Breeding D1-Type Hybrid Japonica Rice in Diverse Upland Rainfed Environments
Abstract
:1. Introduction
2. Results
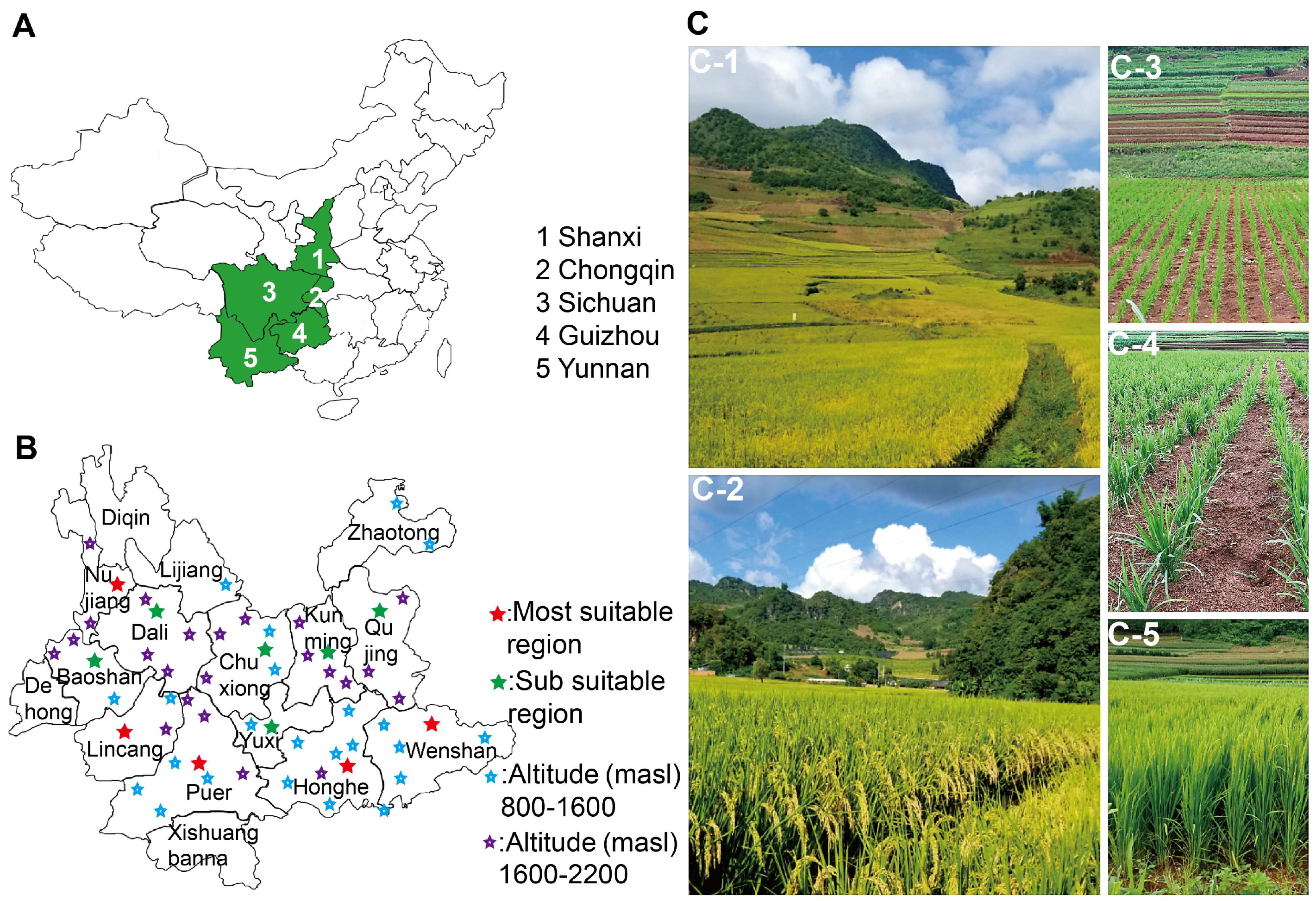
2.1. Ecological Adaptation and Field Performance of ‘DHY615’
| Paddy Field Locations | Altitude (masl) | Yield (t/hm2) | Rainfed Upland Locations | Altitude (masl) | Yield (t/hm2) |
|---|---|---|---|---|---|
| YN, Lincang, Yunxian | 1580 | 8.02 ± 0.78 | YN, Wenshan, Maguan | 1260 | 7.18 ± 0.56 |
| YN, Qujing, Qilin | 2000 | 10.85 ± 1.21 * | YN, Wenshan, Guangnan | 1280 | 7.90 ± 0.25 |
| YN, Qujing, Luliang | 1800 | 12.75 ± 0.45 *** | YN, Kunming, Yiliang | 1801 | 7.20 ± 1.23 |
| YN, Dali, Xiagua | 1970 | 12.15 ± 0.75 *** | YN, Kunming, Xishan | 1905 | 7.56 ± 0.34 |
| YN, Dali, Heqing | 2100 | 11.32 ± 1.66 ** | YN, Kunming, Luquan | 1900 | 7.63 ± 0.67 |
| YN, Dali, Yongping | 1690 | 10.73 ± 0.76 * | YN, Kunming, Shilin | 1870 | 7.64 ± 0.99 |
| YN, Dali, Jianchuan | 2200 | 11.50 ± 0.88 ** | YN, Nujiang, Fugong | 1800 | 8.78 ± 1.26 |
| YN, Kunming, Xundian | 1553 | 11.64 ± 0.92 ** | YN, Nujiang, Lushui | 1637 | 8.97 ± 0.94 |
| YN, Kunming, Yiliang | 1540 | 12.07 ± 1.33 *** | YN, Chuxiong, Yuanmou | 1445 | 7.68 ± 0.92 |
| YN, Wenshan, Maguang | 1337 | 10.28 ± 1.07 | YN, Chuxiong, Lufeng | 1584 | 9.09 ± 1.33 |
| YN, Dehong, Mangshi | 1516 | 9.46 ± 1.23 | YN, Lincang, Linxiang | 1813 | 7.91 ± 0.45 |
| YN, Chuxiong, Lufeng | 1548 | 12.79 ± 1.33 *** | YN, Lincang, Yunxian | 1580 | 8.77 ± 0.72 |
| YN, Hehong, Jianshui | 1700 | 12.27 ± 0.56 *** | YN, Honghe, Hekou | 1075 | 7.71 ± 1.35 |
