Matrix Metalloproteinase-9 Enhances Osteoclastogenesis: Insights from Transgenic Rabbit Bone Marrow Models and In Vitro Studies
Abstract
:1. Introduction
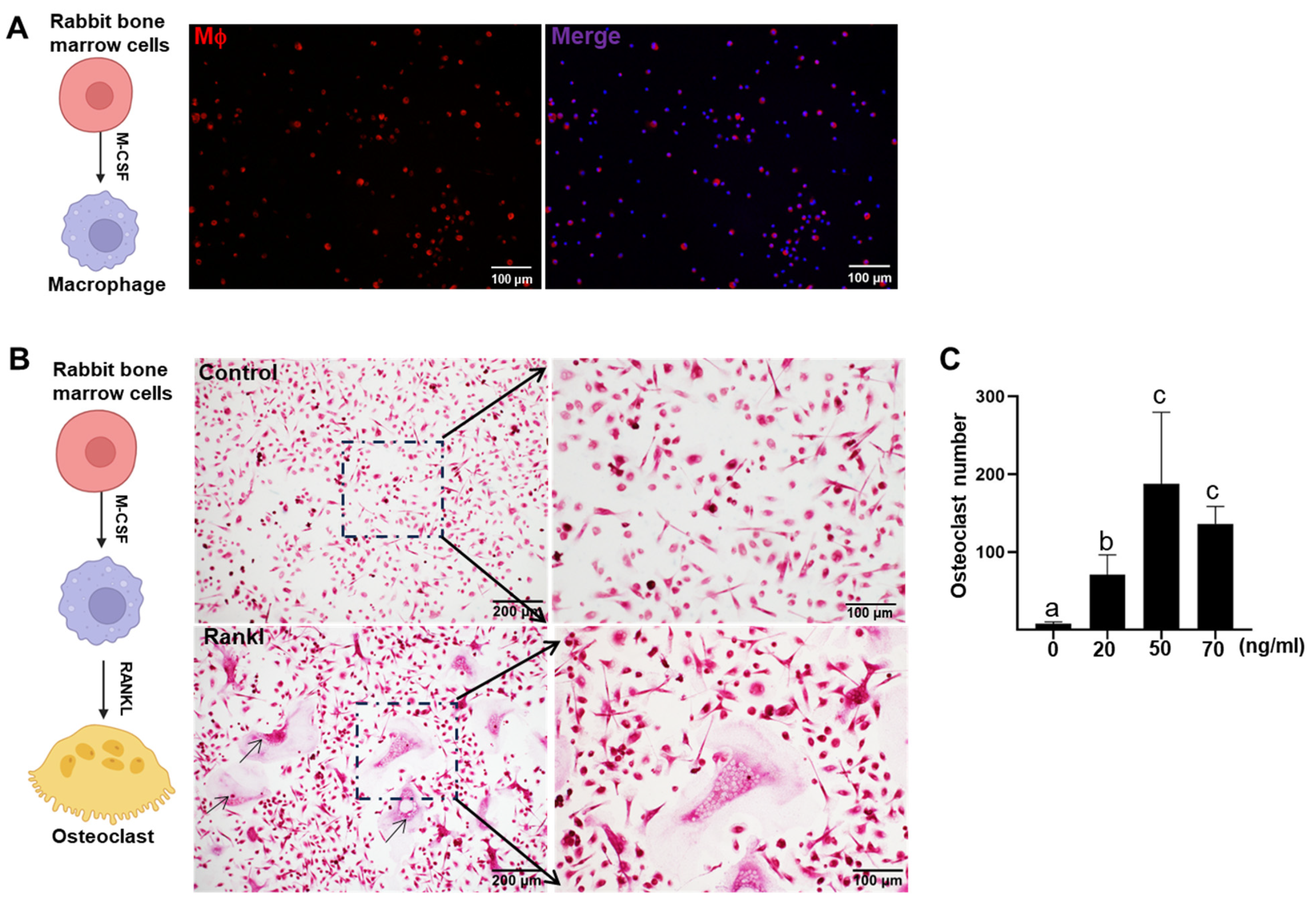
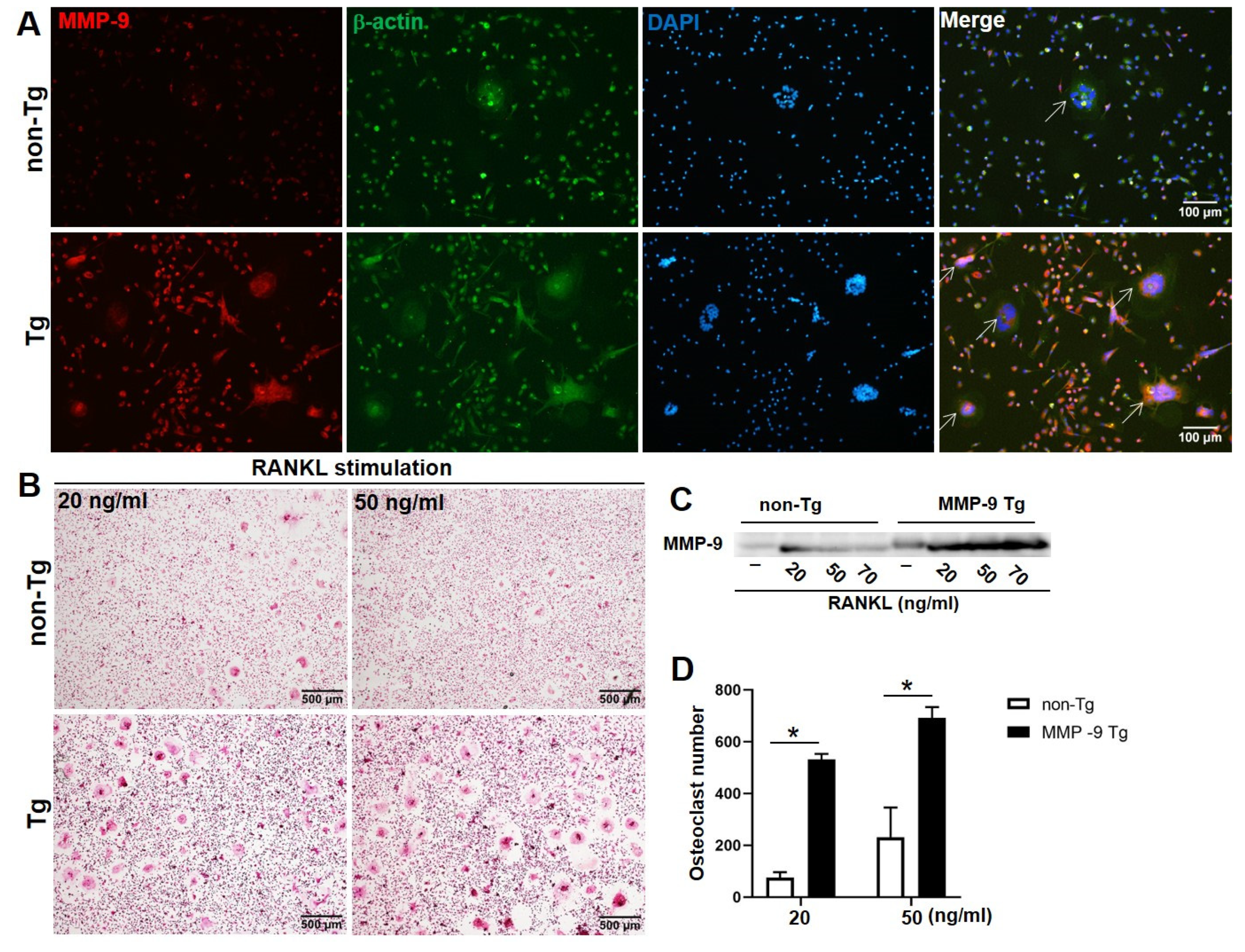
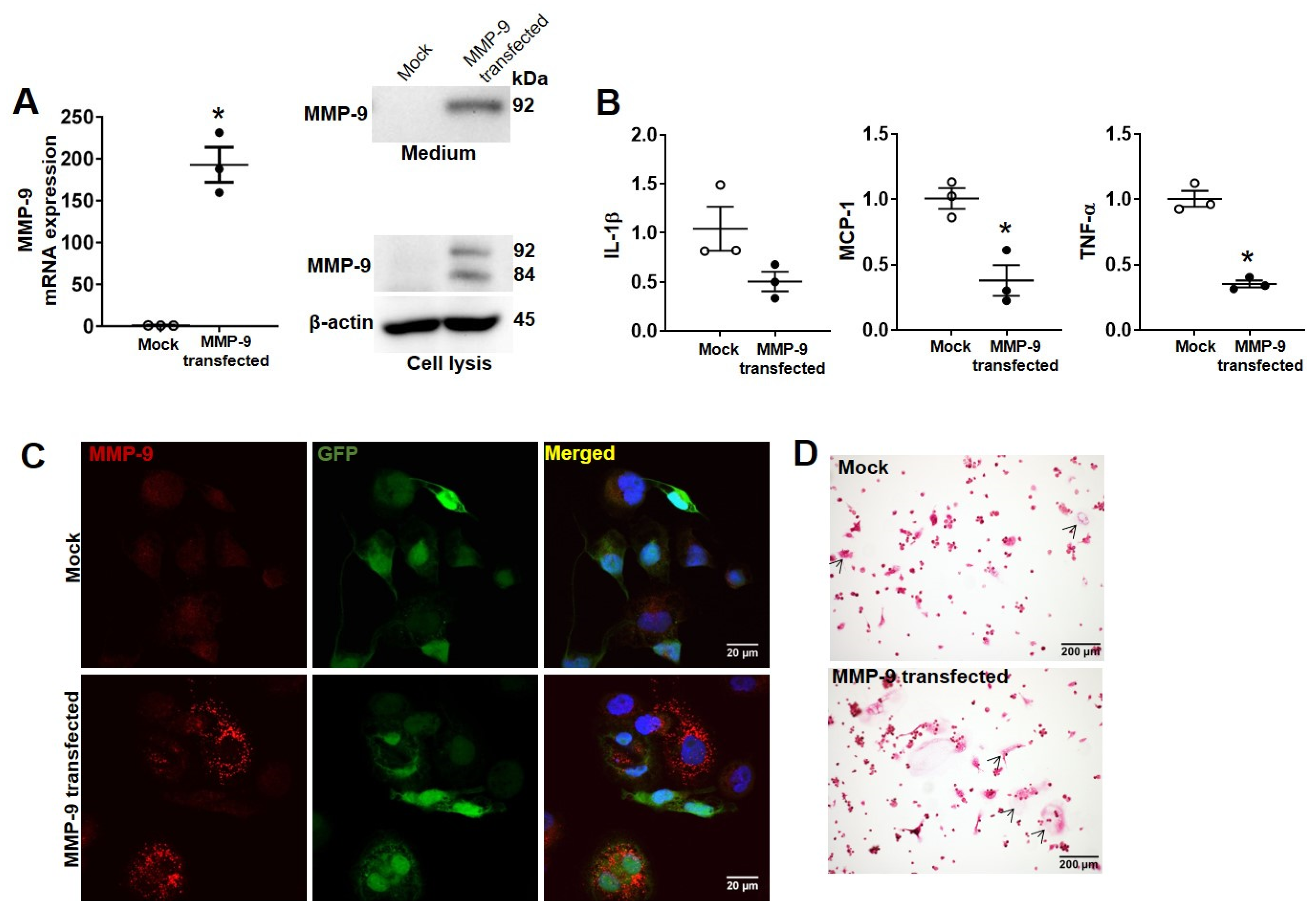
2. Results
3. Discussion
4. Materials and Methods
4.1. Isolation of Rabbit Bone Marrow Cells
4.2. In Vitro Osteoclasts Differentiation
4.3. TRAP Staining
4.4. Immunoblotting Analysis
4.5. Immunofluorescence Staining
4.6. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.7. RAW264.7 Cell-Line Culture
4.8. Lentivirus-Mediated Expression of MMP-9
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast differentiation and activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Alles, N.; Aoki, K.; Ohya, K. Osteoclast formation and differentiation: An overview. J. Med. Dent. Sci. 2012, 59, 65–74. [Google Scholar]
- Henriksen, K.; Bollerslev, J.; Everts, V.; Karsdal, M.A. Osteoclast activity and subtypes as a function of physiology and pathology--implications for future treatments of osteoporosis. Endocr. Rev. 2011, 32, 31–63. [Google Scholar] [CrossRef]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic bone disease: Pathogenesis and therapeutic options: Up-date on bone metastasis management. J. Bone Oncol. 2019, 15, 100205. [Google Scholar] [CrossRef]
- Venetis, K.; Piciotti, R.; Sajjadi, E.; Invernizzi, M.; Morganti, S.; Criscitiello, C.; Fusco, N. Breast Cancer with Bone Metastasis: Molecular Insights and Clinical Management. Cells 2021, 10, 1377. [Google Scholar] [CrossRef]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Krane, S.M.; Inada, M. Matrix metalloproteinases and bone. Bone 2008, 43, 7–18. [Google Scholar] [CrossRef]
