Role of Endothelin-1 and Nitric Oxide in Acute Ischemic Stroke Leptomeningeal Collateral Activation
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics and Clinical Outcome
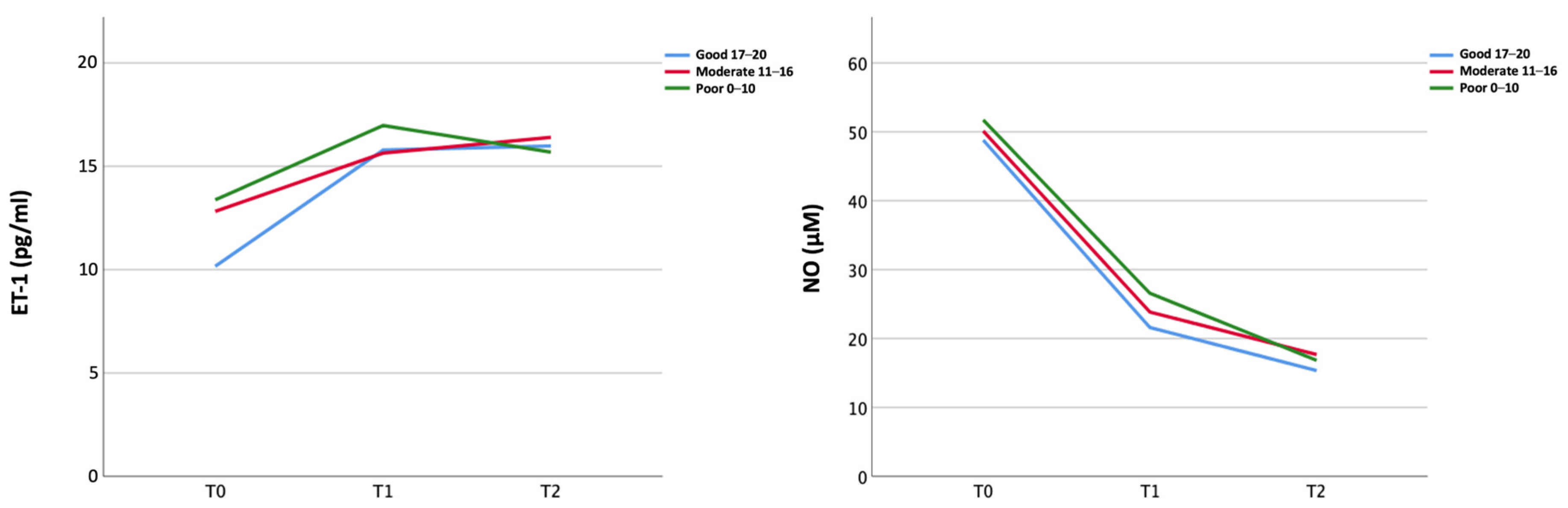
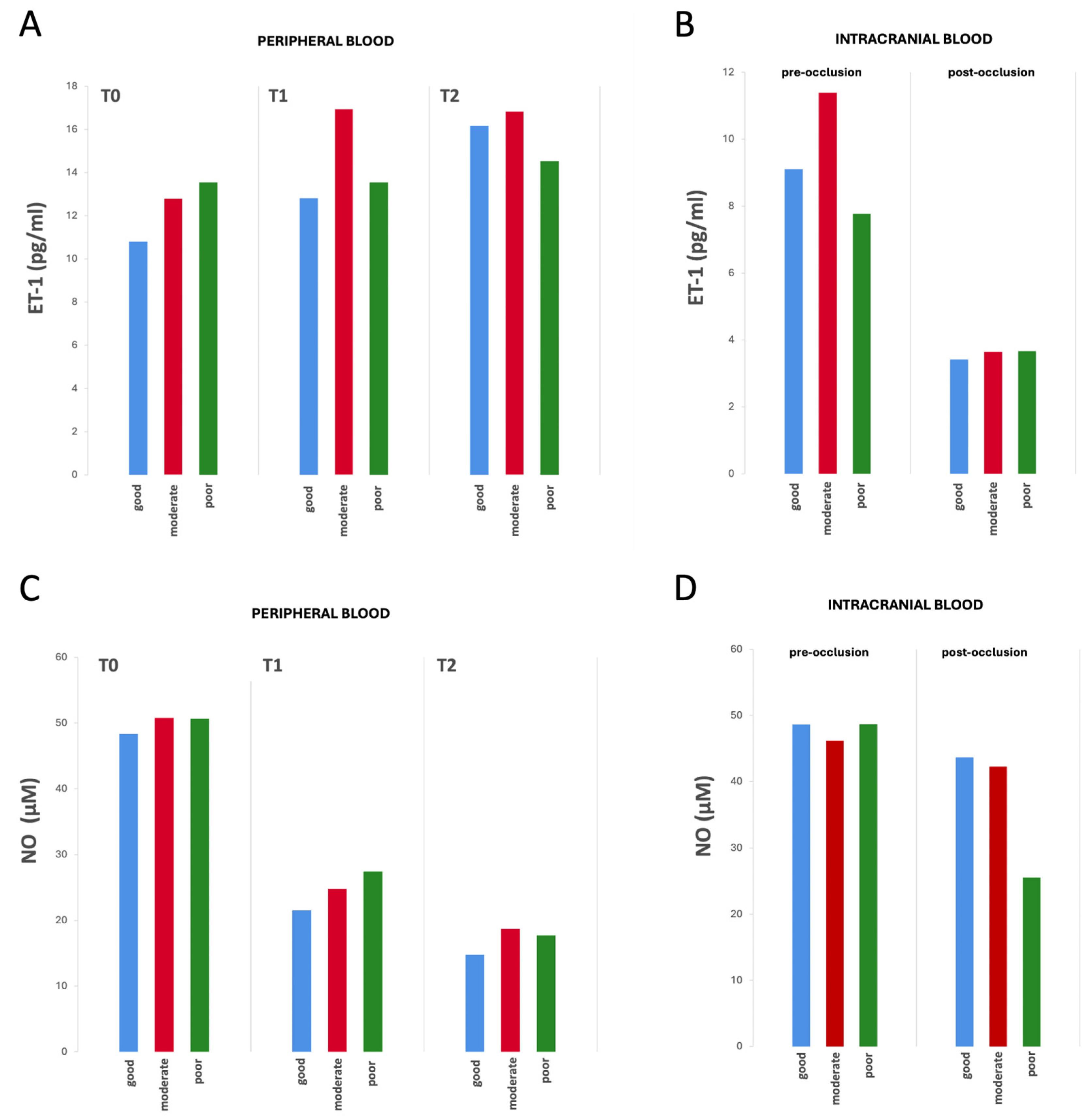
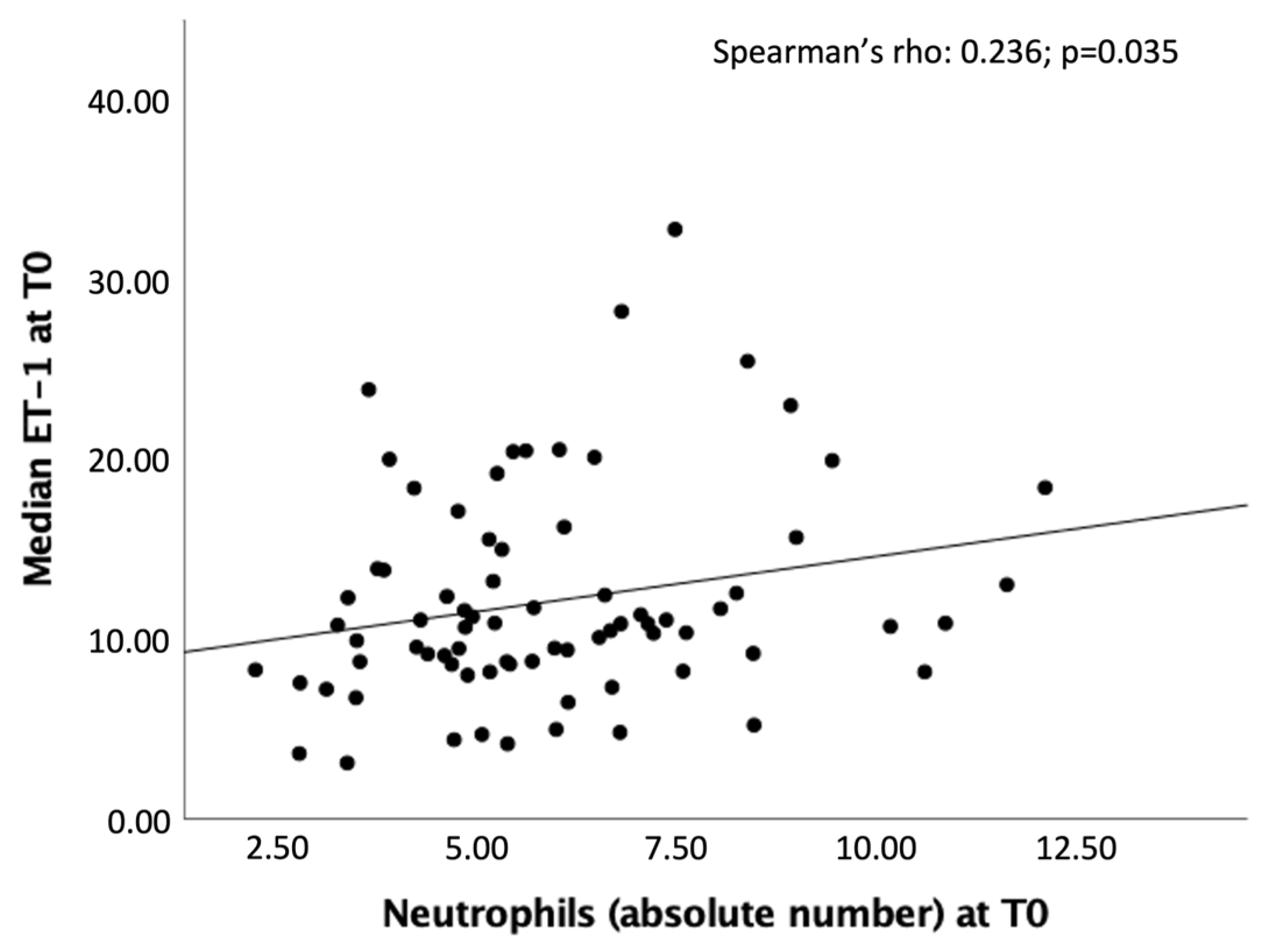
2.2. Biomarkers
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Laboratory Data
4.2.1. ET-1
4.2.2. NO
4.3. Radiological Data
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vagal, A.; Aviv, R.; Sucharew, H.; Reddy, M.; Hou, Q.; Michel, P.; Jovin, T.; Tomsick, T.; Wintermark, M.; Khatri, P. Collateral Clock Is More Important Than Time Clock for Tissue Fate. Stroke 2018, 49, 2102–2107. [Google Scholar] [CrossRef]
- De Michele, M.; Lorenzano, S.; Bertuccini, L.; Iosi, F.; Toni, D. “Time lost is clot resolution lost”: The neglected perspective of the therapeutic time window for ischemic stroke. Front. Neurol. 2023, 14, 1177609. [Google Scholar] [CrossRef]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Albers, G.W.; Marks, M.P.; Kemp, S.; Christensen, S.; Tsai, J.P.; Ortega-Gutierrez, S.; McTaggart, R.A.; Torbey, M.T.; Kim-Tenser, M.; Leslie-Mazwi, T.; et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018, 378, 708–718. [Google Scholar] [CrossRef]
- Rocha, M.; Jovin, T.G. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: Clinical and research implications. Stroke 2017, 48, 2621–2627. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Jin, F.; Li, S.; Ren, C.; Ruchi, M.; Ding, Y.; Zhao, W.; Ji, X. No-reflow after stroke reperfusion therapy: An emerging phenomenon to be explored. CNS Neurosci. Ther. 2024, 30, e14631. [Google Scholar] [CrossRef]
- Liebeskind, D.S. Collateral circulation. Stroke 2003, 34, 2279–2284. [Google Scholar] [CrossRef]