| YN, Lijiang | 2195 | 10.84 ± 2.1 * | YN, Honghe, Luchun | 1306 | 7.51 ± 2.55 |
| YN, Yuxi, Eshan | 1845 | 11.84 ± 0.12 *** | YN, Honghe, Yuanyang | 1700 | 7.69 ± 1.54 |
| YN, Zhaotong, Ludian | 1850 | 12.23 ± 2.04 *** | YN, Honghe, Kaiyuan | 1450 | 9.65 ± 1.72 |
| YN, Baoshan, Longyang | 1650 | 12.55 ± 0.33 *** | YN, Honghe, Gejiu | 1499 | 8.93 ± 3.29 |
| GZ, Bijie | 1520 | 8.93 ± 0.54 | YN, Puer, Mojiang | 1700 | 7.53 ± 0.36 |
| GZ, Qiannan, Huish | 1400 | 11.13 ± 1.95 ** | YN, Puer, Ninger | 1600 | 9.20 ± 1.79 |
| SC, Liangshan, Xichang | 1480 | 10.86 ± 1.34 * | YN, Puer, Lancang | 1460 | 9.99 ± 1.81 |
| SC, Liangshan, Mianning | 1620 | 12.39 ± 0.76 *** | GZ, Anshun | 2132 | 7.01 ± 0.38 |
| SX, Weinan, Fuping | 934 | 7.47 ± 0.50 | SC, Jingchuan | 1300 | 7.37 ± 1.47 |
| Mean | 11.09 | 8.13 | |||
| SD | 1.44 | 0.85 | |||
| Coefficient variation (%) | 13.05 | 10.43 |
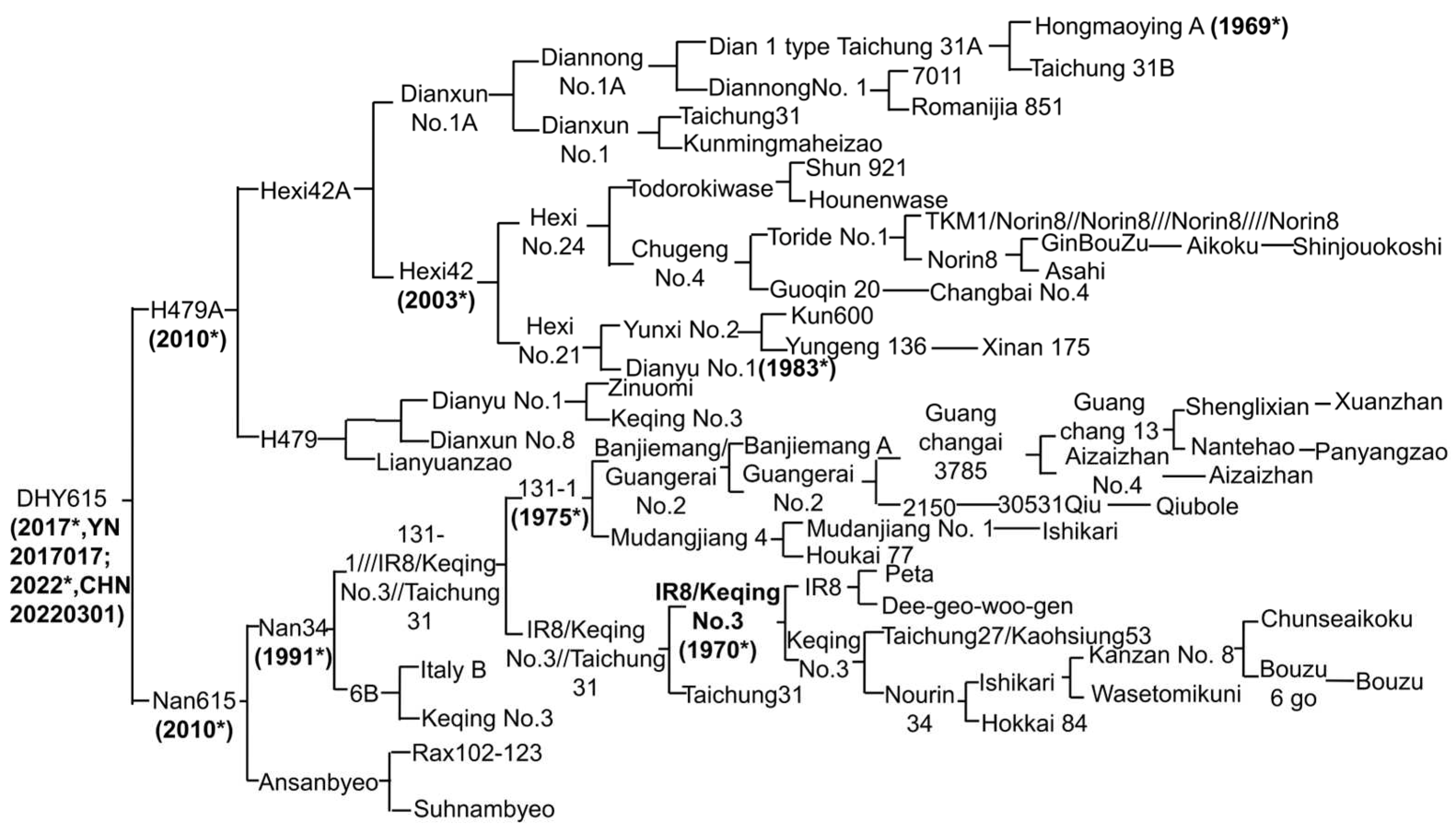
2.2. Genealogy and Phenotypic Characterization of ‘DHY615’
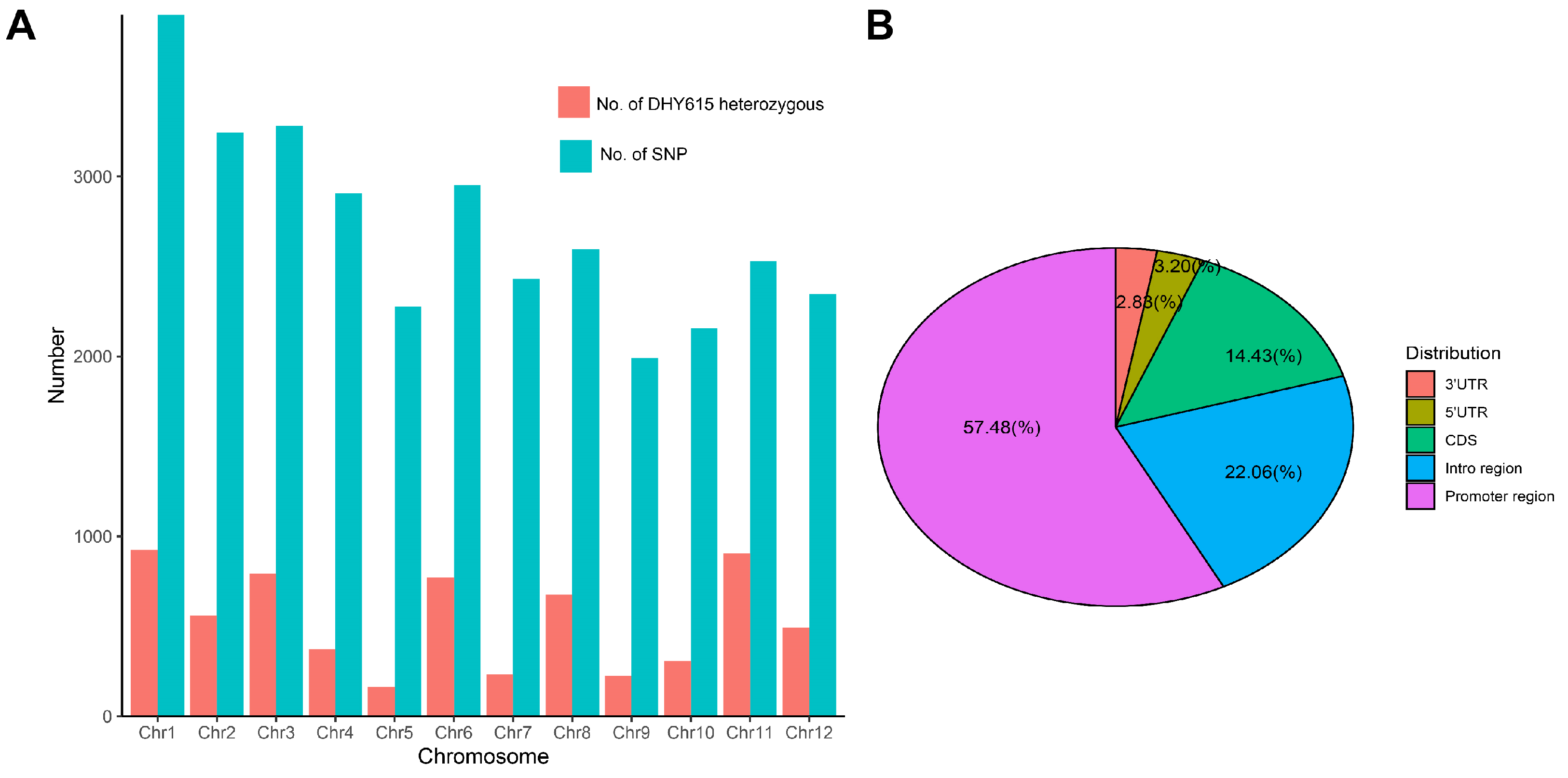
2.3. Indica–Japonica Component and Genomic Differences
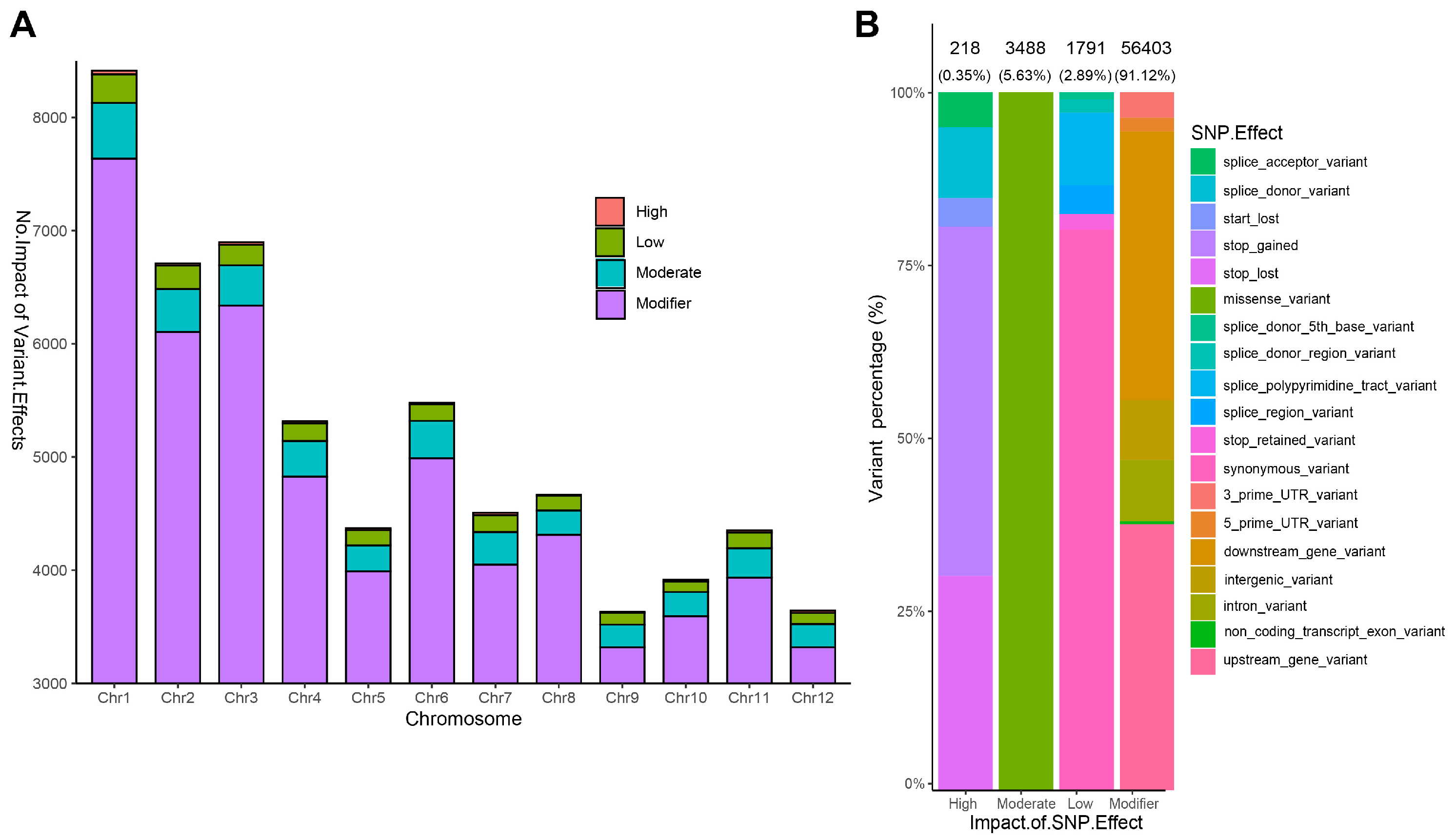
2.4. Functional Impact of SNPs Variations
2.5. Functional Locus Analysis of ‘H479A’, ‘Nan615’, and ‘DHY615’
3. Discussion
4. Materials and Methods
4.1. Research Materials
4.2. Breeding of ‘DHY615’
4.3. Phenotyping and Assessment of Field Performance
4.4. DNA Extraction and High-Density Genome-Wide SNP Microarray Analysis
