The Apple Mitogen-Activated Protein Kinase MdMAPK6 Increases Drought, Salt, and Disease Resistance in Plants
Abstract
:1. Introduction
2. Results
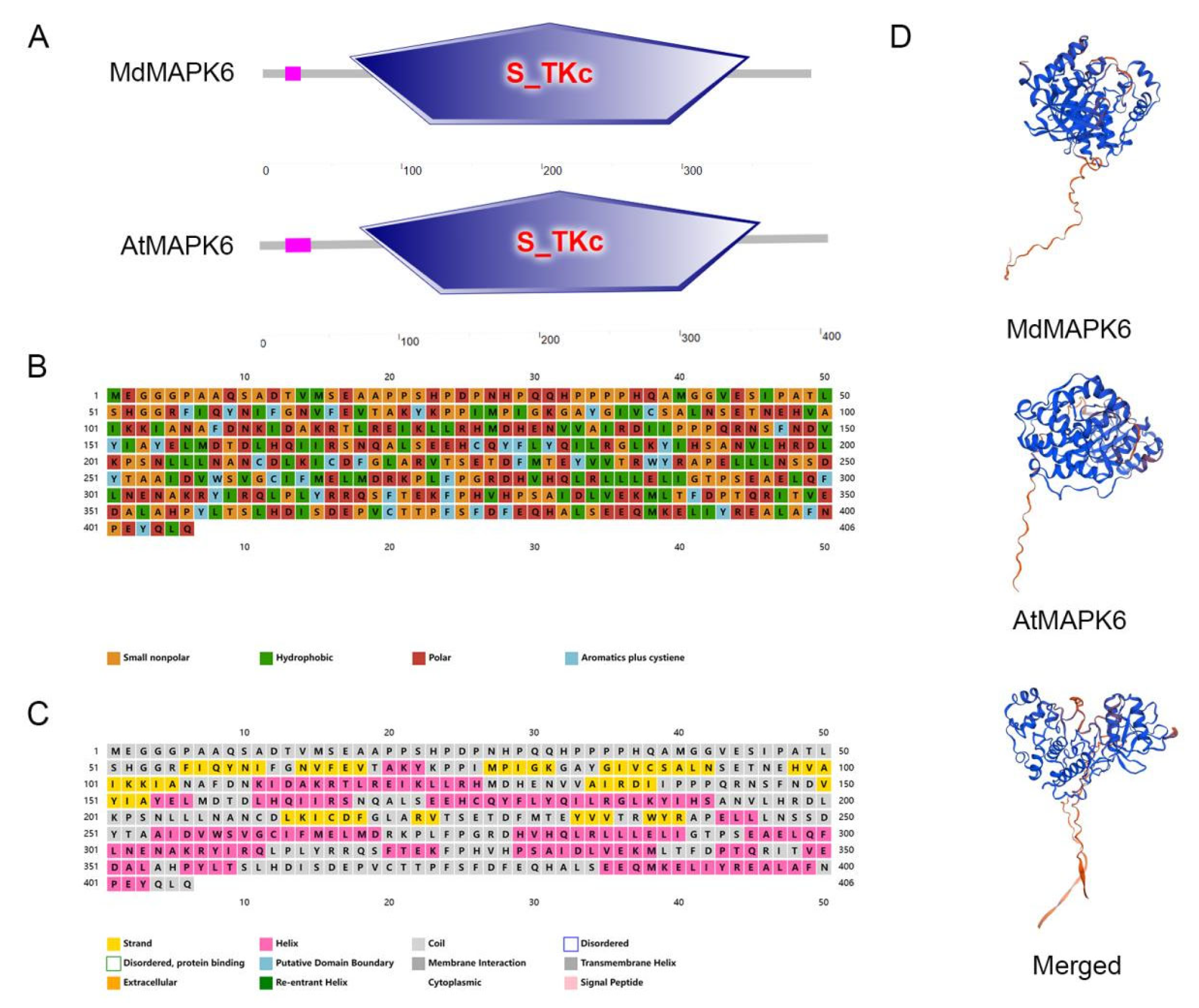
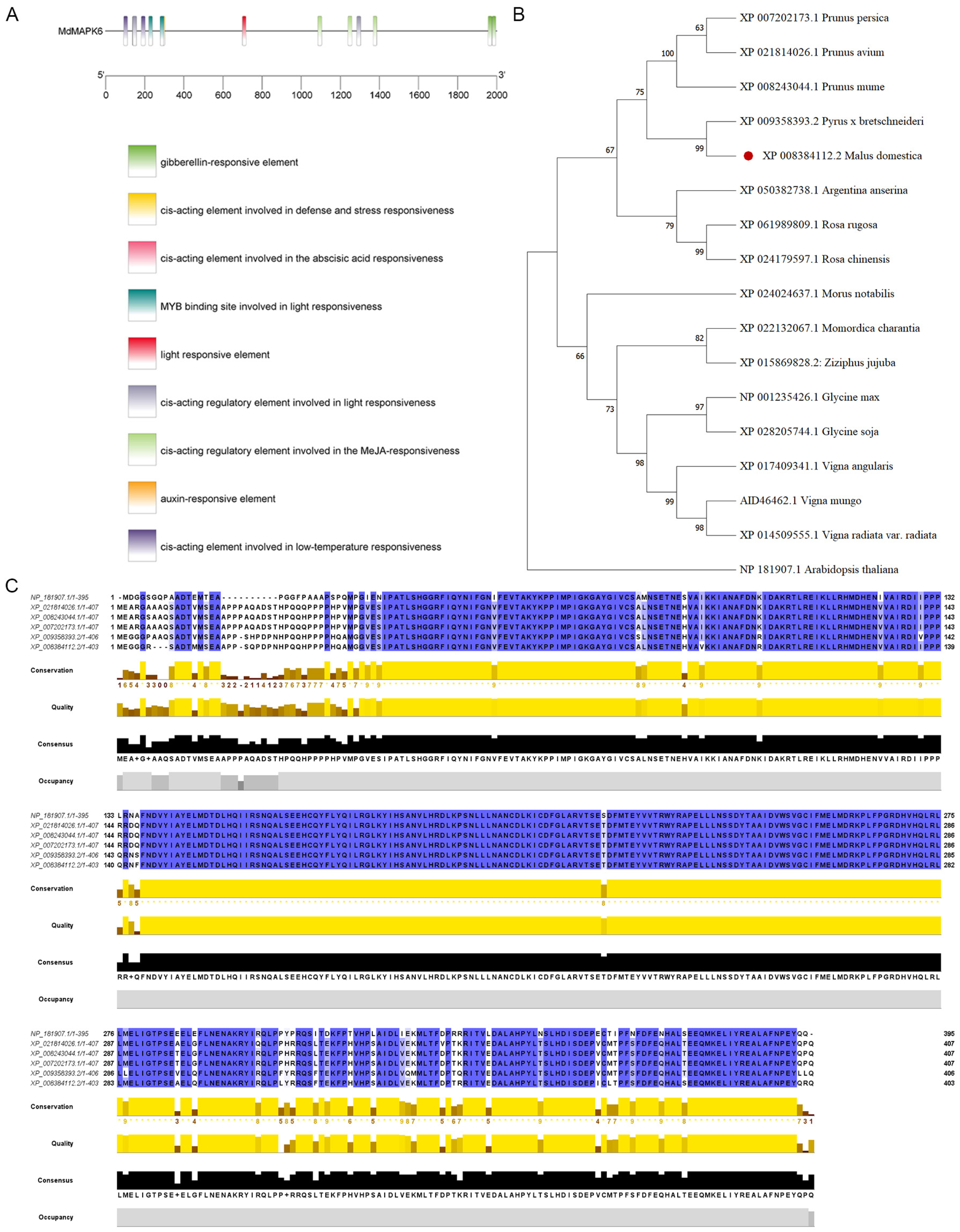
2.1. Bioinformatics Analysis of the MdMAPK6 Gene in Apple
2.2. Expression Profiles of MdMAPK6 in Different Apple Tissues
2.3. Responses of MdMAPK6 to PEG 6000, NaCl, and ABA Treatments
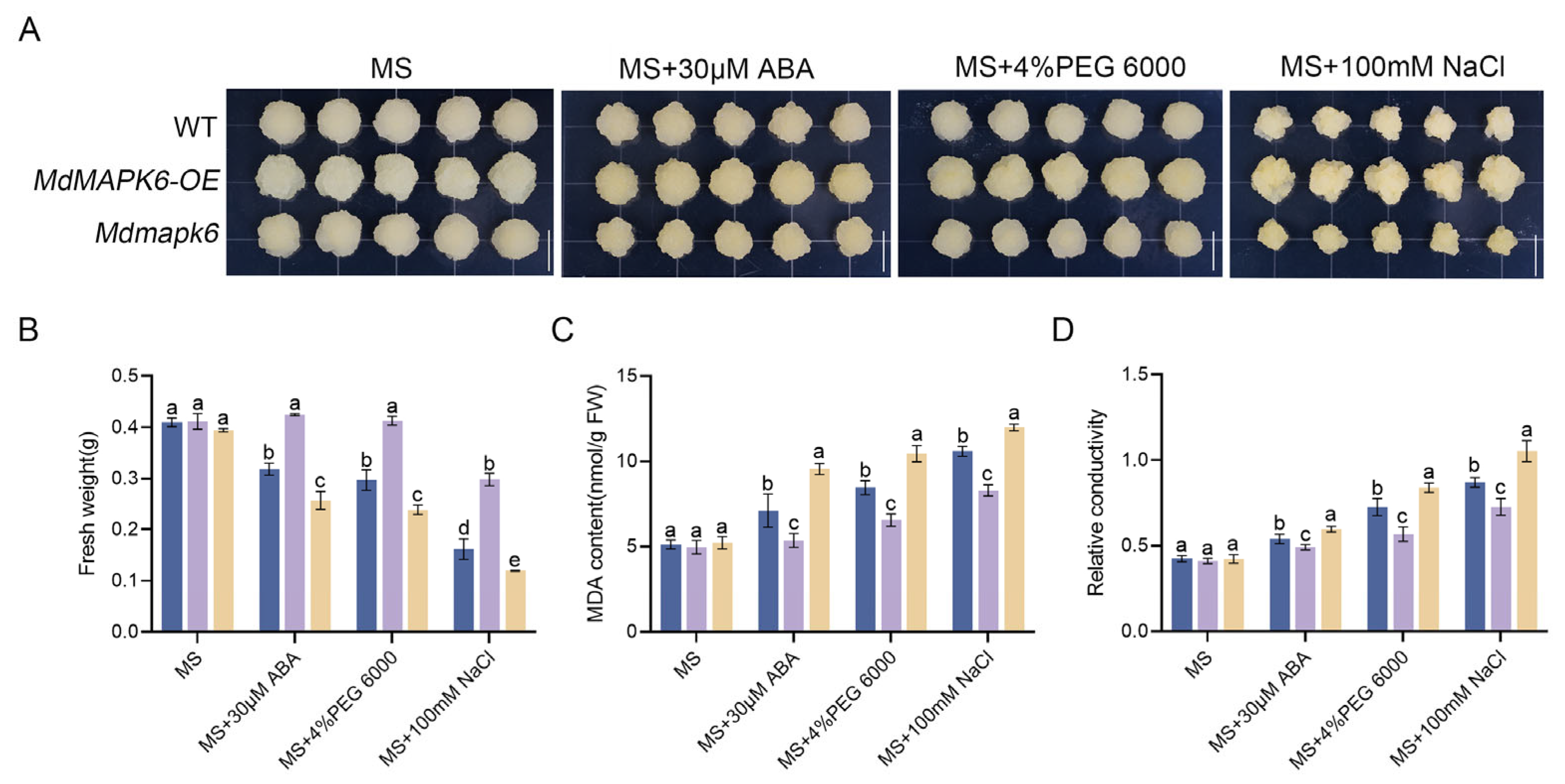
2.4. Overexpression of MdMAPK6 Enhances Drought Salt Tolerance While Decreasing the Sensitivity of Apple Calli to ABA
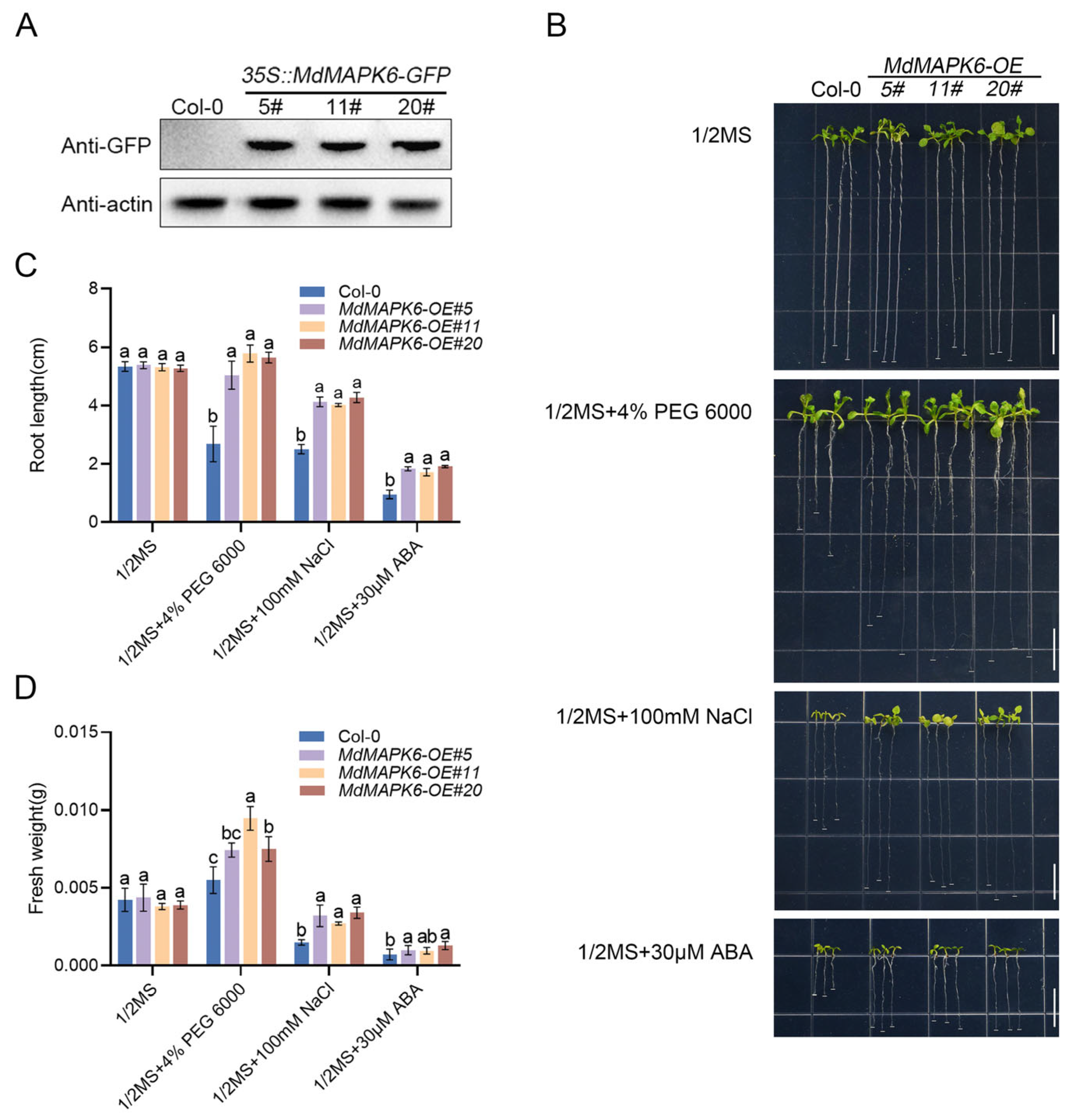
2.5. Overexpression of MdMAPK6 Enhances Drought and Salt Tolerance and Decreases the Sensitivity of A. thaliana to ABA
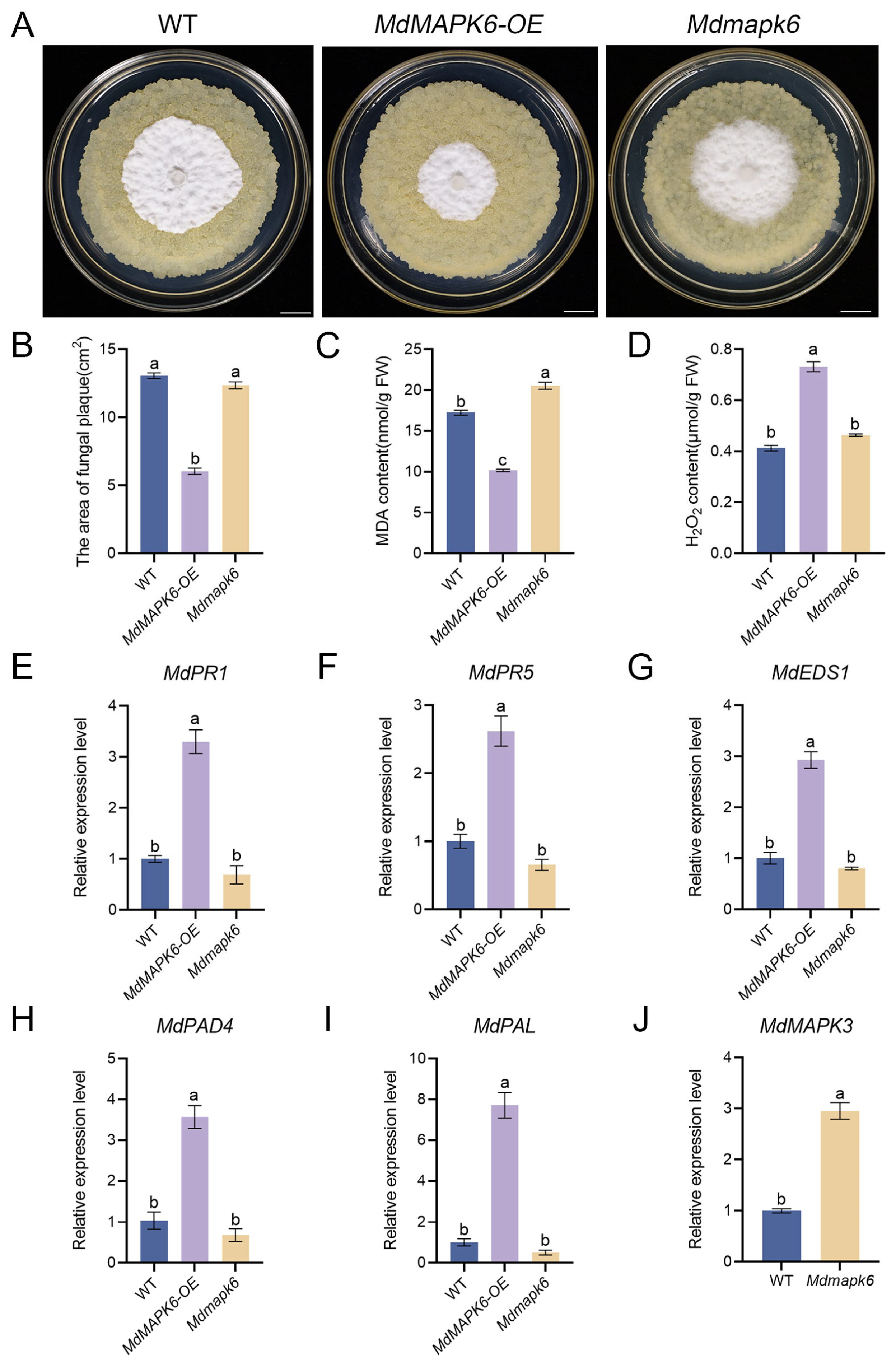
2.6. Overexpression of MdMAPK6 Enhances Resistance of Apple Calli to B. dothidea
2.7. Overexpression of MdMAPK6 Enhances the Resistance of Apple Fruit to B. dothidea
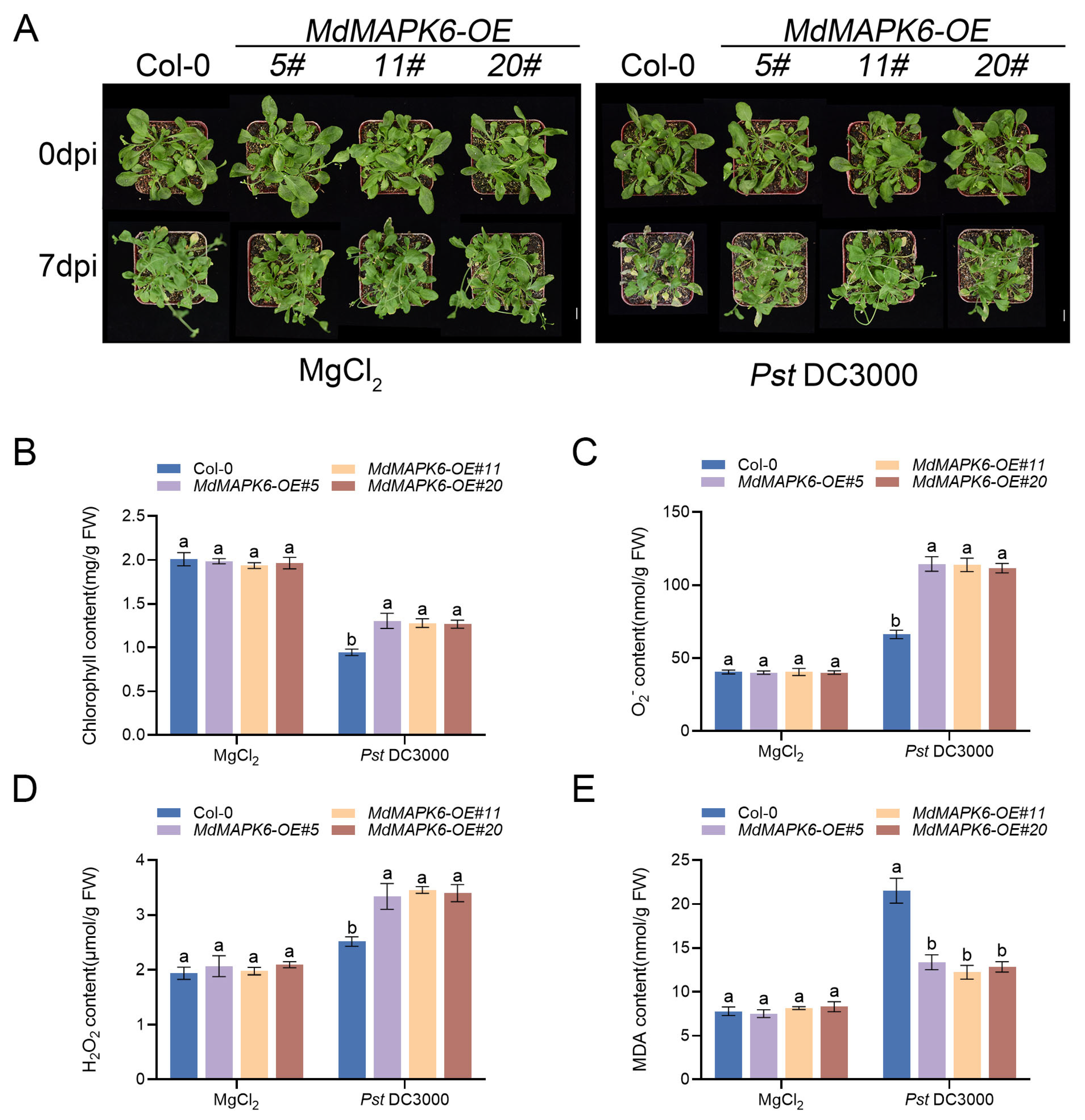
2.8. Overexpression of MdMAPK6 Enhances Resistance to Pst DC3000 in A. thaliana
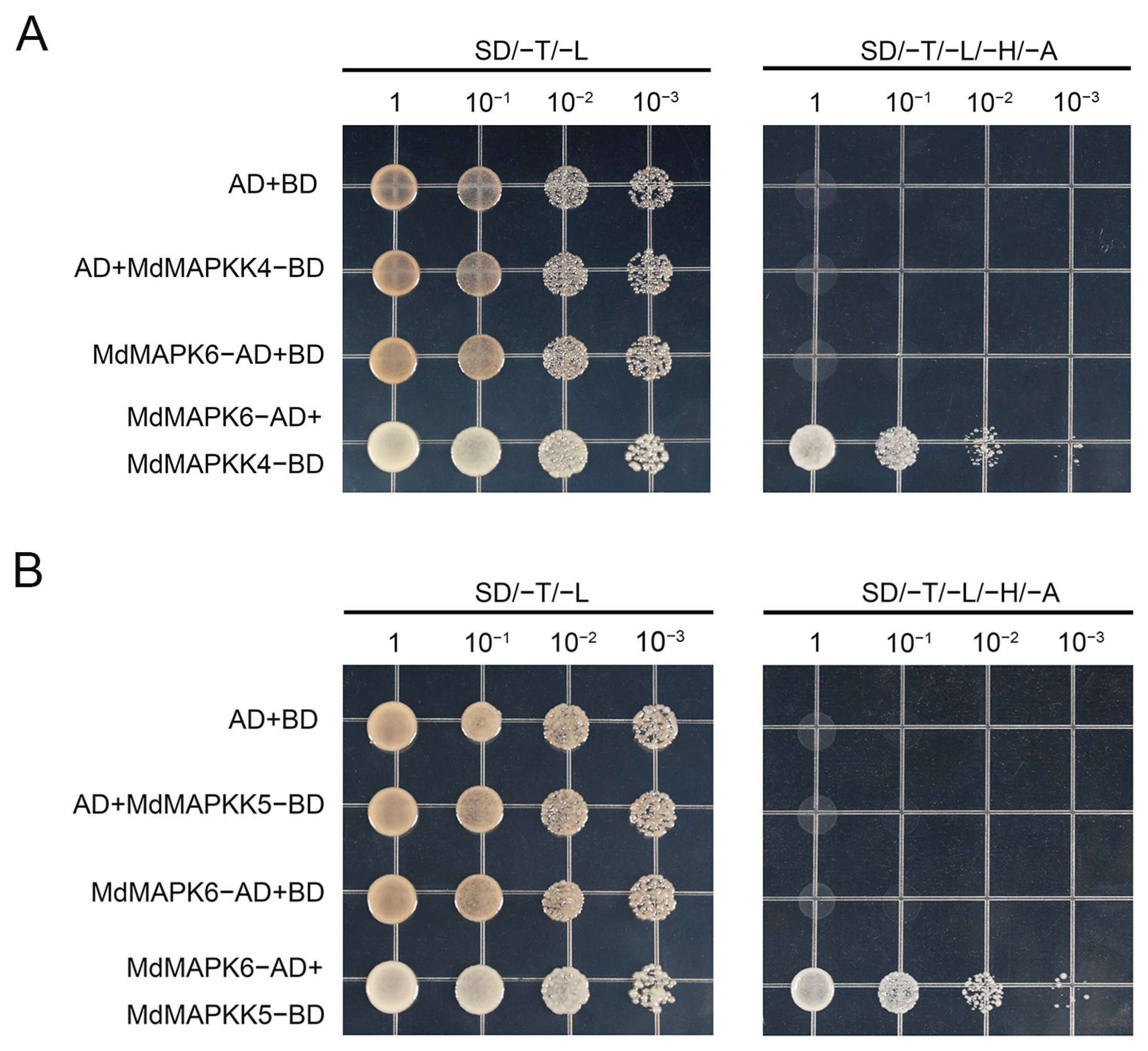
2.9. MdMAPKK4 and MdMAPKK5 Interact with MdMAPK6
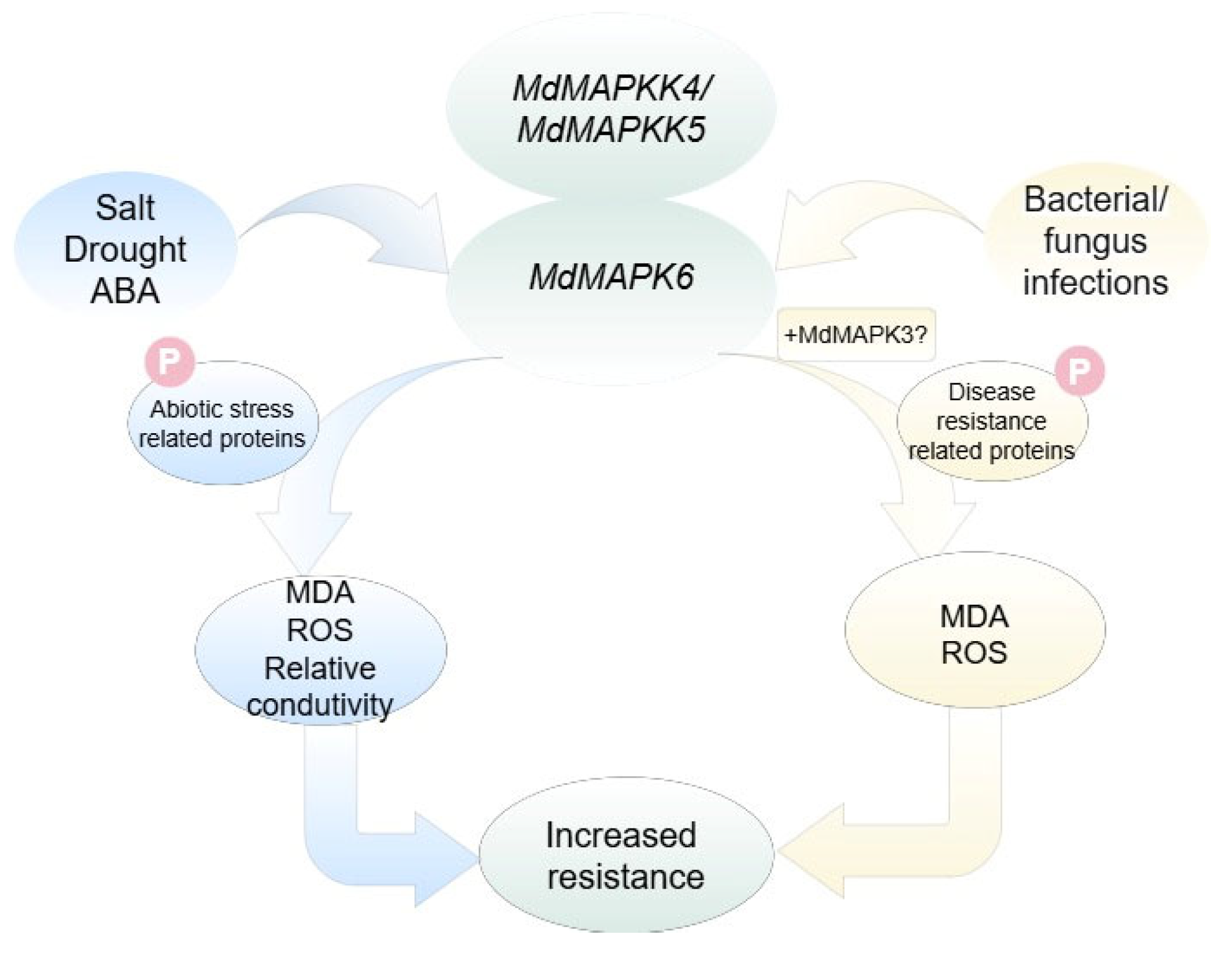
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Bioinformatics Analysis of the MdMAPK6
4.3. Cis-Acting Element Analysis, Phylogenetic Tree, and Multiple Sequence Alignment
4.4. Construction of MdMAPK6 Expression Vector and Genetic Transformation of Transgenic Materials
4.5. Plant Total RNA Extraction and Quantitative Real-Time PCR (qRT-PCR) Analysis
4.6. DNA Extraction
4.7. Protein Extraction
4.8. Western Blot
4.9. The 30 µM ABA, 4% PEG 6000, and 100 mM NaCl Treatments in Apple Calli and A. thaliana
4.10. The Apple Calli and A. thaliana Pathogen Inoculation
4.11. Transient Transfection of Apple Fruit and Inoculation of B. dothidea
4.12. Relevant Physiological Indicator Detection
4.13. Yeast Two-Hybrid (Y2H) Assays
4.14. Data Presentation and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MAPK | mitogen-activated protein kinase |
| MAPKK | mitogen-activated protein kinase kinase |
| MAPKKK | mitogen-activated protein kinase kinase kinase |
| B. dothidea | Botryosphaeria dothidea |