- Paiva, K.B.S.; Granjeiro, J.M. Matrix Metalloproteinases in Bone Resorption, Remodeling, and Repair. Prog. Mol. Biol. Transl. Sci. 2017, 148, 203–303. [Google Scholar] [CrossRef]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, J.; Lee, H.; Shin, W.R.; Lee, S.; Lee, J.; Park, J.I.; Jhun, B.H.; Kim, Y.H.; Yi, S.J.; et al. Tetracycline Analogs Inhibit Osteoclast Differentiation by Suppressing MMP-9-Mediated Histone H3 Cleavage. Int. J. Mol. Sci. 2019, 20, 4038. [Google Scholar] [CrossRef]
- Ishibashi, O.; Niwa, S.; Kadoyama, K.; Inui, T. MMP-9 antisense oligodeoxynucleotide exerts an inhibitory effect on osteoclastic bone resorption by suppressing cell migration. Life Sci. 2006, 79, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, K.; Nishimura, R.; Senn, J.; Youssef, R.F.; London, S.D.; Reddy, S.V. RANK ligand signaling modulates the matrix metalloproteinase-9 gene expression during osteoclast differentiation. Exp. Cell Res. 2007, 313, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Schafrum Macedo, A.; Cezaretti Feitosa, C.; Yoiti Kitamura Kawamoto, F.; Vinicius Tertuliano Marinho, P.; Dos Santos Dal-Bo, I.; Fiuza Monteiro, B.; Prado, L.; Bregadioli, T.; Antonio Covino Diamante, G.; Ricardo Auada Ferrigno, C. Animal modeling in bone research-Should we follow the White Rabbit? Anim. Models Exp. Med. 2019, 2, 162–168. [Google Scholar] [CrossRef]
- Fan, J.; Kitajima, S.; Watanabe, T.; Xu, J.; Zhang, J.; Liu, E.; Chen, Y.E. Rabbit models for the study of human atherosclerosis: From pathophysiological mechanisms to translational medicine. Pharmacol. Ther. 2015, 146, 104–119. [Google Scholar] [CrossRef]
- Arzi, B.; Wisner, E.R.; Huey, D.J.; Kass, P.H.; Hu, J.; Athanasiou, K.A. A proposed model of naturally occurring osteoarthritis in the domestic rabbit. Lab Anim. 2011, 41, 20–25. [Google Scholar] [CrossRef]