4.5. Functional Locus and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, J.; Li, Q. Developing Dian-type japonica hybrid rice. China Seed Ind. 2019, 5, 48–49. [Google Scholar] [CrossRef]
- Hua, Y.; Song, X.; Lin, J.; Wu, M. Breeding and application of Yunnan late Japonica rice male sterile line Chunjiang 29A. Hybrid Rice 2019, 34, 12. [Google Scholar]
- Ma, R.; Wang, X.; Lu, Y.; Zhou, H.; Cai, K.; Li, X.; Zhang, Z. Breeding and application of late japonica male sterile line Yongjing 2A and its late indica-japonica hybrid rice combinations. Hybrid Rice 2010, 185–189. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, M. Historical development of Li Zhengyou’s creation of Dian-type hybrid rice. Food Saf. Guide 2017, 76. [Google Scholar]
- Hu, X.; Qian, Q. Revisiting and thinking on research of Japonica hybrid rice in China. China Rice 2021, 27, 9–11. [Google Scholar]
- Xu, Y.; Yu, D.; Chen, J.; Duan, M. A review of rice male sterility types and their sterility mechanisms. Heliyon 2023, 9, e18204. [Google Scholar]
- He, H. Studies on fertility inheritance and sterility mechanism of Dian-type and BT hybrid rice. J. Yunnan Agric. Univ. 1988, 1, 154–168. [Google Scholar] [CrossRef]
- Tan, X.; Tan, Y.; Zhao, Y.; Zhang, X.; Hong, R.; Jin, S.; Liu, X.; Huang, D. Identification of the Rf gene conferring fertility restoration of the CMS Dian-type 1 in rice by using simple sequence repeat markers and advanced inbred lines of restorer and maintainer. Plant Breed. 2004, 123, 338–341. [Google Scholar]
