The Role of Triterpenoids in Gastric Ulcer: Mechanisms and Therapeutic Potentials
Abstract
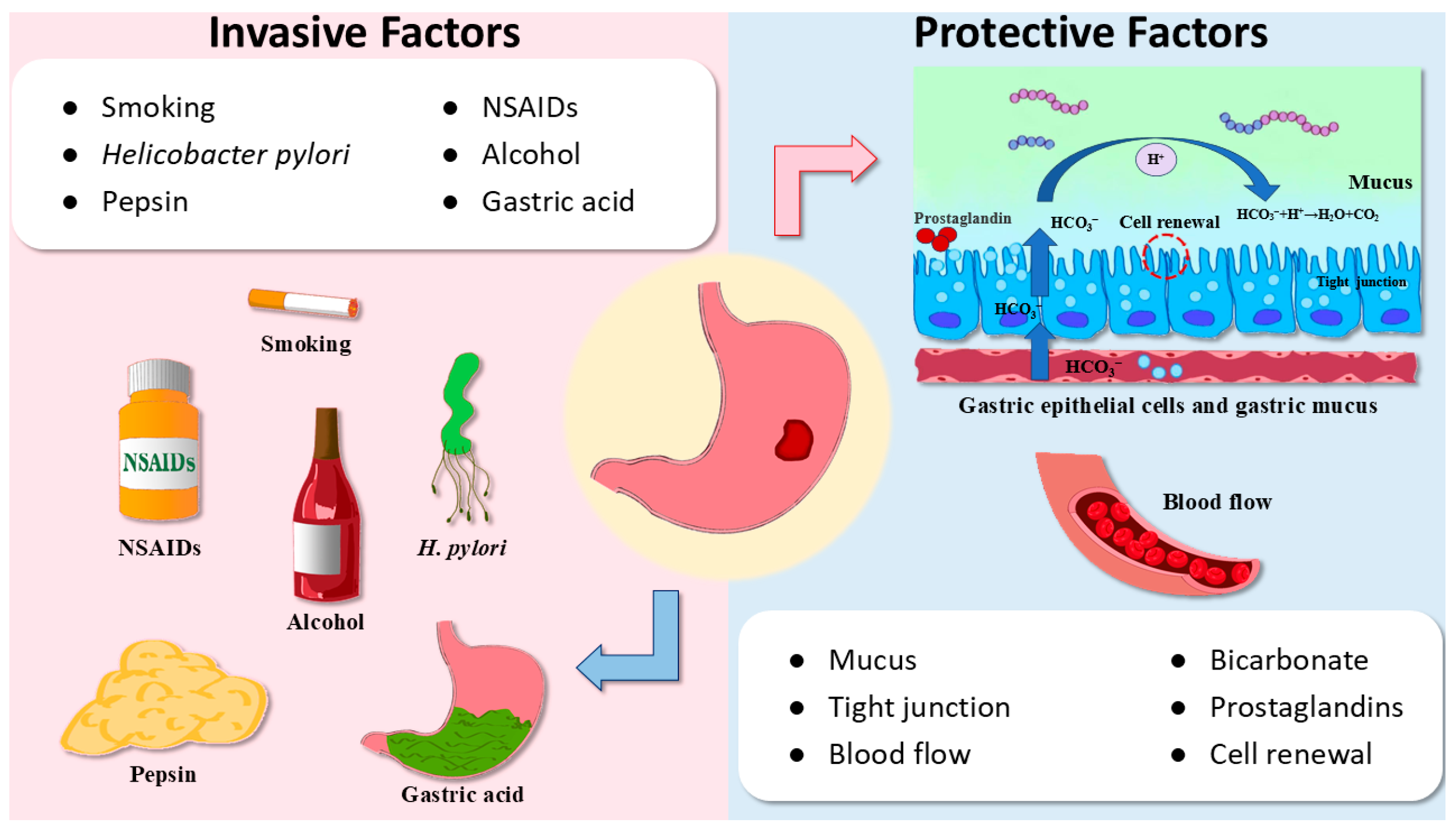
1. Introduction
2. The Activity of Triterpenoids in GU
2.1. Dammarane-Type Triterpenoids
2.1.1. Ginsenoside Rb1, Ginsenoside Rd, and Ginsenoside Rg3
2.1.2. Ginsenoside Rh4 and Protopanaxatriol
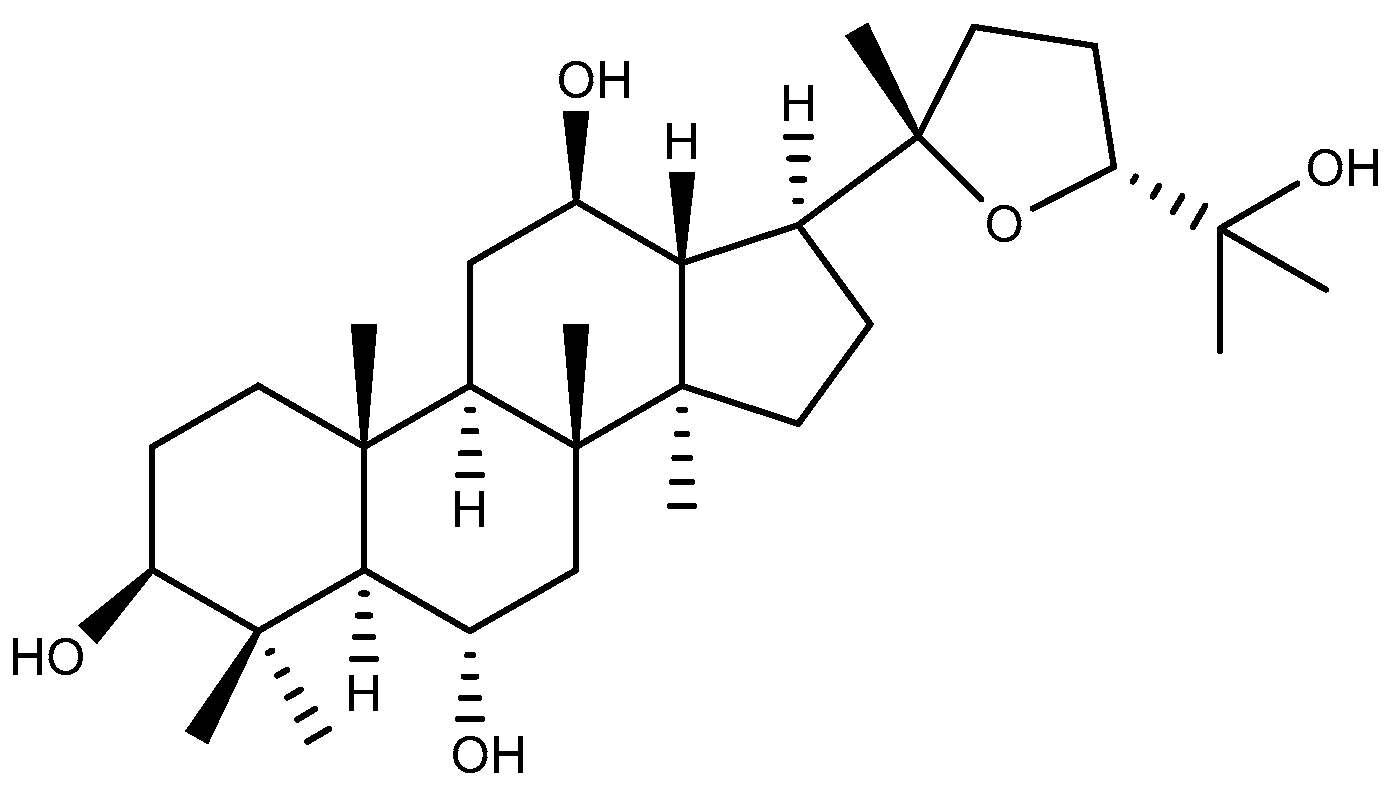
2.1.3. Ocotillol
2.2. Cycloartane-Type Triterpenoid
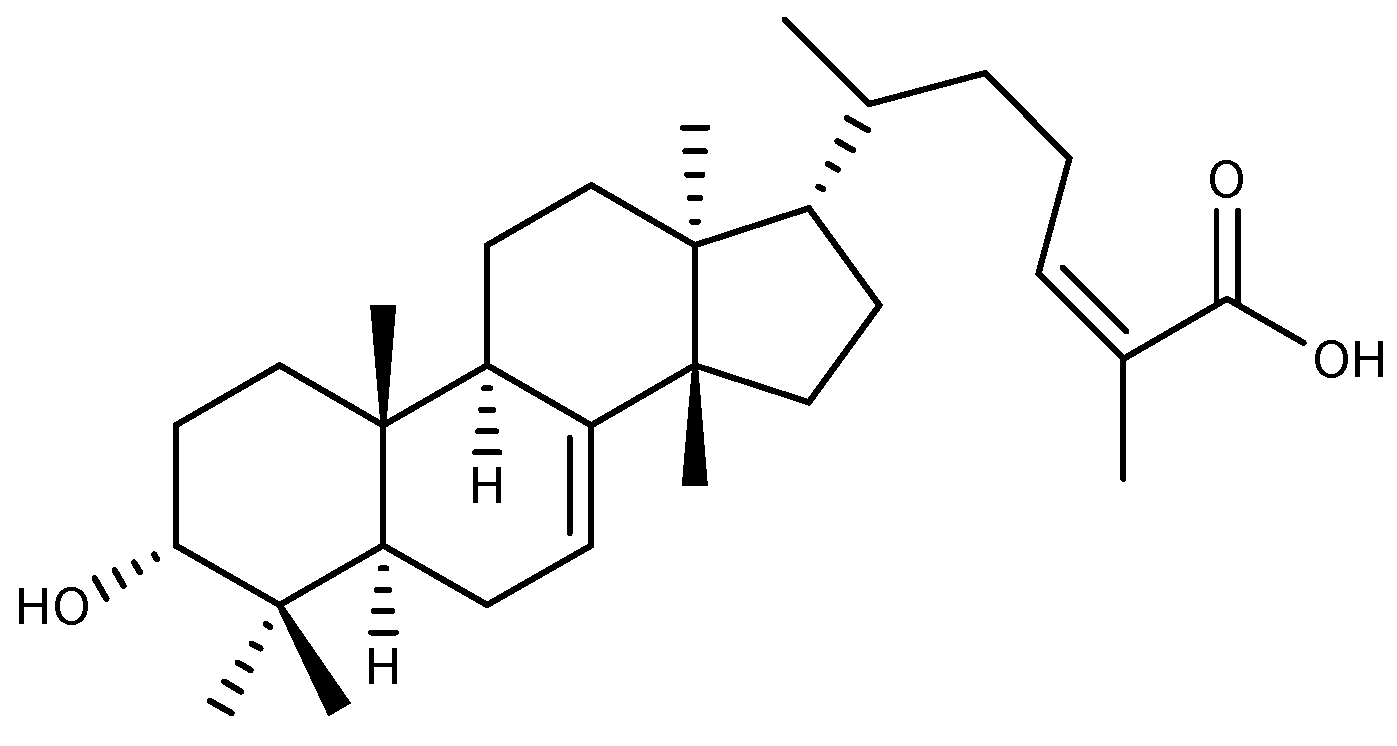
2.3. Tirucallane-Type Triterpenoid
2.4. Oleanane-Type Triterpenoids
2.4.1. Oleanolic Acid
2.4.2. Araloside A
2.4.3. 18β-Glycyrrhetinic Acid
2.4.4. Soyasaponin Bb
2.4.5. δ-Amyrone
2.4.6. Maslinic Acid
2.4.7. α-Boswellic Acid
2.5. Ursane-Type Triterpenoids
2.5.1. Ursolic Acid
2.5.2. Tormentic Acid
2.5.3. Asiaticoside
2.5.4. Niga-Ichigoside F1
2.6. Lupane-Type Triterpenoids
2.6.1. Lupeol
2.6.2. Betulinic Acid
2.7. Friedelane-Type Triterpenoid
2.8. Other Triterpenoids
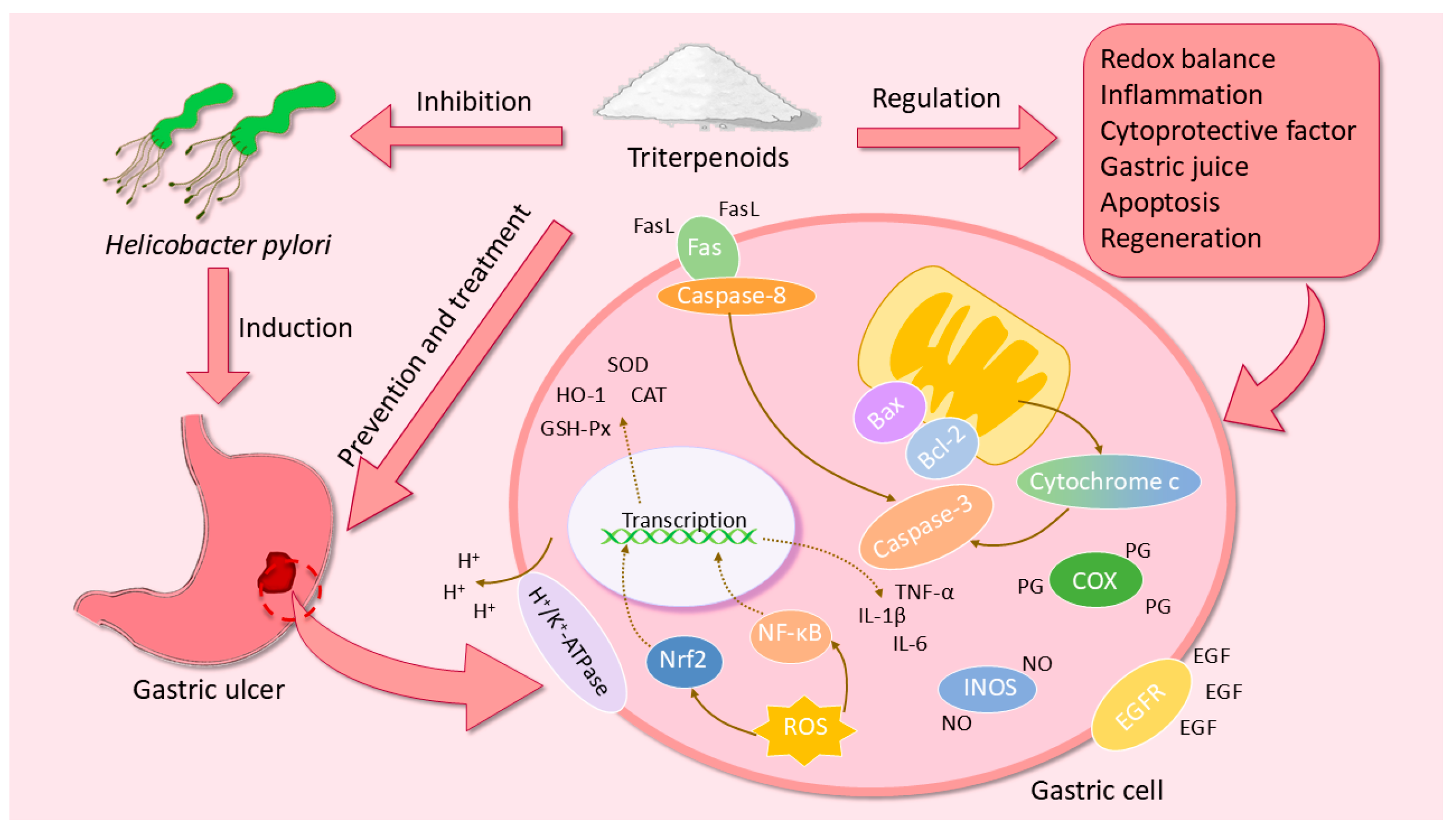
3. The Pharmacological Mechanisms of Triterpenoids in GU
3.1. Regulation of Redox Balance
3.2. Regulation of Inflammatory Cytokines
3.3. Regulation of Gastric Mucosal Cytoprotective Factor
3.4. Regulation of the Acidity and Viscosity of Gastric Juice
3.5. Inhibition of Apoptosis
3.6. Inhibition of Helicobacter Pylori
3.7. Promotion of GU Healing
4. The Animal Models Employed in Research on Triterpenoids in GU
5. The Current Limitations and Challenges of Triterpenoids in GU
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Bax | Bcl-2-associated X protein |
| Bcl-2 | B-cell lymphoma-2 |
| CAT | Catalase |
| COX-1 | Cyclooxygenase-1 |
| COX-2 | Cyclooxygenase-2 |
| EGF | Epidermal growth factor |
| EGFR | Epidermal growth factor receptor |
| ET-1 | Endothelin-1 |
| EtOH | Ethanol |
| Fas | Factor-related Apoptosis |
| FasL | Factor-related Apoptosis Ligand |
| GSH | Glutathione |
| GSH-Px | Glutathione peroxidase |
| GU | Gastric ulcer |
| H. pylori. | Helicobacter pylori |
| H2S | Hydrogen sulfide |
| HCl | Hydrochloric acid |
| HO-1 | Heme oxygenase-1 |
| HSP 70 | Heat shock protein 70 |
| IL-10 | Interleukin-10 |
| IL-1β | Interleukin-1β |
| IL-4 | Interleukin-4 |
| IL-6 | Interleukin-6 |
| iNOS | Inducible nitric oxide synthase |
| LTB4 | Leukotriene B4 |
| MCP-1 | Monocyte chemoattractant protein-1 |
| MDA | Malondialdehyde |
| MPO | Myeloperoxidase |
| NF-κB | Nuclear factor kappa-B |
| NO | Nitric oxide |
| NP-SH | Non-protein sulfhydryl groups |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSAIDs | Non-steroidal anti-inflammatory drugs |
| PGE2 | Prostaglandin E2 |
| PGs | Prostaglandins |
| ROS | Reactive Oxygen Species |
| SOD | Superoxide dismutase |
| TNF-α | Tumor necrosis factor α |
References
- Gong, H.; Zhao, N.; Zhu, C.; Luo, L.; Liu, S. Treatment of gastric ulcer, traditional Chinese medicine may be a better choice. J. Ethnopharmacol. 2024, 324, 117793. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.M.; Alatawi, N.M.; Bagalagel, A.; Diri, R.; Noor, A.; Almasri, D.; Bakhsh, H.T.; Kutbi, H.I.; Ashy, N.; Al-Gayyar, M.M.H. Genistein ameliorated experimentally induced gastric ulcer in rats via inhibiting gastric tissues fibrosis by modulating Wnt/β-catenin/TGF-β/PKB pathway. Redox Rep. 2023, 28, 2218679. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, M.K.; Bodakhe, S.H.; Gupta, S.K. Assessment of the antiulcer potential of Moringa oleifera root-bark extract in rats. J. Acupunct. Meridian Stud. 2013, 6, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Yang, Q.; Tian, T.; Chang, Y.; Li, Y.; Duan, L.R.; Li, H.; Wang, S.W. Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: Involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed. Pharmacother. 2020, 126, 110075. [Google Scholar] [CrossRef]
- Guo, Y.; Wu, Y.; Huang, T.; Huang, D.; Zeng, Q.; Wang, Z.; Hu, Y.; Liang, P.; Chen, H.; Zheng, Z.; et al. Licorice flavonoid ameliorates ethanol-induced gastric ulcer in rats by suppressing apoptosis via PI3K/AKT signaling pathway. J. Ethnopharmacol. 2024, 325, 117739. [Google Scholar] [CrossRef]
- Feng, L.; A, L.; Li, H.; Mu, X.; Ta, N.; Bai, L.; Fu, M.; Chen, Y. Pharmacological Mechanism of Aucklandiae Radix against Gastric Ulcer Based on Network Pharmacology and In Vivo Experiment. Medicina 2023, 59, 666. [Google Scholar] [CrossRef]
- Lanas, A.; Chan, F.K.L. Peptic ulcer disease. Lancet 2017, 390, 613–624. [Google Scholar] [CrossRef]
- Liu, Q.; Tang, J.; Chen, S.; Hu, S.; Shen, C.; Xiang, J.; Chen, N.; Wang, J.; Ma, X.; Zhang, Y.; et al. Berberine for gastric cancer prevention and treatment: Multi-step actions on the Correa’s cascade underlie its therapeutic effects. Pharmacol. Res. 2022, 184, 106440. [Google Scholar] [CrossRef]
- Paragomi, P.; Dabo, B.; Pelucchi, C.; Bonzi, R.; Bako, A.T.; Sanusi, N.M.; Nguyen, Q.H.; Zhang, Z.F.; Palli, D.; Ferraroni, M.; et al. The Association between Peptic Ulcer Disease and Gastric Cancer: Results from the Stomach Cancer Pooling (StoP) Project Consortium. Cancers 2022, 14, 4905. [Google Scholar] [CrossRef]
- Mousavi, T.; Nikfar, S.; Abdollahi, M. The pharmacotherapeutic management of duodenal and gastric ulcers. Expert Opin. Pharmacother. 2022, 23, 63–89. [Google Scholar] [CrossRef]
- Begg, M.; Tarhuni, M.; Fotso, M.N.; Gonzalez, N.A.; Sanivarapu, R.R.; Osman, U.; Latha Kumar, A.; Sadagopan, A.; Mahmoud, A.; Khan, S. Comparing the Safety and Efficacy of Proton Pump Inhibitors and Histamine-2 Receptor Antagonists in the Management of Patients with Peptic Ulcer Disease: A Systematic Review. Cureus 2023, 15, e44341. [Google Scholar] [CrossRef] [PubMed]
- Clarke, K.; Adler, N.; Agrawal, D.; Bhakta, D.; Sata, S.S.; Singh, S.; Gupta, A.; Pahwa, A.; Pherson, E.; Sun, A.; et al. Indications for the Use of Proton Pump Inhibitors for Stress Ulcer Prophylaxis and Peptic Ulcer Bleeding in Hospitalized Patients. Am. J. Med. 2022, 135, 313–317. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, A.; O’Morain, C.A.; Ford, A.C. Population screening and treatment of Helicobacter pylori infection. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 230–240. [Google Scholar] [CrossRef]
- Luo, J.H.; Zou, W.S.; Li, J.; Liu, W.; Huang, J.; Wu, H.W.; Shen, J.L.; Li, F.; Yuan, J.S.; Tao, A.K.; et al. Untargeted serum and liver metabolomics analyses reveal the gastroprotective effect of polysaccharide from Evodiae fructus on ethanol-induced gastric ulcer in mice. Int. J. Biol. Macromol. 2023, 232, 123481. [Google Scholar] [CrossRef]
