Hydroxyurea Mitigates Heme-Induced Inflammation and Kidney Injury in Humanized Sickle Cell Mice
Abstract
1. Introduction
2. Results
2.1. Hydroxyurea Modulates Markers of Systemic Hemolysis and Inflammation Relevant to Kidney Injury in Townes Humanized Sickle Cell Mice
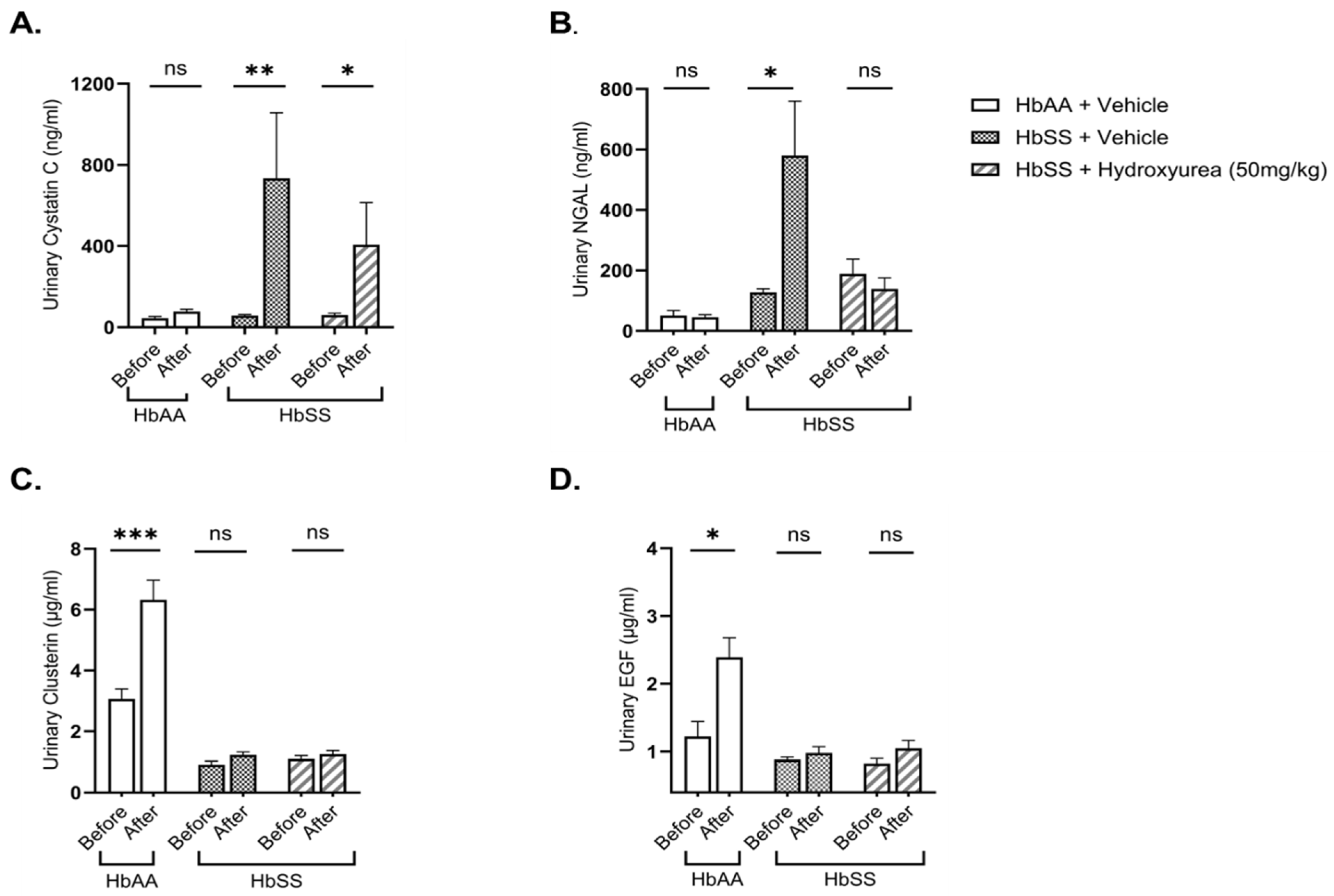
2.2. Hydroxyurea Treatment Mitigates Kidney Injury in Townes Humanized Sickle Cell Mice
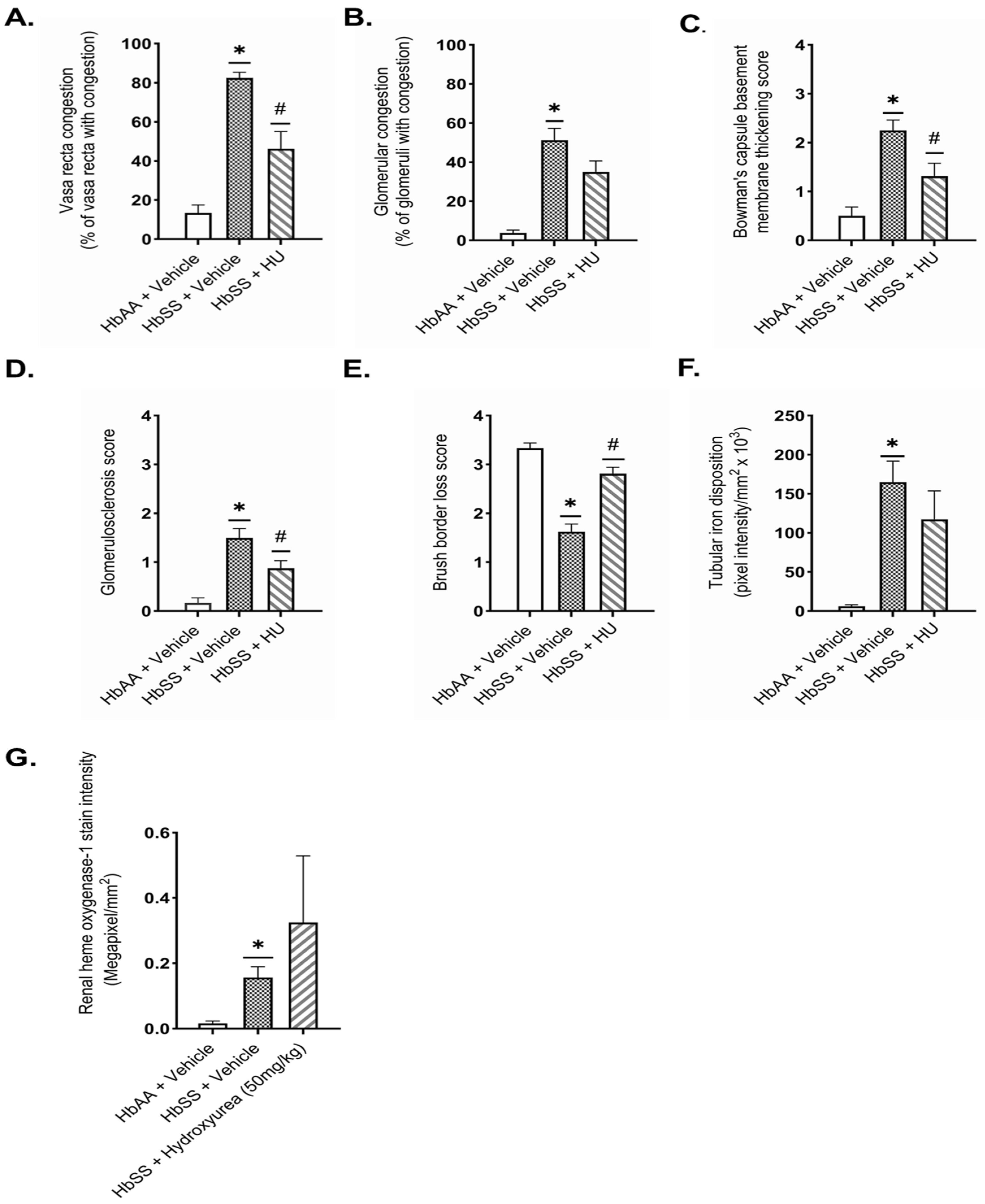
2.3. Hydroxyurea Ameliorates Renal Histopathologic Changes in Townes Humanized Sickle Cell Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Study Design
4.3. Blood and Urine Analyses
4.4. Histology
4.5. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ofori-Acquah, S.F. Sickle Cell Disease as a Vascular Disorder. Expert. Rev. Hematol. 2020, 13, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.M.; McHugh, T.A.; Oron, A.P.; Teply, C.; Lonberg, N.; Vilchis Tella, V.; Wilner, L.B.; Fuller, K.; Hagins, H.; Aboagye, R.G.; et al. Global, Regional, and National Prevalence and Mortality Burden of Sickle Cell Disease, 2000–2021: A Systematic Analysis from the Global Burden of Disease Study 2021. Lancet Haematol. 2023, 10, e585–e599. [Google Scholar] [CrossRef]
- McCormick, M.; Richardson, T.; Warady, B.A.; Novelli, E.M.; Kalpatthi, R. Acute Kidney Injury in Paediatric Patients with Sickle Cell Disease Is Associated with Increased Morbidity and Resource Utilization. Br. J. Haematol. 2020, 189, 559–565. [Google Scholar] [CrossRef]
- Batte, A.; Menon, S.; Ssenkusu, J.; Kiguli, S.; Kalyesubula, R.; Lubega, J.; Mutebi, E.I.; Opoka, R.O.; John, C.C.; Starr, M.C.; et al. Acute Kidney Injury in Hospitalized Children with Sickle Cell Anemia. BMC Nephrol. 2022, 23, 110. [Google Scholar] [CrossRef]
- Roy, N.B.; Carpenter, A.; Dale-Harris, I.; Dorée, C.; Estcourt, L.J. Interventions for Chronic Kidney Disease in People with Sickle Cell Disease. Cochrane Database Syst. Rev. 2023, 8, CD012380. [Google Scholar] [CrossRef]
- Ofori-Acquah, S.F.; Hazra, R.; Orikogbo, O.O.; Crosby, D.; Flage, B.; Ackah, E.B.; Lenhart, D.; Tan, R.J.; Vitturi, D.A.; Paintsil, V.; et al. Hemopexin Deficiency Promotes Acute Kidney Injury in Sickle Cell Disease. Blood 2020, 135, 1044–1048. [Google Scholar] [CrossRef]