- Luan, J.; Liu, T.; Luo, W.; Liu, W.; Peng, M.; Li, W.; Dai, X.; Liang, M.; Chen, L. Mitochondrial DNA genetic polymorphism in thirteen rice cytoplasmic male sterile lines. Plant Cell Rep. 2013, 32, 545–554. [Google Scholar]
- Zheng, X.; Wei, F.; Cheng, C.; Qian, Q. A historical review of hybrid rice breeding. J. Integr. Plant Biol. 2024, 66, 532–545. [Google Scholar]
- Mei, F.; Wu, X.; Yao, C.; Li, L.; Wang, L.; Chen, Q. Rice Cropping Regionalization in China. Chin. J. Rice Sci. 1988, 2, 97–110. [Google Scholar]
- Cheng, K. Cheng Kansheng’s Selected Papers on Rice Farming. Yunnan Academy of Agricultural Sciences Press: Kunming, China, 1987. [Google Scholar]
- Jiang, Z. Rice Farming in Yunnan; Yunnan Science and Technology Press: Kunming, China, 1995; p. 266. [Google Scholar]
- Chen, R.; Deng, Y.; Ding, Y.; Guo, J.; Qiu, J.; Wang, B.; Wang, C.; Xie, Y.; Zhang, Z.; Chen, J. Rice functional genomics: Decades’ efforts and roads ahead. Sci. China Life Sci. 2022, 65, 33–92. [Google Scholar] [PubMed]
- Xiong, L.; Uga, Y.; Li, Y. Rice functional genomics: Theories and practical applications. Mol. Breed. 2020, 40, 72. [Google Scholar]
- Yu, S.; Ali, J.; Zhou, S.; Ren, G.; Xie, H.; Xu, J.; Yu, X.; Zhou, F.; Peng, S.; Ma, L. From green super rice to green agriculture: Reaping the promise of functional genomic research. Mol. Plant 2022, 15, 9–26. [Google Scholar]
- Zhang, Q. Strategies for developing green super rice. Proc. Natl. Acad. Sci. USA 2007, 104, 16402–16409. [Google Scholar]
