Harnessing Apple Cell Suspension Cultures in Bioreactors for Triterpene Production: Transcriptomic Insights into Biomass and Triterpene Biosynthesis
Abstract
:1. Introduction
2. Results and Discussion
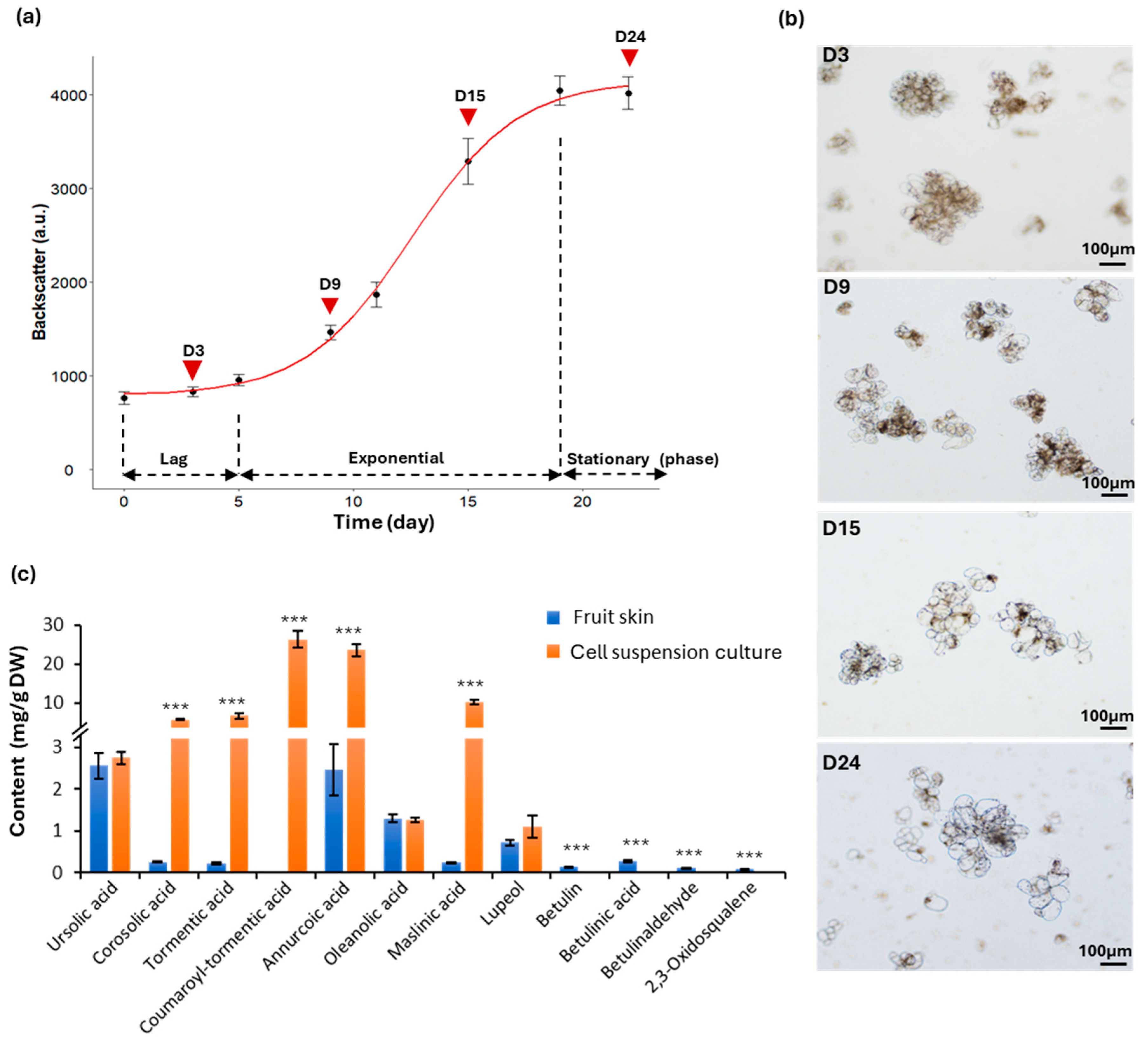
2.1. Development of Cell Suspension Cultures in Shake Flasks
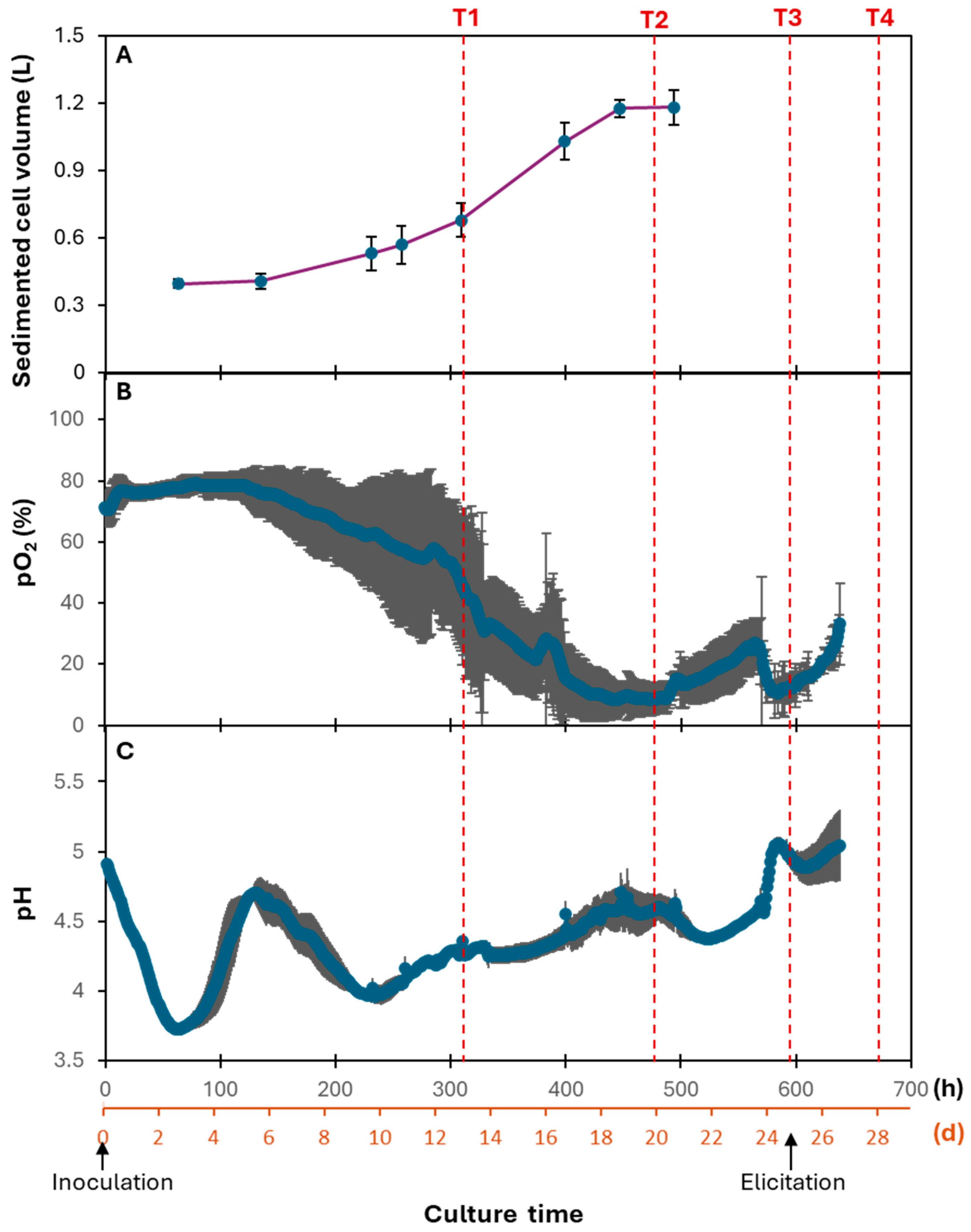
