Generation of the Krt24-CreERT2 Mouse Line Targeting Outer Bulge Hair Follicle Cells
Abstract
1. Introduction
2. Results
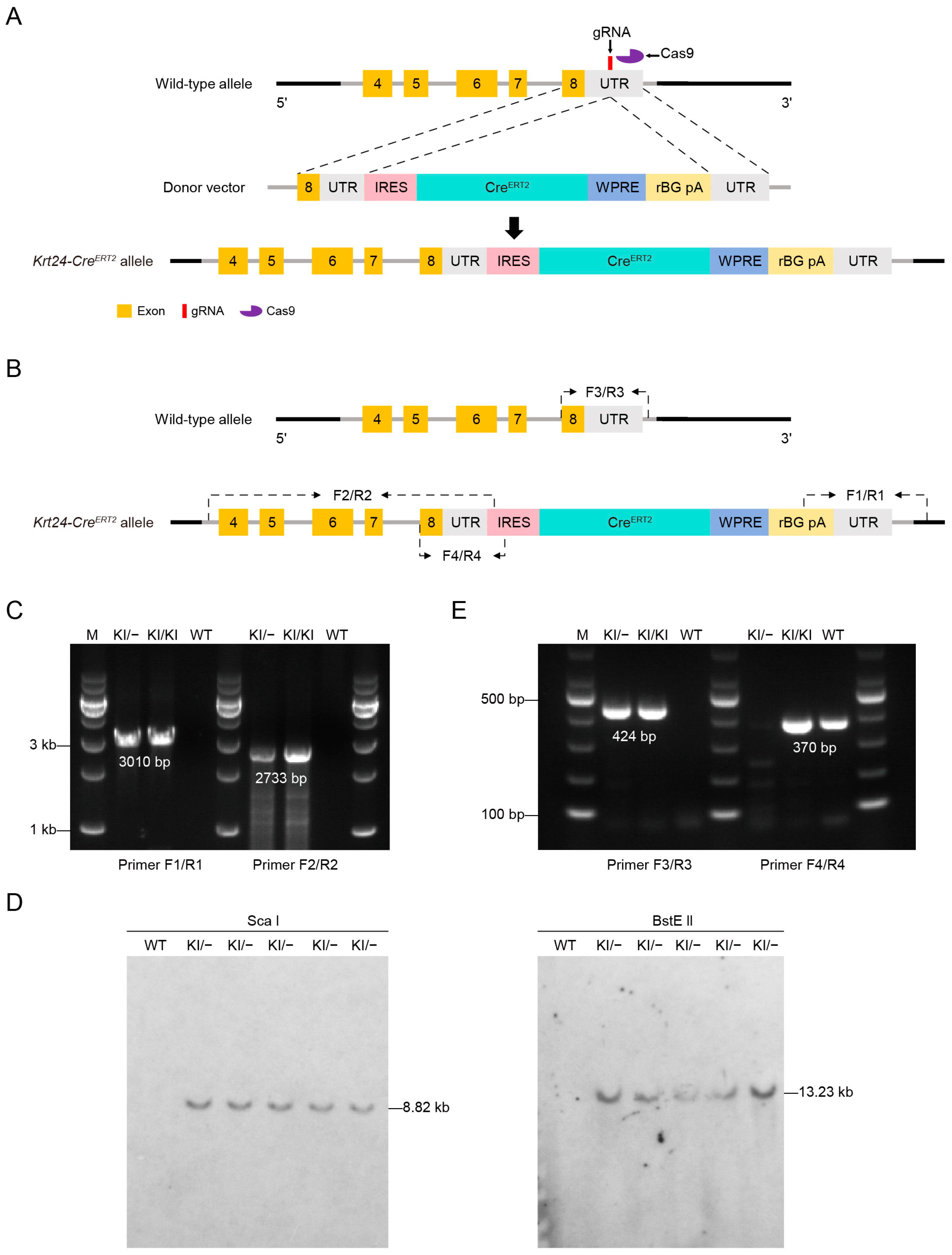
2.1. Generation of the Krt24-CreERT2 Mouse Line Targeting Outer Bulge Hair Follicle Cells
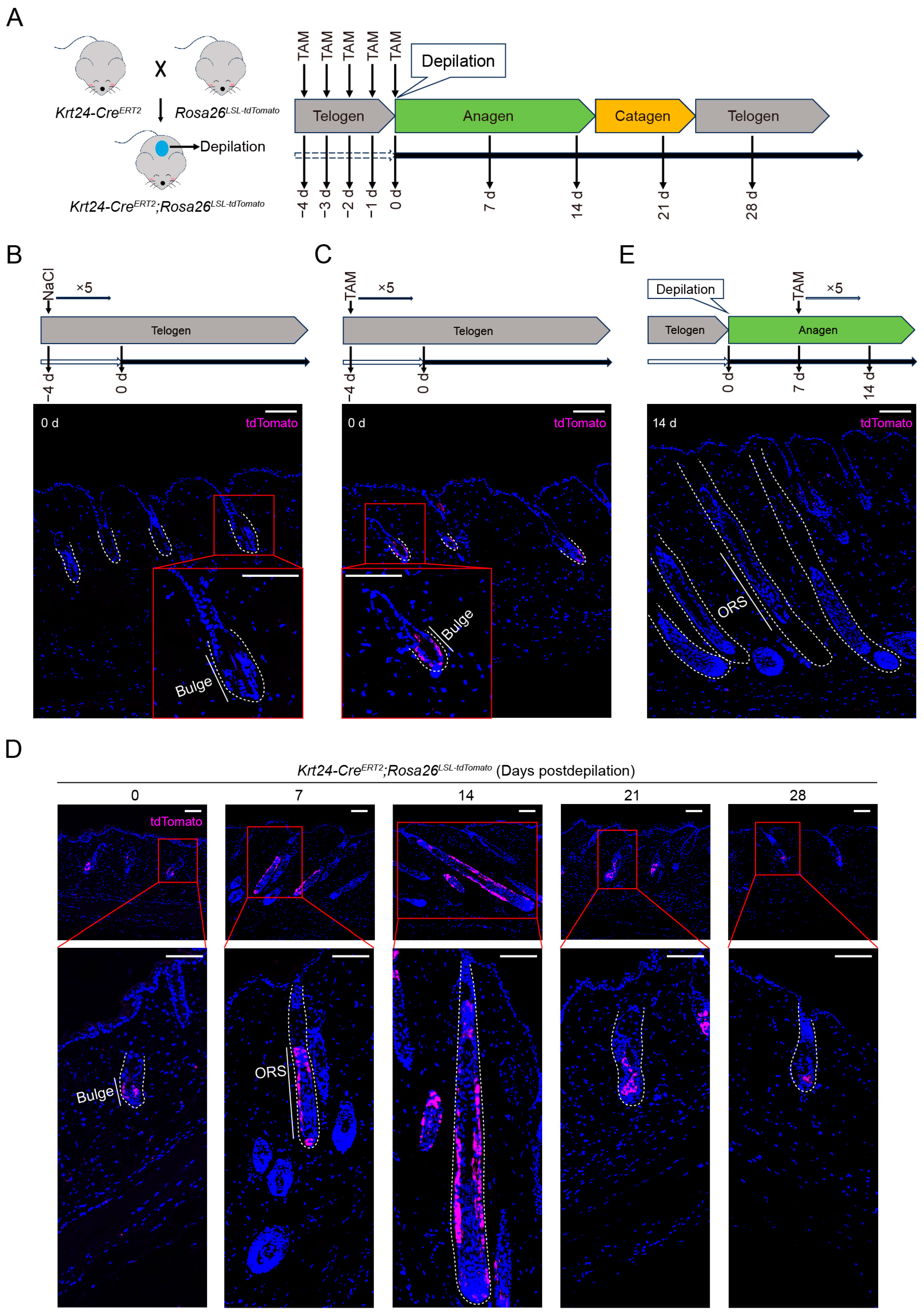
2.2. TdTomato-Labeled Krt24+ Cells Are Located in the Outer Bulge Region and Persist into the Next Hair Follicle Development Cycle
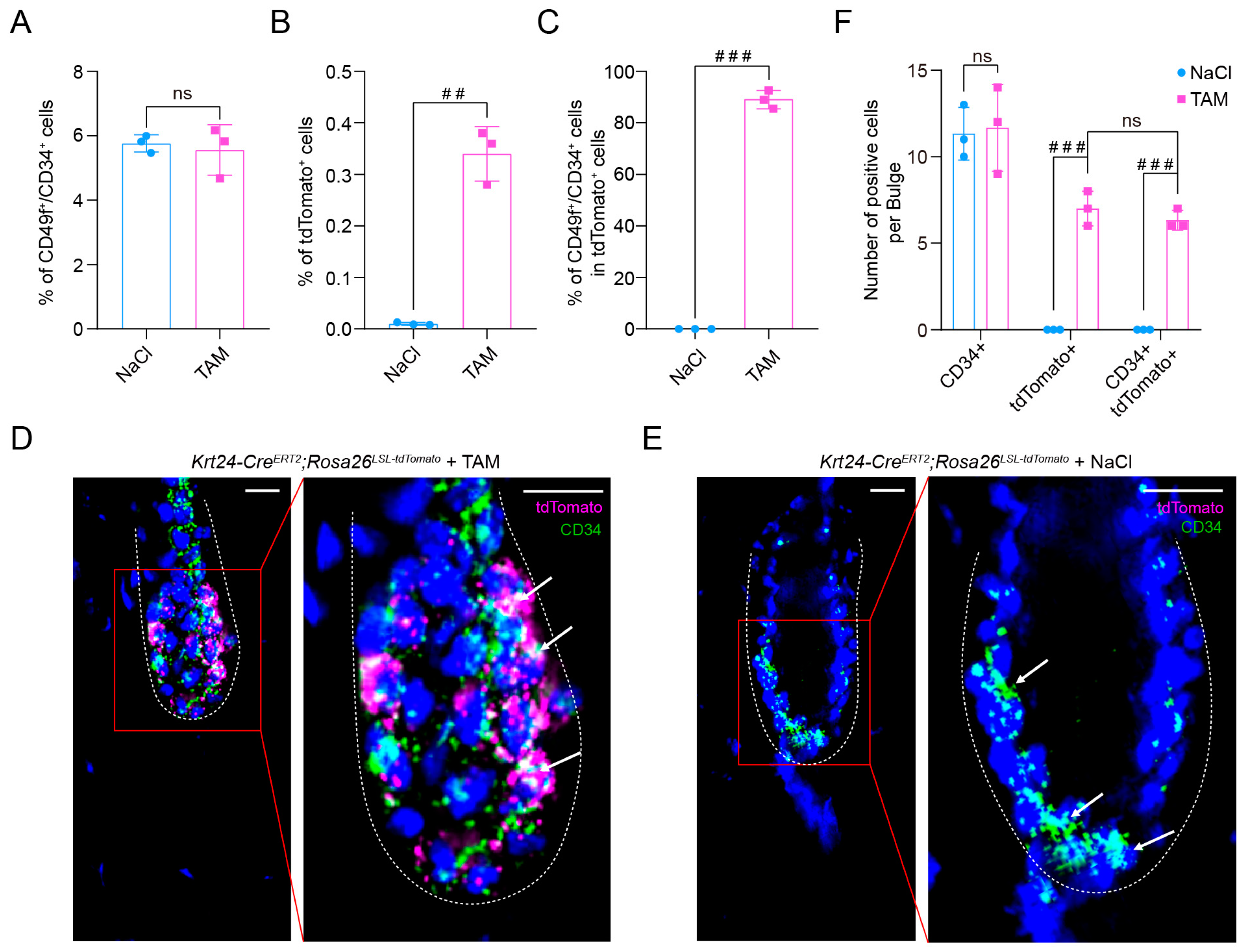
2.3. TdTomato-Labeled Krt24+ Cells Belong to Outer Bulge Hair Follicle Stem Cells
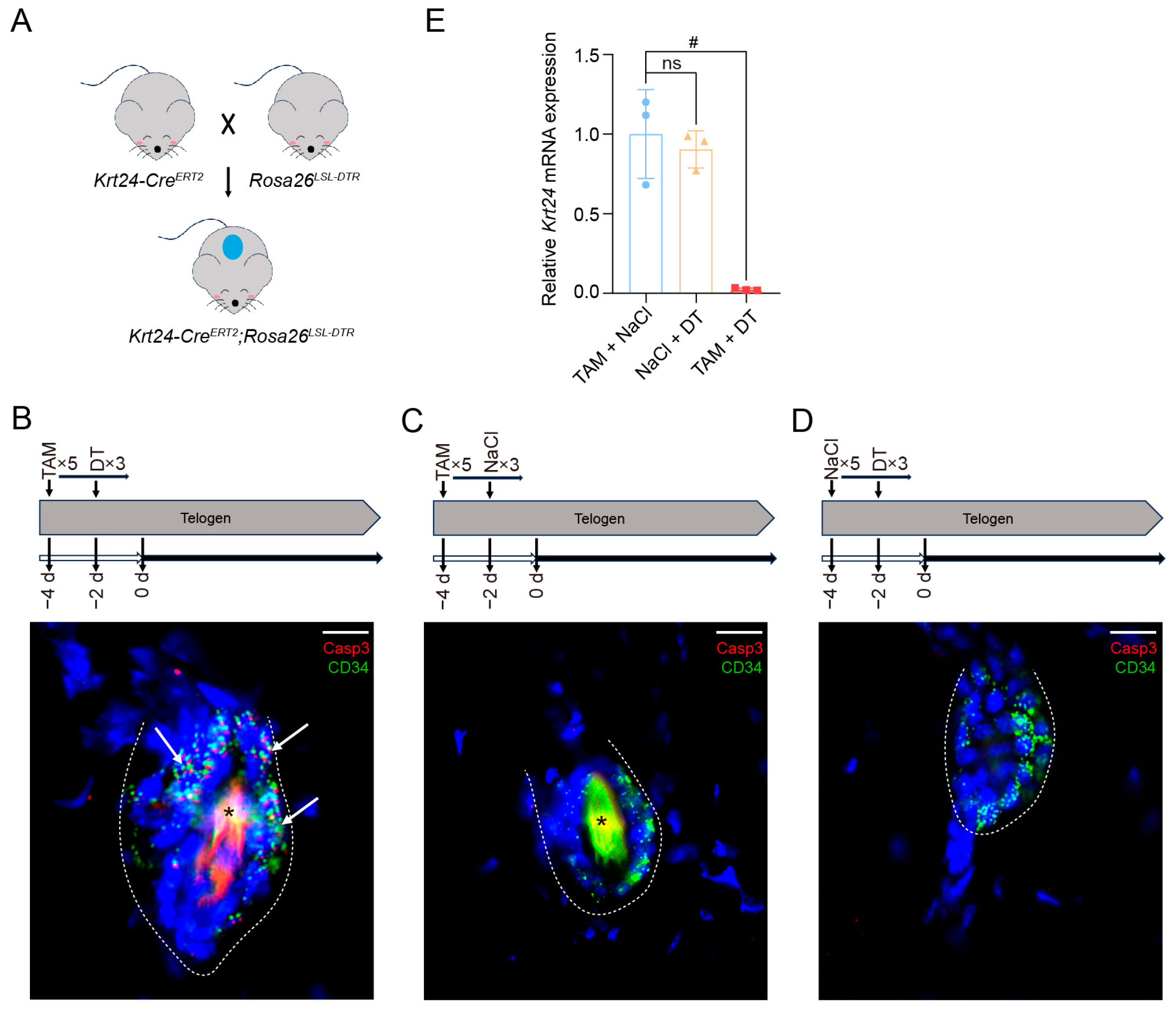
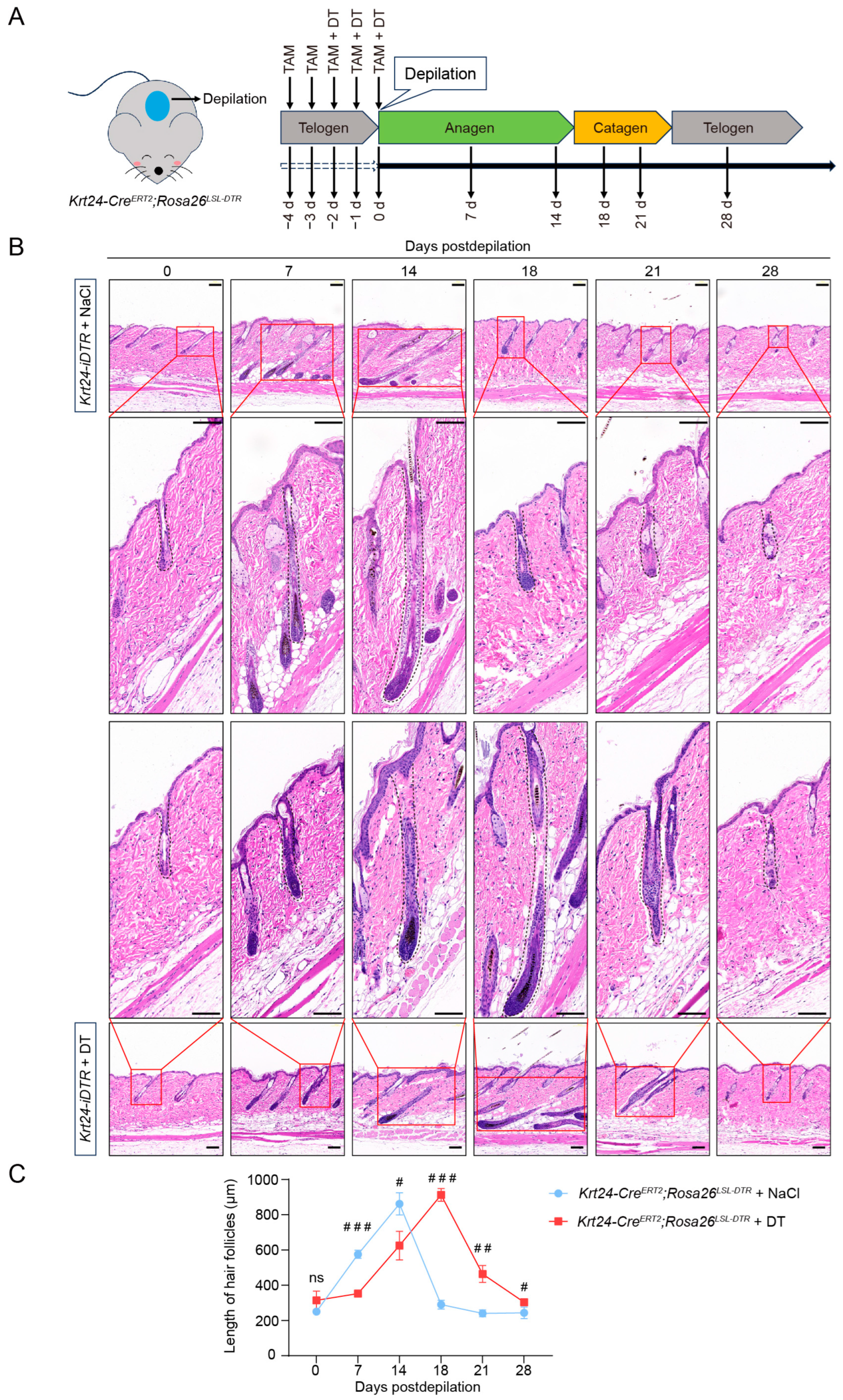
2.4. Generation of the Krt24-CreERT2;Rosa26LSL-DTR Mouse Model
2.5. Ablation of Krt24+ Cells Significantly Delays the Telogen-to-Anagen Transition in Hair Follicle Cycling
2.6. Krt24+ OB HFSC Response to Ionizing-Radiation-Induced Skin Damage
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Generation and Genotyping of Krt24-CreERT2 Knock-in Mice
4.3. Genomic DNA Extraction and Genotyping Analysis
4.4. Southern Blot Assays
4.5. Tamoxifen Induction and Diphtheria Toxin-Induced Cell Ablation
4.6. Tissue Collection
4.7. Immunofluorescence Staining
4.8. Hematoxylin and Eosin (H&E) Staining
4.9. Flow Cytometry Assays
4.10. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR) Assays
4.11. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, B.; Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat. Rev. Mol. Cell Biol. 2024, 25, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.A.U.; Fuchs, E. Skin and Its Regenerative Powers: An Alliance between Stem Cells and Their Niche. Dev. Cell 2017, 43, 387–401. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.Z.; Guo, H.Y.; Xiang, F.; Li, Y.H. Recent progress in hair follicle stem cell markers and their regulatory roles. World J. Stem Cells 2024, 16, 126–136. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Li, L.; Fuchs, E. Emerging interactions between skin stem cells and their niches. Nat. Med. 2014, 20, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Chovatiya, G.; Ghuwalewala, S.; Walter, L.D.; Cosgrove, B.D.; Tumbar, T. High-resolution single-cell transcriptomics reveals heterogeneity of self-renewing hair follicle stem cells. Exp. Dermatol. 2021, 30, 457–471. [Google Scholar] [CrossRef]