- Zhang, Q.; Li, J.; Xue, Y.; Han, B.; Deng, X. Rice 2020: A call for an international coordinated effort in rice functional genomics. Mol. Plant 2008, 1, 715–719. [Google Scholar]
- Thomson, M.J.; Singh, N.; Dwiyanti, M.S.; Wang, D.R.; Wright, M.H.; Perez, F.A.; DeClerck, G.; Chin, J.H.; Malitic-Layaoen, G.A.; Juanillas, V.M. Large-scale deployment of a rice 6K SNP array for genetics and breeding applications. Rice 2017, 10, 40. [Google Scholar]
- Yu, H.; Xie, W.; Li, J.; Zhou, F.; Zhang, Q. A whole-genome SNP array (RICE 6K) for genomic breeding in rice. Plant Biotechnol. 2014, 12, 28–37. [Google Scholar]
- Chen, H.; Xie, W.; He, H.; Yu, H.; Chen, W.; Li, J.; Yu, R.; Yao, Y.; Zhang, W.; He, Y. A high-density SNP genotyping array for rice biology and molecular breeding. Mol. Plant 2014, 7, 541–553. [Google Scholar]
- Huang, X.; Yang, S.; Gong, J.; Zhao, Q.; Feng, Q.; Zhan, Q.; Zhao, Y.; Li, W.; Cheng, B.; Xia, J. Genomic architecture of heterosis for yield traits in rice. Nature 2015, 537, 629–633. [Google Scholar]
- Wang, C.; Wang, Z.; Cai, Y.; Zhu, Z.; Yu, D.; Hong, L.; Wang, Y.; Lv, W.; Zhao, Q.; Si, L.; et al. A higher-yield hybrid rice is achieved by assimilating a dominant heterotic gene in inbred parental lines. Plant Biotechnol. 2024, 22, 1669–1680. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Han, B. Unlocking the mystery of heterosis opens the era of intelligent rice breeding. Plant Physiol. 2024, 196, 735–744. [Google Scholar] [CrossRef] [PubMed]
- NY/T 593-2013; Quality of Edible Rice Varieties. China Agriculture Press: Beijing, China, 2013.