- Yi, L.; Lu, Y.; Yu, S.; Cheng, Q.; Yi, L. Formononetin inhibits inflammation and promotes gastric mucosal angiogenesis in gastric ulcer rats through regulating NF-κB signaling pathway. J. Recept. Signal Transduct. Res. 2022, 42, 16–22. [Google Scholar] [CrossRef]
- Abd-Alla, H.I.; Ibrahim Fouad, G.; Ahmed, K.A.; Shaker, K. Alloimperatorin from Ammi majus fruits mitigates Piroxicam-provoked gastric ulcer and hepatorenal toxicity in rats via suppressing oxidative stress and apoptosis. Biomarkers 2022, 27, 727–742. [Google Scholar] [CrossRef]
- Ren, S.; Wei, Y.; Niu, M.; Li, R.; Wang, R.; Wei, S.; Wen, J.; Wang, D.; Yang, T.; Chen, X.; et al. Mechanism of rutaecarpine on ethanol-induced acute gastric ulcer using integrated metabolomics and network pharmacology. Biomed. Pharmacother. 2021, 138, 111490. [Google Scholar] [CrossRef]
- Szlasa, W.; Ślusarczyk, S.; Nawrot-Hadzik, I.; Abel, R.; Zalesińska, A.; Szewczyk, A.; Sauer, N.; Preissner, R.; Saczko, J.; Drąg, M.; et al. Betulin and Its Derivatives Reduce Inflammation and COX-2 Activity in Macrophages. Inflammation 2023, 46, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Guo, Y.Y.; Cen, X.F.; Qiu, H.L.; Chen, S.; Zeng, X.F.; Zeng, Q.; Xu, M.; Tang, Q.Z. Lupeol protects against cardiac hypertrophy via TLR4-PI3K-Akt-NF-κB pathways. Acta Pharmacol. Sin. 2022, 43, 1989–2002. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhao, Y.; Fu, X.; Chen, Q.; Tang, Y.; Gao, X. Naturally occurring triterpene Lupane exerts anticancer effects on colorectal cancer cells via induction of apoptosis and autophagy and suppresses cell migration and invasion by targeting MMP-9. J. BUON 2020, 25, 884–889. [Google Scholar]
- Chung, P.Y.K.; Gan, M.Y.; Chin, B.Y. Pentacyclic Triterpenoids as Antibiofilm Agents against Methicillinresistant and Biofilm-forming Staphylococcus aureus (MRSA). Curr. Pharm. Biotechnol. 2022, 23, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Abdullah; Khan, M.A.; Adhikari, A. Radical Scavenging, Anti-Inflammatory, and Hepatoprotective Activities of Pentacyclic Triterpene isolated from Rosa webbiana. Curr. Drug Targets 2023, 24, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Farina, C.; Pinza, M.; Pifferi, G. Synthesis and anti-ulcer activity of new derivatives of glycyrrhetic, oleanolic and ursolic acids. Farmaco 1998, 53, 22–32. [Google Scholar] [CrossRef]
- Yao, W.; Guan, Y. Ginsenosides in cancer: A focus on the regulation of cell metabolism. Biomed. Pharmacother. 2022, 156, 113756. [Google Scholar] [CrossRef]
- Shah, M.A.; Abuzar, S.M.; Ilyas, K.; Qadees, I.; Bilal, M.; Yousaf, R.; Kassim, R.M.T.; Rasul, A.; Saleem, U.; Alves, M.S.; et al. Ginsenosides in cancer: Targeting cell cycle arrest and apoptosis. Chem. Biol. Interact. 2023, 382, 110634. [Google Scholar] [CrossRef]
- Jeong, C.S.; Hyun, J.E.; Kim, Y.S. Ginsenoside Rb1: The anti-ulcer constituent from the head of Panax ginseng. Arch. Pharm. Res. 2003, 26, 906–911. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Sugimoto, S.; Nakamura, S.; Sakumae, H.; Matsuda, H. Medicinal flowers. XVI. New dammarane-type triterpene tetraglycosides and gastroprotective principles from flower buds of Panax ginseng. Chem. Pharm. Bull. 2007, 55, 1034–1038. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Wang, C.; Li, J.; Xiong, L.; Wang, Z.; Liu, J.; Li, P. Evaluation of the gastroprotective effects of 20 (S)-ginsenoside Rg3 on gastric ulcer models in mice. J. Ginseng Res. 2019, 43, 550–561. [Google Scholar] [CrossRef]
- Chen, J.; Duan, Z.; Liu, Y.; Fu, R.; Zhu, C. Ginsenoside Rh4 Suppresses Metastasis of Esophageal Cancer and Expression of c-Myc via Targeting the Wnt/β-Catenin Signaling Pathway. Nutrients 2022, 14, 3042. [Google Scholar] [CrossRef]
- Dong, F.; Qu, L.; Duan, Z.; He, Y.; Ma, X.; Fan, D. Ginsenoside Rh4 inhibits breast cancer growth through targeting histone deacetylase 2 to regulate immune microenvironment and apoptosis. Bioorg. Chem. 2023, 135, 106537. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, Z.; Qu, L.; Zhang, Y.; Zhu, C.; Fan, D. Gastroprotective effects of ginsenoside Rh4 against ethanol-induced gastric mucosal injury by inhibiting the MAPK/NF-κB signaling pathway. Food Funct. 2023, 14, 5167–5181. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Chu, S.F.; Li, J.P.; Zhang, Z.; Yan, J.Q.; Wen, Z.L.; Xia, C.Y.; Mou, Z.; Wang, Z.Z.; He, W.B.; et al. Protopanaxtriol protects against 3-nitropropionic acid-induced oxidative stress in a rat model of Huntington’s disease. Acta Pharmacol. Sin. 2015, 36, 311–322. [Google Scholar] [CrossRef]
- Lu, B.; Wang, D.; Xie, D.; Wu, C.; Sun, M. 20(S)-Protopanaxatriol ameliorates MAFLD by inhibiting NLRP3 inflammasome. Eur. J. Pharmacol. 2023, 940, 175468. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Tan, L.; Liu, J.; Fu, D.; Wang, C.; Li, P.; Li, Z.; Liu, J. Integrated Metabolomics and Network Pharmacology to Decipher the Latent Mechanisms of Protopanaxatriol against Acetic Acid-Induced Gastric Ulcer. Int. J. Mol. Sci. 2022, 23, 12097. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Yang, J.; Wang, W.; Zhang, J.; Zhang, R.; Meng, Q. Discovery, semisynthesis, biological activities, and metabolism of ocotillol-type saponins. J. Ginseng Res. 2017, 41, 373–378. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, K.; Xu, S.; Kong, L.; Bi, Y.; Li, X. Recent Advances in the Semisynthesis, Modifications and Biological Activities of Ocotillol-Type Triterpenoids. Molecules 2020, 25, 5562. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yuan, Y.; Pan, H.; Hsu, A.C.; Chen, J.; Liu, J.; Li, P.; Wang, F. Protective Effect of Ocotillol, the Derivate of Ocotillol-Type Saponins in Panax Genus, against Acetic Acid-Induced Gastric Ulcer in Rats Based on Untargeted Metabolomics. Int. J. Mol. Sci. 2020, 21, 2577. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, C.; Gao, L.; Du, G.; Qin, X. Astragaloside IV derived from Astragalus membranaceus: A research review on the pharmacological effects. Adv. Pharmacol. 2020, 87, 89–112. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, B.; Liang, D.; Quan, X.; Gu, R.; Meng, Z.; Gan, H.; Wu, Z.; Sun, Y.; Liu, S.; et al. Pharmacological Effects of Astragaloside IV: A Review. Molecules 2023, 28, 6118. [Google Scholar] [CrossRef]
- Navarrete, A.; Arrieta, J.; Terrones, L.; Abou-Gazar, H.; Calis, I. Gastroprotective effect of Astragaloside IV: Role of prostaglandins, sulfhydryls and nitric oxide. J. Pharm. Pharmacol. 2005, 57, 1059–1064. [Google Scholar] [CrossRef]
- Mao, S.; Yang, G.; Li, W.; Zhang, J.; Liang, H.; Li, J.; Zhang, M. Gastroprotective Effects of Astragaloside IV against Acute Gastric Lesion in Rats. PLoS ONE 2016, 11, e0148146. [Google Scholar] [CrossRef]