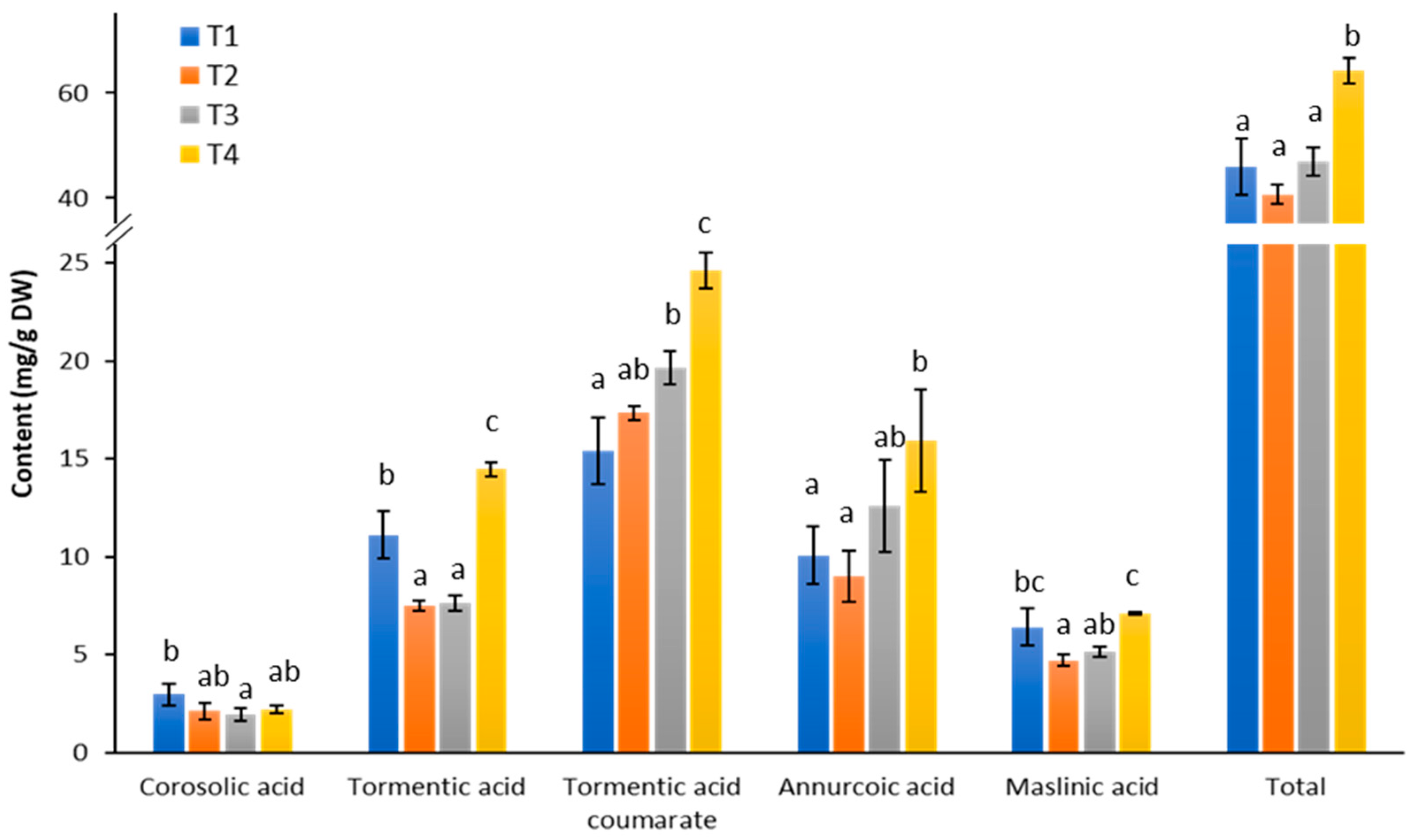
2.2. Upscaling in Bioreactor and Triterpene Analysis of Cells
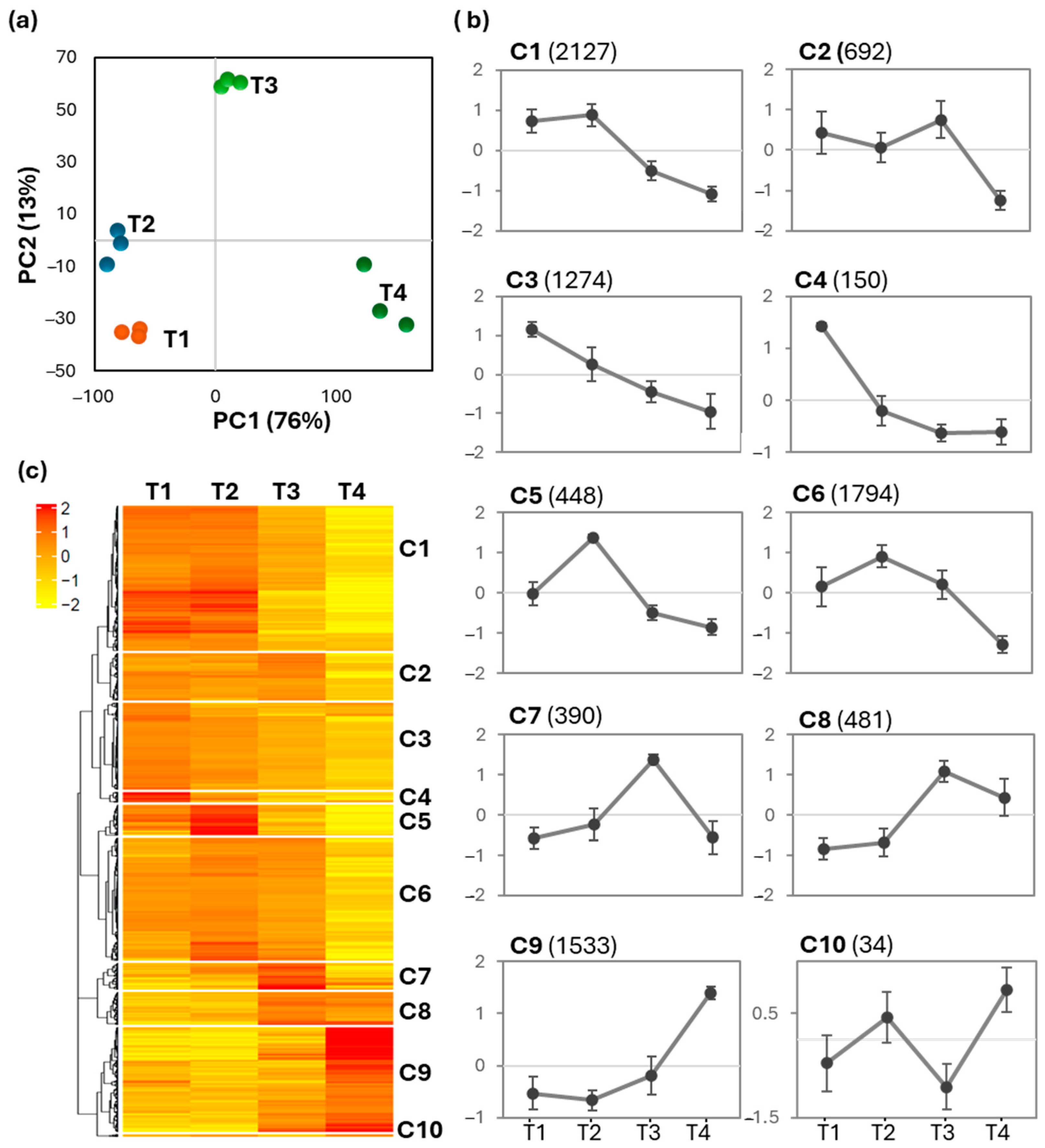
2.3. Transcriptomic Profiles of Cell Suspension Cultures Across Time Points
2.4. GOE Analysis
2.5. Cell Cycle-Related Process and Its Regulation in Suspension Cell Cultures
2.6. Cell Wall-Related Process
- Callose
- 2.
