The Role of α7-Nicotinic Acetylcholine Receptors in the Pathophysiology and Treatment of Parkinson’s Disease
Abstract
1. Introduction
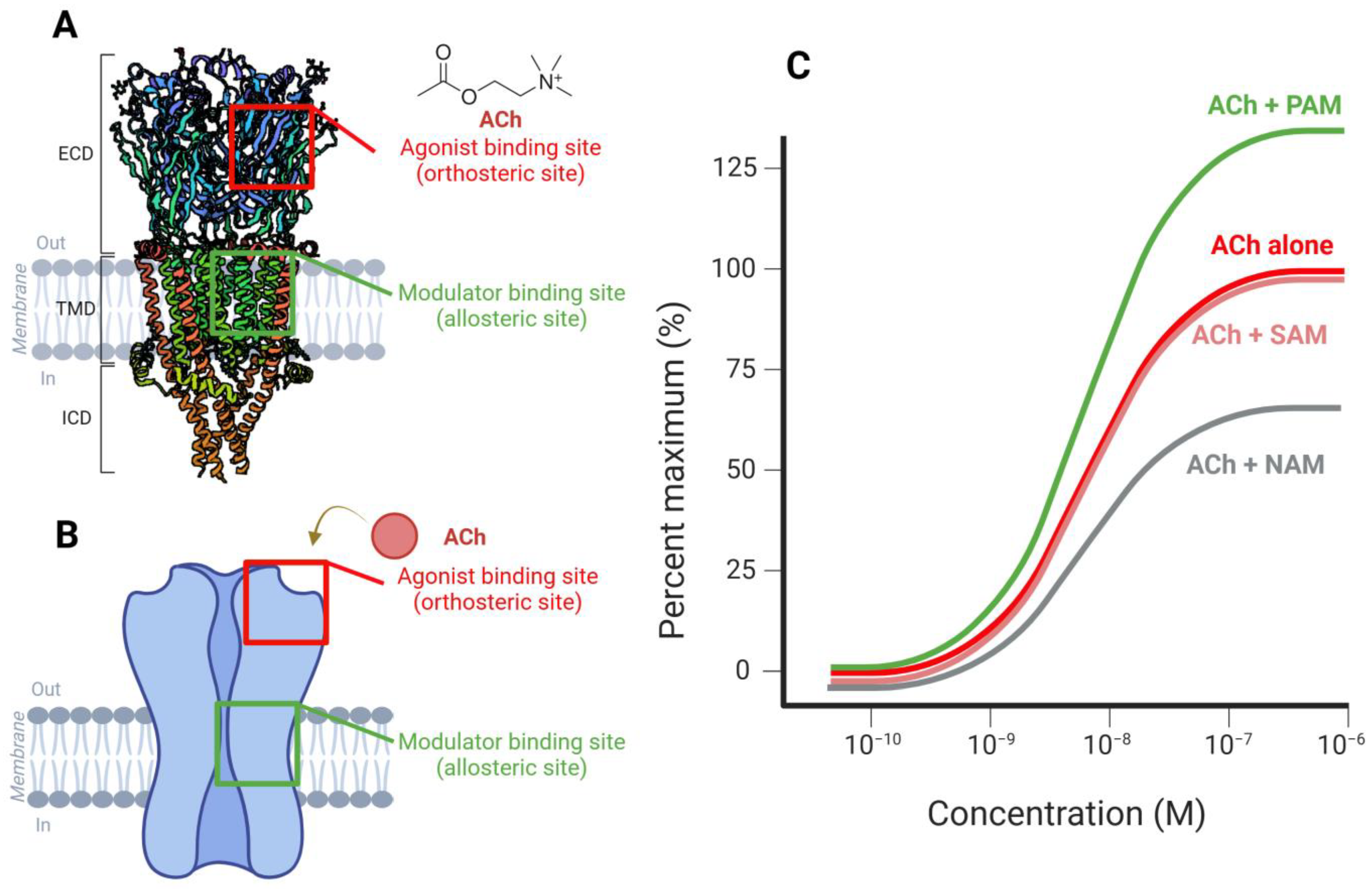
2. Structure of α7-nAChRs
Genetic Variants and Functional Implications
3. Parkinson’s Disease: Pathophysiology and Role of α7-nAChRs
3.1. The Role of Nicotine and α7-Nicotinic Receptors in Neuroprotection
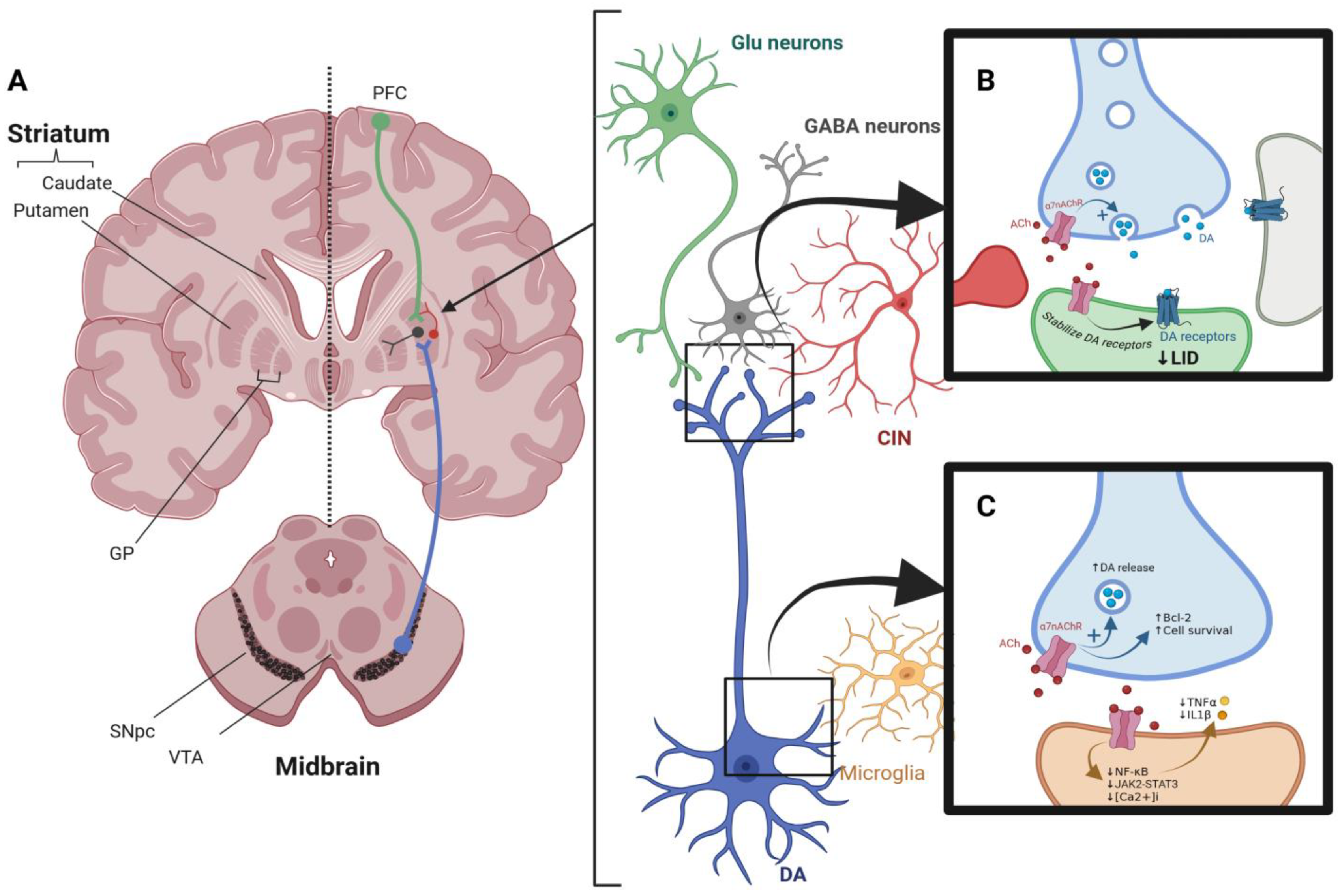
3.2. Role of α7-nAChRs in PD
3.2.1. Dopaminergic and Cholinergic Systems Correlation and Dopamine Release
3.2.2. Immune Modulation via Nicotinic Receptors
3.2.3. Effect of Nicotinic Receptors on L-Dopa-Induced Dyskinesia
3.3. Receptor Cross-Talk and Systems-Level Integration
4. Preclinical Evidence
5. Current Clinical Therapeutic Status
6. Challenges and Future Directions
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACh | Acetylcholine |
| α7 nAChR | α7 nicotinic acetylcholine receptor |
| 6-OHDA | 6-hydroxydopamine |
| LID | L-dopa-induced dyskinesia |
| mAChRs | muscarinic acetylcholine receptors |
| MLA | methyllycaconitine |
| NAMs | Negative allosteric modulators |
| nAChRs | nicotinic acetylcholine receptors |
| PAMs | Positive allosteric modulators |
| PD | Parkinson’s disease |
| SAMs | Silent allosteric modulators |
References
- Nagori, K.; Pradhan, M.; Sharma, M.; Ajazuddin; Badwaik, H.R.; Nakhate, K.T. Current Progress on Central Cholinergic Receptors as Therapeutic Targets for Alzheimer’s Disease. Curr. Alzheimer Res. 2024, 21, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Hung, S.-Y. Physiologic Functions and Therapeutic Applications of A7 Nicotinic Acetylcholine Receptor in Brain Disorders. Pharmaceutics 2022, 15, 31. [Google Scholar] [CrossRef] [PubMed]
- Bye, L.J.; Finol-Urdaneta, R.K.; Tae, H.-S.; Adams, D.J. Nicotinic Acetylcholine Receptors: Key Targets for Attenuating Neurodegenerative Diseases. Int. J. Biochem. Cell Biol. 2023, 157, 106387. [Google Scholar] [CrossRef]
- Jakubík, J.; Randáková, A.; El-Fakahany, E.E.; Doležal, V. Analysis of Equilibrium Binding of an Orthosteric Tracer and Two Allosteric Modulators. PLoS ONE 2019, 14, e0214255. [Google Scholar] [CrossRef]
- Oz, M.; Kury, L.A.; Sadek, B.; Mahgoub, M.O. The Role of Nicotinic Acetylcholine Receptors in the Pathophysiology and Pharmacotherapy of Autism Spectrum Disorder: Focus on A7 Nicotinic Receptors. Int. J. Biochem. Cell Biol. 2024, 174, 106634. [Google Scholar] [CrossRef]
- Antonio-Tolentino, K.; Hopkins, C.R. Selective A7 Nicotinic Receptor Agonists and Positive Allosteric Modulators for the Treatment of Schizophrenia—A Review. Expert Opin. Investig. Drugs 2020, 29, 603–610. [Google Scholar] [CrossRef]
- Yang, T.; Xiao, T.; Sun, Q.; Wang, K. The Current Agonists and Positive Allosteric Modulators of α 7 nAChR for CNS Indications in Clinical Trials. Acta Pharm. Sin. B 2017, 7, 611–622. [Google Scholar] [CrossRef]
- Manetti, D.; Dei, S.; Arias, H.R.; Braconi, L.; Gabellini, A.; Teodori, E.; Romanelli, M.N. Recent Advances in the Discovery of Nicotinic Acetylcholine Receptor Allosteric Modulators. Molecules 2023, 28, 1270. [Google Scholar] [CrossRef]
- Ihnatovych, I.; Saddler, R.-A.; Sule, N.; Szigeti, K. Translational Implications of CHRFAM7A, an Elusive Human-Restricted Fusion Gene. Mol. Psychiatry 2024, 29, 1020–1032. [Google Scholar] [CrossRef]
- Andersen, N.D.; Nielsen, B.E.; Corradi, J.; Tolosa, M.F.; Feuerbach, D.; Arias, H.R.; Bouzat, C. Exploring the Positive Allosteric Modulation of Human A7 Nicotinic Receptors from a Single-Channel Perspective. Neuropharmacology 2016, 107, 189–200. [Google Scholar] [CrossRef]
- Yang, J.-S.; Seo, S.W.; Jang, S.; Jung, G.Y.; Kim, S. Rational Engineering of Enzyme Allosteric Regulation Through Sequence Evolution Analysis. PLoS Comput. Biol. 2012, 8, e1002612. [Google Scholar] [CrossRef]
- Kalappa, B.I.; Sun, F.; Johnson, S.R.; Jin, K.; Uteshev, V.V. A Positive Allosteric Modulator of A7 nAChRs Augments Neuroprotective Effects of Endogenous Nicotinic Agonists in Cerebral Ischaemia. Br. J. Pharmacol. 2013, 169, 1862–1878. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Jin, K.; Uteshev, V.V. A Type-II Positive Allosteric Modulator of A7 nAChRs Reduces Brain Injury and Improves Neurological Function After Focal Cerebral Ischemia in Rats. PLoS ONE 2013, 8, e73581. [Google Scholar] [CrossRef]
- Uteshev, V.V. The Therapeutic Promise of Positive Allosteric Modulation of Nicotinic Receptors. Eur. J. Pharmacol. 2014, 727, 181–185. [Google Scholar] [CrossRef]
- Christensen, D.Z.; Mikkelsen, J.D.; Hansen, H.H.; Thomsen, M.S. Repeated administration of α7 nicotinic acetylcholine receptor (nAChR) agonists, but not positive allosteric modulators, in-creases α7 nAChR levels in the brain. J. Neurochem. 2010, 114, 1205–1216. [Google Scholar] [CrossRef]
- Gowayed, M.A.; El-Sayed, N.S.; Matar, N.A.; Afify, E.A.; El-Ganainy, S.O. The A7 nAChR Allosteric Modulator PNU-120596 Amends Neuroinflammatory and Motor Consequences of Parkinsonism in Rats: Role of JAK2/NF-κB/GSk3β/TNF-α Pathway. Biomed. Pharmacother. Biomed. Pharmacother. 2022, 148, 112776. [Google Scholar] [CrossRef]