- Fan, D.D.; Lin, S.; Song, Y.P.; Wang, Z.Y.; Liu, B.; Gao, S.N.; Fan, Y.H.; Zhu, S.; Li, S.; Jiang, L. Astragaloside IV protects rat gastric mucosa against aspirin-induced damage. Int. Immunopharmacol. 2016, 41, 47–55. [Google Scholar] [CrossRef]
- Navarrete, A.; Martínez-Uribe, L.; Reyes-Trejo, B. Gastroprotective Activity of the Stem Bark of Amphipterygium adstringens in Rats. Phytoteraphy Res. 1998, 12, 1–4. [Google Scholar]
- Arrieta, J.; Benitez, J.; Flores, E.; Castillo, C.; Navarrete, A. Purification of gastroprotective triterpenoids from the stem bark of Amphipterygium adstringens; role of prostaglandins, sulfhydryls, nitric oxide and capsaicin-sensitive neurons. Planta Med. 2003, 69, 905–909. [Google Scholar] [CrossRef]
- Pineda-Peña, E.A.; Orona-Ortiz, A.; Velázquez-Moyado, J.A.; Tavares-Carvalho, J.C.; Chávez-Piña, A.E.; Balderas-López, J.L.; Navarrete, A. Anti-inflammatory, antioxidant, and gaso-protective mechanism of 3α-hydroxymasticadienoic acid and diligustilide combination on indomethacin gastric damage. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1501–1513. [Google Scholar] [CrossRef]
- Pollier, J.; Goossens, A. Oleanolic acid. Phytochemistry 2012, 77, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic Acid: Extraction, Characterization and Biological Activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, L.; Rodriguez, J.A.; Schmeda-Hirschmann, G. Gastroprotective activity of oleanolic acid derivatives on experimentally induced gastric lesions in rats and mice. J. Pharm. Pharmacol. 2002, 54, 583–588. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Astudillo, L.; Schmeda-Hirschmann, G. Oleanolic acid promotes healing of acetic acid-induced chronic gastric lesions in rats. Pharmacol. Res. 2003, 48, 291–294. [Google Scholar] [CrossRef]
- Sánchez, M.; Theoduloz, C.; Schmeda-Hirschmann, G.; Razmilic, I.; Yáñez, T.; Rodríguez, J.A. Gastroprotective and ulcer-healing activity of oleanolic acid derivatives: In Vitro-In Vivo relationships. Life Sci. 2006, 79, 1349–1356. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, X.; Du, M.; Li, F.; Xiao, M.; Zhang, W. Synergistic Antioxidant Effects of Araloside A and L-Ascorbic Acid on H(2)O(2)-Induced HEK293 Cells: Regulation of Cellular Antioxidant Status. Oxid. Med. Cell. Longev. 2021, 2021, 9996040. [Google Scholar] [CrossRef]
- Lee, E.B.; Kim, O.J.; Kang, S.S.; Jeong, C. Araloside A, an antiulcer constituent from the root bark of Aralia elata. Biol. Pharm. Bull. 2005, 28, 523–526. [Google Scholar] [CrossRef]
- He, H.; Li, X.; Yu, H.; Zhu, S.; He, Y.; Komatsu, K.; Guo, D.; Li, X.; Wang, J.; Luo, H.; et al. Gastroprotective effect of araloside A on ethanol- and aspirin-induced gastric ulcer in mice: Involvement of H(+)/K(+)-ATPase and mitochondrial-mediated signaling pathway. J. Nat. Med. 2019, 73, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Brand, E.; Wang, W.; Zhao, Z. Licorice: Resources, applications in ancient and modern times. J. Ethnopharmacol. 2022, 298, 115594. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Gong, J.; Yong, X.; Li, Y.; Wang, S. A review of typical biological activities of glycyrrhetinic acid and its derivatives. RSC Adv. 2024, 14, 6557–6597. [Google Scholar] [CrossRef]
- Yano, S.; Harada, M.; Watanabe, K.; Nakamaru, K.; Hatakeyama, Y.; Shibata, S.; Takahashi, K.; Mori, T.; Hirabayashi, K.; Takeda, M.; et al. Antiulcer activities of glycyrrhetinic acid derivatives in experimental gastric lesion models. Chem. Pharm. Bull. 1989, 37, 2500–2504. [Google Scholar] [CrossRef] [PubMed]
- Dehpour, A.R.; Zolfaghari, M.E.; Samadian, T.; Kobarfard, F.; Faizi, M.; Assari, M. Antiulcer activities of liquorice and its derivatives in experimental gastric lesion induced by ibuprofen in rats. Int. J. Pharm. 1995, 119, 133–138. [Google Scholar] [CrossRef]
- Krausse, R.; Bielenberg, J.; Blaschek, W.; Ullmann, U. In vitro anti-Helicobacter pylori activity of Extractum liquiritiae, glycyrrhizin and its metabolites. J. Antimicrob. Chemother. 2004, 54, 243–246. [Google Scholar] [CrossRef]
- Cao, D.; Jiang, J.; You, L.; Jia, Z.; Tsukamoto, T.; Cai, H.; Wang, S.; Hou, Z.; Suo, Y.E.; Cao, X. The Protective Effects of 18β-Glycyrrhetinic Acid on Helicobacter pylori-Infected Gastric Mucosa in Mongolian Gerbils. Biomed Res. Int. 2016, 2016, 4943793. [Google Scholar] [CrossRef]
- MacDonell, E.C.; Rajcan, I. Identification of quantitative trait loci associated with soyasaponin I concentration in soybean seed. Theor. Appl. Genet. 2018, 131, 2157–2165. [Google Scholar] [CrossRef]
- Morsi, A.A.; Shawky, L.M.; Shawky, T.M.; Bahr, M.H.; Alnasr, M.T.A.; El Bana, E. Targeting NF-κB/COX-2 signaling by soyasaponin I alleviates diclofenac-induced gastric ulceration in male albino rats. Cell Biochem. Funct. 2024, 42, e3927. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Yao, H.; Li, W.; Mu, Q.; Li, H.; Hu, H.; Li, Y.; Huang, H. δ-Amyrone, a specific inhibitor of cyclooxygenase-2, exhibits anti-inflammatory effects in vitro and in vivo of mice. Int. Immunopharmacol. 2014, 21, 112–118. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Niu, X.; Wang, Y.; Zhang, H.; Li, H.; Mu, Q. Protective effect of δ-amyrone against ethanol-induced gastric ulcer in mice. Immunobiology 2015, 220, 798–806. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Mena, G.; Sánchez-González, M.; Juan, M.E.; Planas, J.M. Maslinic acid, a natural phytoalexin-type triterpene from olives—A promising nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef]
- da Rosa, R.L.; Nesello LÂ, N.; Mariano, L.N.B.; Somensi, L.B.; Campos, A.; Pinheiro, A.M.; Costa, S.; Rial, M.; Tozzo, M.; Cechinel-Filho, V.; et al. Gastroprotective activity of the methanol extract from peels of Plinia edulis (Vell.) Sobral fruits and its isolated triterpenes: Maslinic and ursolic acids. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 95–101. [Google Scholar] [CrossRef]
- Kosolapov, D.; Jáč, P.; Riasová, P.; Poušková, J.; Polášek, M.; Nováková, L. Advances and Challenges in the Analysis of Boswellic Acids by Separation Methods. Crit. Rev. Anal. Chem. 2024, 10, 1–27. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, J.; Ding, Y.; Ma, Y.; Shang, P.; Liu, T.; Hui, G.; Wang, L.; Wang, M.; Zhu, Z.; et al. Alpha-boswellic acid protects against ethanol-induced gastric injury in rats: Involvement of nuclear factor erythroid-2-related factor 2/heme oxygenase-1 pathway. J. Pharm. Pharmacol. 2016, 68, 514–522. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.W.; No, M.H.; Rhee, B.D.; Ko, K.S.; Kwak, H.B.; Han, J. Ursolic acid in health and disease. Korean J. Physiol. Pharmacol. 2018, 22, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Kashyap, D.; Tuli, H.S.; Sharma, A.K. Ursolic acid (UA): A metabolite with promising therapeutic potential. Life Sci. 2016, 146, 201–213. [Google Scholar] [CrossRef]