- Lad, S.E.; Anderson, R.J.; Cortese, S.A.; Alvarez, C.E.; Danison, A.D.; Morris, H.M.; Ravosa, M.J. Bone remodeling and cyclical loading in maxillae of New Zealand white rabbits (Oryctolagus cuniculus). Anat. Rec. 2021, 304, 1927–1936. [Google Scholar] [CrossRef]
- Chen, Y.; Waqar, A.B.; Nishijima, K.; Ning, B.; Kitajima, S.; Matsuhisa, F.; Chen, L.; Liu, E.; Koike, T.; Yu, Y.; et al. Macrophage-derived MMP-9 enhances the progression of atherosclerotic lesions and vascular calcification in transgenic rabbits. J. Cell. Mol. Med. 2020, 24, 4261–4274. [Google Scholar] [CrossRef]
- Takeshita, S.; Kaji, K.; Kudo, A. Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclasts. J. Bone Miner. Res. 2000, 15, 1477–1488. [Google Scholar] [CrossRef]
- Karst, M.; Gorny, G.; Galvin, R.J.; Oursler, M.J. Roles of stromal cell RANKL, OPG, and M-CSF expression in biphasic TGF-beta regulation of osteoclast differentiation. J. Cell. Physiol. 2004, 200, 99–106. [Google Scholar] [CrossRef]
- Engsig, M.T.; Chen, Q.J.; Vu, T.H.; Pedersen, A.C.; Therkidsen, B.; Lund, L.R.; Henriksen, K.; Lenhard, T.; Foged, N.T.; Werb, Z.; et al. Matrix metalloproteinase 9 and vascular endothelial growth factor are essential for osteoclast recruitment into developing long bones. J. Cell Biol. 2000, 151, 879–890. [Google Scholar] [CrossRef]
- Bruni-Cardoso, A.; Johnson, L.C.; Vessella, R.L.; Peterson, T.E.; Lynch, C.C. Osteoclast-derived matrix metalloproteinase-9 directly affects angiogenesis in the prostate tumor-bone microenvironment. Mol. Cancer Res. 2010, 8, 459–470. [Google Scholar] [CrossRef] [PubMed]
- Romeo, S.G.; Alawi, K.M.; Rodrigues, J.; Singh, A.; Kusumbe, A.P.; Ramasamy, S.K. Endothelial proteolytic activity and interaction with non-resorbing osteoclasts mediate bone elongation. Nat. Cell Biol. 2019, 21, 430–441. [Google Scholar] [CrossRef]