- Cellulose
- 3.
- Pectin
- 4.
- Hemicelluloses
- 5.
- Expansins
2.7. Lipids
2.8. Biological Processes Related to Early-Exponential Phase
2.9. Biological Processes Related to Late-Exponential Phase
2.10. Biological Processes Related to Stationary Phase
2.11. Biological Processes Induced upon Elicitation
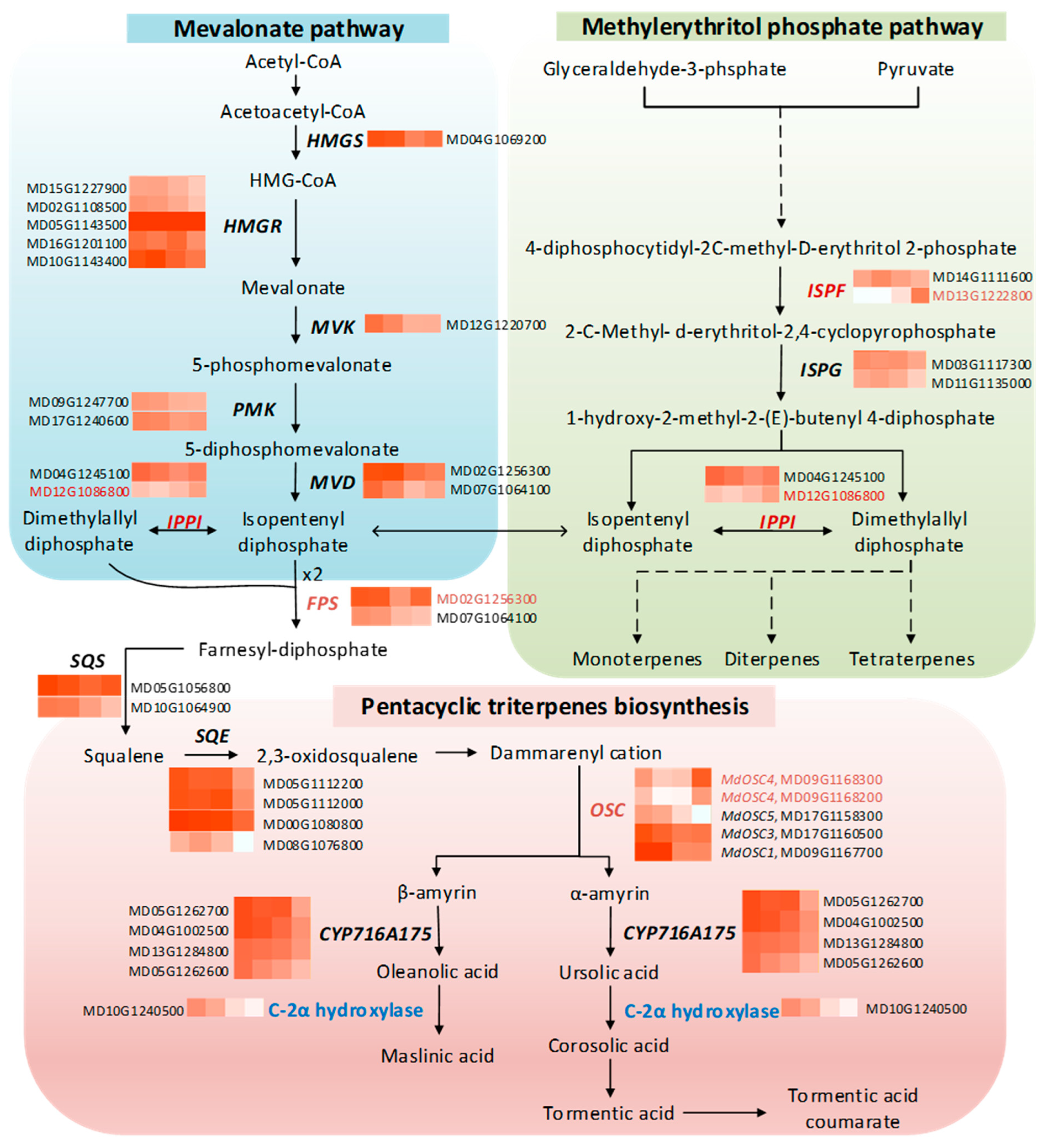
2.12. Transcriptional Profile Related to Triterpene Biosynthesis
3. Materials and Methods
3.1. Callus Induction, Cell Suspension Culture, and Cell Suspension Maintenance
3.2. Growth Kinetics in Flasks
3.3. Bioreactor Operations and Elicitation
3.4. Triterpenes Extraction and Analysis
3.5. RNA Extraction
3.6. Library Preparation, Sequencing, RNA-Seq Analysis, and Validation
3.7. Stastical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abdulhafiz, F.; Mohammed, A.; Reduan, M.F.H.; Kari, Z.A.; Wei, L.S.; Goh, K.W. Plant cell culture technologies: A promising alternatives to produce high-value secondary metabolites. Arab. J. Chem. 2022, 15, 104161. [Google Scholar]
- Howat, S.; Park, B.; Oh, I.S.; Jin, Y.-W.; Lee, E.-K.; Loake, G.J. Paclitaxel: Biosynthesis, production and future prospects. New Biotechnol. 2014, 31, 242–245. [Google Scholar]
- Yadav, S.; Sharma, A.; Nayik, G.A.; Cooper, R.; Bhardwaj, G.; Sohal, H.S.; Mutreja, V.; Kaur, R.; Areche, F.O.; AlOudat, M.; et al. Review of shikonin and derivatives: Isolation, chemistry, biosynthesis, pharmacology and toxicology. Front. Pharmacol. 2022, 13, 905755. [Google Scholar]
- de Freitas Lima, M.; Eloy, N.B.; de Siqueira, J.A.B.; Inzé, D.; Hemerly, A.S.; Ferreira, P.C.G. Molecular mechanisms of biomass increase in plants. Biotechnol. Res. Innov. 2017, 1, 14–25. [Google Scholar]
- Guerriero, G.; Hausman, J.-F.; Cai, G. No stress! Relax! Mechanisms governing growth and shape in plant cells. Int. J. Mol. Sci. 2014, 15, 5094–5114. [Google Scholar] [CrossRef]
- Kelly, A.A.; Feussner, I. Oil is on the agenda: Lipid turnover in higher plants. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2016, 1861, 1253–1268. [Google Scholar]
- Jeyasri, R.; Muthuramalingam, P.; Karthick, K.; Shin, H.; Choi, S.H.; Ramesh, M. Methyl jasmonate and salicylic acid as powerful elicitors for enhancing the production of secondary metabolites in medicinal plants: An updated review. Plant Cell Tissue Organ Cult. (PCTOC) 2023, 153, 447–458. [Google Scholar] [PubMed]
- Mendoza, D.; Cuaspud, O.; Arias, J.P.; Ruiz, O.; Arias, M. Effect of salicylic acid and methyl jasmonate in the production of phenolic compounds in plant cell suspension cultures of Thevetia peruviana. Biotechnol. Rep. 2018, 19, e00273. [Google Scholar]
- Ho, T.-T.; Murthy, H.N.; Park, S.-Y. Methyl jasmonate induced oxidative stress and accumulation of secondary metabolites in plant cell and organ cultures. Int. J. Mol. Sci. 2020, 21, 716. [Google Scholar] [CrossRef]
- Andre, C.M.; Greenwood, J.M.; Walker, E.G.; Rassam, M.; Sullivan, M.; Evers, D.l.; Perry, N.B.; Laing, W.A. Anti-inflammatory procyanidins and triterpenes in 109 apple varieties. J. Agric. Food Chem. 2012, 60, 10546–10554. [Google Scholar]
- Belding, R.D.; Blankenship, S.M.; Young, E.; Leidy, R.B. Composition and variability of epicuticular waxes in apple cultivars. J. Am. Soc. Hortic. Sci. 1998, 123, 348–356. [Google Scholar] [CrossRef]
- Jäger, S.; Trojan, H.; Kopp, T.; Laszczyk, M.N.; Scheffler, A. Pentacyclic triterpene distribution in various plants–rich sources for a new group of multi-potent plant extracts. Molecules 2009, 14, 2016–2031. [Google Scholar] [CrossRef] [PubMed]