- Qian, Q.; Nath, K.A.; Wu, Y.; Daoud, T.M.; Sethi, S. Hemolysis and Acute Kidney Failure. Am. J. Kidney Dis. 2010, 56, 780–784. [Google Scholar] [CrossRef] [PubMed]
- May, O.; Merle, N.S.; Grunenwald, A.; Gnemmi, V.; Leon, J.; Payet, C.; Robe-Rybkine, T.; Paule, R.; Delguste, F.; Satchell, S.C.; et al. Heme Drives Susceptibility of Glomerular Endothelium to Complement Overactivation Due to Inefficient Upregulation of Heme Oxygenase-1. Front. Immunol. 2018, 9, 3008. [Google Scholar] [CrossRef]
- Rubio-Navarro, A.; Sanchez-Niño, M.D.; Guerrero-Hue, M.; García-Caballero, C.; Gutiérrez, E.; Yuste, C.; Sevillano, Á.; Praga, M.; Egea, J.; Román, E.; et al. Podocytes Are New Cellular Targets of Haemoglobin-Mediated Renal Damage. J. Pathol. 2018, 244, 296–310. [Google Scholar] [CrossRef]
- Belisário, A.R.; Vieira, É.L.M.; de Almeida, J.A.; Mendes, F.G.; Miranda, A.S.; Rezende, P.V.; Viana, M.B.; Simões E Silva, A.C. Evidence for Interactions between Inflammatory Markers and Renin-Angiotensin System Molecules in the Occurrence of Albuminuria in Children with Sickle Cell Anemia. Cytokine 2020, 125, 154800. [Google Scholar] [CrossRef]
- Juncos, J.P.; Grande, J.P.; Croatt, A.J.; Hebbel, R.P.; Vercellotti, G.M.; Katusic, Z.S.; Nath, K.A. Early and Prominent Alterations in Hemodynamics, Signaling, and Gene Expression Following Renal Ischemia in Sickle Cell Disease. Am. J. Physiol. Ren. Physiol. 2010, 298, F892–F899. [Google Scholar] [CrossRef] [PubMed][Green Version]
- dos Santos, T.E.d.J.; Gonçalves, R.P.; Barbosa, M.C.; da Silva, G.B.; Daher, E.D.F. Monocyte Chemoatractant Protein-1: A Potential Biomarker of Renal Lesion and Its Relation with Oxidative Status in Sickle Cell Disease. Blood Cells. Mol. Dis. 2015, 54, 297–301. [Google Scholar] [CrossRef]
- López Rubio, M.; Argüello Marina, M. The Current Role of Hydroxyurea in the Treatment of Sickle Cell Anemia. J. Clin. Med. 2024, 13, 6404. [Google Scholar] [CrossRef] [PubMed]
- Lebensburger, J.D.; Pestina, T.I.; Ware, R.E.; Boyd, K.L.; Persons, D.A. Hydroxyurea Therapy Requires HbF Induction for Clinical Benefit in a Sickle Cell Mouse Model. Haematologica 2010, 95, 1599–1603. [Google Scholar] [CrossRef]
