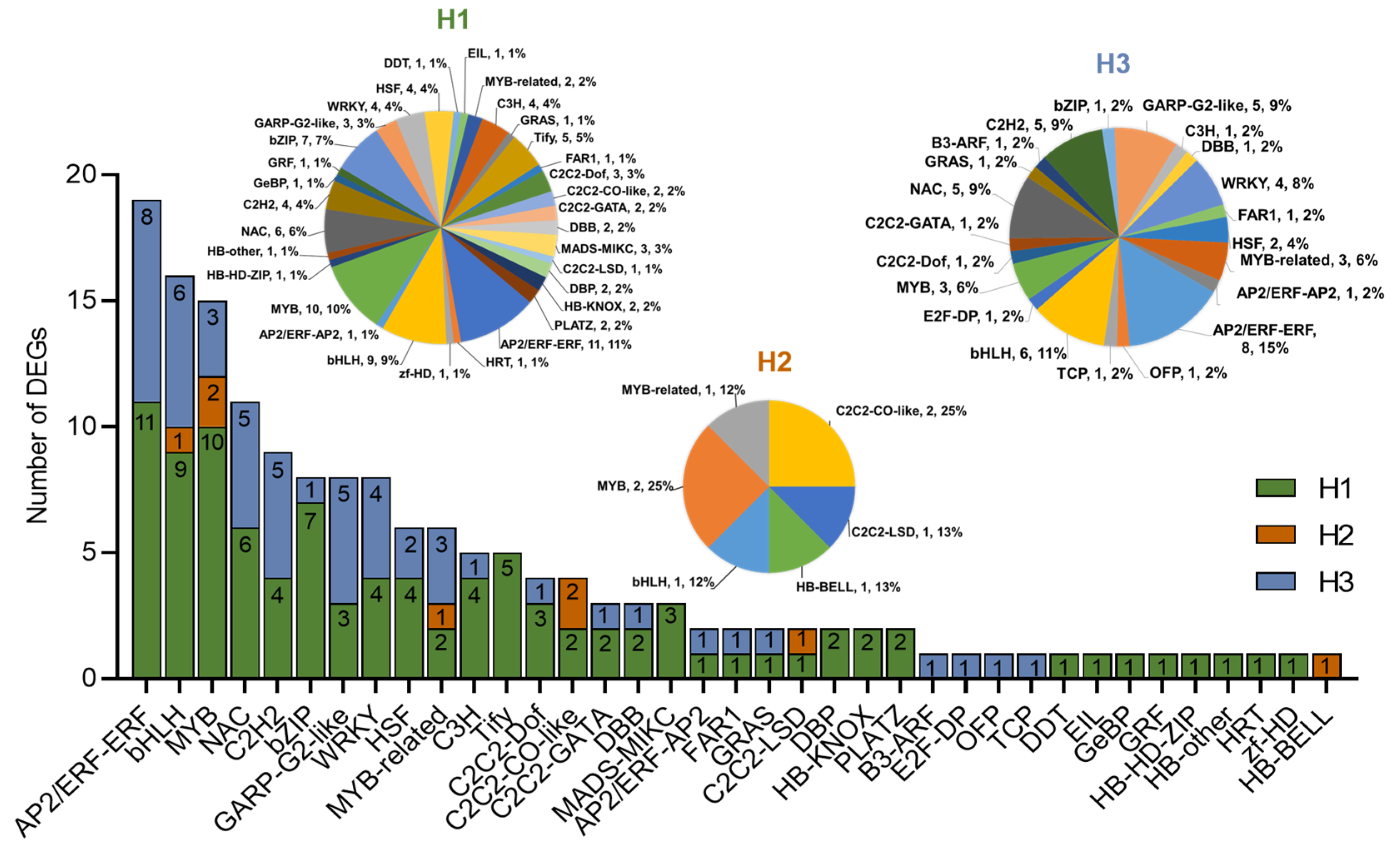
2.5. Identification of TFs in DEGs
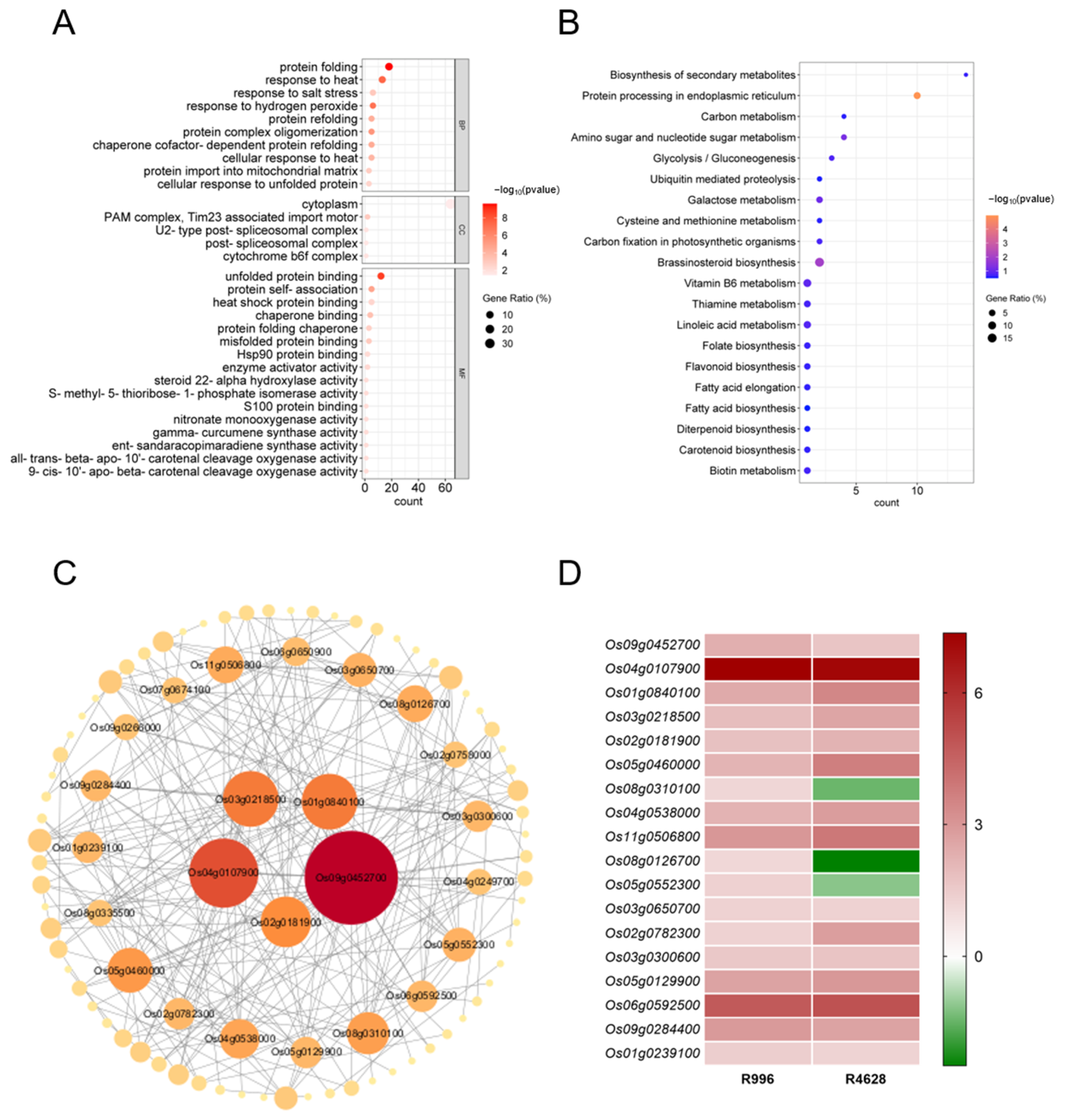
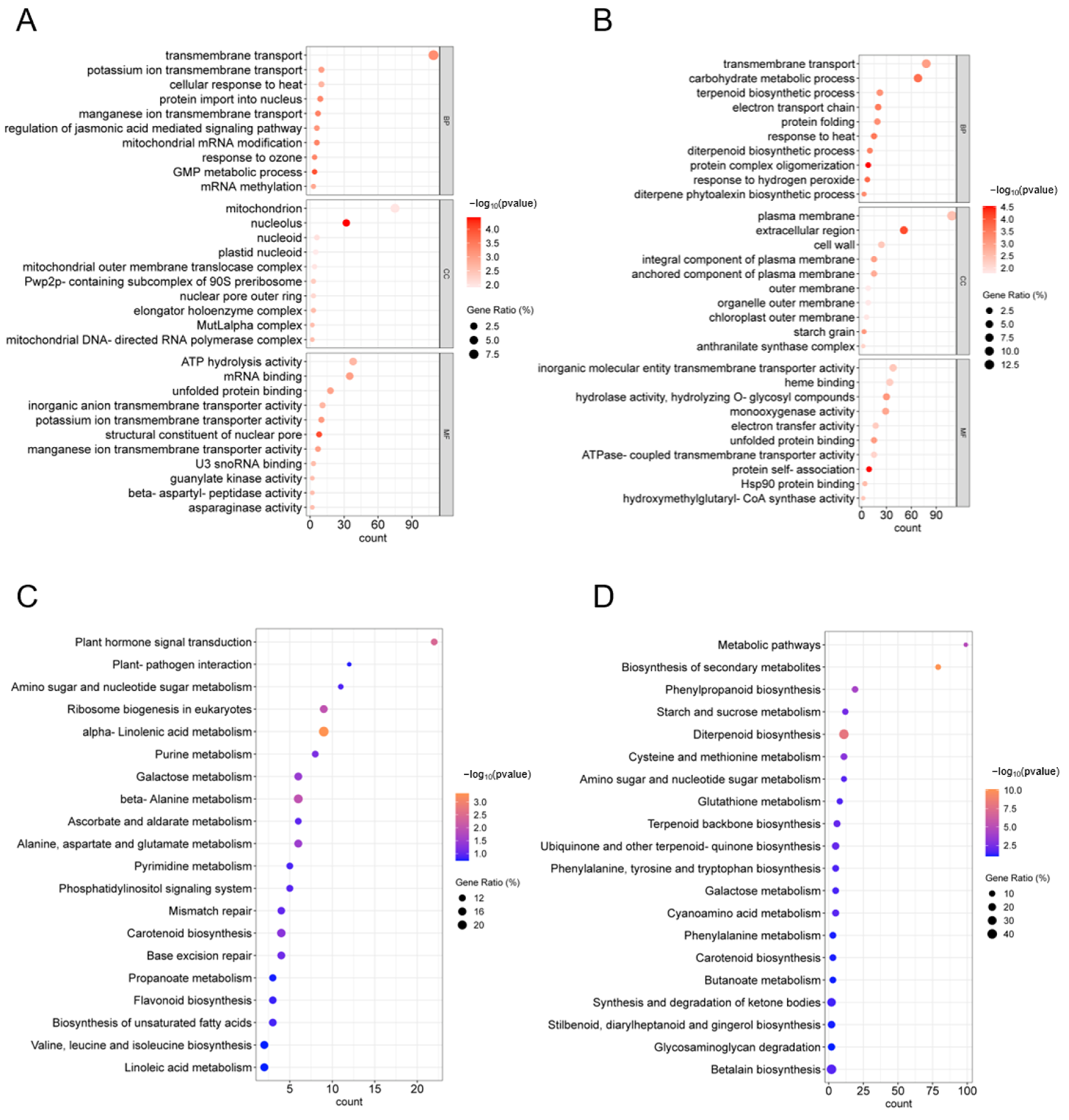
2.6. GO and KEGG Enrichment Analysis and PPI Network Construction of H2 DEGs
2.7. GO and KEGG Analysis of DEGs in H1 and H3
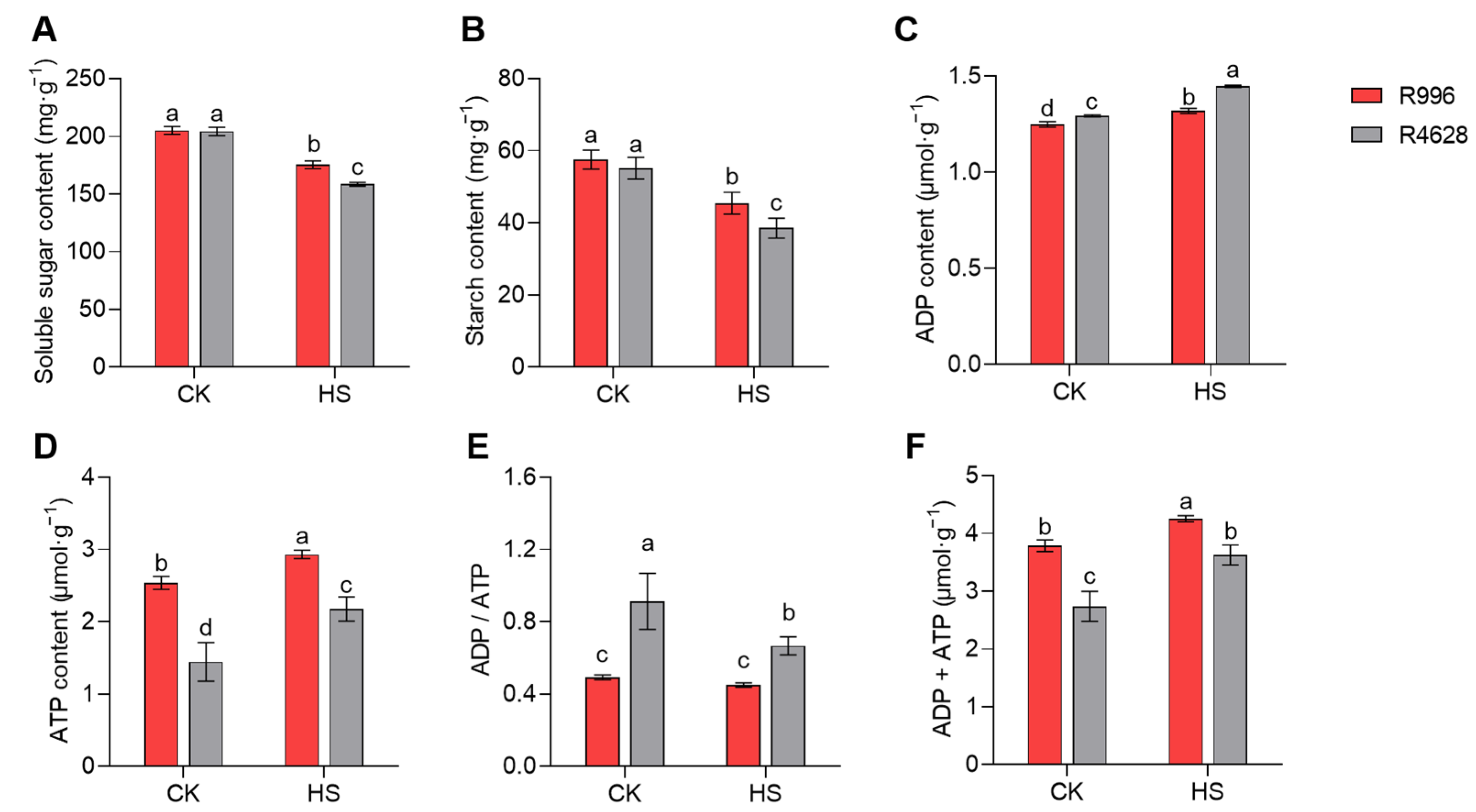
2.8. Non-Structural Carbohydrates and ATP Content
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Pollen Viability Determination
4.3. Number of Pollens on Stigma Determination
4.4. Anther Dehiscence Rate Determination
4.5. Seed Setting Rate Determination
4.6. RNA Extraction
4.7. RNA-Seq Data Processing
4.8. Analysis of DEGs
4.9. Validation of DEGs by qRT–PCR
4.10. Non-Structural Carbohydrate Measurements
4.11. ATP and ADP Content Measurements
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Chang. 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Jagadish, S.; Craufurd, P.; Wheeler, T. High temperature stress and spikelet fertility in rice (Oryza sativa L.). J. Exp. Bot. 2007, 58, 1627–1635. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Chen, T.; Feng, B.; Fu, W.; Yan, J.; Islam, M.R.; Jin, Q.; Tao, L.; Fu, G. Heat stress induces spikelet sterility in rice at anthesis through inhibition of pollen tube elongation interfering with auxin homeostasis in pollinated pistils. Rice 2018, 11, 14. [Google Scholar] [CrossRef]
- Jagadish, S.V.K.; Muthurajan, R.; Oane, R.; Wheeler, T.R.; Heuer, S.; Bennett, J.; Craufurd, P.Q. Physiological and proteomic approaches to address heat tolerance during anthesis in rice (Oryza sativa L.). J. Exp. Bot. 2010, 61, 143–156. [Google Scholar] [CrossRef]