- Kofler, N.; Corti, F.; Rivera-Molina, F.; Deng, Y.; Toomre, D.; Simons, M. The Rab-effector protein RABEP2 regulates endosomal trafficking to mediate vascular endothelial growth factor receptor-2 (VEGFR2)-dependent signaling. J. Biol. Chem. 2018, 293, 4805–4817. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, N.K.; Pauls, J.; Augath, M.; Trinath, T.; Oeltermann, A. Neurophysiological investigation of the basis of the fMRI signal. Nature 2001, 412, 150–157. [Google Scholar] [CrossRef]
- Morita, Y.; Fukuuchi, Y.; Koto, A.; Suzuki, N.; Isozumi, K.; Gotoh, J.; Shimizu, T.; Takao, M.; Aoyama, M. Rapid Changes in Pial Arterial Diameter and Cerebral Blood Flow Caused by Ipsilateral Carotid Artery Occlusion in Rats. Keio J. Med. 1997, 46, 120–127. [Google Scholar] [CrossRef]
- Maguida, G.; Shuaib, A. Collateral Circulation in Ischemic Stroke: An Updated Review. J. Stroke 2023, 25, 179–198. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, G.; Chen, Z.-Q.; Mou, R.-T.; Feng, D.-X. The role of nitric oxide in stroke. Med. Gas. Res. 2017, 7, 194–203. [Google Scholar] [CrossRef]
- Iadecola, C.; Pelligrino, D.A.; Moskowitz, M.A.; Lassen, N.A. Nitric oxide synthase inhibition and cerebrovascular regulation. J. Cereb. Blood Flow Metab. 1994, 14, 175–192. [Google Scholar] [CrossRef]
- Atochin, D.N.; Yuzawa, I.; Li, Q.; Rauwerdink, K.M.; Malhotra, R.; Chang, J.; Brouckaert, P.; Ayata, C.; Moskowitz, M.A.; Bloch, K.D.; et al. Soluble Guanylate Cyclase α1β1 Limits Stroke Size and Attenuates Neurological Injury. Stroke 2010, 41, 1815–1819. [Google Scholar] [CrossRef]
- Ohkita, M.; Takaoka, M.; Shiota, Y.; Nojiri, R.; Matsumura, Y. Nitric oxide inhibits endothelin-1 production through the suppression of nuclear factor kB. Clin. Sci. 2002, 103, 68–71. [Google Scholar] [CrossRef]
- Kuruppu, S.; Rajapakse, N.W.; Dunstan, R.A.; Smith, A.I. Nitric oxide inhibits the production of soluble endothelin con-verting enzyme-1. Mol. Cell. Biochem. 2014, 396, 49–54. [Google Scholar] [CrossRef]