- Khargekar, N.; Banerjee, A.; Athalye, S.; Mahajan, N.; Kargutkar, N.; Tapase, P.; Madkaikar, M. Role of Hydroxyurea Therapy in the Prevention of Organ Damage in Sickle Cell Disease: A Systematic Review and Meta-Analysis. Syst. Rev. 2024, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Bartolucci, P.; Habibi, A.; Stehlé, T.; Di Liberto, G.; Rakotoson, M.G.; Gellen-Dautremer, J.; Loric, S.; Moutereau, S.; Sahali, D.; Wagner-Ballon, O.; et al. Six Months of Hydroxyurea Reduces Albuminuria in Patients with Sickle Cell Disease. J. Am. Soc. Nephrol. 2016, 27, 1847–1853. [Google Scholar] [CrossRef]
- Laurin, L.-P.; Nachman, P.H.; Desai, P.C.; Ataga, K.I.; Derebail, V.K. Hydroxyurea Is Associated with Lower Prevalence of Albuminuria in Adults with Sickle Cell Disease. Nephrol. Dial. Transplant. 2014, 29, 1211–1218. [Google Scholar] [CrossRef]
- Park, F.; Soni, H.; Pressly, J.D.; Adebiyi, A. Acute Hydroxyurea Treatment Reduces Tubular Damage Following Bilateral Ischemia-Reperfusion Injury in a Mouse Model of Sickle Cell Disease. Biochem. Biophys. Res. Commun. 2019, 515, 72–76. [Google Scholar] [CrossRef]
- Lanaro, C.; Franco-Penteado, C.F.; Albuqueque, D.M.; Saad, S.T.O.; Conran, N.; Costa, F.F. Altered Levels of Cytokines and Inflammatory Mediators in Plasma and Leukocytes of Sickle Cell Anemia Patients and Effects of Hydroxyurea Therapy. J. Leukoc. Biol. 2009, 85, 235–242. [Google Scholar] [CrossRef]
- Almeida, C.B.; Souza, L.E.B.; Leonardo, F.C.; Costa, F.T.M.; Werneck, C.C.; Covas, D.T.; Costa, F.F.; Conran, N. Acute Hemolytic Vascular Inflammatory Processes Are Prevented by Nitric Oxide Replacement or a Single Dose of Hydroxyurea. Blood 2015, 126, 711–720. [Google Scholar] [CrossRef]
- Santana, S.S.; Pitanga, T.N.; de Santana, J.M.; Zanette, D.L.; Vieira, J.d.J.; Yahouédéhou, S.C.M.A.; Adanho, C.S.A.; Viana, S.d.M.; Luz, N.F.; Borges, V.M.; et al. Hydroxyurea Scavenges Free Radicals and Induces the Expression of Antioxidant Genes in Human Cell Cultures Treated With Hemin. Front. Immunol. 2020, 11, 1488. [Google Scholar] [CrossRef] [PubMed]
- Keikhaei, B.; Mohseni, A.R.; Norouzirad, R.; Alinejadi, M.; Ghanbari, S.; Shiravi, F.; Solgi, G. Altered Levels of Pro-Inflammatory Cytokines in Sickle Cell Disease Patients during Vaso-Occlusive Crises and the Steady State Condition. Eur. Cytokine Netw. 2013, 24, 45–52. [Google Scholar] [CrossRef]
- Zahran, A.M.; Nafady, A.; Saad, K.; Hetta, H.F.; Abdallah, A.-E.M.; Abdel-Aziz, S.M.; Embaby, M.M.; Abo Elgheet, A.M.; Darwish, S.F.; Abo-Elela, M.G.M.; et al. Effect of Hydroxyurea Treatment on the Inflammatory Markers Among Children With Sickle Cell Disease. Clin. Appl. Thromb. 2020, 26, 1076029619895111. [Google Scholar] [CrossRef]