- Kalkman, H.O.; Feuerbach, D. Modulatory Effects of A7 nAChRs on the Immune System and Its Relevance for CNS Disorders. Cell. Mol. Life Sci. CMLS 2016, 73, 2511–2530. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Li, W.; Benedetti, N.J.; Lee, D.H.S. Alpha 7 Nicotinic Acetylcholine Receptors Mediate Beta-Amyloid Peptide-Induced Tau Protein Phosphorylation. J. Biol. Chem. 2003, 278, 31547–31553. [Google Scholar] [CrossRef]
- Quik, M.; Zhang, D.; McGregor, M.; Bordia, T. Alpha7 Nicotinic Receptors as Therapeutic Targets for Parkinson’s Disease. Biochem. Pharmacol. 2015, 97, 399–407. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E.; Yang, K.-H.S.; Petroianu, G. On the Interaction of β-Amyloid Peptides and A7-Nicotinic Acetylcholine Receptors in Alzheimer’s Disease. Curr. Alzheimer Res. 2013, 10, 618–630. [Google Scholar] [CrossRef]
- Posadas, I.; Lopez-Hernandez, B.; Cena, V. Nicotinic Receptors in Neurodegeneration. Curr. Neuropharmacol. 2013, 11, 298–314. [Google Scholar] [CrossRef]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and Gating Mechanism of the A7 Nicotinic Acetylcholine Receptor. Cell 2021, 184, 2121–2134.e13. [Google Scholar] [CrossRef] [PubMed]
- Toulorge, D.; Guerreiro, S.; Hild, A.; Maskos, U.; Hirsch, E.C.; Michel, P.P. Neuroprotection of Midbrain Dopamine Neurons by Nicotine Is Gated by Cytoplasmic Ca2+. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2011, 25, 2563–2573. [Google Scholar] [CrossRef]
- Millar, N.S.; Gotti, C. Diversity of Vertebrate Nicotinic Acetylcholine Receptors. Neuropharmacology 2009, 56, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.A.; Frolund, B.; Liljefors, T.; Krogsgaard-Larsen, P. Neuronal Nicotinic Acetylcholine Receptors: Structural Revelations, Target Identifications, and Therapeutic Inspirations. J Med Chem 2005, 48, 4705–4745. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Picciotto, M.R. Genetics of Nicotinic Acetylcholine Receptors: Relevance to Nicotine Addiction. Biochem. Pharmacol. 2008, 75, 323–333. [Google Scholar] [CrossRef]
- Vetel, S.; Vercouillie, J.; Buron, F.; Vergote, J.; Tauber, C.; Busson, J.; Chicheri, G.; Routier, S.; Sérrière, S.; Chalon, S. Longitudinal PET Imaging of A7 Nicotinic Acetylcholine Receptors with [18F]ASEM in a Rat Model of Parkinson’s Disease. Mol. Imaging Biol. 2020, 22, 348–357. [Google Scholar] [CrossRef]
- Echeverria, V.; Yarkov, A.; Aliev, G. Positive Modulators of the A7 Nicotinic Receptor Against Neuroinflammation and Cognitive Impairment in Alzheimer’s Disease. Prog. Neurobiol. 2016, 144, 142–157. [Google Scholar] [CrossRef]
- Zoli, M.; Léna, C.; Picciotto, M.R.; Changeux, J.-P. Identification of Four Classes of Brain Nicotinic Receptors Using Β2 Mutant Mice. J. Neurosci. 1998, 18, 4461–4472. [Google Scholar] [CrossRef]
- Letsinger, A.C.; Gu, Z.; Yakel, J.L. A7 Nicotinic Acetylcholine Receptors in the Hippocampal Circuit: Taming Complexity. Trends Neurosci. 2022, 45, 145–157. [Google Scholar] [CrossRef]
- Pan, Y.; Zhu, J.; Cong, L.; Bai, Y.; Ma, Y.; Yang, Y. Expression of nAChRα7 Receptor in Model Rats with Parkinson’s Disease Dementia. Biotechnol. Biotechnol. Equip. 2021, 35, 117–123. [Google Scholar] [CrossRef]
- Banerjee, C.; Nyengaard, J.R.; Wevers, A.; de Vos, R.A.; Jansen Steur, E.N.; Lindstrom, J.; Pilz, K.; Nowacki, S.; Bloch, W.; Schröder, H. Cellular Expression of Alpha7 Nicotinic Acetylcholine Receptor Protein in the Temporal Cortex in Alzheimer’s and Parkinson’s Disease—A Stereological Approach. Neurobiol. Dis. 2000, 7, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Matsuura, Y.; Hosoda, R.; Saji, H. In Vivo Imaging of Nicotinic Acetylcholine Receptors in the Central Nervous System. In Nicotinic Acetylcholine Receptor Signaling in Neuroprotection; Akaike, A., Shimohama, S., Misu, Y., Eds.; Springer: Singapore, 2018; ISBN 978-981-10-8487-4. [Google Scholar]
- Blanco-Lezcano, L.; Alberti-Amador, E.; González-Fraguela, M.E.; de Larrea, G.Z.-L.; Pérez-Serrano, R.M.; Jiménez-Luna, N.A.; Serrano-Sánchez, T.; Francis-Turner, L.; Camejo-Rodriguez, D.; Vega-Hurtado, Y. Nurr1, Pitx3, and A7 nAChRs mRNA Expression in Nigral Tissue of Rats with Pedunculopontine Neurotoxic Lesion. Med. Kaunas Lith. 2019, 55, 616. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Du, Y.; Rosenthal, H.B.; Slania, S.; Min Koo, S.; Park, A.; Solomon, G.; Vranesic, M.; Antonsdottir, I.; Speck, C.L.; et al. The Distribution of the Alpha7 Nicotinic Acetylcholine Receptor in Healthy Aging: An in Vivo Positron Emission Tomography Study with [18F]ASEM. NeuroImage 2018, 165, 118–124. [Google Scholar] [CrossRef]
- Coughlin, J.M.; Rubin, L.H.; Du, Y.; Rowe, S.P.; Crawford, J.L.; Rosenthal, H.B.; Frey, S.M.; Marshall, E.S.; Shinehouse, L.K.; Chen, A.; et al. High Availability of the A7-Nicotinic Acetylcholine Receptor in Brains of Individuals with Mild Cognitive Impairment: A Pilot Study Using 18F-ASEM PET. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2020, 61, 423–426. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Zhou, Y.; Zhang, M.; Chen, H.; Eric Xu, H.; Sun, D.; Liu, L.; Tian, C. Structural Basis of Human A7 Nicotinic Acetylcholine Receptor Activation. Cell Res. 2021, 31, 713–716. [Google Scholar] [CrossRef]