- Zhu, G.; Chen, W.; Tang, C.Y.; McVicar, A.; Edwards, D.; Wang, J.; McConnell, M.; Yang, S.; Li, Y.; Chang, Z.; et al. Knockout and Double Knockout of Cathepsin K and Mmp9 reveals a novel function of Cathepsin K as a regulator of osteoclast gene expression and bone homeostasis. Int. J. Biol. Sci. 2022, 18, 5522–5538. [Google Scholar] [CrossRef]
- Shin, Y.; Kim, S.; Liang, G.; An, W. MMP-9-dependent proteolysis of the histone H3 N-terminal tail: A critical epigenetic step in driving oncogenic transcription and colon tumorigenesis. Mol. Oncol. 2024, 18, 2001–2019. [Google Scholar] [CrossRef]
- Nissinen, L.; Kahari, V.M. Matrix metalloproteinases in inflammation. Biochim. Biophys. Acta 2014, 1840, 2571–2580. [Google Scholar] [CrossRef]
- Zamilpa, R.; Ibarra, J.; de Castro Bras, L.E.; Ramirez, T.A.; Nguyen, N.; Halade, G.V.; Zhang, J.; Dai, Q.; Dayah, T.; Chiao, Y.A.; et al. Transgenic overexpression of matrix metalloproteinase-9 in macrophages attenuates the inflammatory response and improves left ventricular function post-myocardial infarction. J. Mol. Cell. Cardiol. 2012, 53, 599–608. [Google Scholar] [CrossRef]
- Toba, H.; Cannon, P.L.; Yabluchanskiy, A.; Iyer, R.P.; D’Armiento, J.; Lindsey, M.L. Transgenic overexpression of macrophage matrix metalloproteinase-9 exacerbates age-related cardiac hypertrophy, vessel rarefaction, inflammation, and fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, H375–H383. [Google Scholar] [CrossRef]
- Ying, W.; Cheruku, P.S.; Bazer, F.W.; Safe, S.H.; Zhou, B. Investigation of macrophage polarization using bone marrow derived macrophages. J. Vis. Exp. 2013, 76, 50323. [Google Scholar] [CrossRef]
- Canalis, E.; Mazziotti, G.; Giustina, A.; Bilezikian, J.P. Glucocorticoid-induced osteoporosis: Pathophysiology and therapy. Osteoporos. Int. 2007, 18, 1319–1328. [Google Scholar] [CrossRef]