- NY/T 789-2004; Technical Specification for Rice Variety Trials. China Agriculture Press: Beijing, China, 2004.
- Huang, L.; Zhang, H.; Hong, Y.; Liu, S.; Li, D.; Song, F. Stress-responsive expression, subcellular localization and protein-protein interactions of the rice Metacaspase family. Int. J. Mol. Sci. 2015, 16, 16216–16241. [Google Scholar] [CrossRef]
- Li, G.; Wang, Z.; Yokosho, K.; Ding, B.; Fan, W.; Gong, Q.; Li, G.; Wu, Y.; Yang, J.; Ma, J. Transcription factor WRKY22 promotes aluminum tolerance via activation of OsFRDL4 expression and enhancement of citrate secretion in rice (Oryza sativa). New Phytol. 2018, 219, 149–162. [Google Scholar] [CrossRef]
- Meng, W.; Hsiao, A.-S.; Gao, C.; Jiang, L.; Chye, A.-L. Subcellular localization of rice acyl Coa-binding proteins (ACBPs) indicates that OsACBP6: GFP is targeted to the peroxisomes. New Phytol. 2014, 203, 469–482. [Google Scholar] [CrossRef]
- Tran, T.T.T.; Le, L.H.M.; Nguyen, T.T.; Nguyen, T.C.; Hoang, T.T.H.; Do, P.T.; To, H.T.M. QTL-seq identifies genomic region associated with the crown root development under jasmonic acid response. Funct. Integr. Genom. 2024, 24, 141. [Google Scholar] [CrossRef]
- Peng, Y.; Tang, N.; Zou, J.; Ran, J.; Chen, X. Rice MYB transcription factor OsMYB1R1 negatively regulates drought resistance. Plant Growth Regul. 2023, 99, 515–525. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, H.; Huang, H.; Duan, K.; Wu, Z.; Wu, P. Regulation of OsSPX1 and OsSPX3 on expression of OsSPX domain genes and Pi-starvation signaling in rice. J. Integr. Plant Biol. 2009, 51, 663–674. [Google Scholar] [CrossRef]
- Ma, J.; Lei, C.; Xu, X.; Hao, K.; Wang, J.; Cheng, Z.; Ma, X.; Ma, J.; Zhou, K.; Zhang, X. Pi64, encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice. Mol. Plant Microbe Interact. 2015, 28, 558–568. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Q.; Hillwig, M.L.; Peters, R.J. Picking sides: Distinct roles for CYP76M6 and CYP76M8 in rice oryzalexin biosynthesis. Biochem. J. 2013, 454, 209–216. [Google Scholar]
- Yu, S.; Luo, X.; Lian, L.; Xu, H.; Chen, L.; Wei, Y.; Cai, Q.; Xie, H.; Zhang, J. Identification of CIPK family in rice and qRT-PCR analysis on OsCIPK5 induced by Magnaporthe oryzae. Fujian J. Agric. Sci. 2019, 34, 1237–1245. [Google Scholar]
- Zhou, X.; Liao, H.; Chern, M.; Yin, J.; Chen, Y.; Wang, J.; Zhu, X.; Chen, Z.; Yuan, C.; Zhao, W.; et al. Loss of function of a rice TPR-domain RNA-binding protein confers broad-spectrum disease resistance. Proc. Natl. Acad. Sci. USA 2018, 115, 3174–3179. [Google Scholar] [PubMed]
- Raghuvanshi, R.; Srivastava, A.K.; Verulkar, S.; Suprasanna, P. Unlocking allelic diversity for sustainable development of salinity stress tolerance in rice. Curr. Genom. 2021, 22, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Sato, T.; Hidema, J.; Hirouchi, T.; Yamamoto, K.; Kumagai, T.; Yano, M. qUVR10, a major quantitative trait locus for Ultraviolet-B resistance in rice, encodes cyclobutane pyrimidine dimer photolyase. Genetics 2005, 171, 1941–1950. [Google Scholar] [CrossRef]
- Zhou, H.; Li, P.; Xie, W.; Hussain, S.; Li, Y.; Xia, D.; Zhao, H.; Sun, S.; Chen, J.; Ye, H. Genome-wide association analyses reveal the genetic basis of stigma exertion in rice. Mol. Plant 2017, 10, 634–644. [Google Scholar]
- Matsubara, K.; Ogiso-Tanaka, E.; Hori, K.; Ebana, K.; Ando, T.; Yano, M. Natural variation in Hd17, a homolog of Arabidopsis ELF3 that is involved in rice photoperiodic flowering. Plant Cell Physiol. 2012, 53, 709–716. [Google Scholar] [CrossRef]
- Adegoke, T.V.; Wang, Y.; Chen, L.; Wang, H.; Liu, W.; Liu, X.; Cheng, Y.-C.; Tong, X.; Ying, J.; Zhang, J. Posttranslational modification of waxy to genetically improve starch quality in rice grain. Int. J. Mol. Sci. 2021, 22, 4845. [Google Scholar] [CrossRef]