- Sinkus, M.L.; Graw, S.; Freedman, R.; Ross, R.G.; Lester, H.A.; Leonard, S. The Human CHRNA7 and CHRFAM7A Genes: A Review of the Genetics, Regulation, and Function. Neuropharmacology 2015, 96, 274–288. [Google Scholar] [CrossRef]
- Terry, A.V.; Jones, K.; Bertrand, D. Nicotinic Acetylcholine Receptors in Neurological and Psychiatric Diseases. Pharmacol. Res. 2023, 191, 106764. [Google Scholar] [CrossRef]
- Nakaizumi, K.; Ouchi, Y.; Terada, T.; Yoshikawa, E.; Kakimoto, A.; Isobe, T.; Bunai, T.; Yokokura, M.; Suzuki, K.; Magata, Y.; et al. In Vivo Depiction of A7 Nicotinic Receptor Loss for Cognitive Decline in Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 61, 1355–1365. [Google Scholar] [CrossRef]
- Isaias, I.U.; Spiegel, J.; Brumberg, J.; Cosgrove, K.P.; Marotta, G.; Oishi, N.; Higuchi, T.; Küsters, S.; Schiller, M.; Dillmann, U.; et al. Nicotinic Acetylcholine Receptor Density in Cognitively Intact Subjects at an Early Stage of Parkinson’s Disease. Front. Aging Neurosci. 2014, 6, 213. [Google Scholar] [CrossRef]
- Liu, C. Targeting the Cholinergic System in Parkinson’s Disease. Acta Pharmacol. Sin. 2020, 41, 453–463. [Google Scholar] [CrossRef]
- Recio-Barbero, M.; Segarra, R.; Zabala, A.; González-Fraile, E.; González-Pinto, A.; Ballesteros, J. Cognitive Enhancers in Schizophrenia: A Systematic Review and Meta-Analysis of Alpha-7 Nicotinic Acetylcholine Receptor Agonists for Cognitive Deficits and Negative Symptoms. Front. Psychiatry 2021, 12, 631589. [Google Scholar] [CrossRef] [PubMed]
- Kouli, A.; Torsney, K.M.; Kuan, W.-L. Parkinson’s Disease: Etiology, Neuropathology, and Pathogenesis. In Parkinson’s Disease: Pathogenesis and Clinical Aspects; Stoker, T.B., Greenland, J.C., Eds.; Codon Publications: Brisbane, Australia, 2018; ISBN 978-0-9944381-6-4. [Google Scholar]
- Orlando, I.F.; Shine, J.M.; Robbins, T.W.; Rowe, J.B.; O’Callaghan, C. Noradrenergic and Cholinergic Systems Take Centre Stage in Neuropsychiatric Diseases of Ageing. Neurosci. Biobehav. Rev. 2023, 149, 105167. [Google Scholar] [CrossRef]
- Pattanaik, B.; Hammarlund, M.; Mjörnstedt, F.; Ulleryd, M.A.; Zhong, W.; Uhlén, M.; Gummesson, A.; Bergström, G.; Johansson, M.E. Polymorphisms in Alpha 7 Nicotinic Acetylcholine Receptor Gene, CHRNA7, and Its Partially Duplicated Gene, CHRFAM7A, Associate with Increased Inflammatory Response in Human Peripheral Mononuclear Cells. FASEB J. 2022, 36, e22271. [Google Scholar] [CrossRef]
- Jiang, Y.; Cao, Z.; Ma, H.; Wang, G.; Wang, X.; Wang, Z.; Yang, Y.; Zhao, H.; Liu, G.; Li, L.; et al. Auricular Vagus Nerve Stimulation Exerts Antiinflammatory Effects and Immune Regulatory Function in a 6-OHDA Model of Parkinson’s Disease. Neurochem. Res. 2018, 43, 2155–2164. [Google Scholar] [CrossRef]
- Mhyre, T.R.; Boyd, J.T.; Hamill, R.W.; Maguire-Zeiss, K.A. Parkinson’s Disease. Subcell. Biochem. 2012, 65, 389–455. [Google Scholar] [CrossRef]
- Isaacson, S.H. Early Recognition and Diagnosis of Parkinson Disease and Ongoing Assessments. J. Clin. Psychiatry 2019, 81, MS18003BR1C. [Google Scholar] [CrossRef]
- Franco-Iborra, S.; Vila, M.; Perier, C. The Parkinson Disease Mitochondrial Hypothesis: Where Are We At? Neurosci. Rev. J. Bringing Neurobiol. Neurol. Psychiatry 2016, 22, 266–277. [Google Scholar] [CrossRef]
- Olanow, C.W.; McNaught, K. Parkinson’s Disease, Proteins, and Prions: Milestones. Mov. Disord. 2011, 26, 1056–1071. [Google Scholar] [CrossRef]
- Schapira, A.H. Neurobiology and Treatment of Parkinson’s Disease. Trends Pharmacol. Sci. 2009, 30, 41–47. [Google Scholar] [CrossRef]
- Brotchie, J.; Jenner, P. New Approaches to Therapy. Int. Rev. Neurobiol. 2011, 98, 123–150. [Google Scholar] [CrossRef]
- Huot, P.; Johnston, T.H.; Koprich, J.B.; Fox, S.H.; Brotchie, J.M. The Pharmacology of L-DOPA-Induced Dyskinesia in Parkinson’s Disease. Pharmacol. Rev. 2013, 65, 171–222. [Google Scholar] [CrossRef] [PubMed]
- Iravani, M.M.; McCreary, A.C.; Jenner, P. Striatal Plasticity in Parkinson’s Disease and L-Dopa Induced Dyskinesia. Park. Relat. Disord. 2012, 18 (Suppl. S1), S123–S125. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Srivanitchapoom, P. Levodopa-Induced Dyskinesia: Clinical Features, Pathophysiology, and Medical Management. Ann. Indian Acad. Neurol. 2017, 20, 190. [Google Scholar] [CrossRef] [PubMed]
- Allam, M.F.; Campbell, M.J.; Hofman, A.; Del Castillo, A.S.; Fernandez-Crehuet Navajas, R. Smoking and Parkinson’s Disease: Systematic Review of Prospective Studies. Mov. Disord. 2004, 19, 614–621. [Google Scholar] [CrossRef]
- Elbaz, A.; Moisan, F. Update in the Epidemiology of Parkinson’s Disease. Curr. Opin. Neurol. 2008, 21, 454–460. [Google Scholar] [CrossRef]
- Gorell, J.M.; Rybicki, B.A.; Johnson, C.C.; Peterson, E.L. Smoking and Parkinson’s Disease: A Dose-Response Relationship. Neurology 1999, 52, 115–119. [Google Scholar]
- Noyce, A.J.; Bestwick, J.P.; Silveira-Moriyama, L.; Hawkes, C.H.; Giovannoni, G.; Lees, A.J.; Schrag, A. Meta-Analysis of Early Nonmotor Features and Risk Factors for Parkinson Disease. Ann. Neurol. 2012, 72, 893–901. [Google Scholar] [CrossRef]
- Tanner, C.M. Advances in Environmental Epidemiology. Mov. Disord. 2010, 25 (Suppl. S1), S58–S62. [Google Scholar] [CrossRef]