- Choi, Y.S.; Zhang, Y.; Xu, M.; Yang, Y.; Ito, M.; Peng, T.; Cui, Z.; Nagy, A.; Hadjantonakis, A.K.; Lang, R.A.; et al. Distinct functions for Wnt/β-catenin in hair follicle stem cell proliferation and survival and interfollicular epidermal homeostasis. Cell Stem Cell 2013, 13, 720–733. [Google Scholar] [CrossRef]
- Lee, J.; Tumbar, T. Hairy tale of signaling in hair follicle development and cycling. Semin. Cell Dev. Biol. 2012, 23, 906–916. [Google Scholar] [CrossRef]
- Ehrlich, F.; Laggner, M.; Langbein, L.; Burger, P.; Pollreisz, A.; Tschachler, E.; Eckhart, L. Comparative genomics suggests loss of keratin K24 in three evolutionary lineages of mammals. Sci. Rep. 2019, 9, 10924. [Google Scholar] [CrossRef]
- Sprecher, E.; Itin, P.; Whittock, N.V.; McGrath, J.A.; Meyer, R.; DiGiovanna, J.J.; Bale, S.J.; Uitto, J.; Richard, G. Refined mapping of Naegeli-Franceschetti- Jadassohn syndrome to a 6 cM interval on chromosome 17q11.2-q21 and investigation of candidate genes. J. Investig. Dermatol. 2002, 119, 692–698. [Google Scholar] [CrossRef]
- Langbein, L.; Schweizer, J. Keratins of the human hair follicle. Int. Rev. Cytol. 2005, 243, 1–78. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Chen, M.; Xu, Z.; Chen, Y.; Yang, Q.; Lu, R.; Dong, Y.; Li, X.; Xie, J.; Xu, R.H.; Jia, H.; et al. EGFR marks a subpopulation of dermal mesenchymal cells highly expressing IGF1 which enhances hair follicle regeneration. J. Cell. Mol. Med. 2023, 27, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Segre, J.A. Transcriptional control of epidermal specification and differentiation. Curr. Opin. Genet. Dev. 2004, 14, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Dry, I.R.; Frampton, D.; Singh, M.; Kanda, R.K.; Yee, M.B.; Kellam, P.; Hollinshead, M.; Kinchington, P.R.; O’Toole, E.A.; et al. RNA-seq analysis of host and viral gene expression highlights interaction between varicella zoster virus and keratinocyte differentiation. PLoS Pathog. 2014, 10, e1003896. [Google Scholar] [CrossRef]