- Pandey, D.; Joshi, A.; Hemalatha, S. Anti-Ulcer Study of Standardized Ethanol Root Extract of Aganosma Dichotoma and Isolated Ursolic Acid. Int. J. Pharm. Pharm. Sci. 2017, 9, 172. [Google Scholar] [CrossRef][Green Version]
- Elshamy, A.I.; Farrag, A.-R.H.; Mohamed, S.H.; Ali, N.A.; Mohamed, T.A.; Menshawy, M.M.; Zaglool, A.W.; Efferth, T.; Hegazy, M.-E.F. Gastroprotective effects of ursolic acid isolated from Ochrosia elliptica on ethanol-induced gastric ulcer in rats. Med. Chem. Res. 2020, 29, 113–125. [Google Scholar] [CrossRef]
- Olech, M.; Ziemichód, W.; Nowacka-Jechalke, N. The Occurrence and Biological Activity of Tormentic Acid-A Review. Molecules 2021, 26, 3797. [Google Scholar] [CrossRef] [PubMed]
- He, J.Y.; Li, J.; Zhang, Y.Y.; He, H.B.; He, Y.M.; Xu, D.X.; Wang, X.; Wu, H.Y.; Zhang, J.H.; Jahid, H.; et al. Tormentic acid, a triterpenoid isolated from the fruits of Chaenomeles speciose, protected indomethacin-induced gastric mucosal lesion via modulating miR-139 and the CXCR4/CXCL12/PLC/PKC/Rho a/MLC pathway. Pharm. Biol. 2023, 61, 1343–1363. [Google Scholar] [CrossRef]
- Sun, B.; Wu, L.; Wu, Y.; Zhang, C.; Qin, L.; Hayashi, M.; Kudo, M.; Gao, M.; Liu, T. Therapeutic Potential of Centella asiatica and Its Triterpenes: A Review. Front. Pharmacol. 2020, 11, 568032. [Google Scholar] [CrossRef]
- He, Z.; Hu, Y.; Niu, Z.; Zhong, K.; Liu, T.; Yang, M.; Ji, L.; Hu, W. A review of pharmacokinetic and pharmacological properties of asiaticoside, a major active constituent of Centella asiatica (L.) Urb. J. Ethnopharmacol. 2023, 302 Pt A, 115865. [Google Scholar] [CrossRef]
- Cheng, C.L.; Guo, J.S.; Luk, J.; Koo, M.W. The healing effects of Centella extract and asiaticoside on acetic acid induced gastric ulcers in rats. Life Sci. 2004, 74, 2237–2249. [Google Scholar] [CrossRef]
- Guo, J.S.; Cheng, C.L.; Koo, M.W. Inhibitory effects of Centella asiatica water extract and asiaticoside on inducible nitric oxide synthase during gastric ulcer healing in rats. Planta Med. 2004, 70, 1150–1154. [Google Scholar] [CrossRef]
- Tonin, T.D.; Thiesen, L.C.; de Oliveira Nunes, M.L.; Broering, M.F.; Donato, M.P.; Goss, M.J.; Petreanu, M.; Niero, R.; Machado, I.D.; Santin, J.R. Rubus imperialis (Rosaceae) extract and pure compound niga-ichigoside F1: Wound healing and anti-inflammatory effects. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 1235–1244. [Google Scholar] [CrossRef]
- Xia, S.F.; Shao, J.; Zhao, S.Y.; Qiu, Y.Y.; Teng, L.P.; Huang, W.; Wang, S.S.; Cheng, X.R.; Jiang, Y.Y. Niga-ichigoside F1 ameliorates high-fat diet-induced hepatic steatosis in male mice by Nrf2 activation. Food Funct. 2018, 9, 906–916. [Google Scholar] [CrossRef]
- Nam, J.H.; Jung, H.J.; Choi, J.; Lee, K.T.; Park, H.J. The anti-gastropathic and anti-rheumatic effect of niga-ichigoside F1 and 23-hydroxytormentic acid isolated from the unripe fruits of Rubus coreanus in a rat model. Biol. Pharm. Bull. 2006, 29, 967–970. [Google Scholar] [CrossRef]
- Berté, P.E.; da Silva Lopes, J.; Comandulli, N.G.; Rangel, D.W.; Monache, F.D.; Filho, V.C.; Niero, R.; de Andrade, S.F. Evaluation of the gastroprotective activity of the extracts, fractions, and pure compounds obtained from aerial parts of Rubus imperialis in different experimental models. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Sohag, A.A.M.; Hossain, M.T.; Rahaman, M.A.; Rahman, P.; Hasan, M.S.; Das, R.C.; Khan, M.K.; Sikder, M.H.; Alam, M.; Uddin, M.J.; et al. Molecular pharmacology and therapeutic advances of the pentacyclic triterpene lupeol. Phytomedicine 2022, 99, 154012. [Google Scholar] [CrossRef] [PubMed]
- Lira, S.R.; Rao, V.S.; Carvalho, A.C.; Guedes, M.M.; de Morais, T.C.; de Souza, A.L.; Trevisan, M.T.; Lima, A.F.; Chaves, M.H.; Santos, F.A. Gastroprotective effect of lupeol on ethanol-induced gastric damage and the underlying mechanism. Inflammopharmacology 2009, 17, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Oluwasegun, A.; Ogochukwu, U.; Ugochukwu, O.; Mussaddiq, I.; Bunyamin, A. Lupeol: A Triterpenoid Isolated from the Stem Bark of Hymenocardia Acida (tul.) Exhibits a van der Waal Antagonism on the Alpha Subunit of Gastric H+K+Atpase—A Promising Antiulcer Principle. Drug Res. 2023, 73, 448–458. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef]
- Onwuchekwa, C.; Oluwole, F.S. Anti-Gastric Ulcer Effect of Betulinic Acid in Male Albino Rats. Niger. J. Physiol. Sci. 2015, 30, 33–37. [Google Scholar]
- Singh, S.K.; Shrivastava, S.; Mishra, A.K.; Kumar, D.; Pandey, V.K.; Srivastava, P.; Pradhan, B.; Behera, B.C.; Bahuguna, A.; Baek, K.H. Friedelin: Structure, Biosynthesis, Extraction, and Its Potential Health Impact. Molecules 2023, 28, 7760. [Google Scholar] [CrossRef]
- Antonisamy, P.; Duraipandiyan, V.; Aravinthan, A.; Al-Dhabi, N.A.; Ignacimuthu, S.; Choi, K.C.; Kim, J.H. Protective effects of friedelin isolated from Azima tetracantha Lam. against ethanol-induced gastric ulcer in rats and possible underlying mechanisms. Eur. J. Pharmacol. 2015, 750, 167–175. [Google Scholar] [CrossRef]
- Lin, M.; Yang, S.; Huang, J.; Zhou, L. Insecticidal Triterpenes in Meliaceae: Plant Species, Molecules and Activities: Part I (Aphanamixis-Chukrasia). Int. J. Mol. Sci. 2021, 22, 13262. [Google Scholar] [CrossRef]
- Singh, R.; Mishra, V.; Pandeti, S.; Palit, G.; Barthwal, M.K.; Pandey, H.P.; Narender, T. Cytoprotective and Anti-secretory Effects of Azadiradione Isolated from the Seeds of Azadirachta indica (neem) on Gastric Ulcers in Rat Models. Phytother. Res. 2015, 29, 910–916. [Google Scholar] [CrossRef]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2020, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ermis, A.; Aritici Colak, G.; Acikel-Elmas, M.; Arbak, S.; Kolgazi, M. Ferulic Acid Treats Gastric Ulcer via Suppressing Oxidative Stress and Inflammation. Life 2023, 13, 388. [Google Scholar] [CrossRef]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Sun, L.; Zhang, Y.; Wang, Y.; Zheng, J. Imbalanced GSH/ROS and sequential cell death. J. Biochem. Mol. Toxicol. 2022, 36, e22942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.F.; Xie, J.H.; Xu, Y.F.; Liang, Y.Z.; Mo, Z.Z.; Jiang, W.W.; Chen, X.Y.; Liu, Y.H.; Yu, X.D.; Huang, P.; et al. Gastroprotective effect and mechanism of patchouli alcohol against ethanol, indomethacin and stress-induced ulcer in rats. Chem. Biol. Interact. 2014, 222, 27–36. [Google Scholar] [CrossRef]