- Bero, J.; Frédérich, M.; Quetin-Leclercq, J. Antimalarial compounds isolated from plants used in traditional medicine. J. Pharm. Pharmacol. 2009, 61, 1401–1433. [Google Scholar] [CrossRef] [PubMed]
- Salvador, J.A.; Moreira, V.M.; Gonçalves, B.M.; Leal, A.S.; Jing, Y. Ursane-type pentacyclic triterpenoids as useful platforms to discover anticancer drugs. Nat. Prod. Rep. 2012, 29, 1463–1479. [Google Scholar] [CrossRef]
- Moses, T.; Pollier, J.; Thevelein, J.M.; Goossens, A. Bioengineering of plant (tri) terpenoids: From metabolic engineering of plants to synthetic biology in vivo and in vitro. New Phytol. 2013, 200, 27–43. [Google Scholar] [CrossRef]
- McGhie, T.K.; Hudault, S.; Lunken, R.C.; Christeller, J.T. Apple peels, from seven cultivars, have lipase-inhibitory activity and contain numerous ursenoic acids as identified by LC-ESI-QTOF-HRMS. J. Agric. Food Chem. 2012, 60, 482–491. [Google Scholar] [CrossRef]
- Legay, S.; Cocco, E.; André, C.M.; Guignard, C.; Hausman, J.F.; Guerriero, G. Differential Lipid Composition and Gene Expression in the Semi-Russeted “Cox Orange Pippin” Apple Variety. Front. Plant Sci. 2017, 8, 1656. [Google Scholar] [CrossRef]
- Andre, C.M.; Larsen, L.; Burgess, E.J.; Jensen, D.J.; Cooney, J.M.; Evers, D.l.; Zhang, J.; Perry, N.B.; Laing, W.A. Unusual immuno-modulatory triterpene-caffeates in the skins of russeted varieties of apples and pears. J. Agric. Food Chem. 2013, 61, 2773–2779. [Google Scholar] [CrossRef]
- Phillips, D.R.; Rasbery, J.M.; Bartel, B.; Matsuda, S.P. Biosynthetic diversity in plant triterpene cyclization. Curr. Opin. Plant Biol. 2006, 9, 305–314. [Google Scholar] [CrossRef]
- Carelli, M.; Biazzi, E.; Panara, F.; Tava, A.; Scaramelli, L.; Porceddu, A.; Graham, N.; Odoardi, M.; Piano, E.; Arcioni, S. Medicago truncatula CYP716A12 is a multifunctional oxidase involved in the biosynthesis of hemolytic saponins. Plant Cell 2011, 23, 3070–3081. [Google Scholar] [CrossRef]
- Leboeuf, E.; Thoiron, S.; Lahaye, M. Physico-chemical characteristics of cell walls from Arabidopsis thaliana microcalli showing different adhesion strengths. J. Exp. Bot. 2004, 55, 2087–2097. [Google Scholar]
- Matsuoka, K.; Demura, T.; Galis, I.; Horiguchi, T.; Sasaki, M.; Tashiro, G.; Fukuda, H. A comprehensive gene expression analysis toward the understanding of growth and differentiation of tobacco BY-2 cells. Plant Cell Physiol. 2004, 45, 1280–1289. [Google Scholar] [PubMed]
- Catteau, L.; Schioppa, L.; Beaufay, C.; Girardi, C.; Hérent, M.-F.; Frédérich, M.; Quetin-Leclercq, J. Antiprotozoal activities of triterpenic acids and ester derivatives isolated from the leaves of Vitellaria paradoxa. Planta Medica 2021, 87, 860–867. [Google Scholar]
- Taniguchi, S.; Imayoshi, Y.; Kobayashi, E.; Takamatsu, Y.; Ito, H.; Hatano, T.; Sakagami, H.; Tokuda, H.; Nishino, H.; Sugita, D.; et al. Production of bioactive triterpenes by Eriobotrya japonica calli. Phytochemistry 2002, 59, 315–323. [Google Scholar] [CrossRef]
- Kikuchi, T.; Akazawa, H.; Tabata, K.; Manosroi, A.; Manosroi, J.; Suzuki, T.; Akihisa, T. 3-O-(E)-p-coumaroyl tormentic acid from Eriobotrya japonica leaves induces caspase-dependent apoptotic cell death in human leukemia cell line. Chem. Pharm. Bull. 2011, 59, 378–381. [Google Scholar]
- Andre, C.M.; Leclercq, J.; Legay, S.; Beaufay, C.; Catteau, L. Method for Producing a Composition Comprising a 3-O-p-Coumaroyl Ester of Tormentic Acid from a Plant Cell Culture, Applications Thereof as an Antiprotozoal Agent for the Treatment of Trypanosomiasis. WO2020161221A1 2020. Available online: https://patentimages.storage.googleapis.com/63/92/33/ee8bb1a69f6df9/WO2020161221A1.pdf (accessed on 27 March 2025).