| Pst DC3000 | Pseudomonas syringae pv. tomato DC3000 |
| PAMPs | pathogen-associated molecular patterns |
| Col-0 | Columbia-0 |
| WT | wild type |
| MS | Murashige and Skoog |
| PEG 6000 | polyethylene glycol 6000 |
| ABA | abscisic acid |
| PDA | potato dextrose agar |
| MDA | malondialdehyde |
| FW | fresh weight |
| PAL | phenylalanine ammonia lyase |
| PR1 | pathogenesis-related 1 |
| PR5 | pathogenesis-related 5 |
| EDS1 | enhanced disease susceptibility 1 |
| PAD4 | peptidylarginine deiminase 4 |
| qRT-PCR | quantitative reverse transcription polymerase chain reaction |
| Y2H | yeast two-hybrid |
References
- Kissoudis, C.; van de Wiel, C.; Visser, R.G.; van der Linden, G. Enhancing crop resilience to combined abiotic and biotic stress through the dissection of physiological and molecular crosstalk. Front. Plant Sci. 2014, 5, 207. [Google Scholar] [CrossRef]
- Dresselhaus, T.; Hückelhoven, R. Biotic and Abiotic Stress Responses in Crop Plants. Agronomy 2018, 8, 267. [Google Scholar] [CrossRef]
- Manna, M.; Rengasamy, B.; Sinha, A.K. Revisiting the role of MAPK signalling pathway in plants and its manipulation for crop improvement. Plant Cell Environ. 2023, 46, 2277–2295. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, C.; Wang, H.; Li, L.; Wang, C. The Function of MAPK Cascades in Response to Various Stresses in Horticultural Plants. Front. Plant Sci. 2020, 11, 952. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, S. Mitogen-activated protein kinase cascades in plant signaling. J. Integr. Plant Biol. 2022, 64, 301–341. [Google Scholar] [CrossRef] [PubMed]
- Jonak, C.; Okrész, L.; Bögre, L.; Hirt, H. Complexity, cross talk and integration of plant MAP kinase signalling. Curr. Opin. Plant Biol. 2002, 5, 415–424. [Google Scholar] [CrossRef]
- Tena, G.; Asai, T.; Chiu, W.L.; Sheen, J. Plant mitogen-activated protein kinase signaling cascades. Curr. Opin. Plant Biol. 2001, 4, 392–400. [Google Scholar] [CrossRef]
- Bigeard, J.; Hirt, H. Nuclear Signaling of Plant MAPKs. Front. Plant Sci. 2018, 9, 469. [Google Scholar] [CrossRef]