- Hori, K.; Suzuki, K.; Ishikawa, H.; Nonoue, Y.; Nagata, K.; Fukuoka, S.; Tanaka, J. Genomic regions involved in differences in eating and cooking quality other than Wx and Alk genes between indica and japonica Rice cultivars. Rice 2021, 14, 8. [Google Scholar] [CrossRef]
- Tamaki, S.; Matsuo, S.; Wong, H.; YoKol, S.; Shimamoto, K. Hd3a protein is a mobile flowering signal in Rice. Science 2007, 316, 1033–1036. [Google Scholar]
- Xie, Y.; Niu, B.; Long, Y.; Li, G.; Tang, J.; Zhang, Y.; Ren, D.; Liu, Y.-G.; Chen, L. Suppression or knockout of SaF/SaM overcomes the Sa-mediated hybrid male sterility in rice. J. Integr. Plant Biol. 2017, 59, 669–679. [Google Scholar] [CrossRef]
- Zhu, Y.; Ellstrand, N.C.; Lu, B. Sequence polymorphisms in wild, weedy, and cultivated rice suggest seed-shattering locus sh4 played a minor role in Asian rice domestication. Ecol. Evol. 2012, 2, 2106–2113. [Google Scholar] [PubMed]
- Li, L.; Ye, L.; Kong, Q.; Shou, H. A vacuolar membrane Ferric-Chelate Reductase, OsFRO1, alleviates Fe toxicity in rice (Oryza sativa L.). Front. Plant Sci. 2019, 10, 700. [Google Scholar]
- Pidon, H.; Chéron, S.; Ghesquière, A.; Albar, L. Allele mining unlocks the identification of RYMV resistance genes and alleles in African cultivated rice. BMC Plant Biol. 2020, 20, 222. [Google Scholar]
- Tong, J.; Han, Z.; Han, A.; Liu, X.; Zhang, S.; Fu, B.; Hu, J.; Su, J.; Li, S.; Wang, S.; et al. Sdt97: A point mutation in the 5′ untranslated region confers Semi-dwarfism in rice. G3-Genes Genom. Gene. 2016, 6, 1491–1502. [Google Scholar]
- Wang, T.; Li, J.; Luo, D.; Zhu, Q.; Wang, C.; Liu, F.; Li, X.; Li, J.; Su, P.; He, Y.; et al. Population genetic structure analysis of japonica rice in Yunnan Plateau based on 56K SNP chip. Mol. Plant Breed. 2024, 22, 5630–5639. [Google Scholar]
- Ji, H.; Shin, Y.; Lee, C.; Oh, H.; Yoon, I.S.; Baek, J.; Cha, Y.-S.; Lee, G.-S.; Kim, S.L.; Kim, K.-H. Genomic variation in Korean japonica rice varieties. Genes 2021, 12, 1749. [Google Scholar] [CrossRef]
- Hu, B.; Wang, W.; Ou, S.; Tang, J.; Li, H.; Che, R.; Zhang, Z.; Chai, X.; Wang, H.; Wang, Y. Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat. Genet. 2015, 47, 834–838. [Google Scholar]
- Liu, C.; Wang, Z.; Yu, J.; Zhang, Y.; Zhu, S.; Li, M.; Tan, Y.; Xu, j. The molecular basis of rice fragrance in the high quality Japonica hybrid rice variety Dianheyou34. J. Yunnan Univ. Nat. Sci. Ed. 2020, 35, 371–376. [Google Scholar]
- Luo, D.; Wang, T.; Li, J.; Zhu, Q.; He, Y.; Li, J.; Jiang, C.; Huang, D.; Chen, L.; Lee, D. Identification of drought resistance and difference analysis of related gene loci of japonica rice in Yunnan Plateau. Mol. Plant Breed. 2024, 22, 6033–6045. [Google Scholar]
- Li, X.; Zhang, S.; Wang, H.; Cai, X.; Zhou, Q.; Zhou, N. Effects of different planting methods on yield, appearance quality and economic benefit of water-saving and drought-resistant rice. Jiangsu Agric. Sci. 2021, 49, 78–82. [Google Scholar]
- Luo, L.; Mei, H.; Yu, X.; Xia, H.; Chen, L.; Liu, H.; Zhang, A.; Xu, K.; Wei, H.; Liu, G. Water-saving and drought-resistance rice: From the concept to practice and theory. Mol. Breed. 2019, 39, 145. [Google Scholar]
- DB53/T 1318.1-2024; Technical specifications for highland japonica rice variety testing—Part 1: Regional trials. Yunnan Provincial Standard: Kunming, China, 2024.
- DB42/T 1404-2018; Technical specifications for rice variety field trials. Standard Press of Hubei: Wuhan, China, 2018.
- NY/T 2863-2015; Technical code of practice for regional field trials of rice varieties. China Agriculture Press: Beijing, China, 2015.