- Wiley, K.E.; Davenport, A.P. Novel nitric oxide donors reverse endothelin-1-mediated constriction in human blood vessels. J. Cardiovasc. Pharmacol. 2000, 36, S151–S152. [Google Scholar] [CrossRef]
- Wiley, K.E.; Davenport, A.P. Physiological antagonism of endothelin-1 in human conductance and resistance coronary artery. Br. J. Pharmacol. 2001, 133, 568–574. [Google Scholar] [CrossRef]
- Iadecola, C. The Neurovascular Unit Coming of Age: A Journey through Neurovascular Coupling in Health and Disease. Neuron 2017, 96, 17–42. [Google Scholar] [CrossRef]
- Džoljić, E.; Grbatinić, I.; Kostić, V. Why is nitric oxide important for our brain? Funct. Neurol. 2015, 30, 159–163. [Google Scholar]
- Shah, R. Endothelins in health and disease. Eur. J. Intern. Med. 2007, 18, 272–282. [Google Scholar] [CrossRef]
- Franceschini, R.; Gandolfo, C.; Cataldi, A.; Del Sette, M.; Rolandi, A.; Corsini, G.; Rolandi, E.; Barreca, T. Twenty-four-hour endo-thelin-1 secretory pattern in stroke patients. Biomed. Pharmacother. 2001, 55, 272–276. [Google Scholar]
- Farré, A.L.; Riesco, A.; Espinosa, G.; Digiuni, E.; Cernadas, M.R.; Alvarez, V.; Montón, M.; Rivas, F.; Gallego, M.J.; Egido, J. Effect of endothelin-1 on neutrophil adhesion to endothelial cells and perfused heart. Circulation 1993, 88, 1166–1171. [Google Scholar] [CrossRef]
- Hofman, F.M.; Chen, P.; Jeyaseelan, R.; Incardona, F.; Fisher, M.; Zidovetzki, R. Endothelin-1 induces production of the neutrophil chemotactic factor interleukin-8 by human brain-derived endothelial cells. Blood 1998, 92, 3064–3072. [Google Scholar] [CrossRef]
- Koehl, B.; Nivoit, P.; El Nemer, W.; Lenoir, O.; Hermand, P.; Pereira, C.; Brousse, V.; Guyonnet, L.; Ghinatti, G.; Benkerrou, M.; et al. The endothelin B receptor plays a crucial role in the adhesion of neutrophils to the endothelium in sickle cell disease. Haematologica 2017, 102, 1161–1172. [Google Scholar] [CrossRef]