- Tan, H.; Sonam, T.; Shimizu, K. The Potential of Triterpenoids from Loquat Leaves (Eriobotrya japonica) for Prevention and Treatment of Skin Disorder. Int. J. Mol. Sci. 2017, 18, 1030. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Tang, L.; Wei, F.; Chen, H.; Sheng, L.; Yang, Y.; Zhou, X.; Li, Y.; Xu, X.; Zhang, B.; et al. Pentacyclic triterpene compounds from loquat leaves reduce skin inflammation and epidermal hyperplasia in psoriasis via inhibiting the Th17 cells. Mol. Immunol. 2021, 132, 30–40. [Google Scholar]
- Agra, L.C.; Ferro, J.N.; Barbosa, F.T.; Barreto, E. Triterpenes with healing activity: A systematic review. J. Dermatol. Treat. 2015, 26, 465–470. [Google Scholar]
- Veerashree, V.; Anuradha, C.; Kumar, V. Elicitor-enhanced production of gymnemic acid in cell suspension cultures of Gymnema sylvestre R. Br. Plant Cell Tissue Organ Cult. 2012, 108, 27–35. [Google Scholar]
- Wang, J.; Qian, J.; Yao, L.; Lu, Y. Enhanced production of flavonoids by methyl jasmonate elicitation in cell suspension culture of Hypericum perforatum. Bioresour. Bioprocess. 2015, 2, 5. [Google Scholar]
- Batz, O.; Logemann, E.; Reinold, S.; Hahlbrock, K. Extensive reprogramming of primary and secondary metabolism by fungal elicitor or infection in parsley cells. Biol. Chem. 1998, 379, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Reddy, M.S.; Naoumkina, M.; Aziz, N.; May, G.D.; Huhman, D.V.; Sumner, L.W.; Blount, J.W.; Mendes, P.; Dixon, R.A. Methyl jasmonate and yeast elicitor induce differential transcriptional and metabolic re-programming in cell suspension cultures of the model legume Medicago truncatula. Planta 2005, 220, 696–707. [Google Scholar] [CrossRef] [PubMed]
- Logemann, E.; Tavernaro, A.; Schulz, W.; Somssich, I.E.; Hahlbrock, K. UV light selectively coinduces supply pathways from primary metabolism and flavonoid secondary product formation in parsley. Proc. Natl. Acad. Sci. USA 2000, 97, 1903–1907. [Google Scholar] [CrossRef] [PubMed]
- Krzyzanowska, J.; Czubacka, A.; Pecio, L.; Przybys, M.; Doroszewska, T.; Stochmal, A.; Oleszek, W. The effects of jasmonic acid and methyl jasmonate on rosmarinic acid production in Mentha× piperita cell suspension cultures. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 108, 73–81. [Google Scholar] [CrossRef]
- Rahimi, S.; Kim, Y.-J.; Yang, D.-C. Production of ginseng saponins: Elicitation strategy and signal transductions. Appl. Microbiol. Biotechnol. 2015, 99, 6987–6996. [Google Scholar] [CrossRef]
- Lee, H.; Bae, E.K.; Park, S.Y.; Sjödin, A.; Lee, J.S.; Noh, E.W.; Jansson, S. Growth-phase-dependent gene expression profiling of poplar (Populus alba × Populus tremula var. glandulosa) suspension cells. Physiol. Plant. 2007, 131, 599–613. [Google Scholar] [CrossRef]
- Menges, M.; Hennig, L.; Gruissem, W.; Murray, J.A. Genome-wide gene expression in an Arabidopsis cell suspension. Plant Mol. Biol. 2003, 53, 423–442. [Google Scholar] [CrossRef]
- Ge, S.X.; Son, E.W.; Yao, R. iDEP: An integrated web application for differential expression and pathway analysis of RNA-Seq data. BMC Bioinform. 2018, 19, 534. [Google Scholar] [CrossRef]
- Dewitte, W.; Murray, J.A. The plant cell cycle. Annu. Rev. Plant Biol. 2003, 54, 235–264. [Google Scholar] [CrossRef]
- Richard, C.; Granier, C.; Inzé, D.; Veylder, L.D. Analysis of cell division parameters and cell cycle gene expression during the cultivation of Arabidopsis thaliana cell suspensions. J. Exp. Bot. 2001, 52, 1625–1633. [Google Scholar] [CrossRef]
- John, P.C.; Mews, M.; Moore, R. Cyclin/Cdk complexes: Their involvement in cell cycle progression and mitotic division. Protoplasma 2001, 216, 119–142. [Google Scholar] [PubMed]
- Menges, M.; Murray, J.A. Synchronous Arabidopsis suspension cultures for analysis of cell-cycle gene activity. Plant J. 2002, 30, 203–212. [Google Scholar]
- Sorrell, D.A.; Menges, M.; Healy, J.S.; Deveaux, Y.; Amano, C.; Su, Y.; Nakagami, H.; Shinmyo, A.; Doonan, J.H.; Sekine, M. Cell cycle regulation of cyclin-dependent kinases in tobacco cultivar Bright Yellow-2 cells. Plant Physiol. 2001, 126, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Fobert, P.R.; Gaudin, V.; Lunness, P.; Coen, E.S.; Doonan, J.H. Distinct classes of cdc2-related genes are differentially expressed during the cell division cycle in plants. Plant Cell 1996, 8, 1465–1476. [Google Scholar]
- Magyar, Z.; Mészáros, T.; Miskolczi, P.; Deák, M.; Fehér, A.; Brown, S.; Kondorosi, É.; Athanasiadis, A.; Pongor, S.; Bilgin, M. Cell cycle phase specificity of putative cyclin-dependent kinase variants in synchronized alfalfa cells. Plant Cell 1997, 9, 223–235. [Google Scholar]
- Boruc, J.; Van den Daele, H.; Hollunder, J.; Rombauts, S.; Mylle, E.; Hilson, P.; Inze, D.; De Veylder, L.; Russinova, E. Functional modules in the Arabidopsis core cell cycle binary protein–protein interaction network. Plant Cell 2010, 22, 1264–1280. [Google Scholar]
- Magyar, Z.; Bakó, L.; Bögre, L.; Dedeoglu, D.; Kapros, T.; Dudits, D. Active cdc2 genes and cell cycle phase-specific cdc2-related kinase complexes in hormone-stimulated alfalfa cells. Plant J. 1993, 4, 151–161. [Google Scholar] [CrossRef]
- Van Leene, J.; Stals, H.; Eeckhout, D.; Persiau, G.; Van De Slijke, E.; Van Isterdael, G.; De Clercq, A.; Bonnet, E.; Laukens, K.; Remmerie, N. A tandem affinity purification-based technology platform to study the cell cycle interactome in Arabidopsis thaliana. Mol. Cell. Proteom. 2007, 6, 1226–1238. [Google Scholar]
- Zhao, X.A.; Harashima, H.; Dissmeyer, N.; Pusch, S.; Weimer, A.K.; Bramsiepe, J.; Bouyer, D.; Rademacher, S.; Nowack, M.K.; Novak, B. A general G1/S-phase cell-cycle control module in the flowering plant Arabidopsis thaliana. PLoS Genet. 2012, 8, e1002847. [Google Scholar]
- Verkest, A.; Weinl, C.; Inzé, D.; De Veylder, L.; Schnittger, A. Switching the cell cycle. Kip-Relat. Proteins Plant Cell Cycle Control. Plant Physiol. 2005, 139, 1099–1106. [Google Scholar]