- Li, X.-M.; Chao, D.-Y.; Wu, Y.; Huang, X.; Chen, K.; Cui, L.-G.; Su, L.; Ye, W.-W.; Chen, H.; Chen, H.-C.; et al. Natural alleles of a proteasome α2 subunit gene contribute to thermotolerance and adaptation of African rice. Nat. Genet. 2015, 47, 827–833. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, J.-F.; Kan, Y.; Shan, J.-X.; Ye, W.-W.; Dong, N.-Q.; Guo, T.; Xiang, Y.-H.; Yang, Y.-B.; Li, Y.-C.; et al. A genetic module at one locus in rice protects chloroplasts to enhance thermotolerance. Science 2022, 376, 1293–1300. [Google Scholar] [CrossRef]
- Chen, F.; Dong, G.; Wang, F.; Shi, Y.; Zhu, J.; Zhang, Y.; Ruan, B.; Wu, Y.; Feng, X.; Zhao, C.; et al. A β-ketoacyl carrier protein reductase confers heat tolerance via the regulation of fatty acid biosynthesis and stress signaling in rice. New Phytol. 2021, 232, 655–672. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, Y.; Zhang, Y.; Xiao, Y.; Liu, X.; Deng, H.; Lu, X.; Tang, W.; Zhang, G. Comparative analysis of heat-tolerant and heat-susceptible rice highlights the role of OsNCED1 gene in heat stress tolerance. Plants 2022, 11, 1062. [Google Scholar] [CrossRef]
- Cao, Z.; Tang, H.; Cai, Y.; Zeng, B.; Zhao, J.; Tang, X.; Lu, M.; Wang, H.; Zhu, X.; Wu, X.; et al. Natural variation of HTH5 from wild rice, Oryza rufipogon Griff., is involved in conferring high-temperature tolerance at the heading stage. Plant Biotechnol. J. 2022, 20, 1591–1605. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef]