- Wirdefeldt, K.; Adami, H.O.; Cole, P.; Trichopoulos, D.; Mandel, J. Epidemiology and Etiology of Parkinson’s Disease: A Review of the Evidence. Eur. J. Epidemiol. 2011, 26 (Suppl. S1), S1–S58. [Google Scholar] [CrossRef]
- Xu, Z.-Q.; Zhang, W.-J.; Su, D.-F.; Zhang, G.-Q.; Miao, C.-Y. Cellular Responses and Functions of A7 Nicotinic Acetylcholine Receptor Activation in the Brain: A Narrative Review. Ann. Transl. Med. 2021, 9, 509. [Google Scholar] [CrossRef]
- Mizrachi, T.; Vaknin-Dembinsky, A.; Brenner, T.; Treinin, M. Neuroinflammation Modulation via A7 Nicotinic Acetylcholine Receptor and Its Chaperone, RIC-3. Molecules 2021, 26, 6139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Yakel, J.L. The Effect of A7 Nicotinic Receptor Activation on Glutamatergic Transmission in the Hippocampus. Biochem. Pharmacol. 2015, 97, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Pifl, C.; Wolf, A.; Elevado, M.; Scholze, P. α7-Nicotinic Acetylcholine Receptor and Mutated α-Synuclein Interact in Motor Behavior and Nigrostriatal Dopamine—Findings with Potential Relevance for a Protective Effect of Cigarette Smoking and Parkinson’s Disease. Eur. J. Neurosci. 2025, 61, e70063. [Google Scholar] [CrossRef] [PubMed]
- Egea, J.; Buendia, I.; Parada, E.; Navarro, E.; León, R.; Lopez, M.G. Anti-Inflammatory Role of Microglial Alpha7 nAChRs and Its Role in Neuroprotection. Biochem. Pharmacol. 2015, 97, 463–472. [Google Scholar] [CrossRef]
- Lai, J.I.; Porcu, A.; Romoli, B.; Keisler, M.; Manfredsson, F.P.; Powell, S.B.; Dulcis, D. Nicotine-Mediated Recruitment of GABAergic Neurons to a Dopaminergic Phenotype Attenuates Motor Deficits in an Alpha-Synuclein Parkinson’s Model. Int. J. Mol. Sci. 2023, 24, 4204. [Google Scholar] [CrossRef]
- Al-Nusaif, M.; Lin, Y.; Li, T.; Cheng, C.; Le, W. Advances in NURR1-Regulated Neuroinflammation Associated with Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 16184. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Guo, X.; Mailman, R.B.; Park, Y.; Kamel, F.; Umbach, D.M.; Xu, Q.; Hollenbeck, A.; Schatzkin, A.; et al. Smoking Duration, Intensity, and Risk of Parkinson Disease. Neurology 2010, 74, 878–884. [Google Scholar] [CrossRef]
- Chuang, Y.-H.; Paul, K.C.; Sinsheimer, J.S.; Bronstein, J.M.; Bordelon, Y.M.; Ritz, B. Genetic Variants in Nicotinic Receptors and Smoking Cessation in Parkinson’s Disease. Parkinsonism Relat. Disord. 2019, 62, 57–61. [Google Scholar] [CrossRef]
- Quik, M. Smoking, Nicotine and Parkinson’s Disease. Trends Neurosci. 2004, 27, 561–568. [Google Scholar] [CrossRef]
- Quik, M.; O’Leary, K.; Tanner, C.M. Nicotine and Parkinson’s Disease: Implications for Therapy. Mov. Disord. 2008, 23, 1641–1652. [Google Scholar] [CrossRef]
- Quik, M.; Huang, L.Z.; Parameswaran, N.; Bordia, T.; Campos, C.; Perez, X.A. Multiple Roles for Nicotine in Parkinson’s Disease. Biochem. Pharmacol. 2009, 78, 677. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Liu, Y.; Neumann, S.; Gao, X. Nicotine from Cigarette Smoking and Diet and Parkinson Disease: A Review. Transl. Neurodegener. 2017, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, A.; Lockhart, T.E.; Olson, M.C.; Smith Hussain, V.A.; Frames, C.W.; Sadreddin, A.; McCauley, M.; Ludington, E. Nicotine Bitartrate Reduces Falls and Freezing of Gait in Parkinson Disease: A Reanalysis. Front. Neurol. 2019, 10, 424. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.Z.; Grady, S.R.; Quik, M. Nicotine Reduces L-DOPA-Induced Dyskinesias by Acting at Β2* Nicotinic Receptors. J. Pharmacol. Exp. Ther. 2011, 338, 932–941. [Google Scholar] [CrossRef]
- Vetel, S.; Foucault-Fruchard, L.; Tronel, C.; Buron, F.; Vergote, J.; Bodard, S.; Routier, S.; Sérrière, S.; Chalon, S. Neuroprotective and Anti-Inflammatory Effects of a Therapy Combining Agonists of Nicotinic A7 and Σ1 Receptors in a Rat Model of Parkinson’s Disease. Neural Regen. Res. 2021, 16, 1099–1104. [Google Scholar] [CrossRef]
- Threlfell, S.; Cragg, S.J. Dopamine Signaling in Dorsal Versus Ventral Striatum: The Dynamic Role of Cholinergic Interneurons. Front. Syst. Neurosci. 2011, 5, 11. [Google Scholar] [CrossRef]
- Menegas, W.; Bergan, J.F.; Ogawa, S.K.; Isogai, Y.; Umadevi Venkataraju, K.; Osten, P.; Uchida, N.; Watabe-Uchida, M. Dopamine Neurons Projecting to the Posterior Striatum Form an Anatomically Distinct Subclass. eLife 2015, 4, e10032. [Google Scholar] [CrossRef]
- Lim, S.A.O.; Kang, U.J.; McGehee, D.S. Striatal Cholinergic Interneuron Regulation and Circuit Effects. Front. Synaptic Neurosci. 2014, 6, 22. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Tan, Y.; Luo, H.; Lu, C.; Gao, C.; Shen, X.; Cai, F.; Hu, J.; Chen, S. Enhancing Striatal Acetylcholine Facilitates Dopamine Release and Striatal Output in Parkinsonian Mice. Cell Biosci. 2024, 14, 146. [Google Scholar] [CrossRef]
- Assous, M. Striatal Cholinergic Transmission. Focus on Nicotinic Receptors’ Influence in Striatal Circuits. Eur. J. Neurosci. 2021, 53, 2421–2442. [Google Scholar] [CrossRef]