- Ichimura, K.; Shinozaki, K.; Tena, G.; Sheen, J.; Henry, Y.; Champion, A.; Kreis, M.; Zhang, S.; Hirt, H.; Wilson, C.; et al. Mitogen-activated protein kinase cascades in plants: A new nomenclature. Trends Plant Sci. 2002, 7, 301–308. [Google Scholar] [CrossRef]
- Moustafa, K.; AbuQamar, S.; Jarrar, M.; Al-Rajab, A.J.; Trémouillaux-Guiller, J. MAPK cascades and major abiotic stresses. Plant Cell Rep. 2014, 33, 1217–1225. [Google Scholar] [CrossRef]
- Danquah, A.; de Zelicourt, A.; Colcombet, J.; Hirt, H. The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol. Adv. 2014, 32, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Smékalová, V.; Doskočilová, A.; Komis, G.; Samaj, J. Crosstalk between secondary messengers, hormones and MAPK modules during abiotic stress signalling in plants. Biotechnol. Adv. 2014, 32, 2–11. [Google Scholar] [CrossRef]
- Zhang, H.; Zhu, J.; Gong, Z.; Zhu, J.-K. Abiotic stress responses in plants. Nat. Rev. Genet. 2021, 23, 104–119. [Google Scholar] [CrossRef] [PubMed]
- Jammes, F.; Song, C.; Shin, D.; Munemasa, S.; Takeda, K.; Gu, D.; Kwak, J.M. MAP kinases MPK9 and MPK12 are preferentially expressed in guard cells and positively regulate ROS-mediated ABA signaling. Proc. Natl. Acad. Sci. USA 2009, 106, 20520–20525. [Google Scholar]
- Ma, H.; Chen, J.; Zhang, Z.; Ma, L.; Yang, Z.; Zhang, Q.; Li, X.; Xiao, J.; Wang, S. MAPK kinase 10.2 promotes disease resistance and drought tolerance by activating different MAPKs in rice. Plant J. 2017, 92, 557–570. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, N.; Liu, X.; Li, S.; Yang, J.; Hong, X.; Wang, F.; Si, H. Mitogen-activated protein kinase 11 (MAPK11) maintains growth and photosynthesis of potato plant under drought condition. Plant Cell Rep. 2021, 40, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wei, J.-M.; Feng, W.-Z.; Luo, Q.; Tan, G.-F.; Li, Y.-Z. Interaction between SlMAPK3 and SlASR4 regulates drought resistance in tomato (Solanum lycopersicum L.). Mol. Breed. 2023, 43, 73. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, D.; Li, S.; Gao, Z.; Zhao, S.; Shi, J.; Wu, C.; Guo, X. A cotton group C MAP kinase gene, GhMPK2, positively regulates salt and drought tolerance in tobacco. Plant Mol. Biol. 2011, 77, 17–31. [Google Scholar] [CrossRef]