- Ziv, I.; Fleminger, G.; Djaldetti, R.; Achiron, A.; Melamed, E.; Sokolovsky, M. Increased plasma endothelin-1 in acute ischemic stroke. Stroke 1992, 23, 1014–1016. [Google Scholar] [CrossRef]
- Estrada, V.; Téllez, M.J.; Moya, J.; Fernández-Durango, R.; Egido, J.; Cruz, A.F. High Plasma Levels of Endothelin-1 and Atrial Natriuretic Peptide in Patients with Acute Ischemic Stroke. Am. J. Hypertens. 1994, 7, 1085–1089. [Google Scholar] [CrossRef]
- Suzuki, H.; Sato, S.; Suzuki, Y.; Takekoshi, K.; Ishihara, N.; Shimoda, S. Increased endothelin concentration in CSF from patients with subarachnoid hemorrhage. Acta Neurol. Scand. 1990, 81, 553–554. [Google Scholar] [CrossRef]
- Lampl, Y.; Fleminger, G.; Gilad, R.; Galron, R.; Sarova-Pinhas, I.; Sokolovsky, M. Endothelin in cerebrospinal fluid and plasma of patients in the early stage of ischemic stroke. Stroke 1997, 28, 1951–1955. [Google Scholar] [CrossRef]
- Hamann, G.; Isenberg, E.; Strittmatter, M.; Moili, R.; Schimrigk, K. Big-endothelin in acute ischemic stroke. J. Stroke Cerebrovasc. Dis. 1993, 3, 256–260. [Google Scholar] [CrossRef]
- Haapaniemi, E.; Tatlisumak, T.; Hamel, K.; Soinne, L.; Lanni, C.; Opgenorth, T.J.; Kaste, M. Plasma Endothelin-1 Levels Neither Increase nor Correlate with Neurological Scores, Stroke Risk Factors, or Outcome in Patients with Ischemic Stroke. Stroke 2000, 31, 720–725. [Google Scholar] [CrossRef]
- Adams, H.P., Jr.; Bendixen, B.H.; Kappelle, L.J.; Biller, J.; Love, B.B.; Gordon, D.L.; Marsh, E.E., 3rd. Classification of Subtype of Acute Ischemic Stroke. Definitions for Use in a Multicenter Clinical Trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993, 24, 35–41. [Google Scholar] [CrossRef]
- Lauer, T.; Preik, M.; Rassaf, T.; Strauer, B.E.; Deussen, A.; Feelisch, M.; Kelm, M. Plasma nitrite rather than nitrate reflects regional endothelial nitric oxide synthase activity but lacks intrinsic vasodilator action. Proc. Natl. Acad. Sci. USA 2001, 98, 12814–12819. [Google Scholar] [CrossRef]