- Wu, L.-C.; Sun, C.-W.; Ryan, T.M.; Pawlik, K.M.; Ren, J.; Townes, T.M. Correction of Sickle Cell Disease by Homologous Recombination in Embryonic Stem Cells. Blood 2006, 108, 1183–1188. [Google Scholar] [CrossRef]
- Kasztan, M.; Grover, S.P.; Trebak, F.; Sako, M.O.; Key, N.S.; Pawlinski, R. Validation of Townes As Mice As a Model of Chronic Kidney Disease and Venous Thrombosis Associated with Sickle Cell Trait. Blood 2023, 142, 564. [Google Scholar] [CrossRef]
- Kasztan, M.; Fox, B.M.; Lebensburger, J.D.; Hyndman, K.A.; Speed, J.S.; Pollock, J.S.; Pollock, D.M. Hyperfiltration Predicts Long-Term Renal Outcomes in Humanized Sickle Cell Mice. Blood Adv. 2019, 3, 1460–1475. [Google Scholar] [CrossRef]
- Agrawal, R.K.; Patel, R.K.; Shah, V.; Nainiwal, L.; Trivedi, B. Hydroxyurea in Sickle Cell Disease: Drug Review. Indian J. Hematol. Blood Transfus. 2014, 30, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Aygun, B.; Mortier, N.A.; Smeltzer, M.P.; Shulkin, B.L.; Hankins, J.S.; Ware, R.E. Hydroxyurea Treatment Decreases Glomerular Hyperfiltration in Children with Sickle Cell Anemia. Am. J. Hematol. 2013, 88, 116–119. [Google Scholar] [CrossRef]
- Gurkan, S.; Scarponi, K.J.; Hotchkiss, H.; Savage, B.; Drachtman, R. Lactate Dehydrogenase as a Predictor of Kidney Involvement in Patients with Sickle Cell Anemia. Pediatr. Nephrol. Berl. Ger. 2010, 25, 2123–2127. [Google Scholar] [CrossRef]
- Hamideh, D.; Raj, V.; Harrington, T.; Li, H.; Margolles, E.; Amole, F.; Garcia-Buitrago, M.; Ruiz, P.; Zilleruelo, G.; Alvarez, O. Albuminuria Correlates with Hemolysis and NAG and KIM-1 in Patients with Sickle Cell Anemia. Pediatr. Nephrol. 2014, 29, 1997–2003. [Google Scholar] [CrossRef]
- Haymann, J.-P.; Stankovic, K.; Levy, P.; Avellino, V.; Tharaux, P.-L.; Letavernier, E.; Grateau, G.; Baud, L.; Girot, R.; Lionnet, F. Glomerular Hyperfiltration in Adult Sickle Cell Anemia: A Frequent Hemolysis Associated Feature. Clin. J. Am. Soc. Nephrol. 2010, 5, 756. [Google Scholar] [CrossRef]
- Maier-Redelsperger, M.; Lévy, P.; Lionnet, F.; Stankovic, K.; Haymann, J.-P.; Lefèvre, G.; Avellino, V.; Perol, J.-P.; Girot, R.; Elion, J. Strong Association between a New Marker of Hemolysis and Glomerulopathy in Sickle Cell Anemia. Blood Cells Mol. Dis. 2010, 45, 289–292. [Google Scholar] [CrossRef] [PubMed]