- Vezina, C.; Kudelski, A.; Sehgal, S.N. Rapamycin (AY-22,989), a New Antifungal Antibiotic. I. Taxonomy of the Producing Streptomycete and Isolation of the Active Principle. J. Antibiot. Tokyo 1975, 28, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Mena-Segovia, J.; Winn, P.; Bolam, J.P. Cholinergic Modulation of Midbrain Dopaminergic Systems. Brain Res. Rev. 2008, 58, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Blaha, C.D.; Winn, P. Modulation of Dopamine Efflux in the Striatum Following Cholinergic Stimulation of the Substantia Nigra in Intact and Pedunculopontine Tegmental Nucleus-Lesioned Rats. J. Neurosci. 1993, 13, 1035–1044. [Google Scholar] [CrossRef]
- Blaha, C.D.; Allen, L.F.; Das, S.; Inglis, W.L.; Latimer, M.P.; Vincent, S.R.; Winn, P. Modulation of Dopamine Efflux in the Nucleus Accumbens After Cholinergic Stimulation of the Ventral Tegmental Area in Intact, Pedunculopontine Tegmental Nucleus-Lesioned, and Laterodorsal Tegmental Nucleus-Lesioned Rats. J. Neurosci. 1996, 16, 714–722. [Google Scholar] [CrossRef]
- Ztaou, S.; Amalric, M. Contribution of Cholinergic Interneurons to Striatal Pathophysiology in Parkinson’s Disease. Neurochem. Int. 2019, 126, 1–10. [Google Scholar] [CrossRef]
- Forster, G.L.; Blaha, C.D. Pedunculopontine Tegmental Stimulation Evokes Striatal Dopamine Efflux by Activation of Acetylcholine and Glutamate Receptors in the Midbrain and Pons of the Rat. Eur. J. Neurosci. 2003, 17, 751–762. [Google Scholar] [CrossRef]
- Quik, M.; Parameswaran, N.; McCallum, S.E.; Bordia, T.; Bao, S.; McCormack, A.; Kim, A.; Tyndale, R.F.; Langston, J.W.; Di Monte, D.A. Chronic Oral Nicotine Treatment Protects Against Striatal Degeneration in MPTP-Treated Primates. J. Neurochem. 2006, 98, 1866–1875. [Google Scholar] [CrossRef]
- Brimblecombe, K.R.; Threlfell, S.; Dautan, D.; Kosillo, P.; Mena-Segovia, J.; Cragg, S.J. Targeted Activation of Cholinergic Interneurons Accounts for the Modulation of Dopamine by Striatal Nicotinic Receptors. eNeuro 2018, 5, ENEURO.0397-17.2018. [Google Scholar] [CrossRef]
- Quarta, D.; Naylor, C.G.; Barik, J.; Fernandes, C.; Wonnacott, S.; Stolerman, I.P. Drug Discrimination and Neurochemical Studies in Alpha7 Null Mutant Mice: Tests for the Role of Nicotinic Alpha7 Receptors in Dopamine Release. Psychopharmacology 2009, 203, 399–410. [Google Scholar] [CrossRef]
- Hoffman, A.F.; Spivak, C.E.; Lupica, C.R. Enhanced Dopamine Release by Dopamine Transport Inhibitors Described by a Restricted Diffusion Model and Fast-Scan Cyclic Voltammetry. ACS Chem. Neurosci. 2016, 7, 700–709. [Google Scholar] [CrossRef]
- Bono, F.; Mutti, V.; Devoto, P.; Bolognin, S.; Schwamborn, J.C.; Missale, C.; Fiorentini, C. Impaired Dopamine D3 and Nicotinic Acetylcholine Receptor Membrane Localization in iPSCs-Derived Dopaminergic Neurons from Two Parkinson’s Disease Patients Carrying the LRRK2 G2019S Mutation. Neurobiol. Aging 2021, 99, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Colonna, M.; Butovsky, O. Microglia Function in the Central Nervous System During Health and Neurodegeneration. Annu. Rev. Immunol. 2017, 35, 441–468. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in Neurodegenerative Diseases: Mechanism and Potential Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Wang, H.; Yu, M.; Ochani, M.; Amella, C.A.; Tanovic, M.; Susarla, S.; Li, J.H.; Wang, H.; Yang, H.; Ulloa, L.; et al. Nicotinic Acetylcholine Receptor Alpha7 Subunit Is an Essential Regulator of Inflammation. Nature 2003, 421, 384–388. [Google Scholar] [CrossRef]
- Shytle, R.D.; Mori, T.; Townsend, K.; Vendrame, M.; Sun, N.; Zeng, J.; Ehrhart, J.; Silver, A.A.; Sanberg, P.R.; Tan, J. Cholinergic Modulation of Microglial Activation by Alpha 7 Nicotinic Receptors. J. Neurochem. 2004, 89, 337–343. [Google Scholar] [CrossRef]
- de Jonge, W.J.; Ulloa, L. The Alpha7 Nicotinic Acetylcholine Receptor as a Pharmacological Target for Inflammation. Br. J. Pharmacol. 2007, 151, 915–929. [Google Scholar] [CrossRef]
- Café-Mendes, C.C.; Garay-Malpartida, H.M.; Malta, M.B.; de Sá Lima, L.; Scavone, C.; Ferreira, Z.S.; Markus, R.P.; Marcourakis, T. Chronic Nicotine Treatment Decreases LPS Signaling Through NF-κB and TLR-4 Modulation in the Hippocampus. Neurosci. Lett. 2017, 636, 218–224. [Google Scholar] [CrossRef]
- Park, J.-E.; Leem, Y.-H.; Park, J.-S.; Kim, D.-Y.; Kang, J.L.; Kim, H.-S. Anti-Inflammatory and Neuroprotective Mechanisms of GTS-21, an A7 Nicotinic Acetylcholine Receptor Agonist, in Neuroinflammation and Parkinson’s Disease Mouse Models. Int. J. Mol. Sci. 2022, 23, 4420. [Google Scholar] [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia Activation in Central Nervous System Disorders: A Review of Recent Mechanistic Investigations and Development Efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.-H. Neuroinflammation in Neurodegenerative Disorders: The Roles of Microglia and Astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Nourse, J.B.; Harshefi, G.; Marom, A.; Karmi, A.; Cohen Ben-Ami, H.; Caldwell, K.A.; Caldwell, G.A.; Treinin, M. Conserved Nicotine-Activated Neuroprotective Pathways Involve Mitochondrial Stress. iScience 2021, 24, 102140. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 1–32. [Google Scholar] [CrossRef]