- Mukherjee, A.; Das, B. The role of inflammatory mediators and matrix metalloproteinases (MMPs) in the progression of osteoarthritis. Biomater. Biosyst. 2024, 13, 100090. [Google Scholar] [CrossRef]
- Chevalier, X.; Ravaud, P.; Maheu, E.; Baron, G.; Rialland, A.; Vergnaud, P.; Roux, C.; Maugars, Y.; Mulleman, D.; Lukas, C.; et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2015, 74, 1697–1705. [Google Scholar] [CrossRef] [PubMed]
- Knapik, M.; Zelazo, D.A.; Osowiecka, K.; Krajewska-Wlodarczyk, M. Efficacy of Anti-Interleukin-1 Therapeutics in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials from the Years 2000 to 2023. J. Clin. Med. 2024, 13, 2859. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Yang, X.; Lei, Y.; Zhang, Z.; Smith, W.; Yan, J.; Kong, L. Evaluation of efficacy on RANKL induced osteoclast from RAW264.7 cells. J. Cell. Physiol. 2019, 234, 11969–11975. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Chen, Y.; Yan, H.; Niimi, M.; Wang, Y.; Liang, J. Principles and Applications of Rabbit Models for Atherosclerosis Research. J. Atheroscler. Thromb. 2018, 25, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Lang, P.; Nareoja, T.; Windahl, S.H.; Andersson, G. RANKL-dependent osteoclast differentiation and gene expression in bone marrow-derived cells from adult mice is sexually dimorphic. Bone Rep. 2023, 19, 101697. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, N.; Wang, S.; Wang, Y.; Yang, X.; Fan, J.; Chen, Y. Effects of Follicular Fluid on Physiological Characteristics and Differentiation of Fallopian Tube Epithelial Cells Implicating for Ovarian Cancer Pathogenesis. Int. J. Mol. Sci. 2023, 24, 10154. [Google Scholar] [CrossRef]

| Genes | Primer Sequences (5′–3′) | |
|---|---|---|