- Bandeira, I.C.J.; Rocha, L.B.S.; Barbosa, M.C.; Elias, D.B.D.; Querioz, J.A.N.; Freitas, M.V.C.; Gonçalves, R.P. Chronic Inflammatory State in Sickle Cell Anemia Patients Is Associated with HBB*S Haplotype. Cytokine 2014, 65, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.A.; Grande, J.P.; Croatt, A.J.; Frank, E.; Caplice, N.M.; Hebbel, R.P.; Katusic, Z.S. Transgenic Sickle Mice Are Markedly Sensitive to Renal Ischemia-Reperfusion Injury. Am. J. Pathol. 2005, 166, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Olaniran, K.O.; Allegretti, A.S.; Zhao, S.H.; Nigwekar, S.U.; Kalim, S. Acute Kidney Injury among Black Patients with Sickle Cell Trait and Sickle Cell Disease. Clin. J. Am. Soc. Nephrol. 2021, 16, 348–355. [Google Scholar] [CrossRef]
- Cecchini, J.; Lionnet, F.; Djibré, M.; Parrot, A.; Stojanovic, K.S.; Girot, R.; Fartoukh, M. Outcomes of Adult Patients With Sickle Cell Disease Admitted to the ICU: A Case Series. Crit. Care Med. 2014, 42, 1629. [Google Scholar] [CrossRef]
- Weng, X.; Zhao, H.; Guan, Q.; Shi, G.; Feng, S.; Gleave, M.E.; Nguan, C.C.; Du, C. Clusterin Regulates Macrophage Expansion, Polarization and Phagocytic Activity in Response to Inflammation in the Kidneys. Immunol. Cell Biol. 2021, 99, 274–287. [Google Scholar] [CrossRef]
- He, J.; Dijkstra, K.L.; Bakker, K.; Bus, P.; Bruijn, J.A.; Scharpfenecker, M.; Baelde, H.J. Glomerular Clusterin Expression Is Increased in Diabetic Nephropathy and Protects against Oxidative Stress-Induced Apoptosis in Podocytes. Sci. Rep. 2020, 10, 14888. [Google Scholar] [CrossRef]
- Zhou, W.; Guan, Q.; Kwan, C.C.H.; Chen, H.; Gleave, M.E.; Nguan, C.Y.C.; Du, C. Loss of Clusterin Expression Worsens Renal Ischemia-Reperfusion Injury. Am. J. Physiol. Ren. Physiol. 2010, 298, F568–F578. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.-K.; Harris, R.C. Deletion of the Epidermal Growth Factor Receptor in Renal Proximal Tubule Epithelial Cells Delays Recovery from Acute Kidney Injury. Kidney Int. 2012, 82, 45–52. [Google Scholar] [CrossRef]