- Zhang, D.; Jiang, S.; Pan, J.; Kong, X.; Zhou, Y.; Liu, Y.; Li, D.; Mendel, R. The overexpression of a maize mitogen-activated protein kinase gene (ZmMPK5) confers salt stress tolerance and induces defence responses in tobacco. Plant Biol. 2013, 16, 558–570. [Google Scholar] [CrossRef]
- Wu, L.; Zu, X.; Zhang, H.; Wu, L.; Xi, Z.; Chen, Y. Overexpression of ZmMAPK1 enhances drought and heat stress in transgenic Arabidopsis thaliana. Plant Mol. Biol. 2015, 88, 429–443. [Google Scholar] [CrossRef]
- Hong, K.; Zhao, Y.; Yin, X.; Xi, H.; Liu, C.; Wen, C.; Rui, Z. Pathogenic function analysis of pectin lyase gene Bdpl1 of Botryosphaeria dothidea in apple tree. Acta Phytopathol. Sin. 2019, 49, 314–325. [Google Scholar] [CrossRef]
- Copes, W.E.; Hendrix, F.F., Jr. Effect of Temperature on Sporulation of Botryosphaeria dothidea, B. obtusa, and B. rhodina. Plant Dis. 2004, 88, 292–296. [Google Scholar] [CrossRef]
- Du, H.F.; Zhang, Y.H.; Li, W.; Zhu, H.; Pang, S.; Song, D.B.; Liu, Z.; Pittman, C.U., Jr.; Cao, F. Antifungal Activity and Mechanism of Diaporthein B against Botryosphaeria dothidea in Prevention of Apple Ring Rot. J. Agric. Food Chem. 2024, 72, 20892–20904. [Google Scholar] [CrossRef] [PubMed]
- Marsberg, A.; Kemler, M.; Jami, F.; Nagel, J.H.; Postma-Smidt, A.; Naidoo, S.; Wingfield, M.J.; Crous, P.W.; Spatafora, J.W.; Hesse, C.N.; et al. Botryosphaeria dothidea: A latent pathogen of global importance to woody plant health. Mol. Plant Pathol. 2017, 18, 477–488. [Google Scholar] [CrossRef] [PubMed]
- Andersen, E.J.; Ali, S.; Byamukama, E.; Yen, Y.; Nepal, M.P. Disease Resistance Mechanisms in Plants. Genes 2018, 9, 339. [Google Scholar] [CrossRef]
- Mao, G.; Meng, X.; Liu, Y.; Zheng, Z.; Chen, Z.; Zhang, S. Phosphorylation of a WRKY transcription factor by two pathogen-responsive MAPKs drives phytoalexin biosynthesis in Arabidopsis. Plant Cell 2011, 23, 1639–1653. [Google Scholar] [CrossRef]