| Forward | Reverse | |
| Mouse GAPDH | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
| Mcie IL-1b | TTCAGGCAGGCAGTATCACTC | GAAGGTCCACGGGAAAGACAC |
| Mcie TNF-a | ACGGCATGGATCTCAAAGAC | AGATAGCAAATCGGCTGACG |
| Mcie MCP-1 | CTTCTGGGCCTGCTGTTCA | CCAGCCTACTCATTGGGATCA |
| Rabbit GAPDH | ATCACTGCCACCCAGAAGAC | GTGAGTTTCCCGTTCAGCTC |
| Rabbit IL-1b | GGAGAGCTCTTTCCCACCAG | TGGGTACCAAGGTTCTTTGAA |
| Rabbit TNF-a | ATGGTCACCCTCAGATCAGC | CTGGTTGTCCGTGAGCTTC |
| Rabbit MCP-1 | AGCACCAAGTGTCCCAAAGA | TGTGTTCTTGGGTTGTGGAA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zou, J.; Niimi, M.; Qiu, X.; Zhang, S.; Yang, H.; Zhu, M.; Fan, J. Matrix Metalloproteinase-9 Enhances Osteoclastogenesis: Insights from Transgenic Rabbit Bone Marrow Models and In Vitro Studies. Int. J. Mol. Sci. 2025, 26, 3194. https://doi.org/10.3390/ijms26073194
Chen Y, Zou J, Niimi M, Qiu X, Zhang S, Yang H, Zhu M, Fan J. Matrix Metalloproteinase-9 Enhances Osteoclastogenesis: Insights from Transgenic Rabbit Bone Marrow Models and In Vitro Studies. International Journal of Molecular Sciences. 2025; 26(7):3194. https://doi.org/10.3390/ijms26073194
Chicago/Turabian StyleChen, Yajie, Jialun Zou, Manabu Niimi, Xuan Qiu, Shuang Zhang, Han Yang, Maobi Zhu, and Jianglin Fan. 2025. "Matrix Metalloproteinase-9 Enhances Osteoclastogenesis: Insights from Transgenic Rabbit Bone Marrow Models and In Vitro Studies" International Journal of Molecular Sciences 26, no. 7: 3194. https://doi.org/10.3390/ijms26073194
APA StyleChen, Y., Zou, J., Niimi, M., Qiu, X., Zhang, S., Yang, H., Zhu, M., & Fan, J. (2025). Matrix Metalloproteinase-9 Enhances Osteoclastogenesis: Insights from Transgenic Rabbit Bone Marrow Models and In Vitro Studies. International Journal of Molecular Sciences, 26(7), 3194. https://doi.org/10.3390/ijms26073194