- Oda, Y.; Hu, L.; Nguyen, T.; Fong, C.; Zhang, J.; Guo, P.; Bikle, D.D. Vitamin D Receptor Is Required for Proliferation, Migration, and Differentiation of Epidermal Stem Cells and Progeny during Cutaneous Wound Repair. J. Investig. Dermatol. 2018, 138, 2423–2431. [Google Scholar] [CrossRef]
- Geyfman, M.; Gordon, W.; Paus, R.; Andersen, B. Identification of telogen markers underscores that telogen is far from a quiescent hair cycle phase. J. Investig. Dermatol. 2012, 132, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Joost, S.; Zeisel, A.; Jacob, T.; Sun, X.; La Manno, G.; Lönnerberg, P.; Linnarsson, S.; Kasper, M. Single-Cell Transcriptomics Reveals that Differentiation and Spatial Signatures Shape Epidermal and Hair Follicle Heterogeneity. Cell Syst. 2016, 3, 221–237.e229. [Google Scholar] [CrossRef]
- Gur-Cohen, S.; Yang, H.; Baksh, S.C.; Miao, Y.; Levorse, J.; Kataru, R.P.; Liu, X.; de la Cruz-Racelis, J.; Mehrara, B.J.; Fuchs, E. Stem cell-driven lymphatic remodeling coordinates tissue regeneration. Science 2019, 366, 1218–1225. [Google Scholar] [CrossRef]
- Beeler, J.S.; Marshall, C.B.; Gonzalez-Ericsson, P.I.; Shaver, T.M.; Santos Guasch, G.L.; Lea, S.T.; Johnson, K.N.; Jin, H.; Venters, B.J.; Sanders, M.E.; et al. p73 regulates epidermal wound healing and induced keratinocyte programming. PLoS ONE 2019, 14, e0218458. [Google Scholar] [CrossRef]
- Adam, R.C.; Yang, H.; Ge, Y.; Infarinato, N.R.; Gur-Cohen, S.; Miao, Y.; Wang, P.; Zhao, Y.; Lu, C.P.; Kim, J.E.; et al. NFI transcription factors provide chromatin access to maintain stem cell identity while preventing unintended lineage fate choices. Nat. Cell Biol. 2020, 22, 640–650. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, D.; Wang, J.; Wang, L.; Qiu, W.; Kume, T.; Dowell, R.; Yi, R. Escape of hair follicle stem cells causes stem cell exhaustion during aging. Nat. Aging 2021, 1, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Joost, S.; Annusver, K.; Jacob, T.; Sun, X.; Dalessandri, T.; Sivan, U.; Sequeira, I.; Sandberg, R.; Kasper, M. The Molecular Anatomy of Mouse Skin during Hair Growth and Rest. Cell Stem Cell 2020, 26, 441–457.e7. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.J.; Liu, Y.; Marles, L.; Yang, Z.; Trempus, C.; Li, S.; Lin, J.S.; Sawicki, J.A.; Cotsarelis, G. Capturing and profiling adult hair follicle stem cells. Nat. Biotechnol. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Myung, P.S.; Takeo, M.; Ito, M.; Atit, R.P. Epithelial Wnt ligand secretion is required for adult hair follicle growth and regeneration. J. Investig. Dermatol. 2013, 133, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.C.E.; Dai, Z.; Ferrante, A.W.; Drake, C.G.; Christiano, A.M. A Subset of TREM2(+) Dermal Macrophages Secretes Oncostatin M to Maintain Hair Follicle Stem Cell Quiescence and Inhibit Hair Growth. Cell Stem Cell 2019, 24, 654–669.e6. [Google Scholar] [CrossRef]
- Brownell, I.; Guevara, E.; Bai, C.B.; Loomis, C.A.; Joyner, A.L. Nerve-derived sonic hedgehog defines a niche for hair follicle stem cells capable of becoming epidermal stem cells. Cell Stem Cell 2011, 8, 552–565. [Google Scholar] [CrossRef]
- Harfe, B.D.; Scherz, P.J.; Nissim, S.; Tian, H.; McMahon, A.P.; Tabin, C.J. Evidence for an expansion-based temporal Shh gradient in specifying vertebrate digit identities. Cell 2004, 118, 517–528. [Google Scholar] [CrossRef]
- Lapouge, G.; Youssef, K.K.; Vokaer, B.; Achouri, Y.; Michaux, C.; Sotiropoulou, P.A.; Blanpain, C. Identifying the cellular origin of squamous skin tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 7431–7436. [Google Scholar] [CrossRef]
- Moon, H.; Donahue, L.R.; Choi, E.; Scumpia, P.O.; Lowry, W.E.; Grenier, J.K.; Zhu, J.; White, A.C. Melanocyte Stem Cell Activation and Translocation Initiate Cutaneous Melanoma in Response to UV Exposure. Cell Stem Cell 2017, 21, 665–678.e6. [Google Scholar] [CrossRef]