- Menon, B.; Smith, E.; Modi, J.; Patel, S.; Bhatia, R.; Watson, T.; Hill, M.D.; Demchuk, A.; Goyal, M. Regional Leptomeningeal Score on CT Angiography Predicts Clinical and Imaging Outcomes in Patients with Acute Anterior Circulation Occlusions. Am. J. Neuroradiol. 2011, 32, 1640–1645. [Google Scholar] [CrossRef]
- Aviv, R.; Mandelcorn, J.; Chakraborty, S.; Gladstone, D.; Malham, S.; Tomlinson, G.; Fox, A.; Symons, S. Alberta Stroke Program Early CT Scoring of CT Perfusion in Early Stroke Visualization and Assessment. Am. J. Neuroradiol. 2007, 28, 1975–1980. [Google Scholar] [CrossRef]
- Zaidat, O.O.; Yoo, A.J.; Khatri, P.; Tomsick, T.A.; von Kummer, R.; Saver, J.L.; Marks, M.P.; Prabhakaran, S.; Kallmes, D.F.; Fitzsimmons, B.F.; et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: A consensus statement. Stroke 2013, 44, 2650–2663. [Google Scholar] [CrossRef]
- Sims, J.R.; Gharai, L.R.; Schaefer, P.W.; Vangel, M.; Rosenthal, E.S.; Lev, M.H.; Schwamm, L. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology 2009, 72, 2104–2110. [Google Scholar] [CrossRef]

| All Patients n = 105 | Good n = 44 | Moderate n = 36 | Poor n = 22 | p-Value | |
|---|---|---|---|---|---|
| Demographics and Clinical Characteristics | |||||
| Age (years), mean (SD) | 76.0 (12.8) | 75.0 (11.1) | 77.1 (14.9) | 77.8 (10.1) | 0.604 |
| Sex (females) (%) | 66 (62.9) | 32 (72.7) | 20 (55.6) | 13 (59.1) | 0.248 |
| Pre-stroke mRS (%) | |||||
| 77 (73.3) | 38 (86.4) | 23 (63.9) | 14 (63.6) | 0.132 |
| 8 (7.6) | 2 (4.5) | 3 (8.3) | 3 (13.6) | |
| 6 (5.7) | 3 (6.8) | 3 (8.3) | 0 | |
| 11 (10.5) | 1 (2.3) | 6 (16.7) | 3 (13.6) | |
| 2 (1.9) | 0 | 1 (2.8) | 1 (4.5) | |
| 1 (1.0) | 0 | 0 | 1 (4.5) | |
| Pre-stroke mRS 0–1 (%) | 85 (81) | 40 (90.9) | 26 (72.2) | 17 (77.3) | 0.087 |