- Mejia-Vilet, J.M.; Shapiro, J.P.; Zhang, X.L.; Cruz, C.; Zimmerman, G.; Méndez-Pérez, R.A.; Cano-Verduzco, M.L.; Parikh, S.V.; Nagaraja, H.N.; Morales-Buenrostro, L.E.; et al. Association Between Urinary Epidermal Growth Factor and Renal Prognosis in Lupus Nephritis. Arthritis Rheumatol. 2021, 73, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Norvik, J.V.; Harskamp, L.R.; Nair, V.; Shedden, K.; Solbu, M.D.; Eriksen, B.O.; Kretzler, M.; Gansevoort, R.T.; Ju, W.; Melsom, T. Urinary Excretion of Epidermal Growth Factor and Rapid Loss of Kidney Function. Nephrol. Dial. Transpl. 2021, 36, 1882–1892. [Google Scholar] [CrossRef]
- Taylor, C.; Kasztan, M.; Tao, B.; Pollock, J.S.; Pollock, D.M. Combined Hydroxyurea and ETA Receptor Blockade Reduces Renal Injury in the Humanized Sickle Cell Mouse. Acta Physiol. 2019, 225, e13178. [Google Scholar] [CrossRef]
- Taylor, C.M.; Kasztan, M.; Sedaka, R.; Molina, P.A.; Dunaway, L.S.; Pollock, J.S.; Pollock, D.M. Hydroxyurea Improves Nitric Oxide Bioavailability in Humanized Sickle Cell Mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 320, R630–R640. [Google Scholar] [CrossRef]
- Johnson, C.; Telen, M.J. Adhesion Molecules and Hydroxyurea in the Pathophysiology of Sickle Cell Disease. Haematologica 2008, 93, 481–485. [Google Scholar] [CrossRef]
- Saleh, A.W.; Duits, A.J.; Gerbers, A.; de Vries, C.; Hillen, H.F. Cytokines and Soluble Adhesion Molecules in Sickle Cell Anemia Patients during Hydroxyurea Therapy. Acta Haematol. 1998, 100, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Zahr, R.S.; Yee, M.E.; Weaver, J.; Twombley, K.; Matar, R.B.; Aviles, D.; Sreedharan, R.; Rheault, M.N.; Malatesta-Muncher, R.; Stone, H.; et al. Kidney Biopsy Findings in Children with Sickle Cell Disease: A Midwest Pediatric Nephrology Consortium Study. Pediatr. Nephrol. 2019, 34, 1435–1445. [Google Scholar] [CrossRef]
- McKie, K.T.; Hanevold, C.D.; Hernandez, C.; Waller, J.L.; Ortiz, L.; McKie, K.M. Prevalence, Prevention, and Treatment of Microalbuminuria and Proteinuria in Children with Sickle Cell Disease. J. Pediatr. Hematol. Oncol. 2007, 29, 140–144. [Google Scholar] [CrossRef] [PubMed]
- Denton, C.C.; Detterich, J.A.; Coates, T.D.; Wood, J.C. Kidney Iron Deposition by R2* Is Associated with Hemolysis and Urinary Iron. Br. J. Haematol. 2021, 193, 633–636. [Google Scholar] [CrossRef]