- Dash, U.C.; Bhol, N.K.; Swain, S.K.; Samal, R.R.; Nayak, P.K.; Raina, V.; Panda, S.K.; Kerry, R.G.; Duttaroy, A.K.; Jena, A.B. Oxidative Stress and Inflammation in the Pathogenesis of Neurological Disorders: Mechanisms and Implications. Acta Pharm. Sin. B 2024, 15, 15–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, Y.; Li, Y.; Xu, S.; Tao, T.; Hua, Y.; Zhang, J.; Fan, Y. Activation of A7-nAChRs Promotes the Clearance of α-Synuclein and Protects Against Apoptotic Cell Death Induced by Exogenous α-Synuclein Fibrils. Front. Cell Dev. Biol. 2021, 9, 637319. [Google Scholar] [CrossRef]
- Hua, Y.; Yang, B.; Chen, Q.; Zhang, J.; Hu, J.; Fan, Y. Activation of A7 Nicotinic Acetylcholine Receptor Protects Against 1-Methyl-4-Phenylpyridinium-Induced Astroglial Apoptosis. Front. Cell. Neurosci. 2019, 13, 507. [Google Scholar] [CrossRef]
- Liu, Y.; Zeng, X.; Hui, Y.; Zhu, C.; Wu, J.; Taylor, D.H.; Ji, J.; Fan, W.; Huang, Z.; Hu, J. Activation of A7 Nicotinic Acetylcholine Receptors Protects Astrocytes Against Oxidative Stress-Induced Apoptosis: Implications for Parkinson’s Disease. Neuropharmacology 2015, 91, 87–96. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, J.; Zhu, C.; Hui, Y.; Han, Y.; Huang, Z.; Ellsworth, K.; Fan, W. A7 Nicotinic Acetylcholine Receptor-Mediated Neuroprotection Against Dopaminergic Neuron Loss in an MPTP Mouse Model via Inhibition of Astrocyte Activation. J. Neuroinflamm. 2012, 9, 98. [Google Scholar] [CrossRef]
- Zhu, S.; Huang, S.; Xia, G.; Wu, J.; Shen, Y.; Wang, Y.; Ostrom, R.S.; Du, A.; Shen, C.; Xu, C. Anti-Inflammatory Effects of A7-Nicotinic ACh Receptors Are Exerted Through Interactions with Adenylyl Cyclase-6. Br. J. Pharmacol. 2021, 178, 2324–2338. [Google Scholar] [CrossRef]
- Garg, B.K.; Loring, R.H. GTS-21 Has Cell-Specific Anti-Inflammatory Effects Independent of A7 Nicotinic Acetylcholine Receptors. PLoS ONE 2019, 14, e0214942. [Google Scholar] [CrossRef]
- Figge, D.A.; de Amaral, H.O.; Crim, J.; Cowell, R.M.; Standaert, D.G.; Eskow Jaunarajs, K.L. Differential Activation States of Direct Pathway Striatal Output Neurons During L-DOPA-Induced Dyskinesia Development. J. Neurosci. Off. J. Soc. Neurosci. 2024, 44, e0050242024. [Google Scholar] [CrossRef]
- Rascol, O.; Fabbri, M.; Poewe, W. Amantadine in the Treatment of Parkinson’s Disease and Other Movement Disorders. Lancet Neurol. 2021, 20, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Rissardo, J.P.; Vora, N.M.; Tariq, I.; Mujtaba, A.; Caprara, A.L.F. Deep Brain Stimulation for the Management of Refractory Neurological Disorders: A Comprehensive Review. Medicina 2023, 59, 1991. [Google Scholar] [CrossRef] [PubMed]
- Zhong, C.; Akmentin, W.; Role, L.W.; Talmage, D.A. Axonal α7* Nicotinic Acetylcholine Receptors Modulate Glutamatergic Signaling and Synaptic Vesicle Organization in Ventral Hippocampal Projections. Front. Neural Circuits 2022, 16, 978837. [Google Scholar] [CrossRef]
- Quik, M.; Campos, C.; Bordia, T.; Strachan, J.-P.; Zhang, J.; McIntosh, J.M.; Letchworth, S.; Jordan, K. A4β2 Nicotinic Receptors Play a Role in the nAChR-Mediated Decline in L-Dopa-Induced Dyskinesias in Parkinsonian Rats. Neuropharmacology 2013, 71, 191–203. [Google Scholar] [CrossRef]
- Tan, R.; Bölükbaşi Hatip, F.; Açikalin, Ö.; Yamauchi, A.; Kataoka, Y.; Hatip-Al-Khatib, I. Effect of Varenicline on Behavioral Deficits in a Rat Model of Parkinson’s Disease Induced by Unilateral 6-Hydroxydopamine Lesion of Substantia Nigra. Behav. Pharmacol. 2018, 29, 327–335. [Google Scholar] [CrossRef]
- Bordia, T.; McGregor, M.; Papke, R.L.; Decker, M.W.; McIntosh, J.M.; Quik, M. The Alpha7 Nicotinic Receptor Agonist ABT-107 Protects against Nigrostriatal Damage in Rats with Unilateral 6-Hydroxydopamine Lesions. Exp. Neurol. 2015, 263, 277–284. [Google Scholar] [CrossRef]
- Zhang, D.; Bordia, T.; McGregor, M.; McIntosh, J.M.; Decker, M.W.; Quik, M. ABT-089 and ABT-894 Reduce Levodopa-Induced Dyskinesias in a Monkey Model of Parkinson’s Disease. Mov. Disord. 2014, 29, 508–517. [Google Scholar] [CrossRef]
- Kulak, J.M.; Schneider, J.S. Differences in Alpha7 Nicotinic Acetylcholine Receptor Binding in Motor Symptomatic and Asymptomatic MPTP-Treated Monkeys. Brain Res. 2004, 999, 193–202. [Google Scholar] [CrossRef]
- Paul, S.; Candelario-Jalil, E. Emerging Neuroprotective Strategies for the Treatment of Ischemic Stroke: An Overview of Clinical and Preclinical Studies. Exp. Neurol. 2021, 335, 113518. [Google Scholar] [CrossRef]
- Florian, H.; Meier, A.; Gauthier, S.; Lipschitz, S.; Lin, Y.; Tang, Q.; Othman, A.A.; Robieson, W.Z.; Gault, L.M. Efficacy and Safety of ABT-126 in Subjects with Mild-to-Moderate Alzheimer’s Disease on Stable Doses of Acetylcholinesterase Inhibitors: A Randomized, Double-Blind, Placebo-Controlled Study. J. Alzheimers Dis. JAD 2016, 51, 1237–1247. [Google Scholar] [CrossRef]
- Gault, J.; Robinson, M.; Berger, R.; Drebing, C.; Logel, J.; Hopkins, J.; Moore, T.; Jacobs, S.; Meriwether, J.; Choi, M.J.; et al. Genomic Organization and Partial Duplication of the Human Alpha7 Neuronal Nicotinic Acetylcholine Receptor Gene (CHRNA7). Genomics 1998, 52, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Barreto, G.E.; Iarkov, A.; Moran, V.E. Beneficial Effects of Nicotine, Cotinine and Its Metabolites as Potential Agents for Parkinson’s Disease. Front. Aging Neurosci. 2015, 6, 123750. [Google Scholar] [CrossRef]