- Balan, T.; Mohd Sani, M.H.; Suppaiah, V.; Mohtarrudin, N.; Suhaili, Z.; Ahmad, Z.; Zakaria, Z.A. Antiulcer activity of Muntingia calabura leaves involves the modulation of endogenous nitric oxide and nonprotein sulfhydryl compounds. Pharm. Biol. 2013, 52, 410–418. [Google Scholar] [CrossRef]
- Hobani, Y.H.; Mohan, S.; Shaheen, E.; Abdelhaleem, A.; Faruque Ahmad, M.; Bhatia, S.; Abou-Elhamd, A.S. Gastroprotective effect of low dose Eugenol in experimental rats against ethanol induced toxicity: Involvement of antiinflammatory and antioxidant mechanism. J. Ethnopharmacol. 2022, 289, 115055. [Google Scholar] [CrossRef]
- Yanaka, A. Role of NRF2 in protection of the gastrointestinal tract against oxidative stress. J. Clin. Biochem. Nutr. 2018, 63, 18–25. [Google Scholar] [CrossRef]
- Ye, H.Y.; Shang, Z.Z.; Zhang, F.Y.; Zha, X.Q.; Li, Q.M.; Luo, J.P. Dendrobium huoshanense stem polysaccharide ameliorates alcohol-induced gastric ulcer in rats through Nrf2-mediated strengthening of gastric mucosal barrier. Int. J. Biol. Macromol. 2023, 236, 124001. [Google Scholar] [CrossRef]
- Sallam, A.M.; Darwish, S.F.; El-Dakroury, W.A.; Radwan, E. Olmesartan niosomes ameliorates the Indomethacin-induced gastric ulcer in rats: Insights on MAPK and Nrf2/HO-1 signaling pathway. Pharm. Res. 2021, 38, 1821–1838. [Google Scholar] [CrossRef]
- Yang, T.; Wang, R.; Liu, H.; Wang, L.; Li, J.; Wu, S.; Chen, X.; Yang, X.; Zhao, Y. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis. Life Sci. 2021, 266, 118903. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.C. Macrophage polarization: Reaching across the aisle? J. Allergy Clin. Immunol. 2019, 143, 1348–1350. [Google Scholar] [CrossRef]
- Wang, C.; Peng, D.; Liu, Y.; Wu, Y.; Guo, P.; Wei, J. Agarwood Alcohol Extract Protects against Gastric Ulcer by Inhibiting Oxidation and Inflammation. Evid. Based Complement. Alternat. Med. 2021, 2021, 9944685. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Anshita, D.; Ravichandiran, V. MCP-1: Function, regulation, and involvement in disease. Int. Immunopharmacol. 2021, 101, 107598. [Google Scholar] [CrossRef]
- Watanabe, T.; Higuchi, K.; Hamaguchi, M.; Shiba, M.; Tominaga, K.; Fujiwara, Y.; Matsumoto, T.; Arakawa, T. Monocyte chemotactic protein-1 regulates leukocyte recruitment during gastric ulcer recurrence induced by tumor necrosis factor-alpha. Am. J. Physiol. Gastrointest. Liver Physiol. 2004, 287, G919–G928. [Google Scholar] [CrossRef]
- Wan, M.; Tang, X.; Stsiapanava, A.; Haeggström, J.Z. Biosynthesis of leukotriene B(4). Semin. Immunol. 2017, 33, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Peña, E.A.; Martínez-Pérez, Y.; Galicia-Moreno, M.; Navarrete, A.; Segovia, J.; Muriel, P.; Favari, L.; Castañeda-Hernández, G.; Chávez-Piña, A.E. Participation of the anti-inflammatory and antioxidative activity of docosahexaenoic acid on indomethacin-induced gastric injury model. Eur. J. Pharmacol. 2018, 818, 585–592. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.K.; Wang, Q.; Zhang, Z.; Li, B.; Zhou, Z.D.; Zhang, J.F.; Lin, C.; Chen, T.X.; Jin, Z.; et al. Diclofenac and eugenol hybrid with enhanced anti-inflammatory activity through activating HO-1 and inhibiting NF-κB pathway in vitro and in vivo. Eur. J. Med. Chem. 2023, 259, 115669. [Google Scholar] [CrossRef]
- Minatel, I.O.; Francisqueti, F.V.; Corrêa, C.R.; Lima, G.P. Antioxidant Activity of γ-Oryzanol: A Complex Network of Interactions. Int. J. Mol. Sci. 2016, 17, 1107. [Google Scholar] [CrossRef]
- Ren, S.; Wei, Y.; Wang, R.; Wei, S.; Wen, J.; Yang, T.; Chen, X.; Wu, S.; Jing, M.; Li, H.; et al. Rutaecarpine Ameliorates Ethanol-Induced Gastric Mucosal Injury in Mice by Modulating Genes Related to Inflammation, Oxidative Stress and Apoptosis. Front. Pharmacol. 2020, 11, 600295. [Google Scholar] [CrossRef]
- Yu, L.; Li, R.; Liu, W.; Zhou, Y.; Li, Y.; Qin, Y.; Chen, Y.; Xu, Y. Protective Effects of Wheat Peptides against Ethanol-Induced Gastric Mucosal Lesions in Rats: Vasodilation and Anti-Inflammation. Nutrients 2020, 12, 2355. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, W.; Lu, L.; Zheng, J.; Hu, X.; Yu, Z.; Zhu, L. Study on the antiulcer effects of Veronicastrum axillare on gastric ulcer in rats induced by ethanol based on tumor necrosis factor-α (TNF-α) and endothelin-1 (ET-1). Asian Pac. J. Trop Biomed. 2013, 3, 925–930. [Google Scholar] [CrossRef] [PubMed]
- Nishida, T.; Tsuji, S.; Kimura, A.; Tsujii, M.; Ishii, S.; Yoshio, T.; Shinzaki, S.; Egawa, S.; Irie, T.; Yasumaru, M.; et al. Endothelin-1, an ulcer inducer, promotes gastric ulcer healing via mobilizing gastric myofibroblasts and stimulates production of stroma-derived factors. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 290, G1041–G1050. [Google Scholar] [CrossRef] [PubMed]
- Banecki, K.; Dora, K.A. Endothelin-1 in Health and Disease. Int. J. Mol. Sci. 2023, 24, 11295. [Google Scholar] [CrossRef] [PubMed]
- Isik, M.; Ozbayer, C.; Donmez, D.B.; Colak, E.; Ustuner, M.C.; Erol, K.; Degirmenci, I. Effects of the probiotic, Lactobacillus rhamnosus GG, on ulcer pathogenesis, HSP70 stress protein and nitric oxide levels in stress induced ulcer. Biotech. Histochem. 2022, 97, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Abo-Elsoud, R.; Ahmed Mohamed Abdelaziz, S.; Attia Abd Eldaim, M.; Hazzaa, S.M. Moringa oleifera alcoholic extract protected stomach from bisphenol A-induced gastric ulcer in rats via its anti-oxidant and anti-inflammatory activities. Environ. Sci. Pollut. Res. Int. 2022, 29, 68830–68841. [Google Scholar] [CrossRef]
- Chen, G.; Xie, X.; Peng, F.; Wang, T.; Chen, J.; Li, G.; Liu, J.; Peng, C. Protective effect of the combination of essential oil from patchouli and tangerine peel against gastric ulcer in rats. J. Ethnopharmacol. 2022, 282, 114645. [Google Scholar] [CrossRef]
- Mabrok, H.B.; Mohamed, M.S. Induction of COX-1, suppression of COX-2 and pro-inflammatory cytokines gene expression by moringa leaves and its aqueous extract in aspirin-induced gastric ulcer rats. Mol. Biol. Rep. 2019, 46, 4213–4224. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, H.; Albayrak, A.; Bilici, M.; Cadirci, E.; Halici, Z. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 2010, 33, 224–234. [Google Scholar] [CrossRef]
- Takeuchi, K.; Amagase, K. Roles of Cyclooxygenase, Prostaglandin E2 and EP Receptors in Mucosal Protection and Ulcer Healing in the Gastrointestinal Tract. Curr. Pharm. Des. 2018, 24, 2002–2011. [Google Scholar] [CrossRef]