- Sun, Q.; Lee, W.; Mohri, Y.; Takeo, M.; Lim, C.H.; Xu, X.; Myung, P.; Atit, R.P.; Taketo, M.M.; Moubarak, R.S.; et al. A novel mouse model demonstrates that oncogenic melanocyte stem cells engender melanoma resembling human disease. Nat. Commun. 2019, 10, 5023. [Google Scholar] [CrossRef]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The hair follicle as a dynamic miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef]
- Trempus, C.S.; Morris, R.J.; Bortner, C.D.; Cotsarelis, G.; Faircloth, R.S.; Reece, J.M.; Tennant, R.W. Enrichment for living murine keratinocytes from the hair follicle bulge with the cell surface marker CD34. J. Investig. Dermatol. 2003, 120, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Müller-Röver, S.; Handjiski, B.; van der Veen, C.; Eichmüller, S.; Foitzik, K.; McKay, I.A.; Stenn, K.S.; Paus, R. A comprehensive guide for the accurate classification of murine hair follicles in distinct hair cycle stages. J. Investig. Dermatol. 2001, 117, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.A.U.; Polak, L.; Matos, I.; Tierney, M.T.; Gola, A.; Wong, E.; Infarinato, N.R.; Nikolova, M.; Luo, S.; Liu, S.; et al. Stem cells expand potency and alter tissue fitness by accumulating diverse epigenetic memories. Science 2021, 374, eabh2444. [Google Scholar] [CrossRef] [PubMed]
- Lisse, T.S.; Sharma, M.; Vishlaghi, N.; Pullagura, S.R.; Braun, R.E. GDNF promotes hair formation and cutaneous wound healing by targeting bulge stem cells. NPJ Regen. Med. 2020, 5, 13. [Google Scholar] [CrossRef]
- Han, J.; Lin, K.; Choo, H.; Chen, Y.; Zhang, X.; Xu, R.H.; Wang, X.; Wu, Y. Distinct bulge stem cell populations maintain the pilosebaceous unit in a β-catenin-dependent manner. iScience 2023, 26, 105805. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Li, L.; Fuchs, E. Transit-amplifying cells orchestrate stem cell activity and tissue regeneration. Cell 2014, 157, 935–949. [Google Scholar] [CrossRef]