| Obesity (%) | 16/100 (16.0) | 8/42 (19.0) | 3/34 (8.8) | 5/21 (23.8) | 0.291 |
| Smoking (%) | 16/99 (16.2) | 8/43 (18.6) | 3/33 (9.1) | 5/20 (25.0) | 0.289 |
| Alcohol consumption (%) | 6/104 (5.8) | 1 (2.3) | 4/35 (11.4) | 1 (4.5) | 0.221 |
| Drug abuse (%) | 3/104 (2.9) | 0 | 2/35 (5.7) | 1 (4.5) | 0.294 |
| Hypertension (%) | 84/103 (81.6) | 33/43 (76.7) | 30/35 (85.7) | 19 (86.4) | 0.493 |
| Hyperlipidemia (%) | 56/100 (56.0) | 29/43 (67.4) | 17/34 (50.0) | 8/20 (40.0) | 0.089 |
| Atrial fibrillation (%) | 63/102 (61.8) | 25/43 (58.1) | 20/35 (57.1) | 18/21 (85.7) | 0.060 |
| Ischemic cardiopathy (%) | 32/104 (30.8) | 10 (22.7) | 11/35 (31.4) | 9 (40.9) | 0.301 |
| Diabetes mellitus (%) | 25/104 (24.0) | 8 (18.2) | 11/35 (31.4) | 6 (27.3) | 0.381 |
| Previous stroke (%) | 18/102 (97.1) | 5 (11.4) | 8/34 (23.5) | 4/21 (19.0) | 0.357 |
| Previous TIA (%) | 8/103 (7.8) | 2 (4.5) | 5/35 (14.3) | 1/21 (4.8) | 0.236 |
| Previous CEA (%) | 4/104 (3.8) | 1 (2.3) | 3/35 (8.6) | 0 | 0.203 |
| Carotid artery stenosis < 50% | 35/103 (34.0) | 15/43 (34.9) | 13/35 (37.1) | 7 (31.8) | 0.919 |
| Carotid artery stenosis (50–70%) | 7/103 (6.8) | 4/43 (9.3) | 3/35 (8.6) | 0 | 0.343 |
| Carotid artery stenosis > 70% | 15/104 (14.4) | 4/43 (9.3) | 5/35 (14.3) | 6 (27.3) | 0.146 |
| Stroke Characteristics | |||||
| Stroke onset on | 30/104 (28.8) | 14 (31.8) | 12/35 (34.3) | 3 (13.6) | 0.204 |
| |||||
| 22/105 (21.0) | 10/44 (22.7) | 9 (25.0) | 2 (9.1) | 0.312 |
| NIHSS, median (IQR) | |||||
| 15 (10–20) | 14 (10–20) | 15 (9–17) | 18 (14–22.50) | 0.035 |
| 10 (4–17) | 8 (2–14) | 10.50 (5.50–20) | 14 (10–17.25) | 0.090 |
| 9.50 (3–17) | 6 (2–16) | 10.50 (4–18) | 13.9 (7.8) | 0.095 |
| 5 (2–9) | 4 (1–9) | 5 (2–11) | 5 (4.50–14.25) | 0.281 |
| ASPECTS | |||||
| 8.22 (1.32) | 8.48 (1.19) | 8.24 (1.23) | 7.86 (1.21) | 0.155 |
| 8 (7–9) | 9 (8–9) | 8 (7.75–9) | 8 (7–8.25) | |
| Vessel occlusion site (%) | |||||