- Dejban, P.; Eslami, F.; Rahimi, N.; Takzare, N.; Jahansouz, M.; Dehpour, A.R. Involvement of nitric oxide pathway in the anti-inflammatory effect of modafinil on indomethacin-, stress-, and ethanol-induced gastric mucosal injury in rat. Eur. J. Pharmacol. 2020, 887, 173579. [Google Scholar] [CrossRef]
- Liang, T.Y.; Deng, R.M.; Li, X.; Xu, X.; Chen, G. The role of nitric oxide in peptic ulcer: A narrative review. Med. Gas Res. 2021, 11, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.M.; Lim, J.Y.; Kim, Y.; Kim, Y.J.; Choi, H.S.; Kim, E.S.; Keum, B.; Seo, Y.S.; Jeen, Y.T.; Lee, H.S.; et al. Benexate hydrochloride betadex modulates nitric oxide synthesis and cytokine expression in gastric ulcers. Exp. Ther. Med. 2016, 12, 573–580. [Google Scholar] [CrossRef]
- Lima-Júnior, R.C.; Figueiredo, A.A.; Freitas, H.C.; Melo, M.L.; Wong, D.V.; Leite, C.A.; Medeiros, R.P.; Marques-Neto, R.D.; Vale, M.L.; Brito, G.A.; et al. Involvement of nitric oxide on the pathogenesis of irinotecan-induced intestinal mucositis: Role of cytokines on inducible nitric oxide synthase activation. Cancer Chemother. Pharmacol. 2012, 69, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Magierowski, M.; Magierowska, K.; Kwiecien, S.; Brzozowski, T. Gaseous mediators nitric oxide and hydrogen sulfide in the mechanism of gastrointestinal integrity, protection and ulcer healing. Molecules 2015, 20, 9099–9123. [Google Scholar] [CrossRef] [PubMed]
- Jabbar, A.A.J.; Mothana, R.A.; Ameen Abdulla, M.; Othman Abdullah, F.; Abdul-Aziz Ahmed, K.; Rizgar Hussen, R.; Hawwal, M.F.; Fantoukh, O.I.; Hasson, S. Mechanisms of anti-ulcer actions of Prangos pabularia (L.) in ethanol-induced gastric ulcer in rats. Saudi Pharm. J. 2023, 31, 101850. [Google Scholar] [CrossRef]
- Abdoulrahman, K. Anti-ulcer effect of Ranunculus millefoliatus on absolute alcohol-induced stomach ulceration. Saudi J. Biol. Sci. 2023, 30, 103711. [Google Scholar] [CrossRef]
- El-Shiekh, R.A.; Salama, A.; Al-Mokaddem, A.K.; Bader, A.; Abdel-Sattar, E.A.; Russelioside, B. A pregnane glycoside for treatment of gastric ulcer via modulation of heat shock protein-70 and vascular endothelial growth factor. Steroids 2021, 165, 108759. [Google Scholar] [CrossRef]
- Ishihara, T.; Suemasu, S.; Asano, T.; Tanaka, K.; Mizushima, T. Stimulation of gastric ulcer healing by heat shock protein 70. Biochem. Pharmacol. 2011, 82, 728–736. [Google Scholar] [CrossRef]
- Herszényi, L.; Bakucz, T.; Barabás, L.; Tulassay, Z. Pharmacological Approach to Gastric Acid Suppression: Past, Present, and Future. Dig. Dis. 2020, 38, 104–111. [Google Scholar] [CrossRef]
- Engevik, A.C.; Kaji, I.; Goldenring, J.R. The Physiology of the Gastric Parietal Cell. Physiol. Rev. 2020, 100, 573–602. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.F.; Abdo, W.; Nabil, M.; Drissi, B.; El-Shazly, A.M.; Abdelfattah, M.A.O.; Sobeh, M. Apple (Malus domestica Borkh) leaves attenuate indomethacin-induced gastric ulcer in rats. Biomed. Pharmacother. 2023, 160, 114331. [Google Scholar] [CrossRef]
- Pelaseyed, T.; Bergström, J.H.; Gustafsson, J.K.; Ermund, A.; Birchenough, G.M.; Schütte, A.; van der Post, S.; Svensson, F.; Rodríguez-Piñeiro, A.M.; Nyström, E.E.; et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol. Rev. 2014, 260, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Somensi, L.B.; Costa, P.; Boeing, T.; Bolda Mariano, L.N.; de Gregório, E.; ATM, E.S.; Longo, B.; Locatelli, C.; de Souza, P.; Magalhães, C.G.; et al. Lupeol Stearate Accelerates Healing and Prevents Recurrence of Gastric Ulcer in Rodents. Evid. Based Complement. Alternat. Med. 2022, 2022, 6134128. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Hu, J.; Meng, Q.; Dong, X.; Wang, K.; Qi, Y.; Chu, C.; Zhang, X.; Hou, L. Daidzein induced apoptosis via down-regulation of Bcl-2/Bax and triggering of the mitochondrial pathway in BGC-823 cells. Cell Biochem. Biophys. 2013, 65, 197–202. [Google Scholar] [CrossRef]
- Ma, N.; Sun, Y.; Yi, J.; Zhou, L.; Cai, S. Chinese sumac (Rhus chinensis Mill.) fruits alleviate indomethacin-induced gastric ulcer in mice by improving oxidative stress, inflammation and apoptosis. J. Ethnopharmacol. 2022, 284, 114752. [Google Scholar] [CrossRef]
- Sahoo, G.; Samal, D.; Khandayataray, P.; Murthy, M.K. A Review on Caspases: Key Regulators of Biological Activities and Apoptosis. Mol. Neurobiol. 2023, 60, 5805–5837. [Google Scholar] [CrossRef]
- Luo, Y.; Fu, X.; Ru, R.; Han, B.; Zhang, F.; Yuan, L.; Men, H.; Zhang, S.; Tian, S.; Dong, B.; et al. CpG Oligodeoxynucleotides Induces Apoptosis of Human Bladder Cancer Cells via Caspase-3-Bax/Bcl-2-p53 Axis. Arch. Med. Res. 2020, 51, 233–244. [Google Scholar] [CrossRef]
- Cho, J.; Prashar, A.; Jones, N.L.; Moss, S.F. Helicobacter pylori Infection. Gastroenterol. Clin. N. Am. 2021, 50, 261–282. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Q.L.; Cheng, D.D.; Xu, W.T.; Lu, N.H. Adhesion and Invasion of Gastric Mucosa Epithelial Cells by Helicobacter pylori. Front. Cell. Infect. Microbiol. 2016, 6, 159. [Google Scholar] [CrossRef]
- Ansari, S.; Yamaoka, Y. Helicobacter pylori Virulence Factors Exploiting Gastric Colonization and its Pathogenicity. Toxins 2019, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- da Costa, D.M.; Pereira Edos, S.; Rabenhorst, S.H. What exists beyond cagA and vacA? Helicobacter pylori genes in gastric diseases. World J. Gastroenterol. 2015, 21, 10563–10572. [Google Scholar] [CrossRef]
- Wu, Y.; Guo, Y.; Huang, T.; Huang, D.; Liu, L.; Shen, C.; Jiang, C.; Wang, Z.; Chen, H.; Liang, P.; et al. Licorice flavonoid alleviates gastric ulcers by producing changes in gut microbiota and promoting mucus cell regeneration. Biomed. Pharmacother. 2023, 169, 115868. [Google Scholar] [CrossRef]
- Tarnawski, A.S.; Ahluwalia, A. The Critical Role of Growth Factors in Gastric Ulcer Healing: The Cellular and Molecular Mechanisms and Potential Clinical Implications. Cells 2021, 10, 1964. [Google Scholar] [CrossRef] [PubMed]
- Selim, H.M.; Negm, W.A.; Hawwal, M.F.; Hussein, I.A.; Elekhnawy, E.; Ulber, R.; Zayed, A. Fucoidan mitigates gastric ulcer injury through managing inflammation, oxidative stress, and NLRP3-mediated pyroptosis. Int. Immunopharmacol. 2023, 120, 110335. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, M.; Fan, X. Ethanol induces necroptosis in gastric epithelial cells in vitro. J. Food Biochem. 2021, 45, e13692. [Google Scholar] [CrossRef]
- Shams, S.G.E.; Eissa, R.G. Amelioration of ethanol-induced gastric ulcer in rats by quercetin: Implication of Nrf2/HO1 and HMGB1/TLR4/NF-κB pathways. Heliyon 2022, 8, e11159. [Google Scholar] [CrossRef]