- Hasan, M.Y.; Roslan, A.H.M.; Azmi, N.; Ibrahim, N.M.; Arulsamy, A.; Lee, V.L.L.; Siran, R.; Vidyadaran, S.; Chua, E.W.; Mahadi, M.K. A7-Nicotinic Acetylcholine Receptor Activation Modulates BV2 Microglial Plasticity via miR-21/TNF-α/NFκB in Oxygen–Glucose Deprivation/Reoxygenation. J. Mol. Neurosci. 2024, 75, 2. [Google Scholar] [CrossRef]
- Zamanian, M.Y.; Golmohammadi, M.; Gardanova, Z.R.; Rahimi, M.; Khachatryan, L.G.; Khazaei, M. The Roles of Neuroinflammation in L-DOPA-Induced Dyskinesia: Dissecting the Roles of NF-κB and TNF-α for Novel Pharmacological Therapeutic Approaches. Eur. J. Neurosci. 2025, 61, e70034. [Google Scholar] [CrossRef]
- Piovesana, R.; Salazar Intriago, M.S.; Dini, L.; Tata, A.M. Cholinergic Modulation of Neuroinflammation: Focus on A7 Nicotinic Receptor. Int. J. Mol. Sci. 2021, 22, 4912. [Google Scholar] [CrossRef]
- Cai, Y.; Zhang, X.; Zhou, X.; Wu, X.; Li, Y.; Yao, J.; Bai, J. Nicotine Suppresses the Neurotoxicity by MPP+/MPTP Through Activating α7nAChR/PI3K/Trx-1 and Suppressing ER Stress. Neurotoxicology 2017, 59, 49–55. [Google Scholar] [CrossRef]
- Morissette, M.; Morin, N.; Grégoire, L.; Rajput, A.; Rajput, A.H.; Di Paolo, T. Brain A7 Nicotinic Acetylcholine Receptors in MPTP-Lesioned Monkeys and Parkinsonian Patients. Biochem. Pharmacol. 2016, 109, 62–69. [Google Scholar] [CrossRef]
- McGranahan, T.M.; Patzlaff, N.E.; Grady, S.R.; Heinemann, S.F.; Booker, T.K. A4β2 Nicotinic Acetylcholine Receptors on Dopaminergic Neurons Mediate Nicotine Reward and Anxiety Relief. J. Neurosci. 2011, 31, 10891–10902. [Google Scholar] [CrossRef]
- Quik, M.; Perez, X.A.; Grady, S.R. Role of Alpha6 Nicotinic Receptors in CNS Dopaminergic Function; Relevance to Addiction and Neurological Disorders. Biochem. Pharmacol. 2011, 82, 873–882. [Google Scholar] [CrossRef]
- Hajy Heydary, Y.; Castro, E.M.; Lotfipour, S.; Leslie, F.M. Unraveling the Role of CHRNA6, the Neuronal A6 Nicotinic Acetylcholine Receptor Subunit. Receptors 2025, 4, 1. [Google Scholar] [CrossRef]
- Drenan, R.M.; Grady, S.R.; Whiteaker, P.; McClure-Begley, T.; McKinney, S.; Miwa, J.M.; Bupp, S.; Heintz, N.; McIntosh, J.M.; Bencherif, M.; et al. In Vivo Activation of Midbrain Dopamine Neurons via Sensitized, High-Affinity A6* Nicotinic Acetylcholine Receptors. Neuron 2008, 60, 123–136. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W. Relationships and Interactions between Ionotropic Glutamate Receptors and Nicotinic Receptors in the CNS. Neuroscience 2021, 468, 321–365. [Google Scholar] [CrossRef] [PubMed]
- Maex, R.; Grinevich, V.P.; Grinevich, V.; Budygin, E.; Bencherif, M.; Gutkin, B. Understanding the Role A7 Nicotinic Receptors Play in Dopamine Efflux in Nucleus Accumbens. ACS Chem. Neurosci. 2014, 5, 1032–1040. [Google Scholar] [CrossRef]
- Vallés, A.S.; Barrantes, F.J. Dysregulation of Neuronal Nicotinic Acetylcholine Receptor–Cholesterol Crosstalk in Autism Spectrum Disorder. Front. Mol. Neurosci. 2021, 14, 744597. [Google Scholar] [CrossRef]
- Xu, M.; Wong, A.H.C. GABAergic Inhibitory Neurons as Therapeutic Targets for Cognitive Impairment in Schizophrenia. Acta Pharmacol. Sin. 2018, 39, 733–753. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Soares, A.R.; Etherington, I.M.; Abdulla, Z.I.; Picciotto, M.R. Pathophysiology of nAChRs: Limbic Circuits and Related Disorders. Pharmacol. Res. 2023, 191, 106745. [Google Scholar] [CrossRef]
- Bhatt, S.; Devadoss, T.; Manjula, S.N.; Rajangam, J. 5-HT3 Receptor Antagonism: A Potential Therapeutic Approach for the Treatment of Depression and Other Disorders. Curr. Neuropharmacol. 2021, 19, 1545–1559. [Google Scholar] [CrossRef]
- Fakhfouri, G.; Rahimian, R.; Dyhrfjeld-Johnsen, J.; Zirak, M.R.; Beaulieu, J.-M. 5-HT3 Receptor Antagonists in Neurologic and Neuropsychiatric Disorders: The Iceberg Still Lies beneath the Surface. Pharmacol. Rev. 2019, 71, 383–412. [Google Scholar] [CrossRef]
- Bono, F.; Fiorentini, C.; Mutti, V.; Tomasoni, Z.; Sbrini, G.; Trebesova, H.; Marchi, M.; Grilli, M.; Missale, C. Central Nervous System Interaction and Crosstalk Between nAChRs and Other Ionotropic and Metabotropic Neurotransmitter Receptors. Pharmacol. Res. 2023, 190, 106711. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, H.; Wang, X.; Wang, Z.; Yang, Y.; Li, L.; Feng, T. Protective Effect of the A7 Nicotinic Receptor Agonist PNU-282987 on Dopaminergic Neurons Against 6-Hydroxydopamine, Regulating Anti-Neuroinflammatory and the Immune Balance Pathways in Rat. Front. Aging Neurosci. 2020, 12, 606927. [Google Scholar] [CrossRef]
- Stuckenholz, V.; Bacher, M.; Balzer-Geldsetzer, M.; Alvarez-Fischer, D.; Oertel, W.H.; Dodel, R.C.; Noelker, C. The A7 nAChR Agonist PNU-282987 Reduces Inflammation and MPTP-Induced Nigral Dopaminergic Cell Loss in Mice. J. Park. Dis. 2013, 3, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Coronel, J.C.; Loaiza, A.E.; Díaz, J.E.; Cabezas, R.; Ashraf, G.M.; Sahebkar, A.; Echeverria, V.; González, J.; Barreto, G.E. (E)-Nicotinaldehyde O-Cinnamyloxime, a Nicotine Analog, Attenuates Neuronal Cells Death Against Rotenone-Induced Neurotoxicity. Mol. Neurobiol. 2019, 56, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Sérrière, S.; Doméné, A.; Vercouillie, J.; Mothes, C.; Bodard, S.; Rodrigues, N.; Guilloteau, D.; Routier, S.; Page, G.; Chalon, S. Assessment of the Protection of Dopaminergic Neurons by an A7 Nicotinic Receptor Agonist, PHA 543613 Using [(18)F]LBT-999 in a Parkinson’s Disease Rat Model. Front. Med. 2015, 2, 61. [Google Scholar] [CrossRef][Green Version]