- Yanhua, C.; Yaliang, W.; Huizhe, C.; Jing, X.; Yikai, Z.; Zhigang, W.; Defeng, Z.; Yuping, Z. Brassinosteroids mediate endogenous phytohormone metabolism to alleviate high temperature injury at panicle initiation stage in rice. Rice Sci. 2023, 30, 70–86. [Google Scholar]
- Al-Zahrani, H.S.; Alharby, H.F.; Fahad, S. Antioxidative defense system, hormones, and metabolite accumulation in different plant parts of two contrasting rice cultivars as influenced by plant growth regulators under heat stress. Front. Plant Sci. 2022, 13, 911846. [Google Scholar] [CrossRef]
- Alvi, A.F.; Khan, S.; Khan, N.A. Hydrogen sulfide and ethylene regulate sulfur-mediated stomatal and photosynthetic responses and heat stress acclimation in rice. Plant Physiol. Biochem. 2024, 207, 108437. [Google Scholar] [CrossRef]
- He, Q.; Deng, H.; Sun, P.; Zhang, W.; Shu, F.; Xing, J.; Peng, Z. Hybrid Rice. Engineering 2020, 6, 967–973. [Google Scholar] [CrossRef]
- Das, S.; Krishnan, P.; Nayak, M.; Ramakrishnan, B. High temperature stress effects on pollens of rice (Oryza sativa L.) genotypes. Environ. Exp. Bot. 2014, 101, 36–46. [Google Scholar] [CrossRef]
- Cao, Z.-Z.; Zhao, Q.; Huang, F.-D.; Wei, K.-S.; Zaidi, S.-H.; Zhou, W.-J.; Cheng, F.-M. Effects of high temperature at anthesis on spikelet fertility and grain weight in relation to floral positions with in a panicle of rice (Oryza sativa L.). Crop. Pasture Sci. 2015, 66, 922. [Google Scholar] [CrossRef]
- Coast, O.; Murdoch, A.J.; Ellis, R.H.; Hay, F.R.; Jagadish, K.S. Resilience of rice (Oryza spp.) pollen germination and tube growth to temperature stress. Plant Cell Environ. 2016, 39, 26–37. [Google Scholar] [CrossRef]