- Tamura, M.; Ito, H.; Matsui, H.; Hyodo, I. Acetaldehyde is an oxidative stressor for gastric epithelial cells. J. Clin. Biochem. Nutr. 2014, 55, 26–31. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, Z.; Chai, J.; Zhu, D.; Miao, X.; Zhou, J.; Gu, X. ALDH2 ameliorates ethanol-induced gastric ulcer through suppressing NLPR3 inflammasome activation and ferroptosis. Arch. Biochem. Biophys. 2023, 743, 109621. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Mahmoud, Y.I.; Abd El-Ghffar, E.A. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed. Pharmacother. 2019, 109, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hurtado, P.A.; Garduño-Siciliano, L.; Domínguez-Verano, P.; Balderas-Cordero, D.; Gorgua-Jiménez, G.; Canales-Álvarez, O.; Canales-Martínez, M.M.; Rodríguez-Monroy, M.A. Propolis and Its Gastroprotective Effects on NSAID-Induced Gastric Ulcer Disease: A Systematic Review. Nutrients 2021, 13, 3169. [Google Scholar] [CrossRef]
- Musumba, C.; Pritchard, D.M.; Pirmohamed, M. Review article: Cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment. Pharmacol. Ther. 2009, 30, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J. Gastroenterol. 2012, 18, 2147–2160. [Google Scholar] [CrossRef]
- Al-Gabri, N.; Elnagar, G.M.; Saghir, S.A.M.; El-Shaibany, A.; Alnomasy, S.F.; Althafar, Z.M.; Elkomy, N.; Elaasser, M.M.; Abdoh, M.S.; Yosri, M. Preliminary Study of Gastroprotective Effect of Aloe perryi and Date Palm Extracts on Pyloric Ligation-Induced Gastric Ulcer in Experimental Rats. Biomed Res. Int. 2022, 2022, 9246785. [Google Scholar] [CrossRef]
- Asaad, G.F.; Saleh, D.O.; Mostafa, R.E.; Hassan, A.; Jaleel, G.A. Pylorus ligation-induced hyperacidity: Synergistic prophylactic effects of linagliptin and L-arginine via up-regulation of EP4 receptor subtype and improvement of vascular endothelial damage. Naunyn Schmiedebergs Arch. Pharmacol. 2024, 397, 1127–1139. [Google Scholar] [CrossRef]
- De Sales, I.R.P.; Formiga, R.O.; Machado, F.D.F.; Nascimento, R.F.; Pessoa, M.M.B.; Barros, M.; Vieira, G.C.; Gadelha, F.; Marinho, A.F.; Barbosa Filho, J.M.; et al. Cytoprotective, antioxidant and anti-inflammatory mechanism related to antiulcer activity of Cissampelos sympodialis Eichl. in animal models. J. Ethnopharmacol. 2018, 222, 190–200. [Google Scholar] [CrossRef]
- Wang, X.Y.; Yin, J.Y.; Zhao, M.M.; Liu, S.Y.; Nie, S.P.; Xie, M.Y. Gastroprotective activity of polysaccharide from Hericium erinaceus against ethanol-induced gastric mucosal lesion and pylorus ligation-induced gastric ulcer, and its antioxidant activities. Carbohydr. Polym. 2018, 186, 100–109. [Google Scholar] [CrossRef]
- Zaghlool, S.; Shehata, B.; Abo-Seif, A.; Abd El-Latif, H. Comparison between the Protective Effects of Famotidine, Ginger and Marshmallow on Pyloric Ligation-Induced Peptic Ulcer in Rats. J. Bioequiv. Availab. 2015, 7, 170–178. [Google Scholar] [CrossRef]
- Lu, Q.; Tang, H. Overexpression of HSP27 accelerates stress-induced gastric ulcer healing via the CXCL12/CXCR4 axis. Clin. Exp. Pharmacol. Physiol. 2024, 51, e13857. [Google Scholar] [CrossRef]
- Jia, Y.T.; Ma, B.; Wei, W.; Xu, Y.; Wang, Y.; Tang, H.T.; Xia, Z.F. Sustained activation of nuclear factor-kappaB by reactive oxygen species is involved in the pathogenesis of stress-induced gastric damage in rats. Crit. Care Med. 2007, 35, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.Q.; Xue, H.; Sun, H.J. Nervous mechanisms of restraint water-immersion stress-induced gastric mucosal lesion. World J. Gastroenterol. 2020, 26, 2533–2549. [Google Scholar] [CrossRef] [PubMed]
- Akmal, M.N.; Abdel Aziz, I.; Nur Azlina, M.F. Piper sarmentosum Roxb. methanolic extract prevents stress-induced gastric ulcer by modulating oxidative stress and inflammation. Front. Pharmacol. 2022, 13, 971443. [Google Scholar] [CrossRef]
- Yu, Y.; Jia, T.Z.; Cai, Q.; Jiang, N.; Ma, M.Y.; Min, D.Y.; Yuan, Y. Comparison of the anti-ulcer activity between the crude and bran-processed Atractylodes lancea in the rat model of gastric ulcer induced by acetic acid. J. Ethnopharmacol. 2015, 160, 211–218. [Google Scholar] [CrossRef]
- Wang, X.Y.; Wang, M.; Yin, J.Y.; Song, Y.H.; Wang, Y.X.; Nie, S.P.; Xie, M.Y. Gastroprotective activity of polysaccharide from the fruiting body of Hericium erinaceus against acetic acid-induced gastric ulcer in rats and structure of one bioactive fraction. Int. J. Biol. Macromol. 2022, 210, 455–464. [Google Scholar] [CrossRef]
- Xue, Z.; Shi, G.; Fang, Y.; Liu, X.; Zhou, X.; Feng, S.; Zhao, L. Protective effect of polysaccharides from Radix Hedysari on gastric ulcers induced by acetic acid in rats. Food Funct. 2019, 10, 3965–3976. [Google Scholar] [CrossRef]
- Kowalska, A.; Kalinowska-Lis, U. 18β-Glycyrrhetinic acid: Its core biological properties and dermatological applications. Int. J. Cosmet. Sci. 2019, 41, 325–331. [Google Scholar] [CrossRef]
- Mancuso, C.; Santangelo, R. Panax ginseng and Panax quinquefolius: From pharmacology to toxicology. Food Chem. Toxicol. 2017, 107, 362–372. [Google Scholar] [CrossRef]
- Cao, Y.; Tao, F.; Yu, Y.; Song, L.; Zhang, R.; Feng, J.; Zhai, Q.; Xue, P. Safety evaluation of rare ginsenosides of stems and leaves from American ginseng: 90-day exposure toxicity study combined with intestinal flora analysis and metabonomics in rats. Ecotoxicol. Environ. Saf. 2023, 264, 115429. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Y. Drug Metabolism and Pharmacokinetics of Dammarane Triterpenoids. Curr. Drug Metab. 2016, 17, 836–848. [Google Scholar] [CrossRef]
- Jing, L.; Shuang, G.; Jing-bo, L. Acute and Genetic Toxicity of Ursolic Acid Extract from Ledum pulastre L. Food Sci. 2009, 30, 250. [Google Scholar]
- Sun, Q.; He, M.; Zhang, M.; Zeng, S.; Chen, L.; Zhou, L.; Xu, H. Ursolic acid: A systematic review of its pharmacology, toxicity and rethink on its pharmacokinetics based on PK-PD model. Fitoterapia 2020, 147, 104735. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Lin, S.; Yan, W.; Chen, D.; Zeng, Z.; Chen, L.; Li, Y.; He, B. Enhanced Water Solubility and Anti-Tumor Activity of Oleanolic Acid through Chemical Structure Modification. Int. J. Mol. Sci. 2022, 23, 13291. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Shetty, S.; Mutalik, S.; Chandrashekar, H.R.; Nandakumar, K.; Mathew, E.M.; Jha, A.; Mishra, B.; Rajpurohit, S.; Ravi, G.; et al. Treatment of H. pylori infection and gastric ulcer: Need for novel Pharmaceutical formulation. Heliyon 2023, 9, e20406. [Google Scholar] [CrossRef]