- Schein, A.; Enriquez, C.; Coates, T.D.; Wood, J.C. Magnetic Resonance Detection of Kidney Iron Deposition in Sickle Cell Disease: A Marker of Chronic Hemolysis. J. Magn. Reson. Imaging 2008, 28, 698–704. [Google Scholar] [CrossRef]
- Vasavda, N.; Gutiérrez, L.; House, M.J.; Drašar, E.; St Pierre, T.G.; Thein, S.L. Renal Iron Load in Sickle Cell Disease Is Influenced by Severity of Haemolysis. Br. J. Haematol. 2012, 157, 599–605. [Google Scholar] [CrossRef]
- Stehlé, T.; Bartolucci, P.; Bouanane, M.; Galacteros, F.; Dudreuilh, C.; Grimbert, P.; Deux, J.-F.; Audard, V. Reversible Kidney Iron Accumulation in a Patient with Sickle Cell Disease Treated with Hydroxyurea. Am. J. Hematol. 2016, 91, 1283–1284. [Google Scholar] [CrossRef] [PubMed]
- Grunenwald, A.; Roumenina, L.T.; Frimat, M. Heme Oxygenase 1: A Defensive Mediator in Kidney Diseases. Int. J. Mol. Sci. 2021, 22, 2009. [Google Scholar] [CrossRef] [PubMed]
- Lever, J.M.; Boddu, R.; George, J.F.; Agarwal, A. Heme Oxygenase-1 in Kidney Health and Disease. Antioxid. Redox Signal 2016, 25, 165–183. [Google Scholar] [CrossRef]
- Nath, K.A.; Balla, G.; Vercellotti, G.M.; Balla, J.; Jacob, H.S.; Levitt, M.D.; Rosenberg, M.E. Induction of Heme Oxygenase Is a Rapid, Protective Response in Rhabdomyolysis in the Rat. J. Clin. Investig. 1992, 90, 267–270. [Google Scholar] [CrossRef]
- Baddam, S.; Aban, I.; Hilliard, L.; Howard, T.; Askenazi, D.; Lebensburger, J.D. Acute Kidney Injury during a Pediatric Sickle Cell Vaso-Occlusive Pain Crisis. Pediatr. Nephrol. 2017, 32, 1451–1456. [Google Scholar] [CrossRef]
- Dutta, S.; Sengupta, P. Men and Mice: Relating Their Ages. Life Sci. 2016, 152, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Kasztan, M.; Fox, B.M.; Speed, J.S.; De Miguel, C.; Gohar, E.Y.; Townes, T.M.; Kutlar, A.; Pollock, J.S.; Pollock, D.M. Long-Term Endothelin-A Receptor Antagonism Provides Robust Renal Protection in Humanized Sickle Cell Disease Mice. J. Am. Soc. Nephrol. 2017, 28, 2443–2458. [Google Scholar] [CrossRef]
- Akingbola, T.S.; Tayo, B.O.; Ezekekwu, C.A.; Sonubi, O.; Zhang, X.; Saraf, S.L.; Molokie, R.; Hsu, L.L.; Han, J.; Cooper, R.S.; et al. “Maximum Tolerated Dose” vs. “Fixed Low-Dose” Hydroxyurea for Treatment of Adults with Sickle Cell Anemia. Am. J. Hematol. 2019, 94, E112–E115. [Google Scholar] [CrossRef]
- Walker, A.L.; Crosby, D.; Miller, V.; Weidert, F.; Ofori-Acquah, S. Hydroxyurea Decouples Persistent F-Cell Elevation and Induction of γ-Globin. Exp. Hematol. 2022, 112–113, 15–23.e1. [Google Scholar] [CrossRef]