- Kadaja, M.; Keyes, B.E.; Lin, M.; Pasolli, H.A.; Genander, M.; Polak, L.; Stokes, N.; Zheng, D.; Fuchs, E. SOX9: A stem cell transcriptional regulator of secreted niche signaling factors. Genes Dev. 2014, 28, 328–341. [Google Scholar] [CrossRef]
- Hsu, Y.C.; Pasolli, H.A.; Fuchs, E. Dynamics between stem cells, niche, and progeny in the hair follicle. Cell 2011, 144, 92–105. [Google Scholar] [CrossRef]
- Fuchs, E. The tortoise and the hair: Slow-cycling cells in the stem cell race. Cell 2009, 137, 811–819. [Google Scholar] [CrossRef]
- Niu, Y.L.; Li, Y.K.; Gao, C.X.; Li, W.W.; Li, L.; Wang, H.; Shen, W.; Ge, W. Melatonin promotes hair regeneration by modulating the Wnt/β-catenin signalling pathway. Cell Prolif. 2024, 57, e13656. [Google Scholar] [CrossRef]
- Xu, Z.; Wang, W.; Jiang, K.; Yu, Z.; Huang, H.; Wang, F.; Zhou, B.; Chen, T. Embryonic attenuated Wnt/β-catenin signaling defines niche location and long-term stem cell fate in hair follicle. Elife 2015, 4, e10567. [Google Scholar] [CrossRef]
- Peters, F.; Vorhagen, S.; Brodesser, S.; Jakobshagen, K.; Brüning, J.C.; Niessen, C.M.; Krönke, M. Ceramide synthase 4 regulates stem cell homeostasis and hair follicle cycling. J. Investig. Dermatol. 2015, 135, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tsai, P.C.; Gonzalez-Celeiro, M.; Chung, O.; Boumard, B.; Perdigoto, C.N.; Ezhkova, E.; Hsu, Y.C. Hair follicles’ transit-amplifying cells govern concurrent dermal adipocyte production through Sonic Hedgehog. Genes Dev. 2016, 30, 2325–2338. [Google Scholar] [CrossRef] [PubMed]
- Genander, M.; Cook, P.J.; Ramsköld, D.; Keyes, B.E.; Mertz, A.F.; Sandberg, R.; Fuchs, E. BMP signaling and its pSMAD1/5 target genes differentially regulate hair follicle stem cell lineages. Cell Stem Cell 2014, 15, 619–633. [Google Scholar] [CrossRef]
- Choi, S.; Zhang, B.; Ma, S.; Gonzalez-Celeiro, M.; Stein, D.; Jin, X.; Kim, S.T.; Kang, Y.L.; Besnard, A.; Rezza, A.; et al. Corticosterone inhibits GAS6 to govern hair follicle stem-cell quiescence. Nature 2021, 592, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Brown, S.; Gorenc, T.; Rodriguez, J.; Fuchs, E.; Steller, H. Sept4/ARTS regulates stem cell apoptosis and skin regeneration. Science 2013, 341, 286–289. [Google Scholar] [CrossRef]