- Meng, X.; Xu, J.; He, Y.; Yang, K.-Y.; Mordorski, B.; Liu, Y.; Zhang, S. Phosphorylation of an ERF Transcription Factor by Arabidopsis MPK3/MPK6 Regulates Plant Defense Gene Induction and Fungal Resistance. Plant Cell 2013, 25, 1126–1142. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.Y.; Liu, Y.; Zhang, S. Activation of a mitogen-activated protein kinase pathway is involved in disease resistance in tobacco. Proc. Natl. Acad. Sci. USA 2001, 98, 741–746. [Google Scholar] [CrossRef]
- Gao, H.; Jiang, L.; Du, B.; Ning, B.; Ding, X.; Zhang, C.; Song, B.; Liu, S.; Zhao, M.; Zhao, Y.; et al. GmMKK4-activated GmMPK6 stimulates GmERF113 to trigger resistance to Phytophthora sojae in soybean. Plant J. 2022, 111, 473–495. [Google Scholar] [CrossRef]
- Mi, X.; Li, W.; Chen, C.; Xu, H.; Wang, G.; Jin, X.; Zhang, D.; Guo, W. GhMPK9-GhRAF39_1-GhWRKY40a Regulates the GhERF1b- and GhABF2-Mediated Pathways to Increase Cotton Disease Resistance. Adv. Sci. 2024, 11, 2404400. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Dong, C.; Zhang, Y.; Bai, S. MdMAPKKK1 Regulates Apple Resistance to Botryosphaeria dothidea by Interacting with MdBSK1. Int. J. Mol. Sci. 2022, 23, 4415. [Google Scholar] [CrossRef]
- Colcombet, J.; Hirt, H. Arabidopsis MAPKs: A complex signalling network involved in multiple biological processes. Biochem. J. 2008, 413, 217–226. [Google Scholar] [CrossRef]
- Zhang, M.; Su, J.; Zhang, Y.; Xu, J.; Zhang, S. Conveying endogenous and exogenous signals: MAPK cascades in plant growth and defense. Curr. Opin. Plant Biol. 2018, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Ma, J.; Zhang, M.; Li, X.; Sun, Y.; Zhang, M.; Ding, Z. Auxin promotes hypocotyl elongation by enhancing BZR1 nuclear accumulation in Arabidopsis. Sci. Adv. 2023, 9, eade2493. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, S. MAPK Cascades in Plant Disease Resistance Signaling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Shah, S.T.; Basit, A.; Mohamed, H.I.; Li, Y. Mitogen-Activated Protein Kinase: A Potent Signaling Protein that Combats Biotic and Abiotic Stress in Plants. J. Plant Growth Regul. 2024, 43, 1762–1786. [Google Scholar] [CrossRef]
- Son, S.; An, C.S.; Yoon, W.-J.; Jeong, Y.; Han, S.-C.; Kim, Y.B.; Lee, J.-D.; Oh, E.; Choi, H.; Kwon, M.; et al. Soybean mitogen-activated protein kinase GmMPK6 enhances drought tolerance. Biochem. Biophys. Res. Commun. 2025, 745, 151170. [Google Scholar] [CrossRef]