- Dai, C.; Dai, X.; Qu, H.; Men, Q.; Liu, J.; Yu, L.; Gu, M.; Xu, G. The rice phosphate transporter OsPHT1;7 plays a dual role in phosphorus redistribution and anther development. Plant Physiol. 2022, 188, 2272–2288. [Google Scholar] [CrossRef]
- Rahman, M.A.; Fennell, H.; Ullah, H. Receptor for Activated C Kinase1B (OsRACK1B) Impairs Fertility in Rice through NADPH-Dependent H2O2 Signaling Pathway. Int. J. Mol. Sci. 2022, 23, 8455. [Google Scholar] [CrossRef]
- Xie, E.; Chen, J.; Wang, B.; Shen, Y.; Tang, D.; Du, G.; Li, Y.; Cheng, Z. The transcribed centromeric gene OsMRPL15 is essential for pollen development in rice. Plant Physiol. 2023, 192, 1063–1079. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-K.; Eom, J.-S.; Hwang, S.-K.; Shin, D.; An, G.; Okita, T.W.; Jeon, J.-S. Plastidic phosphoglucomutase and ADP-glucose pyrophosphorylase mutants impair starch synthesis in rice pollen grains and cause male sterility. J. Exp. Bot. 2016, 67, 5557–5569. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhao, Y.; Zhang, Y.; Chen, S.; Ying, Q.; Lv, Z.; Che, X.; Wang, D. Heat stress may cause a significant reduction of rice yield in China under future climate scenarios. Sci. Total. Environ. 2022, 818, 151746. [Google Scholar] [CrossRef]
- Kan, Y.; Mu, X.-R.; Gao, J.; Lin, H.-X.; Lin, Y. The molecular basis of heat stress responses in plants. Mol. Plant 2023, 16, 1612–1634. [Google Scholar] [CrossRef]
- Moon, J.-C.; Ham, D.J.; Hwang, S.-G.; Park, Y.C.; Lee, C.; Jang, C.S. Molecular characterization of a heat inducible rice gene, OsHSP1, and implications for rice thermotolerance. Genes Genom. 2014, 36, 151–161. [Google Scholar] [CrossRef]