- Sun, Q.; Lee, W.; Hu, H.; Ogawa, T.; De Leon, S.; Katehis, I.; Lim, C.H.; Takeo, M.; Cammer, M.; Taketo, M.M.; et al. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature 2023, 616, 774–782. [Google Scholar] [CrossRef]
- Ge, M.; Liu, C.; Li, L.; Lan, M.; Yu, Y.; Gu, L.; Su, Y.; Zhang, K.; Zhang, Y.; Wang, T.; et al. miR-29a/b1 Inhibits Hair Follicle Stem Cell Lineage Progression by Spatiotemporally Suppressing WNT and BMP Signaling. Cell Rep. 2019, 29, 2489–2504.e84. [Google Scholar] [CrossRef]
- Kim, D.J.; Kataoka, K.; Rao, D.; Kiguchi, K.; Cotsarelis, G.; Digiovanni, J. Targeted disruption of stat3 reveals a major role for follicular stem cells in skin tumor initiation. Cancer Res. 2009, 69, 7587–7594. [Google Scholar] [CrossRef]
- Li, S.; Park, H.; Trempus, C.S.; Gordon, D.; Liu, Y.; Cotsarelis, G.; Morris, R.J. A keratin 15 containing stem cell population from the hair follicle contributes to squamous papilloma development in the mouse. Mol. Carcinog. 2013, 52, 751–759. [Google Scholar] [CrossRef]
- Salvo, N.; Barnes, E.; van Draanen, J.; Stacey, E.; Mitera, G.; Breen, D.; Giotis, A.; Czarnota, G.; Pang, J.; De Angelis, C. Prophylaxis and management of acute radiation-induced skin reactions: A systematic review of the literature. Curr. Oncol. 2010, 17, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.J.; Yue, Z.; Paus, R. Clinical Pathobiology of Radiotherapy-Induced Alopecia: A Guide toward More Effective Prevention and Hair Follicle Repair. J. Investig. Dermatol. 2023, 143, 1646–1656. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.Y.; Lai, S.F.; Chiu, H.Y.; Chang, M.; Plikus, M.V.; Chan, C.C.; Chen, Y.T.; Tsao, P.N.; Yang, T.L.; Lee, H.S.; et al. Mobilizing Transit-Amplifying Cell-Derived Ectopic Progenitors Prevents Hair Loss from Chemotherapy or Radiation Therapy. Cancer Res. 2017, 77, 6083–6096. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Candi, A.; Mascré, G.; De Clercq, S.; Youssef, K.K.; Lapouge, G.; Dahl, E.; Semeraro, C.; Denecker, G.; Marine, J.C.; et al. Bcl-2 and accelerated DNA repair mediates resistance of hair follicle bulge stem cells to DNA-damage-induced cell death. Nat. Cell Biol. 2010, 12, 572–582. [Google Scholar] [CrossRef]