| 57/97 (58.8) | 24/41 (58.5) | 17/34 (50.0) | 13/19 (68.4) | |
| 29/97 (29.9) | 12/41 (29.3) | 14/34 (41.2) | 3/19 (15.8) | |
| 2/97 (2.1) | 0 | 1/34 (2.9) | 1/19 (5.3) | |
| 9/97 (9.3) | 5/41 (12.2) | 2/34 (5.9) | 2/19 (19.5) | 0.424 |
| Collateral status | <0.001 | ||||
| 14.65 (4.18) | 18.45 (1.0) | 13.72 (1.73) | 8.50 (2.33) | |
| 15.50 (11–18) | 18 (18–19) | 14 (12–15) | 8.50 (6.75–10) | |
| Recanalization treatment (%) | |||||
| 53 (50.5) | 20 (45.5) | 20 (55.6) | 12 (54.5) | 0.622 |
| 90/104 (85.3) | 38 (86.4) | 32/35 (91.4) | 18 (81.8) | 0.562 |
| 42 (41.2) | 15 (34.1) | 18 (50.0) | 9 (40.9) | 0.355 |
| MT technique | |||||
| 41/80 (51.2) | 17/36 (47.2) | 14/28 (50.0) | 10/15 (66.7) | 0.202 |
| 11/80 (13.8) | 3/36 (8.3) | 7/28 (25.0) | 1/15 (6.7) | |
| 23/80 (28.7) | 12/36 (33.3) | 7/28 (25.0) | 3/15 (20.0) | |
| |||||
| 5/80 (6.3) | 4736 (11.1) | 0 | 1/15 (6.7) | |
| Onset to IVT time (min), | 150.0 | 150.0 | 151.0 | 135.0 | |
| Median (IQR) | (120–190) | (131.25–205.0) | (110–190) | (100.0–177.0) | 0.635 |
| Onset to MT time (min), | 265.0 | 265.0 | 286.0 | 208.50 | 0.300 |
| Median (IQR) | (197.50–350) | (213–347.50) | (194.50–438.50) | (177.0–332.0) | |
| TICI (%) | |||||
| 7/83 (8.4) | 1/36 (2.8) | 3/30 (10.0) | 3/16 (18.8) | 0.090 |
| 5/83 (6.0) | 0 | 4/30 (13.3) | 0 | |
| 11/83 (13.3) | 5/36 (13.9) | 5/30 (16.7) | 1/16 (6.3) | |
| 17/83 (20.5) | 10/36 (27.8) | 5/30 (16.7) | 2/16 (12.5) | |
| 43/83 (51.8) | 20/36 (55.6) | 13/30 (43.3) | 10/16 (62.5) | |
| TICI 2b–3 (%) | 60/83 (72.3) | 30/36 (83.3) | 18/30 (60.0) | 12/16 (75.0) | 0.102 |
| Imaging for V measurement: | |||||
| 85/103 (82.5) | 36 (81.8) | 31/35 (88.6) | 17/21 (81.0) | |
| 18/103 (17.5) | 8 (18.2) | 4/35 (11.4) | 4/21 (19.0) | 0.655 |
| Infarct volume, median | 9.70 | 5.90 | 13.35 | 14.60 | 0.482 |
| (IQR) | (3.60–20.50) | (2.80–12.25) | (5.08–20.50) | (8.85–41.80) | |
| Stroke etiopathogenesis (%) | |||||