- Jin, Z.H.; Qiu, W.; Liu, H.; Jiang, X.H.; Wang, L. Enhancement of oral bioavailability and immune response of Ginsenoside Rh2 by co-administration with piperine. Chin. J. Nat. Med. 2018, 16, 143–149. [Google Scholar] [CrossRef]



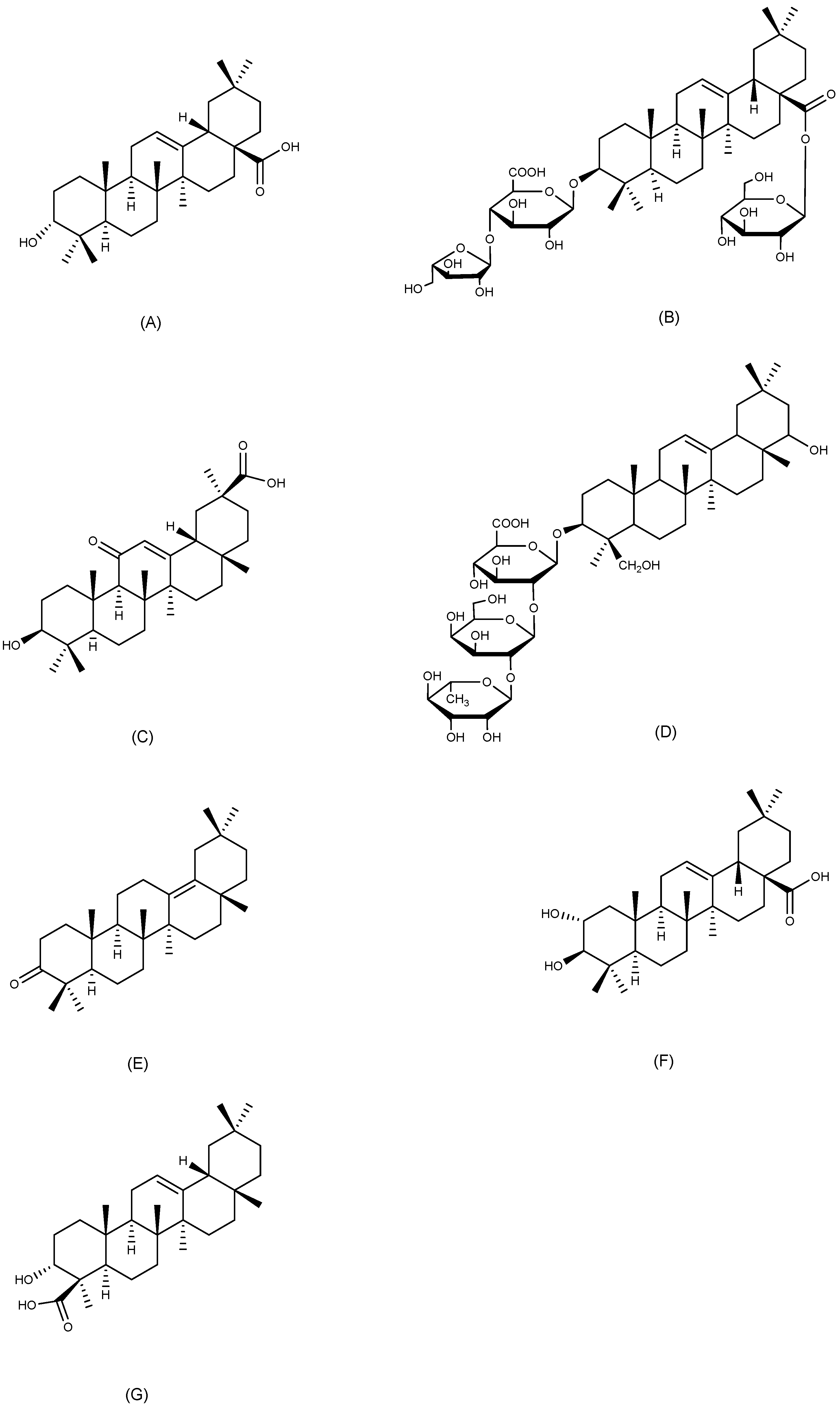

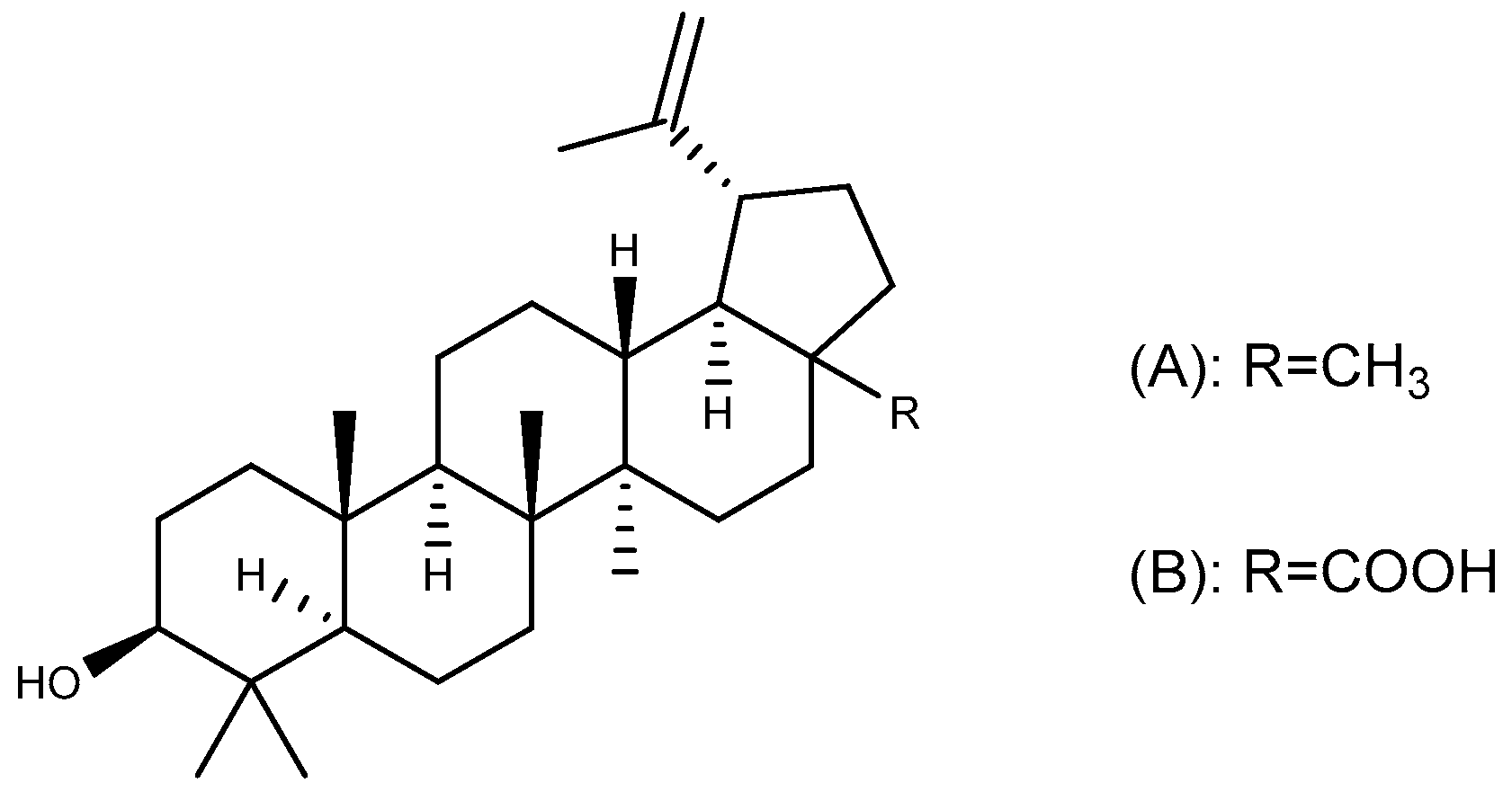
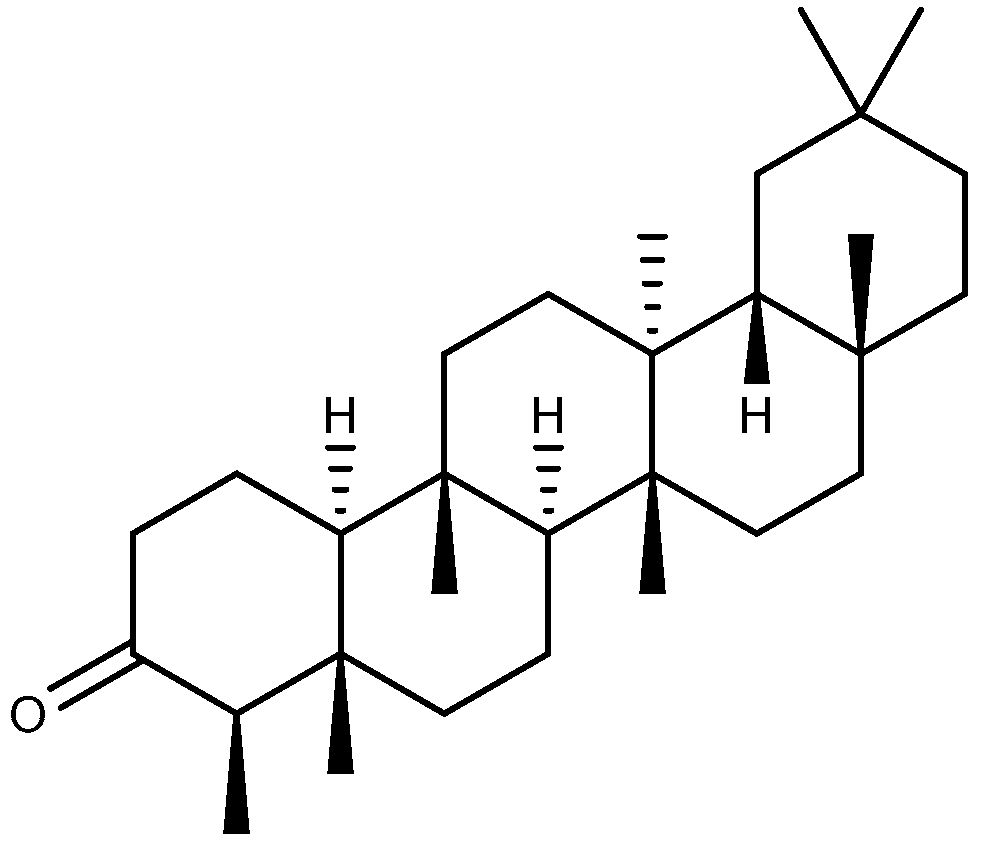
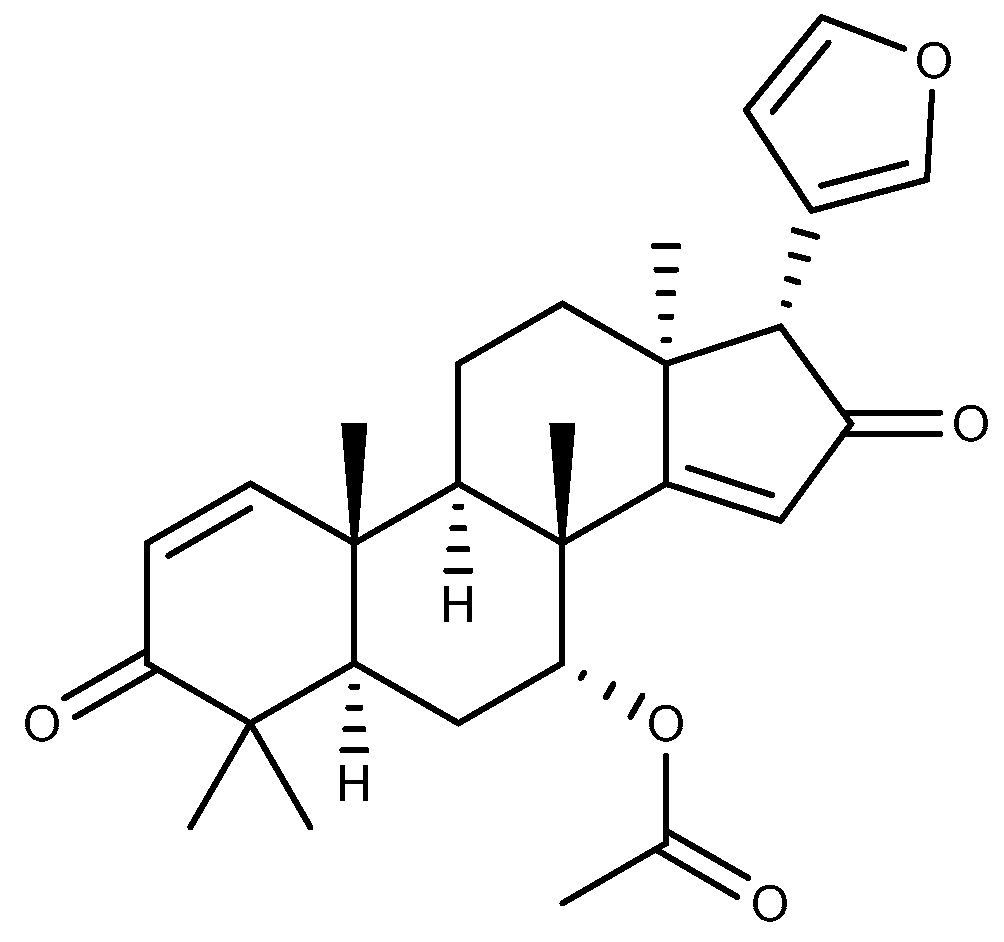
| Compound | Plant Source | Type | Model | Efficacy | Effect or Mechanism | Ref. |
|---|---|---|---|---|---|---|
| Ginsenoside Rb1 | Panax ginseng C. A. Mey. | Dammarane | Male SD rats (oral EtOH/HCl (60%/150 mM, 5 mL/kg)) | 60.6% 1 (300 mg/kg) | ↑ mucus | [26] |
| Ginsenoside Rd | Panax ginseng C. A. Mey. | Dammarane | Rats (oral ethanol (20 mg/kg)) | 57.6% 2 (100 mg/kg) | [27] | |
| Rats (oral indomethacin (20 mg/kg)) | 52.1% 2 (100 mg/kg) | |||||
| Ginsenoside Rg3 | Panax ginseng C. A. Mey. | Dammarane | Male Wistar rats (oral ethanol (5 mg/kg)) | 63.1% 1 (20 mg/kg) | ↑ SOD, NO, EGF, EGFR ↓ iNOS, ET-1 | [28] |
| Male Wistar rats (pyloric ligation) | 64.8% 1 (20 mg/kg) | |||||
| Male Wistar rats (inject 0.3 mL indomethacin) | 62.7% 1 (20 mg/kg) | |||||
| Ginsenoside Rh4 | Panax notoginsen (Burkill) F. H. Chen ex C. Y. Wu & K. M. Feng | Dammarane | Male SD rats (absolute ethanol (5 mg/kg)) | 92.57% 2 (60 mg/kg) | ↑ NO, PGE2, COX-2, Bcl-2 ↓ MAPK/NF-κB signaling pathway, Bax, Fas | [31] |
| Protopanaxatriol | Panax ginseng C. A. Mey. | Dammarane | Male Wistar rats (inject 0.3 mL indomethacin) | ↑ SOD, EGF, EGFR ↓ TNF-α, IL-6, ET-1, MDA | [34] | |
| Ocotillol | Panax plants | Ocotillol | Male Wistar rats (inject 0.3 mL indomethacin) | ↑ NO, SOD, EGF, EGFR ↓ ET-1 | [37] | |
| Astragaloside IV | Astragalus membranaceus (Fisch.) Bunge | Cycloartane | Male Wistar rats (oral absolute ethanol (1 mg/rat)) | 52.3% 2 (30 mg/kg) | ↑ NO | [40] |
| Male SD rats (water immersion and restraint stress) | 70.79% 2 (50 mg/kg) | ↑ PH, mucus, SOD, HSP70 ↓ MDA, TNF-α, MCP1 | [41] | |||
| Wistar rats (oral aspirin (150 mg/kg)) | ↑ COX-1, PGE2, NO, SOD | [42] | ||||
| 3α-Hydroxymasticadienoic acid | Amphipterygium adstringens (Schltdl.) Schiede ex Standl | Tirucallane | Male Wistar rats (oral indomethacin (30 mg/kg)) | 70% 2 (30 mg/kg) | ↑ SOD, PGE2, NO, H2S ↓ TNF-α, LTB4 | [45] |
| Oleanolic acid | Oleaceae plants | Oleanane | Male SD rats (inject 0.05 mL of 30% acetic acid) | 76.0% 2 (100 mg/kg) | ↑ mucus | [49] |
| AGS (10 mM NaT for 30 min) | ↑ PGE2 | [50] | ||||
| Araloside A | Aralia plants | Oleanane | Male SD rats (oral EtOH/HCl (60%/150 mM, 1.5 mL/rat)) | 51.4% 1 (100 mg/kg) | ↑ pH | [52] |
| Male SD rats (aspirin (100 mg/kg)) | 80.7% 1 (100 mg/kg) | |||||
| Male SD rats (water immersion stress) | 84.3% 1 (100 mg/kg) | |||||
| Male SD rats (pyloric ligation) | 73.9% 1 (100 mg/kg) | |||||
| Male Kunming mice (oral 80% ethanol containing 15 mg/mL aspirin (10.0 mL/kg)) | About 40% 1 (40 mg/kg) | ↑ pH, mucus, Bcl-2 ↓ H+/K+-ATPase, cytochrome c, caspase-3, caspase-9, Bax | [53] | |||
| Soyasaponin Bb | Fabaceae plants | Oleanane | Male Wistar albino rats (diclofenac sodium (5 mg/mL)) | ↑ PGE2, mucus, CAT, SOD ↓ COX-2, MDA, TNF-α, IL-6, NF-κB | [61] | |
| δ-Amyrone | Sedum lineare Thunb. | Oleanane | Male Kunming mice (oral 75% ethanol (0.5 mL/100 g)) | ↑ pH, mucus ↓ MPO, TNF-α, IL-6, NO, NF-κB | [63] | |
| Maslinic acid | Loquat, patchouli, hawthorn, spinach, and eggplant | Oleanane | Female Swiss mice (oral EtOH/HCl (60%/0.3 M, 10 mL/kg)) | 97.12% 2 (10 mg/kg) | ↓ H+/K+-ATPase | [65] |
| Female Swiss mice (oral indomethacin (80 mg/kg)) | 96.28% 2 (10 mg/kg) | |||||
| α-Boswellic acid | Boswellia plants | Oleanane | Male SD rats (oral absolute ethanol (5 mL/kg)) | 42.45% 1 (200 mg/kg) | ↑ pH, mucus, PGE2, NO, CAT, SOD, Nrf 2/HO-1 ↓ MDA | [67] |
| Ursolic acid | Apple peel, rosemary, and lavender | Ursane | Female Wistar albino rats (oral 95% ethanol (1 mL/rat)) | 96.9% 1 (100 mg/kg) | ↓ MDA, caspase-3, H+/K+-ATPase | [71] |
| Tormentic acid | Rosaceae plants | Ursane | Male SD rats (oral indomethacin (100 mg/kg)) | ↑ GSH-Px, SOD, CAT, IL-10 ↓ MDA, TNF-a, IL-1b, IL-6, IL-4 | [73] | |
| GES-1 (700 μM indomethacin for 18 h) | ↑ cell migration ↓ cell apoptosis | [73] | ||||
| Asiaticoside | Centella asiatica (L.) Urban | Ursane | Male SD rats (inject 60% acetic acid (0.12 mL/rat)) | ↓ MPO | [76] | |
| Male SD rats (inject 60% acetic acid (0.12 mL/rat)) | ↓ iNOS | [77] | ||||
| Niga-ichigoside F1 | Rubus plants | Ursane | Male SD rats (oral ethanol (4 mL/kg) and sodium salicylate (200 mg/kg) | ↑ SOD, GSH-Px | [80] | |
| Male Swiss mice (oral EtOH/HCl (60%/0.3 M, 0.5 mL/rat)) | 98.45% 2 (30 mg/kg) | [81] | ||||
| Lupeol | Cucumber, carrot, mango, strawberries, and olive | Lupane | Male Swiss albino mice (oral absolute ethanol (0.2 mL/mice)) | 69.3% 2 (30 mg/kg) | ↑ NP-SH | [83] |
| Betulinic acid | Betula platyphylla Sukaczev | Lupane | Male Wistar albino rats (indomethacin (40 mg/kg)) | ↑ mucus, pH ↓ MDA | [86] | |
| Friedelin | Celastraceae, Asteraceae, Fabaceae, and Myrtaceous plants | Friedelane | Wistar albino rats (oral 96% ethanol (5 mL/kg)) | 88.21% 1 (35 mg/kg) | ↑ PGE2, NO, SOD, GSH-px, CAT, GSH, IL-10, mucus, pH ↓ MPO, MDA, TNF-α, IL-6, caspase-3 | [88] |
| Azadiradione | Azadirachta indica A. Juss. | limonoids | SD rats (cold restraint) | 58.5% 1 (40 mg/kg) | ↑ pH, mucus, PGE2 ↓ H+/K+-ATPase | [90] |
| SD rats (oral absolute ethanol (5 mL/kg)) | 71.67% 1 (20 mg/kg) | |||||
| SD rats (pyloric ligation) | 50.0% 1 (20 mg/kg) | |||||
| SD rats (aspirin (150 mg/kg)) | 55.53% 1 (20 mg/kg) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, C.; Zhang, S.; Di, H.; Wang, S.; Wang, Y.; Guan, F. The Role of Triterpenoids in Gastric Ulcer: Mechanisms and Therapeutic Potentials. Int. J. Mol. Sci. 2025, 26, 3237. https://doi.org/10.3390/ijms26073237
Shen C, Zhang S, Di H, Wang S, Wang Y, Guan F. The Role of Triterpenoids in Gastric Ulcer: Mechanisms and Therapeutic Potentials. International Journal of Molecular Sciences. 2025; 26(7):3237. https://doi.org/10.3390/ijms26073237
Chicago/Turabian StyleShen, Congcong, Shengyu Zhang, Han Di, Shuang Wang, Yanhong Wang, and Feng Guan. 2025. "The Role of Triterpenoids in Gastric Ulcer: Mechanisms and Therapeutic Potentials" International Journal of Molecular Sciences 26, no. 7: 3237. https://doi.org/10.3390/ijms26073237
APA StyleShen, C., Zhang, S., Di, H., Wang, S., Wang, Y., & Guan, F. (2025). The Role of Triterpenoids in Gastric Ulcer: Mechanisms and Therapeutic Potentials. International Journal of Molecular Sciences, 26(7), 3237. https://doi.org/10.3390/ijms26073237