- Gorbsky, G.J. The spindle checkpoint and chromosome segregation in meiosis. FEBS J. 2015, 282, 2471–2487. [Google Scholar] [CrossRef]
- Komaki, S.; Schnittger, A. The spindle assembly checkpoint in Arabidopsis is rapidly shut off during severe stress. Dev. Cell 2017, 43, 172–185.e5. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, W.K.; Paules, R.S. DNA damage and cell cycle checkpoints. FASEB J. 1996, 10, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Nikitaki, Z.; Hellweg, C.E.; Georgakilas, A.G.; Ravanat, J.-L. Stress-induced DNA damage biomarkers: Applications and limitations. Front. Chem. 2015, 3, 35. [Google Scholar] [CrossRef]
- Takeda, T.; Seki, M.; Furusaki, S. Hydrodynamic damage of cultured cells of Carthamus tinctorius in a stirred tank reactor. J. Chem. Eng. Jpn. 1994, 27, 466–471. [Google Scholar] [CrossRef]
- Jay, V.; Genestier, S.; Courduroux, J.C. Bioreactor studies on the effect of dissolved oxygen concentrations on growth and differentiation of carrot (Daucus carota L.) cell cultures. Plant Cell Rep. 1992, 11, 605–608. [Google Scholar] [CrossRef] [PubMed]
- De Schutter, K.; Joubès, J.; Cools, T.; Verkest, A.; Corellou, F.; Babiychuk, E.; Van Der Schueren, E.; Beeckman, T.; Kushnir, S.; Inze, D. Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 2007, 19, 211–225. [Google Scholar] [CrossRef]
- Gigli-Bisceglia, N.; Hamann, T. Outside-in control–does plant cell wall integrity regulate cell cycle progression? Physiol. Plant. 2018, 164, 82–94. [Google Scholar] [CrossRef]
- Gu, Y.; Rasmussen, C.G. Cell biology of primary cell wall synthesis in plants. Plant Cell 2022, 34, 103–128. [Google Scholar]
- Verma, D.P.S. Cytokinesis and building of the cell plate in plants. Annu. Rev. Plant Biol. 2001, 52, 751–784. [Google Scholar] [CrossRef]
- Samuels, A.L.; Giddings, T.H., Jr.; Staehelin, L.A. Cytokinesis in tobacco BY-2 and root tip cells: A new model of cell plate formation in higher plants. J. Cell Biol. 1995, 130, 1345–1357. [Google Scholar] [PubMed]
- Seguié-Simarro, J.M.; Austin, J.R.; White, E.A.; Staehelin, L.A. Electron Tomographic Analysis of Somatic Cell Plate Formation in Meristematic Cells of Arabidopsis Preserved by High-Pressure Freezing. Plant Cell 2004, 16, 836–856. [Google Scholar]
- Cosgrove, D.J. Growth of the plant cell wall. Nat. Rev. Mol. Cell Biology 2005, 6, 850–861. [Google Scholar]
- Thiele, K.; Wanner, G.; Kindzierski, V.; Jürgens, G.; Mayer, U.; Pachl, F.; Assaad, F.F. The timely deposition of callose is essential for cytokinesis in Arabidopsis. Plant J. 2009, 58, 13–26. [Google Scholar] [PubMed]
- Hill, J.L., Jr.; Hammudi, M.B.; Tien, M. The Arabidopsis cellulose synthase complex: A proposed hexamer of CESA trimers in an equimolar stoichiometry. Plant Cell 2014, 26, 4834–4842. [Google Scholar]
- Persson, S.; Paredez, A.; Carroll, A.; Palsdottir, H.; Doblin, M.; Poindexter, P.; Khitrov, N.; Auer, M.; Somerville, C.R. Genetic evidence for three unique components in primary cell-wall cellulose synthase complexes in Arabidopsis. Proc. Natl. Acad. Sci. USA 2007, 104, 15566–15571. [Google Scholar] [PubMed]
- Desprez, T.; Juraniec, M.; Crowell, E.F.; Jouy, H.; Pochylova, Z.; Parcy, F.; Hofte, H.; Gonneau, M.; Vernhettes, S. Organization of cellulose synthase complexes involved in primary cell wall synthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2007, 104, 15572–15577. [Google Scholar]
- Miart, F.; Desprez, T.; Biot, E.; Morin, H.; Belcram, K.; Hofte, H.; Gonneau, M.; Vernhettes, S. Spatio-temporal analysis of cellulose synthesis during cell plate formation in Arabidopsis. Plant J. 2014, 77, 71–84. [Google Scholar] [CrossRef]
- Beeckman, T.; Przemeck, G.K.; Stamatiou, G.; Lau, R.; Terryn, N.; De Rycke, R.; Inze, D.; Berleth, T. Genetic complexity of cellulose synthase a gene function in Arabidopsis embryogenesis. Plant Physiol. 2002, 130, 1883–1893. [Google Scholar]
- Hu, Z.; Vanderhaeghen, R.; Cools, T.; Wang, Y.; De Clercq, I.; Leroux, O.; Nguyen, L.; Belt, K.; Millar, A.H.; Audenaert, D.; et al. Mitochondrial Defects Confer Tolerance against Cellulose Deficiency. Plant Cell 2016, 28, 2276–2290. [Google Scholar]
- Caffall, K.H.; Pattathil, S.; Phillips, S.E.; Hahn, M.G.; Mohnen, D. Arabidopsis thaliana T-DNA mutants implicate GAUT genes in the biosynthesis of pectin and xylan in cell walls and seed testa. Mol. Plant 2009, 2, 1000–1014. [Google Scholar] [PubMed]
- Krupkova, E.; Immerzeel, P.; Pauly, M.; Schmülling, T. The TUMOROUS SHOOT DEVELOPMENT2 gene of Arabidopsis encoding a putative methyltransferase is required for cell adhesion and co-ordinated plant development. Plant J. 2007, 50, 735–750. [Google Scholar]
- Rybak, K.; Steiner, A.; Synek, L.; Klaeger, S.; Kulich, I.; Facher, E.; Wanner, G.; Kuster, B.; Zarsky, V.; Persson, S. Plant cytokinesis is orchestrated by the sequential action of the TRAPPII and exocyst tethering complexes. Dev. Cell 2014, 29, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, K.; Goto, Y.; Asatsuma, S.; Koizumi, M.; Mitsui, T.; Matsuoka, K. A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 2009, 21, 1212–1229. [Google Scholar] [CrossRef]
- Brabetz, W.; Wolter, F.P.; Brade, H. A cDNA encoding 3-deoxy-D-manno-oct-2-ulosonate-8-phosphate synthase of Pisum sativum L.(pea) functionally complements a kdsA mutant of the Gram-negative bacterium Salmonella enterica. Planta 2000, 212, 136–143. [Google Scholar] [PubMed]
- York, W.S.; Darvill, A.G.; McNeil, M.; Albersheim, P. 3-Deoxy-D-manno-2-octulosonic acid (KDO) is a component of rhamnogalacturonan II, a pectic polysaccharide in the primary cell walls of plants. Carbohydr. Res. 1985, 138, 109–126. [Google Scholar] [CrossRef]
- Matsuura, K.; Miyagawa, I.; Kobayashi, M.; Ohta, D.; Matoh, T. Arabidopsis 3-deoxy-d-manno-oct-2-ulosonate-8-phosphate synthase: cDNA cloning and expression analyses. J. Exp. Bot. 2003, 54, 1785–1787. [Google Scholar] [CrossRef]
- Delmas, F.; Séveno, M.; Northey, J.G.; Hernould, M.; Lerouge, P.; McCourt, P.; Chevalier, C. The synthesis of the rhamnogalacturonan II component 3-deoxy-D-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. J. Exp. Bot. 2008, 59, 2639–2647. [Google Scholar]