- Singh, G.; Sarkar, N.K.; Grover, A. A Hsp70, sHsps and ubiquitin proteins modulate HsfA6a-mediated Hsp101 transcript expression in rice (Oryza sativa L.). Physiol. Plant. 2021, 173, 2055–2067. [Google Scholar]
- Sarkar, N.K.; Kotak, S.; Agarwal, M.; Kim, Y.-K.; Grover, A. Silencing of class I small heat shock proteins affects seed-related attributes and thermotolerance in rice seedlings. Planta 2020, 251, 26. [Google Scholar] [CrossRef]
- Mahmood, A.; Wang, W.; Raza, M.A.; Ali, I.; Liu, B.; Liu, L.; Zhu, Y.; Tang, L.; Cao, W. Quantifying the individual and combined effects of short-term heat stress at booting and flowering stages on nonstructural carbohydrates remobilization in rice. Plants 2024, 13, 810. [Google Scholar] [CrossRef]
- Thalmann, M.; Santelia, D. Starch as a determinant of plant fitness under abiotic stress. New Phytol. 2017, 214, 943–951. [Google Scholar] [CrossRef]
- Afzal, S.; Chaudhary, N.; Singh, N.K. Plant Growth Regulators: Signalling under Stress Conditions. In Role of Soluble Sugars in Metabolism and Sensing Under Abiotic Stress; Aftab, T., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 305–334. [Google Scholar]
- Lin, S.; Liu, Z.; Sun, S.; Xue, F.; Li, H.; Tursun, A.; Cao, L.; Zhang, L.; A Wilson, Z.; Zhang, D.; et al. Rice HEAT SHOCK PROTEIN60-3B maintains male fertility under high temperature by starch granule biogenesis. Plant Physiol. 2023, 192, 2301–2317. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, Y.; Zhou, L.; Asad, M.A.U.; Zhao, Q.; Pan, G.; Cheng, F. Disruptions of sugar utilization and carbohydrate metabolism in rice developing anthers aggravated heat stress-induced pollen abortion. Plant Physiol. Biochem. 