- McArthur, J.G.; Svenstrup, N.; Chen, C.; Fricot, A.; Carvalho, C.; Nguyen, J.; Nguyen, P.; Parachikova, A.; Abdulla, F.; Vercellotti, G.M.; et al. A Novel, Highly Potent and Selective Phosphodiesterase-9 Inhibitor for the Treatment of Sickle Cell Disease. Haematologica 2020, 105, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Meiler, S.E.; Wade, M.; Kutlar, F.; Yerigenahally, S.D.; Xue, Y.; Moutouh-de Parseval, L.A.; Corral, L.G.; Swerdlow, P.S.; Kutlar, A. Pomalidomide Augments Fetal Hemoglobin Production without the Myelosuppressive Effects of Hydroxyurea in Transgenic Sickle Cell Mice. Blood 2011, 118, 1109–1112. [Google Scholar] [CrossRef] [PubMed]
- Chew, J.L.; Chua, K.Y. Collection of Mouse Urine for Bioassays. Lab. Anim. 2003, 32, 48–50. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agbozo, W.K.; Solomon, W.; Lekpor, C.E.; Erskine, I.J.; Oguljahan, B.; Bashi, A.; Harbuzariu, A.; Driss, A.; Adjei, S.; Paemka, L.; et al. Hydroxyurea Mitigates Heme-Induced Inflammation and Kidney Injury in Humanized Sickle Cell Mice. Int. J. Mol. Sci. 2025, 26, 3214. https://doi.org/10.3390/ijms26073214
Agbozo WK, Solomon W, Lekpor CE, Erskine IJ, Oguljahan B, Bashi A, Harbuzariu A, Driss A, Adjei S, Paemka L, et al. Hydroxyurea Mitigates Heme-Induced Inflammation and Kidney Injury in Humanized Sickle Cell Mice. International Journal of Molecular Sciences. 2025; 26(7):3214. https://doi.org/10.3390/ijms26073214
Chicago/Turabian StyleAgbozo, William Kwaku, Wesley Solomon, Cecilia Elorm Lekpor, Isaac Joe Erskine, Babayewa Oguljahan, Alaijah Bashi, Adriana Harbuzariu, Adel Driss, Samuel Adjei, Lily Paemka, and et al. 2025. "Hydroxyurea Mitigates Heme-Induced Inflammation and Kidney Injury in Humanized Sickle Cell Mice" International Journal of Molecular Sciences 26, no. 7: 3214. https://doi.org/10.3390/ijms26073214
APA StyleAgbozo, W. K., Solomon, W., Lekpor, C. E., Erskine, I. J., Oguljahan, B., Bashi, A., Harbuzariu, A., Driss, A., Adjei, S., Paemka, L., Ofori-Acquah, S. F., & Stiles, J. K. (2025). Hydroxyurea Mitigates Heme-Induced Inflammation and Kidney Injury in Humanized Sickle Cell Mice. International Journal of Molecular Sciences, 26(7), 3214. https://doi.org/10.3390/ijms26073214