| 10/99 (10.1) | 5/41 (12.2) | 1/34 (2.9) | 4/21 (19.0) | 0.134 |
| 68/99 (68.7) | 25/41 (61.0) | 25/34 (73.5) | 17/21 (81.0) | |
| 7/99 (14.1) | 4/41 (9.8) | 3/34 (8.8) | 0 | |
| 14/99 (14.1) | 7/41 (17.1) | 5/34 (14.7) | 0 | |
| mRS 0–2 (n = 39) | mRS 3–6 (n = 61) | p-Value | |
|---|---|---|---|
| ET-1 at T1 (pg/mL), median (IQR) | 12.44 (10.61–16.58) | 16.95 (11.81–23.44) | 0.017 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iacobucci, M.; Risitano, A.; Amisano, P.; Berto, I.; Carnevale, R.; Cammisotto, V.; Biraschi, F.; Cirelli, C.; Di Mascio, M.T.; Toni, D.; et al. Role of Endothelin-1 and Nitric Oxide in Acute Ischemic Stroke Leptomeningeal Collateral Activation. Int. J. Mol. Sci. 2025, 26, 3205. https://doi.org/10.3390/ijms26073205
Iacobucci M, Risitano A, Amisano P, Berto I, Carnevale R, Cammisotto V, Biraschi F, Cirelli C, Di Mascio MT, Toni D, et al. Role of Endothelin-1 and Nitric Oxide in Acute Ischemic Stroke Leptomeningeal Collateral Activation. International Journal of Molecular Sciences. 2025; 26(7):3205. https://doi.org/10.3390/ijms26073205
Chicago/Turabian StyleIacobucci, Marta, Angela Risitano, Paolo Amisano, Irene Berto, Roberto Carnevale, Vittoria Cammisotto, Francesco Biraschi, Carlo Cirelli, Maria Teresa Di Mascio, Danilo Toni, and et al. 2025. "Role of Endothelin-1 and Nitric Oxide in Acute Ischemic Stroke Leptomeningeal Collateral Activation" International Journal of Molecular Sciences 26, no. 7: 3205. https://doi.org/10.3390/ijms26073205
APA StyleIacobucci, M., Risitano, A., Amisano, P., Berto, I., Carnevale, R., Cammisotto, V., Biraschi, F., Cirelli, C., Di Mascio, M. T., Toni, D., Lorenzano, S., & De Michele, M. (2025). Role of Endothelin-1 and Nitric Oxide in Acute Ischemic Stroke Leptomeningeal Collateral Activation. International Journal of Molecular Sciences, 26(7), 3205. https://doi.org/10.3390/ijms26073205