- Delmas, F.; Petit, J.; Joubès, J.; Séveno, M.; Paccalet, T.; Hernould, M.; Lerouge, P.; Mouras, A.; Chevalier, C. The gene expression and enzyme activity of plant 3-deoxy-D-manno-2-octulosonic acid-8-phosphate synthase are preferentially associated with cell division in a cell cycle-dependent manner. Plant Physiol. 2003, 133, 348–360. [Google Scholar]
- Deng, Y.; Wang, W.; Li, W.Q.; Xia, C.; Liao, H.Z.; Zhang, X.Q.; Ye, D. MALE GAMETOPHYTE DEFECTIVE 2, encoding a sialyltransferase-like protein, is required for normal pollen germination and pollen tube growth in Arabidopsis. J. Integr. Plant Biol. 2010, 52, 829–843. [Google Scholar]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar]
- Cocuron, J.-C.; Lerouxel, O.; Drakakaki, G.; Alonso, A.P.; Liepman, A.H.; Keegstra, K.; Raikhel, N.; Wilkerson, C.G. A gene from the cellulose synthase-like C family encodes a β-1, 4 glucan synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 8550–8555. [Google Scholar] [PubMed]
- Moore, P.J.; Staehelin, L.A. Immunogold localization of the cell-wall-matrix polysaccharides rhamnogalacturonan I and xyloglucan during cell expansion and cytokinesis inTrifolium pratense L.; implication for secretory pathways. Planta 1988, 174, 433–445. [Google Scholar]
- Rose, J.K.; Braam, J.; Fry, S.C.; Nishitani, K. The XTH family of enzymes involved in xyloglucan endotransglucosylation and endohydrolysis: Current perspectives and a new unifying nomenclature. Plant Cell Physiol. 2002, 43, 1421–1435. [Google Scholar]
- Vissenberg, K.; Oyama, M.; Osato, Y.; Yokoyama, R.; Verbelen, J.P.; Nishitani, K. Differential expression of AtXTH17, AtXTH18, AtXTH19 and AtXTH20 genes in Arabidopsis roots. Physiological roles in specification in cell wall construction. Plant Cell Physiol. 2005, 46, 192–200. [Google Scholar] [PubMed]
- Maris, A.; Suslov, D.; Fry, S.C.; Verbelen, J.-P.; Vissenberg, K. Enzymic characterization of two recombinant xyloglucan endotransglucosylase/hydrolase (XTH) proteins of Arabidopsis and their effect on root growth and cell wall extension. J. Exp. Bot. 2009, 60, 3959–3972. [Google Scholar] [PubMed]
- Miedes, E.; Suslov, D.; Vandenbussche, F.; Kenobi, K.; Ivakov, A.; Van Der Straeten, D.; Lorences, E.P.; Mellerowicz, E.J.; Verbelen, J.-P.; Vissenberg, K. Xyloglucan endotransglucosylase/hydrolase (XTH) overexpression affects growth and cell wall mechanics in etiolated Arabidopsis hypocotyls. J. Exp. Bot. 2013, 64, 2481–2497. [Google Scholar]
- Matsui, A.; Yokoyama, R.; Seki, M.; Ito, T.; Shinozaki, K.; Takahashi, T.; Komeda, Y.; Nishitani, K. AtXTH27 plays an essential role in cell wall modification during the development of tracheary elements. Plant J. 2005, 42, 525–534. [Google Scholar]
- Osato, Y.; Yokoyama, R.; Nishitani, K. A principal role for AtXTH18 in Arabidopsis thaliana root growth: A functional analysis using RNAi plants. J. Plant Res. 2006, 119, 153–162. [Google Scholar]
- McQueen-Mason, S.J.; Cosgrove, D.J. Expansin mode of action on cell walls (analysis of wall hydrolysis, stress relaxation, and binding). Plant Physiol. 1995, 107, 87–100. [Google Scholar]
- Cosgrove, D.J. Loosening of plant cell walls by expansins. Nature 2000, 407, 321–326. [Google Scholar] [CrossRef] [PubMed]
- Link, B.M.; Cosgrove, D.J. Acid-growth response and α-expansins in suspension cultures of bright yellow 2 tobacco. Plant Physiol. 1998, 118, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, P.; Ruiz Duarte, P.; Rance, G.A.; Schubert, M.; Vordermaier, V.; Vu, L.D.; Murphy, E.; Vilches Barro, A.; Swarup, K.; Moirangthem, K.; et al. EXPANSIN A1-mediated radial swelling of pericycle cells positions anticlinal cell divisions during lateral root initiation. Proc. Natl. Acad. Sci. USA 2019, 116, 8597–8602. [Google Scholar] [CrossRef]
- He, M.; Ding, N.Z. Plant Unsaturated Fatty Acids: Multiple Roles in Stress Response. Front. Plant Sci. 2020, 11, 562785. [Google Scholar] [CrossRef]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar]
- Farmer, E.E.; Weber, H.; Vollenweider, S. Fatty acid signaling in Arabidopsis. Planta 1998, 206, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sano, T.; Kuraya, Y.; Amino, S.-i.; Nagata, T. Phosphate as a limiting factor for the cell division of tobacco BY-2 cells. Plant Cell Physiol. 1999, 40, 1–16. [Google Scholar] [CrossRef]
- Chandrika, N.N.P.; Sundaravelpandian, K.; Yu, S.M.; Schmidt, W. ALFIN-LIKE 6 is involved in root hair elongation during phosphate deficiency in Arabidopsis. New Phytol. 2013, 198, 709–720. [Google Scholar] [CrossRef]
- Poirier, Y.; Antonenkov, V.D.; Glumoff, T.; Hiltunen, J.K. Peroxisomal β-oxidation—A metabolic pathway with multiple functions. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Res. 2006, 1763, 1413–1426. [Google Scholar] [CrossRef]
- Aslam, M.; Travis, R.L.; Huffaker, R.C. Comparative induction of nitrate and nitrite uptake and reduction systems by ambient nitrate and nitrite in intact roots of barley (Hordeum vulgare L.) seedlings. Plant Physiol. 1993, 102, 811–819. [Google Scholar] [CrossRef]
- Glass, A.D.; Shaff, J.E.; Kochian, L.V. Studies of the uptake of nitrate in barley: IV. Electrophysiology. Plant Physiol. 1992, 99, 456–463. [Google Scholar]
- Crawford, N.M.; Glass, A.D. Molecular and physiological aspects of nitrate uptake in plants. Trends Plant Sci. 1998, 3, 389–395. [Google Scholar]
- Hidangmayum, A.; Dwivedi, P.; Katiyar, D.; Hemantaranjan, A. Application of chitosan on plant responses with special reference to abiotic stress. Physiol. Mol. Biol. Plants 2019, 25, 313–326. [Google Scholar] [PubMed]
- Jan, R.; Kim, N.; Lee, S.; Khan, M.; Asaf, S.; Park, J.; Asif, S.; Lee, I.; Kim, K. Enhanced Flavonoid Accumulation Reduces Combined Salt and Heat Stress Through Regulation of Transcriptional and Hormonal Mechanisms. Front. Plant Sci. 2021, 12, 796956. [Google Scholar]