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Yang, H.; Wang, K. Genomic variant annotation and prioritization with ANNOVAR and wANNOVAR. Nat. Protoc. 2015, 10, 1556–1566. [Google Scholar] [PubMed]
- Sakai, H.; Lee, S.S.; Tanaka, T.; Numa, H.; Kim, J.; Kawahara, Y.; Wakimoto, H.; Yang, C.C.; Iwamoto, M.; Abe, T.; et al. Rice Annotation Project Database (RAP-DB): An integrative and interactive data-base for rice genomics. Plant Cell Physiol. 2013, 54, e6. [Google Scholar]


| Gene Name | Gene ID | Trait | No. of Variation Sites | Reference |
|---|---|---|---|---|
| OsMC6 | LOC_Os01g58580 | abiotic stress and biotic stress | 1 | [27] |
| OsWRKY22 | LOC_Os01g60490 | abiotic stress and biotic stress | 1 | [28] |
| OsACBP3 | LOC_Os03g37960 | cold tolerance and blast disease | 1 | [29] |
| OsPUB58 | LOC_Os10g40120 | drought and cold tolerance | 1 | [30] |
| OsMYB1R1 | LOC_Os04g49450 | drought resistance | 1 | [31] |
| OsSPX1 | LOC_Os06g40120 | cold tolerance | 1 | [32] |
| Pi64 | LOC_Os01g57280 | leaf blast disease resistance | 1 | [33] |
| CYP76M6 | LOC_Os02g36280 | insect resistance | 1 | [34] |
| OsCIPK05 | LOC_Os01g10890 | blast disease resistance | 1 | [35] |
| OsBSR-K1 | Os10g0548200 | blast disease | 1 | [36] |
| Trait | Gene | Probe Type | Chr | Representative Variety | Phenotype | H479A | Nan615 | DHY615 |
|---|---|---|---|---|---|---|---|---|
| TQ | BADH2 (fgr) | SNP | 8 | Suyunuo | Fragrance | √ | √o | |
| ABS | SKC1 | SNP | 1 | Nona Bokra | Salt resistant | √ | √o | |
| ABS | qUVR-10 | SNP | 10 | Sasanishiki | Enhanced phytoremediation activity | √ | √o | |
| ABS | NTR1.1B | SNP | 10 | 9311 | Enhanced nitrogen uptake | √ | √o | |
| BS | STV11 | SNP | 11 | Kasalath | Persistent resistance to rice stripe virus | √ | √o | |
| TQ | OsAAP6 | INDEL | 1 | 9311 | High protein | √ | √o | |
| TQ | GS3 | SNP | 3 | Minghui 63 | Long grain | √ | √o | |
| HD | Hd17 | SNP | 6 | Koshihikari | Delayed flowering | √ | √o | |
| HD | Os11g08410 | SNP | 11 | Haplotype C | Delayed heading | √ | √o | |
| TQ | Waxy | SNP | 6 | Nipponbare | Increase the branched starch content | √ | √ | √ |
| TQ | ALK | SNP | 6 | Minghui 63 | Long branched chain starch and higher pasting temperature | √ | √ | √ |
| HD | Os01g62780 | SNP | 1 | Haplotype B | Delayed heading | √ | √ | √ |
| HD | Hd3a | SNP | 6 | Nipponbare | Photoperiod-sensitive genes | √ | √ | √ |
| FE | SaF | SNP | 1 | Nipponbare | Wide affinity | √ | √ | √ |
| OT | sh4 | SNP | 4 | Nipponbare | Non-shattering | √ | √ | √ |
| ABS | OsFRO1 | SNP | 4 | KDML105 | Iron tolerance | √ | √ | √ |
| BS | Rymv1 | SNP | 4 | Nipponbare | Resistance yellow mottle virus disease | √ | √ | √ |
| PA | Sdt97 | SNP | 6 | Y98149 | Semidwarf | √ | √ | √ |
| PA | Sd-1 | INDEL | 1 | DGWG-type | Semidwarf | √ | ||
| TQ | qSW5/GW5 | INDEL | 5 | Nipponbare | Increase grain width | √ | ||
| ABS | SUB1A | SNP | 9 | FR13A | Flood-resistant | o | o | √o |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Li, J.; Zhu, Q.; Li, J.; Zhang, C.; Hong, R.; Huang, D.; Zhang, Z.; Xu, J.; Li, D.; et al. Breeding D1-Type Hybrid Japonica Rice in Diverse Upland Rainfed Environments. Int. J. Mol. Sci. 2025, 26, 3246. https://doi.org/10.3390/ijms26073246
Wang C, Li J, Zhu Q, Li J, Zhang C, Hong R, Huang D, Zhang Z, Xu J, Li D, et al. Breeding D1-Type Hybrid Japonica Rice in Diverse Upland Rainfed Environments. International Journal of Molecular Sciences. 2025; 26(7):3246. https://doi.org/10.3390/ijms26073246
Chicago/Turabian StyleWang, Chunli, Juan Li, Qian Zhu, Junjie Li, Cui Zhang, Ruke Hong, Dajun Huang, Zhonglin Zhang, Jin Xu, Dandan Li, and et al. 2025. "Breeding D1-Type Hybrid Japonica Rice in Diverse Upland Rainfed Environments" International Journal of Molecular Sciences 26, no. 7: 3246. https://doi.org/10.3390/ijms26073246
APA StyleWang, C., Li, J., Zhu, Q., Li, J., Zhang, C., Hong, R., Huang, D., Zhang, Z., Xu, J., Li, D., Wen, J., Li, C., Zhu, Y., Lee, D., & Chen, L. (2025). Breeding D1-Type Hybrid Japonica Rice in Diverse Upland Rainfed Environments. International Journal of Molecular Sciences, 26(7), 3246. https://doi.org/10.3390/ijms26073246