- Liu, C.; Wei, C.; Zhang, M.; Xu, Y.; Xiang, Z.; Zhao, A. Mulberry MnMAPK1, a group C mitogen-activated protein kinase gene, endowed transgenic Arabidopsis with novel responses to various abiotic stresses. Plant Cell Tissue Organ Cult. (PCTOC) 2017, 131, 151–162. [Google Scholar] [CrossRef]
- Zhou, J.; Mu, Q.; Wang, X.; Zhang, J.; Yu, H.; Huang, T.; He, Y.; Dai, S.; Meng, X. Multilayered synergistic regulation of phytoalexin biosynthesis by ethylene, jasmonate, and MAPK signaling pathways in Arabidopsis. Plant Cell 2022, 34, 3066–3087. [Google Scholar] [CrossRef] [PubMed]
- Jalmi, S.K.; Sinha, A.K. ROS mediated MAPK signaling in abiotic and biotic stress- striking similarities and differences. Front. Plant Sci. 2015, 6, 769. [Google Scholar] [CrossRef]
- Chakraborty, J. Microbiota and the plant immune system work together to defend against pathogens. Arch. Microbiol. 2023, 205, 347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, H.; Zhu, L.; Feng, P.; Fan, M.; Wang, J. WSL214 negatively regulates ROS accumulation and pathogen defense response in rice. Plant Cell Rep. 2023, 42, 449–460. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, X.; Lv, H.; Cao, M.; Li, Y.; Yuan, X.; Zhang, X.; Guo, Y.D.; Zhang, N. Anabolism and signaling pathways of phytomelatonin. J. Exp. Bot. 2022, 73, 5801–5817. [Google Scholar] [CrossRef]
- Schweighofer, A.; Meskiene, I. Regulation of stress hormones jasmonates and ethylene by MAPK pathways in plants. Mol. Biosyst. 2008, 4, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Taj, G.; Agarwal, P.; Grant, M.; Kumar, A. MAPK machinery in plants: Recognition and response to different stresses through multiple signal transduction pathways. Plant Signal Behav. 2010, 5, 1370–1378. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, Y.; Yang, T.; Zhang, L.; Xu, S.; Xue, L.; An, L. Diverse signals converge at MAPK cascades in plant. Plant Physiol. Biochem. 2006, 44, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Asano, T.; Nguyen, T.H.; Yasuda, M.; Sidiq, Y.; Nishimura, K.; Nakashita, H.; Nishiuchi, T. Arabidopsis MAPKKK δ-1 is required for full immunity against bacterial and fungal infection. J. Exp. Bot. 2020, 71, 2085–2097. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Zhang, C.; Zhu, P.; Dai, H.; Yu, N.; He, Z.; Xu, L.; Wang, E. OsCERK1-Mediated Chitin Perception and Immune Signaling Requires Receptor-like Cytoplasmic Kinase 185 to Activate an MAPK Cascade in Rice. Mol. Plant 2017, 10, 619–633. [Google Scholar] [CrossRef]
- Tena, G.; Boudsocq, M.; Sheen, J. Protein kinase signaling networks in plant innate immunity. Curr. Opin. Plant Biol. 2011, 14, 519–529. [Google Scholar] [CrossRef]
- Shao, Y.; Yu, X.; Xu, X.; Li, Y.; Yuan, W.; Xu, Y.; Mao, C.; Zhang, S.; Xu, J. The YDA-MKK4/MKK5-MPK3/MPK6 Cascade Functions Downstream of the RGF1-RGI Ligand-Receptor Pair in Regulating Mitotic Activity in Root Apical Meristem. Mol. Plant 2020, 13, 1608–1623. [Google Scholar] [CrossRef]
- Liu, S.; Hua, L.; Dong, S.; Chen, H.; Zhu, X.; Jiang, J.; Zhang, F.; Li, Y.; Fang, X.; Chen, F. OsMAPK6, a mitogen-activated protein kinase, influences rice grain size and biomass production. Plant J. 2015, 84, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Gaur, M.; Tiwari, A.; Chauhan, R.P.; Pandey, D.; Kumar, A. Molecular modeling, docking and protein-protein interaction analysis of MAPK signalling cascade involved in Camalexin biosynthesis in Brassica rapa. Bioinformation 2018, 14, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Zhang, M.; Zhang, L.; Sun, T.; Liu, Y.; Lukowitz, W.; Xu, J.; Zhang, S. Regulation of Stomatal Immunity by Interdependent Functions of a Pathogen-Responsive MPK3/MPK6 Cascade and Abscisic Acid. Plant Cell 2017, 29, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; He, M.; Mei, E.; Zhang, B.; Tang, J.; Xu, M.; Liu, J.; Li, X.; Wang, Z.; Tang, W.; et al. WRKY53 integrates classic brassinosteroid signaling and the mitogen-activated protein kinase pathway to regulate rice architecture and seed size. Plant Cell 2021, 33, 2753–2775. [Google Scholar] [CrossRef]
- Man, Y.-Y.; Lv, Y.-H.; Lv, H.-M.; Jiang, H.; Wang, T.; Zhang, Y.-L.; Li, Y.-Y. MdDEWAX decreases plant drought resistance by regulating wax biosynthesis. Plant Physiol. Biochem. 2024, 206, 108288. [Google Scholar] [CrossRef]