- Marrs, K.A. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Biol. 1996, 47, 127–158. [Google Scholar] [CrossRef]
- May, A.; Berger, S.; Hertel, T.; Köck, M. The Arabidopsis thaliana phosphate starvation responsive gene AtPPsPase1 encodes a novel type of inorganic pyrophosphatase. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2011, 1810, 178–185. [Google Scholar] [CrossRef]
- Amino, S.i.; Fujimura, T.; Komamine, A. Synchrony induced by double phosphate starvation in a suspension culture of Catharanthus roseus. Physiol. Plant. 1983, 59, 393–396. [Google Scholar] [CrossRef]
- Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef]
- Dao, T.; Linthorst, H.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar]
- Sinha, R.K.; Sharma, S.N.; Verma, S.S.; Zha, J. Effects of lovastin, fosmidomycin and methyl jasmonate on andrographolide biosynthesis in the Andrographis paniculata. Acta Physiol. Plant. 2018, 40, 165. [Google Scholar]
- Pankratov, I.; McQuinn, R.; Schwartz, J.; Bar, E.; Fei, Z.; Lewinsohn, E.; Zamir, D.; Giovannoni, J.J.; Hirschberg, J. Fruit carotenoid-deficient mutants in tomato reveal a function of the plastidial isopentenyl diphosphate isomerase (IDI 1) in carotenoid biosynthesis. Plant J. 2016, 88, 82–94. [Google Scholar]
- Ma, D.; Li, G.; Alejos-Gonzalez, F.; Zhu, Y.; Xue, Z.; Wang, A.; Zhang, H.; Li, X.; Ye, H.; Wang, H. Overexpression of a type-I isopentenyl pyrophosphate isomerase of Artemisia annua in the cytosol leads to high arteannuin B production and artemisinin increase. Plant J. 2017, 91, 466–479. [Google Scholar] [PubMed]
- Liu, J.; Yin, X.; Kou, C.; Thimmappa, R.; Hua, X.; Xue, Z. Classification, biosynthesis and biological function of triterpene esters in plants. Plant Commun. 2024, 5, 100845. [Google Scholar] [CrossRef] [PubMed]
- Jozwiak, A.; Sonawane, P.D.; Panda, S.; Garagounis, C.; Papadopoulou, K.K.; Abebie, B.; Massalha, H.; Almekias-Siegl, E.; Scherf, T.; Aharoni, A. Plant terpenoid metabolism co-opts a component of the cell wall biosynthesis machinery. Nat. Chem. Biol. 2020, 16, 740–748. [Google Scholar] [PubMed]
- Andre, C.M.; Legay, S.; Deleruelle, A.; Nieuwenhuizen, N.; Punter, M.; Brendolise, C.; Cooney, J.M.; Lateur, M.; Hausman, J.F.; Larondelle, Y. Multifunctional oxidosqualene cyclases and cytochrome P450 involved in the biosynthesis of apple fruit triterpenic acids. New Phytol. 2016, 211, 1279–1294. [Google Scholar]
- Sandeep; Misra, R.C.; Chanotiya, C.S.; Mukhopadhyay, P.; Ghosh, S. Oxidosqualene cyclase and CYP716 enzymes contribute to triterpene structural diversity in the medicinal tree banaba. New Phytol. 2019, 222, 408–424. [Google Scholar]
- Linsmaier, E.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant 1965, 18, 100–127. [Google Scholar]
- Blom, T.; Kreis, W.; Van Iren, F.; Libbenga, K. A non-invasive method for the routine-estimation of fresh weight of cells grown in batch suspension cultures. Plant Cell Rep. 1992, 11, 146–149. [Google Scholar]
- Legay, S.; Guerriero, G.; Deleruelle, A.; Lateur, M.; Evers, D.; André, C.M.; Hausman, J.-F. Apple russeting as seen through the RNA-seq lens: Strong alterations in the exocarp cell wall. Plant Mol. Biol. 2015, 88, 21–40. [Google Scholar]
- Daccord, N.; Celton, J.-M.; Linsmith, G.; Becker, C.; Choisne, N.; Schijlen, E.; Van de Geest, H.; Bianco, L.; Micheletti, D.; Velasco, R. High-quality de novo assembly of the apple genome and methylome dynamics of early fruit development. Nat. Genet. 2017, 49, 1099–1106. [Google Scholar]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [PubMed]
- Baggerly, K.A.; Deng, L.; Morris, J.S.; Aldaz, C.M. Differential expression in SAGE: Accounting for normal between-library variation. Bioinformatics 2003, 19, 1477–1483. [Google Scholar] [PubMed]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.-H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar]
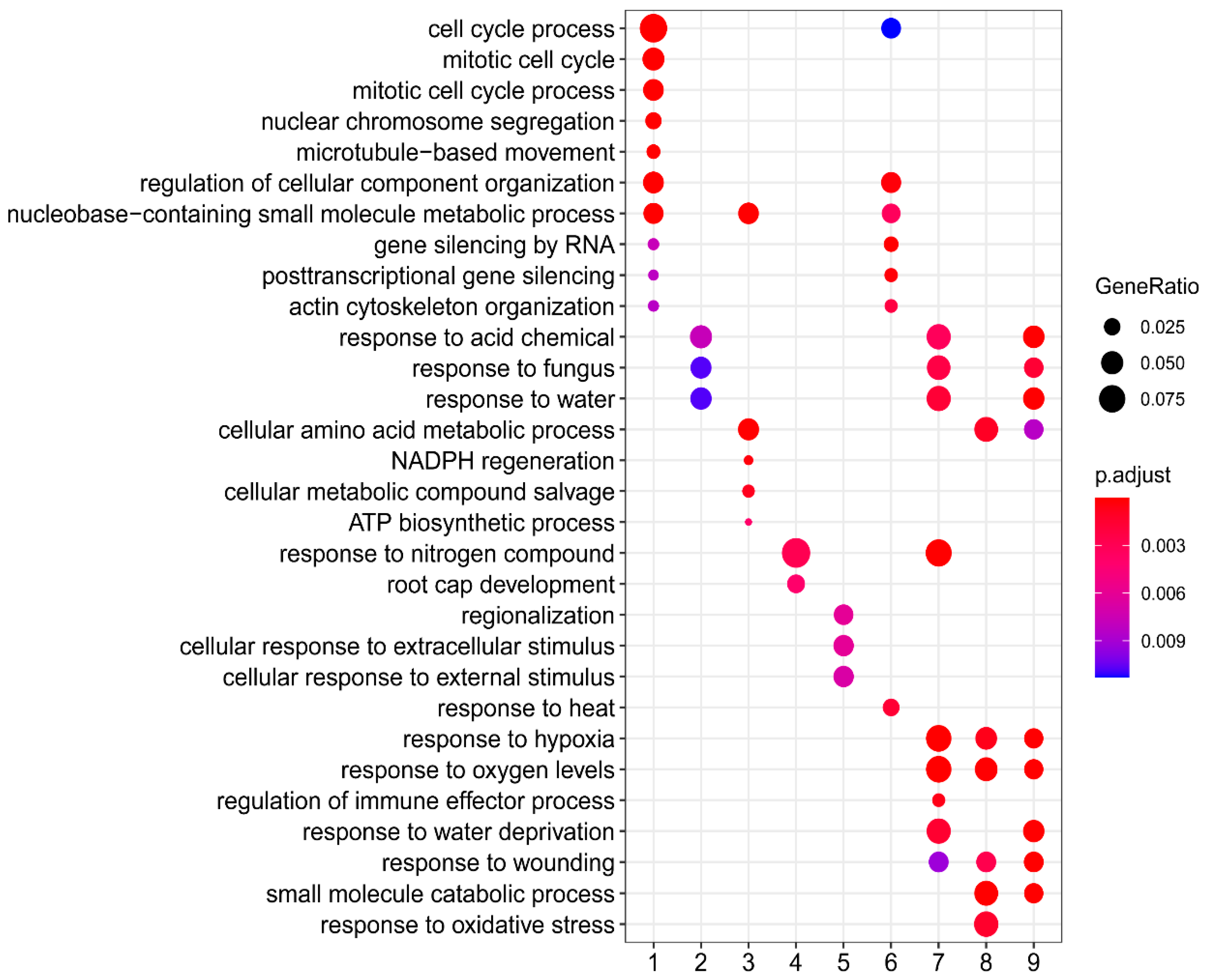
| Principle Component | Biological Process | e-Value |
|---|---|---|
| PC1 | Nuclear division | 4 × 10−8 |
| Organelle organization | 7 × 10−13 | |
| Microtubule-based process | 8 × 10−8 | |
| Cellular component organization | 8 × 10−8 | |
| Chromosome organization | 4 × 10−11 | |
| Cellular component organization or biogenesis | 6 × 10−11 | |
| PC2 | Translation | 2 × 10−6 |
| Peptide metabolic process | 4 × 10−6 | |
| Peptide biosynthetic process | 1 × 10−6 | |
| Cellular amide metabolic process | 1 × 10−4 | |
| Amide biosynthetic process | 5 × 10−5 | |
| Organonitrogen compound biosynthetic process | 2 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Cocco, E.; Guerriero, G.; Sergeant, K.; Jourdan, S.; Renaut, J.; Hausman, J.-F.; Legay, S. Harnessing Apple Cell Suspension Cultures in Bioreactors for Triterpene Production: Transcriptomic Insights into Biomass and Triterpene Biosynthesis. Int. J. Mol. Sci. 2025, 26, 3188. https://doi.org/10.3390/ijms26073188
Xu X, Cocco E, Guerriero G, Sergeant K, Jourdan S, Renaut J, Hausman J-F, Legay S. Harnessing Apple Cell Suspension Cultures in Bioreactors for Triterpene Production: Transcriptomic Insights into Biomass and Triterpene Biosynthesis. International Journal of Molecular Sciences. 2025; 26(7):3188. https://doi.org/10.3390/ijms26073188
Chicago/Turabian StyleXu, Xuan, Emmanuelle Cocco, Gea Guerriero, Kjell Sergeant, Samuel Jourdan, Jenny Renaut, Jean-Francois Hausman, and Sylvain Legay. 2025. "Harnessing Apple Cell Suspension Cultures in Bioreactors for Triterpene Production: Transcriptomic Insights into Biomass and Triterpene Biosynthesis" International Journal of Molecular Sciences 26, no. 7: 3188. https://doi.org/10.3390/ijms26073188
APA StyleXu, X., Cocco, E., Guerriero, G., Sergeant, K., Jourdan, S., Renaut, J., Hausman, J.-F., & Legay, S. (2025). Harnessing Apple Cell Suspension Cultures in Bioreactors for Triterpene Production: Transcriptomic Insights into Biomass and Triterpene Biosynthesis. International Journal of Molecular Sciences, 26(7), 3188. https://doi.org/10.3390/ijms26073188