- Leem, Y.-H.; Park, J.-E.; Park, J.-S.; Kim, D.-Y.; Park, J.-M.; Kim, S.-E.; Kang, J.L.; Kim, H.-S. Activation of α7nAch Receptors Ameliorates α-Synuclein Pathology in the Brain and Gut of a Subacute MPTP Mouse Model of Parkinson’s Disease. Biomed. Pharmacother. Biomed. Pharmacother. 2025, 184, 117871. [Google Scholar] [CrossRef]
- Zhang, D.; McGregor, M.; Decker, M.W.; Quik, M. The A7 Nicotinic Receptor Agonist ABT-107 Decreases L-Dopa-Induced Dyskinesias in Parkinsonian Monkeys. J. Pharmacol. Exp. Ther. 2014, 351, 25–32. [Google Scholar] [CrossRef]
- Zhang, D.; McGregor, M.; Bordia, T.; Perez, X.A.; McIntosh, J.M.; Decker, M.W.; Quik, M. A7 Nicotinic Receptor Agonists Reduce Levodopa-Induced Dyskinesias with Severe Nigrostriatal Damage. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1901–1911. [Google Scholar] [CrossRef]
- Papke, R.L.; Horenstein, N.A. Therapeutic Targeting of A7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2021, 73, 1118–1149. [Google Scholar] [CrossRef]
- El Nebrisi, E.G.; Bagdas, D.; Toma, W.; Al Samri, H.; Brodzik, A.; Alkhlaif, Y.; Yang, K.-H.S.; Howarth, F.C.; Damaj, I.M.; Oz, M. Curcumin Acts as a Positive Allosteric Modulator of A7-Nicotinic Acetylcholine Receptors and Reverses Nociception in Mouse Models of Inflammatory Pain. J. Pharmacol. Exp. Ther. 2018, 365, 190–200. [Google Scholar] [CrossRef]
- Shabbir, W.; Yang, K.-H.S.; Sadek, B.; Oz, M. Apigenin and Structurally Related Flavonoids Allosterically Potentiate the Function of Human A7-Nicotinic Acetylcholine Receptors Expressed in SH-EP1 Cells. Cells 2021, 10, 1110. [Google Scholar] [CrossRef]
- Nebrisi, E.E. Neuroprotective Activities of Curcumin in Parkinson’s Disease: A Review of the Literature. Int. J. Mol. Sci. 2021, 22, 11248. [Google Scholar] [CrossRef]
- Nebrisi, E.E.; Al Kury, L.T.; Yang, K.-H.S.; Jayaprakash, P.; Howarth, F.C.; Kabbani, N.; Oz, M. Curcumin Potentiates the Function of Human A7-Nicotinic Acetylcholine Receptors Expressed in SH-EP1 Cells. Neurochem. Int. 2018, 114, 80–84. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Isaev, D.; Yang, K.-H.S.; Beiram, R.; Oz, M.; Sadek, B. Apigenin Alleviates Autistic-like Stereotyped Repetitive Behaviors and Mitigates Brain Oxidative Stress in Mice. Pharmaceuticals 2024, 17, 482. [Google Scholar] [CrossRef] [PubMed]
- Di Paolo, T.; Grégoire, L.; Feuerbach, D.; Elbast, W.; Weiss, M.; Gomez-Mancilla, B. AQW051, a Novel and Selective Nicotinic Acetylcholine Receptor A7 Partial Agonist, Reduces l-Dopa-Induced Dyskinesias and Extends the Duration of l-Dopa Effects in Parkinsonian Monkeys. Park. Relat. Disord. 2014, 20, 1119–1123. [Google Scholar] [CrossRef]
- King’s College London. Parkinson’s Disease with Mild Cognitive Impairment Treated with Nicotinic Agonist Drug. 2021. Available online: https://clinicaltrials.gov/ (accessed on 5 March 2025).
- Di Lascio, S.; Fornasari, D.; Benfante, R. The Human-Restricted Isoform of the A7 nAChR, CHRFAM7A: A Double-Edged Sword in Neurological and Inflammatory Disorders. Int. J. Mol. Sci. 2022, 23, 3463. [Google Scholar] [CrossRef]
- Vallés, A.S.; Barrantes, F.J. Nicotinic Acetylcholine Receptor Dysfunction in Addiction and in Some Neurodegenerative and Neuropsychiatric Diseases. Cells 2023, 12, 2051. [Google Scholar] [CrossRef]
| Drug Candidate | Developer | Target | Clinical Phase | PD Indication Tested | Trail Status | Notes |
|---|---|---|---|---|---|---|
| AQW051 | Novartis | Partial α7-nAChR agonist | Completed Phase 2a | Motor symptoms and LID in PD | Completed | Evaluated in PD patients; well tolerated but showed no consistent efficacy [156] |
| AZD0328 | AstraZeneca | Selective α7-nAChR agonist | Planned Phase 2a | Parkinson’s disease with mild cognitive impairment | Withdraw before enrolment | Trial NCT04810104; planned but not initiated due to COVID-19 delays [157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
ElNebrisi, E.; Lozon, Y.; Oz, M. The Role of α7-Nicotinic Acetylcholine Receptors in the Pathophysiology and Treatment of Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 3210. https://doi.org/10.3390/ijms26073210
ElNebrisi E, Lozon Y, Oz M. The Role of α7-Nicotinic Acetylcholine Receptors in the Pathophysiology and Treatment of Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(7):3210. https://doi.org/10.3390/ijms26073210
Chicago/Turabian StyleElNebrisi, Eslam, Yosra Lozon, and Murat Oz. 2025. "The Role of α7-Nicotinic Acetylcholine Receptors in the Pathophysiology and Treatment of Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 7: 3210. https://doi.org/10.3390/ijms26073210
APA StyleElNebrisi, E., Lozon, Y., & Oz, M. (2025). The Role of α7-Nicotinic Acetylcholine Receptors in the Pathophysiology and Treatment of Parkinson’s Disease. International Journal of Molecular Sciences, 26(7), 3210. https://doi.org/10.3390/ijms26073210