2023, 202, 107991. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Yu, P.; Fu, W.; Li, G.; Feng, B.; Chen, T.; Li, H.; Tao, L.; Fu, G. Acid invertase confers heat tolerance in rice plants by maintaining energy homoeostasis of spikelets. Plant Cell Environ. 2020, 43, 1273–1287. [Google Scholar] [CrossRef]
- Li, G.; Zhang, C.; Zhang, G.; Fu, W.; Feng, B.; Chen, T.; Peng, S.; Tao, L.; Fu, G. Abscisic Acid Negatively Modulates Heat Tolerance in Rolled Leaf Rice by Increasing Leaf Temperature and Regulating Energy Homeostasis. Rice 2020, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.; Li, J.; Fu, W.; Wang, W.; Tao, L.; Fu, G.; Chen, T.; Feng, B. Abscisic Acid Improves Rice Thermo-Tolerance by Affecting Trehalose Metabolism. Int. J. Mol. Sci. 2022, 23, 10615. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-P.; Ma, X.-C.; Wang, H.; Zhu, Y.; Liu, X.-X.; Li, T.-T.; Zheng, Y.-P.; Zhao, J.-Q.; Zhang, J.-W.; Huang, Y.-Y.; et al. Osa-miR162a fine-tunes rice resistance to Magnaporthe oryzae and Yield. Rice 2020, 13, 38. [Google Scholar] [CrossRef]
- Rezaul, I.M.; Baohua, F.; Tingting, C.; Weimeng, F.; Caixia, Z.; Longxing, T.; Guanfu, F. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2018, 165, 644–663. [Google Scholar] [CrossRef]