- Zhang, C.; Mao, K.; Zhou, L.; Wang, G.; Zhang, Y.; Li, Y.; Hao, Y. Genome-wide identification and characterization of apple long-chain Acyl-CoA synthetases and expression analysis under different stresses. Plant Physiol. Biochem. 2018, 132, 320–332. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Hu, X.; Zhang, Y.; Wang, G.; You, C.; Li, Y.; Hao, Y. An apple AP2/EREBP-type transcription factor, MdWRI4, enhances plant resistance to abiotic stress by increasing cuticular wax load. Environ. Exp. Bot. 2020, 180, 104206. [Google Scholar] [CrossRef]
- Fang, X.; Meng, X.; Zhang, J.; Xia, M.; Cao, S.; Tang, X.; Fan, T. AtWRKY1 negatively regulates the response of Arabidopsis thaliana to Pst. DC3000. Plant Physiol. Biochem. 2021, 166, 799–806. [Google Scholar] [CrossRef]
- Han, P.L.; Wang, C.K.; Liu, X.J.; Dong, Y.H.; Jiang, H.; Hu, D.G.; Hao, Y.J. BTB-BACK Domain E3 Ligase MdPOB1 Suppresses Plant Pathogen Defense against Botryosphaeria dothidea by Ubiquitinating and Degrading MdPUB29 Protein in Apple. Plant Cell Physiol. 2019, 60, 2129–2140. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Gao, H.; Zhou, M.; Zhang, Y.; Jiang, H.; Li, Y. The Apple Mitogen-Activated Protein Kinase MdMAPK6 Increases Drought, Salt, and Disease Resistance in Plants. Int. J. Mol. Sci. 2025, 26, 3245. https://doi.org/10.3390/ijms26073245
Li M, Gao H, Zhou M, Zhang Y, Jiang H, Li Y. The Apple Mitogen-Activated Protein Kinase MdMAPK6 Increases Drought, Salt, and Disease Resistance in Plants. International Journal of Molecular Sciences. 2025; 26(7):3245. https://doi.org/10.3390/ijms26073245
Chicago/Turabian StyleLi, Mengru, Huaina Gao, Minmin Zhou, Yali Zhang, Han Jiang, and Yuanyuan Li. 2025. "The Apple Mitogen-Activated Protein Kinase MdMAPK6 Increases Drought, Salt, and Disease Resistance in Plants" International Journal of Molecular Sciences 26, no. 7: 3245. https://doi.org/10.3390/ijms26073245
APA StyleLi, M., Gao, H., Zhou, M., Zhang, Y., Jiang, H., & Li, Y. (2025). The Apple Mitogen-Activated Protein Kinase MdMAPK6 Increases Drought, Salt, and Disease Resistance in Plants. International Journal of Molecular Sciences, 26(7), 3245. https://doi.org/10.3390/ijms26073245