- Jaks, V.; Barker, N.; Kasper, M.; van Es, J.H.; Snippert, H.J.; Clevers, H.; Toftgård, R. Lgr5 marks cycling, yet long-lived, hair follicle stem cells. Nat. Genet. 2008, 40, 1291–1299. [Google Scholar] [CrossRef]
- Kinoshita, K.; Ishimine, H.; Shiraishi, K.; Kato, H.; Doi, K.; Kuno, S.; Kanayama, K.; Mineda, K.; Mashiko, T.; Feng, J.; et al. Cell and Tissue Damage after Skin Exposure to Ionizing Radiation: Short- and Long-Term Effects after a Single and Fractional Doses. Cells Tissues Organs 2014, 200, 240–252. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Qiu, Y.; Zhu, Y.; Ren, X.; Zhou, X.; Wang, X.; Song, H.; Li, J.; Gao, C.; Zhou, G.; et al. Generation of the Krt24-CreERT2 Mouse Line Targeting Outer Bulge Hair Follicle Cells. Int. J. Mol. Sci. 2025, 26, 3165. https://doi.org/10.3390/ijms26073165
Wang J, Qiu Y, Zhu Y, Ren X, Zhou X, Wang X, Song H, Li J, Gao C, Zhou G, et al. Generation of the Krt24-CreERT2 Mouse Line Targeting Outer Bulge Hair Follicle Cells. International Journal of Molecular Sciences. 2025; 26(7):3165. https://doi.org/10.3390/ijms26073165
Chicago/Turabian StyleWang, Jiao, Yifei Qiu, Yansheng Zhu, Xuejiao Ren, Xiaoqi Zhou, Xia Wang, Huiyang Song, Jianhao Li, Chengming Gao, Gangqiao Zhou, and et al. 2025. "Generation of the Krt24-CreERT2 Mouse Line Targeting Outer Bulge Hair Follicle Cells" International Journal of Molecular Sciences 26, no. 7: 3165. https://doi.org/10.3390/ijms26073165
APA StyleWang, J., Qiu, Y., Zhu, Y., Ren, X., Zhou, X., Wang, X., Song, H., Li, J., Gao, C., Zhou, G., & Cao, P. (2025). Generation of the Krt24-CreERT2 Mouse Line Targeting Outer Bulge Hair Follicle Cells. International Journal of Molecular Sciences, 26(7), 3165. https://doi.org/10.3390/ijms26073165