- Wen, F.; Gao, Y.; Zeng, Y.X.; Li, G.Y.; Feng, B.H.; Li, H.B.; Chen, T.T.; Wang, D.Y.; Tao, L.X.; Xiong, J.; et al. MiR408 balances and heat in rice. Environ. Exp. Bot. 2024, 221, 105717. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Wang, Y.; Li, J.; Song, Z.; Xiao, Y.; Deng, H.; Liu, X.; Chen, Q.; Tang, W.; Zhang, G. Anther Transcriptome Analysis of Two Heat Tolerance-Differentiated Indica Rice Restorer Lines Reveals the Importance of Non-Structural Carbohydrates and ATP in the Regulation of Heat Tolerance. Int. J. Mol. Sci. 2025, 26, 3161. https://doi.org/10.3390/ijms26073161
Zhou J, Wang Y, Li J, Song Z, Xiao Y, Deng H, Liu X, Chen Q, Tang W, Zhang G. Anther Transcriptome Analysis of Two Heat Tolerance-Differentiated Indica Rice Restorer Lines Reveals the Importance of Non-Structural Carbohydrates and ATP in the Regulation of Heat Tolerance. International Journal of Molecular Sciences. 2025; 26(7):3161. https://doi.org/10.3390/ijms26073161
Chicago/Turabian StyleZhou, Jieqiang, Yingfeng Wang, Jiangfeng Li, Zijian Song, Yunhua Xiao, Huabing Deng, Xiong Liu, Qiuhong Chen, Wenbang Tang, and Guilian Zhang. 2025. "Anther Transcriptome Analysis of Two Heat Tolerance-Differentiated Indica Rice Restorer Lines Reveals the Importance of Non-Structural Carbohydrates and ATP in the Regulation of Heat Tolerance" International Journal of Molecular Sciences 26, no. 7: 3161. https://doi.org/10.3390/ijms26073161
APA StyleZhou, J., Wang, Y., Li, J., Song, Z., Xiao, Y., Deng, H., Liu, X., Chen, Q., Tang, W., & Zhang, G. (2025). Anther Transcriptome Analysis of Two Heat Tolerance-Differentiated Indica Rice Restorer Lines Reveals the Importance of Non-Structural Carbohydrates and ATP in the Regulation of Heat Tolerance. International Journal of Molecular Sciences, 26(7), 3161. https://doi.org/10.3390/ijms26073161





